Abstract
Intracellular Listeria monocytogenes actin-based motility is characterized by significant individual variability, which can be influenced by cytoarchitecture. L. monocytogenes was used as a probe to transmit information about structural variation among subcellular domains defined by mitochondrial density. By analyzing the movement of a large population of L. monocytogenes in PtK2 cells, we found that mean speed and trajectory curvature were significantly larger for bacteria moving in mitochondria-containing domains (generally perinuclear) than for bacteria moving in mitochondria-free domains (generally peripheral). Analysis of bacteria that traversed both mitochondria-containing and mitochondria-free domains revealed that these motile differences were not intrinsic to bacteria themselves. Disruption of mitochondrial respiration did not affect bacterial mean speed, speed persistence, or trajectory curvature. In contrast, microtubule depolymerization lead to decreased mean speed per bacterium and increased mean speed persistence of L. monocytogenes moving in mitochondria-free domains compared with untreated cells. L. monocytogenes were also observed to physically collide with mitochondria and push them away from the bacterial path of motion, causing bacteria to slow down before rapidly resuming their speed. Our results show that subcellular domains along with microtubule depolymerization may influence the actin cytoskeleton to affect L. monocytogenes speed, speed persistence, and trajectory curvature.
INTRODUCTION
The cellular cytoplasm is a highly crowded, structured, and heterogeneous environment. The crowded nature of the cytoplasm is due to the high protein concentration in cells, estimated to range from 200 to 300 mg/ml (reviewed by Luby-Phelps, 2000), which more closely resembles protein crystals than a dilute solution (reviewed by Fulton, 1982). Furthermore, polymers comprising the cellular cytoskeleton provide structural networks that may need to be circumvented for subcellular components to move to different locations in the cell (reviewed by Luby-Phelps, 2000). The cytoplasm is a viscoelastic and dynamic material that contains subcellular regions with distinct mechanical properties; for example, lamellar regions are more rigid than perinuclear areas (Yamada et al., 2000; Tseng et al., 2002). Local cytoplasmic mechanical properties have primarily been measured by tracer probe or magnetic bead mobility, which varies nonlinearly with probe size and subcellular domain in living cells (Luby-Phelps et al., 1986; Luby-Phelps and Taylor, 1988; Bausch et al., 1998; Tseng et al., 2002). At present, it is not completely understood how the heterogeneous cytoarchitecture creates variations in mechanical properties that lead to differences in mobility of intracellular components.
Listeria monocytogenes is a gram-positive foodborne facultative pathogen that has been a productive model to dissect the machinery involved in actin-based motility (reviewed by Beckerle, 1998 and Cameron et al., 2000). Within a few hours after L. monocytogenes encounter the host cell cytoplasm during infection, each bacterium propels itself by assembling an elongated structure of actin filaments and associated proteins commonly referred to as an actin “comet tail” (Tilney and Portnoy, 1989; Sanger et al., 1992; Theriot et al., 1992). This form of bacterial actin polymerization-based motility is required for L. monocytogenes to efficiently spread from cell to cell (Tilney and Portnoy, 1989; Mounier et al., 1990). Because L. monocytogenes only require a single surface protein, ActA, to achieve motility (Smith et al., 1995), these bacteria exploit the host cell by recruiting all additional proteins necessary for actin-based motility from the cytoplasm (reviewed by Cameron et al., 2000). The speed of L. monocytogenes has been the primary motility parameter analyzed in different cell types, cell-free extracts, and purified in vitro systems. Bacterial speed has been reported to vary depending on protein concentrations (Loisel et al., 1999), cell type (Dabiri et al., 1990), and physical environment (McGrath et al., 2003). Qualitatively, it has been noted that bacterial speed varies depending on the region of the cell analyzed and even experimental day (Nanavati et al., 1994). Our understanding of what determines variations in L. monocytogenes actin-based motility is incomplete, but a combination of molecular contributors (Theriot et al., 1994; Loisel et al., 1999; Geese et al., 2002) and intracellular physical and macromolecular architecture (Giardini and Theriot, 2001; McGrath et al., 2003) are likely to contribute to variations in parameters of bacterial motility.
Our study has focused on determining whether parameters of L. monocytogenes motility vary as a function of subcellular domain in PtK2 cells. L. monocytogenes offers the advantage of reaching subcellular regions inaccessible to other probes and being able to overcome large cytoplasmic viscoelastic forces (of the order of nanonewtons, Radmacher et al., 1996; Bausch et al., 1999). We have collected a large data set of L. monocytogenes moving in PtK2 cells and systematically measured bacterial speed, speed persistence, and bacterial trajectory curvature and determined that these parameters vary depending on subcellular location relative to mitochondrial density. We found that bacteria moving in mitochondria-containing domains had greater mean speed and trajectory curvature than bacteria moving in mitochondria-free domains and that these motile behaviors were not intrinsic to the bacteria themselves. We have also observed that bacteria slow down after colliding with mitochondria and rapidly resume their speed. Moreover, alterations in the intracellular architecture, triggered by microtubule depolymerization, were readily measured by changes in L. monocytogenes movement in cells. Thus, we have found that variations in cytoarchitecture can be detected using L. monocytogenes as a kinematic probe of subcellular properties.
MATERIALS AND METHODS
Bacterial Culture and Infection
Constitutively green fluorescent protein (GFP)-expressing Listeria monocytogenes (10403S wild-type strain transformed with pMB2044), kindly provided by Dr. Daniel A. Portnoy (University of California, Berkeley, CA), were grown in BHI broth containing 40 μg/ml chloramphenicol at room temperature for 14–18 h without agitation. For infection, Potoroo tridactylis kidney epithelial (PtK2) cells were grown and infected with L. monocytogenes as described previously (Dabiri et al., 1990).
Pharmacological Treatments
To inhibit ATP synthesis in mitochondria, PtK2 cells were treated with 0.1 mM 2,4-dinitrophenol (DNP) (Sigma-Aldrich, St. Louis, MO) in imaging media (L-15 medium lacking phenol red and supplemented with 10% fetal bovine serum) at 37°C for at least 10 min before data collection. In parallel experiments, mitochondrial membrane depolarization upon DNP treatment was confirmed by loss of tetramethylrhodamine ethyl ester (Molecular Probes, Eugene, OR) mitochondrial fluorescence (Loew et al., 1993; Hudman et al., 2002).
For microtubule depolymerization, 1 μg/ml nocodazole was added to PtK2 cells 2 h postinfection. Cells were immediately incubated on ice for 15 min and allowed to recover at 37°C until fixed or imaged in the continuous presence of nocodazole. Microtubule depolymerization was verified by indirect immunostaining by using mouse DM1alpha anti-tubulin antibodies, a kind gift from Dr. Tim Stearns (Stanford University), along with goat anti-mouse IgG (H+L) fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Southern Biotechnology Associates, Birmingham, AL).
Before fixation and F-actin staining (see below), actin stress fiber formation was induced by incubation of infected PtK2 cells with 200 ng/ml lysophosphatidic acid (LPA) sodium salt (Sigma-Aldrich) for 15 min as described previously (Amano et al., 1997).
Fluorescent Labeling and Quantitative Analysis of Infected Cells
PtK2 cells were fixed 4.5 h after infection with L. monocytogenes 146-KKRK-150 ActA mutant 34 (Lauer et al., 2001) or wild-type 10403S strain (kindly provided by Dr. Daniel A. Portnoy) and incubation with 200 nM MitoTracker Red, CM-H2XRos (Molecular Probes) at 37°C in imaging media for 1 h before fixation. For F-actin and vimentin staining, cells were fixed with 4% electron microscopy-grade paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) for 20 min at room temperature. F-actin was fluorescently labeled with 0.5 μg/ml FITC-conjugated phalloidin (Molecular Probes) for 20 min at room temperature. Intermediate filaments were indirectly labeled with mouse monoclonal anti-vimentin antibodies (Sigma-Aldrich), a gift from Dr. Patrick O. Brown, and goat anti-mouse IgG (H+L) FITC-conjugated secondary antibodies (Southern Biotechnology Associates). For microtubule immunostaining, cells were fixed with methanol at –20°C for 15 min and indirectly labeled as mentioned above. DNA was labeled by incubation with 0.5 μg/ml 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) for ∼2 min at room temperature. Images were collected using an Axioplan microscope (Carl Zeiss, Thornwood, NY) and a cooled charge-coupled device camera (MicroMAX 512BFT; Princeton Instruments).
The fluorescent signals from cytoskeletal elements and mitochondria were quantitated using the “measure grid” option available in MetaMorph software (Universal Imaging). A grid consisting of 361 square regions of 4.5 μm x 4.5 μm in size was positioned to fully span each image and the average fluorescent signal was recorded per region. The average background signal obtained from three regions devoid of cells was subtracted from the signal in each region. These values were normalized using the maximum average signal in a region per image. Normalized fluorescence values of cytoskeletal elements lower than 10% of the maximum fluorescence value per image were omitted from analysis.
Time-Lapse Videomicroscopy and Bacterial Tracking
Coverslips with infected PtK2 cells were imaged at 37°C between 3 and 7 h after infection by mounting them on a temperature-controlled chamber connected to a circulating water bath. Cells were covered with imaging media containing 200 nM MitoTracker Red, CM-H2XRos (Molecular Probes). Time-lapse phase and fluorescent images were acquired using a Nikon Diaphot-300 inverted microscope equipped with a cooled charge-coupled device camera (MicroMAX 512BFT; Princeton Scientific Instruments, Monmouth Junction, NJ) by using MetaMorph software (Universal Imaging, Downingtown, PA). For each PtK2 cell, microscopic images were collected at 10-s intervals for 10–30 min. Each time-lapse sequence collected corresponded to a unique cell. Time-lapse sequences of 21 untreated PtK2 cells from 7 d, 13 DNP-treated cells from 3 d, and 10 nocodazole-treated cells from 3 d were analyzed.
The centroids of individual GFP-expressing bacteria were tracked by thresholding their fluorescent signal and using the “track object” function of MetaMorph for each sequence of time-lapse images. Tracking was interrupted if bacteria went out of focus or reached the plasma membrane and made protrusions. Mitochondrial density surrounding each bacterium in the sequential time-lapse images was determined by drawing a circular region with a constant diameter of 5 μm centered on each bacterium in each image of the track. Numbers from zero to three were assigned by eye to each point of the bacterial track, where zero represented no mitochondrial density and three represented the highest mitochondrial density within the constant circular region around the bacterium.
The data set compiled contained full-length tracks consisting of bacteria that moved exclusively in either mitochondria-containing or mitochondria-free domains during the entire length of the time-lapse sequences collected. Bacteria that were assigned mitochondrial density numbers from one to three in their tracks, as described above, were classified as moving in mitochondria-containing domains of cells. Bacteria that were assigned zero mitochondrial density were classified as moving in mitochondria-free domains of cells. Partial tracks consisting of track segments where bacteria passed through mitochondria-containing or mitochondria-free domains during a portion of the time-lapse sequences collected were also included in the analysis. The shortest bacterial track included in any analysis was 10 time-lapse images (90 s) in length. The data set for bacteria moving in mitochondria-containing domains in untreated PtK2 cells contained 43 full-length and 53 partial tracks from 96 bacteria. Eighty-four tracks from different bacteria moving in mitochondria-free domains in untreated cells were compiled from 37 full-length and 47 partial tracks. Thirty-eight bacteria that traversed both mitochondria-containing and mitochondria-free domains produced tracks with segments that were included in both populations of bacteria moving in mitochondria-containing and mitochondria-free domains. In addition, these 38 bacteria were separately used to compare intratrack bacterial speed differences while traversing mitochondria-containing and mitochondria-free domains of host cells. In DNP-treated cells, 39 full-length and 19 partial tracks from 58 bacteria moving in mitochondria-containing domains, and 12 full-length and 19 partial tracks from 31 bacteria in mitochondria-free domains were examined. In nocodazole-treated cells, 18 full-length and 15 track segments from 33 bacteria moving in mitochondria-containing domains and 15 full-length and 12 partial tracks from 27 bacteria mitochondria-free domains of cells were analyzed.
Quantitative Motility Analysis
Interval speed was calculated by dividing the bacterial displacement between consecutive time-lapse images by the time elapsed between those images (Δd/10 s). Mean interval speeds reported consisted of the average of all interval speeds for each bacterial population. The mean speed per bacterium was calculated for each bacterial track (including track segments) by using interval speeds, and the mean for each bacterial population moving either in mitochondria-containing or mitochondria-free domains of cells was reported. Interval speed and mean speed per bacterium were statistically compared using rank sum and Student's t test (p < 0.05). In this report, the White modification of the Wilcoxon rank sum test was used (Ambrose and Ambrose, 1987). Except where noted, statistically significant differences were determined using both rank sum and Student's t test (p < 0.05). When comparing interval speeds of bacteria in DNP- and nocodazole-treated cells to untreated cells, we randomly truncated the total number of interval speed values from untreated cells to correspond to the number of interval speed values from treated cells and compared statistically.
Bacteria that traversed both mitochondria-containing and mitochondria-free domains cells in a single trajectory (n = 38) and used to compare intratrack speed differences were also compared according to their location relative to mitochondria by calculating the mean interval speed and mean speed per bacterium as described above. Partial track segments from these bacteria were also included in the data set of bacterial populations moving either in mitochondria-containing and mitochondria-free domains of untreated cells. The mean speed of each individual while traversing both domains in a single trajectory was calculated and bacteria were separated based on whether they moved faster or slower than the mean speed of this entire group (n = 38). For each bacterium, the intratrack speed difference was calculated by subtracting the mean speed while the bacterium traversed the mitochondria-free segment of the track from the mean speed while the bacterium traversed the mitochondria-containing segment of the track. A positive value of intratrack speed difference reflected faster average bacterial movement in mitochondria-containing domains than in mitochondria-free domains. Then, intratrack differences were averaged for the entire group (n = 38) and for bacteria moving faster (n = 20) than the mean speed of the entire group. Cross-correlation coefficients between interval speed and mitochondrial density, represented with the numbering system described above, were also calculated for each bacterium and averaged for the entire group (n = 38) and for bacteria moving faster (n = 20) than the mean speed of the entire group.
Speed autocorrelation functions were computed for each bacterial track by using pairs of interval speed measurements at all possible time separations in 10-s increments and averaged per time interval, as described previously (Giardini and Theriot, 2001; Auerbuch et al., 2003), for each population of bacteria moving in mitochondria-containing or mitochondria-free domains. Mean speed autocorrelation coefficients were fitted with a single exponential decay function by using GraphPad Prism 3.02 software (GraphPad Software, San Diego, CA). Fitted curves were restricted to equal one at time 0 and plateau to zero. Decay constants (τ) were determined at a mean speed autocorrelation value of 1/e. Bacterial autocorrelation coefficients were compared per time interval and reported as statistically different when p < 0.05 by using both rank sum and Student's t test for at least all time intervals from 10 to 50 s.
For analysis of trajectory curvature, sections of bacterial tracks that were interrupted during data collection and lacked continuous time points were plotted and treated as separate tracks. Therefore, in untreated PtK2 cells, 96 bacteria moving in mitochondria-containing domains generated 123 bacterial tracks, and 84 bacteria moving in mitochondria-free domains generated 90 bacterial tracks. To quantitatively compare directional persistence of bacteria, we calculated the cosine of the angle between velocity vectors for all possible nonoverlapping pairs of track segments in 10-s time interval increments (Auerbuch et al., 2003). Cosine values were then averaged for each time interval and SEs were calculated. Mean cosine values reflect angles between velocity vectors, which extrapolate to bacterial trajectory curvature. Cosine values calculated from tracks of bacteria moving in mitochondria-containing domains compared with those from bacteria in mitochondria-free domains in untreated cells were reported as significantly different if both rank sum and Student's t test determined that p < 0.05 for all time intervals >20 s. Cosine values computed from tracks of bacteria in DNP- and nocodazole-treated cells were statistically compared with cosine values from bacterial tracks from untreated cells by both rank sum and Student's t test (p <0.05) for at least all time intervals from 20 to 50 s.
RESULTS
L. monocytogenes Moving in Mitochondria-containing Domains Have Greater Speed
To determine whether bacterial movement varied among subcellular domains, we infected PtK2 cells with L. monocytogenes and tracked bacterial movement as a function of subcellular location by using time-lapse videomicroscopy. As a convenient marker of subcellular location, we used local mitochondrial density because the cellular distribution of mitochondria is heterogeneous in PtK2 cells with high density near the nucleus and lower density in peripheral areas of the cell. To visualize mitochondria, we used a dye that specifically labels mitochondria (MitoTracker Red) and followed bacterial movement by tracking the fluorescent signal of GFP-expressing L. monocytogenes. During each time-lapse sequence, collected bacteria were classified depending on their location relative to mitochondria into subsets of bacteria moving in mitochondria-containing or mitochondria-free domains (Figure 1A; see MATERIALS AND METHODS).
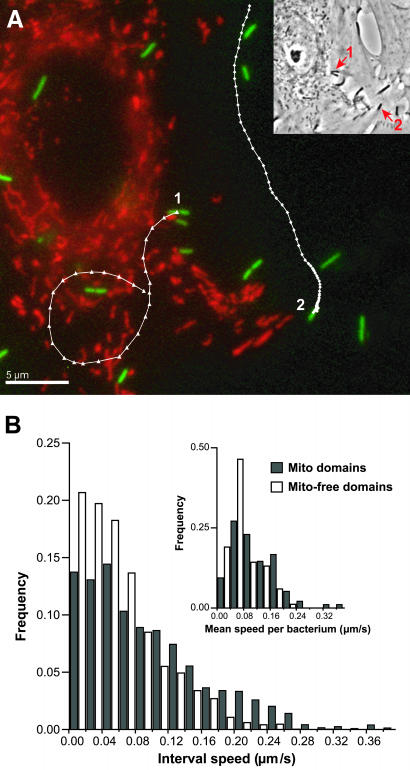
Figure 1.
L. monocytogenes moving in mitochondria-containing domains have greater speed than those moving in mitochondria-free domains in PtK2 cells. (A) PtK2 cells were infected with constitutively GFP-expressing L. monocytogenes (green) and mitochondria were fluorescently labeled with MitoTracker Red (red). Bacterial trajectories (white) with data points indicating position separated by 10 s are superimposed on the fluorescent image. Bacterium 1 moved in mitochondria-containing domains, whereas bacterium 2 moved in mitochondria-free domains of the cell. Trajectories show that bacterium 1 (260 s) moved quickly with high trajectory curvature, whereas bacterium 2 (670 s) moved slower with less trajectory curvature. Inset shows bacteria 1 and 2 (arrows) in the phase image of the infected PtK2 cell. Bar, 5 μm. (B) The interval speed distribution for bacteria moving in mitochondria-containing domains (dark bars) includes a substantial subgroup with speeds above ∼0.16 μm/s not present in the interval speed distribution for bacteria in mitochondria-free domains (white bars) of cells. Interval speed is calculated from the bacterial displacement and the time interval between consecutive time-lapse images (Δd/10 s). The mean interval speed of the entire bacterial population moving in mitochondria-containing domains (0.094 μm/s, SD = 0.074, n = 3244) is significantly larger than the mean interval speed (0.062 μm/s, SD = 0.049, n = 2592) of the bacterial population moving in mitochondria-free domains. Inset shows the distribution of mean speeds per bacterium, where the mean speed per bacterium is also significantly larger for bacteria moving in mitochondria-containing domains (dark bars, 0.114 μm/s, SD = 0.069, n = 96) than for bacteria moving in mitochondria-free domains (white bars, 0.076 μm/s, SD = 0.047, n = 84).
The distribution of interval speeds measured from time-lapse sequences of L. monocytogenes moving in mitochondria-containing domains included a high-speed subpopulation not present in the distribution of interval speeds of L. monocytogenes moving in mitochondria-free domains (Figure 1B). This fast-moving subpopulation consisted of a subset of the bacterial population that had speeds higher than ∼0.16 μm/s during the entire length or part of their movement tracks. The mean interval speed of the entire bacterial population moving in mitochondria-containing domains was 0.094 μm/s (SD = 0.074, n = 3244), which was significantly larger than the mean interval speed (0.062 μm/s, SD = 0.049, n = 2592) of the bacterial population traversing mitochondria-free domains of cells (Table 1; see MATERIALS AND METHODS for statistical analysis). The mean speed per bacterium was also significantly larger for bacteria moving in mitochondria-containing domains (0.114 μm/s, SD = 0.069, n = 96) than for bacteria moving in mitochondria-free domains (0.076 μm/s, SD = 0.047, n = 84) (Figure 1B). The large SD of bacterial speed observed confirms the large individual speed fluctuations and broad speed distribution reported among genetically identical bacteria in live cells as well as in cell-free extracts (Nanavati et al., 1994; Giardini and Theriot, 2001; Auerbuch et al., 2003; Soo and Theriot, unpublished observation). Because the bacterial tracks obtained were not equal in length, we examined a data set where all tracks were truncated to correspond to the shortest track acquired (10 sequential time-lapse images). Comparison of truncated to full-length tracks allowed us rule out the possibility that the speed results observed were weighted by interval speeds corresponding to particularly long bacterial tracks. Results from analysis of truncated tracks gave mean speed values similar to those determined from full-length tracks. The distribution of interval speeds and mean speed per bacterium measured from these truncated tracks were also significantly different between bacteria moving in mitochondria-containing and bacteria moving in mitochondria-free domains (our unpublished results). These results show that L. monocytogenes moved more rapidly in subcellular domains containing mitochondria than in mitochondria-free domains in PtK2 cells.
Table 1.
Comparison of mean speed, speed persistence, and trajectory curvature of L. monocytogenes in subcellular domains relative to mitochondria in PtK2 cells
| n (data points) | Mean interval speed (μm/s ± SD) | n (bacteria) | Mean speed/bacterium (μm/s ± SD) | Mean speed autocorrelation decay constant (τ in s) | n (tracks) | Mean cosine at 30-s interval ± SEM | |
|---|---|---|---|---|---|---|---|
| Untreated cellsa | |||||||
| Mitochondria-containing domains | 3244 | 0.094 ± 0.074c | 96 | 0.114 ± 0.069c | 15.5 | 123 | 0.72 ± 0.02c |
| Mitochondria-free domains | 2592 | 0.062 ± 0.049c | 84 | 0.076 ± 0.047c | 12.8 | 90 | 0.84 ± 0.01c |
| DNP-treated cellsb | |||||||
| Mitochondria-containing domains | 1841 | 0.089 ± 0.076c | 58 | 0.116 ± 0.073 | 11.5 | 73 | 0.70 ± 0.02 |
| Mitochondria-free domains | 774 | 0.074 ± 0.070c | 31 | 0.083 ± 0.068 | 11.4 | 27 | 0.80 ± 0.03 |
| Nocodazole-treated cellsb | |||||||
| Mitochondria-containing domains | 983 | 0.084 ± 0.069c | 33 | 0.088 ± 0.053 | 10.6 | 38 | 0.72 ± 0.03 |
| Mitochondria-free domains | 954 | 0.043 ± 0.047c | 27 | 0.051 ± 0.035c | 31.2c | 27 | 0.78 ± 0.03 |
Compared bacteria moving in mitochondria-containing domains with bacteria moving in mitochondria-free domains.
Compared with the corresponding bacterial population in untreated cells.
Significantly different.
L. monocytogenes Speed Persistence Is Constant Regardless of Subcellular Location Relative to Mitochondria
Regardless of subcellular location relative to mitochondrial density, large speed fluctuations were observed in the bacterial population analyzed. The SD of bacterial speeds measured within a single bacterial track was on average ∼40% of the mean speed for that track. Even though bacterial movement exhibits large speed variability, bacteria show speed memory in cells. In other words, bacterial speed changes slowly rather than being completely uncorrelated from one position to the next, and this phenomenon is represented by slow speed autocorrelation decay (Giardini and Theriot, 2001; Auerbuch et al., 2003). In contrast, speed autocorrelation for L. monocytogenes in cell-free extracts decays rapidly falling to zero in <10 s (Cameron, Robbins, Footer, and Theriot, unpublished observation).
To quantitatively examine the time dependence of speed variation for L. monocytogenes moving in mitochondria-containing and mitochondria-free domains of cells, we calculated autocorrelation decay functions for both populations of bacteria. The correlation between pairs of consecutive speed measurements over increasing time separations was calculated for each bacterium, averaged over the same time interval within each bacterial population and then fitted with an exponential decay function. A single exponential decay function fit the mean speed autocorrelation coefficient well and did not drop immediately to zero, demonstrating that bacteria have speed memory in cells. The speed autocorrelation decay constant for bacteria moving in mitochondria-containing domains (τ = 15.5 s) was not significantly different from that of bacteria moving in mitochondria-free domains of cells (τ = 12.8 s) (Figure 2A). These comparable decay constants show that although bacteria moving in domains with mitochondria were on average faster moving than bacteria traversing mitochondria-free domains, their speed persistence was equivalent. Therefore, differences in subcellular domains relative to mitochondria did not alter the speed persistence of L. monocytogenes in cells.
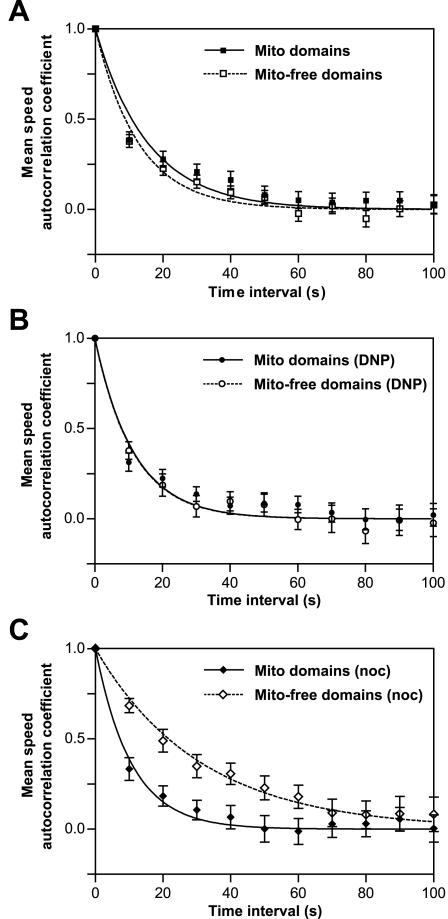
Figure 2.
Mean speed persistence of L. monocytogenes is similar in untreated and DNP-treated cells but increased for bacteria moving in mitochondria-free domains in nocodazole-treated PtK2 cells. Autocorrelation functions were calculated for each individual within each population of bacteria moving in mitochondria-containing or mitochondria-free domains, averaged per time interval, and fitted with single exponential decay functions (solid and dashed curves). (A and B) The mean speed persistence of bacteria moving in mitochondria-containing domains (closed symbols) is comparable with that of bacteria moving in mitochondria-free domains (open symbols) in untreated (A) and both bacterial populations in DNP-treated (B) cells. Decay constants (Mito domains, τ = 15.5 s; Mito-free domains, τ = 12.8 s; Mito domains [DNP], τ = 11.5 s; Mito-free domains [DNP], τ = 11.4 s) are not significantly different between bacterial populations in untreated and DNP-treated cells. Exponential decay curves in DNP-treated PtK2 cells completely overlap in the graph. (C) In nocodazole-treated PtK2 cells, the mean speed persistence of bacteria moving in mitochondria-free domains is significantly larger (Mito-free domains [noc], τ = 31.2 s) than that of to bacteria in mitochondria-containing domains (Mito domains [noc], τ = 10.6 s) and bacteria moving in both domains in untreated and DNP-treated cells. Error bars are SEM.
L. monocytogenes Moving in Mitochondria-containing Domains Have Greater Trajectory Curvature
To perform a more complete characterization of motility, we also examined the directional component of the velocity vector. The direction of bacterial movement was qualitatively characterized by examining bacterial trajectories in a standardized coordinate system. To directly compare these trajectories, we reoriented them so that their initial velocity vectors started at x,y = 0 and pointed in the same direction (+y). Visual inspection of bacterial trajectories by using this approach revealed that bacteria moving in mitochondria-containing domains showed greater curvature in their trajectories compared with bacteria moving in mitochondria-free domains of cells (Figure 3, A and B). The trajectories of bacteria moving in mitochondria-containing domains were curved, dispersed, and spanned all quadrants of the standardized coordinate system. The trajectories of bacteria moving in mitochondria-free domains were straighter and predominantly constrained to the top two quadrants of the standardized coordinate system. The population of bacteria moving in mitochondria-free domains only contained a handful of cases in which bacteria displayed large trajectory curvature similar to bacteria moving in mitochondria-containing subcellular domains.
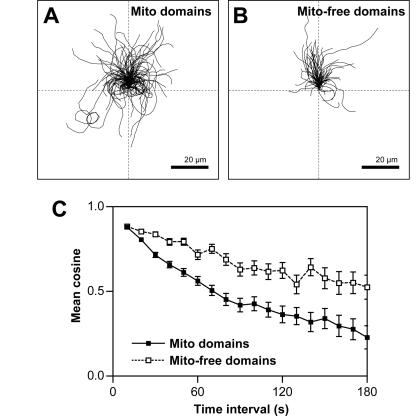
Figure 3.
Trajectories of L. monocytogenes moving in mitochondria-containing domains have greater curvature compared with those of bacteria traversing mitochondria-free domains of cells. Bacterial trajectories are reoriented to start at x,y = 0 in the +y direction in a standardized coordinate system. Discontinuous bacterial tracks are plotted and treated as separate tracks. (A) Trajectories of bacteria moving in mitochondria-containing domains (n = 96 bacteria, 123 tracks) are curved, dispersed, and span all quadrants of the coordinate system. (B) Trajectories of bacteria moving in mitochondria-free domains (n = 84 bacteria, 90 tracks) are straighter and mainly restricted to the top two quadrants of the coordinate system. Bar, 20 μm. (C) Mean cosines of the angles between velocity vectors are significantly smaller (see MATERIALS AND METHODS for statistical analysis) for bacteria moving in mitochondria-containing domains (closed symbols) showing increased trajectory curvature compared with bacteria moving mitochondria-free domains (open symbols). Error bars are SEM.
Bacterial trajectory curvature was also quantitatively compared by calculating the cosine of the angle between all possible nonoverlapping pairs of velocity vectors in 10-s increments and averaged per time interval. Mean cosine values calculated from tracks of bacteria moving in mitochondria-containing or mitochondria-free domains were compared as a function of time interval. These values decayed smoothly to zero, consistent with the hypothesis that bacterial trajectories approximate a persistent random walk. Mean cosine values calculated from bacterial tracks were significantly smaller for bacteria moving in mitochondria-containing domains than for bacteria moving in mitochondria-free domains (Figure 3C). Smaller mean cosine values represent larger angles or greater trajectory curvature during the movement of these bacteria in mitochondria-containing domains. Therefore, in addition to increased mean speed, bacteria traversing mitochondria-containing domains of the cell had greater curvature in their trajectories.
Increased Speed and Trajectory Curvature of L. monocytogenes Traversing Mitochondria-containing Domains Arise from Intracellular Variations
To determine whether the observed variations in L. monocytogenes speed and direction were intrinsic to bacteria themselves or generated by changes in the intracellular environment, we isolated track segments obtained from a group of bacteria (n = 38) that traversed mitochondria-containing as well as mitochondria-free domains of cells in a single trajectory. Track segments of individuals while traversing mitochondria-containing domains were separated from and compared with track segments obtained while the same bacteria moved in mitochondria-free domains.
To follow variations in movement as bacteria moved between subdomains, mitochondrial density in a circle (5 μm in diameter) centered on each bacterium in each time-lapse image was classified numerically from zero to three, where zero represented no mitochondrial density and three represented the highest mitochondrial density. Due to large fluctuations in mitochondrial membrane potential and fluorescence intensity throughout the time-lapse sequences collected (Loew et al., 1993), mitochondrial density was assessed by eye rather than by mean fluorescence intensity. The cross-correlation coefficient between interval speed and mitochondrial density by using this numerical system was calculated for bacterial tracks. An individual bacterium with a statistically significant and positive cross-correlation coefficient for the entire bacterial trajectory (0.54, n = 71, p < 0.0001) is shown in Figure 4A traversing regions with different mitochondrial densities. A significantly positive cross-correlation coefficient (p < 0.05) indicated correlation between interval speed and mitochondrial density better than random chance. In contrast, a cross-correlation coefficient of zero would indicate that interval speed did not correlate with mitochondrial density, whereas a negative coefficient would show that bacterial speed decreased in regions with high mitochondrial density. This particular bacterium also displayed abrupt changes in speed as it moved between regions with different mitochondrial densities. The interval speed of this individual increased as it moved into a region with elevated mitochondrial density, decreased when it entered an area with low mitochondrial density, and rose again as a region with higher mitochondrial density was reached (Figure 4, B and C). This example illustrates the tendency of bacteria to move faster while traversing domains with high mitochondrial density and supports the idea that this tendency is not intrinsic to bacteria themselves.
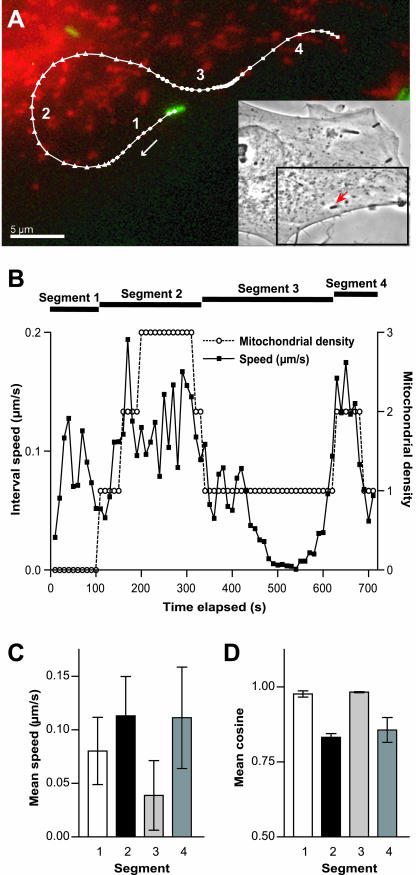
Figure 4.
L. monocytogenes speed and trajectory curvature correlate with mitochondrial density within a single track of a bacterium traversing different subcellular domains. Mitochondrial density around each bacterium was classified numerically from zero to three per time-lapse image, where zero represented no mitochondrial density and three represented the highest mitochondrial density. (A) An individual bacterium (green) that moved between regions of different mitochondrial densities (red) is shown with its corresponding trajectory (white). This bacterium is shown at the beginning of its trajectory and follows the track in the direction of the white arrow. Four sequential track segments (segment 1, diamonds; segment 2, triangles; segment 3, circles; and segment 4, squares) are separated according to mitochondrial density surrounding the bacterium. Time intervals are constant (10 s), thus distances between data points can be compared to reflect speed. The bacterial trajectory shows that segments with greater speed are also more curved. Inset shows the phase image of the infected PtK2 cell with the individual bacterium analyzed (arrow). Bar, 5 μm. (B) Interval speed of this bacterium increases in regions with high mitochondrial density and decreases in regions with fewer mitochondria. The cross-correlation coefficient between interval speed and mitochondrial density for this bacterium is statistically significant (0.54, n = 71, p < 0.0001). (C) Segment 2 has significantly larger mean speed (0.113 μm/s, SD = 0.037, n = 23) than segments 1 and 3 (0.080 μm/s, SD = 0.031, n = 10 and 0.039 μm/s, SD = 0.032, n = 29, respectively). Darker bar colors represent higher mitochondrial density around the bacterium during its movement along these segments. Error bars are SDs. (D) Quantitative comparisons show that segment 2, which has increased mean speed, also has significantly smaller mean cosine (0.83, SD = 0.09, n = 7) at 30-s time interval and thus greater trajectory curvature than segments 1 and 3 (0.98, SD = 0.03, n = 3 and 0.98, SD = 0.02, n = 9, respectively). Error bars are SEM.
Qualitatively, trajectory curvature also increased as this particular bacterium moved from a domain with low mitochondrial density to a domain with high mitochondrial density and vice versa (Figure 4B). Quantitative comparison of the mean cosine values of the angles between velocity vectors separated by 30 s showed that the mean cosine from segment 2 (0.83, SD = 0.09, n = 7) was significantly smaller than the mean cosine from segments 1 and 3 (0.98, SD = 0.03, n = 3; and 0.98, SD = 0.02, n = 9, respectively) (Figure 4D). This result showed that the trajectory of this particular bacterium while traversing segment 2, a mitochondria-rich domain, had greater trajectory curvature than in segments 1 and 3, regions with zero or low mitochondrial density, respectively. Moreover, track segments that had greater curvature also had greater mean speed (Figure 4, C and D). The behavior of this bacterium that traversed both mitochondria-containing and mitochondria-free domains of a cell was consistent with the behavior of bacteria that were only imaged while traversing a single subcellular domain.
To compare the speed of a population of bacteria while they traversed mitochondria-containing domains to the speed while the same bacteria traversed mitochondria-free domains, the intratrack speed difference was calculated for each bacterium by subtracting the mean bacterial speed in the mitochondria-free segment of the track from the mean speed in the mitochondria-containing segment of the track. A positive intratrack speed difference would indicate, on average, faster bacterial movement in mitochondria-containing domains than in mitochondria-free domains of cells. The mean speed of each individual while traversing both domains in a single trajectory was also calculated, and bacteria were separated based on whether they had larger or smaller mean speeds than the average speed of this entire group (0.077 μm/s, SD = 0.037, n = 38). Bacteria with mean speeds greater than the average speed of this entire group had an average intratrack speed difference of 0.040 μm/s (SD = 0.048, n = 20). Furthermore, the mean cross-correlation coefficient between interval speed and mitochondrial density for these bacteria was positive and statistically significant (0.38, SD = 0.37, n = 20, p < 0.05). Eighty-five percent of bacteria in this subgroup moved on average more rapidly in mitochondria-containing than in mitochondria-free domains of cells. Within the subgroup of bacteria with slower means speeds (n = 18) than the average speed of the entire group, only 39% of bacteria moved more rapidly in mitochondria-containing domains than in mitochondria-free domains. These results suggest that the correlation between speed and bacterial location relative to mitochondria was enhanced for fast-moving bacteria.
Even though fast-moving bacteria had a stronger correlation between speed and bacterial location relative to mitochondria, the average intratrack speed difference (0.023 μm/s, SD = 0.043, n = 38) and the mean cross-correlation coefficient between interval speed and mitochondrial density (0.18, SD = 0.43, n = 38) were positive values for the entire group. Furthermore, the mean interval speed (by rank sum and Student's t test, p < 0.05) and mean speed per bacterium (by Student's t test, p < 0.05) of bacteria while traversing mitochondria-containing domains were significantly larger than in track segments in which those same bacteria moved in mitochondria-free domains. Together, these results support the finding that the bacterial population analyzed moved more quickly while traversing mitochondria-containing domains and that this effect was due to variations in the intracellular environment.
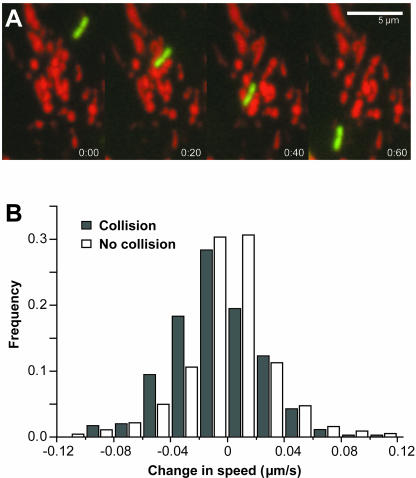
L. monocytogenes Slow Down after Physically Colliding with Mitochondria
As an L. monocytogenes bacterium moves inside the cell, actin-based propulsion must be powerful enough to push it through the cytoplasm, which is a viscoelastic and highly complex medium filled with macromolecules, cytoskeletal elements, and organelles. While analyzing time-lapse sequences of L. monocytogenes movement, we observed that these bacteria physically interacted with mitochondria by colliding with them. Collision events resulted in L. monocytogenes pushing mitochondria away from the bacterial path of motion (Figure 5A; Supplemental Movie 1). In numerous cases, mitochondria lost their fluorescent label presumably due to loss of mitochondrial integrity and transmembrane potential after being struck by bacteria (Supplemental Movie 1). We identified specific time-lapse images that contained single collisions of bacteria with mitochondria and calculated the change in speed between the time-lapse image in which a collision occurred and the following image. This change in speed was compared with the change in speed of bacteria that did not collide with mitochondria in the same subcellular domain. The distribution of speed change of bacteria that did not collide with mitochondria (n = 2734) was symmetrical around zero (Figure 5B). In other words, an equal number of bacteria sped up and slowed down by comparable magnitudes in the absence of collisions with mitochondria. However, bacteria that collided with mitochondria (n = 349) generated a distribution of change in speed shifted by ∼0.02 μm/s toward the negative side, showing that bacteria slowed down slightly after a collision. To determine what happened to bacterial speed shortly after a collision with mitochondria, we calculated the change in speed between the time-lapse image in which a single collision occurred and the second image after this collision. The distribution of change in speed closely resembled that of bacteria that did not collide with mitochondria and was also symmetrical around zero (our unpublished results). These results show that the small decrease in bacterial speed resulting from a single collision with mitochondria was shortly followed by rapid recovery with normal bacterial speed attained in <20 s.
Figure 5.
L. monocytogenes slow down slightly after physically colliding with mitochondria. (A) Bacteria push mitochondria away from the bacterial path of motion as they are propelled through regions with mitochondria. Sequential images show time-lapse images 20 s apart (also see Supplemental Movie 1). Bar, 5 μm. (B) Distribution of change in speed for bacteria that do not collide with mitochondria is symmetrical around zero (white bars). In this case, an equal proportion of bacteria speeds up and slows down by comparable magnitudes. Bacteria that collide with mitochondria (dark bars) have a distribution of change in speed shifted by approximately –0.02 μm/s, showing that bacteria slow down after a collision.
Because the cellular cytoplasm is a complex environment, efficient L. monocytogenes motility should allow them to move through intracellular materials, including macromolecules and organelles. We have directly observed that L. monocytogenes actin-based motility generates enough force for bacteria to push mitochondria aside. Nonetheless, bacterial speed is reduced after collisions with mitochondria only to recover shortly after a collision event. Considering that bacteria moving in domains containing mitochondria have larger mean speeds, physical interactions with mitochondria cannot explain this result because collisions with mitochondria result in a decrease in speed.
Disruption of Mitochondrial ATP Synthesis Does Not Affect L. monocytogenes Movement
After investigating physical interactions of L. monocytogenes with mitochondria and finding that they could not explain the increased bacterial speed observed in mitochondria-containing domains of cells, we explored whether chemical differences in mitochondria-containing domains compared with mitochondria-free subcellular domains may lead to this phenomenon. Because ATP availability has been shown to affect L. monocytogenes speed in cell-free extracts (Marchand et al., 1995), we examined whether mitochondrial respiration that might lead to high local ATP concentration in mitochondria-containing domains could cause the increased bacterial speed measured in these regions. To determine whether this was the case, we analyzed the movement of L. monocytogenes in PtK2 cells treated with DNP, which uncouples mitochondrial respiration from ATP synthesis. Although mitochondrial ATP synthesis would be disrupted by DNP treatment, cellular ATP production via glycolysis would remain unaffected. For simplicity, we only compared the mean speed per bacterium between bacterial populations in untreated and treated PtK2 cells. The mean speed per bacterium of individuals moving in mitochondria-containing (0.116 μm/s, SD = 0.073, n = 58) and of bacteria moving in mitochondria-free domains (0.083 μm/s, SD = 0.068, SEM = 0.012, n = 31) in DNP-treated cells was not significantly different from corresponding bacterial populations in untreated cells (Table 1). In addition, the speed persistence calculated using autocorrelation functions for these two populations of bacteria in DNP-treated cells (Mito domains [DNP], τ = 11.5 s; Mito-free domains [DNP], τ = 11.4 s) was not significantly different from the speed persistence of bacteria in untreated cells (Figure 2B and Table 1).
Qualitative analysis of bacterial tracks in a standardized coordinate system revealed that bacteria in DNP-treated cells exhibited similar trajectory patterns compared with untreated cells. Quantitative comparisons confirmed that the mean cosine values calculated from bacterial tracks of individuals traversing mitochondria-containing or mitochondria-free domains in DNP-treated cells were not significantly different from their corresponding counterparts in untreated cells (Table 1). Alterations in the chemical environment due to respiration in domains with mitochondrial density did not change the behavior of L. monocytogenes while traversing these domains. Therefore, if chemical microenvironments were created in mitochondria-containing domains due to high levels of ATP synthesis, they did not contribute to the increased speed or trajectory curvature of bacteria traversing these domains.
Microtubule Depolymerization Decreases Bacterial Speed but Increases Speed Persistence of L. monocytogenes Moving in Mitochondria-free Domains
After altering the chemical function of mitochondria and examining its effect on L. monocytogenes movement in PtK2 cells, we analyzed the effect of disrupting a structural and regulatory component of the cell linked to mitochondria: the microtubule network. Bacterial cell-to-cell spread has been shown to be independent of microtubule polymerization by inspection of fixed Caco-2 cells (Mounier et al., 1990). Because microtubule disruption could have subtle effects on L. monocytogenes intracellular movement only evident by quantitation of motility parameters in live cells, we analyzed bacterial movement in the absence of microtubules in PtK2 cells. Microtubule depolymerization by nocodazole and ice treatment of infected PtK2 cells was confirmed by immunostaining after image acquisition. After microtubule depolymerization post-L. monocytogenes infection, we found that the mean speed per bacterium was lower (yet not statistically different) for bacteria traversing mitochondria-containing domains in nocodazole-treated cells (0.088 μm/s, SD = 0.053, n = 33) compared with bacteria moving in mitochondria-containing domains in untreated cells. However, the mean speed per bacterium was significantly lower for bacteria traversing mitochondria-free domains in nocodazole-treated cells (0.051 μm/s, SD = 0.035, n = 27) than for bacteria traversing mitochondria-free domains in untreated cells (Table 1). Moreover, in nocodazole-treated cells, bacteria moving in mitochondria-free domains (τ = 31.2 s) had significantly greater mean speed persistence than bacteria moving in mitochondria-containing domains (τ = 10.6 s) and bacterial populations in untreated and DNP-treated cells (Figure 2 and Table 1).
When visually inspecting plotted trajectories of L. monocytogenes moving in nocodazole-treated cells, bacteria moving in mitochondria-containing domains displayed greater trajectory curvature than bacteria moving in mitochondria-free domains similar to bacteria in untreated cells. We quantitatively confirmed that the mean cosine values of bacterial tracks from individuals traversing mitochondria-containing or mitochondria-free domains in nocodazole-treated cells were not significantly different from those of corresponding bacterial populations in untreated cells (Table 1). Our results show that microtubule depolymerization created a significant change in intracellular properties in such a way that decreased mean speed and increased speed persistence along with no significant effect on trajectory curvature were detected in L. monocytogenes moving in mitochondria-free domains of PtK2 cells.
Distribution of Cytoskeletal Elements Varies Relative to Mitochondria
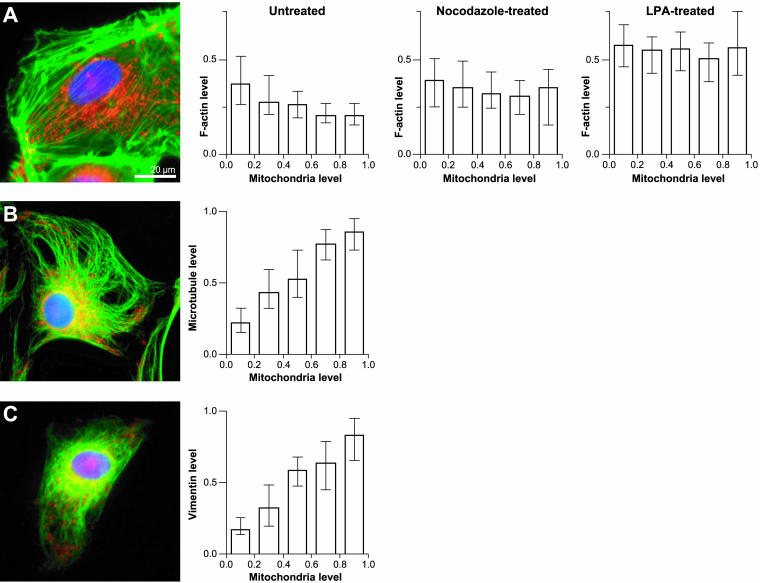
After observing variations in L. monocytogenes motility in different subcellular domains relative to mitochondria, we investigated whether the distribution of mitochondria throughout the cell bore some correlation with the location of cytoskeletal elements that could directly influence bacterial movement. We measured and normalized the fluorescent signal from fluorescently labeled cytoskeletal elements inside small subregions in infected PtK2 cells and compared it with the normalized signal from fluorescently labeled mitochondria in the same subregions. To analyze the distribution of F-actin in relation to mitochondria, we infected PtK2 cells with a strain of L. monocytogenes, 146-KKRK-150 ActA mutant 34, which is unable to form actin comet tails (Lauer et al., 2001). This mutant was used to avoid the fluorescent signal originating from L. monocytogenes actin comet tails from interfering with the quantitation of normal cellular F-actin levels. Overall, we observed that mitochondria were generally localized to regions with low F-actin levels, primarily near the nucleus (Figure 6A). Quantitatively, we confirmed this observation by determining that the spatial distributions of F-actin and mitochondrial density were negatively correlated (n = 15 cells, 914 data points; Figure 6A). The distribution of F-actin in relation to mitochondria in infected cells was comparable to that in uninfected cells (our unpublished results) showing that bacterial infection by itself does not affect F-actin distribution in PtK2 cells.
Figure 6.
Distribution of mitochondria in PtK2 cells infected with L. monocytogenes is negatively correlated with F-actin levels but correlates positively with microtubules and vimentin. (A) PtK2 cells were infected with L. monocytogenes 146-KKRK-150 ActA mutant 34 (Lauer et al., 2001), which fail to assemble comet tails in PtK2 cells, mitochondria were fluorescently labeled with MitoTracker Red (Molecular Probes), and F-actin was stained with FITC-phalloidin after fixation. A representative untreated cell shows lower F-actin (green) levels in areas with higher mitochondrial density (red) generally corresponding to regions proximate to the nucleus (blue). Left graph, normalized F-actin values decrease as the density of mitochondria increases in infected untreated cells (n = 15 cells, 914 data points). Middle graph, the distribution of F-actin is disturbed in infected cells treated with nocodazole and ice to depolymerize microtubules (n = 7 cells, 536 data points). Regions with higher mitochondrial density contain elevated levels of F-actin compared with untreated cells. Right graph, infected cells treated with LPA (n = 6 cells, 484 data points) to stimulate stress-fiber formation show a similar trend to nocodazole-treated cells. (B) PtK2 cells were infected with wild-type L. monocytogenes and mitochondria were fluorescently labeled before indirectly immunostaining microtubules with anti-tubulin antibodies. Mitochondria (red) localize to regions containing microtubules (green) in a representative infected PtK2 cell. Bar graph shows increased normalized microtubule signal in regions with higher mitochondrial density in infected cells (n = 5 cells, 542 data points). (C) PtK2 cells infected with wild-type L. monocytogenes and fluorescently labeled with MitoTracker Red were indirectly immunostained for intermediate filaments using anti-vimentin antibodies. Vimentin (green) is shown generally colocalized with mitochondria (red) in a representative infected PtK2 cell. Similar to the trend observed with microtubules, increasing vimentin levels are present in regions with higher mitochondrial density in infected cells (n = 5 cells, 452 data points). Nuclear DNA was stained with DAPI (blue). Bars show normalized median values with error bars depicting interquartile ranges. All values were normalized to the maximum intensity per image. Bar, 20 μm.
The effect of microtubule depolymerization on the actin cytoskeleton was also examined in infected PtK2 cells. After microtubule depolymerization, infected PtK2 cells contained more stress fibers than untreated cells with stress fibers uniformly distributed through the cell rather than enhanced at the periphery. This was confirmed quantitatively by measuring significantly higher F-actin fluorescence levels in areas with high mitochondrial density (n = 7 cells, 536 data points) in nocodazole-treated cells than in untreated cells (Figure 6A). This trend of increased F-actin levels in regions with low mitochondrial density was also observed in infected PtK2 cells treated with LPA (n = 6 cells, 484 data points), a growth factor known to induce stress fiber formation via the small GTPase Rho (Ridley and Hall, 1992; Amano et al., 1997; Kranenburg et al., 1997; Zhang et al., 1997), but F-actin levels were generally higher (Figure 6A). Although the effect of LPA treatment was more dramatic than microtubule depolymerization, both treatments similarly rearranged the actin cytoskeleton by intensifying stress fiber and F-actin localization to cellular areas with high mitochondrial density.
The distribution of F-actin in relation to mitochondria was complementary to the distribution of microtubules and intermediate filaments. PtK2 cells infected with wild-type L. monocytogenes and fluorescently labeled with MitoTracker Red (Molecular Probes) were indirectly immunostained with anti-tubulin or anti-vimentin antibodies. Regions with high mitochondrial density were generally microtubule rich. This trend was particularly striking when mitochondria were found scattered in peripheral areas of the cell (Figure 6B). Vimentin was more tightly confined to the perinuclear region and generally colocalized with mitochondria (Figure 6C). Overall, we found that the distribution of microtubules (n = 5 cells, 542 data points) and intermediate filaments (n = 5 cells, 452 data points) throughout cells positively correlated with the location of mitochondria (Figure 6, B and C). We concluded that under normal conditions, regions with high mitochondrial density correspond to regions that are F-actin poor, microtubule rich, and vimentin rich in infected PtK2 cells. Microtubule depolymerization and Rho activation via LPA treatment both caused an increase in F-actin density in mitochondria-containing regions of the cell.
DISCUSSION
L. monocytogenes as a Probe for the Heterogeneous Intracellular Environment
Microrheology studies designed to better understand the structural properties of the cytoplasm of living cells have been mainly restricted to the use of inert probes and tracer particles. These studies have fundamentally measured passive diffusion of small tracers, Brownian motion of larger probes, or displacement of magnetic beads exposed to oscillatory fields (Luby-Phelps and Taylor, 1988; Bausch et al., 1998; Tseng et al., 2002). Small tracers (approximately <50 nm) microinjected into cells can passively diffuse throughout most of the cell, whereas larger probes used to examine cellular organization and physical properties become sterically restricted from certain subcellular regions (Luby-Phelps and Taylor, 1988; Provance et al., 1993; Seksek et al., 1997).
L. monocytogenes variable actin-based motility can be due to the intrinsic machinery of bacteria themselves (reviewed by Rao et al., 2002) or to extrinsic determinants associated with the molecular and physical environment in which bacteria move (Dabiri et al., 1990; Loisel et al., 1999; Giardini and Theriot, 2001; McGrath et al., 2003). Even though L. monocytogenes would be considered very large in the realm of cytoplasmic probing particles (1–2 μm in length), in this study we have used them to explore the intracellular environment of infected cells. These self-propelled bacteria have the advantage of reaching many areas of the cell, including thin distal regions of the lamellae devoid of organelles and inaccessible to many large inert probes. L. monocytogenes have been previously used as functional kinematic probes for the cellular cytoplasm in a study that showed that bacterial motility responded to variations in the mechanical properties of their surroundings in cells containing or lacking intermediate filaments (Giardini and Theriot, 2001).
In our study, we specifically used L. monocytogenes to probe intracellular variation in properties of subdomains within living cells. We chose to analyze the movement of L. monocytogenes based on location relative to mitochondria because these organelles are distributed heterogeneously throughout the cell and can be easily labeled. We have shown that speed and trajectory curvature of L. monocytogenes correlate with mitochondrial density but not with mitochondrial ATP synthesis. The consistent variation of L. monocytogenes motility parameters measured while bacteria traversed cytoplasmic regions containing or lacking mitochondria substantiated our perception of the intracellular cytoplasm as a highly heterogeneous yet organized environment. Because mitochondrial respiration could not explain the observed variations in L. monocytogenes movement, we considered physical subcellular components as possible sources of variations in bacterial movement. Compartments in the cell periphery that excluded tracer particles (Luby-Phelps and Taylor, 1988), microtubules, and membrane-bound organelles such as mitochondria have been observed by transmission electron microscopy to contain long F-actin bundles accompanied by a dense F-actin–rich meshwork (Provance et al., 1993). Similarly, we have detected a negative correlation in the distribution of F-actin and mitochondria in our infected PtK2 cell system. F-actin–rich domains that exclude mitochondria have been proposed to sterically hinder the influx of large macromolecular components of the cell (reviewed by Luby-Phelps, 2000). A study in Dictyostelium discoideum supports this idea by showing that actin networks represented the main mechanical barrier even for small protein mobility by diffusion (Potma et al., 2001). How can these structural differences explain the decreased L. monocytogenes speed in mitochondria-free regions of the cell? The speed of L. monocytogenes has been shown to depend on availability of proteins involved in actin-based motility (Theriot et al., 1994; Loisel et al., 1999; Geese et al., 2002). Therefore, it is possible that in our studies, mitochondria-free subcellular regions, which are rich in F-actin, have diminished availability of proteins required for L. monocytogenes motility due to impaired protein mobility. It is also possible that dense F-actin structures in mitochondria-free domains could limit the rate of actin polymerization in L. monocytogenes comet tails by locally depleting proteins required for actin-based motility.
We favor the alternate possibility that mechanical hindrance created by F-actin bundles and meshworks present in mitochondria-free domains could cause L. monocytogenes to slow down by simply impeding their propulsive movement. In living cells, L. monocytogenes can attain speeds up to 4 times greater in J774 macrophages than in PtK2 epithelial cells (Dabiri et al., 1990). This study suggested that bacterial motility might be hampered by the massive cytoskeletal network of microfilaments, intermediate filaments, and microtubules present in PtK2 cells leading to slower speeds compared with J774 macrophages. The presence of intermediate filaments (Giardini and Theriot, 2001) and microtubules (this study) in the cell did not reduce bacterial rate of movement in cells. Therefore, the actin network is the most likely cytoskeletal candidate for this phenomenon. Even though actin-based propulsion of L. monocytogenes generates enough force for them to push through organelles, bacteria slow down or stop when they encounter the actin-dense comet tail of a fellow bacterium in their path of motion (our unpublished observation). This suggests that F-actin-based structures localized to mitochondria-free domains in cells may impair bacterial speed significantly.
L. monocytogenes Movement Can Reflect Changes in Cytoarchitecture
In addition to tethering mitochondria and contributing to maintenance of cellular structure, microtubules are also involved in signaling mechanisms. Microtubule depolymerization regulates the actin cytoskeleton via members of Rho family GTPases (reviewed by Wittmann and Waterman-Storer, 2001). The reduced speed of L. monocytogenes observed in nocodazole-treated PtK2 cells might be explained by two signaling pathways downstream of microtubule depolymerization through the small GTPases Rho and Rac. First, microtubule depolymerization has been shown to indirectly induce stress fiber formation and cellular contractility (Danowski, 1989; Bershadsky et al., 1996) through activation of Rho (Enomoto, 1996; Zhang et al., 1997; Krendel et al., 2002). Microtubule dynamics also modulate lamellipodial protrusion in fibroblasts by activation of Rac1, a member of the Rho family of GTPases (Waterman-Storer et al., 1999).
After microtubule depolymerization in our system of infected PtK2 cells, we have detected rearrangements of Factin similar to the result of Rho activation (Figure 6A). The decrease in overall bacterial speed observed in nocodazole-treated cells (Table 1) may be due to mechanical alterations in the cell cytoplasm contributed by an increase in number and density of stress fibers throughout the entire cell. Microtubules themselves have been shown to contribute very little to the mechanical properties of cells (Tsai et al., 1998), but upon microtubule depolymerization, cellular rigidity rises due to increased actin networks (Tsai et al., 1998; Wu et al., 1998). A more rigid intracellular environment resulting from microtubule depolymerization may impede and thus slow L. monocytogenes movement as they are propelled inside PtK2 cells.
In the absence of microtubules, reduced Rac signaling necessary for lamellipodial protrusion in the periphery of the cell, which generally corresponds to mitochondria-free regions, may detrimentally influence actin comet tail dynamics necessary for L. monocytogenes to move at fast speeds. Conflicting studies have shown that inhibition of Rho family members with clostridial toxins either abolished or did not affect L. monocytogenes comet tail formation (Aullo et al., 1993; Ebel et al., 1999). Therefore, disruption of microtubule dynamics, which has been shown to inhibit Rac signaling, may directly or indirectly lead to decreased L. monocytogenes speed in mitochondria-free domains usually located in the lamellae or periphery of cells. Because an overall decrease in L. monocytogenes speed was observed in nocodazole-treated cells (Table 1), we favor the possibility that rearrangements observed in the actin cytoskeleton after microtubule depolymerization have a greater mechanical effect as hindrance for protein mobility or a physical barrier for L. monocytogenes propulsion. Nonetheless, the increased speed persistence of L. monocytogenes observed in mitochondria-free regions in nocodazole-treated cells may be due to alterations in cytoplasmic elasticity resulting from reduced local Rac signaling. Reduced Rac signaling, which leads to reduced F-actin dynamics, may markedly alter the local elastic properties of mitochondria-free regions in the periphery of cells. A more elastic environment could cause bacterial rate of movement to be more influenced by previous events leading to increased speed persistence as we have observed in mitochondria-free domains in nocodazole-treated cells.
Intracellular bacterial pathogens such as L. monocytogenes that use actin-based motility to move within their host cells have developed a powerful mechanism of self-propulsion to overcome barriers they encounter in the crowded, complex, and viscoelastic cytoplasm. The mechanical forcefulness of bacterial movement is particularly well-illustrated by their ability to shove aside mitochondria with only a slight, brief impedance of their forward motion. In this study, we have provided the first biophysical analysis of direct L. monocytogenes interaction with organelles in living cells during intracellular actin-based motility. We have also found that the mechanics of L. monocytogenes motility as determined by quantitation of speed, speed persistence, and trajectory curvature are sensitive to the variations they encounter while traversing the heterogeneous intracellular milieu. We have used mitochondrial distribution as a convenient marker for cytoplasmic heterogeneity, but it seems likely that examination of other nonuniform cytoplasmic constituents would yield qualitatively similar results and should enable a kinematic mapping of subcellular domain characteristics complementary to studies that have used inert tracer particles as probes. A further advantage of L. monocytogenes is that it readily explores areas of the cell inaccessible to large inert tracer probes. We found that L. monocytogenes movement was particularly affected by cell structural changes after microtubule depolymerization. Thus, we expect that the use of L. monocytogenes as kinematic probe for cytoarchitecture and cytomechanics holds particular promise for exploring large-scale structural changes in the cell consequent to signaling events.
Supplementary Material
Acknowledgments
We are grateful to Daniel A. Portnoy and Tim Stearns for providing L. monocytogenes strains and DM1alpha anti-tubulin antibodies, respectively, and to Paul Berg for suggesting the DNP treatment experiment. We thank members of the Theriot laboratory for stimulating discussions and invaluable support, especially Cyrus Wilson for technical assistance. We also thank Julie B. Sneddon for helpful input in the early stages of this project and for facilitating protocols for vimentin immunostaining. Finally, we thank Susanne Rafelski, Delquin Gong, and Soichiro Yamada for critical review of the manuscript. This work was supported by the National Institute of Health (R01AI36929), the David and Lucile Packard Foundation, and the American Heart Association. C.I.L. is supported by the Cellular and Molecular Biology Training Program grant awarded to Stanford University by the National Institute of Health.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–10–0747. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–10–0747.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Amano, M., Chihara, K., Kimura, K., Fukata, Y., Nakamura, N., Matsuura, Y., and Kozo Kaibuchi. (1997). Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275, 1308–1311. [DOI] [PubMed] [Google Scholar]
- Ambrose, H.W., and Ambrose, K.P. (1987). A Handbook of Biological Investigation, Knoxville, TN: Hunter Textbooks Inc.
- Auerbuch, V., Loureiro, J.J., Gertler, F.B., Theriot, J.A., and Portnoy, D.A. (2003). Ena/VASP proteins contribute to Listeria monocytogenes pathogenesis by controlling temporal and spatial persistence of bacterial actin-based motility. Mol. Microbiol. 49, 1361–1375. [DOI] [PubMed] [Google Scholar]
- Aullo, P., Giry, M., Olsnes, S., Popoff, M.R., Kocks, C., and Boquet, P. (1993). A chimeric toxin to study the role of the 21 kDa GTP binding protein rho in the control of actin microfilament assembly. EMBO J. 12, 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch, A.R., Moller, W., and Sackmann, E. (1999). Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys. J. 76, 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch, A.R., Ziemann, F., Boulbitch, A.A., Jacobson, K., and Sackmann, E. (1998). Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophys. J. 75, 2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle, M.C. (1998). Spatial control of actin filament assembly: lessons from Listeria. Cell 95, 741–748. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A., Chausovsky, A., Becker, E., Lyubimova, A., and Geiger, B. (1996). Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 6, 1279–1289. [DOI] [PubMed] [Google Scholar]
- Cameron, L.A., Giardini, P.A., Soo, F.S., and Theriot, J.A. (2000). Secrets of actin-based motility revealed by a bacterial pathogen. Nat. Rev. Mol. Cell. Biol. 1, 110–119. [DOI] [PubMed] [Google Scholar]
- Dabiri, G.A., Sanger, J.M., Portnoy, D.A., and Southwick, F.S. (1990). Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc. Natl. Acad. Sci. USA 87, 6068–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danowski, B.A. (1989). Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J. Cell Sci. 93, 255–266. [DOI] [PubMed] [Google Scholar]
- Ebel, F., Rohde, M., von Eichel-Streiber, C., Wehland, J., and Chakraborty, T. (1999). The actin-based motility of intracellular Listeria monocytogenes is not controlled by small GTP-binding proteins of the Rho- and Ras-subfamilies. FEMS Microbiol. Lett. 176, 117–124. [DOI] [PubMed] [Google Scholar]
- Enomoto, T. (1996). Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct. Funct. 21, 317–326. [DOI] [PubMed] [Google Scholar]
- Fulton, A.B. (1982). How crowded is the cytoplasm? Cell 30, 345–347. [DOI] [PubMed] [Google Scholar]
- Geese, M., Loureiro, J.J., Bear, J.E., Wehland, J., Gertler, F.B., and Sechi, A.S. (2002). Contribution of Ena/VASP proteins to intracellular motility of Listeria requires phosphorylation and proline-rich core but not F-actin binding or multimerization. Mol. Biol. Cell 13, 2383–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardini, P.A., and Theriot, J.A. (2001). Effects of intermediate filaments on actin-based motility of Listeria monocytogenes. Biophys. J. 81, 3193–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudman, D., Rainbow, R.D., Lawrence, C.L., and Standen, N.B. (2002). The origin of calcium overload in rat cardiac myocytes following metabolic inhibition with 2,4-dinitrophenol. J. Mol. Cell. Cardiol. 34, 859–871. [DOI] [PubMed] [Google Scholar]
- Kranenburg, O., Poland, M., Gebbink, M., Oomen, L., and Moolenaar, W.H. (1997). Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J. Cell Sci. 110, 2417–2427. [DOI] [PubMed] [Google Scholar]
- Krendel, M., Zenke, F.T., and Bokoch, G.M. (2002). Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4, 294–301. [DOI] [PubMed] [Google Scholar]
- Lauer, P., Theriot, J.A., Skoble, J., Welch, M.D., and Portnoy, D.A. (2001). Systematic mutational analysis of the amino-terminal domain of the Listeria monocytogenes ActA protein reveals novel functions in actin-based motility. Mol. Microbiol. 42, 1163–1177. [DOI] [PubMed] [Google Scholar]
- Loew, L.M., Tuft, R.A., Carrington, W., and Fay, F.S. (1993). Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys. J. 65, 2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel, T.P., Boujemaa, R., Pantaloni, D., and Carlier, M.F. (1999). Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613–616. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps, K. (2000). Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 192, 189–221. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps, K., and Taylor, D.L. (1988). Subcellular compartmentalization by local differentiation of cytoplasmic structure. Cell Motil. Cytoskeleton 10, 28–37. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps, K., Taylor, D.L., and Lanni, F. (1986). Probing the structure of cytoplasm. J. Cell Biol. 102, 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand, J.B., Moreau, P., Paoletti, A., Cossart, P., Carlier, M.F., and Pantaloni, D. (1995). Actin-based movement of Listeria monocytogenes: actin assembly results from the local maintenance of uncapped filament barbed ends at the bacterium surface. J. Cell Biol. 130, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J.L., Eungdamrong, N.J., Mahadevan, L., Mitchison, T.J., and Kuo, S.C. (2003). The force-velocity relationship for the actin-based motility of Listeria monocytogenes. Curr. Biol. 13, 329–332. [DOI] [PubMed] [Google Scholar]
- Mounier, J., Ryter, A., Coquis-Rondon, M., and Sansonetti, P.J. (1990). Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocyte like cell line Caco-2. Infect. Immun. 58, 1048–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanavati, D., Ashton, F.T., Sanger, J.M., and Sanger, J.W. (1994). Dynamics of actin and alpha-actinin in the tails of Listeria monocytogenes in infected PtK2 cells. Cell Motil. Cytoskeleton 28, 346–358. [DOI] [PubMed] [Google Scholar]
- Potma, E.O., de Boeij, W.P., Bosgraaf, L., Roelofs, J., van Haastert, P.J., and Wiersma, D.A. (2001). Reduced protein diffusion rate by cytoskeleton in vegetative and polarized Dictyostelium cells. Biophys. J. 81, 2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance, D.W., Jr., McDowall, A., Marko, M., and Luby-Phelps, K. (1993). Cytoarchitecture of size-excluding compartments in living cells. J. Cell Sci. 106, 565–577. [DOI] [PubMed] [Google Scholar]
- Radmacher, M., Fritz, M., Kacher, C.M., Cleveland, J.P., and Hansma, P.K. (1996). Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 70, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, C.V., Wolf, D.M., and Arkin, A.P. (2002). Control, exploitation and tolerance of intracellular noise. Nature 420, 231–237. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., and Hall. A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Sanger, J.M., Sanger, J.W., and Southwick, F.S. (1992). Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect. Immun. 60, 3609–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seksek, O., Biwersi, J., and Verkman, A.S. (1997). Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol. 138, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G.A., Portnoy, D.A., and Theriot, J.A. (1995). Asymmetric distribution of the Listeria monocytogenes ActA protein is required and sufficient to direct actin-based motility. Mol. Microbiol. 17, 945–951. [DOI] [PubMed] [Google Scholar]
- Theriot, J.A., Mitchison, T.J., Tilney, L.G., and Portnoy, D.A. (1992). The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature 357, 257–260. [DOI] [PubMed] [Google Scholar]
- Theriot, J.A., Rosenblatt, J., Portnoy, D.A., Goldschmidt-Clermont, P.J., and Mitchison, T.J. (1994). Involvement of profilin in the actin-based motility of L. monocytogenes in cells and in cell-free extracts. Cell 76, 505–517. [DOI] [PubMed] [Google Scholar]
- Tilney, L.G., and Portnoy, D.A. (1989). Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109, 1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, M.A., Waugh, R.E., and Keng, P.C. (1998). Passive mechanical behavior of human neutrophils: effects of colchicine and paclitaxel. Biophys. J. 74, 3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng, Y., Kole, T.P., and Wirtz, D. (2002). Micromechanical mapping of live cells by multiple-particle-tracking microrheology. Biophys. J. 83, 3162–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., Worthylake, R.A., Liu, B.P., Burridge, K., and Salmon, E.D. (1999). Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1, 45–50. [DOI] [PubMed] [Google Scholar]
- Wittmann, T., and Waterman-Storer, C.M. (2001). Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114, 3795–3803. [DOI] [PubMed] [Google Scholar]
- Wu, H.W., Kuhn, T., and Moy, V.T. (1998). Mechanical properties of L929 cells measured by atomic force microscopy: effects of anticytoskeletal drugs and membrane crosslinking. Scanning 20, 389–397. [DOI] [PubMed] [Google Scholar]
- Yamada, S., Wirtz, D., and Kuo, S.C. (2000). Mechanics of living cells measured by laser tracking microrheology. Biophys. J. 78, 1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Magnusson, M.K., and Mosher, D.F. (1997). Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol. Biol. Cell 8, 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.