Abstract
Although neuronal axons and dendrites with their associated filopodia and spines exhibit a profound cell polarity, the mechanism by which they develop is largely unknown. Here, we demonstrate that specific palmitoylated protein motifs, characterized by two adjacent cysteines and nearby basic residues, are sufficient to induce filopodial extensions in heterologous cells and to increase the number of filopodia and the branching of dendrites and axons in neurons. Such motifs are present at the N-terminus of GAP-43 and the C-terminus of paralemmin, two neuronal proteins implicated in cytoskeletal organization and filopodial outgrowth. Filopodia induction is blocked by mutations of the palmitoylated sites or by treatment with 2-bromopalmitate, an agent that inhibits protein palmitoylation. Moreover, overexpression of a constitutively active form of ARF6, a GTPase that regulates membrane cycling and dendritic branching reversed the effects of the acylated protein motifs. Filopodia induction by the specific palmitoylated motifs was also reduced upon overexpression of a dominant negative form of the GTPase cdc42. These results demonstrate that select dually lipidated protein motifs trigger changes in the development and growth of neuronal processes.
INTRODUCTION
Neurons possess an elaborate plasma membrane architecture that includes axons, dendrites, and synaptic sites (Da Silva and Dotti, 2002). Modulation of the plasma membrane shape and composition regulate processes outgrowth, axonal development, dendritic branching, and the construction of synapses (Jontes and Smith, 2000). These changes are regulated by interactions between the constituents of the plasma membrane, the exo/endocytic vesicles, and the cytoskeleton (Wood and Martin, 2002). In nonneuronal cells, differential modulation of membrane flow can result in the formation of processes such as microspikes, lamellopodia, and filopodia (Wood and Martin, 2002). However, factors that regulate the formation and maintenance of specific classes of membranous processes in neuronal cells remain poorly understood.
Dendritic filopodia, cell surface extensions filled with tight parallel bundles of actin filaments, are thought to be precursors for developing synapses (Dailey and Smith, 1996; Small, 1988). Filopodia are typically 5–35 μm in length and rapidly alter their position and shape. In developing axons, filopodia occur at the tips of growth cones and may aid the axon in finding its appropriate target. In dendrites, filopodia act as precursors to spines, short bulbous protrusions of the dendrite that form excitatory postsynaptic contacts on many neurons (Jontes and Smith, 2000). Thus, changes in membrane structure that result in the formation of filopodia are likely important in axonal guidance and spine formation. In contrast, dendritic branching is thought to be mediated via an actin and/or microtubule-dependent mechanisms (Jan and Jan, 2003). Recent studies showed that in early neuronal development dendrites originate from lamellopodia and short neurites (Jan and Jan, 2003). New branches are then formed through sprouting from an existing branch. Extensive remodeling of the newly formed branches results in the selective stabilization of a limited number of dendritic branches.
Several acylated proteins associated with the cytoskeleton, such as GAP-43 and paralemmin, are implicated in the regulation of membrane dynamics and process outgrowth (Strittmatter et al., 1994a; Resh, 1999; El-Husseini and Bredt, 2002). Studies on the neuronal proteins GAP-43 and paralemmin show that palmitoylation is important for localizing these proteins to filopodia (Zuber et al., 1989a, 1989b; Kutzleb et al., 1998). In contrast, dual palmitoylation of PSD-95 is necessary for appropriate postsynaptic localization to dendritic spines (Craven et al., 1999; El-Husseini et al., 2000; El-Husseini and Bredt, 2002). Overexpression studies showed that the palmitoylated motif of GAP-43 not only targets heterologous proteins to filopodia in neuron-like cells but can also induce filopodia formation (Strittmatter et al., 1994a, 1994b). Moreover, the dually prenylated and palmitoylated motif of paralemmin is essential for its morphogenic activity and concentration at filopodia and microspikes (Kutzleb et al., 1998). Thus, palmitoylation may serve as a signal for delivery of proteins involved in the regulation of cell morphology and membrane dynamics to specific active sites of the plasma membrane.
To test the possibility that specific palmitoylation motifs may be an intrinsic determinant of filopodia formation, we studied the effect of overexpression of various palmitoylation motifs on process outgrowth in COS-7 cells and in primary hippocampal neurons. Here, we find that select dually acylated motifs such as the ones present in GAP-43 and paralemmin can induce filopodia in both heterologous cells and neurons. Surprisingly, these palmitoylated motifs also enhance dendritic and axonal branching. In contrast, the palmitoylation motifs of PSD-95 and PSD-93 are incapable of triggering process outgrowth. We also show that a combination of two adjacent cysteine residues and basic amino acids within two residues of the palmitoylated cysteines are required for filopodia formation. Furthermore, filopodia induction can be reversed by either blocking on-going protein palmitoylation or by activating specific GTPases that regulate bulk membrane cycling and actin dynamics.
MATERIALS AND METHODS
cDNA Cloning and Mutagenesis
Generation of GW1 PSD-95, PSD-95 (C3,5S), PSD-95 (1–26) fused to GFP and mutations within the palmitoylation motifs of GAP-43, paralemmin and PSD-95 were described previously (Craven et al., 1999; El-Husseini et al., 2000). The addition of the GAP-43, NSP, and β2A N-terminal palmitoylation motif to PSD-95 were constructed with oligomers encoding the appropriate wild-type or mutated motifs and restriction sites that were annealed and subcloned into GW1 PSD-95-GFP at a HindIII site upstream of the starter methionine and a silent KpnI site at amino acid 13 of PSD-95. The C-terminal prenyl-palmitoylation motif of paralemmin was added to the C-terminus of PSD-95(C3,5S)-GFP or GFP alone with primers encoding the appropriate wild-type or mutated motif and restrictions sites. Paralemmin was obtained by RT-PCR from mouse brain RNA and subcloned into pEGFP (CLONTECH, Palo Alto, CA) at BglII and HindIII sites. Wild-type and mutant forms of ARF6 were kindly obtained from Dr. Julie G. Donaldson (National Institutes of Health, Bethesda, MD). Cdc42 constructs were obtained from the Guthrie Institute (Sayre, PA).
Primary Neuronal Culture and Transfection
Neuronal cultures were prepared from hippocampi of E18/E19 rats as described in Craven et al. (1999). In brief, hippocampi were dissociated by enzyme digestion with papain followed by brief mechanical trituration. Cells were plated on poly-d-lysine (Sigma, St. Louis, MO) treated glass coverslips (12 mm in diameter) and maintained in Neurobasal media (Life Technologies, Rockville, MD) supplemented with B27, penicillin, streptomycin, and l-glutamine as described in Brewer et al. (1993). Hippocampal cultures were transfected by lipid-mediated gene transfer. Briefly, 2 ìg of DNA and 8 μl of Enhancer (QIAGEN, Valencia, CA) were mixed in 150 μl of EC buffer (Qiagen) and let to stand for 5 min at room temperature (RT). Effectene, 15 μl (Qiagen), was added and incubated at RT for 10 min, followed by 1 ml of Neurobasal medium. The Effectene mix, 135 μl, was added to the cells with 200 μl of Neurobasal medium per well. Cells were incubated for 3 h at 37°C, and the Effectene reagent was replaced with fresh Neurobasal medium. To visualize transfected cells, coverslips were removed from the wells and mounted live onto slides (Frost Plus slides; Fisher Scientific, Pittsburg, PA) with Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL).
Immunofluorescence
Coverslips were removed from culture wells and fixed in 4°C paraformaldehyde for 10–15 min. The cells were washed with phosphate-buffered saline containing 0.1% Triton-X-100 (PBST). COS-7 cells were labeled for F-actin using a rhodamine- or Alexa 488-conjugated phalloidin label (Molecular Probes, Eugene, OR). Antibodies to GFP (Qbiogene, Carlsbad, CA), Cy3 (Jackson ImmunoResearch, West Grove, PA) and Alexa 488 (Molecular Probes) were used. Coverslips were incubated for 1 h at RT with primary antibodies, washed in PBST, and incubated for 1 h at RT with secondary antibodies. Coverslips were then mounted on slides (Frost Plus; Fisher Scientific) with Fluoromount-G (Southern Biotechnology Associates), and images were taken under fluorescence microscopy with a 63× objective affixed to a Zeiss inverted microscope (Thornwood, NY).
COS Cell Labeling and Immunoprecipitation
For labeling with [3H]palmitate, COS cells were labeled for 3 h in media containing 1 mCi/ml [3H]palmitic acid as previously described (El-Husseini et al., 2002). For pulse-chase experiments using [3H]palmitate, cells were incubated for variable times in media containing 100 μM palmitate. Labeled cells were washed with ice-cold PBS and resuspended in 0.1 ml lysis buffer containing TEE (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA), 150 mM NaCl, and 1% SDS. After extracting for 5 min at 4°C, Triton X-100 was added to 1% to neutralize the SDS in a final volume of 0.5 ml, and insoluble material was removed by centrifugation at 10,000 × g for 10 min. For immunoprecipitation, the samples were then incubated with GFP antibodies (anti-guinea pig, 1:1000 dilution) for 1 h at 4°C. After addition of 20 μl of protein A-Sepharose beads (Pharmacia, Piscataway, NJ), samples were incubated for 1 h at 4°C. Immunoprecipitates were washed three times with buffer containing TEE, 150 mM NaCl, and 1% Triton X-100, boiled in SDS-PAGE sample buffer with 1 mM DTT for 2 min, and analyzed by SDS-PAGE. For fluorography, protein samples were separated by SDS-PAGE and dried under vacuum. Gels were exposed to Kodak X-Omat MR (Eastman-Kodak, Rochester, NY) with intensifying screens at –80°C for 5–20 days.
Quantitative Measurement of Filopodia Induction in COS-7 Cells
Images were taken using a 63× objective affixed to a Zeiss inverted microscope and AxioVision software. Cells were scored according to the following criteria: COS-7 cells with a minimum of five filopodia measuring at least 10 μm or cells with 20 filopodia measuring at least 5 μm in length were scored as being “with filopodia.” All other cells were scored as being “without filopodia.” Changes were compared using one-way analysis of variance (ANOVA), and filopodia induction is expressed as the average number of COS-7 cells scored as being “with filopodia.” Error bars on graphs are SEM.
Quantitative Measurement of Dendritic Branching and Filopodia
Images were taken using a 63× objective affixed to a Zeiss inverted microscope and AxioVision software. For dendritic branching, all dendrites within a field of view were counted, including primary and secondary dendrites. All filopodia within the same field of view were counted manually and the dendritic length was measured using Northern Eclipse (Empix Imaging Inc., Mississauga, Ontario, Canada). Both branches and filopodia were counted by an observer blinded to the transfection type. Changes were compared using ANOVA, and filopodia density is expressed as the average number of filopodia per 100 μm. Error bars on graphs are SEM.
Online Supplementary Material
Video1 shows filopodia dynamics in a COS-7 cell transfected with the palmitoylation motif of GAP-43 (GAP 1–14). Video2 shows membrane ruffling in a COS-7 cell transfected with GAP 1–14 and after treatment with 2-bromopalmitate for 4 h. Video3 shows filopodia dynamics in a hippocampal neuron transfected with RFP. Video4 and Video5 show filopodia dynamics in hippocampal neurons transfected with GAP 1–14.
RESULTS
Differential Filopodia Induction by the Palmitoylation Motifs in Heterologous Cells
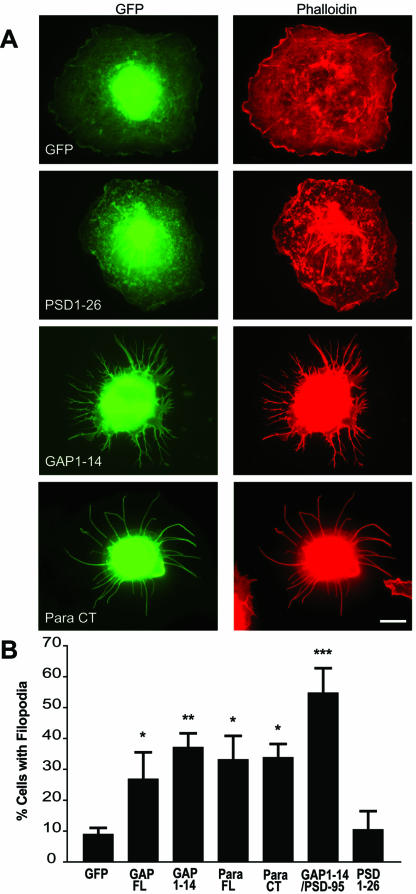
Previous studies have shown that the dually palmitoylated N-terminus of GAP-43 induces filopodia in heterologous cells (Strittmatter et al., 1994b). We first asked whether other dually acylated motifs induce the same effect. For this analysis, we used GFP fusion proteins that contain dually acylated motifs from various neuronal proteins, including the N-terminal palmitoylation motifs of GAP-43, PSD-95, and PSD-93 and the C-terminal motif of paralemmin. Filopodia induction was assessed according to criteria described in MATERIALS AND METHODS. As shown in Figure 1, we find that the palmitoylation motif of PSD-95 fused to GFP (PSD1–26) has no effect on the morphology of transfected cells. Filopodia extensions are found in only 11% of PSD1–26–expressing COS-7 cells, comparable to GFP alone (9%). In contrast, 39% of COS-7 cells transfected with the GAP-43 palmitoylation motif (GAP 1–14) show filopodia. Similarly, 35% of cells transfected with the dually palmitoylated and prenylated motif of paralemmin fused to GFP (Para CT) displays filopodia. Moreover, mutations of the palmitoylated cysteines in GAP-43 and paralemmin blocked the formation of filopodia mediated by these motifs (Table 1). The differential induction of filopodia by specific dually acylated motifs (i.e., GAP-43 and paralemmin, but not PSD-95 and PSD-93) suggests that palmitoylation is necessary but not sufficient to induce filopodia in heterologous cells. These results are surprising because filopodia induction was thought to be a unique property of the dually acylated motif present at the N-terminus of GAP-43 (Strittmatter et al., 1994b). This finding suggests that a larger number of acylated motifs may exhibit this property and that there may be a consensus sequence for filopodia induction
Figure 1.
Filopodia induction in COS-7 cells. (A) COS-7 cells were transiently transfected with various constructs fused to GFP (green) as described in Table 1 and immunolabeled for F-actin with a rhodamine-conjugated phalloidin antibody (red). COS-7 cells transfected with full-length GAP-43 (GAP FL) and full-length paralemmin (Para FL) show increased branching vs. control GFP-transfected cells. When transfected with palmitoylation motifs of GAP-43 (GAP1–14) or of paralemmin (Para CT), COS-7 cells show extensive filopodial outgrowth, compared with cells transfected with the palmitoyaltion motif of PSD-95-GFP (PSD1–26). Filopodia induction was quantified by counting the number of cells showing extensive filopodia outgrowth (at least 5 filopodia of ≥10 μm per cell, or at least 20 filopodia of ≥5 μm) and expressed as a percent of cells “with filopodia.” (B) A graph showing that filopodial induction by GAP1–14, Para FL, Para CT, and GAP1–14/PSD-95 are statistically different from GFP (*p < 0.05; **p < 0.01; ***p < 0.01). Scale bar, 10 μm.
Table 1.
Summary of the chimeric constructs used in this study
| Constructs | Palmitoylation motif | Filopodia induction |
|---|---|---|
| GAP-43 FL | MLCCMRRTKQVEKN | +++ |
| Paralemmin FL | -DMKKHRCKCCSIM | ++++ |
| PSD-95 FL | MDCLCIVTTKKYRY- | - |
| PSD-93 FL | MFFACYCALRTNVKK- | - |
| GAP-43 NT (GAP1-14) | MLCCMRRTKQVEKN- | ++++ |
| PSD-95 NT (PSD1-26) | MDCLCIVTTKKYRY- | - |
| PSD-93 NT (PSD1-64) | MFFACYCALRTNVKK- | - |
| β1a (1-11) | MQCCGLVHRRRY | ++ |
| NSP (416-427) | MLTCCCLWAFKT-KKYR- | ++ |
| Paralemmin CT (Para CT) | -DMKKHRCKCCSIM | ++++ |
| GAP-43 NT (GAP1-14 C3,4S) | MLSSMRRTKQVEKN- | - |
| Paralemmin FL (Para FL C334S) | -DMKKHRSKCCSIM | ++ |
| Paralemmin FL (Para FL C336S) | -DMKKHRCKSCSIM | - |
| Paralemmin FL (Para FL C334,336S) | -DMKKHRSKSCSIM | - |
| GAP-43 To PSD-95 like | ||
| PSD-93 NT/PSD-95 | MFFACYCALRTNVKK- | - |
| GAP-43 NT/PSD-95 | MLCCMRRTKQVEKN- | +++++ |
| GAP-43 NT (InsL4)/PSD-95 | MLCLCMRRTKQVEKN- | +++ |
| GAP-43 NT (R6,7I)/PSD-95 | MLCCMIITKQVEKN- | + |
| GAP-43 NT (C3L)/PSD-95 | MLLCMRRTKQVEKN- | +++ |
| GAP-43 NT (InsL4, R6,7I)/PSD-95 | MLCLCMIITKQVEKN- | - |
| PSD-95 to GAP-43 like | ||
| PSD-95 D2L, Del L4 | MLCCIVTKKYRY- | ++ |
| PSD-95 T8R | MDCLCIVRTKKYRY- | + |
| PSD-95 CCT8R | MLCCIVRTKKYRY- | ++++ |
All constructs are fused to GFP. GAP-43 FL: full-length GAP-43. Paralemmin FL: full-length paralemmin. PSD-95 FL: full-length PSD-95. PSD-93 FL: full-length PSD-93. GAP1-14: residues 1-14 of GAP-43. PSD1-26: residues 1-26 of PSD-95. PSD1-64: residues 1-64 of PSD-93. β2a (1-11): residues 1-11 of the β2a subunit of L-type calcium channel. NSP (416-427): residues 416-427 of the viral nonstructural protein NSP. Para CT: 13 residues from the C-terminus of paralemmin. GAP1-14 C3,4S: palmitoylation-deficient motif of GAP-43. Para FL C334S: Cys 334 Ser mutant full-length paralemmin. Para FL C336S: Cys 336 Ser mutant full-length paralemmin. Para FL C334,336S: Cys 334,336 Ser mutant full-length paralemmin. The following constructs were fused to PSD-95 FL: PSD-93 NT/PSD-95: palmitoylation motif of PSD-93; GAP-43 NT/PSD-95: palmitoylation motif of GAP-43 (GAP1-14); GAP-43 NT (InsL4)/PSD-95: GAP1-14 with a Leu inserted between cys3 and cys4; GAP-43 NT (R6,7I)/PSD-95: GAP1-14 with mutations of Arg 6 and Arg 7; GAP-43 NT (C3L)/PSD-95: Cys3Leu mutant GAP1-14; GAP-43 NT (InsL4, R6,7I)/PSD-95: GAP1-14 with an insertion of Leu at position 4 and mutations of Arg 6 and Arg 7 to Ileu. PSD-95 D2L, Del L4: full-length PSD-95 with Asp 2 mutated to Leu and deletion of Leu 4. PSD-95 T8R: full-length PSD-95 with a mutation of Thr 8 to Arg. PSD-95 CCT8R: full-length PSD-95 with a mutation of Asp 2 to Leu, Thr 8 to Arg and deletion of Leu 4. Constructs were scored from “-” to “++++” according to their relative ability to induce filopodia outgrowth, where “-” means a lack of filopodia inducing properties and “++++” shows maximal filopodia inducing properties. Palmitoylated cysteines are in bold and basic residues are underlined.
Sequence Requirement for Filopodia Induction by Palmitoylation Motifs: Adjacent Cysteines and Nearby Basic Amino Acids
To define the consensus sequence for filopodia induction, we examined the amino acid sequences surrounding specific acylated motifs. Previously, we showed that the palmitoylation motifs of GAP-43 and PSD-95 preferentially but not exclusively target chimeric proteins to axons and dendrites, respectively (El-Husseini et al., 2001). Systematic mutagenesis of these motifs showed that the spacing of the palmitoylated cysteines and the presence of adjacent basic residues determine the differential targeting by these motifs (El-Husseini et al., 2001). To test whether these features are also essential for filopodia induction, we assessed filopodial induction by a panel of chimeric and mutant constructs (Table 1). We first found that a chimeric protein containing the palmitoylation motif of GAP-43 fused to PSD-95 (GAP-43/PSD-95) robustly induces filopodia (Table 1). However, addition of a single amino acid between the contiguous cysteines of the GAP-43 palmitoylation motif fused to PSD-95 (GAP-43 NT (InsL4)/PSD-95), which maintains palmitoylation, reduces its ability to induce filopodia (Table 1). This suggests that the spacing of the cysteines is essential but not sufficient to induce filopodia extension. Consistent with this, palmitoylation motifs such as those present in the viral nonstructural protein NSP (Laakkonen et al., 1996) and the β2a subunit of L-type calcium channel (Chien et al., 1996), which contain two adjacent cysteines, can modestly increase filopodia density (see Table 1). It is important to note that the palmitoylation motif of GAP-43 induced filopodia outgrowth to a greater extent when fused to PSD-95. This is possibly due to a change in the palmitoylation motif conformation, which may have enhanced its accessibility for palmitoylation or its targeting to areas of active membrane rearrangement.
To address whether the two basic amino acids one residue away from the palmitoylated cysteines are important for filopodia induction, we replaced those basic amino acids with the hydrophobic residue Ile (GAP-43 NT (R6,7I)/PSD-95). These changes reduced the ability of the GAP-43 motif to induce filopodia (Table 1). Our results indicate that a combination of two adjacent acylated cysteines and two nearby basic residues are essential for the maximum morphogenic effects induced by the GAP-43 motif.
Next, we tested whether minimal sequence changes to PSD-95 that generate a combination of two adjacent acylated cysteines and a nearby basic residue were sufficient to induce filopodia. For this analysis, we used a PSD-95 mutant with no spacing between the cysteines (del L4) and a T8R mutation (CCT8R). Strikingly, this mutant form induced filopodia as observed with the GAP-43 and paralemmin acylated motifs (Table 1). Conversely, mutating the basic residues of the GAP-43 palmitoylation motif to hydrophobic ones and insertion of an amino acid between the palmitoylated cysteines (GAP43 NT (InsL4;R6,7I)/PSD-95) blocked its ability to induce filopodia. Moreover, mutating the palmitoylated cysteine adjacent to the prenylated one in paralemmin abolished its effects on filopodia induction (Table 1 and unpublished data). Thus, the presence of two adjacent cysteines that either modified by palmitate or by a combination of palmitoyl and prenyl groups can serve the same function on filopodia induction. These results demonstrate that the spacing between the two-acylated cysteines and nearby basic residues are two features critical for creating a filopodia-inducing motif (FIM).
FIMs Induce Filopodia and Enhance Dendritic and Axonal Branching in Neuronal Cells
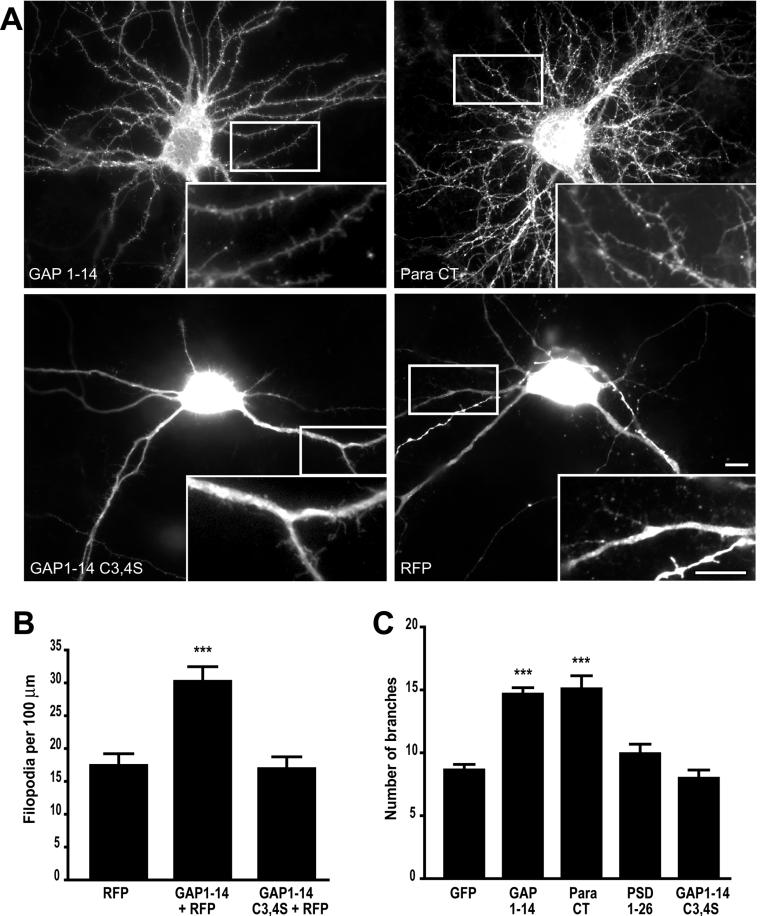
To further characterize the role of dually acylated motifs in the regulation of filopodia extension, we transfected a FIMs into primary cultured hippocampal neurons at 7 days in vitro (DIV 7), a stage at which neurons possess a relatively small number of filopodia and few synapses. We find that FIM transfection significantly increases the number of dendritic filopodia, which is similar to what was observed in COS cells. Hippocampal neurons showed a 79% increase in filopodia density when transfected with GAP1–14 vs. a palmitoylation deficient mutant motif, GAP1–14 (C3,4S) (Figure 2). Similarly, GAP1–14 transfected neurons show a 74% increase in filopodia density when compared with red fluorescent protein (RFP)-transfected cells (Figure 2). For this analysis we cotransfected cells with RFP, which shows a homogenous distribution in neuronal processes and permits visualization of filopodia in neuronal cells.
Figure 2.
Filopodia induction and branching in neuronal cells. (A) Cultured hippocampal neurons transfected at day in vitro 7 (DIV 7) with either the palmitoylation motif of GAP-43 (GAP 1–14), or paralemmin (Para CT) show extensive branching and high filopodial density at DIV 10. In contrast, neurons transfected with RFP alone or RFP together with either the isolated palmitoylation motif of PSD-95 fused to GFP (PSD 1–26) or the palmitoylation-deficient motif of GAP-43 fused to GFP (GAP C3,4S) show less branching and lower filopodial density. Higher magnifications of dendritic branches and filopodia are shown in insets. (B) Graph shows the average number of filopodia per 100 μm and graph. (C) Graph shows the extent of branching in tranfected neurons with various constructs (***p < 0.001). Scale bars, 10 μm.
Surprisingly, we find that FIMs not only increase the number of dendritic filopodia but also promote dendritic branching. Hippocampal neurons overexpressing the palmitoylation motifs of GAP-43 or paralemmin show a 71 and 76% increase in the number of dendritic branches, respectively (Figure 3). In contrast, neurons overexpressing GFP, PSD-95, or the palmitoylation-deficient motif of GAP1–14 (C3,4S) do not show altered dendritic branching (Figure 2). Similarly, mutating the palmitoylated cysteine at position 336 present at the C-terminus of full-length paralemmin abolishes the filopodia formation and dendritic branching induced by paralemmin (Figure S1). These changes in the density of filopodia and branches were not restricted only to dendrites. Axons from neurons overexpressing a FIM also showed enhanced filopodia density and axonal branching, compared with GFP-transfected neurons (Figure S2).
Figure 3.
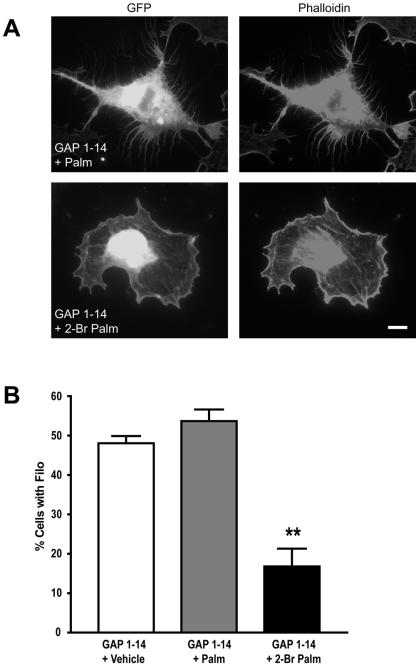
On-going palmitoylation of FIMs regulates filopodia formation in COS-7 cells. (A) COS-7 cells transfected with the GAP-43 palmitoylation motif and treated with 50 μM 2-bromopalmitate (24 h posttransfection), which blocks protein palmitoylation, show a significant decrease in filopodia outgrowth. (B) Graph shows the percentage of cells expressing filopodia for the different treatments; untreated and palmitate-treated cells are statistically different from 2-bromopalmitate-treated cells (**p < 0.01; ***p < 0.001). Scale bar, 10 μm.
Filopodia Induction Is Palmitoylation Dependent and Is Reversible in Heterologous Cells and Neurons
To test whether the palmitoylation of FIMs is dynamic, we metabolically labeled COS cells transfected with various GFP fusion proteins using [3H]palmitate as previously described (El-Husseini et al., 2002). We find that the half-life of palmitate on PSD-95 and FIMs is ∼2–4 h (see supplementary information, Figure S3). To further analyze the role of palmitoylation in filopodia extension, we acutely blocked palmitoylation with 2-bromopalmitate (Coleman et al., 1992; Webb et al., 2000). Treatment with 20 μM 2-bromopalmitate for 6–8 h immediately after transfection inhibits filopodia induced by FIMs in COS cells (unpublished data). Because palmitoylation and filopodia formation are reversible processes (Milligan et al., 1995; Ross, 1995; Mumby, 1997), next we asked whether protein palmitoylation is required for the maintenance of filopodia. To test this, we treated transfected COS-7 cells that had already developed filopodial extensions (24 h posttransfection) with 2-bromopalmitate or palmitate. We find that treatment with 2-bromopalmitate caused a significant decrease in the number of cells with filopodia, from 48 to 18% after 8 h of treatment. In contrast, treatment with palmitate did not significantly change the total number of cells with filopodia (see Figure 3; Video1 and Video2).
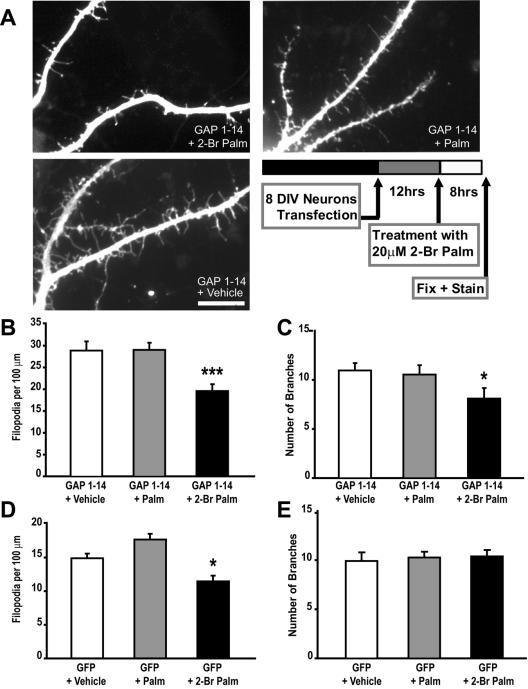
Next, we examined whether FIM-induced filopodia is also reversible in neuronal cells. When compared with untreated or palmitate-treated cells, hippocampal neurons treated with 20 μM 2-bromopalmitate 12 h posttransfection with FIMs showed a 26% decrease in filopodia number (Figure 4, A and B). In contrast, a slight reduction in the number of branches was observed upon 2-bromopalmitate treatment (Figure 4C). These data indicate that palmitoylation is also necessary for maintaining the newly formed neuronal filopodia induced by FIMs.
Figure 4.
Filopodia induction is palmitoylation-dependent and partly reversible in neuronal cells. Treating hippocampal neurons expressing the palmitoylation motif of GAP-43 (GAP 1–14) with 20 μM 2-bromopalmitate for 8 hs significantly reduces filopodia outgrowth. (A) Cells were transfected at DIV 8 and treatments with vehicle, palmitate (Palm) or 2-bromopalmitate (2-Br-Palm) were done 12 h posttransfection. (B) Graph shows the average number of filopodia per 100 μm for the different treatments; vehicle and palmitate-treated cells (GAP 1–14 + Palm) are statistically different from 2-bromopalmitate-treated cells (GAP 1–14 + 2-Br Palm; ***p < 0.001). (C) Graph shows the average number of branches for the different treatments; 2-bromopalmitate–treated cells are slightly but significantly different from vehicle and palmitate treated cells (*p < 0.05). (D) The total number of dendritic filopodia is reduced in GFP transfected neurons upon treatment with 2-Br-Palm treatment but not with Palm or Vehicle. (E) No significant change in dendritic branching was observed in GFP-transfected neurons upon treatment with 2-Br-Palm. Scale bar, 10 μm.
To determine whether palmitoylation is involved in the formation and maintenance of intrinsic dendritic filopodia that are normally formed during early neuronal development, we examined whether treatment with 2-bromopalmitate modulates the total number of filopodia number in GFP-expressing neurons. Indeed, our results show a small but significant decrease in the total filopodia density compared with palmitate-treated or untreated cells (Figure 4D). In contrast, no change in dendritic branching was observed upon acute treatment with 2-bromopalmitate (Figure 4E). These findings indicate that filopodia induction and maintenance in neuronal cells partially involves ongoing protein palmitoylation.
The ADP-Ribosylation Factor (ARF6) Regulates Processes Outgrowth Mediated by Palmitoylated Motifs
Previous studies showed that plasma membrane recycling and reinsertion at defined plasma membrane sites can play a role in cortical actin rearrangements (Song et al., 1998). A candidate protein for the regulation of this process is ARF6, a non-Rho family GTPase that regulates an endosomalplasma membrane cycling compartment and influences cortical actin remodeling. In neurons, recent studies showed that dendritic branching can be regulated by an ARF6-dependent pathway (Hernandez-Deviez et al., 2002). Here we tested whether the changes in membrane dynamics induced by the palmitoylated motifs can be regulated by ARF6.
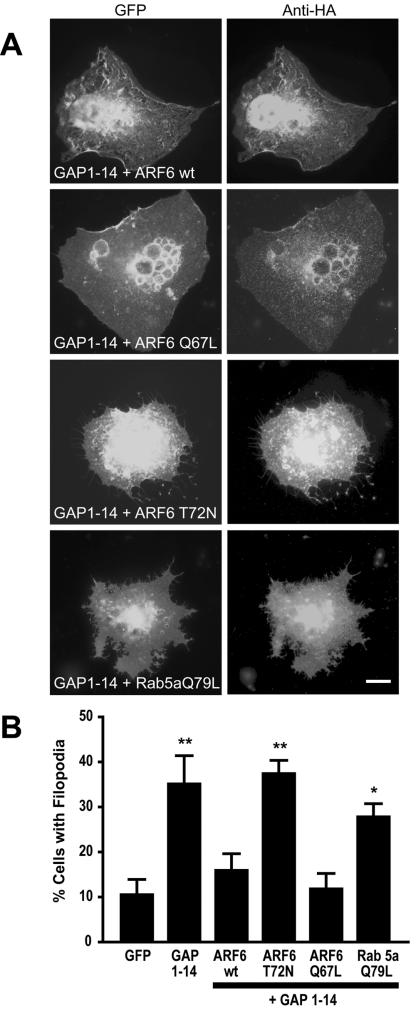
This was first assessed in COS cells by coexpression of a FIM and an HA-tagged ARF6 construct. When compared with cells expressing a FIM alone, we observed a 55% reduction in the number of COS-7 cells with filopodia when cotransfected with the FIM of GAP-43 and wild-type ARF6 (Figure 5). Cotransfection with a constitutively active form of ARF6 (ARF6-Q67L) resulted in a 67% decrease in the number of cells with filopodia. On the other hand, COS-7 cells cotransfected with a dominant negative form of ARF6 (ARF6-T27N) and FIM show no significant change in the number of cells containing filopodia. These results indicate that activation of ARF6 may act as a negative regulator of filopodia induction mediated by the FIM in heterologous cells. To test whether disruption of the conventional endocytic pathway may also block filopodia induced by FIMs, we assessed the effects of overexpression of a constitutively active form of Rab5 (Q79L). Rab5 regulates trafficking of endocytosed membranes in a pathway independent of ARF6. We find that expression of Rab5 (Q79L) had no effect on the filopodia induced by FIM (see Figure 5). Thus, cortical actin rearrangements associated with bulk membrane cycling rather than membrane endocytosis appear to be important for this process.
Figure 5.
ARF6 regulates FIM-induced filopodia extension in COS-7 cells. (A) COS-7 cells cotransfected with the palmitoylation motif of GAP-43 and either wild-type ARF6 (ARF6wt), constitutively active form of ARF6 (ARF6-Q67L), dominant negative ARF6 (ARF6-T72N), or constitutively active Rab5a (Rab5a-Q79L). (B) Filopodia induction is quantified as the percentage of cells cotransfected showing filopodial outgrowth (*p < 0.05; **p < 0.01). Scale bar, 10 μm.
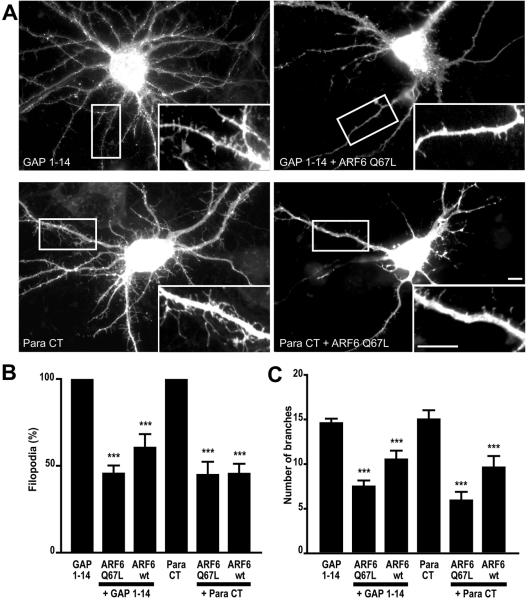
To test whether a similar mechanism may regulate filopodia formation and dendritic branching in neuronal cells, neurons were transfected with FIMs in the presence of HA-tagged, wild-type ARF6 or a constitutively active form of ARF6 (ARF6-Q67L). We find that coexpression of wild-type or ARF6-Q67L with the FIM of GAP43 or paralemmin resulted in a significant decrease in the average number of filopodia and dendritic branches (Figure 6). Neurons cotransfected with the GAP-43-FIM motif and with ARF6-Q67L showed a 54% decrease in filopodia number and a 49% decrease in the number of dendritic branches. Similarly, neuronal cells cotransfected with the paralemmin-FIM and ARF6-Q67L showed a 55% decrease in filopodial extenstions and 61% decrease in the number of dendritic branches. These results indicate that the small GTPase ARF6 contributes to the regulation of dendritic branching and filopodia extension induced by FIMs in neuronal cells.
Figure 6.
ARF6 regulation of dendritic branching and filopodia extension in neuronal cells. (A) Hippocampal neurons were cotransfected with the palmitoylation motif of GAP-43 (GAP 1–14) or the palmitoylation motif of paralemmin (Para CT) and either with wild-type ARF6 (ARF6wt) or with a constitutively active form of ARF6 (ARF6-Q67L). (B) Graph shows the relative filopodia density; data are expressed as a percentage of GAP1–14- or Para CT-transfected cells. (C) Graph shows the extent of branching in transfected neurons. (***p < 0.001). Scale bars, 10 μm.
Filopodia Induction by FIMs Is Also Modulated by the GTPase cdc42
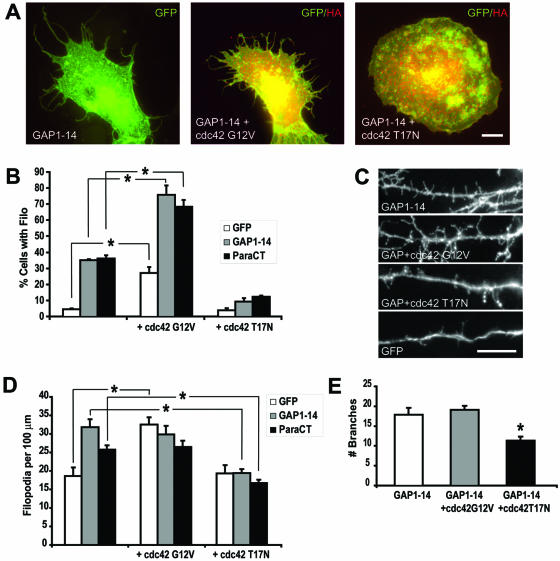
Filopodia extension in heterologous cells is regulated by a variety of proteins associated with the actin cytoskeleton, including members of the Rho family of GTPases (Hall, 1998). In particular, cdc42 has been shown to actively participate in filopodia extension. To assess whether cdc42 is involved in the palmitoylation-dependent induction of filopodia outgrowth mediated by FIMs, we coexpressed the GFP-tagged FIMs with either HA-tagged, wild-type or mutant forms of cdc42 in COS-7 cells. Overexpression of a constitutively active form of cdc42 (cdc42 G12V) resulted in about a fivefold increase in the number of cells with filopodia (Figure 7). Cotransfection of cdc42 G12V with the FIM of GAP-43 or paralemmin further increased the number of cells with filopodia, compared with GFP transfected cells (Figure 7, A and B). In contrast, the number of cells with filopodia was significantly decreased upon cotransfection of FIMs with a dominant negative form of cdc42 (cdc42 T17N) when compared with those expressing FIM alone. These data demonstrate that filopodia induced by FIMs can be modulated by cdc42. Moreover, the cooperative effects of FIMs and cdc42 on filopodial outgrowth suggest that cdc42 may act downstream of FIMs.
Figure 7.
Regulation of filopodia extension and dendritic branching by cdc42. (A) COS-7 cells were transfected with GFP, the palmitoylation motif of GAP-43 (GAP1–14) or the palmitoylation motif of paralemmin (ParaCT) and with either a constitutively active cdc42 (Cdc42 G12V) or a dominant negative cdc42 (Cdc42 T17N). Coexpression of GFP, GAP1–14, or ParaCT with Cdc42 G12V increased the number of cells with filopodia, whereas coexpression with Cdc42 T17N blocked filopodia induced by GAP1–14 and ParaCT. (B) Percentage of COS cells expressing filopodia for the different transfections (*p < 0.05). (C) Hippocampal neurons were cotransfected with GAP1–14 or GFP and either with Cdc42 G12V or Cdc42 T17N. (D) Graph shows the filopodia density for the various transfections. (E) Graph shows the extent of branching in transfected neurons. (*p < 0.05). Scale bars, 10 μm.
Next, we examined whether a similar mechanism regulates filopodia extension in neuronal cells. When compared with GFP transfected cells, neurons expressing a constitutively active cdc42 show a 74% increase in filopodia density (Figure 7, C and D). However, FIM-expressing neurons did not show a further increase in filopodia density when cotransfected with cdc42 G12V. These results suggest that a mechanism of homeostatic regulation may limit the increase in filopodia density. Remarkably, when cotransfected with a dominant negative cdc42, FIM expressing neurons show a 39% decrease in filopodia density and a 34% decrease in the number of dendritic branches (Figure 7, C–E). Similarly, GFP-expressing cells show a decrease in dendritic branching when cotransfected with cdc42 T17N (unpublished data). Taken together, these data indicate that cdc42 participates in the extension and maintenance of FIM-induced neuronal process outgrowth.
Cdc42-induced Filopodia Involves a Palmitoylation-dependent Pathway
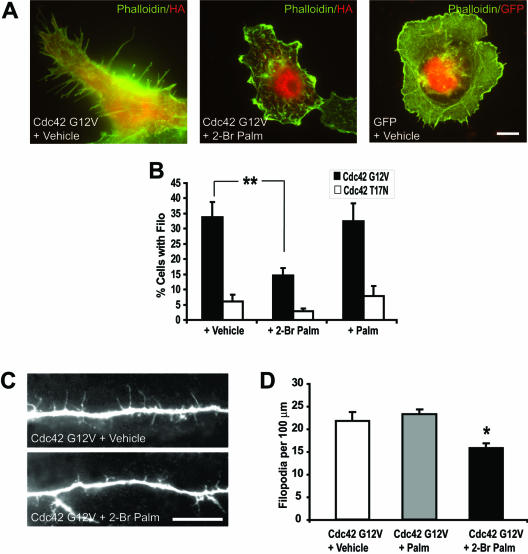
To determine whether filopodia induction mediated by cdc42 requires protein palmitoylation, we treated COS cells transfected with cdc42 G12V, 12 h posttransfection, with 20 μM 2-bromopalmitate or palmitate. We find that 8 h treatment with 2-bromopalmitate, but not with palmitate or vehicle, eliminated filopodia induced by cdc42 G12V (Figure 8, A and B). These results show that filopodia formation by cdc42 in heterologus cells involves a palmitoylation-dependent pathway. To assess whether cdc42-mediated filopodia outgrowth also relies on palmitoylation in hippocampal neurons, we treated cells with 20 μM 2-bromopalmitate 12 h posttransfection. Significantly, neurons transfected with cdc42 G12V showed a 23% decrease in the filopodia density when treated with 2-bromopalmitate compared with untreated or cells treated with palmitate (Figure 8, C and D). These results demonstrate that filopodia extension in neuronal cells is in part dependent on palmitoylation. Furthermore, on-going palmitoylation may participate in the regulation of neuronal processes induced by cdc42.
Figure 8.
Palmitoylation modulates cdc42 induced filopodia extension and dendritic branching. (A) COS-7 cells were transfected with either a constitutively active or inactive cdc42 (Cdc42 G12V or Cdc42 T17N, respectively). Twelve hours posttransfection, cells were treated with either 20 μM palmitate (Palm) or 2-bromopalmitate (2-Br Palm) for 8 h. Results show a significant decrease in the number of cells with filopodia outgrowth compared with vehicle- or palmitate-treated cells. (B) Percentage of cells expressing filopodia for the different treatments; untreated and palmitate-treated cells are statistically different from 2-bromopalmitate–treated cells (**p < 0.01). (C) Images of hippocampal neurons illustrate changes in the density of dendritic filopodia in cells upon transfection with Cdc42 G12V and treatment with 20 μM 2-bromopalmitate or vehicle 12 h posttransfection. (D) Data show a significant decrease in filopodia density after treatment with 2-Br Palm. (*p < 0.05). Scale bars, 10 μm.
DISCUSSION
Recent investigations have revealed several important roles for palmitoylation in protein sorting and targeting to specific cellular compartments. In neuronal cells, palmitoylation serves as a signal for differential sorting of proteins to axons or dendrites and for protein association with specialized lipid microdomains and synaptic membranes (El-Husseini et al., 2001). Here, we define a palmitoylated FIM sufficient to induce filopodia formation in heterologous cells and to increase the number of filopodia and dendritic branches in neurons. We show that the morphological changes induced by this FIM require on-going protein palmitoylation and can be reversed by specific GTPases that regulate bulk membrane cycling and actin dynamics.
Minimal Sequence Required for Induction of Process Outgrowth
The sequence requirement for FIMs strictly relies on the presence of two adjacent acylated cysteines and nearby basic residues. Palmitoylation motifs such as those present in PSD-95 and PSD-93 lack this consensus sequence and fail to induce filopodia. Importantly, the palmitoylation motif of PSD-95 can be converted to a GAP-43 like motif and vice versa by minimal amino acid changes (Table 1). Moreover, similar alterations in the FIM present in paralemmin disrupt its ability to induce filopodia. Because of the degeneracy in the sequence requirements, we predict that FIMs are present in several other neuronal proteins involved in the regulation of cytoskeleton dynamics and processes outgrowth.
Possible Mechanisms for Induction of Process Outgrowth by FIMs
The morphogenic effects of GAP-43 and paralemmin are highly dependent on protein palmitoylation. Our analysis shows that palmitate turnover on GAP-43, paralemmin, and PSD-95 are relatively similar and with a half-life of 2–4 h. Consistent with this, treatment with 2-bromopalmitate, a drug that blocks protein repalmitoylation, significantly disrupts filopodia induction by FIM motifs only 6–8 h after treatment with this drug. Shorter exposures had no effects on the number of FIM-induced filopodia (unpublished data). Moreover, mutating the palmitoylated cysteines of GAP-43 or paralemmin block their ability to induce filopodia (Kutzleb et al., 1998; Aarts et al., 1999). Thus, the presence of FIMs in proteins such as GAP-43 and paralemmin that regulate process outgrowth strongly suggest functional relevance. Previous studies indicate that the first 10 amino acids of GAP-43 are sufficient to induce filopodia and to stimulate Go (Strittmatter et al., 1990; Sudo et al., 1992; Arni et al., 1998). Surprisingly, synthetic di-palmitoylated peptides, which include the N-terminal sequences of GAP-43 abolished Go activity (Strittmatter et al., 1994a). The authors concluded that palmitoylation controls a cycle of GAP-43 between an acylated, membrane associated reservoir of inactive GAP-43 and a depalmitoylated active form of the protein. However, our data show that depleting the pool of palmitoylated protein by 2-bromopalmitate treatment abolishes the effects of FIMs indicating that modification by palmitate mediates the morphogenic effects we observe.
How do specific acylated motifs reorganize the cytoskeleton and generate process outgrowth? We previously showed that the GAP-43 and the paralemmin acylation motifs are efficiently sorted to lipid rafts whereas motifs like PSD-95 lacking the FIM consensus sequence are not (El-Husseini et al., 2001). Lipid rafts are specialized plasma membrane microdomains enriched in sphingolipids that aggregate with cholesterol to form packed raft-like domains within the fluid membrane bilayer. Others recently reported that protein acylation confers localization to cholesterol and sphingolipid-enriched membranes (McCabe and Berthiaume, 2001). In addition, the presence of nearby basic residues has been proposed to stabilize interactions with the negatively charged phospholipids present at the plasma membrane (Resh, 1999). Thus, incorporation into lipid rafts and strong association with the plasma membrane through interactions with positively charged residues may contribute to the regulation of process outgrowth. A possible mechanism for the observed changes in the cell morphology mediated by FIMs is that the incorporation of these lipidated motifs into lipid rafts may create local changes in membrane tension and the extension of filopodia-like structures. Previous studies showed that alteration in the concentration of specific lipids alter membrane dynamics and fluidity. For example, addition of sphingomyelin or phosphatidyl ethanolamine analogs, lipids that expand the plasma membrane, increase the rate of cell spreading and lamellipodia extension and cause a decrease in membrane tension (Harel and Futerman, 1993; Bershadsky and Futerman, 1994; Furuya et al., 1995; Schwarz et al., 1995). It is possible that a similar mechanism is involved whereby the increased rate of addition of palmitate to specific plasma membrane microdomains stimulates process outgrowth by a physical alteration of membrane tension. Alternatively, a change in membrane tension and expansion may stimulate the activation of elements critical for recruitment and anchoring of specific proteins associated with filopodia extension at the plasma membrane.
Filopodia outgrowth is also dependent on proper assembly of signaling proteins that facilitate interaction between receptors and specific downstream signaling components important for the regulation of cytoskeletal dynamics (Meyer and Feldman, 2002). Alteration of protein complex assembly may have major consequences in regulating cell morphology. Under normal conditions the formation of filopodia in many cell types is triggered by the binding of the active GTP bound form of Cdc42 along with phosphatidyl inositol 4,5 bisphosphate (PIP2) to the Wiskott-Aldrich syndrome protein (WASP) family of proteins and the recruitment of profilin and Arp2/3 (actin-related proteins 2 and 3), proteins that regulate nucleation of actin filaments (Miki et al., 1998; Rohatgi et al., 1999; Yarar et al., 1999). The dynamic protrusion and retraction of filopodia is dependent on the regulation of actin binding and capping proteins (Rao et al., 2000). Although one cannot rule out that a direct interaction between FIMs and elements important for regulating cytoskeletal dynamics may trigger changes in cell morphology, the high degeneracy of FIM s makes this possibility less likely.
Regulation of Filopodia Formation and Dendritic Branching by the GTPases ARF6 and cdc42
ARF6, a non-Rho family GTPase regulates an endosomal plasma membrane recycling pathway and influences cortical actin remodeling independent of Rho, Rac, and cdc42 activity (Radhakrishna and Donaldson, 1997; Song et al., 1998). In neuronal cells, dendritic branching is regulated by an ARF6-dependent pathway (Song et al., 1998; Hernandez-Deviez et al., 2002). These findings are consistent with our results that AFR6 plays a critical role in regulating FIM-stimulated process outgrowth and dendritic branching (Figure 5). We find that overexpression of wild-type or constitutively active ARF6 (Q67L) reverses the effects of FIMs both in COS-7 and neuronal cells. In contrast, expression of a constitutively active Rab5 (Q79L), a GTPase that regulate early endosome trafficking in a pathway independent from ARF6, did not affect filopodia induced by FIMs. These results indicate that cytoskeletal rearrangements and membrane dynamics associated with bulk membrane cycling rather than classical membrane endocytosis may contribute to the effects induced by FIMs. Our findings also suggest that ARF6 may act as a negative regulator of filopodia induction mediated by FIMs. The ability of ARF6 to reverse the effects of FIMs in various cell types suggests that a common signaling pathway is involved in this process.
Cdc42 regulates actin dynamics and the formation of filopodia in neuronal and nonneuronal cells (Nobes and Hall, 1995; Hall, 1998). In this study, we define a novel mechanism by which filopdia induction by cdc42 also involves protein palmitoylation. Cdc42 has been shown to be prenylated at cys188, but it is unknown whether this protein is also palmitoylated (Zhang and Casey, 1996). Whether or not cdc42 is palmitoylated, our new findings indicate that palmitoylation plays a role in the regulation of filopodia formation and maintenance induced by cdc42.
Dendritic morphogenesis depends on many factors; for example, several proteins known to regulate microtubule transport or stability have been shown to affect dendritic morphology. MAP2, the microtubule-associated protein is a major component of cross-bridges between the microtubules and is known to stabilize them (Harada et al., 2002). Furthermore, the Rho-family of GTPases play important roles in controlling dendritic morphology (Cline, 2001; Redmond and Ghosh, 2001). Thus, coordinated changes in the assembly of microtubules and actin filaments are important for dendritic morphogenesis. Here we demonstrate that FIM-induced dendritic branching also involves a cdc42-dependent pathway. Although blocking on-going protein palmitoylation by 2-bromo palmitate dramatically reduced the effects of FIMs and cdc42 on process outgrowth in COS cells, these effects were less dramatic in neuronal cells. The smaller change in the number dendritic filopodia is possibly due to differences in the turnover rate of palmitate on various acylated proteins involved in the regulation of process outgrowth and remodeling in neurons.
Possible Roles in Synaptogeneis
Synaptogenesis is a rapid process and appears to rely on the recruitment of synaptic protein complexes to developing dendritic filopodia soon after they are contacted by an axon (Craig et al., 1993; Anderson et al., 1995; Fiala et al., 1998; Zhang and Benson, 2000; Prange and Murphy, 2001). One model for synapse formation predicts that active dendritic filopodia contact axons to induce presynaptic boutons, followed by a period of filopodial maturation into postsynaptic spines (Harris et al., 1992; Ziv and Smith, 1996; Maletic-Savatic et al., 1999; Rao and Craig, 2000). It remains to be clarified whether the increase in filopodia induced by FIMs actually translates into enhanced spine formation and participates in synapse formation.
The enhanced dendritic and axonal branching mediated by FIMs increase the total surface area available for synapse formation. FIMs are present in GAP-43 and paralemmin, two proteins implicated in process outgrowth and synapse formation. GAP-43 is involved in signals that regulate cytoskeletal organization in the nerve ending (Benowitz and Routtenberg, 1997) and GAP-43–deficient mice exhibit restricted axonal pathfinding defects (Strittmatter et al., 1995; Sretavan and Kruger, 1998). Moreover, its expression is dramatically enhanced during neuronal development and after nerve injury (Skene and Willard, 1981; Karns et al., 1987). On the other hand, paralemmin is enriched both in axons and dendrites as well as at pre- and postsynaptic sites (Kutzleb et al., 1998). Interestingly, the expression of a specific isoform of paralemmin is significantly enhanced during a period that correlates with synapse formation (Kutzleb et al., 1998). Also, our data show that overexpression of full-length or the C-terminal palmitoylation motif of paralemmin enhanced the number of filopodia. We propose that in the context of the full protein, FIMs may cooperate with other regulatory domains present in these proteins to coordinate membrane flow and cytoskeleton rearrangements important for process outgrowth.
The reshaping of synaptic contacts is an active process that continues throughout the life of a neuron. Indeed, high levels of expression of proteins containing the FIMs such as GAP-43 and paralemmin persist in the adult nervous system, in regions that have been associated with high plasticity (Routtenberg, 1987; Kutzleb et al., 1998). Recent data show a dramatic decrease in GAP-43 palmitoylation during early neuronal development, suggesting that protein acylation may serve as a dynamic regulator of axonal extension and synapse formation (Patterson and Skene, 1999). Moreover, blocking protein palmitoylation by cerulenin disrupts the morphogenic effects induced by GAP-43 (DeJesus and Bizzozero, 2002). Consistent with these findings, our results show that inhibition of protein palmitoylation by 2-bromopalmitate, blocks filopodia formation induced by FIMs. Thus, regulated protein palmitoylation may also act as a signal for modulating membrane dynamics and protein interactions associated with structural plasticity of synaptic contacts. The reversible nature of protein palmitoylation makes it a good candidate for regulating the dynamic changes within the cell. Our preliminary data indicate that the increase in the number of filopodia induced by FIMs in young neurons correlates with an increase in the number of spine-like structures (unpublished results). Thus, on-going palmitoylation may be necessary for maintaining existing filopodia and for their transformation to spines.
Supplementary Material
Acknowledgments
We thank Alex Trinh and Andrew Barbod for their assistance in neuronal cell culture preparation and transfection. This work was supported by grants from the Canadian Institutes for Health Research (T.H.M., MOP49586; A.E.H., MOP44052), the Michael Smith Foundation for Health Research and the National Association for Research on Schizophrenia and Depression. A.E.H. is a CIHR New investigator and an MSFHR scholar. T.H.M. is a CIHR investigator and MSFHR senior scholar. D.S.B. is an established investigator of the American Heart Association and is supported by grants from the National Institutes of Health and the Human Frontier Science Program.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–07–0493. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–07–0493.
Abbreviations used: ARF6, ADP-ribosylation factor 6; GAP-43, growth-associated protein 43; EGFP, enhanced green fluorescent protein; PSD-95, postsynaptic density 95; DIV, days in vitro.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Aarts, L.H., Verkade, P., van Dalen, J.J., van Rozen, A.J., Gispen, W.H., Schrama, L.H., and Schotman, P. (1999). B-50/GAP-43 potentiates cytoskeletal reorganization in raft domains. Mol. Cell Neurosci. 14, 85–97. [DOI] [PubMed] [Google Scholar]
- Anderson, S.A., Classey, J.D., Conde, F., Lund, J.S., and Lewis, D.A. (1995). Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience 67, 7–22. [DOI] [PubMed] [Google Scholar]
- Arni, S., Keilbaugh, S.A., Ostermeyer, A.G., and Brown, D.A. (1998). Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J. Biol. Chem. 273, 28478–28485. [DOI] [PubMed] [Google Scholar]
- Benowitz, L.I., and Routtenberg, A. (1997). GAP-43, an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 20, 84–91. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A.D., and Futerman, A.H. (1994). Disruption of the Golgi apparatus by brefeldin A blocks cell polarization and inhibits directed cell migration. Proc. Natl. Acad. Sci. USA 91, 5686–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, G.J., Torricelli, J.R., Evege, E.K., and Price, P.J. (1993). Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35, 567–576. [DOI] [PubMed] [Google Scholar]
- Chien, A.J., Carr, K.M., Shirokov, R.E., Rios, E., and Hosey, M.M. (1996). Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J. Biol. Chem. 271, 26465–26468. [DOI] [PubMed] [Google Scholar]
- Cline, H.T. (2001). Dendritic arbor development and synaptogenesis. Curr. Opin. Neurobiol. 11, 118–126. [DOI] [PubMed] [Google Scholar]
- Coleman, R.A., Rao, P., Fogelsong, R.J., and Bardes, E.S. (1992). 2-Bromopalmitoyl-CoA and 2-bromopalmitate: promiscuous inhibitors of membrane-bound enzymes. Biochim. Biophys. Acta 1125, 203–209. [DOI] [PubMed] [Google Scholar]
- Craig, A.M., Blackstone, C.D., Huganir, R.L., and Banker, G. (1993). The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron 10, 1055–1068. [DOI] [PubMed] [Google Scholar]
- Craven, S.E., El-Husseini, A.E., and Bredt, D.S. (1999). Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron 22, 497–509. [DOI] [PubMed] [Google Scholar]
- Da Silva, J.S., and Dotti, C.G. (2002). Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 3, PG-694–704. [DOI] [PubMed] [Google Scholar]
- Dailey, M.E., and Smith, S.J. (1996). The dynamics of dendritic structure in developing hippocampal slices. J. Neurosci. 16, 2983–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus, G., and Bizzozero, O.A. (2002). Effect of 2-fluoropalmitate, cerulenin and tunicamycin on the palmitoylation and intracellular translocation of myelin proteolipid protein. Neurochem. Res. 27, 1669–1675. [DOI] [PubMed] [Google Scholar]
- El-Husseini, A.E., and Bredt, D.S. (2002). Protein palmitoylation: a regulator of neuronal development and function. Nat. Rev. Neurosci. 3, 791–802. [DOI] [PubMed] [Google Scholar]
- El-Husseini, A.E., Craven, S.E., Brock, S.C., and Bredt, D.S. (2001). Polarized targeting of peripheral membrane proteins in neurons. J. Biol. Chem. 276, 44984–44992. [DOI] [PubMed] [Google Scholar]
- El-Husseini, A.E. et al. (2002). Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108, 849–863. [DOI] [PubMed] [Google Scholar]
- El-Husseini, A.E., Craven, S.E., Chetkovich, D.M., Firestein, B.L., Schnell, E., Aoki, C., and Bredt, D.S. (2000). Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J. Cell Biol. 148, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala, J.C., Feinberg, M., Popov, V., and Harris, K.M. (1998). Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 18, 8900–8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya, S., Ono, K., and Hirabayashi, Y. (1995). Sphingolipid biosynthesis is necessary for dendrite growth and survival of cerebellar Purkinje cells in culture. J. Neurochem. 65, 1551–1561. [DOI] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Harada, A., Teng, J., Takei, Y., Oguchi, K., and Hirokawa, N. (2002). MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol. 158, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel, R., and Futerman, A.H. (1993). Inhibition of sphingolipid synthesis affects axonal outgrowth in cultured hippocampal neurons. J. Biol. Chem. 268, 14476–14481. [PubMed] [Google Scholar]
- Harris, K.M., Jensen, F.E., and Tsao, B. (1992). Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 12, 2685–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Deviez, D.J., Casanova, J.E., and Wilson, J.M. (2002). Regulation of dendritic development by the ARF exchange factor ARNO. Nat. Neurosci. 5, 623–624. [DOI] [PubMed] [Google Scholar]
- Jan, Y.N., and Jan, L.Y. (2003). The control of dendrite development. Neuron 40, 229–242. [DOI] [PubMed] [Google Scholar]
- Jontes, J.D., and Smith, S.J. (2000). Filopodia, spines, and the generation of synaptic diversity. Neuron 27, 11–14. [DOI] [PubMed] [Google Scholar]
- Karns, L.R., Ng, S.C., Freeman, J.A., and Fishman, M.C. (1987). Cloning of complementary DNA for GAP-43, a neuronal growth-related protein. Science 236, 597–600. [DOI] [PubMed] [Google Scholar]
- Kutzleb, C., Sanders, G., Yamamoto, R., Wang, X., Lichte, B., Petrasch-Parwez, E., and Kilimann, M.W. (1998). Paralemmin, a prenyl-palmitoyl-anchored phosphoprotein abundant in neurons and implicated in plasma membrane dynamics and cell process formation. J. Cell Biol. 143, 795–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakkonen, P., Ahola, T., and Kaariainen, L. (1996). The effects of palmitoylation on membrane association of Semliki forest virus RNA capping enzyme. J. Biol. Chem. 271, 28567–28571. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic, M., Malinow, R., and Svoboda, K. (1999). Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927. [DOI] [PubMed] [Google Scholar]
- McCabe, J.B., and Berthiaume, L.G. (2001). N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae. Mol. Biol. Cell 12, 3601–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, G., and Feldman, E.L. (2002). Signaling mechanisms that regulate actin-based motility processes in the nervous system. J. Neurochem. 83, 490–503. [DOI] [PubMed] [Google Scholar]
- Miki, H., Sasaki, T., Takai, Y., and Takenawa, T. (1998). Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391, 93–96. [DOI] [PubMed] [Google Scholar]
- Milligan, G., Parenti, M., and Magee, A.I. (1995). The dynamic role of palmitoylation in signal transduction. Trends Biochem. Sci. 20, 181–187. [DOI] [PubMed] [Google Scholar]
- Mumby, S.M. (1997). Reversible palmitoylation of signaling proteins. Curr. Opin. Cell Biol. 9, 148–154. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and Hall, A. (1995). Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem. Soc. Trans. 23, 456–459. [DOI] [PubMed] [Google Scholar]
- Patterson, S.I., and Skene, J.H. (1999). A shift in protein S-palmitoylation, with persistence of growth-associated substrates, marks a critical period for synaptic plasticity in developing brain. J. Neurobiol. 39, 423–437. [DOI] [PubMed] [Google Scholar]
- Prange, O., and Murphy, T.H. (2001). Modular transport of postsynaptic density-95 clusters and association with stable spine precursors during early development of cortical neurons. J. Neurosci. 21, 9325–9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna, H., and Donaldson, J.G. (1997). ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, A., Cha, E.M., and Craig, A.M. (2000). Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J. Neurosci. 20, 8344–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, A., and Craig, A.M. (2000). Signaling between the actin cytoskeleton and the postsynaptic density of dendritic spines. Hippocampus 10, 527–541. [DOI] [PubMed] [Google Scholar]
- Redmond, L., and Ghosh, A. (2001). The role of Notch and Rho GTPase signaling in the control of dendritic development. Curr. Opin. Neurobiol. 11, 111–117. [DOI] [PubMed] [Google Scholar]
- Resh, M.D. (1999). Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys Acta Mol. Cell Res. 1451, 1–16. [DOI] [PubMed] [Google Scholar]
- Rohatgi, R., Ma, L., Miki, H., Lopez, M., Kirchhausen, T., Takenawa, T., and Kirschner, M.W. (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231. [DOI] [PubMed] [Google Scholar]
- Ross, E.M. (1995). Protein modification. Palmitoylation in G-protein signaling pathways. Curr. Biol. 5, 107–109. [DOI] [PubMed] [Google Scholar]
- Routtenberg, A. (1987). Phospholipid and fatty acid regulation of signal transduction at synapses: potential role for protein kinase C in information storage. J. Neural Transm. Suppl. 24, 239–245. [PubMed] [Google Scholar]
- Schwarz, A., Rapaport, E., Hirschberg, K., and Futerman, A.H. (1995). A regulatory role for sphingolipids in neuronal growth. Inhibition of sphingolipid synthesis and degradation have opposite effects on axonal branching. J. Biol. Chem. 270, 10990–10998. [DOI] [PubMed] [Google Scholar]
- Skene, J.H., and Willard, M. (1981). Characteristics of growth-associated polypeptides in regenerating toad retinal ganglion cell axons. J. Neurosci. 1, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, J.V. (1988). The actin cytoskeleton. Electron Microsc. Rev. 1, 155–174. [DOI] [PubMed] [Google Scholar]
- Song, J., Khachikian, Z., Radhakrishna, H., and Donaldson, J.G. (1998). Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J. Cell Sci. 111(Pt 15), PG-2257–2267. [DOI] [PubMed] [Google Scholar]
- Sretavan, D.W., and Kruger, K. (1998). Randomized retinal ganglion cell axon routing at the optic chiasm of GAP-43-deficient mice: association with midline recrossing and lack of normal ipsilateral axon turning. J. Neurosci. 18, 10502–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter, S.M., Fankhauser, C., Huang, P.L., Mashimo, H., and Fishman, M.C. (1995). Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell 80, 445–452. [DOI] [PubMed] [Google Scholar]
- Strittmatter, S.M., Igarashi, M., and Fishman, M.C. (1994a). GAP-43 amino terminal peptides modulate growth cone morphology and neurite outgrowth. J. Neurosci. 14, PG-5503–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter, S.M., Valenzuela, D., and Fishman, M.C. (1994b). An aminoterminal domain of the growth-associated protein GAP-43 mediates its effects on filopodial formation and cell spreading. J. Cell Sci. 107(Pt 1), PG-195–204. [DOI] [PubMed] [Google Scholar]
- Strittmatter, S.M., Valenzuela, D., Kennedy, T.E., Neer, E.J., and Fishman, M.C. (1990). G0 is a major growth cone protein subject to regulation by GAP-43. Nature 344, 836–841. [DOI] [PubMed] [Google Scholar]
- Sudo, Y., Valenzuela, D., Beck-Sickinger, A.G., Fishman, M.C., and Strittmatter, S.M. (1992). Palmitoylation alters protein activity: blockade of G(o) stimulation by GAP-43. EMBO J/11, 2095–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, Y., Hermida-Matsumoto, L., and Resh, M.D. (2000). Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 275, PG-261–270. [DOI] [PubMed] [Google Scholar]
- Wood, W., and Martin, P. (2002). Structures in focus—filopodia. Int. J. Biochem. Cell Biol. 34, 726–730. [DOI] [PubMed] [Google Scholar]
- Yarar, D., To, W., Abo, A., and Welch, M.D. (1999). The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr. Biol. 9, 555–558. [DOI] [PubMed] [Google Scholar]
- Zhang, F.L., and Casey, P.J. (1996). Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65, 241–269. [DOI] [PubMed] [Google Scholar]
- Zhang, W., and Benson, D.L. (2000). Development and molecular organization of dendritic spines and their synapses. Hippocampus 10, 512–526. [DOI] [PubMed] [Google Scholar]
- Ziv, N.E., and Smith, S.J. (1996). Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17, 91–102. [DOI] [PubMed] [Google Scholar]
- Zuber, M.X., Goodman, D.W., Karns, L.R., and Fishman, M.C. (1989a). The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science 244, 1193–1195. [DOI] [PubMed] [Google Scholar]
- Zuber, M.X., Strittmatter, S.M., and Fishman, M.C. (1989b). A membrane-targeting signal in the amino terminus of the neuronal protein GAP-43. Nature 341, 345–348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.