Abstract
Objective
Animal models of combined TBI and hemorrhagic shock (HS) suggest a benefit of HBOC-based resuscitation, but their use remains controversial and little is known of the specific effects of TBI and high-pressure (large arterial injury) bleeding on resuscitation. We examine the effect of TBI and aortic tear injury on low volume HBOC resuscitation in a swine polytrauma model and hypothesize that HBOC-based resuscitation will improve survival in the setting of aortic tear regardless of the presence of TBI.
Methods
Anesthetized swine subjected to HS with aortic tear +/− fluid percussion TBI underwent equivalent limited resuscitation with HBOC, LR, or HBOC+nitroglycerine (NTG) (vasoattenuated HBOC) and were observed for 6 hours.
Results
There was no independent effect of TBI on survival time after adjustment for fluid type and there was no interaction between TBI and resuscitation fluid type. However, total catheter hemorrhage volume required to reach target shock blood pressure was less with TBI (14.0 ml/kg [12.4, 15.6]) vs. HS-only (21.0 ml/kg [19.5, 22.5]), with equivalent lactate accumulation.
Conclusion
TBI did not affect survival in this polytrauma model, but less hemorrhage was required in the presence of TBI to achieve an equivalent degree of shock suggesting globally impaired cardiovascular response to hemorrhage in the presence of TBI. There was also no benefit of HBOC-based fluid resuscitation over LR, contrary to models using liver injury as the source of hemorrhage. Considering wound location is of paramount importance when choosing resuscitation strategy.
Introduction
Trauma is the leading cause of death among younger adults.1 Approximately 40% of these deaths in the U.S. civilian population are due to brain injury (TBI), and as many as 33% to 50% of all traumatic deaths occur prior to hospital arrival.2 Traumatic brain injury is often accompanied by significant blood loss and resulting hypotension from extra cranial injuries. Also of significance, uncontrolled hemorrhage accounts for the preponderance (~60%) of deaths in patients with potentially salvageable injuries.3 These factors support the development of improved strategies for the early initial fluid resuscitation of patients with traumatic brain injury and hemorrhagic shock (HS).
That TBI and HS often occur concomitantly due to multiple organ injuries4–6 is of critical importance because even brief episodes of hypotension and hypoxemia can double TBI mortality.5 This is thought to be due to loss of cerebral auto regulation resulting in secondary ischemic insult to the already vulnerable brain.6–7 Experimental data also suggest that TBI impairs physiologic compensatory mechanisms to HS.8–10 Treatment of patients experiencing both TBI and HS emphasizes aggressive fluid resuscitation through infusion of large volumes of crystalloid with goals of rapid volume expansion with blood pressure (BP) and cerebral perfusion pressure (CPP) restoration in the pre-hospital and hospital settings, respectively.11 However, there is considerable evidence that large volume crystalloid resuscitation is detrimental in the setting of uncontrolled hemorrhage and titrating fluids to achieve normal blood pressures in this setting is not beneficial.12,13 Most recently, studies of combined TBI and uncontrolled hemorrhage demonstrate that aggressive resuscitation, as is currently recommended, may result in failure to optimize cerebrovascular hemodynamics and greater short-term mortality.14–15 Consequently, artificial oxygen-carrying blood alternatives, such as hemoglobin based oxygen carriers (HBOCs), given in low volumes are currently under investigation as potential alternative resuscitation agents for the treatment of trauma and brain injury. These agents might facilitate a resuscitation strategy that minimizes hemorrhage volume while maximizing tissue oxygenation.
Given the promise of the HBOCs for the battlefield and pre-hospital settings, there has been extensive research in this area and several agents are in varying stages of development and pre-clinical and clinical investigation. Several pre-clinical studies suggest that the oxygen-carrying resuscitative fluid, hemoglobin-based oxygen carrier-201 (HBOC-201, Hemopure®, Cambridge, MA), may be of benefit in the setting of HS either with or without concomitant TBI based on its ability to expand the intravascular volume, to stabilize hemodynamics with low-volume resuscitation, to transport and unload O2 with kinetics similar to that of adult human hemoglobin, and to increase tissue oxygenation. 16–22 However, vasoactivity, manifested as vasoconstriction from local scavenging of nitric oxide (NO), has hampered development for trauma use.23 Consequently, much effort to attenuate HBOC-201’s vasoactivity is underway, mainly by modifying the molecular weight (MW) distribution and admixing NO donors such as sodium nitroglycerine (NTG). However, there is limited knowledge of the interaction between TBI-related physiological dysfunction and HBOC-based resuscitation in the prehospital setting. This is an important knowledge gap given the distinct challenges associated with treatment of combined TBI and HS in the prehospital setting. Therefore, the goal of this study is to identify the impact of TBI on a strategy of limited resuscitation of HS using HBOC and vasoattenuated HBOC in a model of large arterial vessel injury with free internal bleeding. We first hypothesize that TBI will impair resuscitation and decrease survival time during low-volume resuscitation of HS using multiple resuscitation fluids. Secondarily, we hypothesize that early limited resuscitation with HBOC-201 in the setting of a high pressure vascular injury inflicted via aortic tear will improve survival over limited resuscitation with standard lactated Ringers (LR) solution. The knowledge gained from this study informs further research into the correct choice of resuscitation fluid strategy for use in the complex and challenging polytrauma patient with TBI and HS.
Materials and Methods
Animals
All protocols were approved by the Animal Care and Use Committee of the University of Washington. Animal housing and all experiments were conducted in compliance with the Animal Welfare Act and in accordance with the principals set forth in the Guide for the Care and Use of Laboratory Animals, National Academy of Science, 2011. Animals were maintained at the University of Washington, Department of Comparative Medicine facility, an AAALAC accredited facility. These experiments were conducted in two phases with each phase lasting approximately 5–6 months. The model used in phase one was combined hemorrhagic shock with TBI, while phase two was hemorrhagic shock alone. During each phase all animals were randomly assigned to receive one of the three resuscitation fluids (HBOC, HBOC+5NTG, or LR).
Instrumentation for Monitoring and Hemorrhage
Immature (4 month old) female mixed breed Yorkshire swine weighing 26.6 kg [95%CI 25.7, 27.5] purchased from the Washington State University Swine Center were fasted overnight prior to surgery with free access to water. The animals were sedated with an intramuscular injection of Ketamine (30mg/kg) (BionichePharma, Galway, Ireland) followed by 3% isoflurane (VetOne, Boise, ID) by nasal cone to facilitate endotracheal intubation. A onetime IM injection of Buprenorphrine (0.01mg/kg) (Ben Venue Laboratories Inc. Bedford, OH) was given to reduce the concentration of isoflurane needed to achieve and maintain a surgical-plane of anesthesia. Animals were allowed to breathe spontaneously; if they became bradypneic or apneic they were placed on a volume cycled ventilator and ventilated (ANESCO SAV 2500 Anesthesia Ventilator, ANESCO, Inc. Georgetown, KY) with a tidal volume of 5 to 10 mL/kg at a rate of 12 to 15 breaths per min adjusted to maintain ETCO2 between 35 and 40 mmHg (Capnomac Ultima, Datex, Madison, WI). Bilateral femoral arteries and femoral veins were isolated and cannulated with PE240 polyethylene catheters for blood pressure monitoring, blood sampling, and controlled hemorrhage and for intravascular infusion. An 8 French introducer sheath was placed into the right external jugular vein and a 7 F Swan-Ganz thermodilution catheter (Edwards Life Sciences, Irvine, CA) was advanced to the pulmonary artery for measurement of cardiac output (CO), pulmonary artery pressure, central venous pressure and sampling for central venous blood gas. Splenectomy and bladder cannulation were then performed via midline laparotomy. The infrarenal aorta was then isolated and a 5-0 monofilament stainless steel surgical wire was inserted traversing 4mm longitudinally for the induction of an aortic tear. The aortotomy wires were externalized through the midline abdominal incision and the abdominal cavity was closed. This and similar aortic tear models of uncontrolled hemorrhage have been utilized in several previous studies.12–14,24
Brain Instrumentation for Fluid percussion/TBI
The scalp was widely incised and reflected posteriorly so that the cranium was exposed. A 16 mm diameter craniotomy was performed in the right parietal region adjacent to the sagittal suture and anterior to the coronal suture. A 3-way bolt was placed into the craniotomy to abut the intact dura. This bolt was then connected to a fluid percussion injury device (FPI) as well as a pressure transducer (SenSym®, Sunnyvale, CA) to quantify brain injury. A second craniotomy was performed in the left posterior parietal region and a neonatal intraventricular catheter (Phoenix Biomedical Corp. Valley Forge PA) was inserted into the left ventricle and connected to a pressure transducer to monitor intracranial pressure (ICP). A third craniotomy was performed on the midline suture, just anterior to the inion, and the sagittal sinus was cannulated with a 16 gauge Angiocath® catheter for sagittal sinus blood sampling. A fourth craniotomy was made in the right frontoparietal region for placement of a Licox® tissue oxygen/temperature probe (Integra Neurosciences Inc. Plainsboro, NJ). Any cortical bleeding was controlled with bone wax and thombin gel. All craniotomies were sealed with dental cement to secure catheters and probes in place and the animal allowed to re-equilibrate for 30 minutes during which fraction of inspired oxygen (FiO2) was maintained at 28–30%, end-tidal CO2 was kept at 35–40 torr, and body temperature, monitored by Swan Ganz Catheter, was maintained at 37±1°C with a warming blanket. All animals underwent brain instrumentation, regardless of whether TBI was inflicted.
Hemorrhage and TBI
Fluid percussion traumatic brain injury (TBI) was inflicted in the standard fashion as follows. The 3-way bolt in the right frontoparietal craniotomy position was connected to a saline-filled column, which is connected on the opposite end to a pendulum arm with a weight at its distal end. The pendulum is pulled back a standard distance and allowed to fall, striking a plexiglass piston which in turn strikes a rubber seal at the end of the fluid-filled cylinder. The resulting fluid wave that is generated in this closed system transmits a 15-msec pressure pulse to the intact dura, which is quantified via the high-pressure transducer, also connected to the 3-way craniotomy bolt.
Immediately following TBI, the animals were rapidly placed in supine position and controlled hemorrhage was initiated from the right femoral artery catheter with a MasterFlex® roller pump (Cole-Parmer, Vernon Hills, IL) to bleed 35ml/kg over 30 minutes, the rate pre-programmed to decrease exponentially over time simulating the natural course of arterial hemorrhage. Once the mean arterial pressure (MAP) reached 50 mmHg, the infrarenal aortotomy wire was pulled, creating a 4mm aortic tear for uncontrolled hemorrhage in the closed abdomen. When MAP reached 30 mmHg, the roller pump was switched to a pause/restart mode to keep the MAP at 25–30 mmHg for 15 minutes. The shed blood from the femoral artery was collected into a blood collection bag containing citrate anticoagulant for blood transfusion. This time period represents simulated injury prior to medic arrival. This method of inducing uncontrolled hemorrhage has been utilized in several previous studies
Resuscitation
Following the 15 minute shock period, animals were randomly assigned to one of three resuscitation strategies based on fluids infused: 1) LR control, 2) HBOC, and 3) HBOC + NTG 5 μg/kg/min. Resuscitation was divided into two phases: I) pre-hospital resuscitation (105 minutes) and II) in-hospital surgical repair of aortic tear at 105 minutes and further care up to 360 minutes. (Figure 1)
Figure 1.
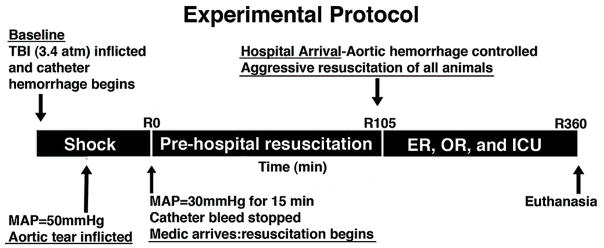
Experimental Protocol.
During the pre-hospital phase, the animals were resuscitated with either LR (20ml/kg), HBOC (10ml/kg), or HBOC+NTG (10ml/kg HBOC plus 5 μg/kg/min NTG) given at seven time points: resuscitation time zero, 15, 30, 45, 60, 75, and 90 minutes as needed if MAP was below 60 mmHg. Each of these fluids was infused over 10 minutes. The NTG (American Regent Inc. Shirley, NY) was infused concomitantly with HBOC for an equal period of time. At simulated hospital arrival (105 minutes), the abdomen was opened and intraperitoneal hemorrhage was measured by soaking pre-weighed gauze. A hemostatic primary repair of the aortic injury was performed using 6-0 prolene suture. The peritoneum and midline fascia was then closed with 0 silk and the skin closed with surgical staples. The animals were then resuscitated to restore normal physiological parameters using a goal-directed protocol using normal saline to achieve MAP ≥ 70 mmHg and shed blood to achieve Hb ≥ 7g/dl. If both ICP > 20mmHg and MAP > 40 mmHg, one bolus of Mannitol (Hospira Inc. Lake Forest, IL) was given, 1g/kg IV, up to a maximum of 3g/kg. Mechanical ventilation was titrated to achieve arterial blood oxygen saturation >92% and end tidal CO2 between 35 to 40 torr. Resuscitation was continued in this manner up to 6 hours after the initial injury or until death. Surviving animals were euthanized humanely under anesthesia using intravenous pentobarbital (Euthanasia III solution, Med-Pharmex, Inc. Pomona, CA) overdose. Animals in the HS only group were instrumented and treated similarly, except that no fluid percussion TBI was inflicted.
Measurements
Hemodynamic parameters, heart rate (HR), blood pressure (BP), mean arterial pressure (MAP), pulmonary arterial pressure (PAP), left ventricle pressure (LVP), central venous pressure (CVP), intracranial pressure (ICP), cerebral perfusion pressure (MAP- ICP= CPP) and EKG were continuously monitored and recorded with a Biopac® multichannel digital data acquisition system (Biopac Systems, Inc. Goleta, CA) throughout the experiment. ETCO2, FiO2, isoflurane concentration, respiration rate (RR) and tidal volume (TV) were monitored continuously with Datex Ultima Capnometer (Datex, Madison, Wis). Cardiac output (CO), core body blood temperature (via Swan-Ganz catheter), brain tissue oxygen (brpO2), transcutaneous oxygen (tcpO2) were recorded every 5 minutes to R180 and every 15 minutes thereafter to R360. Arterial, mixed venous and sagittal sinus blood samples were collected at baseline, time T= 0 and every 15 minutes until 2 hours, then at 30-minute intervals until 4 hours and then every one hour thereafter. Standard metabolic markers including lactate, base excess, blood gas, and glucose were analyzed with Radiometer blood gas analyzer ABL 805 (Radiometer, Copenhagen, Denmark).
Statistical analysis
Continuous data was normally distributed and expressed as mean with 95% confidence intervals. The primary outcome of interest was survival. Cox proportional hazard regression was used as time-to-event-based analyses to compare survival rates for each covariate (type of resuscitation fluid and presence of TBI) thus allowing for adjustment for one another. A Cox proportional hazard was chosen for this analysis because it allows survival time to be adjusted for multiple covariates and gives a conditional risk ratio (or hazard ratio) for each covariate rather than making assumptions based on an overall percentage of survival which can be problematic given that there were only N=7–8 animals in each fluid resuscitation group for each condition. This statistical approach rather allows for scaling of a baseline hazard function by the model’s covariates to give a general hazard function and specific hazard function for each covariate.
Secondary normally distributed continuous outcome variables that were measured at a single point in time (e.g. blood loss, fluid requirement) were compared using two-way analysis of variance (ANOVA) with the type of fluid resuscitation and presence of TBI included as the primary effects. A mixed model was used to compare the effects of resuscitation fluid type and TBI on continuous data measured longitudinally over time (e.g. MAP, HR). In these instances, mixed model repeated measures two-way analysis of variance (rmANOVA) was used to identify parameter estimates for time, treatment groups, and their interaction. For all analyses two-sided P <= 0.05 was considered statistically significant for each overall effect. Individual comparisons were made after adjusting for multiple comparisons by the method of Tukey Kramer.
Based on a previously demonstrated absolute survival benefit of 60% with HBOC-201 versus LR in a similar model, 7–8 animals per group will provide at least 80% power to detect a survival benefit of greater than 20%. This would also allow us to detect a change in lactate concentration of at least 4mmol/l and a change in intraperitoneal blood loss of at least 20ml/kg between groups with 80% power to detect differences. All analyses were performed using JMP-9 statistical package (SAS Inc., Cary, NC).
Results
Survival
Using a Cox proportional hazards model with the covariates of resuscitation fluid type and presence of TBI, we found no independent effect of TBI on survival time after adjusting for resuscitation fluid (TBI effect likelihood ratio, ChiSqr=1.45, DF=1, p=0.22). The calculated relative risk ratio was elevated above the baseline hazard ratio for TBI but not significantly (Relative Risk=1.5, [0.8, 2.8]). However, when examining the effect of resuscitation fluid on survival time in the same model, we found a significant independent effect of resuscitation fluid on survival time (resuscitation fluid effect likelihood ratio, ChiSqr=6.7, DF=1, p=0.03). This difference was attributed only to a significantly increased risk ratio for death for HBOC+NTG versus LR (Relative Risk=2.8, 95%CI=1.3, 6.1). (Table 3.)
Table 3.
Survival time in minutes.
| TBI + Hemorrhage
|
Hemorrhage Only
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resuscitation Fluid | N | Mean | Std Dev | 95% CI | N | Mean | Std Dev | 95% CI | ||
|
|
|
|||||||||
| LR | 7 | 294.1 | 117.1 | 185.8 | 402.4 | 8 | 279.8 | 148.6 | 155.5 | 404.0 |
| HBOC | 7 | 217.1 | 122.7 | 103.7 | 330.6 | 8 | 172.4 | 156.9 | 41.2 | 303.5 |
| HBOC+NTG | 7 | 56.0 | 59.2 | 1.3 | 110.7 | 8 | 179.1 | 154.5 | 50.0 | 308.3 |
Descriptive statistics comparing survival time according to injury pattern and resuscitation fluid. TBI=traumatic brain injury by fluid percussion, LR= lactated ringers solution, HBOC= hemoglobin-based oxygen carrier, HBOC+NTG= hemoglobin-based oxygen carrier with nitroglycerine co-infusion.
Impact of TBI
The TBI group (N=21, 7 per resuscitation fluid) weighed (mean, [95%CI] 27.5 kg [26.3, 28.5] which was statistically greater than the HS only group (N=24, 8 per resuscitation fluid), 25.9 kg [24.9, 27.0], but more likely clinically irrelevant. TBI-specific outcomes are summarized in Table 1. Mean TBI pressure applied to the dura in the TBI group averaged 3.4 atm [3.2, 3.5]. Total catheter hemorrhage volume required to reach goal hypotensive MAP and maintain it for 15 minutes was less with TBI compared to those with HS alone, though a similar degree of shock was attained as demonstrated by mean lactate concentrations at the end of the standard hemorrhage period that were similar between groups. There was no significant independent effect of TBI on volume of fluid resuscitation given, intraperitoneal blood loss, or total hemorrhage volume. Of the TBI animals, two (2) animals in the LR, two (2) in the HBOC, and one (1) in the HBOC/NTG group required mannitol for an elevated ICP. Each of the animals in the LR and HBOC groups received a total of 3 infusions of mannitol (3 g/kg or 15 mL/kg), while the animal in the HBOC/NTG group received only 1 infusion (1 g/kg or 5 mL/kg).
Table 1.
Effect of TBI on outcomes.
| TBI + Hemorrhage
|
Hemorrhage Only
|
|||||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||
|
|
|
|||||
| Total Catheter Hemorrhage Vol (ml/kg) at 30 mmHg for 15 minutes | 14.0 | 12.4 | 15.6 | 21.0* | 19.5 | 22.5 |
| End of Hemorrhage Lactate (mmol/L) | 4.7 | 4.1 | 5.4 | 4.7 | 4.1 | 5.3 |
| No. Pre-hosp boluses | 3.6 | 2.8 | 4.3 | 3.1 | 2.4 | 3.9 |
| Total Prehospital Fluid given (ml/kg) | 48.9 | 38.3 | 59.4 | 42.5 | 32.6 | 52.3 |
| Total NS Infused (ml/kg) | 37.3 | 18.1 | 56.5 | 73.8# | 50.3 | 97.3 |
| Shed Blood Infused (ml/kg) | 3.9 | 0.6 | 7.3 | 8.8 | 4.1 | 13.5 |
| Intraperitoneal bleeding (ml/kg) | 23.7 | 14.6 | 32.8 | 21.1 | 12.6 | 29.6 |
| Total Hemorrhage volume (catheter+intraperitoneal, ml/kg) | 37.7 | 28.7 | 46.7 | 38.9 | 30.4 | 47.3 |
| Survival Time in minutes (%survival to 6 hr) | 189.1(37%) | 130.5 | 247.7 | 210.4(63%) | 155.6 | 265.2 |
Least square mean outcome measurements for hemorrhage and prehospital phase variables after adjusting for fluid resuscitation type.
significantly different than TBI p<0.0001,
p=0.02,
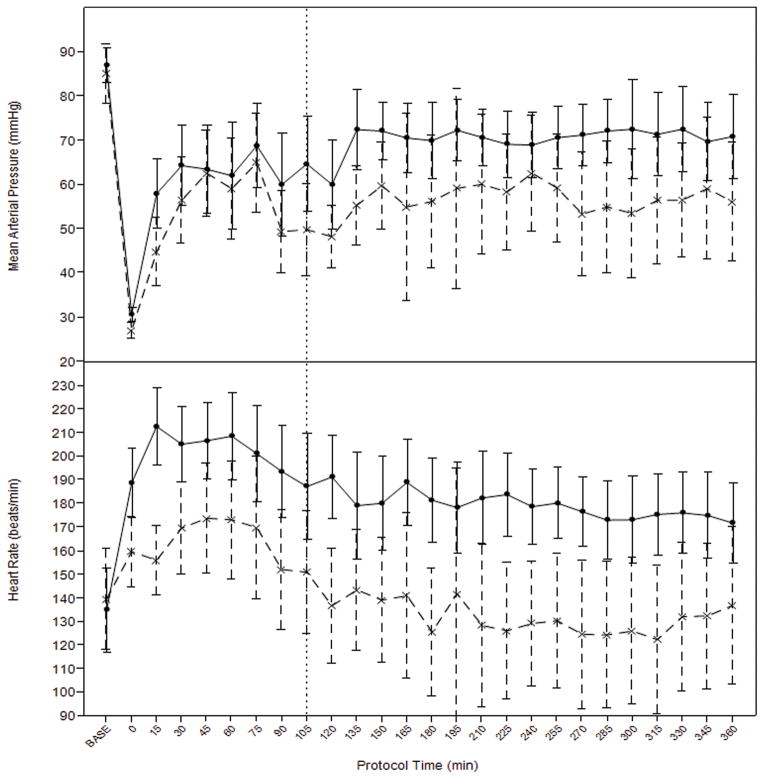
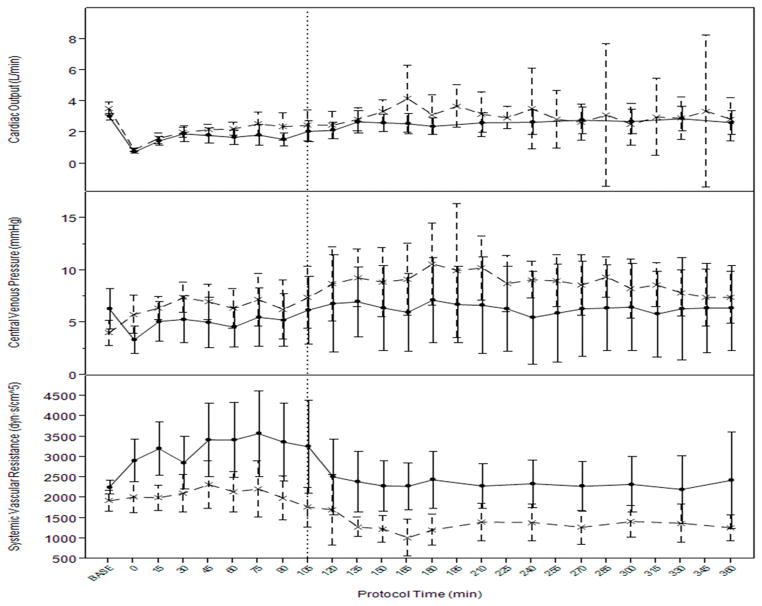
There was a significant independent effect of TBI on MAP (p=0.01), heart rate (p<0.0001), and systemic vascular resistance (p=0.0002), after adjusting for resuscitation fluid and phase of resuscitation (prehospital vs. hospital). (Figure 2.) Overall, MAP, heart rate, and SVR were higher in the HS-only group. Central venous pressure tended to be higher in the TBI+HS group (p=0.068) and cardiac output was not different but tended to be higher in the TBI+HS group. Pulmonary artery pressure was also not different (p=0.11). (Figure 3.)
Figure 2.
Average blood pressure and heart rates over time in TBI (interrupted line) and hemorrhage only (solid line) groups during fluid resuscitation. Vertical interrupted line at 105 denotes time of aortic repair and transition from prehospital to in-hospital resuscitation protocol. Error bars= 95%CI.
Figure 3.
Mean cardiac output by thermodilution, central venous pressure, and systemic vascular resistance during hemorrhage and resuscitation in TBI (interrupted line) and hemorrhage only (solid line). Vertical interrupted line at 105 denotes time of aortic repair and transition from prehospital to in-hospital resuscitation protocol. Error bars= 95%CI.
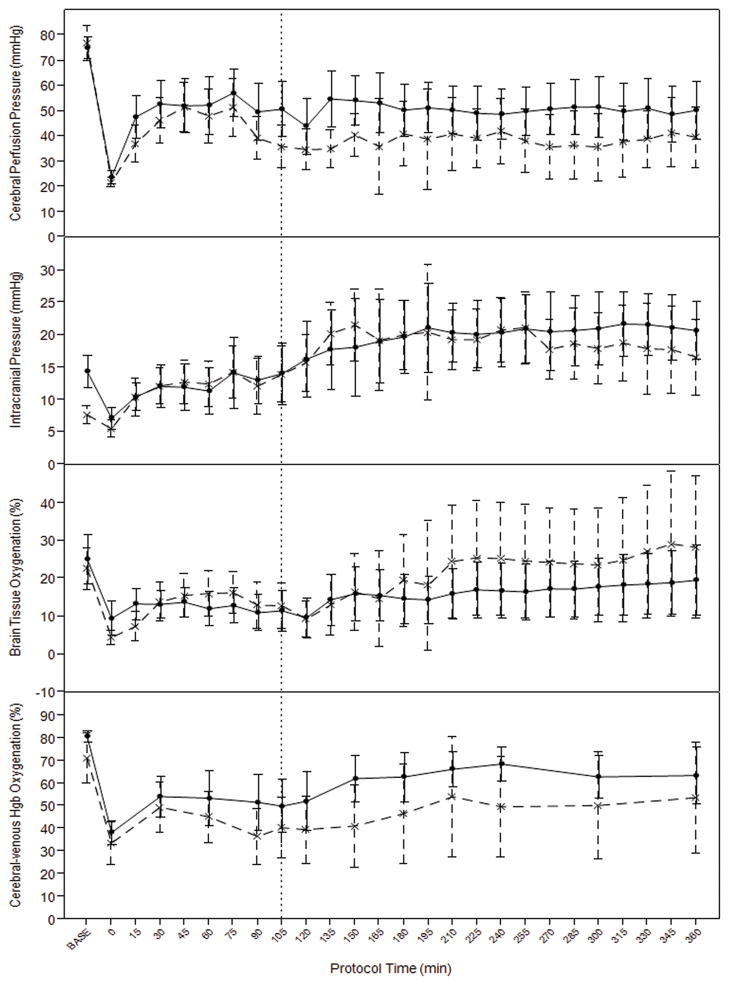
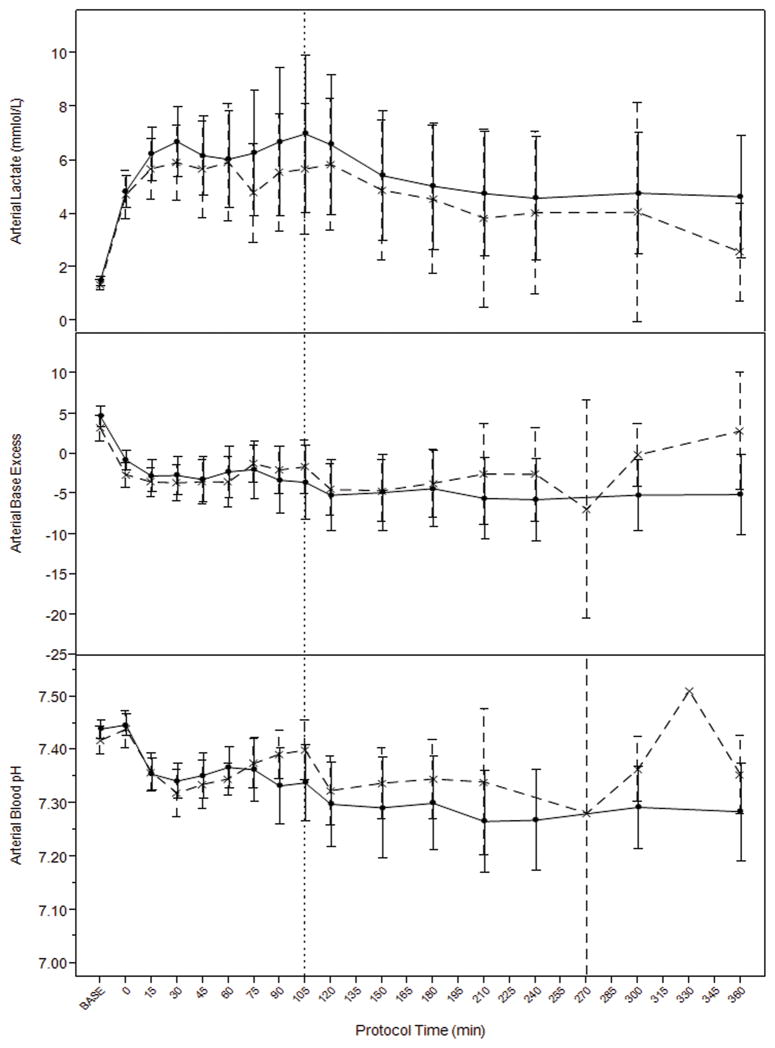
There was no significant independent effect of TBI on ICP (p=0.16), a trend towards overall higher cerebral perfusion pressure in the HS only group (p=0.09), and no difference in brain tissue oxygenation by Licox probe (p=0.7). There was a significant independent effect of TBI on cerebrovascular venous oxygen saturation which was higher in the HS-only group (p=0.0027). (Figure 4.) There were no differences in metabolic markers of resuscitation including overall levels of arterial lactate (p=0.4), arterial pH (p=0.21), or arterial base excess (p=0.49) comparing TBI and HS-only groups during hemorrhage and resuscitation. (Figure 5.)
Figure 4.
Cerebral hemodynamics and contralateral brain tissue oxygenation, and cerebral sagittal venous sinus hemoglobin saturation during hemorrhage and resuscitation in TBI (interrupted line) and hemorrhage only (solid line) models. Vertical interrupted line at 105 denotes time of aortic repair and transition from prehospital to in-hospital resuscitation protocol. Error bars= 95%CI.
Figure 5.
There were no significant differences in systemic metabolic markers of resuscitation including lactate, base excess, and blood pH during hemorrhage and resuscitation between TBI (interrupted line) and hemorrhage only (solid line) models. Vertical interrupted line at 105 denotes time of aortic repair and transition from prehospital to in-hospital resuscitation protocol. Error bars= 95%CI.
Impact of Resuscitation Fluid
Outcomes stratified by resuscitation fluid are summarized in Table 2. End of hemorrhage lactate was higher in the HBOC+NTG group compared to the HBOC group. As expected, the LR group received significantly more resuscitation fluid volume (20ml/kg per bolus) compared to the HBOC-containing fluids (10ml/kg per bolus) during prehospital resuscitation, but the number of boluses required to maintain MAP > 60mmHG was not different. Total NS required during hospital treatment was less in the HBOC+NTG group compared to HBOC.
Table 2.
Effect of resuscitation fluid on outcomes.
| Lactated Ringers
|
HBOC
|
HBOC+NTG
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||
|
|
|
|
|||||||
| Total Cath Hem Vol (ml/kg) at 30 mmHg for 15 minutes | 17.4 | 15.5 | 19.3 | 16.2 | 14.3 | 18.0 | 19.0 | 17.1 | 20.9 |
| End of Hemorrhage Lactate (mmol/L) | 5.1 | 4.3 | 5.9 | 3.8 | 3.1 | 4.6 | 5.3* | 4.5 | 6.0 |
| No. Pre-hosp boluses | 3.8 | 2.9 | 4.7 | 3.7 | 2.8 | 4.7 | 2.5 | 1.6 | 3.5 |
| Total Prehospital Fluid given (ml/kg) | 74.4*C | 61.9 | 86.9 | 37.3 | 24.8 | 49.8 | 25.4 | 12.9 | 37.9 |
| Total NS Infused (ml/kg) | 62.6 | 39.1 | 86.1 | 76.0 | 45.7 | 106.4 | 28.1* | 3.7 | 52.6 |
| Shed Blood Infused (ml/kg) | 8.8 | 4.7 | 12.9 | 7.0 | 0.8 | 13.1 | 3.3 | 0.0 | 7.8 |
| Intraperitoneal bleeding (ml/kg) | 12.1 | 1.3 | 22.9 | 30.4 | 19.6 | 41.2 | 24.8 | 14.0 | 35.6 |
| Total Hemorrhage volume (catheter+intraperitoneal, ml/kg) | 29.4 | 18.7 | 40.1 | 46.5 | 35.8 | 57.2 | 38.9 | 28.2 | 49.6 |
| Survival Time (min) (%survived to 6 hr) | 286.9 (58%) | 217.4 | 356.4 | 194.8 (26%) | 125.3 | 264.3 | 117.6† (16%) | 48.1 | 187.1 |
Outcome measurements grouped according to type of resuscitation fluid administered. LR= Lactated Ringers, HBOC= hemoglobin-based oxygen carrier, HBOC+NTG= HBOC with nitroglycerin co-infusion,
Sig Different than HBOC, p<0.05,
Sig Different than LR, p<0.05,
Sig Different than HBOC+NTG, p<0.05. Two-way ANOVA with Tukey HSD.
There was a significant independent effect of resuscitation fluid type after adjusting for presence of TBI on multiple hemodynamic and metabolic variables measured during the experimental protocol as outlined in Table 4. In general, MAP was highest in the HBOC+NTG group and HR was lowest in the HBOC group. ICP was highest with LR with a correspondingly lower CPP compared to the HBOC groups. However, brain tissue oxygenation was lowest with HBOC and cerebral lactate was elevated with HBOC-containing fluids compared to LR suggesting a detrimental effect of HBOC on cerebral oxygen delivery and metabolism even though CPP was greater. The same was true with central mixed venous oxygen saturation which was lower with HBOC-containing fluids relative to LR. PAP was highest in the HBOC group and was significantly greater than HBOC+NTG and LR suggesting an attenuation of pulmonary vasoactivity with the addition of NTG to HBOC, however SVR was actually highest in the HBOC+NTG relative to HBOC and LR. In summary, these data demonstrate an overall better hemodynamic and metabolic resuscitation with LR compared to HBOC-containing fluids. The addition of NTG may be associated with locally reduced pulmonary vasoreactivity by the noted decrease in PAP, but did not significantly reduce SVR in this model.
Table 4.
Continuous hemodynamic and metabolic variables modified by resuscitation fluid type after adjustment for presence of TBI.
| HBOC
|
HBOC+NTG
|
LR
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
|
|
|
|
|||||||
| Mean Arterial pressure, mmHg | 60.2# | 57.8 | 62.5 | 66.3 | 63.1 | 69.4 | 61.9 | 60.1 | 63.7 |
| Heart Rate, bpm | 150.4 | 145.1 | 156 | 168.2† | 161 | 175.3 | 169.9† | 165.8 | 174 |
| Intracranial Pressure, mmHg | 13.0* | 12.0 | 14.0 | 14.8* | 13.5 | 16.2 | 19.6 | 18.9 | 20.4 |
| Cerebral Perfusion Pressure, mmHg | 47.8* | 45.3 | 50.2 | 51.4† | 48.2 | 54.7 | 43.1# | 41.2 | 45 |
| Pulmonary Artery Pressure, mmHg | 42.0* | 40.7 | 43.2 | 34.1† | 32.4 | 35.8 | 27.7#* | 26.7 | 28.7 |
| Central Venous Pressure, mmHg | 7.4# | 6.7 | 8.2 | 4.8 | 3.8 | 5.8 | 7.5# | 6.9 | 8.1 |
| Systemic Vascular Resistance | 2508.2*# | 2349.3 | 2667 | 2893.6†* | 2673.5 | 3113.8 | 1609.5 | 1479.5 | 1739.6 |
| Brain Tissue O2Sat % (Licox) | 14.5* | 12.7 | 16.2 | 16.4 | 14 | 18.8 | 18.5 | 17.1 | 19.9 |
| Arterial Lactate, mmol/l | 6.0* | 5.5 | 6.5 | 6.0* | 5.3 | 6.7 | 4.1 | 3.7 | 4.6 |
| Cerebral Venous Lactate mmol/l | 6.2* | 5.6 | 6.8 | 5.8* | 5 | 6.6 | 4.1 | 3.6 | 4.6 |
| Mixed Central Venous Hgb O2Sat% | 38.9* | 36.3 | 41.5 | 36.4* | 32.9 | 39.9 | 51.7 | 49.6 | 53.8 |
Two Way ANOVA with Tukey Kramer adjustment for multiple comparisons.
different than HBOC+NTG p<0.05,
different than Lactated Ringers p<0.05,
different than HBOC p<0.05.
Discussion
There are two major important observations from these data. First, in this multiphased treatment model, there was no significant impact of TBI on survival during low-volume resuscitation of uncontrolled hemorrhagic shock, though presence of TBI was associated with a decreased compensatory response to acute hemorrhage. Second, in this high pressure aortic bleeding model limited resuscitation with HBOC did not improve survival which is in contrast to previous studies using solid organ injury as the source of free hemorrhage.
Impact of TBI
The lack of effect of TBI on survival time may be partially explained by inequalities in the volume of blood withdrawn during hemorrhage. In this pressure-guided model, HS-only animals required on average 150% more blood to be withdrawn to meet the pre-specified MAP = 30mmHg. Yet, lactate levels were similar at the end of the shock period, indicating a similar degree of shock and suggesting an exaggerated hypotensive response to blood removal in the TBI+HS group. Therefore, TBI+HS animals likely maintained significantly more blood volume during resuscitation, which is supported by the strong trend towards higher CVP with TBI. However, this logically advantageous retained blood volume did not translate to improved MAP, CO, perfusion, or survival time during low volume resuscitation due to a significantly lower SVR that also accompanied TBI. Taken together, these data suggest that there was TBI-specific impairment of global vascular responsiveness during hemorrhage and resuscitation, thus negating the advantage of increased circulating blood volume. This may also account for the worse outcome in the TBI+HS animals treated with HBOC+NTG. One might hypothesize that in animals with an already compromised vascular response, the administration of an agent known to cause vasodilatation, such as nitroglycerine, might easily further impair the animal’s already compromised physiological response to HS.
Historical study of the cardiovascular responses to progressive isolated hemorrhage report the immediate response as an increase of heart rate, maintaining blood pressure for a period of time as hemorrhage progresses, until a vagally-mediated reduction of sympathetic tone and bradycardia predominate resulting in fainting and hypotension.25 Tissue damage can modify this response by prolonging the initial period of tachycardia and maintaining blood pressure for longer periods, which has been associated with reduced survival experimentally.26,27 McMahon et al, also found a similar association between brain-specific injury and cardiovascular response to hemorrhage in rats where heart rate remained elevated longer and there was a delayed hypotensive response to graded fixed-volume hemorrhage with TBI.28 However, other investigators have found impaired vascular responses similar to our witnessed response during hemorrhage and resuscitation in a rat model where vascular compensation was blunted in the setting of TBI.29 Our data agrees with an immediate attenuation of initial tachycardia and impaired peripheral vasoactivity during hemorrhage and fluid resuscitation in the setting of brain injury. The differences noted between ours and previous models may also be due to uncontrolled hemorrhage, or tissue injury which can also contribute to altered cardiovascular responses during hemorrhage.26
Even brief episodes of hypotension are known to contribute to secondary brain injury during resuscitation of hemorrhagic shock. So the exaggerated hypotensive response to hemorrhage seen with TBI is likely a clinically-relevant phenomenon that deserves further study. Our data also suggest that estimating blood loss according to vital signs may be disadvantageous in the presence of concomitant TBI. Estimating blood loss and degree of shock by vital signs, including heart rate and blood pressure are currently accepted standard methods of classifying shock severity in trauma patients and drive many resuscitative algorithms.30 However, tools such as shock index perform poorly in the setting of combined TBI and hemorrhage.31 Given the data from this study suggesting a TBI-specific exaggerated bradycardic and hypotensive response to hemorrhage, estimation of blood loss and resuscitation based solely on these parameters may lead to less-effective fluid resuscitation.
CPP tended to be higher and cerebral venous O2 saturation (ScvO2) was significantly greater in the HS-only group suggesting a detrimental effect of TBI on cerebral physiology. ScvO2 remained depressed from baseline levels (<60 mmHg) in both groups until late in the hospital phase, when the HS-only group recovered to near baseline levels. This is likely a reflection of both the low-volume resuscitation strategy and increased MAP in the HS-only group because ICP did not differ significantly. Local changes in cerebrovascular resistance and autoregulation with TBI, known to shift the cerebral autoregulatory curve rightward, have been used to explain increased cerebral injury with TBI during resuscitation.32 Our results support an important role for global cardiovascular dysfunction in addition to cerebral deregulation as drivers of cerebral injury during TBI with HS. These findings argue for proper systemic resuscitation, with an emphasis on restoring vascular tone, as a priority for the initial resuscitation of the polytrauma casualty with TBI and HS. Other polytrauma models of combined TBI and HS support the importance of systemic vascular tone during fluid resuscitation by demonstrating that goal CPP could only be achieved with recovery of systemic vascular resistance.33 Of course, restoration of vascular resistance and blood pressure must be balanced with risk for rebleeding from wounds. As is the current standard of care in the Intensive Care Unit, we suggest that there may be a role for early invasive hemodynamic and metabolic monitoring in the Emergency Department to better guide the initial fluid resuscitation of polytrauma patients with hemorrhagic shock and TBI.
Impact of Resuscitation Fluid
A second important observation of these data is the significant impact that type of resuscitation fluid had on outcomes. Limited resuscitation with lactated Ringers was associated with significantly better survival compared to HBOC+NTG treated animals and a trend toward better survival compared to the HBOC group. These differences were most pronounced in the TBI group. Given the evidence for impaired vasomotor response to hemorrhage in the TBI group, we suspect that perhaps the additional vasorelaxation afforded by the addition of NTG led to a further impairment in physiological response to hemorrhage. While the differences did not reach statistical significance, there was a clear trend toward greater intraperitoneal hemorrhage in the HBOC treated animals, which may have also contributed to the shorter survival time.
Our results are contrary to previous studies using similar polytrauma models in which low dose HBOC-201 appeared to be advantageous by improving global hemodynamics, cerebral oxygenation and perfusion, and reducing contralateral neuronal injury compared to LR.16,33 However, much of the benefit of HBOC-201 on cerebral resuscitation in these previous studies appears to be due to its vasopressor effect, increasing central blood pressure and thus improving cerebral perfusion pressure, and much of this previous work was performed without free bleeding. A study by Stern et al using a model of combined TBI and uncontrolled hemorrhage inflicted via liver laceration and a simulated pre-hospital resuscitation time of 75 minutes demonstrated no difference in hemorrhage volume but improved outcome with limited resuscitation with HBOC as compared to LR.34 In that study, cardiac indices, cerebral perfusion pressures and brain tissue oxygen tension were significantly better and lactate and base deficit lower in the HBOC treated animals as compared to the animals treated with LR.
In the current study, animals subjected to combined TBI + HS with aortic tear and provided initially limited resuscitated with HBOC experienced a trend toward greater free bleeding as compared to those resuscitated with LR; total hemorrhage volumes in the HBOC and LR resuscitated animals were 46.5ml/kg [35.8, 57.2] and 29.4ml/kg [18.7, 40.1] respectively. In contrast, in the previous study by Stern et al that utilized a TBI/liver injury model, there was no difference in free bleeding between animals resuscitated with HBOC versus LR; total hemorrhage volumes in the HBOC and LR resuscitated animals were 72.4±14.2 and 74.4±15.1 mL/kg respectively.34 Yet in both the current and the previous study, HBOC infusion resulted in a similar initial hemodynamic response, that is an increase in systemic vascular resistance. It is noteworthy that the current and the previous study by Stern et al were similar in all regards with the exception of injury pattern (aortic tear versus liver laceration) and a slightly longer simulated pre-hospital period in the current model. Therefore, it is reasonable to hypothesize that the cause of the disparate findings in these two studies may be related to the differing injury patterns. Specifically, the data suggest that in the setting of a localized high pressure (large artery) vascular injury, the pressor effect of HBOC may cause an increase in hemorrhage volume as compared to limited resuscitation with an agent that has no vasoactive properties (i.e LR), whereas this response is not observed in a low pressure injury such as a liver laceration. An additional cause may be related to the severity of shock at the start of resuscitation. Mean arterial lactate levels in the current study were 4.7 mmol/L compared to 2.0 mmol/L in the previous study by Stern et al, suggesting greater oxygen debt at onset of resuscitation. It is conceivable that the infusion of a vasoactive agent under these circumstances without additional volume resuscitation may further compromise perfusion to vital organs, including myocardium. That survival was poorest in the TBI+HS group that received NTG infusion in addition to HBOC may also suggest a role for NO production in TBI-induced vascular disturbances.
Limitations
This study has several important limitations. First, the use of the swine model limits extrapolation to the clinical setting. To address this, we have developed a relevant model that closely mimics the time course of events that occur in the pre-hospital period following injury as well as in a simulated in-hospital phase, including an operative intervention. Second, to test both of our hypotheses required that we utilize two differing injury profiles, a model of TBI with HS and a model of HS alone, and that we test our resuscitation strategies in each model. While such a complex model better ensures clinical relevance, it may limit hypothesis testing by the introduction of many uncontrolled variables. We attempted to isolate the specific effects of TBI on physiology and outcomes by adjusting for treatment phase and resuscitation fluid during data analysis. Nevertheless this study reports important effects of TBI on hemorrhage and low volume resuscitation of hemorrhagic shock with standard LR and HBOC that deserve further focused attention. Further study of cardiovascular responses would benefit from volume-controlled hemorrhage models.
A third limitation, also related to the use of two injury models, one with and one without TBI, is that animals in the TBI arm of the study received mannitol infusions as needed to treat elevations in ICP, as would be provided in an ED, ICU or operative setting. Therefore, this represented an asymmetry between animals that sustained HS and TBI and those that sustained HS alone. Given that these infusions were provided only after control of hemorrhage from, and operative repair of, the aortic injury had been accomplished and at a time when aggressive fluid resuscitation was being provided to normalize systemic hemodynamic and metabolic parameters, administration of mannitol in the volumes provided are unlikely to have affected hemodynamic response. Additionally, as noted in the results section, no more than 2 animals from any group received mannitol. And perhaps most importantly, clinical data demonstrate that in the setting of polytrauma, administration of mannitol has no effect on hemodynamic parameters.35
A final limitation is the use of isoflurane anesthesia. While an unanesthetized model may be more clinically relevant, this would not be humane or ethically responsible. In order to reduce the concentration of isoflurane required to achieve and maintain an adequate level of anesthesia, we provided a single dose of buprenorphine. This is consistent with care provided in the clinical setting, where trauma victims often receive opiates in the form of morphine sulfate to control pain.
Conclusion
Survival was not different with combined TBI and HS compared to HS alone during limited resuscitation in this uncontrolled hemorrhage model. However, less hemorrhage was required to produce an equivalent degree of shock in the presence of TBI, and cardiovascular response to fluid resuscitation was blunted during fluid resuscitation when TBI was present. These results suggest a distinct systemic physiology associated with combined TBI and HS that may require targeted resuscitation strategies to improve outcomes. Additionally, data from the current study, which uses a high pressure vascular injury model of uncontrolled hemorrhage, demonstrate decreased survival for animals provided limited resuscitation with HBOC as compared to LR. These findings are different than previously published data from models of uncontrolled hemorrhage inflicted via liver injury that demonstrate improved survival with limited resuscitation with HBOC. These data demonstrate the need to consider wound geometry and type, specifically high pressure vascular versus low pressure solid organ injuries, as well as shock severity when considering resuscitation strategies.
Acknowledgments
This study was supported by the Naval Medical Research Center (NMRC) through CDMRP grant #W81XWH-08-2-0166 and, in part, by Grant Number KL2 TR000421 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). NMRC also provided the hemoglobin-based oxygen carrier, HBOC-201 (Biopure, OPK Biotech).
Footnotes
The authors report no relevant financial disclosures or conflicts of interest associated with this study.
Disclaimers
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Navy, Army, Department of Defense, nor the U.S. Government and do not necessarily represent the official view of NCATS or NIH. Author NJW Funded under NCRR Grant KL2 RR025015.
References
- 1.Trunkey D. Trauma: Accidental and intentional injuries account for more years of life lost in the US than cancer and heart disease-among the prescribed remedies are improved resuscitative efforts, speedier surgery and further research. Scientific Amer. 1983;249:28–35. [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: A reassessment. J Trauma. 1995;38:180–185. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira PG, Inaba K, Hadjizacharia P, Brown C, Salim A, Rhee P, Browder T, Noguchi TT, Demetriades D. Preventable or potentially preventable mortality at a mature trauma center. J Trauma. 2007;63:1338–1347. doi: 10.1097/TA.0b013e31815078ae. [DOI] [PubMed] [Google Scholar]
- 4.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe brain injury. J Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in traumatic coma data bank. Acta Neurochir Suppl (Wein) 1993;59:121–125. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- 6.McMahon CG, Yates DW, Campbell FM, Hollis S, Woodford M. Unexpected contribution of moderate traumatic brain injury to death after major trauma. J Trauma. 1999;47:891–895. doi: 10.1097/00005373-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 7.DeWitt DS, Prough DS, Taylor CL, Whitley JM, Deal DD, Vines SM. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am J Physiol. 1992;263 (4 Pt 2):H1276–H1284. doi: 10.1152/ajpheart.1992.263.4.H1276. [DOI] [PubMed] [Google Scholar]
- 8.Yuan XQ, Wade CE. Influences of traumatic brain injury on the outcomes of delayed and repeated hemorrhages. Circ Shock. 1991;35:23–236. [PubMed] [Google Scholar]
- 9.Yuan XQ, Wade CE, Clifford CB. Suppression by traumatic brain injury of spontaneous hemodynamic recovery from hemorrhagic shock in rats. J Neurosurg. 1991;75:408–414. doi: 10.3171/jns.1991.75.3.0408. [DOI] [PubMed] [Google Scholar]
- 10.Fulton RL, Flynn WJ, Mancino M, Bowles D, Cryer HM. Brain injury causes loss of cardiovascular response to hemorrhagic shock. J Invest Surg. 1993;6:117–131. doi: 10.3109/08941939309141603. [DOI] [PubMed] [Google Scholar]
- 11.Brain Trauma Foundation. Update to guidelines for the management and prognosis of severe traumatic brain injury. Brain Trauma Foundation; 708 3rd Avenue, New York, NY 10017: 2003. http://www2.braintrauma.org/guidelines/downloads/btf_guidelines_cpp_u1.pdf. [Google Scholar]
- 12.Bruttig SP, O’Benar JD, Wade CE, Dubick MA. Benefit of slow infusion of hypertonic saline/dextran in swine with uncontrolled aortotomy hemorrhage. Shock. 2005;24(1):92–6. doi: 10.1097/01.shk.0000168872.37660.d2. [DOI] [PubMed] [Google Scholar]
- 13.Stern SA, Wang X, Mertz M, Chowanski ZP, Remick DG, Kim HM, Dronen SC. Under- resuscitation of near-lethal uncontrolled hemorrhage: Effects on mortality and end-organ failure at 72 hours. Shock. 2001;15:16–23. doi: 10.1097/00024382-200115010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Stern SA, Zink BJ, Mertz M, Wang X, Dronen SC. Effect of initially limited resuscitation in a combined model of fluid-percussion brain injury and severe uncontrolled hemorrhagic shock. J Neurosurg. 2000;93(2):305–14. doi: 10.3171/jns.2000.93.2.0305. [DOI] [PubMed] [Google Scholar]
- 15.Jin G, DeMoya MA, Duggan M, Knightly T, Mejaddam AY, Hwabejire J, Lu J, Smith WM, Kasotakis G, Velmahos GC, Socrate S, Alam HB. Traumatic brain injury and hemorrhagic shock: evaluation of different resuscitation strategies in a large animal model of combined insults. Shock. 2012;8(1):49–56f. doi: 10.1097/SHK.0b013e3182574778. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal G, Morabito D, Cohen M, Roeytenberg A, Derugin N, Panter SS, Knudson MM, Manley G. Use of hemoglobin-based oxygen-carrying solution-201 to improve resuscitation parameters and prevent secondary brain injury in a swine model of traumatic brain injury and hemorrhage: laboratory investigation. J Neurosurg. 2008;108(3):575–87. doi: 10.3171/JNS/2008/108/3/0575. [DOI] [PubMed] [Google Scholar]
- 17.Teranishi K, Scultetus A, Haque A, Stern S, Philbin N, Rice J, Johnson T, Auker C, McCarron R, Freilich D, Arnaud F. Traumatic brain injury and severe uncontrolled haemorrhage with short delay pre-hospital resuscitation in a swine model. Injury. 2010 doi: 10.1016/j.injury.2010.09.042. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Manning JE, Katz LM, Brownstein MR, Pearce LB, Gawryl MS, Baker CC. Bovine hemoglobin-based oxygen carrier (HBOC-201) for resuscitation of uncontrolled, exsanguinating liver injury in swine: Carolina Resuscitation Research Group. Shock. 2000;13:152–159. doi: 10.1097/00024382-200013020-00010. [DOI] [PubMed] [Google Scholar]
- 19.York GB, Eggers JS, Smith DL, Jenkins DH, McNeil JD, Mueller D, Josephs JD, Kerby JD. Low-volume resuscitation with a polymerized bovine hemoglobin-based oxygen-carrying solution (HBOC-201) provides adequate tissue oxygenation for survival in a porcine model of controlled hemorrhage. J Trauma. 2003;55:873–885. doi: 10.1097/01.TA.0000092681.17874.6F. [DOI] [PubMed] [Google Scholar]
- 20.Rice J, Philbin N, McGwin G, Arnaud F, Johnson T, Flournoy WS, Pearce LB, McCarron R, Kaplan L, Handrigan M, Freilich D. Bovine polymerized hemoglobin versus Hextend resuscitation in a swine model of severe controlled hemorrhagic shock with delay to definitive care. Shock. 2006;26:302–310. doi: 10.1097/01.shk.0000226338.48033.c2. [DOI] [PubMed] [Google Scholar]
- 21.Johnson T, Arnaud F, Dong F, Philbin N, Rice J, Asher L, Arrisueno M, Warndorf M, Gurney J, McGwin G, Kaplan L, Flournoy WS, Apple FS, Pearce LB, Ahlers S, McCarron R, Freilich D. Bovine polymerized hemoglobin (HBOC-201) resuscitation in three swine models of hemorrhagic shock with militarily relevant delayed evacuation-effects on histopathology and organ function. Crit Care Med. 2006;34:1464–1474. doi: 10.1097/01.CCM.0000215824.85190.89. [DOI] [PubMed] [Google Scholar]
- 22.Kerby JD, Sainz JG, Zhang F, Hutchings A, Sprague S, Farrokhi FR, Son M. Resuscitation from hemorrhagic shock with HBOC-201 in the setting of traumatic brain injury. Shock. 2007;27:652–656. doi: 10.1097/01.shk.0000248584.10400.dc. [DOI] [PubMed] [Google Scholar]
- 23.Rice J, Philbin N, Handrigan M, Hall C, McGwin G, Ahlers S, Pearce LB, Arnaud F, McCarron R, Freilich D. Vasoactivity of bovine polymerized hemoglobin (HBOC-201) in swine with traumatic hemorrhagic shock with and without brain injury. J Trauma. 2006 Nov;61(5):1085–99. doi: 10.1097/01.ta.0000236640.62893.fa. [DOI] [PubMed] [Google Scholar]
- 24.Sapsford W, Watts S, Cooper G, Kirkman E. Recombinant activated factor VII increases survival time in a model of incompressible arterial hemorrhage in the anesthetized pig. J Trauma. 2007;62(4):868–79. doi: 10.1097/ta.0b013e318034204b. [DOI] [PubMed] [Google Scholar]
- 25.Barcroft H, Edholm OG. On the vasodilatation in human skeletal muscle during post-haemorrhagic fainting. J Physiol. 1945;15;104(2):161–75. doi: 10.1113/jphysiol.1945.sp004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson ID, Little RA, Irving MH. An effect of trauma on human cardiovascular control: Baroreflex suppression. J Trauma. 1990;30:974–981. doi: 10.1097/00005373-199008000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Overman RR, Wang SC. The contributory role of the afferent nervous factor in experimental shock: Sublethal hemorrhage and sciatic nerve stimulation. Am J Physiol. 1947;148:289–295. doi: 10.1152/ajplegacy.1947.148.2.289. [DOI] [PubMed] [Google Scholar]
- 28.McMahon CG, Kenny R, Bennett K, Kirkman E. Modification of acute cardiovascular homeostatic responses to hemorrhage following mild to moderate traumatic brain injury. Crit Care Med. 2008;36(1):216–24. doi: 10.1097/01.CCM.0000295425.41831.85. [DOI] [PubMed] [Google Scholar]
- 29.Law MM, Hovda DA, Cryer HG. Fluid percussion brain injury adversely affects control of vascular tone during hemorrhagic shock. Shock. 1996;6:213–217. [PubMed] [Google Scholar]
- 30.American College of Surgeons Committee on Trauma. ATLS ® for Doctors Student Manual with DVD. 8. American College of Surgeons; Jan, 2008. [Google Scholar]
- 31.McMahon CG, Kenny R, Bennett K, Little R, Kirkman E. The effect of acute traumatic brain injury on the performance of shock index. J Trauma. 2010;69(5):1169–75. doi: 10.1097/TA.0b013e3181cc8889. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins LW, Moszynski K, Lyeth BG, Lewelt W, DeWitt DS, Allen A, Dixon CE, Povlishock JT, Majewski TJ, Clifton GL. Increased vulnerability of the mildly traumatized rat brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Res. 1989;477:211–224. doi: 10.1016/0006-8993(89)91409-1. [DOI] [PubMed] [Google Scholar]
- 33.Dudkiewicz M, Harpaul TA, Proctor KG. Hemoglobin-based oxygen carrying compound-201 as salvage therapy for severe neuro- and polytrauma (Injury Severity Score = 27–41) Crit Care Med. 2008;36(10):2838–48. doi: 10.1097/CCM.0b013e318186f6b3. [DOI] [PubMed] [Google Scholar]
- 34.Stern S, Rice J, Philbin N, McGwin G, Arnaud F, Johnson T, Flournoy WS, Ahlers S, Pearce LB, McCarron R, Freilich D. Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201, in a swine model of severe uncontrolled hemorrhage and traumatic brain injury. Shock. 2009;31(1):64–79. doi: 10.1097/SHK.0b013e3181778dc3. [DOI] [PubMed] [Google Scholar]
- 35.Sayre MR, Daily SW, Stern SA, Storer DL, van Loveren HR, Hurst JM. Out-of-hospital administration of mannitol to head-injured patients does not change systolic blood pressure. Acad Emerg Med. 1996;3(9):840–8. doi: 10.1111/j.1553-2712.1996.tb03528.x. [DOI] [PubMed] [Google Scholar]