Abstract
Alexithymia and increased interoceptive awareness have been associated with affective disorders as well as with altered insula and anterior cingulate cortex (ACC) function. Brain imaging studies have demonstrated an association between neurotransmitter function and affective disorders as well as personality traits. Here, we first examined the relationship between alexithymic facets as assessed with the Toronto Alexithymia Scale (TAS-20) and interoceptive awareness (assessed with the Body Perception Questionnaire) in 18 healthy subjects. Second, we investigated their association with glutamate and gamma-aminobutyric acid (GABA) concentrations in the left insula and the ACC using 3-Tesla proton magnetic resonance spectroscopy. Behaviorally, we found a close association between alexithymia and interoceptive awareness. Furthermore, glutamate levels in the left insula were positively associated with both alexithymia and awareness of autonomic nervous system reactivity, while GABA concentrations in ACC were selectively associated with alexithymia. Although preliminary, our results suggest that increased glutamate-mediated excitatory transmission—related to enhanced insula activity—reflects increased interoceptive awareness in alexithymia. Suppression of the unspecific emotional arousal evoked by increased awareness of bodily responses in alexithymics might thus be reflected in decreased neuronal activity mediated by increased GABA concentration in ACC.
Keywords: alexithymia, interoceptive awareness, neurotransmitter, proton magnetic resonance spectroscopy
INTRODUCTION
The biological underpinnings of individual differences in personality traits are incompletely understood. Although there have been several functional imaging studies investigating the neuronal activity signatures of personality traits (Johnson et al., 1999; Canli et al., 2001, Canli and Amin, 2002; Canli, 2004; Kumari et al., 2004; Deckersbach et al., 2006; Vaidya et al., 2007; Simon et al., 2010; Brühl et al., 2011), less is known about their association with neurotransmitter concentrations. Specific personality traits such as low extraversion, high neuroticism (Watson and Clark, 1997; Kotov et al., 2010) and alexithymia (Luminet, 2010; Leweke et al., 2012) have been linked to increased vulnerability to psychiatric disorders, specifically affective disorders, which are in turn associated with dysfunctional neurotransmission (Mathew et al., 2008; Sanacora et al., 2008; Walter et al., 2009; Hashimoto et al., 2010; Grimm et al., 2012a). Of specific relevance with regard to affective disorders are findings of a negative correlation between prefrontal glutamate (Glu) concentrations, mental perspective taking and extraversion (Montag et al., 2008; Grimm et al., 2012b). In addition, decreased and increased ACC gamma-aminobutyric acid (GABA) concentrations, respectively, have been associated with extraversion and harm avoidance (Kim et al., 2009; Goto et al., 2010) in healthy subjects. Thus, the investigation of alexithymia might be promising, since it is marked by cognitive and affective features including difficulties in identifying and describing feelings as well as in distinguishing feelings from bodily sensations of emotional arousal (Franz et al., 2008). Alexithymics can be characterized by low extraversion, high neuroticism, high harm avoidance, low self-directedness and low perspective taking (Wise et al., 1992; Guttman and Laporte, 2002; Picardi et al., 2005). This personality trait is prevalent in 10% of the general population (Linden et al., 1995; Salminen et al., 1999) and its facets have been identified as a risk factor for affective disorders (Conrad et al., 2009; Luminet, 2010; Leweke et al., 2012). Results from neuroimaging studies indicate a crucial role of insula and ACC in mediating alexithymic features. In both regions, heterogeneous findings with either increased (Berthoz et al., 2002; Mériau et al., 2006; Frewen et al., 2008; Karlsson et al., 2008; Heinzel et al., 2010) or decreased (Leweke et al., 2004; Karlsson et al., 2008; Silani et al., 2008; Bird et al., 2010; Reker et al., 2010) response to emotion stimuli have been reported in alexithymic individuals. Data on structural changes in alexithymia are inconsistent, with studies reporting a correlation between alexithymia and the size of the right ACC (Gündel et al., 2004), smaller volumes of ACC, medial temporal gyrus and anterior insula in alexithymic women (Borsci et al., 2009) as well as no volume difference in alexithymic men (Heinzel et al., 2012). Reduced gray matter volume in the ACC has been recently associated with an interaction between two polymorphisms on the BDNF and DRD2/ANKK1 gene (Montag et al., 2010) which in turn are also associated with alexithymia (Walter et al., 2011). Insula and ACC are implicated in processing of affect, self-awareness and mood and show functional alterations in affective disorders (Bush et al., 2000; Mayberg, 2003; Phillips et al., 2003; Grimm et al., 2009, 2011; Horn et al., 2010; Wiebking et al., 2010). A recently proposed model by Medford and Critchley (2010) states that the conjoint activity of insula and ACC is crucial for the production of subjective feelings and co-ordinating appropriate responses to internal and external stimuli, thereby providing the neural basis of self-awareness. The insula also has a central role in attention to interoceptive states (Critchley et al., 2004; Pollatos et al., 2007; Menon and Uddin, 2010; Terasawa et al., 2011; Simmons et al., 2012). Interoceptive awareness at least partially mediates emotional experience (Bechara et al., 1996; Pollatos et al., 2005; Werner et al., 2009; Dunn et al., 2010) and the degree of interoceptive awareness might modulate the emotional experience (Wiens and Palmer, 2001; Critchley et al., 2004; Werner et al., 2009). In affective disorders, studies have reported alterations in interoceptive awareness and insula activity (Wiebking et al., 2011). Furthermore, glutamate concentrations in ACC predict the resting-state functional connectivity between insula and ACC in depressed patients (Horn et al., 2010). The ACC is not only crucial for emotion processing in general (Bush et al., 2000; Kober et al., 2008) but also for constituting our sense of self and consecutively for becoming aware of one’s self (McKiernan et al., 2006; Northoff et al., 2006). A recent study by Liemburg et al. (2012) reported lower connectivity in anterior cortical midline structures including the ACC in alexithymic subjects. Functional alterations, as reflected in reduced negative BOLD responses in these regions during emotional and self-related processing, have been associated with increased self-focus of depressive patients (Grimm et al., 2009, 2011). Negative BOLD responses in ACC are mediated by GABA in healthy subjects (Northoff et al., 2007), but by glutamate in depressive patients (Walter et al., 2009).
Despite the specific role of both insula and ACC in alexithymia as well as in interoceptive awareness and the behavioral relevance of altered neurotransmission in these regions, the respective relationships have not been elucidated yet. Therefore, we first aimed to investigate the association of interoceptive awareness with alexithymic features. Second, we aimed to examine the association between alexithymic features, interoceptive awareness and glutamate and GABA concentrations in the left insula and ACC of healthy subjects. We hypothesized a relationship between interoceptive awareness and alexithymic features. For the insula, we predicted an association between increased interoceptive awareness, alexithymic features and glutamate concentration. Based on reports of alterations in ACC responses in alexithymia (Berthoz et al., 2002; Karlsson et al., 2008; Heinzel et al., 2010) and findings showing that ACC signal changes are mediated by GABA (Northoff et al., 2007), we predicted alexithymic features to be associated with GABA concentrations in ACC.
MATERIALS AND METHODS
Subjects
Healthy subjects [13 women and 9 men, mean age 27.12 (s.d. 7.6)] were recruited through online study advertisements. Exclusion criteria were major medical illnesses, histories of seizures, head trauma with loss of consciousness and pregnancy. In addition, subjects who met criteria for any psychiatric or neurologic disorder or had a history of substance dependence were excluded from the study. All subjects were right-handed as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). The study was performed in accordance with the latest version of the Declaration of Helsinki and approved by the State of Zurichs’ Review Board. All subjects gave written informed consent. Subjects were investigated with proton magnetic resonance spectroscopy (1H-MRS), the 20-item Toronto Alexithymia Scale (TAS-20; Bagby et al., 1994a, German version by Bach et al., 1996) and the Body Perception Questionnaire (BPQ; Porges, 1993). The TAS-20 is a self-administered questionnaire that captures two affective and one cognitive alexithymic facets, respectively: difficulties identifying feelings (e.g. I am often confused about what emotion I am feeling), difficulties describing feelings (DDF, e.g. it is difficult for me to find the right words for my feelings) and a concrete, externally oriented thinking (EOT) style (e.g. being in touch with emotion is essential; inverted item). The scale has a good psychometric quality (Cronbach’s α > 0.80; Bagby et al., 1994b) and is widely used in emotion research, so that comparability with previous studies is assured. Its psychometric properties have previously been investigated in healthy subjects (Franz et al., 2008). Each item of the TAS-20 is rated on a five-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). The BPQ (Porges, 1993) is a 96 item self-report instrument to assess body perception and interoceptive awareness on four subscales [awareness subscale: subjects are asked to imagine how aware they are of their bodily processes (e.g. swallowing frequently); stress response: subjects are asked to imagine being in a very stressful situation and rate their bodily changes due to that situation (e.g. emotional problems such as more frequent feelings of depression, frustration, rage or anger); autonomic nervous system reactivity: requires that subjects answer items about their own autonomous nervous system reactions (e.g. ‘my heart often beats irregularly’); stress style subscale: evaluates the manner in which the subject responds to stress (e.g. ‘I have difficulty speaking’)]. Each item is rated on a five-point Likert scale ranging from 1 (never) to 5 (always).
Spectroscopic data acquisition and analysis
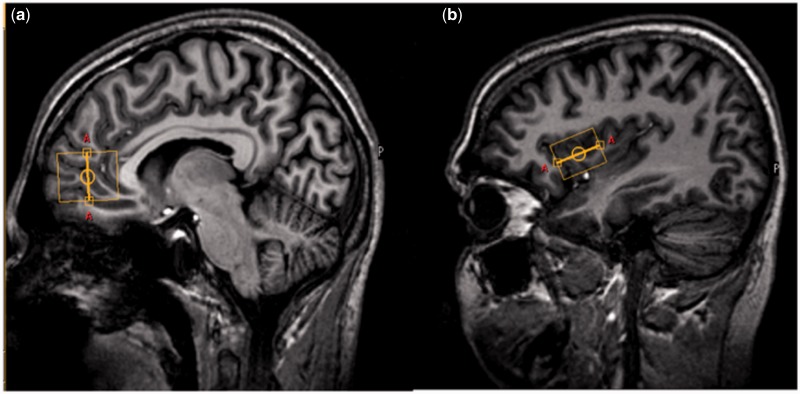
Single voxel 1H-MRS data were acquired at rest from two volumes of interest (VOI) in each subject using a Philips Achieva 3-T whole-body MR unit (Philips Medical Systems, Best, The Netherlands) equipped with a birdcage transmit-receive head coil. One VOI of 32 × 21 × 24 mm3 = 16.128 ml was placed in the left insula, while a second one (25 × 25 × 25 mm3 = 15.625 ml) was placed in the ACC (Figure 1). To enable an unambiguous measurement of metabolites, data from each VOI were acquired using a 2D JPRESS sequence (Schulte and Boesiger, 2006), which encodes the J coupling along the indirect spectral dimension by acquiring data with multiple echo times. This approach allows for a significant reduction of spectral overlap by spreading multiplet resonances along two frequency axes. The sequence was preceded by water suppression using frequency-selective excitation and gradient spoiling followed by adiabatic frequency-selective rephasing and gradient spoiling. The echo times for the JPRESS experiment ranged from 28 to 228 ms with a step size of 2 ms and a phase cycling of 16 for each TE. Other parameters included a bandwidth in the direct dimension of 2 kHz and 2048 sample points. Using 100 encoding steps and eight averages per encoding step at a repetition time of TR = 2000 ms, the acquisition time for one voxel accounted to 24 min. JPRESS data were quantified using ProFit (Schulte and Boesiger, 2006), a two-dimensional fitting procedure, which applies the full amount of prior knowledge by fitting a linear combination of simulated two-dimensional basis metabolite spectra. Simulation of the basis metabolite spectra was performed with GAMMA (Smith et al., 1994). Cramer-Rao lower bounds, an estimate of the fitting error, were used as a quality criterion to exclude data sets with unreliable quantification results. Hence, analyses were restricted to subjects who met strict quality criteria to indicate reliable spectral quantification (Cramer-Rao lower bounds 20%) for each metabolite and four subjects had to be excluded from the analysis. Because determination of absolute metabolite concentrations in millimolars requires a reliable T1 and T2 relaxation correction, while relaxation times of coupled metabolites are hardly known for spectroscopy at 3-T, all metabolite concentrations are given relative to creatine levels. Creatine was proven to be an appropriate internal reference for the ProFit analysis (Schulte and Boesiger, 2006).
Fig. 1.
Placement of the magnetic resonance spectroscopic voxel (orange frame) in (a) the ACC and (b) the left insula.
Statistical analyses
Statistical calculations were carried out as indicated in the ‘Results’ section using SPSS for Windows (Release 18.0; SPSS, Inc., Chicago, IL, USA). Within-group comparisons were performed using paired t-tests. Pearson correlation coefficients were computed to assess the relationship between neurotransmitter concentrations in both regions and the association between these concentrations and TAS-20 and BPQ scores. In an exploratory analysis, median split was used to create two groups of subjects with high and low scores on the TAS-20. Independent-sample t-tests were performed to analyze group differences between these subjects. Bonferroni corrections were used to counteract the problem of multiple comparisons. All tests were performed at a two-tailed level of significance of 5%.
RESULTS
Behavioral data
TAS-20 and BPQ total scores and subscores are summarized in Table 1. The total score of the TAS-20 correlated significantly with the BPQ total score (r = 0.70, P < 0.01) as well as with the BPQ subscales for awareness (r = 0.55, P < 0.05), stress response (r = 0.73, P < 0.05), autonomic nervous system reactivity (r = 0.65, P < 0.05; Figure 2) and stress style (r = 0.66, P < 0.05). There were no effects of age and gender on TAS-20 and BPQ scores.
Table 1.
Questionnaire data
| Mean (s.d.; range) | |
|---|---|
| TAS total Score | 38.33 (7.88; 23–51) |
| TAS—DIF Score | 12.39 (5.03; 7–23) |
| TAS—DDF Score | 10.61 (2.81; 6–17) |
| TAS—EOT Score | 15.11 (3.55; 8–22) |
| BPQ total Score | 172.83 (34.94; 117–223) |
| BPQ—Awareness Score | 1.84 (0.48; 1–2.56) |
| BPQ—Stress Response Score | 2.12 (.62; 1.4–3.3) |
| BPQ—Autonomic Nervous System Reactivity Score | 1.41 (0.27; 1.07–1.93) |
| BPQ—Stress Style Score | 2.53 (0.33; 2.08–3) |
TAS, Toronto Alexithymia Scale; DIF, Difficulty Identifying Feelings; DDF, Difficulty Describing Feelings; EOT, Externally Oriented Thinking; BPQ, Body Perception Questionnaire.
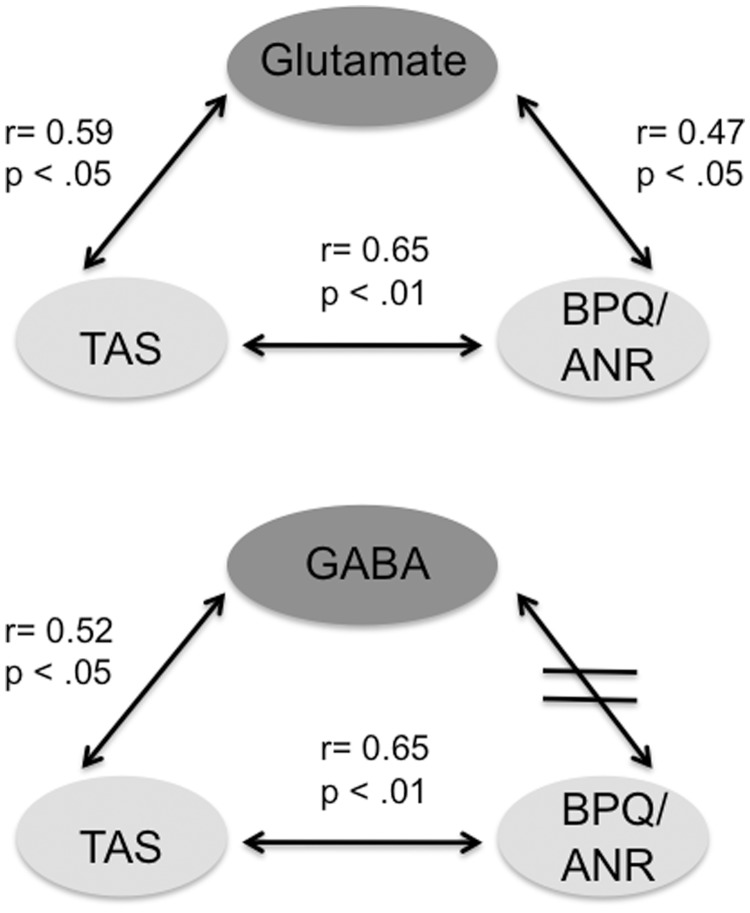
Fig. 2.
Schematic representation of correlations between functional (dark gray) and behavioral markers (light gray) in (a) the ACC and (b) the left insula. There is a significant correlation between alexithymia (TAS-20) and awareness of autonomic nervous system reactivity (BPQ-ANS). Glutamate levels in left insula are positively associated with both alexithymia and awareness of autonomic nervous system reactivity, whereas GABA concentrations in ACC are associated with alexithymia, but not with interoceptive awareness. TAS-20, Toronto Alexithymia Scale; BPQ, Body Perception Questionnaire; ANS, Awareness of Autonomous Nervous System Reactivity and ACC, anterior cingulate cortex.
MRS data
First, concentrations of glutamate (Glu) and GABA did not differ significantly between the two investigated regions (Table 2). Glu concentrations in both regions were correlated (r = 0.74, P < 0.01), whereas GABA concentrations were not (r = 0.33, P > 0.05). Regarding the association of neurotransmitters with alexithymia and measures of sensitivity for bodily processes, we found a significant positive correlation between TAS total score and Glu concentrations in insula (r = 0.59, P < 0.05; Figure 2), which was mainly due to a strong correlation with the DDF (r = 0.49, P < 0.05) and EOT (r = 0.53, P < 0.05) subscores.
Table 2.
Metabolite concentrations in insula and ACC
| Left insula | ACC | |
|---|---|---|
| Glu/Cr | 1.62 (±0.32) | 1.59 (±0.26) |
| GABA/Cr | 0.23 (±0.07) | 0.25 (±0.06) |
ACC, anterior cingulate cortex; Glu, glutamate; GABA, gamma-aminobutyric acid.
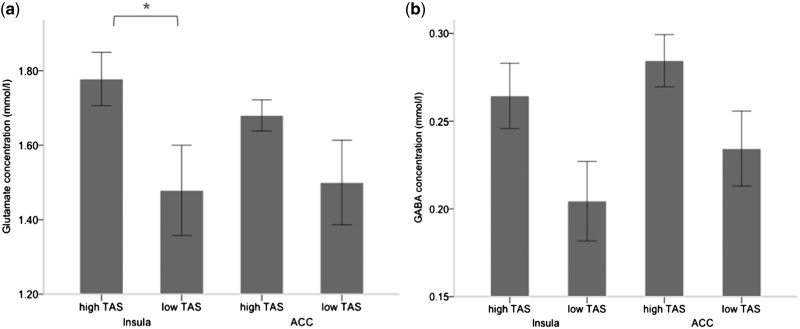
Furthermore, there was a significant positive correlation between TAS total score and GABA concentrations in ACC (r = 0.52, P < 0.05; Figure 2), which was mainly due to a strong correlation with the DDF subscore (r = 0.57, P < 0.05). The BPQ subscore for autonomic nervous system reactivity also correlated with Glu concentration in insula (r = 0.47, P < 0.05), whereas no correlation was found with GABA concentrations in ACC (Figure 2). In an exploratory analysis, we investigated differences between subjects scoring high and low, respectively, on the TAS-20. High scoring subjects not only showed significantly higher BPQ scores (total score: P < 0.01; awareness score: P < 0.05; autonomic nervous system reactivity: P < 0.01) but also significantly higher Glu concentrations in insula, but not in ACC (P < 0.05). Strikingly, these subjects also showed a higher GABA concentration in ACC, but not in insula (P < 0.07; Figure 3).
Fig. 3.
Bar diagrams show (a) glutamate and (b) GABA concentrations in ACC and insula in subjects scoring high and low, respectively, on the TAS-20. Differences in glutamate concentration between these two groups were observed in insula (P < 0.05), but not ACC, whereas differences in GABA concentration were observed in ACC (P < 0.07), but not in insula. TAS-20, Toronto Alexithymia Scale; ACC, anterior cingulate cortex. *P < 0.05.
DISCUSSION
The main goal of this study was to test the interconnection between alexithymic features, interoceptive awareness and concentrations of Glu and GABA in ACC and insula. As hypothesized, alexithymia was closely related to the different facets of interoceptive awareness. Glu levels in left insula and GABA concentrations in ACC were positively associated with alexithymia. Furthermore, there was a double dissociation of GABA and Glu concentrations in insula and ACC as a function of alexithymia: subjects scoring high on the TAS-20 showed high Glu concentration in insula, but not in ACC and high GABA concentration in ACC, but not in insula. Finally, Glu levels in left insula, but not in ACC, were positively associated with the awareness of autonomic nervous system reactivity.
The difficulty in distinguishing feelings from bodily sensations of emotional arousal is a hallmark of alexithymia (Taylor, 2000). Even though previous data suggest a close association between alexithymia and altered interoceptive awareness, this was mainly concluded from the diminished insula response to emotional stimuli in alexithymics (Silani et al., 2008; Reker et al., 2010). Our results complement findings suggesting a significant relationship between interoceptive processes and subjective emotional experience (Wiens and Palmer, 2001; Critchley et al., 2004; Werner et al., 2009) by showing increased interoceptive awareness in subjects with more pronounced alexithymic features. However, we applied a subjective measure of body awareness (Porges, 1993), while previous studies often used interoceptive accuracy, as quantified by measuring an individual’s ability to accurately perceive the own heartbeat (Cameron, 2001; Critchley et al., 2004; Pollatos et al., 2005) as an indicator for interoceptive awareness. Interoceptive accuracy is related to both insula volume and function as well as to subjective body awareness (Critchley et al., 2004). Specifically the awareness of autonomic system reactivity has been shown to predict interoceptive accuracy, which emphasizes the convergence between neurological and subjective traits associated with interoception (Fairclough and Goodwin, 2007). A central role for insula in interoceptive awareness has been suggested by numerous neuroimaging studies (Critchley et al., 2004; Pollatos et al., 2007; Menon and Uddin, 2010; Terasawa et al., 2011; Simmons et al., 2012), whereas previous studies in alexithymia linked decreased insula response to reduced emotional awareness (Silani et al., 2008; Bird et al., 2010). However, these studies investigated the response to emotional stimuli, whereas interoceptive awareness, at least in healthy subjects, has been shown to increase insula activity (Critchley et al., 2004; Terasawa et al., 2011). Alexithymics show increased vulnerability to affective disorders, where studies have reported increased interoceptive awareness and insula activity, which might reflect the patients’ inability to shift the focus of perception/awareness from the own body to the environment (Grimm et al., 2009; Wiebking et al., 2010). Our results show a close association between alexithymia and awareness of bodily and stress responses, which are in turn related to Glu concentration in insula. Elevated Glu levels in insula have been shown in acute and chronic pain and been discussed as an indicator for amplified interoceptive sensory processing (Harris et al., 2008; Gussew et al., 2010; Gutzeit et al., 2011), which is supported by our finding of a relationship between insula Glu concentration and awareness of autonomic nervous system reactivity. High interoceptive awareness, which in turn might be related to a Glu-mediated increase in insula activity, has been associated with higher emotional arousal (Wiens and Palmer, 2001; Pollatos et al., 2005). Likewise, the positive association reported between insula Glu concentration and alexithymia might reflect enhanced insula activity in alexithymia related to increased Glu-mediated excitatory transmission. The role of insula Glu in alexithymia is further emphasized by the region-specific elevation in Glu levels in subjects scoring high on the TAS-20.
Thus, we propose that the interconnection observed between alexithymia, interoceptive awareness and Glu concentration in insula reflects increased awareness of bodily and stress responses as well as enhanced insula activity due to increased Glu-mediated excitatory transmission, which in turn might lead to a high unspecific arousal in alexithymic subjects. To the best of our knowledge, no studies have yet investigated the modulation of alexithymic features by neurotransmitter concentrations. However, alexithymics show increased vulnerability to affective disorders (Luminet, 2010; Leweke et al., 2012), which are characterized by dysfunctional Glu- and GABA-ergic neurotransmission (Sanacora et al., 2008; Mathew et al., 2008; Walter et al., 2009; Hashimoto et al., 2010; Grimm et al., 2012; Scheidegger et al., 2012). Furthermore, alexithymics show low extraversion, high neuroticism, high harm avoidance, low self-directedness and impaired perspective taking (Wise et al., 1992; Guttman and Laporte, 2002; Picardi et al., 2005). The correlation observed between Glu and alexithymic features is therefore well in accordance with previous studies investigating these traits and showing a negative correlation between prefrontal Glu and mental perspective taking as well as extraversion (Montag et al., 2008; Grimm et al., 2012b). Harm avoidance and extraversion have been associated with increased and decreased ACC GABA concentrations (Kim et al., 2009; Goto et al., 2010), respectively, which fits well with the reported correlation between ACC GABA and alexithymia. The ACC is a crucial region for emotion processing and for constituting our sense of self (McKiernan et al., 2006; Northoff et al., 2006). It has been hypothesized that while insula is involved in the generation of all subjective feeling states, combined action of insula and ACC might provide the neural basis of self-awareness (Craig, 2009). Medford and Critchley (2010) proposed that awareness of self, i.e. an integrated awareness of cognitive, affective and physical state is generated by the integrative functions of the insula and then re-represented in ACC as a basis for responses to inner or outer events. Back-projections from the ACC may then allow the insular representation of the feeling state to be modulated by cingulate activity. The proposed reciprocity between ACC and insula is supported by neuroanatomical studies (Nieuwenhuys et al., 2008; Moisset et al., 2010), findings of correlated BOLD signal fluctuations (Taylor et al., 2009; Horn et al., 2010) and joint activity of these areas during emotional experience (Harrison et al., 2008). Menon and Uddin (2010) characterize insula and ACC as a ‘salience network’ that functions to segregate the most relevant among internal and external stimuli in order to guide behavior. Insula activity modulates autonomic reactivity to salient stimuli and Glu-mediated increased insula activity in alexithymia might reflect the misattribution of emotional salience to mundane events or bodily responses. A similar overdrive in the salience network has also been discussed in neuroticism, increased anxiety and depression (Paulus and Stein, 2006; Stein et al., 2007; Horn et al., 2010), all of which are closely related to alexithymia (Picardi et al., 2005; Luminet, 2010; Leweke et al., 2012). Our results show an association between GABA concentrations in ACC and alexithymic features. Since increased GABA transmission mediates a decrease in neuronal activity our finding is therefore well in accordance with previous studies showing lower ACC activation in response to emotion stimuli in alexithymia (Leweke et al., 2004; Moriguchi et al., 2007; Karlsson et al., 2008; Silani et al., 2008; Bird et al., 2010; Reker et al., 2010). Although we investigated Glu and GABA concentrations in the ventral ACC, a region crucially involved in emotional experience (Lane et al., 1998; Larisch et al., 1997; Bush et al., 2000; Northoff et al., 2007; Grimm et al., 2009), several previous studies reported increased activity in dorsal ACC in alexithymia (Berthoz et al., 2002; Mériau et al., 2006; Frewen et al., 2008; Karlsson et al., 2008; Heinzel et al., 2010). The dorsal region of the ACC provides a cognitive processing of emotions (Bush et al., 2000; Beauregard et al., 2001) and is especially relevant for emotion regulation (Ochsner et al., 2002; Phan et al., 2005; Kim and Hamann, 2007; Wager et al., 2008). Alexithymia has been conceptualized as a disorder of emotion regulation (Swart et al., 2009), since it is associated with maladaptive coping strategies, notably emotional inhibition and immature defensive styles (Helmes et al., 2008). Increased activity in dorsal ACC might represent an effort to suppress the unspecific emotional arousal that results from increased interoceptive awareness and Glu-mediated enhanced insula activity (Swart et al., 2009; Heinzel et al., 2010) and eventually lead to GABA-mediated decreased activity in ventral ACC, which prevents excessive experience of negative emotions (Urry et al., 2009; Abler et al., 2010). Increased GABA concentrations in alexithymia might therefore indicate increased inhibitory control of ACC activity as a neuronal correlate of impoverished conscious experience of emotion in alexithymia (blindfeel) (Lane et al., 1997, 1998). This hypothesis is supported first by the region-specific elevation in GABA levels in subjects scoring high on the TAS-20. Second, previous findings show that signal changes in ventral ACC during emotional processing are mediated by GABA (Northoff et al., 2007). Third, a recent study by Kupers et al. (2009) reports that acute pain and associated aversive emotional experience induced a significant increase in GABA in ACC. Finally, a recent study reported lower connectivity in ventral ACC in alexithymia (Liemburg et al., 2012).
There are several limitations to this study. The rather small sample size has to be considered when interpreting the results. We did not control for phase effects within our female participants, which might be of importance since it has been demonstrated that luteal and follicular phases impact MRS metabolites (Batra et al., 2008). Additionally, although we investigated two regions related to alexithymia and interoceptive awareness, future studies should include a further control region and also investigate the right insula, since specifically right insula might support interoceptive awareness and integrate it with other information to form the basis of the subjective experience of an emotional state (Craig, 2003). Future studies should also consider including additional scales to shed further light on the association between other personality dimensions related to increased vulnerability to affective disorders (e.g. neuroticism) and neurotransmitter concentrations. In sum, this study indicates for the first time a close relationship between alexithymia, interoceptive awareness and GABA and Glu concentrations in ACC and insula. Increased Glu-mediated excitatory transmission and related enhanced insula activity might reflect increased interoceptive awareness in alexithymia. We assume that increased awareness of bodily and stress responses in alexithymics results in unspecific emotional arousal. Alexithymics mainly use suppression as a strategy to down-regulate emotional arousal, which might be reflected in neuronal activity decreases as mediated by the here reported increased GABA concentration in ACC. These hypotheses should be tested in further studies, though, that combine neuroimaging during emotional processing and interoceptive awareness with MRS measurements in insula and ACC in healthy subjects as well as in patients with affective disorders.
Conflict of interest
None declared.
Acknowledgments
This work was supported by a grant of the Hope of Depression Research Foundation (to G.N.); the EJLB Michael Smith Foundation and CRC Canada Research Chair (to G.N.); Eli Lilly (Suisse) (to H.B.).
REFERENCES
- Abler B, Hofer C, Walter H, et al. Habitual emotion regulation strategies and depressive symptoms in healthy subjects predict fMRI brain activation patterns related to major depression. Psychiatry Research. 2010;183:105–13. doi: 10.1016/j.pscychresns.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Bach M, Bach D, de Zwaan M, Serim M, Böhmer F. Validierung der deutschen Version der 20-Item Toronto-Alexithymie-Skala bei Normalpersonen und psychiatrischen Patienten. Psychotherapie, Psychosomatik, medizinische Psychologie. 1996;46:23–8. [PubMed] [Google Scholar]
- Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994a;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, Parker JD. The twenty-item Toronto Alexithymia Scale—II. Convergent, discriminant, and concurrent validity. Journal of Psychosomatic Research. 1994b;38:33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Batra NA, Seres-Mailo J, Hanstock C, et al. Proton magnetic resonance spectroscopy measurement of brain glutamate levels in premenstrual dysphoric disorder. Biological Psychiatry. 2008;63:1178–84. doi: 10.1016/j.biopsych.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–25. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Artiges E, van de Moortele PF, et al. Effect of impaired recognition and expression of emotions on frontocingulate cortices: an fMRI study of men with alexithymia. The American Journal of Psychiatry. 2002;159:961–7. doi: 10.1176/appi.ajp.159.6.961. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133:1515–25. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsci G, Boccardi M, Rossi R, et al. Alexithymia in healthy women: a brain morphology study. Journal of Affective Disorders. 2009;114(1–3):208–15. doi: 10.1016/j.jad.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Viebke M-C, Baumgartner T, Kaffenberger T, Herwig U. Neural correlates of personality dimensions and affective measures during the anticipation of emotional stimuli. Brain Imaging and Behavior. 2011;5:86–96. doi: 10.1007/s11682-011-9114-7. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosomatic Medicine. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. Journal of Personality. 2004;72:1105–32. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Amin Z. Neuroimaging of emotion and personality: scientific evidence and ethical considerations. Brain and Cognition. 2002;50:414–31. doi: 10.1016/s0278-2626(02)00517-1. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Conrad R, Wegener I, Imbierowicz K, Liedtke R, Geiser F. Alexithymia, temperament and character as predictors of psychopathology in patients with major depression. Psychiatry Research. 2009;165:137–44. doi: 10.1016/j.psychres.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig ADB. How do you feel—now? The anterior insula and human awareness. Nature Reviews. Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Miller KK, Klibanski A, et al. Regional cerebral brain metabolism correlates of neuroticism and extraversion. Depression and Anxiety. 2006;23:133–38. doi: 10.1002/da.20152. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Evans D, Oliver C, Hawkins A, Dalgleish T. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behaviour Research and Therapy. 2010;48:1133–38. doi: 10.1016/j.brat.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough SH, Goodwin L. The effect of psychological stress and relaxation on interoceptive accuracy: implications for symptom perception. Journal of Psychosomatic Research. 2007;62:289–95. doi: 10.1016/j.jpsychores.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Franz M, Popp K, Schaefer R, et al. Alexithymia in the German general population. Social Psychiatry and Psychiatric Epidemiology. 2008;43:54–62. doi: 10.1007/s00127-007-0265-1. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Lanius RA. Meta-analysis of alexithymia in posttraumatic stress disorder. Journal of Traumatic Stress. 2008;21:243–46. doi: 10.1002/jts.20320. [DOI] [PubMed] [Google Scholar]
- Goto N, Yoshimura R, Moriya J, et al. Critical examination of a correlation between brain gamma-aminobutyric acid (GABA) concentrations and a personality trait of extroversion in healthy volunteers as measured by a 3 Tesla proton magnetic resonance spectroscopy study. Psychiatry Research. 2010;182:53–7. doi: 10.1016/j.pscychresns.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, et al. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. The World Journal of Biological Psychiatry. 2011a;12:627–37. doi: 10.3109/15622975.2010.545145. [DOI] [PubMed] [Google Scholar]
- Grimm S, Schubert F, Jaedke M, Gallinat J, Bajbouj M. Prefrontal cortex glutamate and extraversion. Social Cognitive and Affective Neuroscience. 2011b;7:811–18. doi: 10.1093/scan/nsr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Schubert F, Jaedke M, Gallinat J, Bajbouj M. Prefrontal cortex glutamate and extraversion. Social Cognitive and Affective Neuroscience. 2012b;7:811–8. doi: 10.1093/scan/nsr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Luborzewski A, Schubert F, et al. Region-specific glutamate changes in patients with unipolar depression. Journal of Psychiatric Research. 2012a;46:1059–65. doi: 10.1016/j.jpsychires.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Gündel H, López-Sala A, Ceballos-Baumann AO, et al. Alexithymia correlates with the size of the right anterior cingulate. Psychosomatic Medicine. 2004;66:132–40. doi: 10.1097/01.psy.0000097348.45087.96. [DOI] [PubMed] [Google Scholar]
- Gussew A, Rzanny R, Erdtel M, et al. Time-resolved functional 1H MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. NeuroImage. 2010;49:1895–902. doi: 10.1016/j.neuroimage.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Guttman H, Laporte L. Alexithymia, empathy, and psychological symptoms in a family context. Comprehensive Psychiatry. 2002;43:448–55. doi: 10.1053/comp.2002.35905. [DOI] [PubMed] [Google Scholar]
- Gutzeit A, Meier D, Meier ML, et al. Insula-specific responses induced by dental pain. A proton magnetic resonance spectroscopy study. European Radiology. 2011;21:807–15. doi: 10.1007/s00330-010-1971-8. [DOI] [PubMed] [Google Scholar]
- Harris RE, Sundgren PC, Pang Y, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis and Rheumatism. 2008;58:903–7. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yücel M. Modulation of brain resting-state networks by sad mood induction. PLoS One. 2008;3(3):e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Matsubara T, Lewis DA. Schizophrenia and cortical GABA neurotransmission. Psychiatria et Neurologia Japonica. 2010;112:439–52. [PubMed] [Google Scholar]
- Heinzel A, Minnerop M, Schäfer R, Müller HW, Franz M, Hautzel H. Alexithymia in healthy young men: a voxel-based morphometric study. Journal of Affective Disorders. 2012;136(3):1252–6. doi: 10.1016/j.jad.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Heinzel A, Schäfer R, Müller HW, et al. Increased activation of the supragenual anterior cingulate cortex during visual emotional processing in male subjects with high degrees of alexithymia: an event-related fMRI study. Psychotherapy and Psychosomatics. 2010;79:363–70. doi: 10.1159/000320121. [DOI] [PubMed] [Google Scholar]
- Helmes E, McNeill PD, Holden RR, Jackson C. The construct of alexithymia: associations with defense mechanisms. Journal of Clinical Psychology. 2008;64:318–31. doi: 10.1002/jclp.20461. [DOI] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression—the role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience. 2010;4:33. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DL, Wiebe JS, Gold SM, et al. Cerebral blood flow and personality: a positron emission tomography study. The American Journal of Psychiatry. 1999;156:252–57. doi: 10.1176/ajp.156.2.252. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Näätänen P, Stenman H. Cortical activation in alexithymia as a response to emotional stimuli. The British Journal of Psychiatry. 2008;192:32–38. doi: 10.1192/bjp.bp.106.034728. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim JE, Cho G, et al. Associations between anterior cingulate cortex glutamate and gamma-aminobutyric acid concentrations and the harm avoidance temperament. Neuroscience Letters. 2009;464:103–7. doi: 10.1016/j.neulet.2009.07.087. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bulletin. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Kumari V, Ffytche DH, Williams SCR, Gray JA. Personality predicts brain responses to cognitive demands. The Journal of Neuroscience. 2004;24:10636–41. doi: 10.1523/JNEUROSCI.3206-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Danielsen ER, Kehlet H, Christensen R, Thomsen C. Painful tonic heat stimulation induces GABA accumulation in the prefrontal cortex in man. Pain. 2009;142:89–93. doi: 10.1016/j.pain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Lane RD, Ahern GL, Schwartz GE, Kaszniak AW. Is alexithymia the emotional equivalent of blindsight? Biological Psychiatry. 1997;42:834–44. doi: 10.1016/s0006-3223(97)00050-4. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–35. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Larisch R, Klimke A, Vosberg H, Löffler S, Gaebel W, Müller-Gärtner HW. In vivo evidence for the involvement of dopamine-D2 receptors in striatum and anterior cingulate gyrus in major depression. NeuroImage. 1997;5:251–60. doi: 10.1006/nimg.1997.0267. [DOI] [PubMed] [Google Scholar]
- Leweke F, Leichsenring F, Kruse J, Hermes S. Is alexithymia associated with specific mental disorders? Psychopathology. 2012;45:22–8. doi: 10.1159/000325170. [DOI] [PubMed] [Google Scholar]
- Leweke F, Stark R, Milch W, et al. Neuronale Aktivitätsmuster auf affektinduktive Reize bei Alexithymie. Psychotherapie, Psychosomatik, medizinische Psychologie. 2004;54:437–44. doi: 10.1055/s-2004-828350. [DOI] [PubMed] [Google Scholar]
- Liemburg EJ, Swart M, Bruggeman R, et al. Altered resting state connectivity of the default mode network in alexithymia. Social Cognitive and Affective Neuroscience. 2012;7:660–6. doi: 10.1093/scan/nss048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden W, Wen F, Paulhus DL. Measuring alexithymia: reliability, validity, and prevalence. Advances in Personality Assessment. 1995;10:51–95. [Google Scholar]
- Luminet O. Commentary on the paper “Is alexithymia a risk factor for major depression, personality disorder, or alcohol use disorders? A prospective population-based study”. Journal of Psychosomatic Research. 2010;68:275–77. doi: 10.1016/j.jpsychores.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008;33:2080–92. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. NeuroImage. 2006;29:1185–91. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure & Function. 2010;214:535–49. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mériau K, Wartenburger I, Kazzer P, et al. A neural network reflecting individual differences in cognitive processing of emotions during perceptual decision making. NeuroImage. 2006;33:1016–27. doi: 10.1016/j.neuroimage.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Moisset X, Bouhassira D, Denis D, Dominique G, Benoit C, Sabaté J-M. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. European Journal of Pain. 2010;14:142–48. doi: 10.1016/j.ejpain.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Montag C, Weber B, Jentgens E, Elger C, Reuter M. An epistasis effect of functional variants on the BDNF and DRD2 genes modulates gray matter volume of the anterior cingulate cortex in healthy humans. Neuropsychologia. 2010;48(4):1016–21. doi: 10.1016/j.neuropsychologia.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Montag C, Schubert F, Heinz A, Gallinat J. Prefrontal cortex glutamate correlates with mental perspective-taking. PloS One. 2008;3(12):e3890. doi: 10.1371/journal.pone.0003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, et al. Empathy and judging other’s pain: an fMRI study of alexithymia. Cerebral Cortex. 2007;17:2223–34. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. 4th edn. Heidelberg, Germany: Springer; 2008. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nature Neuroscience. 2007;10:1515–7. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–87. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–19. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Picardi A, Toni A, Caroppo E. Stability of alexithymia and its relationships with the ‘big five’ factors, temperament, character, and attachment style. Psychotherapy and Psychosomatics. 2005;74:371–8. doi: 10.1159/000087785. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, Schandry R. On the relationship between interoceptive awareness, emotional experience, and brain processes. Cognitive Brain Research. 2005;25:948–62. doi: 10.1016/j.cogbrainres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Porges SW. Body Perception Questionnaire. 1993. (German version). Laboratory of Developmental Assessment, University of Maryland. [Google Scholar]
- Reker M, Ohrmann P, Rauch AV, et al. Individual differences in alexithymia and brain response to masked emotion faces. Cortex. 2010;46:658–67. doi: 10.1016/j.cortex.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Salminen JK, Saarijärvi S, Aärelä E, Toikka T, Kauhanen J. Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. Journal of Psychosomatic Research. 1999;46:75–82. doi: 10.1016/s0022-3999(98)00053-1. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews. Drug Discovery. 2008;7:426–37. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7(9):e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte RF, Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR in Biomedicine. 2006;19:255–63. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Social Neuroscience. 2008;3:97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human Brain Mapping. 2012 doi: 10.1002/hbm.22113. ebup ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, et al. Neural reward processing is modulated by approach- and avoidance-related personality traits. NeuroImage. 2010;49:1868–74. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Smith SA, Levante TO, Meier BH, Ernst RR. Computer simulations in magnetic resonance: an object-oriented programming approach. Journal of Magnetic Resonance, Series A. 1994;106:75–105. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American Journal of Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Swart M, Kortekaas R, Aleman A. Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PloS One. 2009;4(6):e5751. doi: 10.1371/journal.pone.0005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ. Recent developments in alexithymia theory and research. Canadian Journal of Psychiatry. 2000;45:134–42. doi: 10.1177/070674370004500203. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2009;30:2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa Y, Fukushima H, Umeda S. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI Study. Human Brain Mapping. 2011;34:598–612. doi: 10.1002/hbm.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47:852–63. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya JG, Paradiso S, Andreasen NC, Johnson DL, Boles Ponto LL, Hichwa RD. Correlation between extraversion and regional cerebral blood flow in response to olfactory stimuli. The American Journal of Psychiatry. 2007;164:339–41. doi: 10.1176/ajp.2007.164.2.339. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Archives of General Psychiatry. 2009;66:478–86. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- Walter NT, Montag C, Markett SA, Reuter M. Interaction effect of functional variants of the BDNF and DRD2/ANKK1 gene is associated with alexithymia in healthy human subjects. Psychosomatic Medicine. 2011;73(1):23–8. doi: 10.1097/PSY.0b013e31820037c1. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Measurement and mismeasurement of mood: recurrent and emergent issues. Journal of Personality Assessment. 1997;68:267–96. doi: 10.1207/s15327752jpa6802_4. [DOI] [PubMed] [Google Scholar]
- Werner NS, Jung K, Duschek S, Schandry R. Enhanced cardiac perception is associated with benefits in decision-making. Psychophysiology. 2009;46:1123–29. doi: 10.1111/j.1469-8986.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me”. The World Journal of Biological Psychiatry. 2010;11:538–49. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- Wiebking C, de Greck M, Duncan NW, Heinzel A, Tempelmann C, Northoff G. Are emotions associated with activity during rest or interoception? An exploratory fMRI study in healthy subjects. Neuroscience Letters. 2011;491:87–92. doi: 10.1016/j.neulet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Wiens S, Palmer SN. Quadratic trend analysis and heartbeat detection. Biological Psychology. 2001;58:159–75. doi: 10.1016/s0301-0511(01)00110-7. [DOI] [PubMed] [Google Scholar]
- Wise TN, Mann LS, Shay L. Alexithymia and the five-factor model of personality. Comprehensive Psychiatry. 1992;33:147–51. doi: 10.1016/0010-440x(92)90023-j. [DOI] [PubMed] [Google Scholar]