Abstract
Studies of healthy adults show that engagement in physical, social, and mental activities is associated with better cognitive outcomes, suggesting these activities may increase cognitive reserve. Given the prevalence and real-world impact of HIV-associated neurocognitive disorders (HAND), the present study examined the association between neurocognitive outcomes and self-reported proxies for physical exercise, social activity, and mental activity (employment was used as a proxy for mental activity) among 139 HIV-infected adults (Mage = 48.7; 48% age 50+). Participants completed a neuromedical and neuropsychological battery and were classified based on the number of self-reported active lifestyle factors (ALFs; 0 to 3), including physical exercise, social activity, and current employment. The association between ALFs and both demographically-adjusted average neuropsychological T-scores and HAND diagnoses were examined. Results revealed that an increased number of ALFs was associated with better global neurocognitive performance as well as a lower prevalence of HAND. These cross-sectional findings suggest that an active engagement in life may bolster neurocognitive functioning, perhaps by enhancing cognitive and/or brain reserve. However, an alternative explanation might be that persons with better neurocognitive functioning are more inclined and able to engage in these life activities. Future studies should utilize neuroimaging methodology, longitudinal data, and interventional approaches to establish cause-effect relationships and uncover the neural mechanisms whereby physical, social, and mental stimulation may protect neurocognition via cognitive reserve among those living with HIV.
Keywords: cognitive reserve, neuroAIDS, cognitive impairment, protective factors
Introduction
Infection with HIV continues to negatively impact the central nervous system despite effective combination antiretroviral therapy (cART) that has improved survival rates (Heaton et al., 2011). Although the prevalence of HIV-associated dementia (HAD) has decreased in the cART era, milder forms of HIV-associated neurocognitive disorders (HAND) that nevertheless commonly interfere with real-world functioning are observed in about half of HIV-infected adults (Heaton et al., 2010). With the increased longevity of HIV-infected individuals (Deeks & Phillips, 2009), and the potential combined effect of HIV and aging on neurocognition (Cherner et al., 2004; Hardy et al., 1999; Wilkie et al., 2003), there is a significant need to examine factors that may help this high-risk population to potentially avoid, or delay the onset of, neurocognitive impairment. Successful cognitive aging occurs in a subset of persons living with HIV and is associated with better everyday functioning (Malaspina et al., 2011), so isolating factors that may further explain the heterogeneity in neurocognitive trajectories in this population is warranted.
Among both HIV-infected and uninfected adults, research to date has shown that higher education, better socioeconomic status, a more cognitively challenging occupation, and a higher Intelligence Quotient (IQ) are associated with better neurocognitive and everyday functioning outcomes (Basso & Bornstein, 2000; La Carret et al., 2003; Morgan et al., 2012; Stern, 2006). These findings support the theory of cognitive reserve, which refers to individual differences in the threshold of neurological insult that a person can withstand before manifestation of cognitive impairment (Stern, 2009). For example, studies have shown that for two individuals with the same neuropathological burden, the person with higher cognitive reserve (e.g., higher education level) would express less neurocognitive impairment than the person with lower reserve (Scarmeas et al., 2003; Stern, Alexander, Prohovnik, & Mayeux, 1992; Thames et al., 2011). Unfortunately, the factors that typically serve as proxies for cognitive reserve are largely static in adulthood. In other words, once past young adulthood, most individuals might not have significant changes in education, socioeconomic status, or IQ. Thus, attempting to change these particular cognitive protective factors via an intervention may be difficult to accomplish in adulthood and do not represent the ideal cognitive intervention targets.
More recently, studies of healthy adults have shown an association between engagement in physical, social, and mental leisure activities and better neurocognitive outcomes, suggesting that these activities may bolster cognitive and/or brain reserve (Verghese et al., 2003). The most consistent finding is that participation in activities that involve both social and mental engagement can positively influence neurocognition (Singh-Manoux, Richards, & Marmot, 2003; Wang et al., 2002; Wang et al., 2006). Some studies have also demonstrated a combined effect of physical, social, and mental activities on better neurocognitive outcomes (e.g., Karp et al., 2006). Studies of healthy adults also suggest that remaining employed for longer in life may also have protective neurocognitive effects, presumably via continued cognitive stimulation on the job (Bonsang, Adam, & Perelman, 2012; Bosma, Boxtel, Ponds, Houx, & Jolles, 2003; Bosma et al., 2003; Mazzonna & Peracchi, 2012; Schwingel, Niti, Tang, & Ng, 2009). Further, depending on one’s occupation remaining employed may also provide continued physical and/or social stimulation. Consequently, employment may serve as a useful proxy for mental activity when other information on leisure mental activity is unavailable.
In addition to cognitive reserve theory, these studies exploring protective lifestyle factors to neurocognition also support the “use it or lose it” or “disuse” theory, which posits that engagement in intellectually stimulating activities may serve as a buffer against normal and abnormal cognitive decline in aging (Hultsch, Hertzog, Small, & Dixon, 1999). While the mechanisms that underlie the potential beneficial effect of physical, social, and mental activities are not yet completely understood, some hypotheses for these mechanisms include: synaptogenesis, neurogenesis, strengthening of neural networks, as well as recruitment of compensatory brain mechanisms and cognitive networks (Fratiglioni, Paillard-Borg, & Winbald, 2004). Altogether, these studies support the idea that modifiable lifestyle factors to protect neurocognitive function exist, and highlight the potential for these factors to guide the development of intervention strategies to improve or maintain neurocognition in persons living with HIV infection.
We are unaware of any previous studies that have examined the combined effect of engagement in physical, social, mental activities on neurocognitive outcomes of HIV-infected persons. Cross-sectional studies with HIV-infected persons support the hypothesis that engagement in physical activity (Dufour et al., 2013; Honn, Para, Whitacre, & Bornstein, 1999) and employment (Heaton et al., 2004) are associated with better neurocognitive outcomes. A few longitudinal studies on employment in HIV have been conducted, showing that neurocognitive function predicts employment (van Gorp et al., 2007), yet the authors of one study examining individuals seeking workforce reentry caution the clinical significance of this predictor in the context of more influential motivational factors such as self-efficacy (Chernoff, Martin, Schrock, & Huy, 2010). Emerging intervention studies with HIV-infected individuals have also demonstrated that cognitive functioning may be improved with restorative neurorehabilitation approaches (Weber, Blackstone, & Woods, 2013). While development of cognitive intervention strategies for this population is the ultimate goal, detailed examination of the potential beneficial effect of mental, physical, and social activities on neurocognition is an important first step in informing future interventions.
Therefore, the purpose of the current study was to determine if a proxy measure of engagement in a greater number of active lifestyle factors (ALFs) using self-reported data on physical exercise, social activity, and mental activity (i.e., employment) was independently associated with better neurocognitive functioning in a well-characterized sample of HIV-infected adults.
Method
Participants
The present study examined a convenience sample of 139 community-dwelling HIV-infected adults from the larger HIV Neurobehavioral Research Center (HNRC) study cohort who had available data for all of the study variables of interest. Participants were appropriately consented via an IRB-approved informed consent document and screened via structured interview. Exclusion criteria included a history of non-HIV related neurologic disorders or any other known conditions that may be associated with impaired neurocognitive performance (e.g., seizure disorder, stroke, head trauma with loss of consciousness greater than 30 minutes, and self-reported history of psychotic disorders). The current study included HIV-infected participants evaluated between 2006 and 2011 who had complete data for the primary variables of interest (i.e., physical exercise, social activity, employment status, and neuropsychological functioning). The participants ranged from 20 to 74 years old (M = 48.7, SD = 10.6; 48% age 50 and above), were mostly male (79.9%), and had an average of one year of college education (M = 13.6 total years of schooling, SD = 2.6). Approximately half of the participants (59.7%) reported their ethnicity as non-Hispanic white. The majority of participants were prescribed ART (87.6%), had an AIDS diagnosis (65.5%), and had an undetectable plasma viral load (71.3%).
Neurocognitive Assessment
Participants completed a standardized neurocognitive test battery that assesses seven cognitive domains commonly affected by HIV, including verbal fluency, working memory, speed of information processing, verbal and visual learning and delayed recall, executive function, and motor function (see Heaton et al., 2010 for further detail on the specific neurocognitive measures). The test scores were adjusted to correct for normal effects of age, gender, education, and race/ethnicity, as indicated (Heaton, Miller, Taylor, & Grant, 2004; Heaton, Taylor, & Manly, 2002; Norman et al., 2011). Each neurocognitive test score was converted to a standard T-score and averaged in order for continuous examination of global neurocognitive function. Additionally, HAND diagnoses (i.e., HIV-associated dementia [HAD], mild neurocognitive disorder [MND], or asymptomatic neurocognitive impairment [ANI]) were calculated by a trained neuropsychologist based on clinical ratings and using standardized and well-validated procedures (i.e., Frascati criteria; Antinori, et al., 2007). Subsequent analyses examined average global neurocognitive T-scores for continuous examination of neurocognitive functioning across groups, as well as HAND diagnoses (HAND vs. neurocognitively normal) in order to examine clinical impairment rates.
Active Lifestyle Factor Classification
In order to quantify presence of active lifestyle factors (ALFs), participants were classified based on the number of self-reported domains in which they engaged, which included physical exercise, social activity, and employment, yielding four possible outcomes ranging from zero to three ALFs. Physical exercise was gathered via a locally-developed, staff-administered questionnaire (which has previously demonstrated an association with neurocognitive impairment in HIV) in which participants reported whether they had engaged in any strenuous exercise (i.e., an activity that makes the heart beat rapidly) in the past 72 hours (Dufour et al., 2013). Based on our previous research (Dufour et al., 2013) showing that strenuous exercise specifically was associated with less neurocognitive impairment, and because we were particularly interested in activities that would be more likely to increase one’s heart rate, any person indicating that they had engaged in any strenuous exercise (e.g., running, jogging, aerobics) in the last 72 hours was classified as having the exercise ALF present. Social activity was determined via the Lawton and Brody Activities of Daily Living questionnaire (Lawton & Brody, 1969). Participants who reported that they “frequently engage in or initiate social activity” were classified as having the social activity ALF, in contrast to those who reported that they “rarely” or “never engage in or initiate social activity”. Engagement in the mental activity ALF was determined based on those who reported either part or full-time employment. Undoubtedly, there are differences in the cognitive demands and complexity of certain occupations (as well as the causes for unemployment); however, we did not have the data to make this more nuanced distinction. We posit that being employed, in most cases, is more mentally (and possibly socially and physically) stimulating than not working. Chi-square tests for independence conducted between each ALF pair revealed that there was a significant association between these factors (social activity and physical exercise, χ2 = 8.02, phi = 0.24, p < 0.01; social activity and employment, χ2 = 10.54, phi = 0.28, p < 0.01; physical exercise and employment, χ2 = 5.57, phi = 0.20, p = 0.02). These data confirm that these constructs are moderately correlated but not collinear, providing justification for our analyses considering these three factors as an overarching active lifestyle construct.
Covariates
We also examined common covariates that may influence the relationship between ALFs and cognition. The variables included demographic factors (i.e., age, gender, education, estimated verbal IQ, race/ethnicity), HIV disease characteristics (i.e., current and nadir CD4+ lymphocyte count, AIDS status, ART status, and plasma viral load), lifetime and current substance use disorder diagnosis, lifetime and current major depressive disorder (MDD) diagnosis, and current depressive mood symptoms. The complete list of potential covariates examined is available in Table 1. Nadir CD4+ count was self-reported unless a study lab value was lower than the self-reported value. AIDS diagnosis was derived using CDC criteria (Castro et al., 1992). Plasma HIV viral load was considered “undetectable” below 48 copies/mL. Current mood was assessed using the Beck Depression Inventory-II (BDI-II) (Beck, Steer, & Brown, 1996). Substance use disorders (i.e., including any current or past diagnosis of substance abuse and/or dependence for the following substances: amphetamine, cocaine, hallucinogen, inhalant, sedative, opioid, PCP, alcohol, marijuana) and MDD diagnoses were assessed through the computer-assisted Composite International Diagnostic Interview, version 2.1 (Wittchen, 1994). The Wide Range Achievement Test-4 Reading (WRAT-4) was used to assess estimated verbal IQ (Wilkinson & Robertson, 2006). Overall physical functioning was assessed using the well-validated Karnofsky Scale of Performance Status in which each participant was assigned an overall functional impairment rating by a certified nurse ranging from 100 to 0 (e.g., 100 = normal, no complaints, and no evidence of disease; 0 = death) (Karnofsky & Burchenal, 1949). Note, in the current sample, the lowest Karnofsky Score was a 50 (i.e., requires considerable assistance and frequent medical care [n = 2]), however 88% (n = 122) of the sample was in the 80 – 100 range (i.e., able to carry on normal activity and to work; no special care needed).
Table 1.
Sample characteristics by active lifestyle factor classification
| Active Lifestyle Factor Classification | |||||
|---|---|---|---|---|---|
| Variable | 0 n = 38 |
1 n = 51 |
2** n = 30 |
3 n = 20 |
Omnibus p- value |
| Active Lifestyle Factors | |||||
| % Social Activity | 0 (0%) | 31 (60.8%) | 29 (96.7%) | 20 (100%) | n/a |
| % Physical Activity | 0 (0%) | 10 (19.6%) | 14 (46.7%) | 20 (100%) | n/a |
| % Currently Employed | 0 (0%) | 10 (19.6%) | 17 (56.7%) | 20 (100%) | n/a |
| Demographics | |||||
| Age | 52.3 (9.7) | 48.2 (10.9) | 47.8 (11.6) | 44.5 (7.9) | 0.05 c |
| Education* | 12.4 (2.5) | 13.6 (2.9) | 13.9 (2.0) | 15.5 (1.9) | <0.01 a, b, c, e, f |
| Estimated Verbal IQ | 97.9 (14.8) | 97.7 (14.8) | 99.3 (12.7) | 107.4 (13.4) | 0.06 c, e |
| % Male* | 33 (86.8%) | 38 (74.5%) | 20 (66.7%) | 20 (100.0%) | <0.01 e, f |
| % White | 23 (60.5%) | 28 (54.9%) | 17 (56.7%) | 15 (75.0%) | 0.46 |
| HIV Disease Indices | |||||
| Current CD4 Count 1 | 581.4 (419.6) | 493.5 (273.8) | 549.8 (297.5) | 642.9 (218.5) | 0.16 |
| Nadir CD4 Count | 150.7 (107.7) | 176.8 (147.6) | 154.5 (148.6) | 258.9 (182.5) | 0.12 c, e, f |
| % with UD PL Viral Load* 2 | 30 (81.1%) | 26 (56.5%) | 24 (82.8%) | 12 (70.6%) | 0.04 a, d |
| % with AIDS | 27 (71.1%) | 35 (68.6%) | 21 (70.0%) | 8 (40%) | 0.08 c, e, f |
| % on ARV 3 | 36 (94.7%) | 39 (78.0%) | 27 (93.1%) | 18 (90.0%) | 0.10 a |
| Mental and Physical Health | |||||
| BDI-II* | 16.1 (10.4) | 9.7 (9.5) | 7.3 (9.5) | 4.7 (4.6) | <0.01 a, b, c, e |
| % Alcohol D× LT4 | 20 (52.6%) | 27 (55.1%) | 13 (43.3%) | 12 (60.0%) | 0.66 |
| % Marijuana D× LT4 | 9 (23.7%) | 20 (40.8%) | 10 (33.3%) | 8 (40.0%) | 0.37 |
| % Other Substance D× LT4 | 22 (57.9%) | 27 (55.1%) | 12 (40.0%) | 12 (60.0%) | 0.41 |
| % Alcohol D× CU4 | 0 (0.0%) | 1 (2.0%) | 0 (0.0%) | 1 (5.0%) | 0.41 |
| % Marijuana D× CU4 | 2 (5.3%) | 2 (4.1%) | 0 (0.0%) | 0 (0.0%) | 0.48 |
| % Other Substance D× CU 4 | 1 (2.6%) | 2 (4.1%) | 1 (3.3%) | 0 (0.0%) | 0.99 |
| % MDD D× Lifetime 4 | 23 (60.5%) | 30 (61.2%) | 13 (43.3%) | 10 (50.0%) | 0.38 |
| % MDD D× Current* 4 | 9 (23.7%) | 8 (16.3%) | 2 (6.7%) | 0 (0.0%) | 0.04 c |
| Karnofsky Performance Status* | 79.7 (14.0) | 88.2 (9.5) | 94.0 (7.2) | 99.0 (3.1) | <0.01 a, b, c, e |
| % with HAND* | 24 (63%) | 26 (51%) | 10 (33%) | 4 (20%) | <0.01 b, c, e |
Note. M(SD) or number (%) reported. PL = Plasma; UD = undetectable; ARV = antiretroviral medications; BDI-II = Beck Depression Inventory II; D× = diagnosis; LT = lifetime; CU = current; MDD = Major Depressive Disorder; HAND = HIV-associated neurocognitive disorder.
= 0 differs from 1;
= 0 differs from 2;
= 0 differs from 3;
= 1 differs from 2;
= 1 differs from 3;
= 2 differs from 3 (ps < 0.05).
Missing data notes:
1 from group 3;
1 from group 0, 5 from group 1, 1 from group 2, 3 from group 3;
1 from group 1, 1 from group 2;
2 from group 1.
Omnibus p < 0.05;
Physical activity & employed, n = 1; Social activity & employed, n = 16; Social activity & physical activity, n = 13.
Statistical Analyses
To assess the association between ALF classification and global neurocognitive functioning (average neurocognitive T-scores) we first examined the bivariate relationship between these two variables using an analysis of variance (ANOVA) to confirm that there was a significant association. To adjust for potential confounds in subsequent analyses, we then compared the four-level ALF classification across a series of potential covariates using ANOVA (or Wilcoxon/Kruskal Wallis test for non-parametric variables) for continuous variables and Chi-Square tests (or Fisher’s Exact Test when appropriate) for categorical variables. Those variables with a p-value < 0.10 between ALF classification categories were considered as potential covariates if they also had a significant bivariate association with our primary dependent variable (i.e., global neurocognitive T-scores). However, as these T-scores were adjusted for age, education, gender and race/ethnicity, these demographic variables were not included as covariates. Relevant covariates were entered into an analysis of covariance (ANCOVA), along with ALF classification to examine their effect on global neurocognitive T-scores. In order to examine the association of ALF classification with HAND (HAND vs. neurocognitively normal), a multivariable logistic regression model predicting HAND diagnosis was performed using the same potential predictors from the ANCOVA model. Odds ratios with 95% confidence intervals were computed to determine the strength of the relationship. All reported p-values were based on two-sided tests with a critical alpha of 0.05.
Results
Preliminary Analyses
The ALF classification yielded the following groupings: presence of 0 (n = 38), 1 (n = 51), 2 (n = 30), and 3 ALFs (n = 20). Preliminary unadjusted ANOVA confirmed that there was a significant positive association between the ALF groups and global neurocognitive T-scores (F [3, 135] = 8.9, p < 0.001, R2 = 0.16, adjusted R2 = 0.15). Furthermore, within this unadjusted model, there were the following significant (p < 0.05) pair-wise comparisons: 0 vs. 2 (Cohen’s d = 0.81); 0 vs. 3 (Cohen’s d = 1.39); 1 vs. 2 (Cohen’s d = 0.56); 1 vs. 3 (Cohen’s d = 1.01). The four groups significantly differed on education, gender, plasma viral load, BDI-II, current MDD diagnosis, and Karnofsky Score, and there was a trend for age, estimated verbal IQ, and AIDS status. Table 1 presents pair-wise results for those groups with significant differences from one another. Estimated verbal IQ, BDI-II, and Karnofsky Score were the only potential covariates that met the following criteria: 1) each differed between the ALF groups (p < 0.10) and, 2) each was correlated with global neurocognitive T-scores. However, given the importance of education in the context of cognitive reserve and that education differed between the ALF groups, education was also forced in as a covariate in all primary models along with estimated verbal IQ, BDI-II, and Karnofsky Score. To confirm that these were the only relevant covariates to consider, we also ran a separate model including the other variables that differed between groups (p < 0.10) and these variables did not influence the association of our independent variable of interest (ALF classification) with global neurocognitive T-scores, confirming our decision to only include estimated verbal IQ, education, BDI-II, and Karnofsky Score.
Global Neurocognitive T-Scores and ALFs
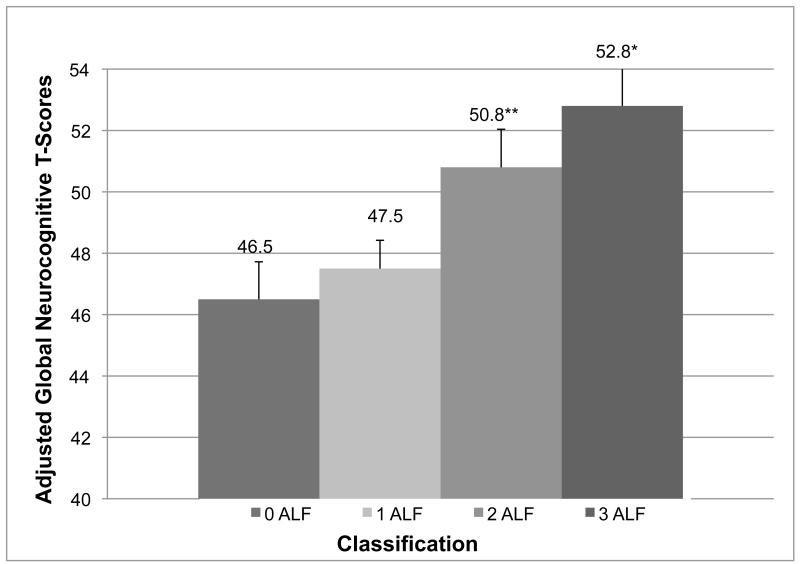
Results of the overall ANCOVA model predicting average neurocognitive T-scores from ALF classification were significant (F [7, 131] = 10.0, p < 0.001, R2 = 0.35, adjusted R2 = 0.31). Estimated verbal IQ, education, and Karnofsky Score all emerged as significant covariates (ps < 0.05). There was a significant main effect for ALF classification (F [3, 131] = 3.9, p = 0.01), such that there was a positive association between more ALFs and better global neurocognitive performance. Post-hoc analyses using Tukey’s Honestly Significant Difference test revealed that the 3 ALF classification significantly differed from the 1 and 0 ALF classifications (ps < 0.05), and there was a trend towards the 2 ALF classification differing from the 0 ALF group (p = 0.09). Covariate-adjusted average T-score group means are presented in Figure 1.
Figure 1.
Covariate Adjusted Neurocognitive Means by Active Lifestyle Factor Classification
Notes. ALF = Active lifestyle factors; *3 > 1 and 0 (p < 0.05); **2 > 0 (p = 0.09). The y-axis is truncated for visual representation of means. Bars represent standard errors.
HAND and ALFs
Results of the overall logistic regression model predicting HAND status including the same potential predictors as the ANCOVA were also significant (df = 5, χ2 = 27.1, p < 0.001). Estimated verbal IQ and education were significant covariates (ps < 0.05). There was a significant independent main effect of ALF classification, such that an increasing number of ALFs was associated with a lower prevalence of HAND (df = 1, χ2 = 5.1, p = 0.02; OR = 0.59; 95% CI = 0.36 – 0.93). Specifically, 63% of the participants with the 0 ALF classification had HAND (34% ANI, 18% MND, 11% HAD), while the other classifications had the following HAND rates: 51% (35% ANI, 14% MND, 2% HAD) of 1 ALF group; 33% (27% ANI, 3% MND, 3% HAD) of 2 ALF group and 20% (15% ANI, 5% MND, 0% HAD) of the 3 ALF group (Table 1).
Post-hoc Analyses
Several post-hoc analyses were performed to further delineate our main findings. In addition to the aforementioned ANCOVA model examining global neurocognitive T-scores, we conducted a confirmatory multiple regression analysis treating ALF classification as a continuous variable, including the same original covariates. Results showed that the overall model was significant (F [5, 133] = 14.0, p < 0.001, R2 = 0.34, adjusted R2 = 0.32) and confirmed a significant main effect of ALF classification (p < 0.01). Moreover, in order to examine the bivariate associations of each of the individual factors comprising the ALF classifications and global neurocognition, independent samples t-tests were performed with each of these factors (i.e., presence/absence of physical exercise, social activity, and employment) as separate independent variables and global neurocognitive T-scores. Results showed that each of these factors were associated with better global cognition (all ps < 0.01). Specifically the effect sizes are as follows: physical exercise, Cohen’s d = 0.58; social activity, Cohen’s d = 0.41; employment, Cohen’s d = 0.57. In order to examine the independent associations with each of these ALF factors relative to one another and in the context of cognitive reserve, a multiple regression was performed including presence or absence of each ALF along with estimated verbal IQ and education as independent variables and global neurocognitive T scores as the dependent variable. Results showed that employment, physical activity, and social activity all remained significant (ps < 0.05). Lastly, follow-up analyses examining domain specific neurocognitive mean T-scores were conducted using the previous ANCOVA model used for global T-scores. Results showed that ALF classification was independently associated with better performance in each neurocognitive domain (i.e., verbal, executive function, speed of information processing, recall, and working memory) (ps < 0.05) except for motor, which approached significance (p = 0.06) and learning, which was non-significant.
In order to examine the ALFs with more granularity, a new summative scale was created using the same three domains (physical, social, cognitive) measured on a broader range (i.e., physical exercise: 0 = no exercise, 1 = moderate exercise [based on lower range on median split of minutes per past 72 hours], 2 = high exercise [based on higher range on median split]; social activity: 0 = never engage in social activities; 1 = rarely engage in social activities; 2 = frequently engage in social activities; employment: 0 = unemployed, 1 = working part-time, 2 = working full-time). This approach yielded an ALF variable with scores ranging from 0 to 6. Note that this new ALF variable was highly correlated with the original version (r = 0.95). Furthermore, as with the aforementioned Chi-square analyses between each of the dichotomous ALF domains in our original variable (i.e., physical, social, cognitive) showing interrelatedness without collinearity, we calculated Cronbach’s alphas for the this new ALF variable and confirmed the relative distinctness of these three domains (α = 0.49). Using the same covariates as the previous analyses (i.e., BDI-II, education, estimated verbal IQ, and Karnofsky Score), linear and logistic multivariable regressions were conducted with this ALF classification as the independent variable and global neurocognitive T-scores and HAND status, respectively, as the dependent variables. Results from the analysis with global neurocognitive T-scores showed that the new ALF variable had a significant positive association (p < 0.001) with neurocognitive function. The analysis with HAND also showed a significant association, with higher ALF scores associated with lower odds of HAND (p < 0.01; OR = 0.64, 95% CI = 0.47 – 0.86).
In addition to the analyses highlighting the reliability of both of our ALF variables (i.e., each component is moderately associated with the others), post-hoc analyses were conducted to provide further construct validity of the ALF determination. Correlations and t-tests were conducted between variables hypothesized to be either conceptually related to (i.e., convergence) or distinct from (i.e., divergence) the original ALF classification. Specifically, as expected, education (rho = 0.36), estimated verbal IQ (rho = 0.17), BDI-II (rho = −0.43), and Karnofsky Score (rho = 0.55) were each significantly associated with ALF classification (ps < 0.05). In contrast, ALF classification diverged from sex and race (ps > 0.05). As an additional validation analysis of the ALF variable, 17 HIV-negative control subjects were examined. As with the HIV+ sample, the ALF distribution showed variability (i.e., 0 ALF n = 3; 1 ALF n = 6; 2 ALF n = 7; 3 ALF n =1), as well as a strong association with global neurocognitive T-scores despite a small sample size (rho = 0.43, p = 0.08).
Discussion
Our findings revealed that a higher number of active lifestyle factors in this HIV-infected adult sample were independently associated with better global neurocognitive performance and less prevalence of HAND, roughly following a stair-step pattern. There were raw mean differences in global neurocognitive T-scores between all ALF groups in the expected direction, such that progression from 0 to 3 ALFs was associated with an increase in neurocognitive performance, although not all were statistically significant. The HAND rates among those with 0 or 1 ALFs are consistent with the published rates of neurocognitive impairment among HIV-infected persons (Heaton et al., 2010), while the rates of impairment in the 2 and 3 ALF classification were somewhat lower. Thus, engagement in a greater number of active lifestyle factors may protect against neurocognitive impairment in HIV. This study extends previous work from our group examining the potential protective effect of physical exercise on neurocognitive function (Dufour et al., 2013) by showing that a multicomponent active lifestyle construct provides added conceptual and statistical value in predicting better neurocognitive function in adults with HIV.
Additionally, ALF classification was associated with better performance in most of the individual cognitive domains, suggesting that an active lifestyle may have beneficial effects for a wide range of neurocognitive functions that are commonly affected in people living with HIV. The post-hoc analysis examining the independent effect of each ALF showed that each factor (physical, social, and employment) was significantly related to neurocognitive functioning when examined separately. When examined in combination along with cognitive reserve, physical exercise, employment, and social activity each remained significant, implying that all three activities may have a unique potential benefit to neurocognitive functioning. Albeit, our analyses showed the combination of either two or three ALFs had a stronger influence on neurocognitive outcomes than having the presence of only one. Therefore, consistent with findings in those without HIV (Karp et al., 2006; Wang et al., 2006), our findings suggest that engaging in multiple lifestyle activities across different domains (i.e., physical, social, mental) may be the most protective for neurocognitive functioning in HIV.
Several post-hoc analyses provide preliminary evidence of the reliability and validity of the ALF determination used in this study. The original and new ALF variable both evidenced reliability via the interrelatedness of each individual component in the absence of collinearity, providing justification for considering the three components within an overarching active lifestyle construct. Furthermore, the ALF classification showed convergence with conceptually related factors such as cognitive reserve, depressive symptoms, and physical functioning, which is consistent with the literature (e.g., Wang et al., 2002). While there is consensus in the non-HIV infected literature on these convergent factors, there is mixed evidence on divergent factors. Evidence suggests that the types of active lifestyle components one participates in may vary by sex and race (e.g., Janke, Davey, & Kleiber, 2006); however, the total number of ALFs women and men participate in does not appear to differ (Singh-Manoux et al., 2003). Moreover, when differences emerge between racial groups in frequency of participation in ALFs, this association may be largely mediated by secondary factors such as education (e.g., Xiaoxing, & Baker, 2005). In the current study, our ALF variable showed divergence with both race and sex. Finally, an analysis conducted among a small sample of HIV-uninfected persons showed variability in ALFs and an association between ALFs and neurocognitive function.
Intervention studies in healthy older adults have shown promising effects of increasing lifestyle activities for improving mild neurocognitive deficits and may be applied to the HIV population. Randomized controlled trials (RCTs) have shown that aerobic exercise interventions are effective in improving neurocognitive function, particularly executive functions (Colcombe & Kramer, 2003). Cognitive training RCTs have also shown robust effects on cognitive outcomes, with improvements that parallel the magnitude of decline expected in normal aging, suggesting that these interventions may allow older adults to avoid or delay neurocognitive impairment (Ball et al., 2002). Cognitive interventions may be particularly beneficial in those who are not working and thus may not be able to stimulate their minds via occupational tasks. To date, there have been no RCTs in the aging literature explicitly examining social activity interventions for neurocognitive protection. However a robust association between social engagement and reduced risk of dementia has been found (Fratiglioni, Paillard-Borg, & Winbald, 2004), providing promising directions for this type of intervention. Improving social activities in an intervention setting per se may not be as directly implementable as other cognitive intervention strategies (e.g., prescribing a physical or cognitive exercise regimen); however, encouraging social activity among patients in a clinical setting may prove beneficial for cognitive function as well as overall mental health and quality of life, especially among those living and aging with HIV. Further research is needed to understand the preventative and/or restorative value of physical, cognitive, and social interventions in older HIV-infected samples, as well as the feasibility, durability, dosage, and underlying mechanisms of their efficacy (Weber, Blackstone, & Woods, 2013).
This study has limitations. First, the current study demonstrates only cross-sectional associations, and thus causation cannot be determined (e.g., perhaps those with poorer neurocognitive functioning are less likely and able to engage in physical exercise, social activity, and employment). As such, results should be interpreted with caution. In order to demonstrate causality, longitudinal prospective studies are needed to examine leisure activities in this population using matched groups and a well-developed questionnaire gathering types of activities (e.g., crossword puzzles), frequency, age of onset for the initiation of each activity, as well as examination of other cofactors (e.g., nutrition, reduction in blood pressure) that may mediate or moderate the association between ALFs and neurocognitive function. Yet, given the dearth of research on protective factors to neurocognition in HIV, and the clinical significance in this vulnerable population, the positive associations that emerged signal the need for longitudinal studies on this topic, as well as informing potential intervention strategies.
Next, we recognize that our ALF classification is relatively crude, and is comprised of self-reported proxies for these factors. The nature of our physical activity questionnaire did not allow for examination of frequency of physical activity (e.g., days per week exercised) or type of activity, nor did it differentiate between what would be considered “strenuous” for a younger adult relative to an older adult. In fact, it has been shown that HIV+ individuals may over-report strenuous activity because they may perceive an activity as strenuous when it may actually be moderate or less (Fillipas, Cicuttini, Holland, and Cherry, 2010). Furthermore, the self-report nature of this variable limits validity because memory problems are a common feature of HAND (e.g., Woods, Moore, Weber, & Grant, 2009) that may lead to inaccurate recall of time spent in physical exercise. While our self-reported exercise questionnaire timeframe was limited to 72-hours based on the available data, there is evidence that 3-day recalls (i.e., 72 hours) converge with 7-day exercise recall and objective physical activity indices (Han & Dinger, 2009). Future studies examining physical exercise and neurocognition in HIV should include both self-report and objective measures of exercise (e.g., heart rate monitors). Despite these limitations, similar to studies conducted among persons without HIV, we found a robust relationship between ALFs and decreased HAND, providing support for the cognitive reserve theory in HIV. Yet, prior studies provide validation of the ALF construct in large samples of healthy control subjects (Karp et al., 2006; Wang et al., 2006), which is a limitation of this study. Nonetheless the ALF variable used in this study does have value as an initial validation study of ALFs in HIV, providing reliability and construct validity in our HIV+ sample. While these findings do indeed support the cognitive reserve theory, the available data do not include measurement of brain pathology, and thus do not allow for direct examination of this theory. Finally, although our overall sample size was adequate to detect associations, our 3 ALF classification group was quite small. Despite this limited statistical power, we still found a robust association between ALFs and neurocognition.
We acknowledge that employment, our available proxy for cognitive stimulation, may not be ideal, as the type of stimulation one receives at work could be cognitive, physical, or social, or some combination of the three, thus making it difficult to disentangle the underlying factors associated with employment. We are also cognizant of the fact that the level of cognitive complexity varies between occupations and this unmeasured element may have influenced the association. Also, using employment as a proxy for cognitive activity in an HIV-infected sample can be problematic, as it is particularly difficult to unravel the causes for unemployment (e.g., physical ability, motivation, strained economic climate), although our available data did allow determination that only three of the unemployed subjects reported retirement as their reason for not working. Nonetheless, being employed represents some basic ability to engage with others and be productive with a skill in some capacity and the direction of our results suggests that it serves as an adequate proxy. Given the research in healthy adults on better neurocognition in those who remain employed, and that neurocognition (particularly when frank impairment is not present) may not be the best predictor of employment in HIV among those who are actively seeking workforce reentry, further research is needed to understand the complex association between employment as a protective factor to neurocognition in HIV. The directionality may be reciprocal, such that neurocognitive impairment may lead to unemployment, whereas unemployment may in turn exacerbate neurocognitive impairment.
These findings suggest that living an active lifestyle may protect neurocognitive functioning in the face of concurrent risk factors among people living with HIV. Similar to studies with healthy adults, these results support the hypothesis that more activities may have an additive beneficial effect to neurocognition. While promising, these preliminary results require further investigation to ascertain their causal validity as well as a true measurement of cognitive reserve. Given the prevalence of HAND, the aging of the HIV population, and the importance of intact neurocognitive functioning for successful aging, future studies should examine active lifestyle factors longitudinally, including the direct and indirect mechanisms underlying the protective effect of these activities on cognitive and/or brain reserve. For example, physical exercise may protect neurocogition indirectly by reducing cardiovascular comorbidities, or may directly impact the brain. Mental stimulation (either at work or in leisure activities) may directly affect neurocognition via neuroplasticity. Social engagement may protect neurocognition via mediation of emotional problems (e.g., depression, apathy), or via some direct mechanism within the brain. In addition to bolstering cognitive reserve, engagement in physical, social, and mental activities may also enhance other components of successful aging, including biological health, length of life, productivity, and life satisfaction (Baltes & Baltes, 1990) as well as positive psychological factors such as resilience and optimism (Moore et al., 2013). Thus, the interplay between active lifestyle factors in aging with HIV at the neural, neurocognitive, functional, and psychosocial levels warrants further elucidation, with the ultimate goal of 1) developing interventions implementing these activities and 2) determining if and how these interventions might help HIV-infected individuals to avoid or delay neurocognitive decline.
Acknowledgements
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH. Dr. Fazeli is supported by R25MH081482 from NIMH (Interdisciplinary Research Fellowship in NeuroAIDS, M. Cherner, PI), ID10-SD-057 from California HIV/AIDS Research Program (CHRP) (Determinants of Successful Aging Among Older HIV+ Persons, D.J. Moore, PI), R01MH099987 from NIMH (Multi-Dimensional Successful Aging Among HIV-Infected Adults, D. Jeste/D.J. Moore, co-PIs), and L30-AG045921 from NIA (Mechanisms of Successful Cognitive and Functional Aging with HIV, P. Fazeli, PI).
Footnotes
Conflicts of Interest
The authors (Drs. Pariya L. Fazeli, Steven Paul Woods, Robert K. Heaton, Raeanne C. Moore, Igor Grant, and David J. Moore, and Ms. Umlauf, Ms. Rosario, and Mr. Gouaux) have no conflicts of interest to report.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Willis SL. Effects of cognitive training interventions with older Adults: A randomized controlled trial. The Journal of the American Medical Association. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM. Psychological perspectives in successful aging: The model of selective optimization with compensation. In: Baltes PB, Baltes MM, editors. Successful Aging: Perspectives from the Behavioral Sciences. Cambridge University Press; Cambridge, UK: 1990. pp. 1–34. [Google Scholar]
- Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals with HIV across 12 months. Journal of Clinical and Experimental Neuropsychology. 2000;22(2):208–218. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. p. 1.p. 82. [Google Scholar]
- Bonsang E, Adam S, Perelman S. Does retirement affect cognitive functioning? Journal of Health Economics. 2012;31(3):490–501. doi: 10.1016/j.jhealeco.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Bosma H, van Boxtel MPJ, Ponds RWHM, Houx PJ, Burdorf A, Jolles J. Mental work demands protect against cognitive impairment: MAAS prospective cohort study. Experimental Aging Research. 2003;29:33–45. doi: 10.1080/03610730303710. [DOI] [PubMed] [Google Scholar]
- Bosma H, van Boxtel MPJ, Ponds RWHM, Houx PJ,H, Jolles J. Education and age-related cognitive decline: The contribution of mental workload. Educational Gerontology. 2003;29:165–173. [Google Scholar]
- Castro KG, Ward JW, Slutsker L, Buehler JW, Jaffe HW, Berkelman RL. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. US Department of Health and Human Services; 1992. [PubMed] [Google Scholar]
- Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, HNRC Group Effects of HIV-1 infection and aging on neurobehavioral functioning: Preliminary findings. AIDS. 2004;18(Suppl 1):S27–S34. [PubMed] [Google Scholar]
- Chernoff RA, Martin DJ, Schrock DA, Huy MP. Neuropsychological functioning as a predictor of employment activity in a longitudinal study of HIV-infected adults contemplating workforce reentry. Journal of the International Neuropsychological Society. 2010;16:38–48. doi: 10.1017/S1355617709990828. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. British Medical Journal. 2009;338:288–292. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- Dufour CA, Marquine MJ, Fazeli PL, Henry B, Ellis RJ, Grant I, Moore DJ, HNRP Group Strenuous exercise is associated with less neurocognitive impairment in HIV-infected adults. Journal of Neurovirology. 2013;19(5):410–417. doi: 10.1007/s13365-013-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillipas S, Cicuttini F, Holland AE, Cherry CL. The International Physical Activity Questionnaire overestimates moderate and vigorous physical activity in HIV-infected individuals compared to accelerometry. Journal of the Association of Nurses in AIDS Care. 2010;21:173–181. doi: 10.1016/j.jana.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially Integrated lifestyle in late life might protect against dementia. The Lancet Neurology. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Han JL, Dinger MK. Validation of a self-administered 3-day physical activity recall in young adults. American Journal of Health Education. 2009;40(1):5–13. [Google Scholar]
- Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, Moore LH. Age differences and neurocognitive performance in HIV-infected adults. New Zealand Journal of Psychology. 1999;28(2):94–101. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, HNRC Group HIV-associated neurocognitive disorders before and after the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, HNRC Group The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources; Lutz, FL: 2004. [Google Scholar]
- Heaton RK, Taylor MJ, Manly JJ. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Tulsky D, Saklofske D, Heaton RK, et al., editors. Clinical Interpretation of the WAIS-III and WMS-III. Academic Press; San Diego, CA: 2002. [Google Scholar]
- Honn VJ, Para MF, Whitacre CC, Bornstein RA. Effect of exercise on neuropsychological performance in asymptomatic HIV infection. AIDS and Behavior. 1999;3(1):67–74. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology & Aging. 1999;14(2):245–263. doi: 10.1037//0882-7974.14.2.245. doi:10.1037/0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Janke M, Davey A, Kleiber D. Modeling change in older adults’ leisure activities. Leisure Sciences. 2006;28:285–303. [Google Scholar]
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Maclead CM, editor. Evaluation of Chemotherapeutic Agents. Columbia University Press; New York: 1949. pp. 191–205. [Google Scholar]
- Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and Geriatric Cognitive Disorders. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Le Carret N, Lafont S, Letenneur L, Dartigues JF, Mayo W, Fabrioule C. The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Developmental Neuropsychology. 2003;23(3):317–337. doi: 10.1207/S15326942DN2303_1. [DOI] [PubMed] [Google Scholar]
- Malaspina L, Woods SP, Moore DJ, Depp CA, Letendre SL, Jeste DV, Grant I, HNRP Group Successful cognitive aging in persons living with HIV infection. Journal of NeuroVirology. 2011;17:110–119. doi: 10.1007/s13365-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzonna F, Peracchi F. Ageing, cognitive abilities and retirement. European Economic Review. 2012;56:691–710. [Google Scholar]
- Moore RC, Moore DJ, Thompson W, Vahia IV, Grant I, Jeste DV. A case-controlled study of successful aging in older HIV-infected adults. Journal of Clinical Psychiatry. 2013;74(5):417–423. doi: 10.4088/JCP.12m08100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND) AIDS & Behavior. 2012;16(8):2279–2285. doi: 10.1007/s10461-012-0229-7. PMID: 22677976 PMCID: PMC3443502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D, Cysique L, Ake C, HNRC Group Demographically corrected norms for African Americans and caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. Journal of Clinical And Experimental Neuropsychology. 2011;33(7):793–804. doi: 10.1080/13803395.2011.559157. PMID: 21547817 PMCID: PMC3154384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, Stern Y. Association of life activities with cerebral blood flow in Alzheimer’s Disease: Implications for the cognitive reserve hypothesis. Archives of Neurology. 2003;60:359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingel A, Niti MM, Tang C, Ng TP. Continued work employment and volunteerism and mental well-being of older adults: Singapore longitudinal ageing studies. Age and Ageing. 2009;38:531–537. doi: 10.1093/ageing/afp089. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Richards M, Marmot M. Leisure activities and cognitive function in middle-age: Evidence from the Whitehall II Study. Journal of Epidemiology and Community Health. 2003;57:907–913. doi: 10.1136/jech.57.11.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive Reserve and Alzheimer’s Disease. Alzheimer Disease & Associated Disorders. 2006;20(2):112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and perfusion deficit in Alzheimer’s disease. Annals of Neurology. 1992;32(3):371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- Thames AD, Foley JM, Panos SE, Singer EJ, El-Saden S, Hinkin C. Cognitive reserve masks neurobehavioral expression of human immunodeficiency virus associated neurological disorder in older patients. Neurobehavioral HIV Medicine. 2011;3:87–93. [Google Scholar]
- van Gorp WG, Rabkin JG, Ferrando SJ, Mintz J, Ryan E, Borkowski T, McElhiney M. Neuropsychiatric predictors of return to work in HIV/AIDS. Journal of the International Neuropsychological Society. 2007;13:80–89. doi: 10.1017/S1355617707070117. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Buschke H. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine. 2003;348:2508–16. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Wang HX, Karp K, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen Project. American Journal of Epidemiology. 2002;155(12):1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Wang JYJ, Zhou DHD, Li J, Zhang M, Deng J, Tang M, Chen M. Leisure activities and risk of cognitive impairment: The Chongqing aging study. Neurology. 2006;66(6):911–913. doi: 10.1212/01.wnl.0000192165.99963.2a. [DOI] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Woods SP. Cognitive neurorehabilitation of HIV-associated neurocognitive disorders: A qualitative review and call to action. Journal of NeuroVirology. 2013;19(1):65–74. doi: 10.1007/s11065-013-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie FL, Goodkin K, Khamis I, van Zuilen MH, Lee D, Lecusay R, Eisdorfer C. Cognitive functioning in younger and older HIV-1-infected adults. Journal of Acquired Immune Deficiency Syndromes. 2003;33:S93–S105. doi: 10.1097/00126334-200306012-00006. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. WRAT 4: Wide Range Achievement Test; Professional Manual. Psychological Assessment Resources, Incorporated; 2006. [Google Scholar]
- Wittchen HU. Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): A critical review. Journal of Psychiatric Research. 1994;28(1):57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaoxing ZH, Baker DW. Differences in leisure-time, household, and work-related physical activity, by race, ethnicity, and education. Journal of General Internal Medicine. 2005;20(3):259–266. doi: 10.1111/j.1525-1497.2005.40198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]