Abstract
Social anxiety disorder (SAD), which is characterized by the fear of being rejected and negatively evaluated, involves altered brain activation during the processing of negative emotions in a social context. Although associated temperament traits, such as shyness or behavioral inhibition, have been studied, there is still insufficient knowledge to support the dimensional approach, which assumes a continuum from subclinical to clinical levels of social anxiety symptoms. This study used functional magnetic resonance imaging (fMRI) to examine the neural bases of individual differences in social anxiety. Our sample included participants with both healthy/subclinical as well as clinical levels of social anxiety. Forty-six participants with a wide range of social anxiety levels performed a gender decision task with emotional facial expressions during fMRI scanning. Activation in the left anterior insula and right lateral prefrontal cortex in response to angry faces was positively correlated with the level of social anxiety in a regression analysis. The results substantiate, with a dimensional approach, those obtained in previous studies that involved SAD patients or healthy and subclinical participants. It may help to refine further therapeutic strategies based on markers of social anxiety.
Keywords: social anxiety disorder, dimensional approach, anger, insula, lateral prefrontal cortex
INTRODUCTION
Social anxiety disorder (SAD) is characterized by the fear and avoidance of social situations (Leray et al., 2011), and by sensitivity to cues of disapproval by others (Stein, 1999; Kessler, 2003; Ashbaugh et al., 2005; Hirsch et al., 2006). Patients suffering from SAD exhibit extreme worry about being embarrassed, humiliated or judged by others (American Psychiatric Association, 2000). High SAD symptom severity is often associated with depression or substance abuse and significantly impaired quality of life (Kaufman and Charney, 2000; Aderka et al., 2012).
There is strong evidence that emotion processing impairments are a core feature of SAD (Turk et al., 2005; Jacobs et al., 2008). Because SAD involves fear of rejection and hypersensitivity to criticism, most studies have used socially threatening stimuli, such as angry or contemptuous faces (Mohlman et al., 2007; Quadflieg et al., 2008; Arrais et al., 2010). SAD patients exhibit heightened sensitivity to angry faces, or are more likely to misinterpret neutral faces as angry ones (Mogg et al., 2004; Mohlman et al., 2007; Hunter et al., 2009; Arrais et al., 2010; Beaton et al., 2010; Bell et al., 2011). Functional magnetic resonance imaging (fMRI) studies addressing the processing of emotional facial expressions in SAD have reported abnormal neural activity in several limbic structures: the amygdala, the insula, the anterior cingulate cortex (ACC); and in the fusiform gyrus and the prefrontal cortex (PFC). Many studies have found SAD patients to display exaggerated responses in the amygdala (Phan et al., 2006; Evans et al., 2008; Miskovic and Schmidt, 2012) and insula (Klumpp et al., 2012; Miskovic and Schmidt, 2012) to threat-related stimuli. Increased activation of the ACC during the perception of disgust (Amir et al., 2005), and an increased activation of the insula in response to threat (Etkin and Wager, 2007; Klumpp et al., 2012) have also been observed. Finally, the lateral, dorsolateral and medial PFC displayed increased activity during the processing of happiness and fear (Campbell et al., 2007).
Using data from a subsample of the National Comorbidity Survey Replication, constituted of adults who had reported excessive social fear in their lifetime, Ruscio (2010) found that multiple taxometric procedures and consistency tests converged on a dimensional solution, suggesting that SAD is continuous with milder social anxiety. Other studies also support the hypothesis of a continuum of social anxiety symptoms from healthy individuals to SAD patients (Kollman et al., 2006; but see also Weeks et al., 2010). Some neuroimaging studies were interested in dimensions close to social anxiety (e.g. healthy individuals exhibiting subclinical levels of shyness or behavioral inhibition), in an attempt to identify the neural underpinnings of social anxiety (Stein et al., 2007; Pujol et al., 2009; Beaton et al., 2010; Carlson et al., 2011; Battaglia et al., 2012). These studies recorded similar patterns of activation to those seen in SAD participants, especially concerning activations of amygdala, fusiform gyrus or insula. For instance, in a non-clinical sample of 22 young healthy adults which varied in terms of social anxiety symptoms intensity, Pujol et al. (2009) found a correlation of amygdala response to happy or fearful faces with social anxiety ratings. Those results were obtained after controlling for fusiform gyrus activation.
In line with a conception of a continuum in social anxiety, Battaglia et al. (2012) aimed to identify trait markers and vulnerability processes by using a longitudinal design. They found that N400 event-related potential amplitudes (which could reflect emotion processing in temporo-frontal regions including amygdala) acquired at age 8–9 among shy children predicted the number of SAD symptoms regarding the Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV, American Psychiatric Association, 2000) at age 14–15, and that fMRI activation in reaction to anger (defined as a prototypical signal of social rejection) in the left amygdala was positively correlated with SAD symptoms.
Those studies, which aimed to address the issue of a continuum between health and psychopathology as regard to social anxiety, had several limitations. (i) Some of them were based on a restricted range of social anxiety symptoms, including either participants with high levels of symptoms (i.e. SAD patients) or with low or no symptom (i.e. healthy participants), thus failing to span the full ‘continuum’ of social anxiety symptoms. (ii) They tended to focus either on a limited range of basic emotions, ignoring some key emotions such as anger (Pujol et al., 2009) or fear (Battaglia et al., 2012), or on a small number of regions of interest (ROIs), ignoring some key regions such as the insula (Pujol et al., 2009; Battaglia et al., 2012). (iii) Some of them may not have enough statistical power (Desmond and Glover, 2002; Murphy and Garavan, 2005; Mumford and Nichols, 2008). (iv) They did not control for general anxiety or depressive symptoms in their statistical analyses, thus failing to ensure the specificity of their results concerning social anxiety.
This study examined the neural bases of individual differences in social anxiety from healthy to clinical individuals, including subclinical participants. The goal was to investigate the potential continuum between healthy and SAD levels, and to address the above-mentioned limitations. More specifically, (i) this study used the Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987) to index social anxiety symptoms across the continuum; (ii) examined the full range of social anxiety using a dimensional and psychometrically informed approach (Krueger and Piasecki, 2002); (iii) examined the range of basic emotions (i.e. anger, fear, happiness and sadness), without clustering negative emotions to examine the specific contribution of emotions relevant to social anxiety (i.e. fear and anger) compared with other basic positive and negative emotions; (iv) examined a larger number of ROIs, including the insula; (v) used a larger sample than previous studies (i.e. 46 participants) including participants with both healthy/subclinical as well as clinical levels of social anxiety; (vi) controlled for state and trait anxiety, as well as depressive symptoms, in the statistical analyses.
In line with the core definition of social anxiety (i.e. heightened sensitivity to cues that may signal a risk of social rejection), we expected a positive and linear association between social anxiety symptoms and activations in response to anger within brain regions that have previously been implicated in anxiety disorders (Etkin and Wager, 2007), namely the amygdala, the fusiform gyrus, the insula, the ACC and the lateral PFC.
METHODS
Participants
Participants were recruited among students and employees of the University of Reims Champagne-Ardenne and healthcare training institutes. A total of 468 participants (184 men, mean age = 23.7 years, s.d. = 4.8; 284 women, mean age = 22.2 years, s.d. =6.4) agreed to fill out a 10-item ad hoc questionnaire comprising six general anxiety items and four specific social anxiety items. This screening questionnaire (Supplementary material 1) was adapted from a questionnaire used by de Lima Osório et al. (2007). We then preselected a sample of 57 right-handed (verified with the Edinburgh Inventory, Oldfield, 1971) participants with a score on the screening questionnaire of either 4 (i.e. with a high level of presumed social anxiety symptoms), or 0 or 1 (i.e. with a low level of social anxiety symptoms) to ensure a sufficient contrast between selected participants.
Procedure and clinical assessment
Participants were individually administered a structured clinical face-to-face interview for current and lifetime psychiatric disorders (The Mini-International Neuropsychiatric Interview, MINI, Sheehan et al., 1998). Their level of social anxiety symptoms was assessed with the LSAS (Safren et al., 1999). According to Mennin et al. (2002), the standard cut-off greater or equal to 30 reflects a clinical level of SAD symptoms. General anxiety was assessed with the State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983). Depressive mood was assessed with the 13-item version of the Beck Depression Inventory (BDI-13; Beck et al., 1988). The trait-anxiety STAI subscale was administered at inclusion only, whereas the state-anxiety STAI subscale was administered twice: at inclusion and immediately before the fMRI session, along with the BDI.
Exclusion criteria were: (i) past or present history of neurological or psychiatric disorders (except SAD), including current or past major depressive episodes, current psychotropic drug use and substance-related disorders (abuse or dependence); (ii) non-compliance with the study protocol or data acquisition failure (such as head movements). According to these criteria, 11 participants were excluded (four due to data acquisition failure). The final sample consisted of 46 participants, with 14 meeting the criteria of SAD (according to MINI) and LSAS scores showing clinical levels of symptomatology (ranging from 30 to 97). Finally, this sample reflected a continuum of participants with clinical and subclinical levels of social phobia, as well as healthy participants.
All participants conformed to standard health and safety regulations regarding the use of MRI. The local ethics committee approved the study. Participants freely gave their written informed consent prior to the study and received €30 for their participation.
Material and stimuli
Stimuli consisted of images drawn from an emotional facial expression battery created specially for this study based on Ekman and Friesen’s criteria (Ekman and Friesen, 1976; Ekman, 2004). Male and female actors were asked to produce emotions of happiness, fear, anger, sadness and ‘emotional neutrality’. As suggested by previous studies, hair and clothing were removed from the images to show only faces (Blair et al., 2001; Minzenberg et al., 2007). Eighty images were rated by an independent sample of 97 volunteers. Faces were selected if agreement about the nature of emotion was above 70%, with a confidence rating above 75% (Supplementary Table S1).
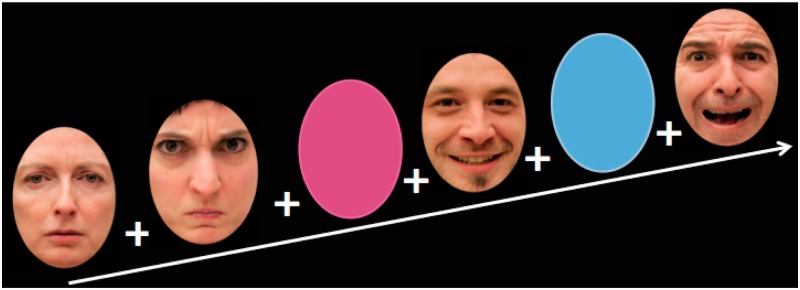
We used an event-related design. Each event encompassed the display of a fixation cross at the center of the screen for 200–900 ms, followed by a male or female face, or a blue or pink oval shape, for 1000 ms, followed by a mask representing a degraded face (sex and emotion unidentifiable) for 150 ms, as suggested by Esteves and Öhman (1993). Faces expressed happiness, fear, anger, sadness or emotional neutrality. Participants performed a task that did not directly make reference to the emotional expression of the faces. That is, they were asked to make a gender/color decision by pressing a button on a two-button response box placed under the right hand (index for male/blue and middle finger for female/pink). The fixation cross was sometimes unexpectedly followed by a blank screen instead of a face or an oval shape, to maximize attention and introduce ‘jittering’ to minimize inter-event correlation: participants were instructed to remain at rest during those blank screens and wait for the next stimulus. The material consisted of 40 pictures of emotional facial expressions, 40 shape stimuli and 40 blank screens (Figure 1). Pictures of emotional facial expressions were as follows: eight fear, eight anger, eight sadness, eight happiness and eight neutral expressions of 16 different actors, with 50% of them presenting a male. The shapes consisted of 20 blue and 20 pink ovals, identical except for color. The different categories of events (emotional facial expressions or shapes followed by masks, blank screens) were pseudo-randomly distributed.
Fig. 1.
Sample of the faces and shape used in the fMRI paradigm.
Stimuli were projected onto a screen (28 in wide and 37 in high) with an Epson EB-G5300 video projector (Epson France, Seiko Epson Corporation) and E-Prime software (Psychology Software Tools, Pittsburgh, PA, USA). Stimuli were viewed through a prismatic mirror mounted on the head coil.
To familiarize the participants with the task, the MRI environment (MRI table, screen and response pad) and the experiment’s timing constraints, a training session took place at the beginning of the fMRI session while participants were on the MRI table. During this training session, participants were given feedback after each response. During the experimental session, participants were instructed to respond as quickly as possible, and no feedback was given.
Image acquisition and fMRI statistical analysis
Images were acquired during a single session, using a 3-T whole-body MRI scanner (Achieva, Philips Medical Systems, Best, The Netherlands) with an eight-channel head coil. Head motions were minimized with a forehead strap and comfortable padding around the participant’s head. For each participant, a T1-weighted anatomical image oriented parallel to the AC-PC was first acquired using a fast field echo sequence (T1-FFE; TR =253 ms, TE =2.30 ms, flip angle =80°, 38 axial slices, slice thickness = 4.50 mm, no interslice gap, FOV = 240 × 240 mm2, matrix =268 × 214 and acquisition voxel size =0.43 × 0.43 × 4.5 mm3). Functional data were acquired using an ascending slice acquisition 2D T2*-weighted EPI sequence sensitive to blood-oxygen-level-dependent contrast in the same axial plane as the T1-weighted structural images (2D-T2*-FFE-EPI; EPI factor = 39, TR =2000 ms, TE =30 ms, flip angle =90°, 38 axial slices, slice thickness =3 mm, no gap, matrix = 80 × 72, FOV = 240 × 216 mm2 and acquisition voxel size = 3 × 3 × 4.5 mm3). To minimize susceptibility artifacts and optimize limbic activity recording, a shim was positioned over the limbic and insular structures, excluding the sinus cavities. The 300 functional volumes were collected during a single functional session (total scan time = 10 min 16 s).
The fMRI data were analyzed using statistical parametric mapping (SPM8, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm/software/spm8. MRIcro software (www.mccauslandcenter.sc.edu/mricro) was used for image conversion. Five initial brain volumes of the functional run were discarded to eliminate non-equilibrium effects of magnetization. Functional images were spatially realigned to the first volume, slice-time corrected and normalized to the standard space of the Montreal Neurological Institute (MNI) brain. Spatial smoothing was performed with an isotropic three-dimensional Gaussian filter with a full width at half maximum of 8 mm. A high-pass filter was implemented using a cut-off period of 128 s to remove low-frequency drift from the time series.
For each participant, first-level contrast images were generated. Regressors of interest were computed by convolving the canonical hemodynamic response function with the onset of the following events: anger, happiness, fear, sadness, neutral and shapes. The six movement parameters estimated during the realignment procedure were entered as covariates into the model to control for the variance caused by micro-movements of the head. This task used novel stimuli. To assess its validity, the contrast neutral faces > shapes and the contrast emotional > neutral faces were first analyzed to identify those brain regions that were involved in face processing and emotion processing, respectively. The results showed expected activations; for example, activations of the fusiform gyrus across all participants contrasts in the contrasts neutral faces > shapes, or amygdala activation in the contrast emotional > neutral faces. Details of these activations are presented in Supplementary Table S2. Then, to examine our hypothesis, we used sensitive contrasts (anger, fear, happiness, sadness and neutral vs shapes). A second-level false discovery rate (FDR) corrected t-test was then conducted for each of these contrasts with a threshold of P= 0.05 (k > 10). Finally, to examine individual differences in brain activation according to levels of social anxiety, we then computed a second-level regression with the LSAS score as the variable of interest. To avoid false positives due to non-independent voxel selection, we ensured that we had a large enough sample (in this analysis, N= 46) when performing the ROI analyses (Lieberman et al., 2009; Vul et al., 2009). ROIs were selected a priori, based on the meta-analysis by Etkin and Wager (2007): fusiform gyrus, amygdala, insula, ACC and lateral PFC (referenced as the middle frontal gyrus). These were defined bilaterally from the AAL brain atlas (Tzourio-Mazoyer et al., 2002) using WFU PickAtlas (Maldjian et al., 2003, 2004). The different emotional conditions (e.g. angry > shape contrasts for each participant) were tested in separate regression models. Significance was set at P< 0.001, uncorrected for multiple comparison to maximize the specificity of within-subjects effects, and optimize the sensitivity for between-subjects effects. Behavioral statistical analyses were processed using Statistica version 9 (StatSoft Inc., Tulsa, OK, USA).
RESULTS
Participants’ characteristics
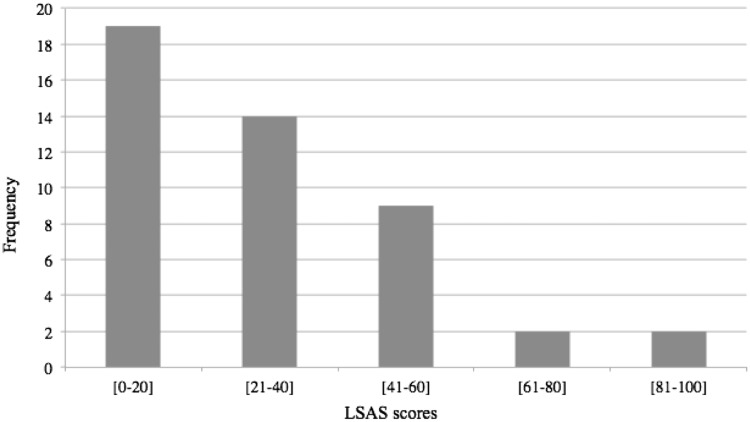
The characteristics of the participants are set out in Table 1. The Shapiro–Wilk test (0.17, P < 0.15) revealed that we could not reject the hypothesis of normality of our sample as regard the LSAS total score. Scores are represented in Figure 2. Levels of state anxiety differed significantly between the clinical interview and the fMRI session (STAI-State 1: mean =33.13, s.d. = 9.18; STAI-State 2: mean = 41.28, s.d. = 6.47; t= −4.94, P < 0.001).
Table 1.
Mean (s.d.) [range] characteristics of participants (N = 46)
| Sex ratio (women/men) | 27/19 |
| Age (years) | 21.00 (2.50) [18–30] |
| Education level (years) | 12.49 (1.18) [12–16] |
| LSAS | 30.47 (23.69) [0–97] |
| STAI-Trait | 36.85 (10.27) [21–61] |
| STAI-State (clinical examination) | 33.13 (9.18) [20–60] |
| STAI-State (fMRI session) | 41.28 (6.47) [28–65] |
| BDI-13 | 3.29 (3.67) [0–10] |
Fig. 2.
Scores of the participants at the LSAS.
Behavioral results
In the gender decision task, the mean reaction time was 730.03 ms (s.d. = 417.30), and the mean accuracy rate was 95% (s.d. = 11.17). There was no significant correlation between the LSAS score and either the accuracy rate (r= −0.26, P= 0.14) or the reaction time (r= 0.18, P= 0.26) during the gender decision task. Behavioral data were missing for two participants because of technical issues.
Brain imaging data
Mapping brain responses to emotional faces (main task effect)
We examined the activation of selected ROIs (see Methods section) for each contrast of interest (i.e. anger vs shape, happiness vs shape, sadness vs shape and fear vs shape) with P < 0.05, FDR corrected t-test for multiple comparisons (Table 2).
Table 2.
Activation of brain ROIs during the emotional facial expressions fMRI paradigm (P < 0.05, FDR corrected, k = 10)
| Anger > shape | Fear > shape | Happiness > shape | Sadness > shape | Neutral > shape | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | MNI (x; y; z) | t | MNI (x; y; z) | t | MNI (x; y; z) | t | MNI (x; y; z) | t | MNI (x; y; z) | |
| Fusiform | ||||||||||
| R | 13.69 (1268) | 28; −86; −12 | 11.70 (1154) | 28; −84; −14 | 12.09 (1045) | 28; −82; −14 | 8.02 (395) | 26; −86; −12 | 10.93 (1050) | 28; −84; −14 |
| L | 10.94 (1126) | −20; −88; −10 | 10.24 (1065) | −36; −86; −14 | 11.30 (918) | −22; −86; −18 | 6.34 (182) | −22; −86; −18 | 10.47 (999) | −36; −86; −14 |
| Amygdala | ||||||||||
| R | 4.68 (180) | 30; 2; −24 | 4.94 (107) | 30; 4; −26 | 3.66 (17) | 20; −2; −18 | 4.18 (44) | 28; 0; −26 | 3.04 (18) | −20; −4; −18 |
| L | 5.18 (306) | −24; 0; −28 | 4.18 (301) | −18; −2; −22 | 3.89 (143) | −18; −6; −18 | 4.70 (67) | −26; −4; −24 | – | – |
| ACC | ||||||||||
| R | – | – | 4.29 (563) | 1; 22; 30 | 3.28 (303) | 2; 54; 10 | – | – | – | – |
| L | – | – | 3.51 (563) | −8; 30; 24 | 3.36 (145) | −1; 32; 30 | – | – | – | – |
| Insula | ||||||||||
| R | 2.86 (15) | 40; 8; 2 | 5.01 (461) | 26; 24; −12 | 3.75 (333) | 38; 26; −6 | – | – | 3.57 (170) | 32; 26; −4 |
| L | 3.70 (122) | −36; 20; −6 | 5.74 (350) | −32; 22; 4 | 3.40 (26) | −40; 18; −8 | – | – | 3.92 (302) | −36; 10; −4 |
| Middle frontal | ||||||||||
| R | 3.55 (64) | 48; −4; 58 | 3.97 (74) | 50; −4; 58 | 4.57 (656) | 28; 38; 32 | 4.16 (51) | 42; −6; 54 | 3.47 (140) | 44; 0; 52 |
| L | 3.31 (26) | −30; −6; 52 | 5.89 (337) | −30; 34; 32 | 5.11 (546) | −32; 44; 38 | – | – | 3.30 (120) | −34; 40; 40 |
R, right; L, left. Scores in parentheses below the t-values represent cluster size.
In all conditions (neutral and emotional faces), the fusiform gyrus was activated, in accordance with its expected role in the processing of faces. Activation in bilateral amygdala was found in all emotional conditions. The middle frontal was activated bilaterally for anger, fear, happiness and neutral conditions, but only activated in the right hemisphere for sadness. The insular cortex was bilaterally activated in all conditions except sadness. Finally, the ACC was activated bilaterally for fear and happiness.
Regressions with the LSAS score
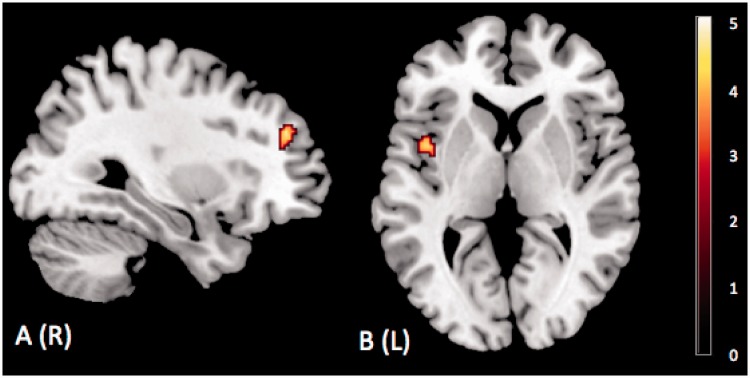
Finally, we examined individual differences in the activation of a priori selected ROIs (see Methods section) for each contrast of interest (anger vs shape, happiness vs shape, sadness vs shape, fear vs shape and neutral vs shape) in relation to the LSAS total score (P < 0.001 uncorrected, k > 10). Using SPM 8, we computed a linear regression analysis. Significant activations were only found for the comparison between anger and shape. This analysis revealed positive correlations between the LSAS score and the activation of the right lateral PFC (x= 30; y = 44; z = 28; k = 40; t = 4.39) and the left anterior insula (x = −42; y = 4; z = 4; k = 77; t = 4.18) (Figure 3). To estimate the proportion of variance explained by the LSAS score at the peak voxel, we calculated the R2 value for each brain area activated using the formula [R2 = sign(t)/sqrt(dof/(t*t) + 1)2] (Hays, 1994). Considering the LSAS total score, the R2 value at the peak voxel was 0.34 for the insular cortex and 0.41 for the PFC (P < 0.05).
Fig. 3.
Regression results. Brain activation in anger > shape condition. (A) Right prefrontal dorsolateral cortex (x = 30; y = 44; z = 28) and (B) left insular cortex (x = −42; y = 4; z = 4) (P < 0.001 uncorrected, extent threshold k = 10).
When we adjusted for state anxiety (fMRI session), the correlation remained significant for the lateral PFC (x = 30; y = 44; z = 28; k = 29; t = 4.25) and the insula (x = −44; y = 6; z = 40; k = 77; t = 3.96). When we adjusted for trait anxiety and depression, the correlation only remained significant for the lateral PFC (STAI-Trait: x = 30; y = 44; z = 28; k = 41; t = 4.34; and BDI: x = 32; y = 44; z = 12; k = 34; t = 3.93).
DISCUSSION
This study investigated the neural bases of individual differences in social anxiety among healthy individuals, subclinical and clinical participants. The primary goal of the study was to show that the relationship between patterns of neural activation and SAD symptoms that have been observed in previous studies using restricted samples of either healthy-only or SAD-only participants exist on a linear level across all people.
The main finding was the positive correlation between the LSAS score and the activation of the left anterior insula and right lateral PFC for the processing of anger. In the literature, the correlation between insular cortex activation during angry face processing and social anxiety has been regarded as reflecting the role of this brain structure in aversive emotion processing and anxiety (Paulus and Stein, 2006; Stein et al., 2007; Klumpp et al., 2012). The insular cortex is involved in integrating emotional information from the limbic system with self-referential processing (Damasio et al., 2000; Paulus and Stein, 2006; Schmidt et al., 2010). Activation of the left insula has been observed in SAD patients, as well as shy and anxiety-prone individuals (Stein et al., 2007; Beaton et al., 2010; Carlson et al., 2011). Activation of the right lateral PFC may correspond to early emotion regulation processes triggered by the insular activation (Morecraft et al., 2004; Stein et al., 2007; Goldin et al., 2009; Vrtička et al., 2011). In the context of social anxiety, the joint activation of the anterior insula and the lateral PFC, as revealed by the processing of angry emotional facial expressions, is consistent with the role of these two regions in processing the emotional distress associated with social rejection (Eisenberger and Lieberman, 2004). The recruitment of the lateral PFC, coupled with the insula, is thought to minimize and regulate personal distress during social rejection vs acceptance. Here, we suggest that SAD symptoms may correlate with an increased distress, as signaled by an increased insula activation, thus requiring down-regulation by the lateral PFC. Although in healthy subjects, lateral PFC activation might eventually lead to less distress (Kross et al., 2007), SAD symptoms may be related to a failure of this process. Further studies are needed to test this hypothesis. Within this framework, the lack of a correlation with the dorsal ACC, which may relate more closely to expectancy violation than to social rejection (Somerville et al., 2006), is not surprising, given that the emotional facial expressions paradigm adopted (gender decision task) did not involve high levels of cognitive control.
This study was conducted among participants from the general population. The lack of linear relationship with the amygdala during the regression analyses may reveal that the insula has a more ‘dose-response’ relationship with the SAD symptomatology during the processing of social rejection cues such as angry faces. Our results provide new arguments about a linear activity in insula and lateral PFC in the whole continuum of social anxiety.
As suggested by Yiend (2010), healthy participants and SAD patients are likely to represent the two extremes of a Gaussian distribution. In this framework, most individuals fall between these two extremes. As our study aimed at testing the hypothesis of a continuum, our choice of population was designed to cover the whole range of social anxiety symptoms severity. As a consequence, our sample included participants with various levels of social anxiety symptoms. Our participant sample was found to have a continuity of scores from healthy to diagnostic levels. Our results thus suggest that emotional response could be qualitatively similar in individuals with low levels of social anxiety and in those with more severe social anxiety symptoms, but with quantitative variations. Nevertheless, a possible limitation of this study is that even if the test of normality of SAD symptoms provides a sufficient foundation for considering this sample to reflect a ‘continuous’ range of social anxiety symptoms, the sampling procedure was based on extreme values of SAD scores.
The paradigm we used included only basic emotions such as fear, sadness and anger. Thus, we did not include stimuli that might be more specifically related to social threat, such as contemptuous or disapproving expressions, or dynamic faces that express social rejection (Stein et al., 2002; Burklund et al., 2007; Heuer et al., 2010; Bell et al., 2011). This point is a limitation of this study, which needs replication with more complex emotional facial expressions. Further research is therefore needed to replicate this type of paradigm with dynamic expressions, to highlight the temporal effects of those expressions.
In conclusion, our study showed that the social anxiety dimension is associated with specific brain responses to angry faces, especially in the continuum from healthy to clinical levels. Social anxiety levels were positively correlated with the activation of the left anterior insula, which is involved in integrating emotions with social cognition, and the right lateral PFC, which mediates executive functions and emotion regulation. Studies of the brain structures involved in reactions to emotional stimuli in non-clinical participants are key to defining the trait markers of anxiety disorders (Stein et al., 2007). Here, we have extended this research to the continuum of social anxiety (Pujol et al., 2009). Given that many mental disorders, including SAD, lie on a continuum from health to clinical forms, the need to improve our understanding of these disorders may warrant the investigation of this continuum at the cerebral level (Lemogne et al., 2011). Such results may help to identify patterns that are specific to clinical cases and to refine therapeutic strategies based on these state and trait markers.
CONTRIBUTORS
A.C., F.G., E.T., L.P. and F.L. designed the study and wrote the protocol. A.C., F.G., E.T., D.R.C. and B.R.P. performed the study. A.C., F.G., C.B.R., C.L. and F.L. conducted the literature searches and analyses. A.C., F.G., C.L., A.K. and C.P. undertook the statistical analyses (behavioral and neuroimaging). A.C. wrote the first draft of the manuscript. All authors significantly participated in interpreting the results and revising the manuscript. All authors contributed to and have approved the final manuscript.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
Part of this study was presented at the 19th Congress of European Psychiatry (Vienna, Austria, March 2011).
The authors wish to thank the teams of the psychiatry and neuroradiology departments (Reims University Hospital) for their assistance, Sylvie Berthoz for her helpful advice and the cultural department of the University of Reims Champagne-Ardenne (SUAC—Françoise Mittelette) for its support in constructing the EFE battery. They also wish to thank Kathleen Smith for her benevolent proofreading of English. This research was funded by the Reims University Hospital research program (CHU de Reims—AOL-2008—Troubles anxieux & émotions). A.C. was supported by a scientific grant from Champagne-Ardenne Regional Council.
REFERENCES
- Aderka IM, Hofmann SG, Nickerson A, Hermesh H, Gilboa-Schechtman E, Marom S. Functional impairment in social anxiety disorder. Journal of Anxiety Disorders. 2012;26:393–400. doi: 10.1016/j.janxdis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (DSM-IV-TR) Diagnostic and Statistical Manual of Mental Disorders. 4th edn. 2000. text revision. Washington, DC: American Psychiatric Press, Inc. [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–81. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Arrais KC, Machado-de-Sousa JP, Trzesniak C, et al. Social anxiety disorder women easily recognize fearful, sad and happy faces: the influence of gender. Journal of Psychiatric Research. 2010;44:535–40. doi: 10.1016/j.jpsychires.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Ashbaugh AR, Antony MM, McCabe RE, Schmidt LA, Swinson RP. Self-evaluative biases in social anxiety. Cognitive Therapy and Research. 2005;29:387–98. [Google Scholar]
- Battaglia M, Zanoni A, Taddei M, et al. Cerebral responses to emotion expressions and the development of social anxiety disorder: a preliminary longitudinal study. Depression and Anxiety. 2012;29:54–61. doi: 10.1002/da.20896. [DOI] [PubMed] [Google Scholar]
- Beaton EA, Schmidt LA, Schulkin J, Hall GB. Neural correlates of implicit processing of facial emotions in shy adults. Personality and Individual Differences. 2010;49:755–61. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Bell C, Bourke C, Colhoun H, Carter F, Frampton C, Porter R. The misclassification of facial expressions in generalised social phobia. Journal of Anxiety Disorders. 2011;25:278–83. doi: 10.1016/j.janxdis.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Colledge E, Murray L, Mitchell DGV. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29:491–8. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Eisenberger NI, Lieberman MD. The face of rejection: rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Social Neuroscience. 2007;2(3–4):238–53. doi: 10.1080/17470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DW, Sareen J, Paulus MP, Goldin PR, Stein MB, Reiss JP. Time-varying amygdala response to emotional faces in generalized social phobia. Biological Psychiatry. 2007;62:455–63. doi: 10.1016/j.biopsych.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Social Cognitive and Affective Neuroscience. 2011;6:74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- de Lima Osório F, Crippa JA, Loureiro SR. A study of the discriminative validity of a screening tool (MINI-SPIN) for social anxiety disorder applied to Brazilian university students. European Psychiatry. 2007;22:239–43. doi: 10.1016/j.eurpsy.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. Journal Of Neuroscience Methods. 2002;118(2):115–28. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman M. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Science. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ekman P. Emotions Revealed. Understanding Faces and Feelings. London: Phoenix; 2004. [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Esteves F, Öhman A. Masking the face: recognition of emotional facial expressions as a function of the parameters of backward masking. Scandinavian Journal of Psychology. 1993;34:1–18. doi: 10.1111/j.1467-9450.1993.tb01096.x. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety. 2008;25(6):496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry. 2009;66:1091–9. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays WL. Statistics. 5th edn. Orlando, FL: Harcourt Brace College Publishers; 1994. p. 682. [Google Scholar]
- Heuer K, Lange W-G, Isaac L, Rinck M, Becker ES. Morphed emotional faces: Emotion detection and misinterpretation in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41(4):418–25. doi: 10.1016/j.jbtep.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Clark DM, Mathews A. Imagery and interpretations in social phobia: support for the combined cognitive biases hypothesis. Behavior Therapy. 2006;37:223–36. doi: 10.1016/j.beth.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hunter LR, Buckner JD, Schmidt NB. Interpreting facial expressions: the influence of social anxiety, emotional valence, and race. Journal of Anxiety Disorders. 2009;23:482–8. doi: 10.1016/j.janxdis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Snow J, Geraci M, et al. Association between level of emotional intelligence and severity of anxiety in generalized social phobia. Journal of Anxiety Disorders. 2008;22:1487–95. doi: 10.1016/j.janxdis.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depression and Anxiety. 2000;12:69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatrica Scandinavica. 2003;108:19–27. doi: 10.1034/j.1600-0447.108.s417.2.x. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological Psychology. 2012;89:273–6. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman DM, Brown TA, Liverant GI, Hofmann SG. A taxometric investigation of the latent structure of social anxiety disorder in outpatients with anxiety and mood disorders. Depression & Anxiety. 2006;23:190–9. doi: 10.1002/da.20158. [DOI] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience. 2007;19:945–56. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Piasecki TM. Toward a dimensional and psychometrically-informed approach to conceptualizing psychopathology. Behaviour Research and Therapy. 2002;40:485–500. doi: 10.1016/s0005-7967(02)00016-5. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Gorwood P, Bergouignan L, Pelissolo A, Lehéricy S, Fossati P. Negative affectivity, self-referential processing, and the cortical midline structures. Social Cognitive and Affective Neuroscience. 2011;6:426–33. doi: 10.1093/scan/nsq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leray E, Camara A, Drapier D, et al. Prevalence, characteristics and comorbidities of anxiety disorders in France: results from the “Mental Health in General Population” survey (MHGP) European Psychiatry. 2011;26:339–45. doi: 10.1016/j.eurpsy.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Berkman ET, Wager TD. Correlations in social neuroscience aren't voodoo: commentary on Vul et al. (2009) Perspectives on Psychological Science. 2009;4:299–307. doi: 10.1111/j.1745-6924.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems of Pharmacopsychiatry. 1987;22:141–73. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach Atlas. NeuroImage. 2004;21:450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM, Heimberg RG, Schneier FR, Davies SO, Liebowitz MR. Screening for social anxiety disorder in the clinical setting: using the Liebowitz Social Anxiety Scale. Journal of Anxiety Disorders. 2002;16(6):661–73. doi: 10.1016/s0887-6185(02)00134-2. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Research: Neuroimaging. 2007;155:231–43. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA. Social fearfulness in the human brain. Neuroscience and Biobehavioral Reviews. 2012;36:459–78. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–5. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Carmin CN, Price RB. Jumping to interpretations: social anxiety disorder and the identification of emotional facial expressions. Behaviour Research & Therapy. 2007;45:591–9. doi: 10.1016/j.brat.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: new insights from neuroscience. The Neurologist. 2004;10:235–49. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Nichols TE. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage. 2008;39(1):261–8. doi: 10.1016/j.neuroimage.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Deriving the optimal number of events for an event-related fMRI study based on the spatial extent of activation. Neuroimage. 2005;27(4):771–7. doi: 10.1016/j.neuroimage.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of Handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Pujol J, Harrison BJ, Ortiz H, et al. Influence of the fusiform gyrus on amygdala response to emotional faces in the non-clinical range of social anxiety. Psychological Medicine. 2009;39:1177–87. doi: 10.1017/S003329170800500X. [DOI] [PubMed] [Google Scholar]
- Quadflieg S, Mohr A, Mentzel H-J, Miltner WHR, Straube T. Modulation of the neural network involved in the processing of anger prosody: the role of task-relevance and social phobia. Biological Psychology. 2008;78:129–37. doi: 10.1016/j.biopsycho.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Ruscio AM. The latent structure of social anxiety disorder: consequences of shifting to a dimensional approach. Journal of Abnormal Psychology. 2010;119:662–71. doi: 10.1037/a0019341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Heimberg RG, Horner KJ, Juster HR, Schneier FR, Liebowitz MR. Factor structure of social fears: the Liebowitz Social Anxiety Scale. Journal of Anxiety Disorders. 1999;13:253–70. doi: 10.1016/s0887-6185(99)00003-1. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Mohr A, Miltner WHR, Straube T. Task-dependent neural correlates of the processing of verbal threat-related stimuli in social phobia. Biological Psychology. 2010;84:304–12. doi: 10.1016/j.biopsycho.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RC, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stein MB. Coming face-to-face with social phobia. American Family Physician. 1999;60:2244. [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American Journal of Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Turk CL, Heimberg RG, Luterek JA, Mennin DS, Frescos DM. Emotion dysregulation in generalized anxiety disorder: a comparison with social anxiety disorders. Cognitive Therapy and Research. 2005;29:89–106. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vrtička P, Sander D, Vuilleumier P. Effects of emotion regulation strategy on brain responses to the valence and social content of visual scenes. Neuropsychologia. 2011;49:1067–82. doi: 10.1016/j.neuropsychologia.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4:274–90. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Weeks JW, Carleton RN, Asmundson GJG, McCabe RE, Antony MM. “Social Anxiety Disorder Carved at its Joints”: evidence for the taxonicity of social anxiety disorder. Journal of Anxiety Disorders. 2010;24:734–42. doi: 10.1016/j.janxdis.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Yiend J. The effects of emotion on attention: a review of attentional processing of emotional information. Cognition and Emotion. 2010;24(1):3–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.