Abstract
Although empathy is crucial for successful social interactions, excessive sharing of others’ negative emotions may be maladaptive and constitute a source of burnout. To investigate functional neural plasticity underlying the augmentation of empathy and to test the counteracting potential of compassion, one group of participants was first trained in empathic resonance and subsequently in compassion. In response to videos depicting human suffering, empathy training, but not memory training (control group), increased negative affect and brain activations in anterior insula and anterior midcingulate cortex—brain regions previously associated with empathy for pain. In contrast, subsequent compassion training could reverse the increase in negative effect and, in contrast, augment self-reports of positive affect. In addition, compassion training increased activations in a non-overlapping brain network spanning ventral striatum, pregenual anterior cingulate cortex and medial orbitofrontal cortex. We conclude that training compassion may reflect a new coping strategy to overcome empathic distress and strengthen resilience.
Keywords: fMRI, social, emotion, insula, medial orbitofrontal cortex
INTRODUCTION
Our capacity to understand others’ feelings through empathy is crucial for successful social interactions (Bird et al., 2010). However, when confronting the suffering of others, intense sharing of the other’s pain can be a primary cause for empathic distress and decreased helping behavior (Batson et al., 1987; Eisenberg et al., 1989). In fact, empathic responses to witnessing another in pain are usually experienced as aversive (Lamm et al., 2011). This may be especially problematic for people working in professions where suffering is routinely encountered. Physicians, for example, have a high prevalence rate of burnout (McCray et al., 2008) and an elevated risk for suicide (Schernhammer and Colditz, 2004). A potential remedy for the excessive sharing of negative affect may be compassion. Compassion is defined as a feeling of concern for the suffering of others that is associated with the motivation to help (Keltner and Goetz, 2007). Recent studies of others and ourselves have shown that training compassion can foster emotional well-being (Fredrickson et al., 2008), positive emotions (Klimecki et al., 2012) and prosocial behavior (Leiberg et al., 2011). Although compassion emerges as a promising strategy to strengthen personal resources, it is, so far, unresolved how compassion can help to overcome the adverse effects related to empathic distress.
Furthermore, it is unresolved whether the neural systems subserving empathy and compassion can be dissociated. Two recent cross-sectional meta-analyses suggest that empathy for pain crucially involves anterior insula (AI) and anterior midcingulate cortex (aMCC) (Fan et al., 2011; Lamm et al., 2011). This is consistent with the observation that negative affect often covaries with activations in AI and aMCC (Lamm et al., 2011). On a more general level, AI and aMCC are key structures for processing salient events (Seeley et al., 2007), and aMCC function has been robustly implicated in cognitive control and pain processing in two recent large-scale meta-analyses (Beckmann et al., 2009; Shackman et al., 2011). Conversely, several cross-sectional studies (Lutz et al., 2008; Beauregard et al., 2009; Immordino-Yang et al., 2009; Kim et al., 2009) and one short-term longitudinal study performed by our group (Klimecki et al., 2012) suggest that compassion is accompanied by activations in regions typically associated with reward, love and affiliation. These regions comprise insula, ventral striatum and medial orbitofrontal cortex (mOFC) (Beauregard et al., 2009; Immordino-Yang et al., 2009). Animal studies suggest that the neurobiology of the ‘care’ system can be clearly dissociated from other emotional–motivational systems such as the ‘panic’ system, as the ‘care’ system relies on distinct brain structures and is mediated by distinct neurotransmitters comprising opioids, oxytocin and dopamine (Panksepp, 2011). In addition, affiliative memories in mammals seem to rely on a circuitry that includes mOFC, ventral striatum and ventral tegmental area (Depue and Morrone-Strupinsky, 2005). Our aim was, thus, to determine whether training empathy and compassion will have distinct effects on neural function and whether training compassion can help overcome excessive levels of distress.
To address these issues, we conducted a prospective training study in which one group of participants was first trained in empathy and subsequently in compassion. We repeatedly acquired functional magnetic resonance imaging (fMRI) measures, while participants were exposed to videos depicting others suffering. To train compassion, we used a contemplative technique from secular compassion training programs that aims at cultivating feelings of benevolence and friendliness in a state of quiet concentration (Salzberg, 2002; for empirical work, see Fredrickson et al., 2008; Leiberg et al., 2011; Klimecki et al., 2012). Similar to strengthening modes of affiliation, compassion training relies on extending caring feelings—which are usually experienced toward close loved persons—to other human beings. The preceding empathy training closely matched the compassion training in form and structure, but focused on resonating with suffering. Unspecific effects introduced by training in groups and by repeated measurements were controlled by including an active control group that received memory training using the Method of Loci (Bower, 1970). A detailed description of the employed training techniques can be found in the Supplementary material.
On the level of subjective experience, we hypothesized that training empathy would increase empathy and negative affect when witnessing the distress of others. Pertaining to neural function, we assumed that training empathy would induce plasticity in AI and aMCC as these structures are robustly involved in cross-sectional studies on empathy for pain (Fan et al., 2011; Lamm et al., 2011). In contrast, we expected that a subsequent compassion training would strengthen positive affect and induce specific functional plasticity in a different neural network. This network includes mOFC, ventral tegmental area/substantia nigra (VTA/SN) and striatum, as compassion-related activation changes in these structures have been observed in our recent longitudinal study (Klimecki et al., 2012).
METHODS
Participants
As gender differences in social emotions were observed in previous neuroscientific research (e.g. Singer et al., 2006), we decided to control for possible gender effects by restricting our sample to female participants only. In the affect group, the study was completed by 25 of an initial group of 30 participants (age: 25.88 ± 4.32 years, mean ± s.d.). In the memory group, 28 of 33 participants completed the study (age: 22.89 ± 4.02 years, mean ± s.d.). Participants for the affect and memory training groups (Figure 1) were recruited and tested sequentially due to temporal and infrastructural constraints (i.e. scanning slots). To avoid any selection bias, participants in both training groups were recruited with advertisements announcing participation in mental training studies. Furthermore, participants were not aware of the specific training content until pre-test measurement was completed and they entered the training phase. The five persons who dropped out in the memory control group had higher scores on the Beck’s Depression Inventory (Beck et al., 1996) than those who completed the study (t31 = 2.31, P < 0.05, dropouts: mean = 7.4, s.d. = 5.6; completers: mean = 3.11, s.d. = 3.49). No other selective dropouts were observed in the memory control group and no selective dropouts occurred in the affect group. To account for selective dropout in the memory group and age differences between both groups (Supplementary Table S1), we included age and depression scores as covariates in all between-group analyses. The study was approved by the Research Ethics Committee of Zurich (‘Kantonale Ethikkommission des Kantons Zürich—Spezialisierte Unterkommission Psychiatrie, Neurologie, Neurochirurgie’; E-25/2008) and carried out in compliance with the Declaration of Helsinki. All subjects gave informed written consent, were paid for their participation and were debriefed after the completion of the study. As participants whose data are reported here were part of a larger study, we specify the relation between this study and other experiments (Klimecki et al., 2012) in Supplementary Figure S1. A description of inclusion criteria, the employed trait questionnaires, data acquisition and data analysis procedures, as well as the training regimes can be found in the Supplementary material.
Fig. 1.
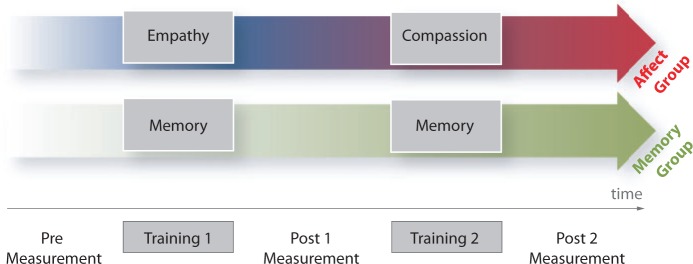
Experimental design. This longitudinal training study consisted of two groups: the affect group, which first received empathy training and subsequently compassion training, and the memory control group, which received two memory trainings. Participants were tested three times while watching videos depicting others suffering: before the first training (Pre) and after each training (Post1 and Post2). Details on the training regimes can be found in the Supplementary material.
Measures
Socio-affective video task
Participants’ affective experiences and blood oxygenation level dependent signals were measured three times in response to the socio-affective video task (SoVT; for more details about the SoVT properties, please see also Klimecki et al., 2012)—before training (Pre), after empathy or memory training (Post1) and after compassion or memory training (Post2). To avoid habituation and repetition, participants saw one of three parallel video sets matched for valence, arousal and empathy at Pre, Post1 and Post2, respectively. Each set contained 12 high emotion (HE) videos and 12 low emotion (LE) videos. Video scenes were taken from footage cast for news or documentaries and depict men, women and children. LE videos showed everyday scenes, whereas HE videos depicted people who were suffering (e.g. due to injuries or natural disasters). After each video (duration 10–18 s), participants rated how much empathy, positive affect and negative affect they had experienced while seeing the video. To assure that all participants had the same basic notion of empathy, they were instructed before each measurement that the empathy rating captures how much they shared the emotion of the depicted persons. Videos were shown in blocks of three HE or LE videos. Each block was followed by a null event (10 s fixation cross). At Post1 and Post2, participants in the affect training group were encouraged to make use of the trained competences when viewing the videos.
Memory task
To test the effectiveness of the memory intervention, participants were seated in front of a computer screen at each measurement point and asked to encode a different matched list of 34 words. Words were presented for 4 s each, followed by a 2 s fixation cross. Subsequently, participants were given 5 min to fill the recollected words into a computer table, if possible in the correct sequence.
RESULTS
Socio-affective video task
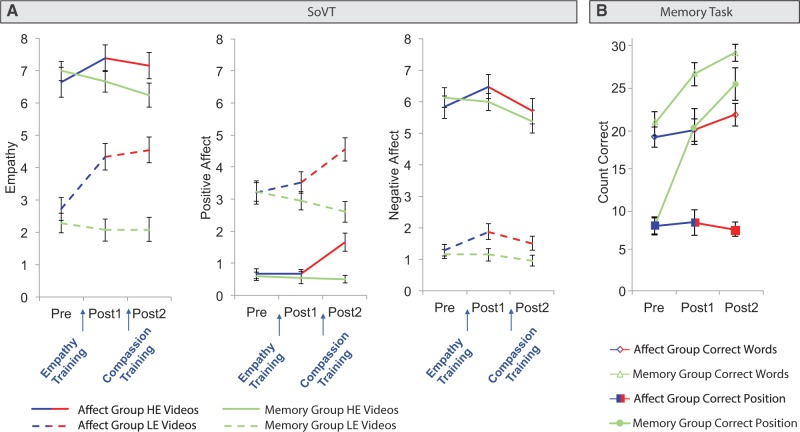
To determine how the different training regimes affected subjective experiences of empathy, positive affect and negative affect in response to the SoVT, we conducted a repeated measures multivariate analysis of variance (MANOVA). Age and depression scores (Beck et al., 1996) were included as covariates to account for selective dropout and between-group differences. The within-subject factors were time (three levels: Pre, Post1 and Post2) and video type (two levels: LE and HE videos). The between-subject factor was group (two levels: affect and memory). The main effect of video type was significant (F3,47 = 8.18, P < 0.001, η2 = 0.34). Significant interactions were observed for video type × group (F3,47 = 2.85, P < 0.05, η2 = 0.15) and time × group (F6,44 = 6.01, P < 0.001, η2 = 0.45). The triple interaction time × video type × group was marginally significant (F6,44 = 2.3, P = 0.05, η2 = 0.24). Univariate ANOVAs determined that all three affect ratings showed the main effect of video type (all F ≥ 5.28, all P < 0.05). Follow-up paired t-tests showed that compared with LE videos, HE videos elicited more negative affect and empathy and less positive affect (all t52 ≥ 15.82, all P < 0.001). The interaction video type × group was significant for empathy (F1,49 = 7.01, P < 0.05). Follow-up independent t-tests revealed that when combining all three time points, empathy ratings for LE videos were higher in the affect group than in the memory group (t51 = 3.68, P < 0.01). Importantly, the time × group interaction was significant for all three affect ratings (all F ≥ 3.44, all P < 0.05). Follow-up independent t-tests comparing the memory and the affect groups at pre- and post-tests showed that the groups did not differ before training (all t ≤ 0.29, all P ≥ 0.77), that empathy was higher in the affect group compared with the memory group after both trainings (both t51 ≥ 3.18, both P < 0.01), that a similar trend was present for negative affect after empathy training (t51 = 1.75, P = 0.09), and that positive affect was higher after compassion training (t51 = 4.37, P < 0.001). Paired t-tests focusing on changes within the affect group between Pre and Post1 and between Post1 and Post2 showed that empathy training increased negative affect (t24 = 3.5, P < 0.01) and empathy (t24 = 4.66, P < 0.001). Conversely, compassion training decreased negative affect (t24 = 3.04, P < 0.01) and augmented positive affect (t24 = 4.25, P < 0.001). Paired t-tests in the memory group showed that negative affect decreased from Post1 to Post2 (t27 = 3.17, P < 0.01). Finally, Pearson correlations between the change in affect ratings and self-reports of practice duration in the affect group revealed no significant relation (all P ≥ 0.1). In summary, training empathy led to increases in subjective reports of negative affect and empathy. Adding compassion training strengthened positive affect and reversed the observed increase in negative affect (Figure 2A).
Fig. 2.
(A) Self-reported empathy and negative affect significantly increased after empathy training. Positive affect only increased after compassion training. (B) Memory, but not affect training, improved the number of correctly remembered words and the number of words remembered in the correct position. Error bars indicate standard error of mean.
Memory task
To validate the effectiveness of the memory control training, we computed a 3 × 2 repeated measures MANOVA with the within-subject factor time (three levels: Pre, Post1 and Post2) and the between-subject factor training group (two levels: affect training and memory training group). Age and depression scores (Beck et al., 1996) were included as covariates. The dependent variables were the number of correctly remembered words and the number of words remembered in the correct position. We found a significant main effect of group (F2,46 = 11.35, P < 0.001, η2 = 0.33) and a significant time × group interaction (F4,44 = 11, P < 0.001, η2 = 0.5). There was a trend for a main effect of time (F4,44 = 2.23, P = 0.08). The time × group interaction was significant for both dependent variables (both F2,94 ≥ 4.67, both P < 0.05). Confirming the effectiveness of the memory intervention (n = 51; Figure 2B), follow-up independent t-tests revealed that, whereas the groups did not differ at Pre (both P ≥ 0.4), the memory group performed better than the affect group at Post1 and at Post2 on both dependent measures (all t ≥ 3.43, all P < 0.01). Paired t-tests showed that memory performance did not change in the affect group (all t ≤ 1.35, all P ≥ 0.19). In contrast, the number of correctly remembered words and words remembered in the correct position increased significantly in participants of the memory group, both, from Pre to Post1 (both t ≥ 4.37, both P < 0.001) and from Post1 to Post2 (both t ≥ 2.35, both P < 0.05). All other effects were not significant.
Functional imaging changes
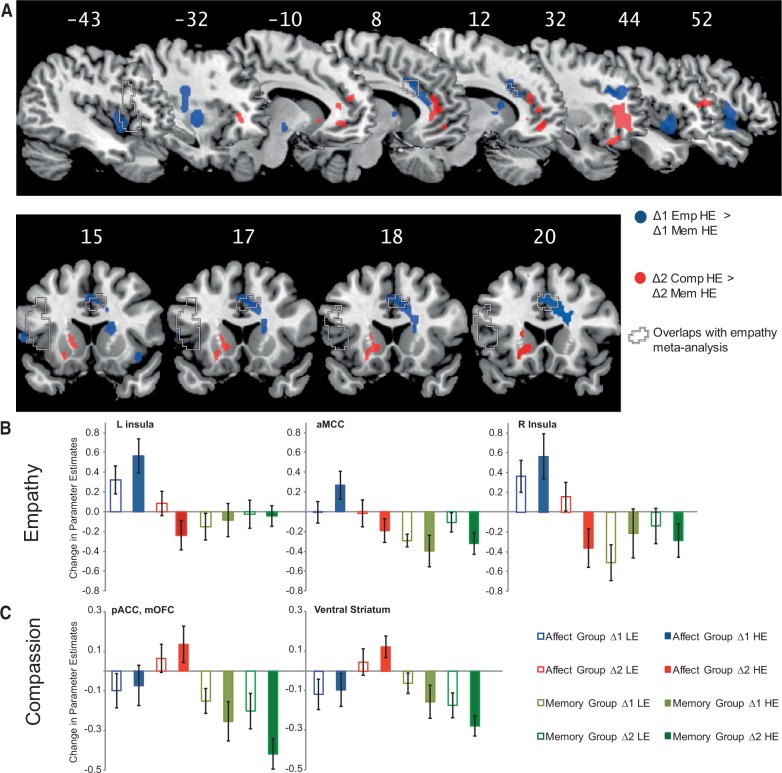
To examine functional neural plasticity induced by training empathy and compassion, we analyzed imaging data using event-related statistics on the whole brain (P < 0.05, FWE corrected using cluster level thresholds; Friston et al., 1994). On the second level, we performed repeated measures ANOVAs with the within-subject factors change (two levels: Pre to Post1, Δ1, and Post1 to Post 2, Δ2) and video type (LE and HE) and the between-subject factor training group (affect and memory) (Supplementary Table S2). Age and depression scores (Beck et al., 1996) were included as covariates. Activations in cingulate cortex were classified and labeled based on Vogt (2005). Paralleling behavioral findings, in which similar changes occurred for HE and LE videos, no significant triple interaction for video type × time × group was observed. Empathy training, but not memory training [Empathy (Δ1 LE and Δ1 HE) > Memory (Δ1 LE and Δ1 HE)], increased activations in insula, temporal gyrus, operculum, posterior putamen, pallidum, thalamus and head of caudate. In response to HE videos (Empathy Δ1 HE > Memory Δ1 HE), empathy training augmented activations in brain areas spanning insula, aMCC, dorsolateral prefrontal cortex (DLPFC), posterior putamen, pallidum and head of caudate (Figure 3). The same contrast for LE videos revealed overlapping changes in right anterior and middle insula, operculum, temporal gyrus and head of caudate. Notably, activation changes for HE videos after empathy training overlapped with meta-analytic findings on empathy for pain (Lamm et al., 2011) in aMCC and left AI (Figure 3A). Conversely, compassion training, but not a second day of memory training [Compassion (Δ2 LE and Δ2 HE) > Memory (Δ2 LE and Δ2 HE)], increased activations in mOFC, pregenual anterior cingulate cortex (pACC), inferior frontal gyrus (IFG) and ventral striatum. Consistently, in response to HE videos, compassion training augmented activity in mOFC, pACC, ventral striatum and right middle frontal gyrus. This overlapped with changes induced by compassion training in response to LE videos in pACC and right IFG. To visualize the change in parameter estimates for each factor, mean activations across all voxels in each cluster were extracted (Figure 3B). None of the contrasts comparing changes induced by memory training with changes induced by affect training revealed significant activations. To test for any overlap between regions showing functional plasticity after empathy and compassion training, we conducted conjunction analyses of equivalent empathy- and compassion-related contrasts (Supplementary Table S2). None of the conjunctions was significant, underlining that patterns of induced functional plasticity after empathy and compassion training were distinct and non-overlapping.
Fig. 3.
(A) Functional neural changes related to empathy (blue) and compassion training (red) in comparison with the memory control group (P < 0.05, FWE corrected). Regions in which changes related to empathy training overlap with a recent empathy for pain meta-analysis (Lamm et al., 2011) are indicated by dashed lines. (B) Bar charts of changes in parameter estimates in the areas related to empathy training (Empathy Δ1 HE > Memory Δ1 HE). (C) Bar charts of changes in parameter estimates of the areas related to compassion training (Compassion Δ2 HE > Memory Δ2 HE). The values represent the mean activation of all voxels in one cluster; error bars depict standard error of mean.
Parametric analyses in the affect group revealed that the increase in empathy ratings after empathy training (Δ1) correlated with the increase in aMCC activity (P < 0.05, FWE corrected, Supplementary Table S3). Activation changes in right AI were also parametrically modulated by increases in negative affect and empathy ratings between Pre and Post1, albeit at an uncorrected threshold of P < 0.001. No significant correlations were found for changes in positive affect ratings after empathy training. Parametric analyses on the changes in subjective ratings and brain activity after compassion training (Δ2) revealed that activity changes in the left supramarginal gyrus (P < 0.05, FWE corrected) were linearly modulated by changes in negative affect ratings. No significant effects were found for changes in empathy ratings or positive affect ratings after compassion training. Practice duration did not parametrically modulate neural changes. The intervention was probably too short to reveal a robust impact of inter-individual practice differences on neural and experiential changes.
In summary, observed changes in brain activation after empathy and compassion training revealed distinct patterns of functional brain plasticity. The effects of empathy training overlapped with previous peak activations in AI and aMCC as identified in a meta-analysis on cross-sectional empathy for pain studies (Lamm et al., 2011). Subsequent compassion training induced activations in a non-overlapping network spanning mOFC, pregenual ACC and ventral striatum.
DISCUSSION
The goal of this short-term affective intervention study with an active memory control group was to dissociate empathy and compassion and to investigate related plasticity on the neural and experiential level. We hypothesized that although these two socio-affective and motivational states may be related, they may have important differential signatures and consequences. Thus, we anticipated that empathizing with the suffering of others might be associated with negative states, distress and activations in brain networks playing a crucial role in empathy for pain. Conversely, compassion should be accompanied by positive feelings of warmth and concern for the other and increased activations in brain networks related to reward and affiliation.
Indeed, we found evidence for different patterns of emotional experiences and neural plasticity associated with the sequential training of these two social emotions within the same participants: a short-term training in empathy increased empathic responses and negative affect in response to others’ distress. In addition, watching others’ suffering after empathy training was associated with activations in a network spanning insula, aMCC, temporal gyrus, DLPFC, operculum and parts of basal ganglia. These results align with and extend previous cross-sectional meta-analytic findings on a crucial role of insula and aMCC in empathy for pain (Fan et al., 2011; Lamm et al., 2011), as well as their involvement in self-experienced pain, and negative affect in general (Beckmann et al., 2009; Lamm et al., 2011; Shackman et al., 2011).
Importantly, compassion training reversed these effects: it decreased negative affect back to baseline levels and increased positive affect. On the neural level, compassion training increased brain activations in mOFC, pregenual ACC and striatum—a network previously associated with positive affect (Kringelbach and Berridge, 2009), affiliation (Strathearn et al., 2009) and reward (Haber and Knutson, 2010). Interestingly, this distinction is paralleled by recent neuroscientific evidence which indicates that social connectedness is typically associated with activations in brain regions that comprise ventromedial prefrontal cortex and ventral striatum, whereas social disconnection is rather associated with activations in AI and dorsal ACC (for review, see Eisenberger and Cole, 2012).
The analyses of subjective ratings revealed that empathy training led to an increase in empathy and negative affect in response to both, LE and HE videos. This suggests that training empathy not only induced a stronger sharing of painful and distressing experiences, but also increased the susceptibility to feel negative affect in response to everyday life situations.
Importantly, compassion training counteracted this effect: it increased positive affect and decreased negative affect back to baseline levels. Remarkably, the increase in positive affect occurred even though participants were still exposed to equally distressing video material. This finding adds to the observation of a previous study in which a similar compassion and loving kindness training increased general levels of positive affect in daily life (Fredrickson et al., 2008). It is also in line with previously observed experiential and neural effects after a short-term compassion training (Klimecki et al., 2012). Taken together, this suggests that the generation of compassion in response to distressing situations is distinct from other emotion regulation strategies, such as suppression or reappraisal, which involve an active down-regulation of negative affect (Gross, 2002). Thus, the generation of compassion focuses on strengthening positive affect, while not ignoring the presence of suffering or changing the negative reality. Future studies may formally compare compassion with existing emotion regulation strategies. As compassion does not rely on the temporal denial of the negative nature of events, one hypothesis would be that compassion training would abolish rebound effects, as observed in the amygdala after effortful emotion regulation (Walter et al., 2009). Furthermore, acknowledging the negative experience of others rather than suppressing it may be a crucial prerequisite for the development of prosocial motivation and helping behavior. Accordingly, it has recently been shown that the frequency of helping behavior can indeed be increased with a similar short-term compassion training (Leiberg et al., 2011).
On the neural level, we obtained evidence that short-term empathy training, but not memory training, induced functional plasticity in a network spanning insula, aMCC, temporal gyrus, operculum, DLPFC, posterior putamen, pallidum and head of caudate. The observed activation increases in DLPFC and middle temporal gyrus align with previous findings on emotion regulation (Kalisch, 2009), cognitive control (Beckmann et al., 2009; Mansouri et al., 2009; Shackman et al., 2011) and pain processing (Beckmann et al., 2009; Shackman et al., 2011). Importantly, and as illustrated in Figure 3A, activations in AI and aMCC were concordant with peak activations identified in a meta-analysis performed over more than 30 cross-sectional studies on empathy for pain (Lamm et al., 2011). Moreover, activation in AI and aMCC has repeatedly been observed to covary with negative affect ratings, both during self-experienced pain and when observing others suffering (Jackson et al., 2005; Cheng et al., 2007; Lamm et al., 2007; Saarela et al., 2007; Singer et al., 2008; Akitsuki and Decety, 2009). Finally, we previously observed that activations in AI and aMCC are parametrically modulated by individual differences in empathic experiences for distressing videos in the SoVT (Klimecki et al., 2012). However, the activation changes stemming from empathy training were not limited to AI, but instead spanned the entire insular cortex. This accords with a key role of insular cortex in integrating interoceptive information (Craig, 2009; Lamm and Singer, 2010).
In contrast to empathy training, cultivating feelings of kindness, warmth and concern induced non-overlapping brain changes in mOFC, pACC and striatum. These findings extend previous functional imaging findings on compassion in cross-sectional studies (Beauregard et al., 2009; Kim et al., 2009) and one short-term intervention study from our group (Klimecki et al., 2012). For example, viewing sad facial expressions with a compassionate stance was observed to activate ventral striatum and VTA/SN (Kim et al., 2009). In addition, activations in the head of caudate and VTA occurred when participants applied unconditional love toward pictures of intellectually disabled individuals (Beauregard et al., 2009). Finally, the present results mirror our previous findings on the effects of short-term compassion training (Klimecki et al., 2012) in a network involving mOFC, striatum and VTS/SN. In general, mOFC, pACC and ventral striatum activations have been shown to be centrally implicated in reward processing (Haber and Knutson, 2010) as well as in the experience of pleasure and positive affect (Kringelbach and Berridge, 2009). In addition to this convergence with previous neuroimaging findings on positive affect and reward, activations in prefrontal cortex and ventral striatum have been related more specifically to maternal affiliation (Strathearn et al., 2009), as well as to maternal and romantic love (Bartels and Zeki, 2004). Similarly, studies in rodents and other mammals suggest that the formation of affiliative memories relies on a circuitry comprising mOFC, ventral striatum and ventral tegmental area (Depue and Morrone-Strupinsky, 2005). Furthermore, animal models distinguish between different affective and motivational systems such as panic and care systems that rely on distinct brain networks and neurotransmitter systems (Panksepp, 2011). In line with this notion, our results suggest that empathy and compassion indeed rely on antagonistic affective systems and that even short-term training of compassion has the potential to counteract empathic distress.
The observed increases in brain activation after compassion and empathy training also differed with respect to their location in the cingulate cortex. Empathy training led to an increase of activation in aMCC. A recent meta-analysis of 939 studies (Shackman et al., 2011) found that aMCC is crucial for processing negative affect, pain and cognitive control. Converging results were provided by a different meta-analysis (Beckmann et al., 2009) which reported that aMCC is implicated in processing pain, conflict monitoring and error detection. In addition, this part of cingulate cortex was found to be highly connected to dorsal prefrontal regions (Beckmann et al., 2009). In keeping with this structural connectivity, training empathy in this study increased activations in both aMCC and DLPFC. The comparison of cingulate cortex locations from fMRI studies on reward processing revealed a more anterior activation (Beckmann et al., 2009), which converges with the present observation of pACC involvement in compassion. Consistent with this notion, this part of the cingulate cortex was shown to be highly connected with ventral striatum and OFC (Beckmann et al., 2009).
In summary, the present findings reveal that already short-term affective intervention programs can induce reliable experiential and neural plasticity. More importantly, we could show that training two seemingly similar social emotions altered brain activation in non-overlapping neural networks and changed affective responses of opposing valence. Whereas empathy training increased negative affect and activation in associated brain circuits, compassion training reversed these effects by strengthening positive affect and activation in networks associated to affiliation and reward. Compassion may, therefore, represent a very potent strategy for preventing burnout. In light of high prevalence rates of burnout and stress-related diseases in Western societies, we anticipate that the present findings will inform other intervention studies on the plasticity of adaptive social emotions. As this study only focused on females, future studies are needed to address whether the observed training effects can also be generalized to the male population. In the long run, the gained insights will hopefully help to design new training programs aimed at increasing resilience and coping strategies in many domains, including health care, educational settings and high-stress environments in general.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
FUNDING
This work was supported by grants from the University of Zurich, the Swiss National Science Foundation and the European Research Council (205557).
Supplementary Material
Acknowledgments
We thank the participants, the people who assisted in conducting the experiments, Claus Lamm for advice and Stephan Liebig for help with the figures. Our gratitude especially goes to the teachers Catherine Felder, Annette Rentz-Lühning, Jotika Hermsen and Ariya Ñani.
REFERENCES
- Akitsuki Y, Decety J. Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage. 2009;47:722–34. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Batson CD, Fultz J, Schoenrade PA. Distress and empathy: two qualitatively distinct vicarious emotions with different motivational consequences. Journal of Personality. 1987;55:19–39. doi: 10.1111/j.1467-6494.1987.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Courtemanche J, Paquette V, St-Pierre EL. The neural basis of unconditional love. Psychiatry Research. 2009;172:93–8. doi: 10.1016/j.pscychresns.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience. 2009;29:1175–90. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133:1515–25. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower GH. Analysis of a mnemonic device. American Scientist. 1970;58:496–510. [Google Scholar]
- Cheng Y, Lin CP, Liu HL, et al. Expertise modulates the perception of pain in others. Current Biology. 2007;17:1708–13. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–50. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Miller PA, et al. Relation of sympathy and personal distress to prosocial behavior: a multimethod study. Journal of Personal and Social Psychology. 1989;57:55–66. doi: 10.1037//0022-3514.57.1.55. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neuropsychological mechanisms linking social ties with physical health. Nature Neuroscience. 2012;15:669–74. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35:903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. Journal of Personal and Social Psychology. 2008;95:1045–62. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8021–6. doi: 10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neuroscience and Biobehavioral Reviews. 2009;33:1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Keltner D, Goetz JL. Compassion. In: Baumeister RF, Vohs KD, editors. Encyclopedia of Social Psychology. Thousand Oaks, CA: Sage Publications; 2007. pp. 159–61. [Google Scholar]
- Kim JW, Kim S-E, Kim J-J, et al. Compassionate attitude towards others' suffering activates the mesolimbic neural system. Neuropsychologia. 2009;47:2073–81. doi: 10.1016/j.neuropsychologia.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Lamm C, Singer T. Functional neural plasticity and associated changes in positive affect after compassion training. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs142. doi:10.1093/cercor/bhs142. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Sciences. 2009;13:479–87. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One. 2007;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214:579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Leiberg S, Klimecki O, Singer T. Short-term compassion training increases prosocial behavior in a newly developed prosocial game. PLoS One. 2011;6:e17798. doi: 10.1371/journal.pone.0017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS One. 2008;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nature Reviews Neuroscience. 2009;10:141–52. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- McCray LW, Cronholm PF, Bogner HR, Gallo JJ, Neill RA. Resident physician burnout: is there hope? Family Medicine. 2008;40:626–32. [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Cross-species affective neuroscience decoding of the primal affective experiences of humans and related animals. PLoS One. 2011;6:e21236. doi: 10.1371/journal.pone.0021236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another's face. Cerebral Cortex. 2007;17:230–7. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Salzberg S. Loving-kindness: The Revolutionary Art of Happiness. Boston, MA: Shambhala; 2002. [Google Scholar]
- Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis) American Journal of Psychiatry. 2004;161:2295–302. doi: 10.1176/appi.ajp.161.12.2295. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Snozzi R, Bird G, et al. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8:781–91. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–66. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, Erk S. The temporal dynamics of voluntary emotion regulation. PLoS One. 2009;4:e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.