Abstract
In many metazoans, germ cells are separated from somatic lineages early in development and maintain their identity throughout life. Here we show that a Polycomb group (PcG) component, Enhancer of Zeste [E(z)] H3K27me3-specific methyltransferase, maintains germline identity in Drosophila adult testes. We find excessive early-stage somatic gonadal cells in E(z) mutant testes, which originate from both overproliferative cyst stem cells and germ cells turning on an early-stage somatic cell marker. Using complementary lineage-tracing experiments in E(z) mutant testes, a portion of excessive early-stage somatic gonadal cells are found to originate from early-stage germ cells, including germline stem cells. Moreover, knocking down E(z) specifically in somatic cells caused this change, suggesting a non-cell autonomous role of E(z) to antagonize somatic identity in germ cells.
The dichotomy of germline and soma represents the earliest lineage specification among many metazoan organisms (1–4). Drosophila has provided a paradigmatic system to study germ cell establishment and maintenance. During Drosophila development, primordial germ cells are specified in early embryogenesis, which requires suppression of somatic gene expression (5–7). Specified primordial germ cells migrate and interact with mesodermal somatic cells to establish germline stem cells (GSCs) (8, 9). In adult testes, GSCs interact with two populations of somatic gonadal cells: post-mitotic hub cells and cyst stem cells (CySCs) (10, 11) (Fig. 1A). Since germline-to-soma conversion has only been reported at the embryonic stage, which leads to germ cell death (12), it is unclear whether germ cells retain plasticity in adult gonads.
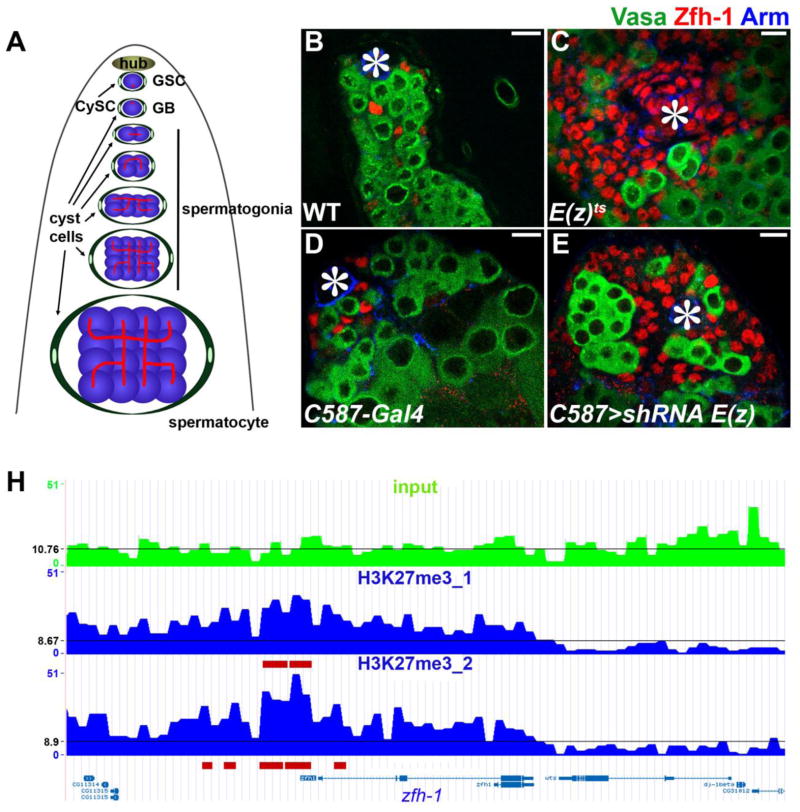
Figure 1. Loss-of-function of E(z) leads to excessive Zfh-1-expressing cells in adult testes.
(A) A schematic diagram of Drosophila adult testis. The spherical spectrosomes (red dots) are present in GSCs and gonialblasts (the daughter cell of GSC which undergoes differentiation) whereas branched fusomes (red lines) appear when germ cells undergo differentiation into both spermatogonia and spermatocytes. Germ cells (blue) are encapsulated by cyst cells (green). (B to E) Immunostaining using α-Zfh-1 (red labeling of early cyst cells, including CySCs), α-Vasa (green labeling of all germ cells), and α-Armadillo (Arm, blue labeling of hub cells, as indicated by asterisks) in testes from (B) WT and (C) E(z)61/E(z)731 males after shifting temperature from 25°C to 29°C for 28 days as adult flies; (D) C587-Gal4 control and (E) C587-Gal4; UAS- shRNA E(z) males after shifting temperature from 18°C to 29°C for 21 days (21 days, 29°C) as adult flies. Scale bars: 10μm. (F) A genome browser snapshot at the zfh-1 gene locus of H3K27me3 ChIP-seq data using nos>shRNA E(z) testes, where H3K27me3 is only detected in somatic cells (Fig. S1, J to L). H3K27me3 is enriched at the zfh-1 gene region in two H3K27me3 ChIP-seq replicates (marked by red lines), but not in the Input. The read density was binned into 1-kb window, and the black line indicates the average read density at chromosome 3R.
Polycomb group (PcG) proteins maintain somatic cell fate decisions, and mutations in PcG genes often lead to transdifferentiation through which one somatic cell type adapts a different somatic identity (13). Here we investigate a PcG gene, Enhancer of Zeste [E(z)], in Drosophila adult testes. E(z) encodes a histone methyltransferase which generates the H3K27me3 repressive histone modification (14). To inactivate E(z) upon temperature shift, a null allele of E(z), E(z)731, was used in trans to a temperature-sensitive (ts) allele, E(z)61 (15). We found that the H3K27me3 mark was not detectable by immunostaining in E(z)61/E(z)731 testes after temperature shift (fig. S1, A to F). We then examined the expression of Zinc-finger homeodomain protein 1 (Zfh-1), a marker for CySCs and early-stage cyst cells (16) (Fig. 1, B and D). The overall Zfh-1-positive CySCs and early cyst cells ranged from 20 to 59 per wild-type (WT) testis, but their numbers increased significantly in ~20% E(z)61/E(z)731 testes under the same condition (Fig. 1C and fig. S2A).
To determine in which cell type loss of E(z) function leads to excessive Zfh-1-positive cells, E(z) was knocked down using a UAS- shRNA E(z) (17) in combination with different cell type-specific Gal4 drivers (18). Subsequent immunostaining experiments demonstrated that knockdown of E(z) in cyst cells [by C587-Gal4 (19)] or germ cells [by nanos/nos-Gal4 (20)] resulted in a cell-type-specific loss of H3K27me3 (fig. S1, G to L). We found a significant overpopulation of Zfh-1-positive cells when UAS-shRNA E(z) was driven by either the C587-Gal4 driver (Fig. 1E and fig. S2B) or another cyst cell-specific eya-Gal4 driver (21) (fig. S2B). By contrast, the same UAS- shRNA E(z), when driven by either the germline-specific nos-Gal4 driver (22) or a hub-specific unpaired-Gal4 (upd-Gal4) driver, did not recapitulate these phenotypes (fig. S2B). A similar phenotype was also detected when another PcG component, Su(z)12, was knocked down in C587>shRNA Su(z)12 testes (fig. S2B). Together, these results suggest that E(z) is required in CySCs and cyst cells to prevent the accumulation of excessive Zfh-1-positive cells in adult testes. Zfh-1 is a known downstream target of the JAK-STAT signaling pathway (18, 21). However, we found no difference of JAK-STAT activity, as represented by Stat92E immunostaining pattern (21, 23), in control testes compared with E(z) mutant testes [both E(z)ts and UAS- shRNA E(z), fig. S3], suggesting that the ectopic expression of Zfh-1 in E(z) mutant testes is not directly caused by hyperactive JAK-STAT signaling. Consequently, we speculated that zfh-1 might be a direct target of PcG in somatic gonadal cells. To test this, we performed an H3K27me3 ChIP-seq experiment (fig. S4) using nos>shRNA E(z) testes where H3K27me3 was only detected in somatic cells (fig. S1, J to L). Enrichment of the H3K27me3 mark was found at the zfh-1 gene locus (Fig. 1F), suggesting that zfh-1 is, in fact, directly bound and repressed by PcG in somatic gonadal cells.
To investigate whether overpopulated Zfh-1-positive cells in E(z) mutant testes are derived from ectopically dividing CySCs, a pulse EdU incorporation was performed to label S-phase cells (24). In WT adult testes, CySCs but not cyst cells (Fig. 1A) are mitotically active (25). Accordingly, EdU signals were detected in Zfh-1-positive CySCs next to the hub in the control testes (arrowheads in Fig. 2A). However, EdU and Zfh-1 double-positive somatic cells were detected away from the hub in E(z) mutant testes (arrows in Fig. 2, B to C). In addition, all Zfh-1-positive cells in the C587-Gal4 control testes expressed an early somatic cell marker Tj (fig. S5, A to C), while most of the overpopulated Zfh-1-positive cells in C587>shRNA E(z) (fig. S5, D to F) and E(z)61/E(z)731(fig. S5, G to I) testes lacked Tj expression. Furthermore, EdU and Vasa double-positive germ cells were detected in the middle, or toward the basal region, of both C587>shRNA E(z) (fig. S6, A to C, and H, 31.7%, n=63) and E(z)61/E(z)731 (fig. S6, D to F, and J, 6.1%, n=33) testes, whereas they were only detectable at the tip of the control testes (fig. S6G, n=44; fig. S6I, n=32). These results were further confirmed using a mitosis-specific marker, serine 10-phosphorylated histone 3 (phH3) (fig. S6, K to N). We also found several unique cellular features of these ectopically dividing germ cells. First, they divided like GSCs or gonialblasts (Fig. 1A), as shown by single germ cells labeled by EdU (white arrowheads in fig. S6, C and F) and germ cells with round spectrosome (yellow arrows in fig. S7, C and D), consistent with previous studies showing that sustained Zfh-1 expression in cyst cells leads to excessive GSC self-renewal away from the niche (21). However, different from the previous report (21), these ectopically dividing germ cells failed to intermingle with Zfh-1-expressing cells (yellow arrowheads in fig. S6, B to C and E to F). It is possible that lack of Tj expression in those overpopulated Zfh-1-positive cells in E(z) mutant testes abolished their proper association with germ cells (26). Second, we found that some ectopically dividing GSC-like cells turned on Zfh-1 expression in E(z) mutant testes, leading to cells with both cytoplasmic Vasa staining and nuclear Zfh-1 staining (Fig. 2, D to I). The Vasa and Zfh-1 double-positive cells could be detected in ectopically dividing germ cell clusters that lacked association with cyst cells in E(z) mutant testes (Fig. 2, D to I). Notably, although the subcellular localization of Vasa and Zfh-1 in these double-positive cells was normal, their immunostaining signals were usually weaker than those of cells with a single signal (Fig. 2G–I), suggesting some type of intermediate cellular state.
Figure 2. E(z) is required in somatic cells to prevent accumulation of excessive Zfh-1-expressing cells and cells with both germline and somatic lineage markers.
(A to C) EdU (green) and Zfh-1 (red) double-positive cells representing dividing somatic cells are detected in CySCs adjacent to hub in (A) C587-Gal4 control, but far from hub in (B) C587>shRNA E(z) (small hairpin RNA) testes (21 days at 29°C) and (C) E(z)61/E(z)731 testes (28 days at 29°C), α-Vasa (blue). Arrowheads indicate dividing CySCs next to hub; arrows indicate dividing cyst cells away from hub. (D to F) Cells with Vasa (D and green in F) and Zfh-1 (E and red in F) signals in a cluster of cells in C587>shRNA E(z) testes (21 days at 29°C) (insets: an enlarged cell indicated by yellow arrowheads). (G to I) Cells with both Vasa (G and green in I) and Zfh-1 (H and red in I) signals in a cluster of cells in E(z)61/E(z)731 mutant testes (28 days at 29°C), α-Arm (blue). Hub: asterisks. Scale bars: 10μm.
The observation of Vasa and Zfh-1 double-positive cells raised a possibility of mixed cell fate. To test this idea, we used a lineage-tracing regimen to permanently label germ cells with the nos-Gal4>UAS-Gal4>UAS-GFP transgene combination, which irreversibly labels all GSCs and their derivatives with GFP (9). Indeed, we detected GFP and Zfh-1 double-positive cells in nos-Gal4>UAS-Gal4>UAS-GFP; E(z)61/E(z)731 testes (Fig. 3, D to I, 18.9%, n=53) after temperature shift, but not in control testes (Fig. 3, A to C, n=47), suggesting ectopic expression of Zfh-1 in germ cells upon inactivation of E(z). We also used the G-TRACE lineage tracing method combining UAS-FLP, UAS-RFP, and ubi-FRT-STOP-FRT-GFP transgenes (27) with the nos-Gal4 driver, which labels all germline lineage cells with GFP and early-stage germ cells with RFP. With G-TRACE, we found Zfh-1 and GFP double-positive cells in E(z)61/E(z)731 testes (fig. S8, E to H; 17.1%, n=76), but not in control testes (fig. S8, A to D, n=95). We also found Zfh-1 and RFP double-positive cells in E(z)61/E(z)731 testes (arrowheads in fig. S8, E to H, 9.2%, n=76), but not in control testes (fig. S8, A to D, n=95). All Zfh-1 and RFP double-positive cells in E(z)61/E(z)731 testes were also labeled by GFP, suggesting that they represent as a subset of germline lineage cells that transiently express both lineage markers, similar to the Vasa and Zfh-1 double-positive cells shown previously (Fig. 2, F and I). Therefore, using two different lineage tracing methods, we found that germ cells turned on a key somatic cell-specific transcription factor, Zfh-1, when E(z) was inactivated in somatic gonadal cells. Notably, the nuclear morphology of Zfh-1 positive cells originated from germline lineage was indistinguishable from that of CySC lineage-derived Zfh-1 positive cells (Fig. 3, E and H; Fig. S8G). Furthermore, no spectrosome or fusome structure could be detected in those overpopulated Zfh-1-positive cells (Fig. S7, C and D), suggesting that the germline-derived Zfh-1-positive cells eventually lose germ cell features. Lineage-tracing experiments were also performed using bam-Gal4 which drives GFP expression in more differentiated germ cells from 4-cell spermatogonia to later stages (28). In bam-Gal4>UAS-Gal4>UAS-GFP; E(z)61/E(z)731 testes, no Zfh-1-positive cells were found to be labeled by GFP (fig. S9, n=76), suggesting that only GSCs, gonialblasts and/or 2-cell spermatogonia are capable of turning on Zfh-1 expression.
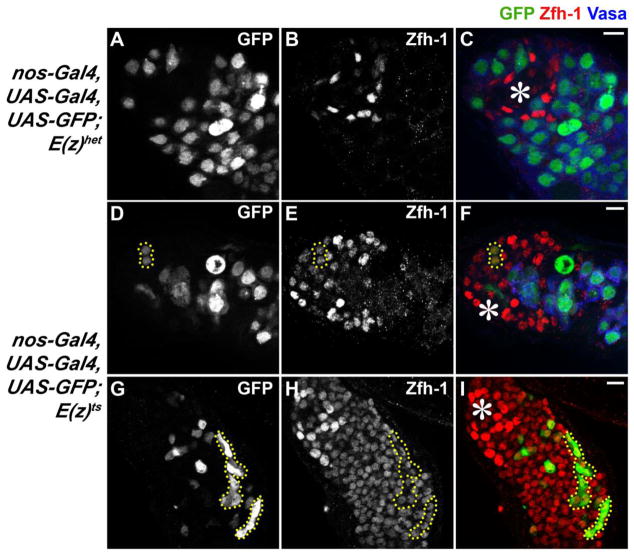
Figure 3. Some Zfh-1-positive cells are derived from germline lineage.
(A to I) Lineage tracing with permanently labeled germline cells using auto-regulatory Gal4-controlled gene expression. The nanos-Gal4>UAS-Gal4>UAS-GFP transgenes in (A to C) E(z) heterozygous (28 days at 29°C) and (D to I) E(z)61/E(z)731 testes (28 days at 29°C). Detection of GFP and Zfh-1 double-positive cells (yellow dotted outlines) suggests that some Zfh-1-expressing cells are derived from germline lineage. Hub: asterisks. Scale bars: 10μm.
In a complementary lineage-tracing experiment, we used a somatic driver to permanently label all CySC lineage cells. In C587-Gal4>UAS-Gal4>UAS-GFP testes, no GFP and Vasa double-positive cells could be detected in either E(z)61/E(z)731(n=32) or C587>shRNA E(z) testes (n=45), suggesting that somatic lineage cells did not express the germline marker. However, we did find that some Zfh-1-positive cells lacked the GFP marker in both E(z)61/E(z)731 (Fig. 4, D to F, yellow outline, 50.0%, n=32) and C587>shRNA E(z) (Fig. 4, G to I, yellow outline, 68.9%, n=45) testes, suggesting that some Zfh-1-expressing cells were not derived from the CySC lineage. By contrast, all Zfh-1-positive cells showed GFP labeling in the controls (Fig. 4, A to C, n=26). Consistent with these lineage tracing results, our H3K27me3 ChIP-seq data showed lack of H3K27me3 at the germline gene loci in somatic gonadal cells (fig. S10), suggesting that germline-specific genes are not direct targets of PcG in somatic cells. Taken together, these results show that germline lineage cells also contribute to Zfh-1-expressing cells in E(z) mutant testes.
Figure 4. Some Zfh-1-positive cells are not derived from somatic gonadal cell lineage.
Lineage tracing with permanently labeled somatic-lineage cells. The C587-Gal4>UAS-Gal4>UAS-GFP transgene in (A to C) E(z) heterozygous (28 days at 29°C); (D to F) E(z)61/E(z)731(28 days at 29°C); and (G to I) UAS- shRNA E(z) testes (21 days at 29°C). Hub: asterisks. Scale bars: 10μm. Detection of GFP-negative and Zfh-1-positive cells (outlined by yellow dotted lines) suggests that these cells are not derived from somatic cell lineage.
Because these results suggest that E(z) has a non-cell-autonomous role, we hypothesize that E(z) regulates signaling pathway(s) for proper communication between germ cells and somatic cells. Our H3K27me3 ChIP-seq data in somatic gonadal cells showed enrichment of H3K27me3 at several signaling pathways, including Wnt (fig. S11) and EGF (fig. S12A) pathway genes’ loci. Furthermore, we found that removal of one copy of Egfr suppresses the E(z)61/E(z)731 phenotype (fig. S12B), suggesting that Egfr is a direct target of PcG in somatic gonadal cells and may connect E(z) activity in soma with the observed germline defects.
In summary, we demonstrate that the PcG gene E(z) is required in somatic gonadal cells to prevent ectopic early-stage somatic and germ cell proliferation. Moreover, E(z) is required in somatic cells to prevent germ cells from expressing a somatic cell marker (fig. S13). In C. elegans, it has been reported that the germline can convert to specific neurons upon ectopic germline expression of neuron-specific transcription factors and loss of a key histone chaperone, LIN-53 (29). Removal of mes-2, the C. elegans homolog of E(z), also makes germ cells more susceptible for conversion to somatic cell types (30). However, it is unclear in which cell type LIN-53 and MES-2 act to maintain germline identity because its function is ubiquitously compromised in those experiments. Here, using cell type-specific knockdown experiments, we demonstrate that the germline-to-soma change relies on the activity of E(z) in somatic gonadal cells, suggesting that cell fate maintenance depends on the microenvironment or niche where the cells reside.
Supplementary Material
Acknowledgments
We thank C. Choi for technical assistance, D. Drummond-Barbosa, M. Van Doren, H. Zhao, Y. Yamashita and Chen lab members for suggestions. ChIP-Seq libraries were sequenced at NHLBI Sequencing and Computation Core facility, the GEO accession number is GSE53238. Supported by NICHD/NIH grants R00HD055052 and R01HD065816, the David & Lucile Packard Foundation, March of Dimes #05-FY09-88, JHU start-up (X.C.), and DIR, NHLBI, NIH (K.Z.).
Footnotes
References
- 1.Strome S, Lehmann R. Science. 2007 Apr 20;316:392. doi: 10.1126/science.1140846. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann R. Cell Stem Cell. 2012 Jun 14;10:729. doi: 10.1016/j.stem.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura A, Seydoux G. Development. 2008 Dec;135:3817. doi: 10.1242/dev.022434. [DOI] [PubMed] [Google Scholar]
- 4.Saitou M, Yamaji M. Reproduction. 2010 Jun;139:931. doi: 10.1530/REP-10-0043. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell TK. Curr Biol. 2004 Mar 23;14:R229. doi: 10.1016/j.cub.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 6.Leatherman JL, Jongens TA. Bioessays. 2003 Apr;25:326. doi: 10.1002/bies.10247. [DOI] [PubMed] [Google Scholar]
- 7.Seydoux G, Braun RE. Cell. 2006 Dec 1;127:891. doi: 10.1016/j.cell.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Richardson BE, Lehmann R. Nat Rev Mol Cell Biol. 2010 Jan;11:37. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bras S, Van Doren M. Dev Biol. 2006 Jun 1;294:92. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Davies EL, Fuller MT. Cold Spring Harb Symp Quant Biol. 2008;73:137. doi: 10.1101/sqb.2008.73.063. [DOI] [PubMed] [Google Scholar]
- 11.de Cuevas M, Matunis EL. Development. 2011 Jul;138:2861. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi Y, Hayashi M, Kobayashi S. Proc Natl Acad Sci U S A. 2004 Jul 13;101:10338. doi: 10.1073/pnas.0401647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringrose L, Paro R. Annu Rev Genet. 2004;38:413. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 14.Muller J, et al. Cell. 2002 Oct 18;111:197. [Google Scholar]
- 15.Jones RS, Gelbart WM. Genetics. 1990 Sep;126:185. doi: 10.1093/genetics/126.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issigonis M, et al. Science. 2009 Oct 2;326:153. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni JQ, et al. Nat Methods. 2011 May;8:405. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leatherman JL, Dinardo S. Nat Cell Biol. 2010 Aug;12:806. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manseau L, et al. Dev Dyn. 1997 Jul;209:310. doi: 10.1002/(SICI)1097-0177(199707)209:3<310::AID-AJA6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Van Doren M, Williamson AL, Lehmann R. Curr Biol. 1998 Feb 12;8:243. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- 21.Leatherman JL, Dinardo S. Cell Stem Cell. 2008 Jul 3;3:44. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iovino N, Ciabrelli F, Cavalli G. Dev Cell. 2013 Aug 26;26:431. doi: 10.1016/j.devcel.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Tarayrah L, Herz HM, Shilatifard A, Chen X. Development. 2013 Mar;140:1014. doi: 10.1242/dev.089433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Insco ML, Leon A, Tam CH, McKearin DM, Fuller MT. Proc Natl Acad Sci U S A. 2009 Dec 29;106:22311. doi: 10.1073/pnas.0912454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonczy P, DiNardo S. Development. 1996 Aug;122:2437. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- 26.Li MA, Alls JD, Avancini RM, Koo K, Godt D. Nat Cell Biol. 2003 Nov;5:994. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- 27.Evans CJ, et al. Nat Methods. 2009 Aug;6:603. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng J, et al. Nature. 2008 Dec 4;456:599. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tursun B, Patel T, Kratsios P, Hobert O. Science. 2011 Jan 21;331:304. doi: 10.1126/science.1199082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel T, Tursun B, Rahe DP, Hobert O. Cell Rep. 2012 Nov 29;2:1178. doi: 10.1016/j.celrep.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.