Abstract
Like varicella zoster virus in humans, simian varicella virus (SVV) becomes latent in ganglionic neurons along the entire neuraxis and reactivates in immunosuppressed monkeys. Five rhesus macaques were inoculated with SVV; 142 days later (latency), 4 monkeys were immunosuppressed and T cells were analyzed for naïve, memory and effector phenotypes and expression of programmed death receptor 1 (PD-1; T cell exhaustion). All T cell subsets decreased during immunosuppression, and except for CD8 effectors, peaked two weeks before zoster. Compared to before immunosuppression, PD-1 expression increased at reactivation. Increased T cells before zoster is likely due to virus reactivation.
Keywords: varicella zoster virus, simian varicella virus, T cells, PD-1
Introduction
Varicella zoster virus (VZV), an exclusively human alphaherpesvirus, causes varicella (chickenpox), after which virus becomes latent in ganglionic neurons along the entire neuraxis. As cell-mediated immunity to VZV declines with age or immunosuppression, virus reactivates to cause zoster (shingles). Simian varicella virus in primates parallels human VZV infection. Primary SVV infection of monkeys produces varicella and a robust SVV-specific T cell response 7-14 days later (Messaoudi et al. 2009; Ouwendijk et al. 2013). After primary infection, virus becomes latent in ganglia and reactivate to produce zoster (Mahalingam et al. 2010). The T cell repertoire (naïve, memory and effector populations) immediately before and during reactivation is yet unknown.
Because VZV-specific T cells decrease before reactivation, a better understanding of T cell phenotypes at reactivation is necessary. T cells may become functionally exhausted, or unresponsive due to age or persistent infection (Day et al. 2006). Exhausted T cells are characterized by an increase in expression of programmed death receptor-1 (PD-1). PD-1 expression has not been studied after varicella or zoster.
Materials and methods
Five rhesus macaques were inoculated intrabronchially with 104 pfu of SVV (n=3) or SVV-GFP (SVV expressing green fluorescent protein) (n=2). All monkeys developed varicella 9-14 days post-inoculation (dpi). Five months later (142 dpi), 4 monkeys were irradiated once (200 cGy) and treated daily with tacrolimus (80 μg/kg/day) and prednisone (2mg/kg/day) for the duration of the experiment; one monkey was not treated, but was subjected to the stress of transportation and isolation. Zoster rash developed in all 4 immunosuppressed monkeys beginning 48 days post-immunosuppression, as well as in the non-immunosuppressed monkey as previously described (Mahalingam et al., 2010). Blood samples obtained weekly were analyzed for T cell repertoire before and after primary infection, during latency and at reactivation. Blood mononuclear cells were isolated and analyzed by flow cytometry for T cell markers CD3 (clone SP34-2), CD4 (clone L200), CD8 (clone SK1) CD28 (clone CD28.2) and CD95 (clone DX2). T cells were identified as naïve (CD28+ CD95−), memory (CD28+ CD95+) or effector (CD28− CD95+) cells. T cells were further analyzed for expression of programmed death receptor-1 (PD-1, clone J105) before immunosuppression and at reactivation. Results of flow cytometry from the 4 immunosuppressed monkeys were analyzed by ANOVA using GraphPad Prism software.
Monkeys were euthanized and necropsy was performed 24-48 h after SVV reactivation, as verified by the appearance of zoster and confirmed by immunohistochemical analysis of skin biopsies. Skin was fixed in 4% paraformaldehyde and paraffin-embedded 5 μm sections were stained as described (Mahalingam et al. 1996) with rabbit polyclonal antibodies directed against SVV glycoproteins H and L (1:5000); normal rabbit serum applied to adjacent sections served as a negative control. Slides were incubated at 4°C for 16 h, washed, incubated with biotin-labeled goat anti-rabbit IgG (1:300), followed by incubation with alkaline phosphatase-conjugated streptavidin and new fuchsia substrate.
Results
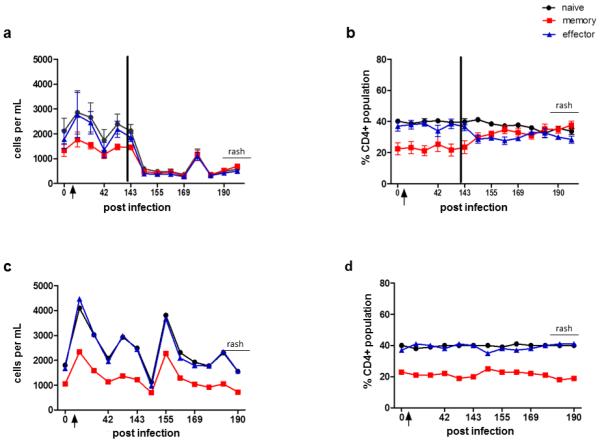
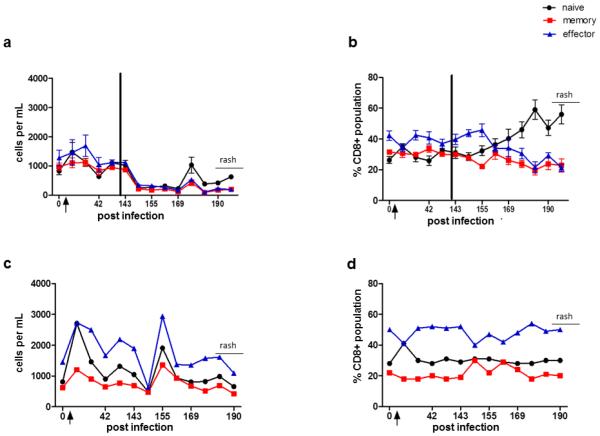
Detection of SVV antigen in skin rash confirmed the presence of zoster (Fig.1). Seven days after immunosuppression (151 dpi), significant decreases in absolute counts of naïve, memory and effector CD4 (p=0.004, 0.003, 0.05, respectively) and CD8 (p= 0.005, 0.01 and 0.02, respectively) T cells were found (Figs. 2a & 3a). Although a decline in all T cell subsets was also seen in the non-immunosuppressed monkey 151 dpi, T cell counts increased immediately thereafter and remained higher than in immunosuppressed monkeys (Fig. 2c & 3c). Importantly, 14 days before zoster, there was a significant increase in CD4 naïve, memory and effector cells (p=0.02, 0.05, 0.009, respectively) and in CD8 naïve and memory cells (p=0.02, 0.02, respectively). This effect was not seen in the non-immunosuppressed monkey (Figs. 2c & 3c).
Fig. 1. Simian varicella virus (SVV) antigen in skin rash during reactivation.
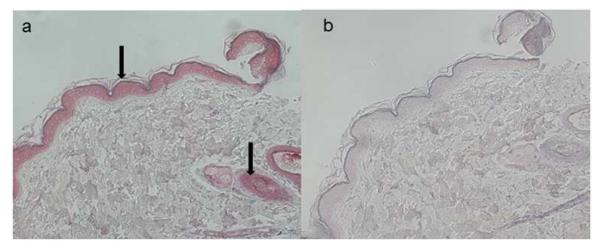
SVV antigens are seen in the epidermis and surrounding hair follicles after staining with rabbit anti-SVV gH and gL antibodies (a, arrows), but not in adjacent sections stained with normal rabbit serum (b).
Fig. 2. CD4 T cells in blood from monkeys before and during experimental SVV infection, after immunosuppression and during virus reactivation (zoster).
At times indicated, blood was obtained and processed as described in Materials and methods. Flow cytometric analysis was used to calculate numbers of naïve (CD28+, CD95−), memory (CD28+, CD95+) and effector (CD28−, CD95+) T cells (a and c) and their percentages of CD4 T cells (b and d). Total CD4 T cell counts increased significantly in naïve, memory and effector populations 14 days post-infection (dpi) in all monkeys compared to those before infection (a and c; p<0.05). After immunosuppression, all T cell subsets declined (a, p<0.05), whereas at 176 dpi (2 weeks before zoster), all CD4 T cells increased (a, p<0.05). The percentage of naïve, memory and effector CD4 T cells remained constant (b). CD4 T cells in the non-immunosuppressed monkey also declined after transportation to the radiation facility, but not to the extent seen in immunosuppressed monkeys (c); the percentages of naïve, memory and effector T cells also remained stable (d). Arrow indicates day of inoculation; black bar indicates time of immunosuppression. Data points represent the mean value +/− SEM.
Fig. 3. CD8 T cells in blood from monkeys before and during experimental SVV infection, after immunosuppression and during virus reactivation (zoster).
At times indicated, blood was obtained and processed as described in Materials and methods. Flow cytometric analysis was used to calculate numbers of naïve (CD28+, CD95−), memory (CD28+, CD95+) and effector (CD28−, CD95+) T cells (a and c) and the total CD4 T cells (b and d). Total CD8 T cell counts increased significantly in naïve, memory and effector populations 14 days post-infection (dpi) in all monkeys compared to those before infection (a and c; p<0.05). After immunosuppression, all T cell subsets immediately declined (a, p<0.05); at 176 dpi (two weeks before zoster), naïve and memory T cells increased (a, p<0.05). The relative percentages of CD8 memory and effector T cells remained similar to each other, while naïve cells increased during latency until zoster (b). CD8 T cells in the non-immunosuppressed monkey also declined after transportation to the radiation facility, but not to the extent seen in immunosuppressed monkeys (c). The relative percentages of CD8+ memory remained constant; while the percentage of naïve and effector cells fluctuated at 42 dpi, they were otherwise consistent throughout the study (d). Arrow indicates day of inoculation; black bar indicates time of immunosuppression. Data points represent the mean value +/− SEM.
Relative percentages of all CD4 T cell subsets remained constant in both immunosuppressed and non-immunosuppressed monkeys during latency and reactivation (Fig. 2b, d). The percentage of CD8 naïve T cells began to increase 5 weeks before reactivation in immunosuppressed monkeys, while memory and effector T cells declined. Percentages of all CD8 T cell populations in the non-immunosuppressed monkey remained constant before rash (Fig. 3b, d).
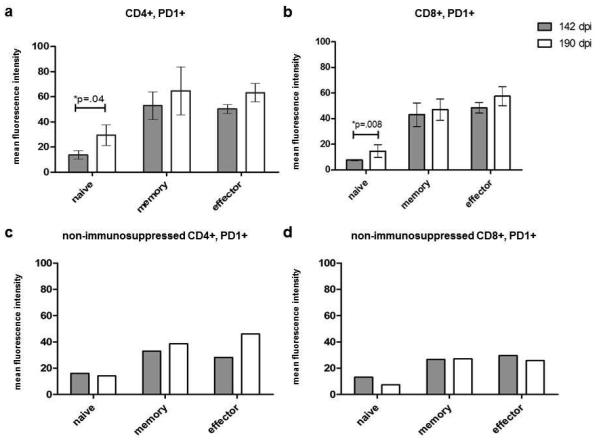
T cells examined before immunosuppression and at the time of reactivation were also evaluated by flow cytometry for expression of PD-1 (a marker of T cell exhaustion), a term used to describe T cells that are unresponsive to antigenic stimulation. At the time of zoster (190 dpi), expression of PD-1 increased in all CD4 T cell phenotypes compared to before immunosuppressive (142 dpi). This increase was statistically significant in CD4 naïve T cells (p=0.04, Fig. 4a). In comparison, PD-1 expression in the non-immunosuppressed monkey increased only in CD4 memory and effector cells (Fig. 4c).
Fig. 4. Expression of programmed death receptor (PD)-1 in blood of SVV-infected animals before immunosuppression and during reactivation (zoster).
As described in Materials and methods, PD-1 expression was determined by flow cytometry in both CD4 (a and c) and CD8 (b and d) T cells before immunosuppression (142 dpi) and 48 days later (190 dpi) when 2 monkeys developed zoster. PD-1 expression increased significantly in CD4 and CD8 naive T cell subsets at the time of zoster in immunosuppressed monkeys (p=0.04 and 0.008, respectively). In the non-immunosuppressed monkey that developed zoster after the stress of isolation and transportation (c and d), PD-1 expression increased in CD4 memory and effector cells (b) but not in other cell types (d). Data points represent the mean value +/− SEM.
PD-1 expression was also significantly increased in the CD8 naive T cells (p=0.008) in immunosuppressed monkeys (Fig. 4b) and only slightly in CD8 memory and effector populations. In contrast, PD-1 expression in the non-immunosuppressed monkey was decreased on CD8 naïve cells at reactivation compared to latency (142 dpi), while PD-1 expression on CD8 memory and effector cells did not differ 142 to 190 dpi (Fig. 4d). PD-1 expression at other times during immunosuppression could not be determined due to low absolute T cell numbers resulting from immunosuppression.
Discussion
At one week after primary SVV infection, the number of all CD4 and CD8 naïve, memory and effector T cells were significantly increased compared to before infection, indicative of a cell-mediated immune response. These findings support those of Messaoudi et al. (2009) which demonstrated an increase in SVV-specific T cells in rhesus macaques 7 days after SVV infection. Herein, SVV-specific T cell responses could not be determined because the number of T cells was too low due to immunosuppression.
Immunosuppression of latently infected monkeys resulted in a significant decrease in total T cell counts as seen in humans receiving chemotherapy (Locksley et al.1985; Bilgrami et al. 1999). The immunosuppression treatment used significantly decreased the total number of T cells; all subsets decreased indicating that it did not affect one type of T cell more than another. However, it is noted that the increase in the percentage of CD8 naïve T cells, and simultaneous decrease in CD8 effector cells, could be a result of immunosuppression and that a proper CD8 effector response is necessary to control reactivation. Importantly, two weeks before zoster, all T cell subsets increased in latently infected immunosuppressed monkeys, most likely in response to virus reactivation, a finding that might have been predicted based on the ability of humans to make a VZV-specific cell-mediated immune response when exposed to VZV (Hayward et al. 1991; Asano et al. 1994; Weinberg et al. 2009). It is not possible to predict when a person will develop zoster; thus there are no reports of T cell counts weeks before zoster in humans. However, VZV does reactivate before rash as evidenced by chronic radicular pain in the same dermatome in which rash eventually develops (Gilden et al. 1991).
Finally, VZV-specific T cells in humans decline with age, possibly due to functional T cell exhaustion with age or immunosuppression. When monkeys developed zoster, PD-1 expression was increased in all CD4 and CD8 T cells compared to latency. While the relationship of PD-1 expression to T cell function during VZV reactivation in humans is unknown, PD-1 expression on T cells is increased in humans chronically infected with HIV and hepatitis C virus (Day et al. 2006; D’Souza et al. 2007; Larrubia et al. 2009; Urbani et al. 2006) as well as in monkeys chronically infected with SIV (Finnefrock et al. 2009). PD-1 expression in SVV-specific T cells remains to be analyzed.
Acknowledgments
This work was supported by Public Health Service grants AG032958 (D.G.). Dr. James was supported by Training Grant NS007321 to Dr. Gilden from the National Institutes of Health and in part with federal funds from National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) through grant P51 RR001464 to the Tulane National Primate Research Center. We thank Marina Hoffman for editorial review and Lori DePriest for manuscript preparation.
Footnotes
Disclosure The authors report no conflicts of interest.
References
- Asano Y, Suga S, Yoshikawa T, Kobayashi I, Yazaki T, Shibata M, Tsuzuki K, Ito S. Experience and reason: twenty-year follow-up of protective immunity of the Oka strain live varicella vaccine. Pediatrics. 1994;94:524–526. [PubMed] [Google Scholar]
- Bilgrami S, Chakraborty NG, Rodriguez-Pinero F, Khan AM, Feingold JM, Bona RD, Edwards RL, Dorsky D, Clive J, Mukherji B, Tutschka PJ. Varicella zoster virus infection associated with high-dose chemotherapy and autologous stem-cell rescue. Bone Marrow Transplant. 1999;23:469–474. doi: 10.1038/sj.bmt.1701594. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker B. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- D'Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- Finnefrock AC, Tang A, Li F, Freed DC, Feng M, Cox KS, Sykes KJ, Guare JP, Miller MD, Olsen DB, Hazuda DJ, Shiver JW, Casimiro DR, Fu TM. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182:980–98. doi: 10.4049/jimmunol.182.2.980. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Dueland AN, Cohrs R, Martin JR, Kleinschmidt-DeMasters BK, Mahalingam R. Preherpetic neuralgia. Neurology. 1991;41:1215–1218. doi: 10.1212/wnl.41.8.1215. [DOI] [PubMed] [Google Scholar]
- Hayward A, Levin M, Wolf W, Angelova G, Gilden D. Varicella-zoster virus-specific immunity after herpes zoster. J Infect Dis. 1991;163:873–875. doi: 10.1093/infdis/163.4.873. [DOI] [PubMed] [Google Scholar]
- Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C. World J Gastroenterol. 2009;15:5129–5140. doi: 10.3748/wjg.15.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Flournoy N, Sullivan KM, Meyers JD. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172–1181. doi: 10.1093/infdis/152.6.1172. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Traina-Dorge V, Wellish M, Deharo E, Singletary ML, Ribka EP, Sanford R, Gilden D. Latent simian varicella virus reactivates in monkeys treated with tacrolimus with or without exposure to irradiation. J Neurovirol. 2010;16:342–354. doi: 10.3109/13550284.2010.513031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden D. Expression of protein encoded by varicella zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Barron A, Wellish M, Engelmann F, Legasse A, Planer S, Gilden D, Nikolich-Zugich J, Mahalingam R. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5(11):e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwendijk WJ, Mahalingam R, de Swart RL, Haagmans BL, van Amerongen G, Getu S, Gilden D, Osterhaus AD, Verjans GM. T-Cell tropism of simian varicella virus during primary infection. PLoS Pathog. 2013;9(5):e1003368. doi: 10.1371/journal.ppat.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schödel F, Morrison VA, Kauffman CA, Straus SE, Schmader KE, Davis LE, Levin MJ, US Department of Veterans Affairs (VA) Cooperative Studies Program Shingles Prevention Study Investigators Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]