Abstract
Anatomical and physiological evidence suggests that common input to motor neurons of hand muscles is an important neural mechanism for hand control. To gain insight into the synaptic input underlying the coordination of hand muscles, significant effort has been devoted to describing the distribution of common input across motor units of extrinsic muscles. Much less is known, however, about the distribution of common input to motor units belonging to different intrinsic muscles and to intrinsic-extrinsic muscle pairs. To address this void in the literature, we quantified the incidence and strength of near-simultaneous discharges of motor units residing in either the same or different intrinsic hand muscles (m. first dorsal, FDI, and m. first palmar interosseus, FPI) during two-digit object hold. To extend the characterization of common input to pairs of extrinsic muscles (previous work) and pairs of intrinsic muscles (present work), we also recorded electromyographic (EMG) activity from an extrinsic thumb muscle (m. flexor pollicis longus, FPL). Motor-unit synchrony across FDI and FPI was weak (common input strength, CIS, mean ± SE: 0.17 ± 0.02). Similarly, motor units from extrinsic-intrinsic muscle pairs were characterized by weak synchrony (FPL-FDI: 0.25 ± 0.02; FPL-FPI: 0.29 ± 0.03) although stronger than FDI-FPI. Last, CIS from within FDI and FPI was more than three times stronger (0.70 ± 0.06 and 0.66 ± 0.06, respectively) than across these muscles. We discuss present and previous findings within the framework of muscle-pair specific distribution of common input to hand muscles based on their functional role in grasping.
INTRODUCTION
Object grasping and manipulation require fine coordination of multiple hand muscles. To simplify control of a complex system such as the hand, it may be advantageous to activate groups of muscles with a neural signal that is distributed to motor neuron pools of different muscles. The role of such common neural input would be to facilitate the coupling of movement and/or forces at the digits. Within this theoretical framework, common input to motor neurons might be an important mechanism for coupling the neural activity of motor units within or across muscles (De Luca and Mambrito 1987; Reilly et al. 2004; Santello and Fuglevand 2004; Santello and Soechting 2000; for review, see Schieber and Santello 2004). Near synchronous activation of motor units belonging to the same muscle would modulate its force output (De Luca and Mambrito 1987; Yao et al. 2000). Synchrony occurring between motor units residing in two different muscles or muscle compartments might play a functional role in the coordination of forces at different digits, for example as it might be required to prevent slipping of an object in grasping (Johnston et al. 2005; Santello and Fuglevand 2004; Winges and Santello 2004; Winges et al. 2006).
Previous studies have used tasks involving either force production by individual digits or holding an object against gravity to determine the distribution of common input to extrinsic hand muscles that are important for grasping and manipulation. These studies have shown that common input to motor units of extrinsic hand muscles is heterogeneously distributed across muscles, i.e., m flexor pollicis longus (FPL) and compartments of m. flexor digitorum profundus (FDP) (Winges and Santello 2004) as well as across compartments of FDP (Reilly et al. 2004; Winges and Santello 2004), m. flexor digitorum superficialis (FDS) (McIsaac and Fuglevand 2007), and m. extensor digitorum communis (EDC) (Keen and Fuglevand 2004).
Intrinsic hand muscles are also important for the coordination of digits in the production of manipulative forces. It is therefore important to understand the organization of synaptic input to intrinsic muscles—both within and across muscles—of the hand. Most studies on motor-unit synchronization within intrinsic hand muscles in humans have used m. first dorsal interosseus (FDI) for its ease of accessibility and its functional role in grasping. These studies have yielded a great deal of information about motor-unit synchronization within FDI such as being mediated by common presynaptic inputs to motor neurons (Datta and Stephens 1990). Furthermore, synchronization of motor-unit activity within FDI appears to be modulated according to the task being performed (Kilner et al. 2002; Semmler et al. 2002) as well as dependent on chronic usage patterns (Milner-Brown et al. 1975; Semmler and Nordstom 1998). However, much less is known about the organization of neural common input across motor units of simultaneously active intrinsic hand muscles as well as across intrinsic-extrinsic muscles.
The primary objective of the present study was to quantify the strength of common input to motor units across two intrinsic muscles, FDI and m. first palmar interosseus (FPI), during a two-digit object hold task. The FDI and FPI are abductors and adductors of the index finger, respectively, hence antagonists. Furthermore, they are also synergists for flexion at the metacarpal-phalangeal joint and extension at interphalangeal joints (Brand and Hollister 1999). Both muscles play an important role for grasping and manipulation as demonstrated by electromyographic (EMG) studies (Long et al. 1970; Valero-Cuevas 2000; Valero-Cuevas et al. 1998). The second objective of the present study was to further improve our understanding of the principles underlying the distribution of common input to motor units of hand muscles. Adding the present analysis of common input to intrinsic muscles to our previous analysis of extrinsic muscles (Winges and Santello 2004; Winges et al. 2006) and using the same object hold task allowed us to compare data from extrinsic with intrinsic muscle pairs. To further extend the characterization of common input to pairs of extrinsic muscles (previous work) and pairs of intrinsic muscles (present work), we also recorded EMG activity from an extrinsic thumb muscle (FPL). This allowed us to examine common input within a third category of muscle pairs, i.e., intrinsic-extrinsic muscles.
METHODS
Experimental procedures
Five subjects (4 males and 1 female; mean age: 32 yr, range: 23–38 yr) with no known neuromuscular disorders or musculoskeletal injuries of the hand took part in the experiments. Three subjects were right-handed, one subject was ambidextrous (but with a right-hand dominance for object grasping and holding), and one subject was left-handed as determined by the Edinburgh questionnaire (Oldfield 1971). None of our subjects had specific training in manual skills. Each subject participated in at least three experimental sessions and gave their informed consent prior to each experimental session. The experimental procedures were approved by the Institutional Review Board at Arizona State University and were in accordance with the declaration of Helsinki.
Subjects sat in an adjustable dental chair with their right forearm resting on a flat platform, the hand in a semipronated position and the wrist slightly extended. We asked subjects to grasp, lift, and hold a manipulandum (total mass: 0.145 kg) in an upright position using a thumb-index finger grip for a minimum period of 3 min (Fig. 1). Subjects were instructed to exert sufficient forces at the fingertips to prevent object slip and maintain the grip device aligned with the vertical during the entire duration of the trial. To attain the second objective, subjects were instructed to use a bull’s-eye level attached to the top of the grip device (Fig. 1) that indicated deviations from the vertical orientation, i.e., pitch and roll. The task requirements are similar to holding a glass filled with water while preventing it from spilling.
FIG. 1.
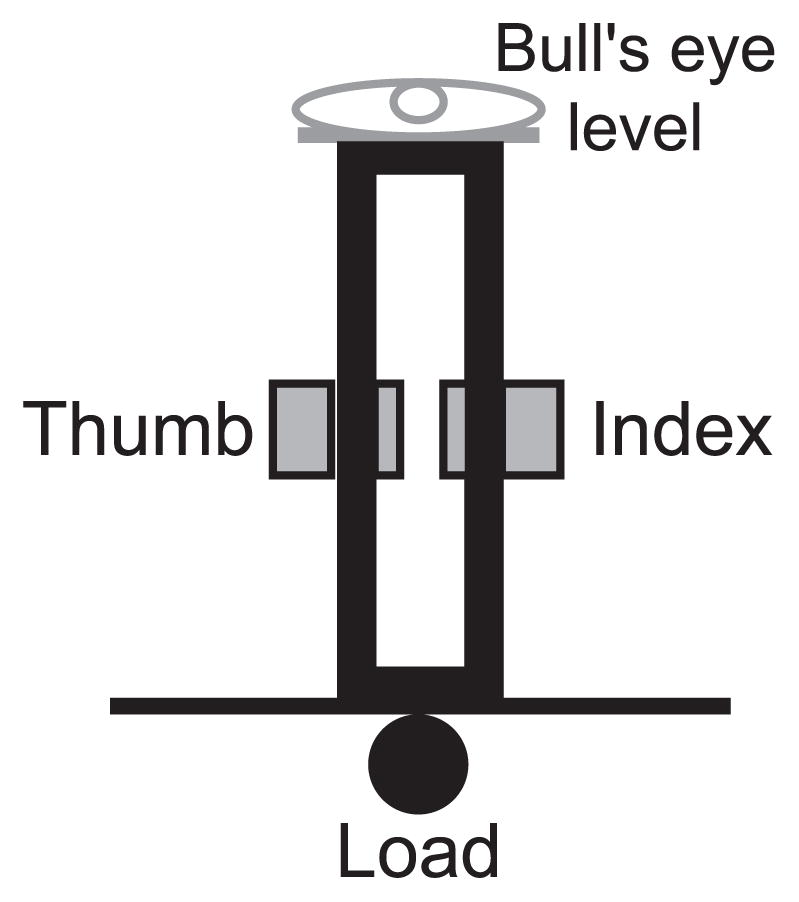
Grip device. The device used to measure normal and tangential forces during a 2-digit object hold task (figure is not to scale).
Before lifting the device, subjects placed the distal pad of the thumb and finger on their respective force sensor plate on either side of the grip device. After establishing a secure grip, subjects were asked to lift the device to a height of ~5 cm from the support surface. After the object was lifted, the experimenter placed a soft support under the forearm and proximal to the ulnar styloid process to prevent fatigue of the elbow flexor muscles. Before starting data recording, the subject aligned and maintained the object vertically using the bull’s eye level as a reference. We gave rest periods of ~5 min between trials to ensure that subjects were fully rested before starting a new trial.
Force and EMG recordings
Normal forces were measured at the thumb and index finger by two Nano17/SI-25-250 force/torque sensors (Fig. 1; ATI Industrial Automation, Apex, NC; nominal resolution: 0.0015 N). The static coefficient of friction (μ) of the contact surfaces was = 0.89 (this was measured as described by Aoki et al. 2007). Intramuscular EMG recordings were obtained over the course of the study using 27-gauge hypodermic needles to insert fine-wire electrodes (25 μm diam; California Fine Wire, Grover Beach CA) into the muscle bellies of two intrinsic index finger muscles (FPI and FDI). For each session, four fine-wire electrodes were inserted into FDI and two were inserted into FPI. One surface electrode (10 mm diam gold-plated silver disc, Model F-E5GH, Grass Instruments; West Warwick, RI) was placed on the radial styloid to serve as a reference electrode for each fine wire electrode and one tungsten microelectrode (see following text).
In addition to the preceding assessment of common input across intrinsic muscles, we also recorded from one extrinsic thumb flexor (FPL) using a tungsten microelectrode (Frederick Haer, Bowdoinham, ME; 1–5 μm tip diameter, 5–10 μm uninsulated length, 50 mm shaft length; 250 μm shaft diameter, ~200 kΩ impedance at 1,000 Hz after insertion). Recording from an extrinsic muscle controlling the thumb allowed us to extend our measurement of common neural input from one intrinsic muscle pair (FDI-FPI) to two extrinsic-intrinsic muscle pairs, i.e., FPL-FDI and FPL-FPI. Adding these two muscle pairs was useful in further characterizing the distribution of common neural input by allowing us to examine a larger number of muscle pairs and combinations. To allow natural physiological modulation of motor-unit discharge rate during an object hold task, subjects did not receive auditory feedback of discharge rate during the trial. Similarly, no visual feedback of the forces exerted on the device was given to allow for a natural distribution and fluctuation of individual fingertip forces during the object hold task (see Winges and Santello 2004 for details).
Normal forces and EMG analysis
Our force analysis focused on quantifying the normal forces exerted by each digit. Maximal voluntary grip force was also measured for each subject during a separate session to provide a relative measure of the normal force elicited by our grasping task. Subjects were asked to produce maximal voluntary grip forces during three 5-s trials separated by rest periods of 3 min. The maximum two-digit grip force across the three trials was used to normalize normal forces measured during object hold.
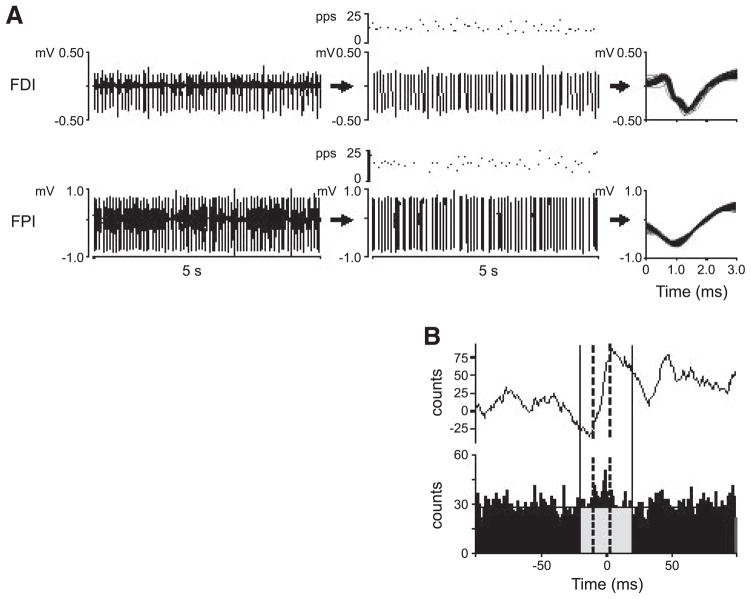
Single motor-unit activity was discriminated into spike trains from the interference EMG signal (Fig. 2A) using a commercially available software package (Spike2 v5.09, Cambridge Electronic Design, Cambridge, U.K). For each discriminated motor-unit spike train, the instantaneous discharge rate was computed as the inverse of the interspike interval (ISI). To assess whether there were any systematic increases or decreases in the discharge rate within each trial, we performed a least-square regression analysis on the instantaneous discharge rate of each motor unit. We then subtracted the slope of the regression line from the data to remove any trend (Laidlaw et al. 2000). The mean and SD of the motor-unit discharge rate were computed on the de-trended data from each trial and used to compute the coefficient of variation (CV) of motor-unit discharge rate. We then computed the geometric mean (GM) of the discharge rate and the GM of the CV of discharge rate for each motor-unit pair (Nordstrom et al. 1992) to quantify the within-trial variability of discharge rate.
FIG. 2.
Electromyograph (EMG), motor-unit synchrony during an object hold task. A: EMG from m. first dorsal interosseus (FDI) and m. first palmar interosseus (FPI) during a 2-digit object hold task. The data shown are from a smaller recording period than the entire duration of the trial (~4 min). Each row shows an interference EMG trace (left), motor units discriminated from the interference EMG trace and their instantaneous discharge rates (pulses per second: pps), and the action potentials of the discriminated motor units (right). B: cumulative sum (CUSUM) of the events of the cross-correlogram and cross-correlogram (top and bottom trace, respectively) for the same 2 motor units. Vertical solid lines denote the ±20-ms time period relative to 0. Vertical dashed lines indicate 10 and 90% of the CUSUM value within ±20 ms used to define the width of the cross-correlogram peak above chance level (horizontal line, bottom row). The number of events below chance level is calculated from the corresponding gray area. All data are from the same subject (2). CIS = 0.56 and peak width is 12.84 ms for this pair.
Analysis of motor-unit synchrony
Custom software was used to quantify motor-unit synchronization within and across muscles. Reference and test spike trains from separate electrodes were defined (arbitrary) and a cross-correlogram (1-ms bin, 201 bins) between the two motor-unit spike trains was computed for ±100 ms from the discharge of the reference unit (Fig. 2B, bottom). A technique using the cumulative sum (CUSUM) (Ellaway 1978) was used to determine the existence of a peak in the cross-correlogram that would indicate near synchronous discharge of the two motor units (Fig. 2B, top). The peak was defined by the area between the 10th and 90th percentiles of the largest inflection in the CUSUM within ±20 ms of the reference unit discharging (time period within the 2 dotted vertical lines in Fig. 2B) (Keen and Fuglevand 2004; Schmied et al. 1993). If a peak could not be defined within this region, a narrowed region of 11 ms centered around time 0 was used for the assessment of the strength of motor-unit synchrony for that motor-unit pair (Semmler et al. 1997). The duration of the cross-correlogram peak has been used to further interpret the mechanisms leading to motor-unit synchrony. Narrow cross-correlogram peaks resulting from motor units discharging within a few milliseconds of each other (short-term synchrony) would arise from shared inputs from branched axons of single last-order neurons (Kirkwood 1979). Broader cross-correlogram peaks would reflect synchrony of separate presynaptic inputs to the motor neurons (Semmler et al. 2002). Therefore to assess possible differences in the way common input is delivered to motor neurons, we computed the width of the cross-correlogram peak for each motor-unit pair.
We used the common input strength index (CIS) (Nordstrom et al. 1992) to quantify the strength of motor-unit synchrony. The value of the CIS index represents the frequency of synchronous discharges for a motor-unit pair above chance level. Chance level (horizontal line, Fig. 2B, bottom) is defined as the mean number of counts (spikes) per bin occurring in time bins from −100 to −40 ms and from 40 to 100 ms. CIS was computed as the ratio of the total counts in the peak of the cross-correlogram defined by the CUSUM minus the counts due to chance, normalized by the trial duration, i.e., the time within which both motor units were tonically active. The criteria for including a motor unit in the computation of the CIS was a tonic discharge of ≥800 discharges occurring without large gaps (>1 s). CIS was preferred over other synchrony measures due to its lower sensitivity to across-trial differences in discharge rate (Nordstrom et al. 1992). This was an important factor for our study because motor-unit discharge rate was not constrained. The preceding criteria for inclusion of motor units for analysis of synchrony significantly reduced the number of usable motor units within FDI and FPI for analysis of within-muscle synchrony because these muscles tended to be concurrently active for shorter period than the minimum required for analysis.
Statistical analysis
ANOVA was used to determine the effect of muscle pair (independent variable) on CIS (dependent variable). As differences in CIS may result from differences in discharge rate variability (Nordstrom et al. 1992), we used separate ANOVAs to assess whether GM of motor-unit discharge rate or the GM of the CV of motor-unit discharge rate (dependent variables) differed across muscle pairs (independent variable). We also performed linear regression analysis to assess the extent to which GM of motor-unit discharge rate, GM of the CV of discharge rate, and sum of the normal forces produced by the thumb and index finger influenced the CIS. For the regression analysis on normal forces (3rd analysis in the previous sentence), we used the sum of normal forces exerted by two digits instead of the force of individual digits because our measures of common input are based on correlating activity of motor units across muscles of two digits for two of the three muscle pairs studied (FPL-FDI and FPL-FPI).
We also used ANOVA to assess whether the peak width of the cross-correlogram (dependent variable) differed across muscle pairs (independent variables) to assess how the common input is delivered to pairs of motor units (see preceding text). When appropriate, Bonferroni-corrected post hoc tests were used to locate statistical differences. A significance level of P ≤ 0.05 was used for all comparisons. All data are reported as means ± SE unless otherwise noted.
RESULTS
Analysis of 198 across-muscle motor-unit pairs during our two-digit object hold task revealed weak across-muscle synchrony for the intrinsic-intrinsic muscle pair (FDI-FPI). Motor units from both extrinsic-intrinsic muscle pairs (FPL-FDI, FPL-FPI) were characterized by slightly stronger synchrony than FDI-FPI. Last, the strength of within-FDI and -FPI motor-unit synchrony was more than three times stronger than that measured across FDI and FPI.
Digit forces during object hold
Mean thumb and index finger normal forces were 2.61 ± 0.32 and 2.66 ± 0.32 n, respectively. These forces were <5% of maximal voluntary grip force (range: 3.1–4.4%). Therefore as our two-digit object hold task elicited small forces, the motor units sampled in this study were restricted to presumably small, low-threshold motor units.
Motor-unit discharge properties
The range of motor-unit discharge rates was broad and similar to that reported by our previous study, i.e., 7–15 Hz (Winges and Santello 2004). The de-trended discharge rate data (see METHODS) varied only slightly from the raw data, i.e., mean difference of 0.011 ± 0.004 (SD) Hz with a maximum difference of 0.035 Hz. Hence no systematic increase or drift in motor-unit discharge rate occurred during our object hold task.
The GM of motor-unit discharge rates was significantly higher for FPL-FPI compared with FDI-FPI [Table 1; F(4,218) = 5.195; P < 0.001]; however, this difference was very small, i.e., 1.0 Hz. The GM of the CV of motor-unit discharge rate ranged from ~28 to ~32% (Table 1). This range is comparable, but higher than, that reported by our previous studies of motor units from extrinsic hand muscles during five-digit grasping (~20 to ~28%) (Winges and Santello 2004) and two-digit grasping (~20–24%) (Winges et al. 2006). Significant main effects of muscle pair on the GM of the CV of motor-unit discharge rate were found such that this variable was higher for FDI-FPI than FPL-FDI and FPL-FPI [F(4,218) = 4.526; P < 0.01], although these differences were both <3.5%.
TABLE 1.
Motor-unit discharge rates, discharge rate variability, and CIS
| MU Pairs | GM Discharge Rate, pps | GM CV Discharge Rate, % | CIS | Peak Width, ms | |
|---|---|---|---|---|---|
| Across muscle | |||||
| FDI-FPI | 74 | 9.81 ± 0.17 | 31.56 ± 0.63 | 0.17 ± 0.02 | 16.98 ± 1.19 |
| FPL-FDI | 67 | 10.24 ± 0.18 | 28.29 ± 0.66 | 0.25 ± 0.02 | 15.46 ± 1.08 |
| FPL-FPI | 57 | 10.82 ± 0.19 | 28.35 ± 0.72 | 0.29 ± 0.03 | 18.64 ± 1.08 |
| Within muscle | |||||
| FDI-FDI | 11 | 9.30 ± 0.43 | 30.47 ± 1.64 | 0.70 ± 0.06 | 16.00 ± 2.29 |
| FPI-FPI | 10 | 9.92 ± 0.45 | 31.54 ± 1.72 | 0.66 ± 0.06 | 20.74 ± 2.54 |
The number of motor-unit (MU) pairs used for the analysis is given together with the geometric mean (GM) of motor-unit firing rate and the coefficient of variation (CV) of motor-unit firing rate, the common input strength index (CIS), and the duration of the cross-correlogram peak. All values are means ± SE. FDI, m. first dorsal interosseus; FPI, m. first palmar interosseus; FPL, m. flexor pollicis longus.
Across-muscle motor-unit synchrony
A typical experimental record of EMG is shown in Fig. 2. Seventy experimental trials yielded 198 discriminated motor-unit pairs (74 from FDI-FPI, 67 from FPL-FDI, 57 from FPL-FPI) used for the analysis of across-muscle motor-unit synchrony (Table 1). The number of motor-unit pairs included in the analysis from each subject is given in Table 2.
TABLE 2.
Number of motor-unit pairs per subject
| Subject
|
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Across muscle | |||||
| FDI-FPI | 10 | 15 | 11 | 26 | 12 |
| FPL-FDI | 14 | 14 | 12 | 16 | 11 |
| FPL-FPI | 10 | 10 | 10 | 15 | 12 |
| Within muscle | |||||
| FDI-FDI | 2 | 3 | 1 | 5 | 0 |
| FPI-FPI | 1 | 1 | 0 | 7 | 1 |
The mean number of counts used to generate the cross-correlograms (Fig. 2B) for each motor-unit pair was 2,952 ± 53. The widths of peaks that could be defined in the cross-correlogram ranged from 2.76 to 35.69 ms (mean values for each muscle pair are shown in Table 1). Only 1% of motor-unit pairs had narrow peaks (±5 ms), and 30% had no definable peak in the cross-correlogram. The largest proportion of motor-unit pairs (69%) had broad peaks (≥ ±5 ms) in the cross-correlogram. There was no effect of muscle pair on cross-correlogram peak width [F(4,158) = 1.759; P > 0.05].
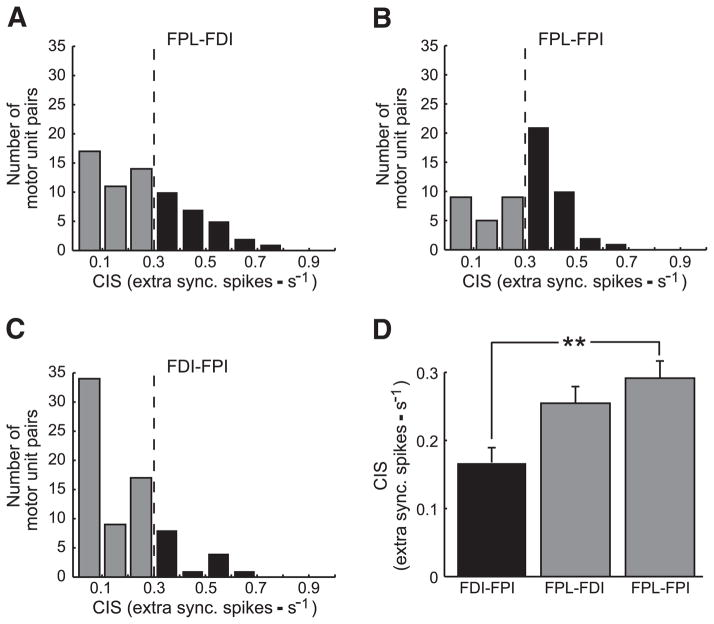
Figure 3 shows the values of common input strength (CIS; see Experimental procedures) from each muscle pair. Across-muscle CIS values ranged from 0 to 0.79 with the majority of the motor-unit pairs having small CIS values, i.e., ≤0.3 (Fig. 3, A–C). Note that the CIS of 0.3 is an arbitrary cut-off based on the results of a simulation study the purpose of which was to determine a CIS value that would be capable of affecting the coordination of forces (Santello and Fuglevand 2004) and is used here to separate “weak” from “moderate” motor-unit synchrony. As discussed in Winges and Santello (2004), a CIS value of 0.6 measured from motor units of hand muscles would indicate relatively strong synchrony, whereas values >0.3 would denote moderate but still significant level. CIS values <0.3 are associated with weak motor-unit synchrony as often no clear peaks in the cross-correlogram can be detected (see following text; see also Fig. 2 in Semmler and Nordstrom 1998).
FIG. 3.
Common input strength (CIS) distributions. Distributions of CIS values for the extrinsic-intrinsic muscle pairs [m. flexor pollicis longus (FPL)-FDI and FPL-FPI; A and B, respectively] and the intrinsic muscle pair (FDI-FPI; C). For graphical purposes, CIS data were binned into 0.1 intervals. Gaps in the distributions indicate no motor-unit pairs with that CIS value. Vertical lines indicate an arbitrary cut-off (CIS: 0.3) between weak and moderate to strong motor-unit synchrony. D: mean CIS values (±SE) for each muscle pair are shown (**, significant difference at P < 0.001).
The extrinsic-intrinsic muscle pairs (FPL-FDI and FPL-FPI) had a larger number of motor-unit pairs above the 0.3 cut-off level (black bars; Fig. 3, A and B) than the intrinsic-intrinsic muscle pair (FDI-FPI; Fig. 3C). CIS computed on motor units across intrinsic-extrinsic muscles (FPL-FDI and FPL-FPI) was larger than CIS across intrinsic muscles (FPI-FDI) in three of five and five of five subjects, respectively. CIS amplitude was significantly different across these muscle pairs [Fig. 3D; F(4,218) = 28.929; P < 0.001]. The intrinsic muscle pair exhibited the weakest across-muscle motor-unit synchrony compared with the other two muscle pairs, whereas motor units from FPL-FPI were characterized by the strongest synchrony (Fig. 3D). Overall, however, across-muscle motor-unit synchrony was weak for each of the muscle pairs studied.
Common input relative to force and motor-unit characteristics
To summarize, the intrinsic muscle pair (FDI-FPI) exhibited weaker synchrony than the extrinsic-intrinsic muscle pairs. To assess the extent to which this finding might have resulted from differences in digit force or motor-unit discharge characteristics, we performed linear regression analysis (see METHODS).
FORCE CHARACTERISTICS
A significant negative linear correlation was found between the sum of the normal forces and the magnitude of CIS values for the FPL-FDI muscle pair only, although this correlation was very weak (n = 67, r2 = 0.10, P < 0.01). Correlations were not significant between the sum of the normal forces and the magnitude of CIS values from FPL-FDI (n = 57, r2 = 0.01, P > 0.05) or FDI-FPI (n = 74, r2 = 0.01, P > 0.05).
MOTOR-UNIT DISCHARGE CHARACTERISTICS
A significant positive effect of geometric mean (GM; see Experimental procedures) of discharge rate was found on the magnitude of CIS values for each muscle pair (FDI-FPI: n = 74, r2 = 0.299, P < 0.001; FPL-FDI: n = 67, r2 = 0.099, P ≤ 0.01; FPL-FPI: n = 57, r2 = 0.092, P < 0.05). However, the magnitude of CIS computed on each muscle pair was minimally (~30% of the variance; FDI-FPI) or not affected (<10% of the variance; FPL-FDI and FPL-FDI) by variability in motor-unit discharge rate. Therefore for the muscle pair with the strongest linear correlation (FDI-FPI), the mean CIS value (Table 1) is slightly inflated by motor units with higher discharge rate. Hence, the weak CIS values found across motor units of FDI and FPI might have been slightly weaker had we pooled motor-unit pairs with a more homogenous discharge rate. Last, no significant correlation was found between CIS and the GM of the CV of motor-unit discharge rate for any muscle pair (P > 0.05).
Within-muscle motor-unit synchrony
As more than one fine-wire electrode was used on each intrinsic muscle (see METHODS), in some experimental sessions, we were able to record and isolate concurrently active motor-unit pairs within FDI and FPI. The within-muscle analysis of motor-unit synchrony was performed on 11 motor-unit pairs from FDI (4 subjects) and 10 motor-unit pairs from FPI (4 subjects). Peak width of the cross-correlograms computed on motor units from either muscle were comparable to those computed on motor units across muscles (see Table 1). Regression analysis between CIS and the sum of normal forces revealed a significant positive linear correlation for the FDI-FDI muscle pair only (n = 11, r2 = 0.40, P < 0.05). No statistically significant relation was found between CIS values and GM of discharge rate or GM of the CV of motor-unit discharge rate (P > 0.05). Note that the strength of synchrony of motor units within FDI and FPI was stronger (~3-fold difference) than that computed across motor units belonging to any of the three muscle pairs studied (Table 1). Examination of data from individual subjects showed that CIS computed on FDI-FDI and FPI-FPI was larger than CIS computed on FPI-FDI, FPL-FDI, and FPL-FPI for all subjects and for three of four subjects, respectively.
DISCUSSION
We found that motor-unit synchrony across intrinsic muscles was weaker than within intrinsic muscles as well as previously reported across-extrinsic muscle motor-unit synchrony (Winges and Santello 2004; Winges et al. 2006). Motor-unit synchrony across intrinsic-extrinsic muscles was also weak but stronger than across intrinsic muscles. Combining present and previous results suggests that common neural input to intrinsic and extrinsic hand muscles is distributed in a muscle-pair specific fashion.
Common neural input to motor units within and across intrinsic hand muscles
In agreement with other studies (Nordstrom et al. 1992; Semmler et al. 1997, 2000), we found strong common neural input to single motor-unit pairs within intrinsic hand muscles (Table 1). In contrast to the strong common input delivered to motor units of either FDI or FPI, motor-unit activity across these muscles was characterized by weak synchrony (Fig. 3; Table 1). This might be interpreted as an expectable finding as it has been reported that motor-unit synchrony across hand muscles is relatively weak compared with that found within a muscle (e.g., Bremner et al. 1991a; Gibbs et al. 1995; Huesler et al. 2000). However, the magnitude of CIS values obtained from motor units of FPL and the index compartment of FDP is more than double the magnitude of the present values of CIS across intrinsic muscles (Hockensmith et al. 2005; McIsaac and Fuglevand 2006; Winges and Santello 2004; Winges et al. 2006). Therefore the fact that motor-unit synchrony was measured across muscles cannot fully account for the weak common input across FDI and FPI. Further, it suggests that weak motor-unit synchrony across these intrinsic muscles may originate from factors others than those associated with weaker motor-unit synchrony for pairs across different synergists or muscle compartments. Note that a comparison between our present and previous results is reasonable as data were collected using the same object hold task. We speculate that the stronger common input across extrinsic versus intrinsic muscles reflects a muscle-pair specific organization (see following text).
Organization of inputs to motor units of intrinsic and extrinsic hand muscles
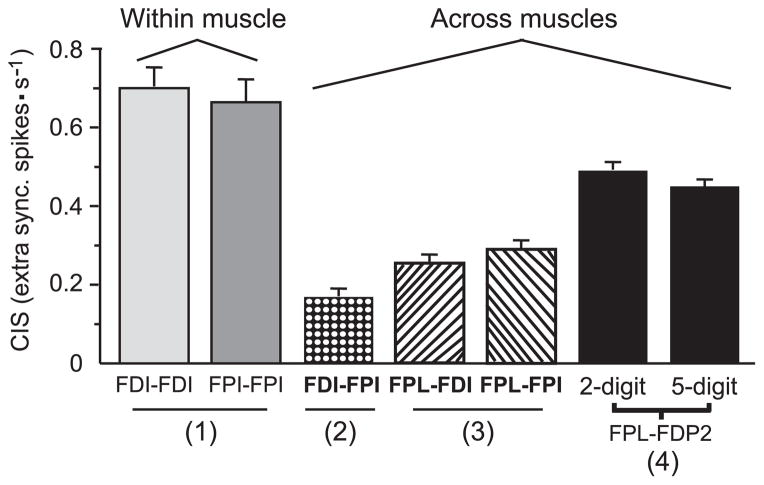
Strong motor-unit synchrony within intrinsic muscles of the hand is consistent with previous research (e.g., Kim et al. 2001). Within the framework of our previous work, however, the results on within-muscle synchrony measured from FDI and FPI are of particular significance as they provide further insight on the coordination of neural activity of multiple hand muscles during an object-hold task. Specifically, the strength of within-muscle synchrony was three times larger than that measured across the same muscles (see above; Fig. 4, dotted bar). The weaker common input across versus within muscles suggests that the neural coupling of these muscles is organized to maintain a higher degree of independence across FDI and FPI.
FIG. 4.
Distribution of CIS for thumb and index muscles measured during an object-hold task. The figure shows mean CIS values (±SE) computed from motor-unit pairs within intrinsic muscles (1), across intrinsic muscles (2), across intrinsic and extrinsic muscles (3), and across extrinsic muscles (4). Data 1–3 refer to the present 2-digit object hold task. Data 4 are from Winges and Santello (2004) (5-digit object hold) and Winges et al. (2006) (2-digit object hold) and are shown for comparison with the results of the present study.
A comparison with our previous studies of motor-unit activity during an object hold task (Fig. 4) (Winges and Santello 2004; Winges et al. 2006) provides further understanding of the distribution of common input to extrinsic and intrinsic hand muscles. These studies found that the strength of motor-unit synchrony across a thumb and index finger extrinsic muscle (FPL and FDP2, respectively) was more than twice the magnitude of that reported here across intrinsic muscles. We speculate that the difference in the strength of motor-unit synchrony between FPL-FDP2 and FDI-FPI might reflect differences in the long-term adaptations to their role in coordinating grip forces during object grasping. FPL and FDP2 act as synergists for maintaining equilibrium of normal forces necessary to prevent object slip. In contrast, while FDI and FPI share a synergist action with index finger flexors and extensors, they also have opposite mechanical actions in the abduction-adduction plane. Using an index finger force production task, Valero-Cuevas et al. (1998) reported that FDI and FPI are co-activated when generating forces in the ulnar and radial direction. However, these authors also reported that the level of FDI and FPI activation differed depending on force direction (Valero-Cuevas et al. 1998). Therefore fine modulation of the direction of tangential forces (opposite to gravitational force in our object hold task) might be better served by a relatively independent control of FDI relative to FPI rather than by coupling their activation through common neural input. Similar considerations might apply to neural coupling of FPL and FDI or FPI, as FPL plays a significant role in directing thumb force in opposition pinch (Johanson et al. 2001).
Last, we found that motor-unit synchrony across an extrinsic thumb muscle, FPL, and either intrinsic index finger muscle, FDI or FPI, was generally weak (Fig. 3, A and B, respectively). However, motor-unit synchrony across FPL-FDI and FPL-FPI was stronger than across FDI-FPI (Fig. 3C) and weaker than motor-unit synchrony across extrinsic muscles during either object hold or force production tasks (Fig. 4) (Hockensmith et al. 2005; Winges and Santello 2004; Winges et al. 2006). Huesler et al. (2000) also found that the incidence of synchrony was larger for extrinsic muscle pairs than for intrinsic and mixed intrinsic/extrinsic muscle pairs, although these samples included motor-unit pairings within and across muscles. Together these results might reflect a different organization of the inputs to motor neuron pools as these intrinsic and extrinsic muscles are innervated by different nerves. Specifically, the two extrinsic-intrinsic muscle pairs studied, FPL-FDI and FPL-FPI, receive different innervations (FPL: median nerve; FPI and FDI: ulnar nerve). As suggested by Maier and Hepp-Reymond (1995), the motor neuron pool associated with different innervation might be associated with a weaker degree of last-order branching of presynaptic common input relative to motor neuron pools that share the same innervation.1 This might also account for the weaker CIS from FPL-FDI and FPL-FPI than previously reported from extrinsic muscle pairs and compartments as these are innervated by the same nerve (Winges and Santello 2004; Winges et al. 2006). Note, however, that this explanation does not account for the differences found between across- and within-intrinsic muscles discussed in the preceding text.
The work by Bremner et al. (1991a,b) is also relevant to the discussion of the present results. Specifically, these authors reported that the incidence (Bremner et al. 1991a) and strength (Bremner et al. 1991b) of motor-unit synchrony was higher for hand muscles that had similar actions on different digits than muscles with different actions inserting on the same digit. A more direct comparison with our findings, however, can be made when considering Bremner et al.’s (1991b) finding that motor units innervating muscles with different actions inserting on different digits (index abductor-thumb extensor) exhibit weaker synchrony than muscles with different actions on the same digit (index abductor-index extensor; their Fig. 5C, page 390). This finding is relevant to our comparison of FPL-FDI and FPL-FPI (different action, different digit) with FDI-FPI (different action, same digit). Note, however, that we are reporting opposite results, i.e., the former groups of muscle pairs exhibited stronger synchrony than the latter muscle pair. As there are many methodological differences (e.g., task, method to quantify synchrony, muscles used for analysis) between our study and the work by Bremner and colleagues, we are unable to identify the factors underlying these differences. Therefore further work is needed to determine the extent to which the weak synchrony across motor units of FDI and FPI demonstrates a general principle of organization of neural control of intrinsic hand muscles.
In summary, we interpret the weak synchrony across motor units of FDI and FPI to be related to the functional role played by these muscles (i.e., to prevent object slip vs. modulation of the tangential forces) and that the organization of common neural input to hand muscles is muscle-pair specific.
Conclusions
The present and previous work supports the notion of common inputs being distributed nonuniformly across hand muscle pairs. The muscle-pair specific distribution appears to reflect the different role that given muscle pairs play in object grasping and manipulation.
Acknowledgments
The authors thank Dr. Jamie Johnston and L. Bobich for helpful comments on the manuscript.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 2RO1 AR47301 to M. Santello and National Science Foundation-Integrated Graduate Education and Research Training Grant 9987619 to S. Winges.
Footnotes
Note, however, that the association between the incidence of motor-unit synchrony (significant peak in the cross-correlograms) and innervation type for motor-unit pairs across different muscles was not observed in a later study from the same laboratory (Huesler et al. 2000).
References
- Aoki T, Latash ML, Zatsiorsky VM. Adjustments to local friction in multifinger prehension. J Mot Behav. 2007;39:276–290. doi: 10.3200/JMBR.39.4.276-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand PW, Hollister A. Clinical Mechanics of the Hand. 3. St. Louis, MO: Mosby; 1999. [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Correlation between the discharges of motor units recorded from the same and from different finger muscles in man. J Physiol. 1991a;432:355–380. doi: 10.1113/jphysiol.1991.sp018389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Variation in the degree of synchronization exhibited by motor units lying in different finger muscles in man. J Physiol. 1991b;432:381–399. doi: 10.1113/jphysiol.1991.sp018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Stephens JA. Synchronization of motor unit activity during voluntary contractions in man. J Physiol. 1990;442:397–419. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Mambrito B. Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J Neurophysiol. 1987;58:525–542. doi: 10.1152/jn.1987.58.3.525. [DOI] [PubMed] [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol. 1978;45:302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Organization of inputs to motoneurone pools in man. J Physiol. 1995;485:245–256. doi: 10.1113/jphysiol.1995.sp020727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockensmith GB, Lowell SY, Fuglevand AJ. Common input across motor nuclei mediating precision grip in humans. J Neurosci. 2005;25:4560–4564. doi: 10.1523/JNEUROSCI.0046-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesler EJ, Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. III. Synchronization of single motor units. Exp Brain Res. 2000;134:441–455. doi: 10.1007/s002210000484. [DOI] [PubMed] [Google Scholar]
- Johanson ME, Valero-Cuevas FJ, Hentz VR. Activation patterns of the thumb muscles during stable and unstable pinch tasks. J Hand Surg. 2001;26A:698–705. doi: 10.1053/jhsu.2001.26188. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Winges SA, Santello M. Periodic modulation of motor-unit activity in extrinsic hand muscles during multidigit grasping. J Neurophysiol. 2005;94:206–218. doi: 10.1152/jn.01134.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol. 2004;91:57–62. doi: 10.1152/jn.00650.2003. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Alonso-Alonso M, Fisher R, Lemon RN. Modulation of synchrony between single motor units during precision grip tasks in humans. J Physiol. 2002;541:937–948. doi: 10.1113/jphysiol.2001.013305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Masakado Y, Tomita Y, Chino N, Pae YS, Lee KE. Synchronization of single motor units during voluntary contractions in the upper and lower extremities. Clin Neurophysiol. 2001;112:1243–1249. doi: 10.1016/s1388-2457(01)00549-1. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979;1:107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve. 2000;23:600–612. doi: 10.1002/(sici)1097-4598(200004)23:4<600::aid-mus20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Long C, Conrad PW, Hall EA, Furler SL. Intrinsic-extrinsic muscle control of the hand in power grip and precision handling. J Bone Joint Surg Am. 1970;52:853–867. [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. II. Muscular synergies in the spatial and temporal domain. Exp Brain Res. 1995;103:123–136. doi: 10.1007/BF00241970. [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Influence of tactile afferents on the coordination of muscles during a simulated precision grip. Exp Brain Res. 2006;174:769–774. doi: 10.1007/s00221-006-0643-z. [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Motor unit synchrony within and across compartments of the human flexor digitorum superficialis. J Neurophysiol. 2007;97:550–556. doi: 10.1152/jn.01071.2006. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Lee RG. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalogr Clin Neurophysiol. 1975;38:245–254. doi: 10.1016/0013-4694(75)90245-x. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol. 1992;453:547–574. doi: 10.1113/jphysiol.1992.sp019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly KT, Nordstrom MA, Schieber MH. Short-term synchronization between motor units in different functional subdivisions of the human flexor digitorum profundus muscle. J Neurophysiol. 2004;92:734–742. doi: 10.1152/jn.00027.2004. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Santello M, Fuglevand AJ. Role of across-muscle motor unit synchrony for the coordination of forces. Exp Brain Res. 2004;159:501–508. doi: 10.1007/s00221-004-1975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Soechting JF. Force synergies for multifingered grasping. Exp Brain Res. 2000;133:457–467. doi: 10.1007/s002210000420. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. 2004;96:2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- Schmied A, Ivarsson C, Fetz EE. Short-term synchronization of motor units in human extensor digitorum communis muscle: relation to contractile properties and voluntary control. Exp Brain Res. 1993;97:159–172. doi: 10.1007/BF00228826. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Kornatz KW, Dinenno DV, Zhou S, Enoka RM. Motor unit synchronisation is enhanced during slow lengthening contractions of a hand muscle. J Physiol. 2002;545:681–695. doi: 10.1113/jphysiol.2002.026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG, Nordstom MA. Motor unit discharge and force tremor in skill-and strength-trained individuals. Exp Brain Res. 1998;119:27–38. doi: 10.1007/s002210050316. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Nordstrom MA, Wallace CJ. Relationship between motor unit short-term synchronization and common drive in human first dorsal interosseous muscle. Brain Res. 1997;767:314–320. doi: 10.1016/s0006-8993(97)00621-5. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Steege JW, Kornatz KW, Enoka RM. Motor-unit synchronization is not responsible for larger motor-unit forces in old adults. J Neurophysiol. 2000;84:358–366. doi: 10.1152/jn.2000.84.1.358. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol. 2000;83:1469–1479. doi: 10.1152/jn.2000.83.3.1469. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Zajac FE, Burgar CG. Large index-fingertip forces are produced by subject independent patterns of muscle excitation. J Biomech. 1998;31:693–703. doi: 10.1016/s0021-9290(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Winges SA, Johnston JA, Santello M. Muscle-pair specific distribution and grip type modulation of neural common input to extrinsic digit flexors. J Neurophysiol. 2006;96:1258–1266. doi: 10.1152/jn.00327.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winges SA, Santello M. Common input to motor units of digit flexors during multi-digit grasping. J Neurophysiol. 2004;92:3210–3220. doi: 10.1152/jn.00516.2004. [DOI] [PubMed] [Google Scholar]
- Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol. 2000;83:441–452. doi: 10.1152/jn.2000.83.1.441. [DOI] [PubMed] [Google Scholar]