Abstract
The vast majority of attempts to quit smoking cigarettes are unsuccessful. Negative affect (NA) is one of the primary factors contributing to smoking relapse, in part because it interferes with psychological processes that are essential for self-regulation and coping. Converging evidence suggests that NA may be less of a problem for smokers with high relative to low dispositional self-control, but very little is known about the mechanisms that underlie this effect. We used functional magnetic resonance imaging to address this issue by examining the associations between trait self-control, state levels of NA and patterns of brain activation in nicotine-deprived smokers (n = 117) during the performance of a verbal n-back paradigm (a task requiring cognitive processes that support self-regulation). While the activation of several brain regions linked to executive control correlated positively and negatively with state NA and trait self-control, respectively, an interaction between these factors was identified in only one region: the ventromedial prefrontal cortex (vmPFC). We conclude that the functions supported by the vmPFC are an important source of variability in smokers’ self-regulatory functioning and propose that the region may contribute to the use of implicit forms of self-control under demanding circumstances.
Keywords: individual differences, negative affect, self-control, smoking, ventromedial prefrontal cortex
INTRODUCTION
Successfully quitting smoking is a supremely challenging endeavor, and efforts to understand this sustained self-regulatory effort likely require a multidisciplinary approach integrating the perspectives of emotion, cognition and neuroscience. Such comprehensive methods may be particularly useful for characterizing the mechanisms through which cessation attempts are derailed by negative affect (NA), one of the primary factors contributing to smoking relapse (Kassel et al., 2003; Baker et al., 2004). NA is thought to undermine attempts to quit smoking in part because it interferes with psychological processes that play a central role in behavioral regulation (Shiffman, 2005; McCarthy et al., 2010). According to one prominent model (the self-regulatory strength model of self-control; Baumeister and Heatherton, 1996), NA is problematic because it prompts emotion regulation, which in turn draws from a limited pool of domain-general resources supporting self-control.1 That is, attempts to manage unpleasant emotions temporarily ‘deplete’ resources necessary for additional acts of self-control (e.g. managing craving), thereby increasing vulnerability to relapse. Broadly consistent with this view, research suggests that NA does not affect the probability that quitting smokers engage in coping during high-risk moments but does substantially reduce the effectiveness of coping at such times (Shiffman, 2005).
The self-regulatory strength model holds that individuals vary widely in the capacity for self-control (Tangney et al., 2004). From this perspective, some smokers are likely to be more successful at self-regulation in the face of strong NA than are others, an idea supported by research examining constructs related to self-control. Brown et al. have conducted a series of studies investigating individual differences in distress tolerance (the ability to tolerate NA and psychological/physical discomfort) as a risk factor for smoking relapse and a potential target for intervention (for review, see Brown et al., 2005, 2008). In conceptually related work, Brandon et al. have examined task persistence (the act of persisting in an effortful or unpleasant task) as a predictor of cessation outcomes (e.g. Brandon et al., 2003; Steinberg et al., 2012). Collectively, these interrelated lines of research have demonstrated that individual differences in the ability to persevere at cognitive and behavioral tasks despite distress are strongly associated with the likelihood of success at quitting smoking. Distress tolerance and task persistence—themselves similar constructs (Brandon et al., 2007)—overlap substantially with the core features of most theories of self-control, including the self-regulatory strength model. It therefore may be inferred that NA is less of an impediment for smokers with high relative to low levels of self-control. More generally, the findings are consistent with the idea that smokers with high self-control are better able to regulate their behavior while experiencing unpleasant emotions because they have greater self-regulatory resources, compared to those with low self-control.
A fundamental question remains, however—precisely what are the putative resources that underlie such individual differences in self-regulatory ability? Recently, a small number of studies have addressed this question indirectly by attempting to link self-control depletion effects to physiological and neurobiological processes. Gailliot et al. (2007) observed that the depletion of self-control was associated with reductions in blood glucose levels. Using electroencephalography, Inzlicht and Gutsell (2007) linked depletion to an attenuation of the error-related negativity, a waveform associated with activation of the anterior cingulate cortex (ACC). Similarly, in a study employing functional magnetic resonance imaging (fMRI), Hedgcock et al. (2012) found that choice-related activation of the dorsolateral prefrontal cortex (dlPFC) was reduced following depletion. Finally, using fMRI, Wagner and Heatherton (2013) demonstrated that amygdala reactivity to negative emotional scenes was enhanced subsequent to the depletion of self-control resources and that the magnitude of this change was inversely related to trait self-control.
While these innovative studies have yielding promising results, much remains unknown about the neural mechanisms that underlie individuals differences in the ability to exert self-control under taxing conditions. The goal of this study was to address this issue, with a focus on characterizing the neurobiological substrates of individual differences in the ability to regulate behavior during elevated NA among smokers. More specifically, we used fMRI to investigate the associations between dispositional self-control, state NA and brain activity during effortful cognitive processing in moderate-to-heavy daily smokers following acute nicotine deprivation. For regular smokers, brief abstinence from nicotine results in significant increases in NA (Shiffman et al., 2006; Hughes, 2007), with the magnitude of this effect varying across individuals (Piasecki et al., 2003; McClernon et al., 2008). Participants were scanned while performing a verbal n-back task, and ideal paradigm for exploring the connections between self-control and NA for two reasons. First, the n-back requires use of working memory and attentional control—core cognitive processes essential for higher order self-regulatory functions (Kane and Engle, 2002; Patterson et al., 2010; Hofmann et al., 2012). Second, patterns of brain activation during the n-back are influenced by both task difficulty (Owen et al., 2005) and negative emotional states (e.g. Weerda et al., 2010). When combined with fMRI, the n-back, therefore, offers a useful framework for investigating the brain mechanisms through which high self-control reduces the detrimental impact of NA on smokers’ ability to utilize attention and working memory to support self-regulation, more broadly.
In sum, the overarching goal of this work was to provide a deeper understanding of what makes some smokers more successful at regulating their behavior in the face of NA than others. Toward this end, we sought to identify brain regions for which dispositional self-control influenced the relationship between state NA and activation during the n-back, with a particular interest in frontal cortical areas implicated in executive control and regulatory processing both broadly (Heatherton and Wagner, 2011; Mischel et al., 2011) and in the context of smoking, specifically (Curtin et al., 2006; Brody et al., 2007; Kober et al., 2010; Berkman et al., 2011; Nestor et al., 2011). By including a large sample, this study provided a distinctively well-powered opportunity to identify such nuanced associations.
METHODS
Participants
Participants were drawn from two fMRI studies. Study 1 (Wilson et al., 2012) examined the effects of quitting motivation and smoking opportunity on cue-elicited neural responses; the study included both males and females and smokers who were and who were not motivated to quit smoking. Study 2 (Wilson et al., 2013) examined neural responses associated with different strategies for coping with a smoking cue coupled with the opportunity to smoke; the study included male smokers who were motivated to quit smoking. For both studies, participants had to report smoking an average of 15–40 cigarettes per day for the past 24 months and had to be right-handed. All participants from Study 1 and Study 2 who completed relevant questionnaires and tasks were pooled to form the sample used herein. A total of 117 participants (77 from Study 1 and 40 from Study 2) were included in the present analyses (see Table 1 for sample characteristics). Informed consent was obtained, and procedures were approved by the local Institutional Review Board. Individuals were paid for their participation.
Table 1.
Means (s.d.) for select sample characteristics
| Full sample (n = 117) | Study 1 (n = 77) | Study 2 (n = 40) | |
|---|---|---|---|
| Percent male | 74 | 60 | 100 |
| Age | 30.8 (7.9) | 29.7 (7.3) | 33.0 (8.7) |
| Years of formal education | 12.8 (2.1) | 12.8 (2.4) | 12.7 (1.6) |
| Number of cigarettes per day | 19.8 (4.9) | 19.7 (5.2) | 20.0 (4.3) |
| FTND score | 4.9 (1.6) | 5.0 (1.6) | 4.6 (1.6) |
Self-control scale
The Self-Control Scale (SCS) consists of 36 self-descriptive items (e.g. ‘I have a hard time breaking bad habits’) rated on five-point scale anchored by 1 (‘Not at all like me’) and 5 (‘Very much like me’) (Tangney et al., 2004). Scores on the SCS have been found to correlate with various behaviors thought to require self-control (e.g. regulation of eating behavior; Tangney et al., 2004). A total score indexing trait self-control was obtained by summing all 36 responses (minimum and maximum possible score of 36 and 180, respectively; higher scores indicate higher trait self-control).
Positive and negative affect schedule
The Positive and Negative Affect Schedule (PANAS) consists of 10 adjectives describing positive affective states (e.g. excited) and 10 adjectives describing negative affective states (e.g. irritable), each rated along a five-point scale from 1 (‘Very slightly or not at all’) to 5 (‘Extremely’) (Watson et al., 1988). The PANAS may be used to assess affect over various time frames by altering the instructions. To measure state affect, participants completed the PANAS with the following instructions: ‘Indicate to what extent you feel this way at the present moment’. This study focused specifically on the NA subscale of the PANAS. A total score indexing state NA was calculated by summing ratings for the relevant adjectives (minimum and maximum possible score of 10 and 50, respectively; higher scores indicate stronger state NA).
Verbal n-back task
The n-back task consisted of 36 s blocks, during which 12 randomly selected English letters were presented individually (500 ms stimulus duration and 2500 ms interstimulus interval). Participants performed two versions of the task that varied in working memory load: a version with minimal memory requirements (0-back), during which participants were instructed to press a button with their right index finger if a specific target (the letter ‘X’) appeared; and a version with comparatively high memory load (3-back), during which participants were instructed to press a button with their right index finger if the currently presented letter matched the letter presented three items previously. For the 3-back, participants were encouraged to rehearse the three most recently presented letters while continuously updating their list as each new letter appeared. For both the 0-back and 3-back, participants were instructed to push a button with their right middle finger for all non-target items. For both versions, the probability of an item being a target, new distracter or repeat distracter was 33%, 47% and 20%, respectively. Participants also were given 36 s rest periods (Rest) during which they were asked to relax and view a central fixation cross. Participants performed a single 288 s run consisting of the following sequence of blocks: 0-back, 3-back, Rest, 0-back, 3-back, Rest, 0-back, 3-back.
Procedure
Prior to the experiment, participants attended a separate baseline screening session that consisted of the completion of several tasks and questionnaires, including the SCS (for details, see Wilson et al., 2012, 2013). They also provided a baseline carbon monoxide (CO) sample (BreathCo, Vitalograph, Lenexa, KS, USA). Participants were instructed to abstain from smoking and using any other nicotine-containing products for at least 12 h before the experimental session.
Upon arriving for the experimental visit, a second CO sample was collected to assess compliance with deprivation instructions. Participants had to have a CO level that was at least 50% lower than their baseline, a cutoff established based upon research using similar procedures (e.g. Sayette et al., 2008). Participants then completed the PANAS and additional questionnaires. Next, participants were informed about whether or not they would be permitted to smoke during the study, rated their urge to smoke on a 0–100 scale and then were placed in the scanner. After the collection of anatomical images, participants completed the verbal n-back task. Subsequently, participants completed an fMRI-based cigarette cue exposure paradigm (see Wilson et al., 2012, 2013), and then were debriefed and paid.
fMRI data acquisition and analysis
Scanning was conducted using a 3-T Siemens Allegra magnet (Siemens Corporation, New York, NY, USA) equipped with a standard transmit/receive head coil. Prior to functional scanning, an anatomical series was obtained using a T2-weighted pulse sequence (repetition time (TR) = 6600 ms, echo time (TE) = 73 ms, flip angle = 150°, 3.125 × 3.125 × 3.0 mm voxels and 40 oblique axial slices). In addition, a high-resolution three-dimensional structural volume was collected using a magnetization prepared rapid acquisition gradient echo sequence (TR = 1540 ms, TE = 3.04 ms, flip angle = 8°, 1 × 1 × 1 mm voxels and 160 slices). Next, functional images were acquired in the same plane as the 40-slice anatomical series using a one-shot echo-planar imaging pulse sequence (TR = 2000 ms, TE = 25 ms, field of view (FOV) = 20 cm, flip angle = 79°, 3.125 × 3.125 × 3.0 mm voxels).
Analysis of fMRI data was conducted using utilities from the following software pages: Analysis of Functional NeuroImages (AFNI, Version 2.6; Cox, 1996), Automated Image Registration (AIR, Version 3.08; Woods et al., 1992), FMRIB’s Software Library (FSL, Release 4.1; Smith et al., 2004) and the NeuroImaging Software Package (NIS 3.5; Laboratory for Clinical Cognitive Neuroscience, University of Pittsburgh and the Neuroscience of Cognitive Control Laboratory, Princeton University). Software integration and image format conversion was implemented using the Functional Imaging Software Widgets graphical computing environment (Fissell et al., 2003). Before analysis, functional images were corrected for head motion and adjusted for drift within and between runs. Each participant’s anatomical image was co-registered to a common reference anatomy using a six-parameter rigid-body automated registration algorithm and the transformation matrix generated during this step then was applied to the corresponding functional images. Subsequently, functional images were globally mean normalized and smoothed using a three-dimensional Gaussian filter (8-mm full width at half maximum). Group-based statistical maps were transformed into Montreal Neurological Institute (MNI) stereotaxic space for anatomical localization.
The primary aim of analysis was to examine the relationship between trait self-control, state NA and the degree to which brain activation changed with increasing demands during n-back task performance. fMRI data were analyzed using a random effects general linear model (GLM) implemented on a voxel-wise (i.e. whole brain) basis. First, predictors for each n-back condition (i.e. 0-back and 3-back) were entered into a GLM to obtain parameter estimates for each participant. Next, contrast images (3-back minus 0-back) were generated for each participant. Finally, these contrast images were entered into a voxel-wise multiple regression to examine the associations between self-control, NA and task-related brain activation. The regression model included a total of six covariates: three dummy coded control variables [smoking expectancy condition (expecting to smoke during study; not expecting to smoke during study); sex (male, female) and motivational status (quitting-motivated, quitting-unmotivated)] and three covariates of interest [trait self-control (scores on SCS; mean-centered); state NA (scores on PANAS; mean-centered) and the trait self-control by state NA interaction (product of the mean-centered trait self-control and state NA covariates)]. We were particularly interested in identifying regions for which trait self-control modulated the association between state NA and task-related brain activation (i.e. those for which the interaction of trait self-control and state negative was significant).
Monte Carlo simulations indicated that a combined per-voxel threshold of P < 0.005 and cluster-extent threshold of 41 or more contiguous voxels (i.e. 1201 mm3) would yield a corrected map-wise false positive rate of P < 0.05. These parameters were applied to all statistical maps.
RESULTS
Levels of trait self-control and state NA
The mean score on the SCS was 113.58 (s.d. = 19.94; range = 62–165). The mean score on the PANAS was 17.00 (s.d. = 5.52; range = 10–38). Scores on the SCS and PANAS were negatively correlated, r(117) = −0.39, P < 0.001. (Regression diagnostics indicated no evidence of problems with multicollinearity between these variables.)
We also examined the association between the primary factors of interest (i.e. trait self-control and state NA) and the smoking-related variables that were measured, including number of cigarettes smoked per day, level of nicotine dependence [as assessed using the Fagerstrom Test for Nicotine Dependence (FTND); Heatherton et al., 1991] and self-reported urge to smoke immediately preceding the scan session. State NA was positively correlated with level of nicotine dependence [r(117) = 0.26, P = 0.005] and pre-scan urge [r(117) = 0.24, P = 0.009]. All remaining associations were non-significant (P’s > 0.1). [All fMRI results reported below remain significant when controlling for level of nicotine dependence (FTND scores) and pre-scan urge ratings. Results also remain significant when controlling for trait levels of NA.]
N-back performance
Response accuracy and reaction time data were collected during the n-back. To quantify participants’ ability to distinguish targets from non-targets, accuracy was converted to the signal detection metric d-prime (d'). Performance was above chance for both the 0-back and 3-back conditions (P’s < 0.001). As expected, participants performed better (as indexed by d') in the 0-back condition (mean = 3.76, s.d. = 0.92) than in the 3-back condition (mean = 2.00, s.d. = 1.17), t(116) = 13.79, P < 0.001. In addition, participants responded more quickly in the 0-back condition (mean = 670.65 ms, s.d. = 198.53) than in the 3-back condition (mean = 840.15 ms, s.d. = 208.12), t(116) = 12.27, P < 0.001. There was a marginally significant negative correlation between state NA and 3-back d' scores, r(117) = −0.16, P = 0.09. None of the remaining correlations between state NA, trait self-control and n-back performance was significant (P’s > 0.1).
Activation during N-back task
Details regarding brain regions exhibiting a main effect of task load are presented elsewhere (Nichols et al., 2013). Briefly, consistent with previous research using the n-back, activation during the 3-back condition was significantly greater than activation during the 0-back condition bilaterally in the dlPFC, premotor cortex and inferior parietal lobule, as well as in the dorsal ACC.
Neural correlates of trait self-control, state NA and their interaction
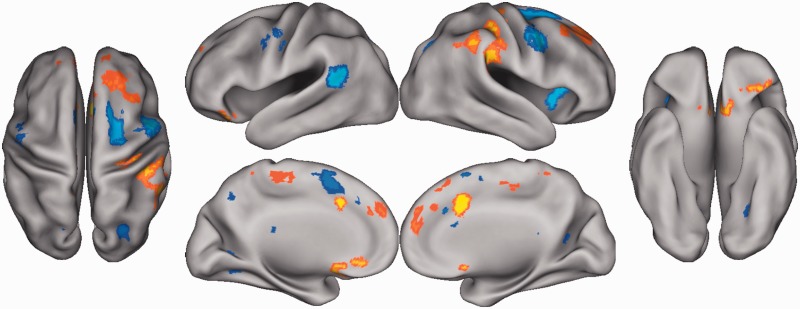
Brain regions for which activation during the n-back task (3-back minus 0-back) was significantly associated with state NA (scores on the PANAS) are presented in Table 2 and depicted in Figure 1. As shown, state NA was positively associated with activation in several regions, including the dorsal ACC and a large portion of the ventromedial prefrontal cortex (vmPFC) encompassing the subgenual ACC. No negative correlations between state NA and activation during the n-back task were found.
Table 2.
Regions for which activation during the n-back task (3-back minus 0-back) was positively associated with state NA
| Region | MNI coordinates |
|||||
|---|---|---|---|---|---|---|
| BA | Size (mm3) | x | y | z | Average F ratio | |
| R medial frontal g/paracentral lobule | 5/6 | 8719 | 11 | −23 | 44 | 9.70 |
| R medial frontal g/superior frontal g | 8/9 | 8128 | 8 | 48 | 11 | 9.17 |
| Dorsal ACC | 32 | 1238 | 9 | 17 | 29 | 9.77 |
| vmPFC/vlPFC (subgenual ACC/medial frontal g/inferior frontal g) | 11/25/47 | 3966 | −27 | 25 | −18 | 9.65 |
| R inferior parietal lobule | 40 | 6553 | 57 | −32 | 17 | 12.46 |
Stereotaxic coordinates are given for local maxima of activation cluster in MNI atlas space. g, gyrus; R, right hemisphere; vlPFC, ventrolateral prefrontal cortex.
Fig. 1.
Areas of the brain for which activation during the n-back (3-back minus 0-back) was positively associated with state NA (depicted in yellow/orange) and negatively associated with trait self-control (depicted in blue). Activation maps are shown on an inflated surface map (neurological orientation) in Caret (Van Essen, 2005).
As presented in Table 3 and Figure 1, a significant negative association between activation during the n-back task and trait self-control (scores on the SCS) was observed in numerous areas, including the dorsal ACC and bilateral middle frontal gyrus. No positive correlations between trait self-control and activation during the n-back task were found.
Table 3.
Regions for which activation during the n-back task (3-back minus 0-back) was negatively associated with trait self-control
| Region | MNI coordinates |
|||||
|---|---|---|---|---|---|---|
| BA | Size (mm3) | x | y | z | Average F ratio | |
| R superior frontal g/middle frontal g | 6 | 3375 | 21 | 3 | 48 | 10.08 |
| R middle frontal g | 6 | 2644 | 52 | 4 | 34 | 9.94 |
| L middle frontal g | 6 | 1744 | −41 | −6 | 29 | 10.33 |
| L middle frontal g | 10 | 1294 | −30 | 41 | 5 | 10.25 |
| Dorsal ACC/supplementary motor area | 6/32 | 3459 | −9 | 30 | 42 | 9.54 |
| R insula | 13 | 2644 | 41 | 12 | −3 | 8.76 |
| L superior temporal g/supramarginal g | 22/40 | 1153 | −52 | −48 | 12 | 9.74 |
| R angular g | 39 | 6666 | 35 | −61 | 37 | 9.34 |
| L precuneus | 7 | 7481 | −13 | −60 | 38 | 9.89 |
| L lingual g | 19 | 3488 | −30 | −63 | −4 | 9.53 |
Stereotaxic coordinates are given for local maxima of activation cluster in MNI atlas space. L, left hemisphere.
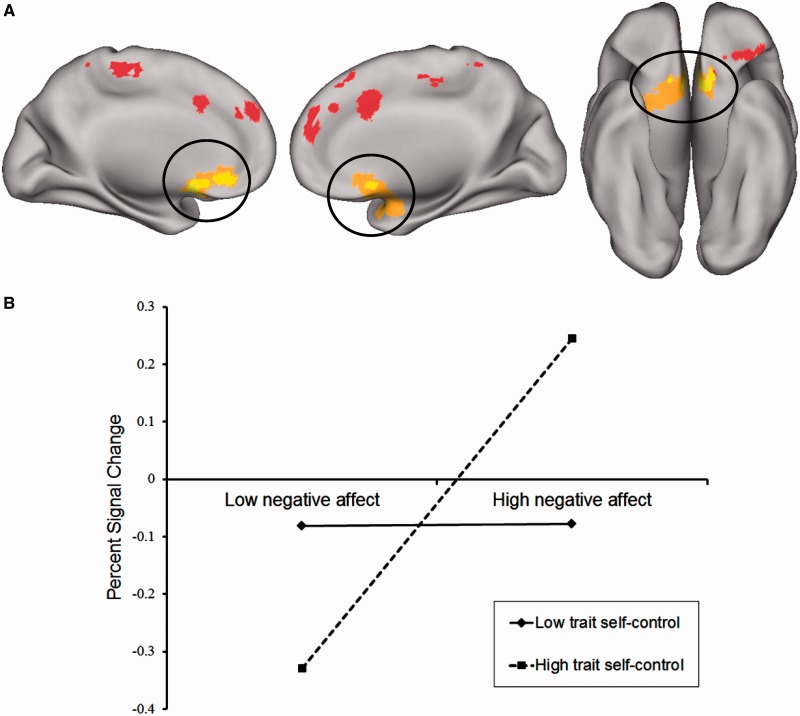
Our primary goal was to identify regions exhibiting activation associated with the interaction between trait self-control and state NA. We observed such an effect in a large region of the vmPFC including the subgenual ACC [MNI coordinates x = 6, y = 14, z = −17; size = 4078 mm3; Brodmann’s areas (BAs) 11 and 25; average F ratio = 11.24]. As depicted in Figure 2A, this region subsumed the portion of the vmPFC for which activation during the n-back was positively associated with state NA. We probed the nature of the observed interaction by evaluating simple slopes at high (mean + 1 s.d.) and low (mean – 1 s.d.) levels of trait self-control, as plotted in Figure 2B. Among those with high self-control, there was a strong positive association between state NA and activation of the vmPFC during n-back task performance (β = 0.77, P < 0.001). In contrast, among those with low self-control, state NA and activation of the vmPFC were not significantly related (β = 0.01, P = 0.90).
Fig. 2.
(A) The main effect of state NA and the interaction of state NA and trait self-control were associated with activation during the n-back in partially overlapping portions of vmPFC (circled). Voxels exhibiting activation associated only with state NA and state NA X trait self-control are depicted in red and orange, respectively, whereas overlapping voxels are depicted in yellow. Activation maps are shown on an inflated surface map (neurological orientation) in Caret (Van Essen, 2005). (B) The association between state NA and activation of the vmPFC during the n-back task (3-back minus 0-back) plotted at high (mean + 1 s.d.) and low (mean – 1 s.d.) values of trait self-control.
We also conducted follow-up analysis to determine whether activation of the vmPFC was related to n-back task performance. Specifically, we assessed the correlation between load-related changes in activation of the vmPFC (3-back minus 0-back) and load-related change in performance (i.e. 0-back d' score minus 3-back d' score; larger values indicate a greater decrease in performance as load increased). We found a significant negative correlation between vmPFC activation and change in n-back performance [r(117) = −0.25, P = 0.006]. Thus, greater increases in activation of the vmPFC from the 0-back to 3-back were associated with smaller load-related decreases in task performance.
DISCUSSION
We examined the associations between trait self-control, state NA and brain responses in nicotine-deprived smokers during the performance of a difficult task requiring the use of working memory and attention (core cognitive processes supporting self-regulation and coping). Our primary aim was to shed light on potential mechanisms through which high trait self-control reduces the impact of NA on regulatory functioning. Behaviorally, it has been shown that stable individual differences in self-control interact with state levels of NA. Our results link these behavioral observations to a neural region—the vmPFC—that exhibits the same interaction pattern. More specifically, high, relative to low, dispositional self-control appears to be related to greater engagement of the processes supported by the vmPFC when it is necessary to perform an effortful cognitive task while simultaneously experiencing a high degree of NA. This interpretation is supported by the observation that there was a robust positive correlation between state NA and vmPFC activity in smokers with relatively high trait self-control, whereas NA and vmPFC activity were not related for smokers with comparatively low trait self-control. Also of interest, greater activation of the vmPFC was associated with better performance as memory load increased, suggesting that the processes supported by the region facilitated the ability to meet the demands of the more challenging n-back condition.
While efforts to develop a unifying theory of vmPFC function are ongoing (Roy et al., 2012), it is clear that the vmPFC plays a key role in affect-related processing. Of particular relevance to this study, findings from several areas of research indicate that the set of interconnected regions that comprise the vmPFC are critical for emotion regulation (e.g. Delgado et al., 2006; Lane, 2008; Phillips et al., 2008). Moreover, individual differences in the ability to regulate emotions are linked to variability in the structure (Milad et al., 2005) and function (e.g. Zald et al., 2002; van Reekum et al., 2007) of the vmPFC. For instance, van Reekum et al. (2007) observed a strong positive correlation between psychological well-being and the degree to which the vmPFC was activated by negative stimuli in a non-clinical sample of older adults, suggesting that the effective recruitment of the vmPFC in response to aversive events is associated with better mental health.
Recent evidence suggests that the vmPFC may be particularly important for automatic or implicit (rather than non-automatic or effortful) efforts to modulate affect (Lane, 2008; Phillips et al., 2008; Berkman and Lieberman, 2009; Etkin et al., 2010; Gyurak et al., 2011). In this study, smokers were not instructed to try to change their emotional state. In addition, the demands associated with performing the n-back task presumably reduced the capacity to simultaneously engage in effortful forms of emotion regulation (i.e. attempts to regulate affect using non-automatic or explicit approaches would be expected to ‘deplete’ the resources necessary for performing a difficult cognitive task, and vice versa). Indeed, we did not observe a significant interaction between trait self-control and state NA in brain areas associated with non-automatic emotion regulation strategies (Ochsner and Gross, 2005; Phillips et al., 2008; Gyurak et al., 2011). This pattern suggests that the benefits of greater self-control may not stem exclusively from having more non-automatic resources to divide between cognitive task performance and the explicit/effortful regulation of affect. Rather, one intriguing possibility is that, among those who were experiencing significant levels of NA, only smokers with relatively high self-control implemented automatic emotion regulation processes (resulting in a corresponding increase in vmPFC activation) while concurrently performing the n-back task. If so, the use of an implicit approach to emotion regulation by those with high self-control, mediated in part by the vmPFC, may have spared the use of explicit control resources, leaving more available for 3-back task performance.
As noted earlier, recent studies have linked the depletion of self-control to changes in the activation of the dorsal ACC (Inzlicht and Gutsell, 2007) and dlPFC (Hedgcock et al., 2012), regions strongly implicated in ‘cold’ cognitive control and, more recently, effortful forms of emotion regulation (Kane and Engle, 2002; Wilson et al., 2004; Ochsner and Gross, 2005). Whereas we observed significant main effects of trait self-control and state NA in regions supporting executive functioning, including the dorsal ACC, our findings indicate that stable individual differences in the ability to utilize self-control under demanding circumstances are associated instead with the vmPFC. Interestingly, Wagner and Heatherton (2013) found that the depletion of self-control was related to a decrease in functional connectivity between the vmPFC and amygdala during the processing of emotional stimuli, suggesting that exhausting regulatory capacity may reduce the ability to use the processes supported by the vmPFC to regulate affective responses. Our findings point towards the possibility that, in addition to such state effects, the vmPFC may be an important locus for stable individual differences in the ability to regulate affect under challenging circumstances.
As noted, several regions exhibited a significant main effect of trait self-control or state NA, including multiple brain areas that reliably exhibit increases in activation during the n-back task (e.g. dorsal ACC/supplementary motor area, lateral premotor cortex, inferior parietal cortex; Owen et al., 2005). A clear pattern emerged across this set of regions. Specifically, trait self-control and state NA were negatively and positively associated with activation during the n-back task, respectively. Previous work indicates that brain activation during the n-back and related tasks increases concomitant with escalations in task demands or difficulty (Owen et al., 2005), at least until capacity limitations are reached and/or the motivation to perform the task ceases (e.g. Jaeggi et al., 2003). In other words, as the n-back becomes harder, activation rises in several task-related regions (e.g. the dlPFC) and vice versa. The observation of a positive correlation between state NA and task-related activation therefore suggests that smokers with higher levels of NA may have had to work harder to perform the n-back than those with lower levels of NA, in accord with prior work in non-clinical samples (e.g. Weerda et al., 2010). In contrast, the negative correlation between trait self-control and activation in several regions supporting task performance indicates that, relative to those with fewer resources, smokers with greater dispositional self-regulatory capacity may have required less effort to successfully perform the task, a pattern that is consistent with previous research (Jaeggi et al., 2007). However, given the complexity of load-effort–activation associations (Callicott et al., 1999; Jaeggi et al., 2003; Nichols et al., 2013), future research is needed to determine precisely how state NA and trait self-control relate to subjective experience and accuracy during cognitive task performance.
It would be particularly useful to assess how these variables relate across a broader range of difficulty, as this study included only two memory load conditions. A second limitation of the study was that we did not assess participants’ affective state during or immediately following the n-back task. Additional research using a finer-grained sampling of affective experience (e.g. by measuring facial expressions, a relatively continuous non-verbal index of affect; Sayette et al., 2003), as well as work focusing on dynamic changes in neural activity as a function of emotion regulation, would be informative. A third limitation is that this study did not include direct manipulations of self-control depletion and NA. While it is likely that participants’ self-regulatory resources were taxed by acute nicotine withdrawal (and, as noted, effects remained significant after controlling for relevant variables, including trait levels of NA), future research assessing behavioral performance and brain activation before and after laboratory-based manipulations designed to exhaust regulatory resources and/or increase NA is indicated. Similarly, although this study focused on NA and we attempted to control for other factors that may have contributed to observed effects (e.g. level of nicotine dependence), we cannot completely rule out the possibility that our findings were driven to some extent by related variables (e.g. other aspects of nicotine withdrawal). Finally, it is worth noting that the study included only moderate-to-heavy smokers who were nicotine-deprived. Whereas we believe that this an important population upon which to focus, future research including additional groups (e.g. non-deprived heavy smokers, light smokers and non-smokers) would be beneficial for determining the extent to which the present results were influenced by smoking status and associated factors.
In summary, we found that trait self-control and state NA exert robust influences on nicotine-deprived smokers’ brain activation while performing a challenging cognitive task. Of particular interest, the current results suggest that the protective effects of high self-control are related to the ability to utilize the processes supported by the vmPFC under demanding conditions, such as when it is necessary to perform a difficult task in the context of strong NA. Efforts to extend these findings will provide important data for understanding individual differences in relapse vulnerability among quitting smokers. Such research also may prove beneficial for refining interventions that focus on bolstering self-control through practice to facilitate smoking cessation and other forms of behavior change (Muraven, 2010; Friese et al., 2011).
Acknowledgments
We thank Deidra Rendinell, Alex Ciuca and Maryam Khatami for their assistance with data collection and Corrine Durisko, Kate Fissel, Scott Kurdilla and Deborah Viszlay for technical assistance. This work was supported by the National Institutes of Health (R01DA02463 to J.A.F., R03DA029675 to S.J.W.).
Footnotes
1 We use both self-regulation and self-control to refer to the capacity to alter or override one’s responses (Baumeister and Heatherton, 1996; Vohs and Baumeister, 2011), although we recognize that distinctions between these terms have been made (e.g. the use of self-regulation as a broader term encompassing both conscious and unconscious processes, but self-control to refer more narrowly to conscious efforts; Baumeister, 2002). We use emotion regulation to refer to the use of non-automatic or automatic processes to changes affective responses (Ochsner and Gross, 2005).
REFERENCES
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. Ego depletion and self-control failure: an energy model of the self's executive function. Self and Identity. 2002;1(2):129–36. [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: an overview. Psychological Inquiry. 1996;7(1):1–15. [Google Scholar]
- Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychological Science. 2011;22(4):498–506. doi: 10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Using neuroscience to broaden emotion regulation: theoretical and methodological considerations. Social and Personality Psychology Compass. 2009;3(4):475–93. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology. 2003;112(3):448–56. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annual Review of Clinical Psychology. 2007;3:257–84. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, et al. Neural substrates of resisting craving during cigarette cue exposure. Biological Psychiatry. 2007;62(6):642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clinical Psychology Review. 2005;25(6):713–33. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, et al. Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. Behavior Modification. 2008;32(3):302–32. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex. 1999;9(1):20–6. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional resonance neuroimages. Computational and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: a model of boundary conditions. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA: Sage Publications, Inc; 2006. pp. 233–50. [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biological Psychology. 2006;73(1):39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry. 2010;167(5):545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissell C, Tseytlin E, Cunningham D, et al. A graphical computing environment for neuroimaging analysis. Neuroinformatics. 2003;1:111–25. doi: 10.1385/ni:1:1:111. [DOI] [PubMed] [Google Scholar]
- Friese M, Hofmann W, Wiers RW. On taming horses and strengthening riders: recent developments in research on interventions to improve self-control in health behaviors. Self and Identity. 2011;10(3):336–51. [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall CN, et al. Self-control relies on glucose as a limited energy source: willpower is more than a metaphor. Journal of Personality and Social Psychology. 2007;92(2):325–36. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cognition and Emotion. 2011;25(3):400–12. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Science. 2011;15(3):132–9. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgcock WM, Vohs KD, Rao AR. Reducing self-control depletion effects through enhanced sensitivity to implementation: evidence from fMRI and behavioral studies. Journal of Consumer Psychology. 2012;22(4):486–95. [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends in Cognitive Science. 2012;16(3):174–80. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine and Tobacco Research. 2007;9(3):315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Gutsell JN. Running on empty: neural signals for self-control failure. Psychological Science. 2007;18(11):933–7. doi: 10.1111/j.1467-9280.2007.02004.x. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Etienne A, Ozdoba C, Perrig WJ, Nirkko AC. On how high performers keep cool brains in situations of cognitive overload. Cognitive, Affective and Behavioral Neuroscience. 2007;7(2):75–89. doi: 10.3758/cabn.7.2.75. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Seewer R, Nirkko AC, et al. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. Neuroimage. 2003;19(2 Pt 1):210–25. doi: 10.1016/s1053-8119(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychonomic Bulletin and Review. 2002;9(4):637–71. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD. Neural substrates of implicit and explicit emotional processes: a unifying framework for psychosomatic medicine. Psychosomatic Medicine. 2008;70(2):214–31. doi: 10.1097/PSY.0b013e3181647e44. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Curtin JJ, Piper ME, Baker TB. Negative reinforcement: possible clinical implications of an integrative model. In: Kassel JD, editor. Substance Abuse and Emotion. Washington, DC: American Psychological Association; 2010. pp. 15–42. [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–57. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10706–11. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, et al. ‘Willpower’ over the life span: decomposing self-regulation. Social Cognitive and Affective Neuroscience. 2011;6(2):252–6. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M. Practicing self-control lowers the risk of smoking lapse. Psychology of Addictive Behaviors. 2010;24(3):446–52. doi: 10.1037/a0018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage. 2011;56(4):2258–75. doi: 10.1016/j.neuroimage.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Nichols TT, Gates KM, Molenaar PCM, Wilson SJ. Greater BOLD activity but more efficient effective connectivity is associated with better cognitive performance within a sample of nicotine-deprived smokers. Addiction Biology. 2013 doi: 10.1111/adb.12060. April 9 (doi:10.1111/adb.12060; epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence. 2010;106(1):61–4. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13(9):829, 833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Experimental and Clinical Psychopharmacology. 2003;11(4):276–85. doi: 10.1037/1064-1297.11.4.276. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Science. 2012;16(3):147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Loewenstein G, Griffin KM, Black JJ. Exploring the cold-to-hot empathy gap in smokers. Psychological Science. 2008;19(9):926–32. doi: 10.1111/j.1467-9280.2008.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Experimental and Clinical Psychopharmacology. 2003;11(3):218–27. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73(6):1715–48. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, et al. Natural history of nicotine withdrawal. Addiction. 2006;101(12):1822–32. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Williams JM, Gandhi KK, Foulds J, Epstein EE, Brandon TH. Task persistence predicts smoking cessation in smokers with and without schizophrenia. Psychology of Addictive Behaviors. 2012;26(4):850–58. doi: 10.1037/a0028375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–62. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, et al. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience. 2007;19(2):237–48. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Baumeister RF, editors. Handbook of Self-Regulation: Research, Theory, and Applications. 2nd edn. New York: Guilford Press; 2011. [Google Scholar]
- Wagner DD, Heatherton TF. Self-regulatory depletion increases emotional reactivity in the amygdala. Social Cognitive and Affective Neuroscience. 2013;8(4):410–17. doi: 10.1093/scan/nss082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weerda R, Muehlhan M, Wolf OT, Thiel CM. Effects of acute psychosocial stress on working memory related brain activity in men. Human Brain Mapping. 2010;31(9):1418–29. doi: 10.1002/hbm.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nature Neuroscience. 2004;7(3):211–4. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Quitting-unmotivated and quitting-motivated cigarette smokers exhibit different patterns of cue-elicited brain activation when anticipating an opportunity to smoke. Journal of Abnormal Psychology. 2012;121(1):198–211. doi: 10.1037/a0025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Neural correlates of self-focused and other-focused strategies for coping with cigarette cue exposure. Psychology of Addictive Behaviors. 2013 doi: 10.1037/a0027055. January 30 (doi:10.1037/a0027055; epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Cherry S, Mazziotta J. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–33. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):2450–4. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]