Abstract
Background
Both HIV-1 infection and illicit stimulant use can adversely impact neurocognitive functioning, and these effects can be additive. However, significant variability exists such that as-of-yet unidentified exogenous and endogenous factors affect ones risk for neurocognitive impairment. Both HIV and stimulant literature indicates that host genetic variants in immunologic and dopamine-related genes are one such factor. In this study the individual and interactive effects of HIV status, stimulant use, and genotype upon neurocognitive functioning was examined longitudinally over a 10 year period.
Methods
952 Caucasian HIV+ and HIV− cases from the Multicenter AIDS Cohort Study were included. All cases had at least two comprehensive neurocognitive evaluations between 1985 and 1995. Pre-HAART data was examined in order to avoid the confounding effect of variable drug regimens. Linear mixed models were used, with neurocognitive domain scores as the outcome variables.
Results
No 4-way interactions were found, indicating that HIV and stimulant use do not interact over time to affect neurocognitive functioning as a function of genotype. Multiple 3-way interactions were found that involved genotype and HIV status. All immunologic-related genes found to interact with HIV status affected neurocognitive functioning in the expected direction; however, only CCL2 and CCL3 affected HIV+ individuals specifically. Dopamine-related genetic variants generally affected HIV-negative individuals only. Neurocognitive functioning among HIV+ individuals who also used stimulants was not significantly different from those who did not use stimulants.
Conclusion
The findings support the role of immunologic-related genetic differences in CCL2 and CCL3 in neurocognitive functioning among HIV+ individuals; however their impact is minor. Consistent with findings from another cohort, DA-related genetic differences do not appear to impact the longitudinal neurocognitive functioning of HIV+ individuals.
Keywords: HIV-associated neurocognitive disorder, HAND, genetic, chemokine, dopamine, stimulant abuse
INTRODUCTION
The prevalence of neurocognitive dysfunction among people infected with Human Immunodeficiency Virus-1 (HIV) remains high, with recent prevalence estimates between 40%–50%.(Heaton et al 2011, McArthur et al 2005, McArthur et al 2003) Chronic HIV infection may result in a persistent neuroinflammatory state, even with relatively effective peripheral viral suppression.(Brew 2004, Cysique et al 2005, Cysique et al 2004, Kraft-Terry et al 2009) The result of this has been an increase of mild forms of HIV-associated neurocognitive disorder, or HAND.(Antinori et al 2007, Heaton et al 2010) Common comorbidities, such as substance abuse, can increase the risk for neurologic and neurocognitive dysfunction(Rippeth et al 2004). With lifetime substance abuse rates ranging between 20–50% among the HIV-1 infected (HIV+), understanding the interactive effects of substance use and HIV upon neuropathogenesis has great importance.(Carey et al 2006, Ferrando et al 1998, Ferrando & Batki 2000, Levine et al 2006, Martin et al 2004, Rabkin et al 1997, Rabkin et al 2004, Rippeth et al 2004) Perhaps most germane in the context of HIV is stimulant use (e.g., methamphetamine and cocaine), which is one of the more commonly abused classes of drugs among infected and at-risk individuals in the United States(Klinkenberg & Sacks 2004, Stall et al 2001), and an established neurotoxin known to exacerbate the effects of HIV-1.(Aksenov et al 2006, Gaskill et al 2009, Kuczenski et al 2007, Levine et al 2006, Rippeth et al 2004, Silverstein et al 2011, Zhang et al 1998)
Evidence from neuroimaging, animal model, and in vitro studies suggests that stimulants and HIV have overlapping neuroanatomical targets(Avison et al 2004, Aylward et al 1993, Berger & Nath 1997, Cass 1997, Dal Pan et al 1992, Itoh et al 2000, Little et al 1999, Power et al 1993) and result in additive or even synergistic adverse impact upon neurophysiology.(Aksenov et al 2006, Cass et al 2003, Flora et al 2003, Martin-Thormeyer & Paul 2009, Nath et al 2002, Nath et al 2001, Theodore et al 2007) However, while laboratory studies strongly indicate additive or synergistic adverse neurobiological effects of combined stimulant use and HIV, findings from clinical and epidemiological studies so far have yielded mixed results. Cross-sectional studies indicate increased neurocognitive impairment with the combined effects of HIV and stimulant use.(Carey et al 2006, Rippeth et al 2004) For example, Rippeth and colleagues (Rippeth et al 2004) examined four groups with various combinations of the risk factors of HIV-infection and methamphetamine use (HIV+/drug using, HIV+/non-drug using, HIV-/drug-using, and HIV-/non-drug using) and found an incremental increase in depression and neurocognitive impairment among those with more risk factors. However, other cross-sectional investigations have not found either an additive or synergistic relationship between HIV and stimulants(Basso & Bornstein 2003, Durvasula et al 2000), suggesting that additional, unidentified factors likely determine the extent to which stimulant use results in neurobehavioral impairment among individuals with HIV.
Conspicuously lacking are longitudinal studies of neurocognitive outcomes in HIV+ individuals who are abusing stimulants. Such a dynamic model of HAND neuropathogenesis could prove useful in predicting outcomes, identifying salient biological factors, and potentially allowing for the prioritizing of treatments. Towards such an end, consideration of endogenous biological factors can enhance the utility of such studies by identifying viable targets for treatment. Because of the natural variation in host genotype, and the known influence of such variation upon neurophysiology and immunology, consideration of genes suspected to contribute to HIV disease progression or to affect neurophysiological functioning is an important dimension to consider in any longitudinal analysis of HIV and stimulant use. There is evidence that sequence variants of chemokine and other immune-related genes results in differences in susceptibility for HIV infection and disease progression, and in some instances HAND. These include the C-C chemokine receptor type-5 (CCR5)(Liu et al 1999, Samson et al 1996), CCR2(Singh et al 2004, Smith et al 1997), C-X-C motif chemokine 12 (CXCL12)(Winkler et al 1998) (also called stromal cell-derived factor-1), C-C chemokine ligand-2 (CCL2)(Gonzalez et al 2002) (also called monocyte chemotactic protein-1), CCL3(Gonzalez et al 2001) (also called macrophage inflammatory protein-1α), CCL5(Liu et al 1999, McDermott et al 2000) (also called Regulated on Activation, Normal T cell Expressed and Secreted, or RANTES), tumor necrosis factor-alpha (TNF-α)(Quasney et al 2001), mannose binding lectin-2 (MBL-2)(Spector et al 2010), and apolipoprotein-E (ApoE)(Burt et al 2008, Chang et al 2011, Corder et al 1998, Soontornniyomkij et al 2012, Valcour et al 2004) genes, among others. However, with regards to HAND, none of these allelic associations have been consistently replicated.
In addition to polymorphisms within these largely immunologic-related genes, there exist a number of variants of neurobiologically-related genes that may impact neurocognitive functioning in the context of HIV and stimulant use. Because HAND has been associated with dopamine (DA) dysfunction, genetic variants that further affect DA availability may augment neurobehavioral impairment. Among these is the val158met allele within the catechol-O-methyl-transferase (COMT) gene, which is the result of a single nucleotide polymorphism (SNP; rs4680). COMT is an enzyme that catabolizes DA within the prefrontal cortex, an area crucial for a variety of cognitive abilities. This SNP results in a sequence modification and amino acid substitution of methionine (Met) for valine (Val). The Met allele reduces the enzymatic activity of COMT (Lachman et al 1996), leading to greater availability of DA within the prefrontal cortex. Individuals who are homozygous for the Met allele perform better on neuropsychological tests of working memory and other executive ability tests(Egan et al 2001, Goldberg et al 2003, Mattay et al 2003). Such findings are particularly relevant to HIV, as a recent neuroimaging study found reduced neural processing capacity in working memory networks in those with HIV(Tomasi et al 2006). Another gene of interest produces dopamine-β-hydroxylase (DBH), which catalyzes the conversion of DA to norepinephrine. A SNP within the DBH gene accounts for 35–52% of the variation in plasma-DBH activity(Zabetian et al 2001). In addition, another polymorphism within this gene has been associated with biochemical variability in the catecholamine pathway(Wei et al 1997) and ADHD(Daly et al 1999, Roman et al 2002). Dopamine receptors have also been implicated as a bridge between stimulants and HIV progression. For example, Gaskill et al.(Gaskill et al 2009) found that extracellular DA acts through dopamine receptor 2 (DR2) on monocytes to increase HIV replication, and a SNP in the DR3 gene (rs6280) was recently found affect risk for neurocognitive impairment among HIV+ stimulant addicts(Gupta et al 2011). Finally, brain derived neurotrophic factor (BDNF) is a neurotrophin that promotes neuronal survival and regulates the production and differentiation of neurons. BDNF plays a regulatory role in the DA and serotonin systems(Guillin et al 2001, Mossner et al 2000). Both in vitro and in vivo evidence suggests that BDNF may reduce the neurotoxic effects of gp120 in those with HIV(Nosheny et al 2005). A SNP (rs6265) resulting in the Val or Met is associated with neurobehavioral disorders(Zhang et al 2006). The Met allele is also associated with diminished episodic memory, abnormal activation, and decreased levels of n-acetyl aspartate in the hippocampus as determined via brain MRS(Egan et al 2003).
We propose that there is compelling evidence that some individuals possess genotypes that make them more vulnerable to neurocognitive deficits in the face of environmental stressors such as HIV infection and stimulant abuse. While the results of cross-sectional studies have been equivocal, a number of methodological improvements may elucidate the true relationship. In the current study, we modeled the individual and interactive effects of HIV status, stimulant use, and host genotype upon neurocognitive functioning over a time period of up to 10 years, while controlling for the effect of other important factors known to increase risk of HAND. We hypothesized that both DA-related genes and immunologically-related genes would significantly modify the effects of HIV status and/or stimulant use on neurocognitive functioning over time.
METHODS
Participants
Genotype, behavioral, medical, and virologic data were obtained from participants in the Multicenter AIDS Cohort Study (MACS). The MACS is a multicenter epidemiological study of the natural history of HIV infection in homosexual men, conducted in four U.S. cities (Baltimore, Chicago, Pittsburgh, and Los Angeles). Recruitment procedures have been described in detail elsewhere (Miller et al 1990). MACS participants are generally evaluated at semiannual intervals. Evaluations included physical examinations, HIV testing, laboratory testing, structured clinical interviews, and neuropsychological testing, as well as collection of information about illicit substance use. From the larger MACS cohort, cases for the current study were selected according to the following criteria: 1) Only Caucasian individuals (including Hispanic) were chosen in order to avoid the confounds of population stratification and different neurocognitive test normative data, 2) We excluded individuals with history of HIV-associated dementia, AIDS, loss of consciousness of greater than one hour (per self-report), learning disability (per self-report), brain neoplasm, stroke, current injection drug use, or current use of the following drugs: ethyl chloride, GHB, MDMA, PCP, hallucinogens, downers, heroin/opiates. 3) We included only those individuals with at least two comprehensive neurocognitive evaluations between November 1985 and May 1995. This timeframe was chosen to avoid the possible confounding effects of highly active antiretroviral therapy (HAART), allowing us to better delineate the interactive effects of stimulant use, HIV status, and genotype. After applying these strict criteria, 1110 individuals qualified. Following genotyping quality control, the final sample included 952 individuals (914 Caucasian/non-Hispanic and 38 Caucasian/Hispanic). Participant characteristics are summarized in Table 1.
Table 1.
Group characteristics
| Variable | HIV− | HIV+ |
|---|---|---|
| Age | 37.3 (7.9) | 35.7 (6.6) |
| Education | ||
| <High School | 1 (<1%) | 6 (1%) |
| High School | 21 (5%) | 55 (10%) |
| Some College | 108 (27%) | 161 (29%) |
| College | 107 (26%) | 133 (24%) |
| Post Graduate | 169 (42%) | 191 (35%) |
| CD4+ | 991 (329) | 589 (282) |
| Viral Load | n/a | 44263 (67674) |
| History of IVDU | 13 (3%) | 67 (12%) |
| Time between evaluations | 6.5 (2.4) | 5.5 (2.3) |
Primary Variables
Neuropsychological Functioning
A comprehensive neuropsychological exam is administered every two years and consists of a 40-minute conventional and computerized neuropsychological test battery designed to cover a broad range of cognitive domains. Raw scores are converted into age and education-adjusted T-scores using normative data derived from HIV-seronegative MACS participants. T-scores for all tests comprising a domain are averaged to obtain a domain T-score. A total of six domain T-scores were derived, including Learning & Memory (derived from the learning and delayed recall trials of the Rey-Osterrieth Complex Figure Test(Osterrieth 1944, Rey 1964) and Rey Auditory Verbal Learning Test(Rey 1941)), Processing Speed (Stroop Color Naming(Comalli et al 1962) and Symbol Digit Modalities Test(Smith 1982)), Motor (Grooved Pegboard(Klove & Matthews 1966)), Executive (Stroop Interference(Comalli et al 1962) and Trail Making Test-Form B(Reitan 1958)), and Sustained Attention (CalCAP(Miller 1990)). Domain scores were used as neurocognitive phenotypes, each in separate analyses.
Stimulant Use
Stimulant use was recorded at each visit based on self-reported use during the six months prior to the visit. Stimulant use was classified as Use vs. No Use of powder cocaine, crack cocaine, and amphetamines (methamphetamine, speed, ice, crystal).
Genotype
We chose those markers that are directly implicated or suspected to tag genetic susceptibility loci for HAND. They included two SNPs within the CCL3 (rs1719134 and rs1130371)(Levine 2009), a three-SNP haplotype within the MBL-2 gene (rs1800450, rs1800451, and rs5030737)(Spector et al), and single SNPs within coding or non-coding regions of the CCL2 gene (rs1024611)(Gonzalez et al 2002), TNF-α (rs1800629)(Mathis et al 2008), COMT (rs4680)(Bousman et al 2010, Kumar et al 2011), BDNF (rs6265)(Ahmed et al 2008, Nosheny et al 2007), DBH (rs1611115)(Kumar et al 2011), DR2/ANKK1 - rs1800497)(Kumar et al 2011), DR3 (rs6280), and CXCL12 (rs1801157)(Langford et al 2002, Peng et al 2006).
Time
Time was determined as the number of years from baseline testing and was calculated in years.
Additional Covariates
Marijuana, injection drug, and alcohol use was recorded at each visit in terms of frequency of use in the last six months as well as average amount used each time. We examined history of injection drug use (IDU), as well as current marijuana and alcohol use. Participants were classified based on their pattern of substance use at each visit examined. Marijuana use was determined at each visit based on self-report, and classified as Use vs. No Use. For alcohol, participants were classified as having no use, 1–3 drinks/week, 4–13 drinks/week, or more than 13 drinks/week. Visits in which IVD use was reported were excluded. History of IVD use was included as a co-variate (Yes vs. No).
CD4
For HIV+ cases, nadir CD4 was included as a co-variate. For HIV- cases, mean CD4 for all prior visits was used as a co-variate.
Hepatitis C Infection (HCV) status in MACS participants was determined via blood testing. Participants were classified as HCV-negative if antibody testing was negative. Participants were classified as HCV-positive if they were found to be in the process of seroconversion, acute infection, chronic infection, clearing (between RNA+ and RNA−), or previously HCV-positive but now being clear of HCV RNA.
Depression
Depression was determined indirectly with the Center for Epidemiologic Studies Depression Scale (CES-D)(Radloff 1977). This measure assessed symptoms that are often associated with depression. Scores on the CES-D are entered for each visit in a continuous manner.
Practice effects
The effect of repeated testing was controlled for by the effects of first follow-up, second follow-up, and later follow-up.
DNA extraction and genotyping
For most HIV-seronegative participants, DNA was obtained from pelleted peripheral blood mononuclear cells. The Autopure LS™ nucleic acid purification instrument was used for extracting DNA. Samples were quantitated using OD 260/280. DNA was then genotyped with the Sequenom iPlex. Samples submitted for genotyping with the Sequenom iPlex assay were quanted using a RiboGreen fluorescent assay and normalized to 10 ng per ul. 5 ul iPlex reactions were set up in 384 well PCR plates using 1 ul (10 ng) of templates DNA and using pooled custom PCR primers normalized to 100um per primer using a Tecan Evo 100 workstation and cycled in PE 9700 dual 384 PCR machines. PCR reactions were prepared for genotyping by treatment with SAP. iPlex primer extension reactions were prepared using custom, mass tuned, extension primer pools and cycled in PE 9700 dual 384 PCR machines. Extension products were prepared for detection by resin deionization and spotted onto Spectrochip II chips using the Sequemom NanoDispenser. MALDI-TOF detection was done on the Sequenom MassArray Compact system. Genotype data was reviewed and extracted using Typer v4.0 software and exported to the investigators for analysis. Genotype for most HIV+ participants was obtained from a genome-wide genotyping database, described previously(Levine et al 2012a). Briefly, genotyping was conducted on the Illumina 1M, 1MDuo, or 550K platform, and was either directly obtained or was imputed. Imputation was performed using MACH (Li et al 2010), and all data were imputed to the forward/positive strand. Strand ambiguous AT/GC SNPs were excluded. HapMap2 was used as the reference population (Frazer et al 2007). SNPs with an imputation quality score (r2) greater than 0.3 were retained for analysis. All SNPs were examined for Hardy Weinberg equilibrium (HWE). It was found that rs1130371 in the MIP1α gene was not in HWE, so it was excluded from analyses.
Statistical Analysis
Our basic approach involved the use of generalized linear mixed models to take account of repeated observations on the same subjects. One advantage of this family of models is that well developed procedures exist for diagnosing violations of assumptions and lack of fit of the proposed model(Atkinson 1985, Chatterjee & Hadi 1988, McCullagh & Nelder 1989). These procedures provide checks of the error assumptions, e.g., normally distributed errors with constant variance for continuous outcomes, and can suggest data transformations, such as the log transformation, when these are needed. Procedures are also available to check for outliers and influential points, as well as nonlinear trends, interactions, and multicollinearity.
Linear mixed models with domain specific T-scores as the outcome variables were used (Table 2). Independent variables in these models included variables for the genotype of interest, stimulant use, HIV serostatus (positive vs. negative), Hepatitis C serostatus (positive vs. negative), CD4 (nadir for HIV+, mean CD4 for HIV), marijuana use, alcohol use, history of IVD use, depression, time since first test administration, and practice effects (between baseline and 1st follow-up, 2nd follow-up, and any follow-up thereafter) at each visit. All were time-varying covariates in these models. The covariance structure included a random intercept for each subject. Each SNP listed in Table 3 was examined in a separate model with individual cognitive domains. A total of 60 models (10 SNPs × 6 domains) were run.
Table 2.
COMT and Learning Functioning: Genotype Interaction with HIV Status
| Group* | Estimate† | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| HIV− Val/Val 1 | −0.1197 | 0.1552 | 4695 | −0.77 | 0.4405 |
| HIV− Val/Met 4 | 0.1483 | 0.1146 | 4719 | 1.29 | 0.1957 |
| HIV− Met/Met 5 | 0.4163 | 0.1527 | 4696 | 2.73 | 0.0064 |
| HIV+ Val/Val 2 | 0.1711 | 0.1154 | 4811 | 1.48 | 0.1380 |
| HIV+ Val/Met 9 | 0.1051 | 0.07869 | 4893 | 1.34 | 0.1817 |
| HIV+ Met/Met 10 | 0.0390 | 0.1135 | 4770 | 0.34 | 0.7306 |
|
| |||||
| Post Hoc Comparisons** | |||||
| HIV− Val/Val vs. HIV− Val/Met | −0.2680 | 0.1028 | 4665 | −2.61 | 0.0092 |
| HIV− Val/Val vs. HIV− Met/Met | −0.5360 | 0.2056 | 4665 | −2.61 | 0.0092 |
| HIV− Met/Met vs. HIV+ Met/Met | 0.3772 | 0.1579 | 4776 | 2.39 | 0.0169 |
All cases included regardless of stimulant use status (i.e. the estimates are averaged over stimulant use)
Only significant results are shown. Positive estimates indicate that the regression coefficient (slope) for time in the first group is significantly larger than that of the second group
Estimates based on a significant three-way interaction (HIV × genotype × Time)
Table 3.
MBL and Learning Functioning: Genotype Interaction with HIV Status
| Group* | Estimate | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| HIV− A/A | 0.3130 | 0.1479 | 3387 | 2.12 | 0.0345 |
| HIV+ A/A | 0.08419 | 0.1227 | 3499 | 0.69 | 0.4925 |
| HIV− O/O|A/O | −0.00128 | 0.1388 | 3428 | −0.01 | 0.9927 |
| HIV+ O/O|A/O | 0.1504 | 0.1306 | 3528 | 1.15 | 0.2492 |
|
| |||||
| Post Hoc Comparisons** | |||||
|
| |||||
| HIV− A/A vs. HIV− O/O|A/O | 0.3143 | 0.1547 | 3379 | 2.03 | 0.0423 |
All cases included regardless of stimulant use status
Only significant results are shown
The focus of the analysis was the estimation of time trends in the domain specific test scores, so that time (years from first administration) was treated as a continuous variable. In addition, we examined interactions between time and HIV serostatus (positive vs. negative), current stimulant use (yes or no), and genotype. Genotypes were coded as positive/negative in accordance with a dominant model in most instances. In the case of rs4680 and rs6280 additive models were used, with values 0, 1, and 2 corresponding to the number of minor alleles present, so that only a single parameter was required. Our strategy involved fitting a full model for each of 60 combinations of genotype and domain score. Each model included the four-way interaction between time and the three exposures of interest, as well as all four three-way interactions, six two-way interactions and the four individual main effects. The resulting p-values for the significance tests for all of these effects involving time (8) for all 60 full models were adjusted for multiplicity using the false discovery rate. Using the adjusted p-values with a cutoff of 0.1, we then determined a reduced model for each of the 60 analyses using the hierarchy principle. That is, the highest order significant interaction was determined for each model, and all interactions of the same or lower order were included in the reduced model. This was done primarily to help with the interpretation of the results, which involved the highest order interactions that were significant in the reduced model. The resulting reduced models were of two types, those with all three-way (and two-way) interactions and the remainder with only two-way interactions. Models with only two-way interactions can be directly interpreted; for the significant three-way interactions we estimated time trends (slopes) for each of the groups implied by the interaction. Pairwise comparisons among these groups were also examined.
RESULTS
In the reduced models, a p-value threshold of 0.05 was used. We did not correct for multiple comparisons at this step, as the False Discovery Rate correction was already applied when determining significant interactions in the full model. Using this approach, no 4-way interactions were found, indicating that HIV status and stimulant use status do not interact over time to affect neurocognitive functioning as a function of genotype. However, multiple 3-way interactions were found that involved genotype and HIV status. Of the 10 genetic markers examined, 7 had significant interactions with HIV status upon the trajectory of performances on learning, memory, information processing speed, and working memory over time. No effect on motor functioning, executive functioning, or sustained attention was found. Only one of the 10 genetic markers was found to have a significant interaction with stimulant use over time, affecting the information processing domain. These results are described next.
3-Way Interactions
Time × HIV Status × Genotype Interactions
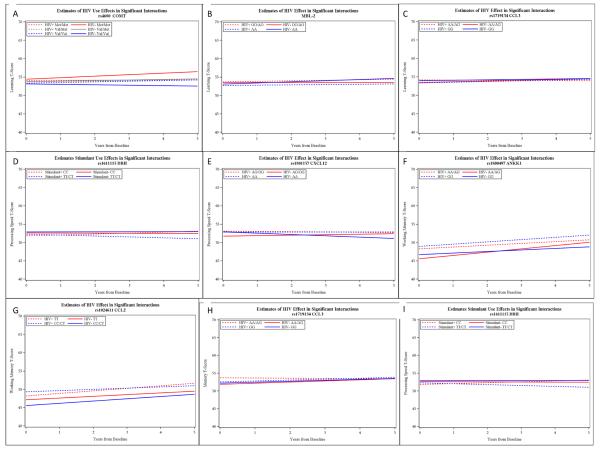
For the Learning domain, the COMT genotype had a significant interaction with time and HIV status (P=.0004). Post hoc comparisons shown in Table 2 indicate that the learning ability of HIV+ individuals did not differ across genotypes. However, a dosage effect for the Met allele was observed for the HIV-negative group, such that the Val/Val group declined slightly over time, the Val/Met group remained generally stable, and the Met/Met group improved (Figure 1, Panel A). Also within the Learning domain, MBL had a significant interaction with time and HIV status (P=.0093) (Table 3). Post hoc testing revealed that the HIV-negative A/A genotype group differed significantly from the HIV-negative combined O/O and A/O genotype group, in that the former showed greater improvement over time (Table 3 and Figure 1, Panel B). Finally, CCL3 genotype (rs1719134) also had a significant interaction with time and HIV status in affecting Learning ability (p=0.0081). Posthoc analysis did not reveal any significant group differences (Table 4); however Figure 1 (Panel C) suggests that the HIV+ AA/AG group declined relative to the other groups over time.
Figure 1.
Slope estimates for significant 3-way interactions
Table 4.
CCL3 and Learning Functioning: Genotype Interaction with HIV Status
| Group* | Estimate | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| HIV− GG | 0.1173 | 0.1204 | 4732 | 0.97 | 0.3300 |
| HIV+ GG | 0.1976 | 0.09153 | 4910 | 2.16 | 0.0310 |
| HIV− AA/AG | 0.2092 | 0.1543 | 4784 | 1.36 | 0.1751 |
| HIV+ AA/AG | −0.04407 | 0.1113 | 4802 | −0.40 | 0.6922 |
|
| |||||
| Post Hoc Comparisons** | |||||
|
| |||||
| HIV+ GG vs. HIV+ AA/AG | 0.2416 | 0.1246 | 4698 | 1.94 | 0.0526 |
All cases included regardless of stimulant use status
Only near-significant results are shown
For the Information Processing domain, DBH (rs1611115) had a significant interaction with time and HIV status (p=0.0168). Post hoc testing revealed that the HIV- CC group had significantly greater improvement in these scores over time as compared to the other groups (Table 5 and Figure 1, Panel D). CXCL12 (rs1801157) also interacted with HIV status to affect Information Processing Speed (p=.0305). While none of the post hoc tests were significant, it appears from Figure 1 (Panel E) that the HIV-negative combined AG & GG group had a trend towards improvement relative to the HIV-negative AA group (Table 6).
Table 5.
DBH and Information Processing Speed: Genotype Interaction with HIV Status
| Group* | Estimate | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| HIV− TT/CT | −0.05803 | 0.1173 | 7745 | −0.49 | 0.6209 |
| HIV+ TT/CT | −0.1742 | 0.1085 | 7893 | −1.61 | 0.1084 |
| HIV− CC | 0.3033 | 0.1066 | 7703 | 2.84 | 0.0045 |
| HIV+ CC | −0.08448 | 0.07922 | 8089 | −1.07 | 0.2863 |
|
| |||||
| Post Hoc Comparisons** | |||||
|
| |||||
| HIV− TT/CT vs. HIV− CC | −0.3613 | 0.1250 | 7718 | −2.89 | 0.0038 |
| HIV+ TT/CT vs. HIV− CC | −0.4775 | 0.1632 | 7808 | −2.93 | 0.0034 |
| HIV+ CC vs. HIV− CC | 0.3878 | 0.1188 | 7944 | 3.27 | 0.0011 |
All cases included regardless of stimulant use status
Only near-significant results are shown
Table 6.
CXCL12 and Information Processing Speed: Genotype Interaction with HIV Status
| Group* | Estimate | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| HIV− AA | −0.3597 | 0.2852 | 7646 | −1.26 | 0.2073 |
| HIV+ AA | 0.00412 | 0.2029 | 7665 | 0.02 | 0.9838 |
| HIV− AG/GG | 0.1364 | 0.09435 | 7714 | 1.45 | 0.1482 |
| HIV+ AG/GG | −0.07033 | 0.07500 | 8141 | −0.94 | 0.3484 |
|
| |||||
| Post Hoc Comparisons** | |||||
|
| |||||
| HIV− AA vs. HIV− AG/GG | −0.4961 | 0.2774 | 7649 | −1.79 | 0.0738 |
| HIV− AG/GG vs. HIV+ AG/GG | 0.2068 | 0.1122 | 7948 | 1.84 | 0.0654 |
All cases included regardless of stimulant use status
Only near-significant results are shown
Two genetic markers showed an associated with Working Memory performance over time. ANKK1 (rs1800497) had a significant interaction with time and HIV status (p=0.0007). Post hoc analysis revealed that the HIV-negative AA/AG group had significantly better improvement over time compared to the HIV-negative GG group, as well as their HIV+ counterparts (Table 7 and Figure 1, Panel F). CCL2 (rs1024611) also affected working memory performance as a function of HIV status and time (p=0.0021). Post hoc testing revealed that the HIV+ TT group improved at a faster rate than the HIV+ CC/CT group, but not faster than the HIV-negative groups (Table 8 and Figure 1, Panel G).
Table 7.
ANKK1 and Working Memory: Genotype Interaction with HIV Status
| Group* | Estimate | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| HIV− GG | 0.4262 | 0.1213 | 3987 | 3.51 | 0.0004 |
| HIV+ GG | 0.6181 | 0.1329 | 4204 | 4.65 | <.0001 |
| HIV− AA/AG | 0.9042 | 0.1938 | 4032 | 4.66 | <.0001 |
| HIV+ AA/AG | 0.4951 | 0.1728 | 4163 | 2.87 | 0.0042 |
|
| |||||
| Post Hoc Comparisons** | |||||
|
| |||||
| HIV− GG vs. HIV− AA/AG | −0.4780 | 0.1934 | 4032 | −2.47 | 0.0135 |
| HIV− AA/AG vs. HIV+ AA/AG | 0.4091 | 0.2004 | 4116 | 2.04 | 0.0413 |
All cases included regardless of stimulant use status
Only significant results are shown
Table 8.
CCL2 and Working Memory: Genotype Interaction with HIV Status
| Group* | Estimate | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| HIV− CC/CT | 0.6180 | 0.1374 | 3958 | 4.50 | <.0001 |
| HIV+ CC/CT | 0.3337 | 0.1710 | 4176 | 1.95 | 0.0511 |
| HIV− TT | 0.4715 | 0.1448 | 4023 | 3.26 | 0.0011 |
| HIV+ TT | 0.6915 | 0.1309 | 4171 | 5.28 | <.0001 |
|
| |||||
| Post Hoc Comparisons** | |||||
|
| |||||
| HIV+ CC/CT vs. HIV+ TT | −0.3577 | 0.1771 | 4011 | −2.02 | 0.0434 |
All cases included regardless of stimulant use status
Only significant results are shown
Finally, Memory functioning was affected as a function of CCL3 (rs1719134) genotype and HIV status (p=0.0062). It was the HIV AA/AG group that differed relative to the other groups, showing relative decline in memory ability (Table 9 and Figure 1, Panel H).
Table 9.
CCL3 and Memory: Genotype Interaction with HIV Status
| Group* | Estimate | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| HIV− GG | 0.2630 | 0.1270 | 4461 | 2.07 | 0.0384 |
| HIV+ GG | 0.2640 | 0.1052 | 4661 | 2.51 | 0.0121 |
| HIV− AA/AG | 0.3186 | 0.1637 | 4524 | 1.95 | 0.0517 |
| HIV+ AA/AG | −0.05818 | 0.1233 | 4546 | −0.47 | 0.6370 |
|
| |||||
| Post Hoc Comparisons** | |||||
|
| |||||
| HIV+ GG vs. HIV+ AA/AG | 0.3222 | 0.1381 | 4452 | 2.33 | 0.0197 |
| HIV− AA/AG vs. HIV+ AA/AG | 0.3768 | 0.1630 | 4577 | 2.31 | 0.0208 |
All cases included regardless of stimulant use status
Only significant results are shown
Time × Stimulant Use × Genotype Interactions
DBH (rs1611115) had an interaction with stimulant use and time (P=0.0096). Specifically, the stimulant users with CC genotype had a more significant improvement in information processing speed over time (Table 10 and Figure 1, Panel I).
Table 10.
DBH and Information Processing Speed: Genotype Interaction with Stimulant Use
| Group* | Estimate | Standard Error | df | T | p-value |
|---|---|---|---|---|---|
| Non-Stimulant TT/CT | 0.01934 | 0.05366 | 7817 | 0.36 | 0.7186 |
| Stimulant TT/CT | −0.2516 | 0.1709 | 7755 | −1.47 | 0.1410 |
| Non-Stimulant CC | −0.03136 | 0.04725 | 7937 | −0.66 | 0.5070 |
| Stimulant CC | 0.2502 | 0.1306 | 7730 | 1.92 | 0.0555 |
|
| |||||
| Post Hoc Comparisons** | |||||
|
| |||||
| Stimulant TT/CT vs. Stimulant CC | −0.5018 | 0.2052 | 7711 | −2.45 | 0.0145 |
| Non-Stimulant CC vs. Stimulant CC | −0.2815 | 0.1319 | 7713 | −2.13 | 0.0328 |
All cases included regardless of HIV status
Only significant results are shown
Time × HIV Status × Stimulant Use
There were no significant interactions between HIV status and stimulant use status over time. In only one model was a three-way interaction involving HIV status, stimulant use, and genotype observed. This occurred in the COMT (rs4680) gene for the learning domain (p = .0439).
Two-Way Interactions
In those models in which no three-way interactions were found, there were a total of 21 two-way interactions involving time. Twelve involved HIV status, and consistently showed that HIV+ status was associated with relative decline in motor functioning over time, but improvement of information processing speed over time. In 6 models, stimulant use was also consistently associated with motor functioning decline over time, and to a greater extent than HIV+ status. In one instance, stimulant use was associated with a very marginal improvement in working memory. In two instances, genotype (DR3 C and BDNF-GG) was associated with poorer motor functioning over time. Results of the two-way interactions are shown in Table 11.
Table 11.
Main effect and interaction estimates for models with no three-way or four-way interactions.
| Domain (Gene) | Interaction | Time estimate* | Interaction estimate** | Standard Error | p-value |
|---|---|---|---|---|---|
| Motor (DRD3) | Time | 0.7441 | |||
| Motor | Time × DRD3 | −0.1106 | 0.04146 | 0.0077 | |
| Motor | Time × Stimulant | −0.2549 | 0.09969 | 0.0106 | |
| Motor | Time × HIV+ | 0.2175 | 0.06197 | 0.0005 | |
|
| |||||
| Speed† (BDNF) | Time | −0.02840 | |||
| Speed | Time × HIV+ | 0.3205 | 0.06075 | <.0001 | |
|
| |||||
| Motor (BDNF) | Time | 0.7181 | |||
| Motor | Time × BDNF | −0.1322 | 0.05997 | 0.0275 | |
| Motor | Time × Stimulant | −0.2646 | 0.09934 | 0.0077 | |
| Motor | Time × HIV+ | 0.2080 | 0.06130 | 0.0007 | |
|
| |||||
| Speed (MBL-2) | Time | 0.01543 | |||
| Speed | Time × HIV+ | 0.3590 | 0.07094 | <.0001 | |
|
| |||||
| Motor (MBL-2) | Time | 0.7425 | |||
| Motor | Time × Stimulant | −0.3528 | 0.1189 | 0.0030 | |
| Motor | Time × HIV+ | 0.2781 | 0.07062 | <.0001 | |
|
| |||||
| Working Memory (DBH) | Time | 0.3002 | |||
| Working Memory | Time × Stimulant | 0.4535 | 0.1458 | 0.0019 | |
|
| |||||
| Speed (ANKK1) | Time | −0.06188 | |||
| Speed | Time × HIV+ | 0.3116 | 0.05994 | <.0001 | |
|
| |||||
| Motor (ANKK1) | Time | 0.6667 | |||
| Motor | Time × Stimulant | −0.2620 | 0.09921 | 0.0083 | |
| Motor | Time × HIV+ | 0.2250 | 0.06092 | 0.0002 | |
|
| |||||
| Speed (CCL2) | Time | −0.03545 | |||
| Speed | Time × HIV+ | 0.3215 | 0.06040 | <.0001 | |
|
| |||||
| Speed (TNF-α) | Time | −0.1018 | |||
| Speed | Time × HIV+ | 0.3108 | 0.05994 | <.0001 | |
|
| |||||
| Motor (TNF-α) | Time | 0.6896 | |||
| Motor | Time × Stimulant | −0.2651 | 0.09920 | 0.0075 | |
| Motor | Time × HIV+ | 0.2223 | 0.06095 | 0.0003 | |
|
| |||||
| Speed (CCL3) | Time | −0.1197 | |||
| Speed | Time × HIV+ | 0.3166 | 0.06045 | <.0001 | |
|
| |||||
| Motor (CCL3) | Time | 0.7116 | |||
| Motor | Time × Stimulant | −0.2751 | 0.09945 | 0.0057 | |
| Motor | Time × HIV+ | 0.2165 | 0.06114 | 0.0004 | |
Time estimate indicates T-score change per year
Negative interaction estimates indicate greater decline relative to the overall estimate. Positive estimates indicate relative improvement.
Speed = Information Processing Speed
DISCUSSION
Variability in HAND risk, with or without concurrent stimulant abuse, suggests that inherent factors such as genotype may protect some individuals against neurocognitive impairment. Cross-sectional studies and some short-duration longitudinal studies have found genetic markers associated with HIV-related neurocognitive deficits; however, very few findings have been replicated. We hypothesized that certain alleles of DA and immunologically-related genes would confer protection against neurocognitive deficits resulting from HIV and stimulant use, and that this relationship would bear out over time. Our findings indicate that genotype does affect neurocognitive functioning over time as a function of HIV status, and to a lesser extent stimulant use. However, these effects are insubstantial.
Multiple lines of research implicate that activation of immunologic-related factors, such as cytokines and chemokines, is an important component of HAND neuropathogenesis. Our analyses revealed that all immunologic-related genetic markers found to interact with HIV status affected neurocognitive functioning in the expected direction; however, some of these effects were observed in the HIV-negatives only. This included MBL, which influenced learning ability over time. An adverse impact of the `O' genotype was observed among the HIV-negative cases only. This genotype was previously reported to increase risk of neurocognitive impairment at a one-year follow-up in HIV+ Chinese individuals(Spector et al 2010). Note that the previous study did not include HIV-negative individuals; therefore it was not possible to determine if the previously observed decline in neurocognitive status was due to genotype alone or in combination with HIV serostatus. Our findings suggest that it was the former. CXCL12 genotype (rs1801157) affected information processing speed among HIV-negatives only. This interaction appears to have been driven largely by the relative improvement of the HIV-negative AG/GG individuals relative to the HIV-negative AA. As such, it does not appear that this allele plays a significant role in neurocognitive functioning among HIV+ individuals. The AA genotype was previously associated with faster disease progression, including development of neurocognitive impairment, in a cohort of HIV+ children(Singh et al 2003). Conversely, this genotype has been associated with slower disease progress in adults, based on a study involving the MACS cohort(Winkler et al 1998). No association between this allele and HIV-related neurocognitive impairment was found in other studies(Levine et al 2009, Spector et al 2010). The CCL2 (MCP-1) rs1024611 marker validated previous findings. In our sample, HIV+ individuals possessing a C allele did not demonstrate improvement in working memory functioning, as compared to HIV+ individuals with a TT genotype or HIV-negative individuals as a whole (regardless of genotype). The C allele has previously been associated with increased risk for HIV-associated dementia(Gonzalez et al 2002) and higher HIV DNA in the CSF of children(Shiramizu et al 2006), although other studies have not observed a relationship between this allele and neurocognitive functioning in HIV(Levine et al 2009, Singh et al 2004, Spector et al 2010). Finally, those individuals homozygous or heterozygous for the A allele at rs1719134 of the CCL3 gene demonstrated relatively greater decline in learning and memory ability as compared to HIV-negative individuals with this genotype, or individuals with the GG genotype. This SNP is in high linkage disequilibrium with rs1130371, previously shown by our group to be associated with increased risk of HIV-associated dementia. (As mentioned in the Methods section, we did not use rs1130371 genotype in this analysis due to its violation of Hardy Weinberg Equilibrium, possibly indicating genotyping error.) Specifically, in Levine et al (2009)(Levine et al 2009) we found that the TT (equivalent to AA, due to the strand of DNA read by the technology) genotype at rs1130371 in the CCL3 gene was associated with a two-fold greater risk for HIV-associated dementia in the National NeuroAIDS Tissue Consortium cohort(Morgello et al 2001). The current results may validate the previous finding in this second cohort.
The hypothesis that variation in dopamine-related genes would affect neurocognitive functioning as a function of stimulant use was not supported. In addition, and somewhat surprisingly, two of the three DA-related SNPs showed significant interaction with HIV status or stimulant use in the opposite direction than expected. The DBH (rs1611115) CC genotype, which is associated with greater DBH activity and therefore increased DA catabolism into norepinephrine(Zabetian et al 2001), was associated with faster information processing speed for HIV-negatives only. This same relationship was observed for stimulant users, with CC genotype showing greater improvement than those with CT or TT genotype. It should be noted that DBH is limited neuroanatomically to the adrenal medulla and synaptic vesicles of postganglionic sympathetic neurons.(Kim et al 2002) Therefore, our findings may indicate that it is increased norepinephrine availability in the adrenal medulla and sympathetic neurons that is related to improved processing speed performance among CC carriers, and that whatever decrease in DA availability resulting from this does not influence neurocognitive functioning. Variation at the ANKK1 (rs1800497) SNP, which has been linked to striatal DR2 receptor density and neuropsychiatric illness(Jonsson et al 1999, Neville et al 2004), modified working memory performance over time in HIV-negatives. Our analysis found that HIV-negative individuals who possessed one or two of the ancestral A alleles had greater improvement in working memory ability over time when compared to HIV+ A carriers and HIV-negative GG carriers. As suggested by Figure 1, Panel F, both HIV+ genotype groups and the HIV-negative GG carriers has similar trajectories, suggesting that whatever beneficial effects there were of the A allele were not manifested in HIV+ individuals. Of the DA-related genes examined, only COMT (rs4680) showed an expected association. Specifically, the Met allele was associated with stronger learning ability; however, this was observed only for HIV-negative individuals.
Perhaps most notable is that even in those models that demonstrated significant genetic interactions, the influence of genotype was minor. The largest effect due to genotype amounted to 3 or 4 T-score points over a five-year period, while in most models the effect was indiscernible. Also notable are the results of models that involved two-way interactions, which consistently showed a relative decline in motor functioning among HIV+ individuals, and even more so among stimulant users. Conversely and unexpectedly HIV+ individuals demonstrated improvement in information processing speed over time. Due to the large number of comparisons, even with the corrections for multiple testing, some results are likely to represent false positive errors. One such likely instance was the finding that stimulant use was associated with very marginal improvement in working memory over time. This finding was seen in only one model, and the degree of improvement combined with lack of replication suggests that the finding was spurious. In two instances, variation in DR3 (C) and BDNF (GG) was associated with poorer motor functioning over time, regardless of HIV status or stimulant use.
Contrary to expectation, the trajectory of neurocognitive functioning among HIV+ individuals who also used stimulants was not significantly different from those who did not use stimulants. Note that our design differed from previous studies (e.g., (Carey et al 2006, Rippeth et al 2004)) in that we did not have defined substance use groups, but rather considered substance use on a visit-by-visit basis. As such, the same individual may have been considered a stimulant user at one visit, but not at another. This approach was necessary considering the long duration of the retrospective data capture, and may better reflect the actual drug use habits of stimulant users. Regardless, the lack of significant interactions may be due to the varying inclusion of substance users, abusers, and dependent individuals across studies. It is also noted that our analysis included only men, and that these findings may not generalize to females, who are may be more vulnerable to stimulant dependence(Lynch 2009) and who may experience different neurocognitive consequences from stimulant use(Meyer et al 2013). Finally, drug use was determined via participant self-report. While this is the only feasible method for obtaining such information in the population studied, self-report does have inherent flaws, including under and over-reporting.
To summarize, our findings support the notion that immunologic-related genetic differences in the chemokines CCL2 and CCL3 affect the neurocognitive functioning of HIV+ individuals over time, although their influence appears to be minor. This likely reflects the multifaceted nature of HAND pathogenesis, which is influenced by numerous cytokines, chemokines, and other immunological factors. Consistent with findings from another cohort(Levine et al 2012b), DA-related genetic differences do not appear to influence the longitudinal neurocognitive functioning of HIV+ individuals, despite significant evidence for the role of DA dysfunction in HAND. Further, in the pre-HAART era, improvement of processing speed and decline of motor functioning over time among HIV+ individuals was consistently observed, and an unrelated decline in motor functioning due to stimulant use (regardless of HIV status) was also consistently observed. Finally, in addition to the very small effects observed here, we suggest that the inconsistencies in previous cross sectional genetic association studies of HAND may also be due to a number of factors, including survival bias, use of different neurocognitive phenotypes and/or psychometric measures, and problems inherent in genetic analyses (e.g., population stratification).
ACKNOWLEDGEMENTS
Our deepest gratitude to the volunteers enrolled in the Multicenter AIDS Cohort Study (MACS). This study was funded through the National Institute for Drug Abuse grant R03DA026099 (Levine) and UCLA-AIDS Institute/Center for AIDS Research grant AI28697 (Freimer). Susan Service and Nelson Freimer (UCLA) assisted with data acquisition and analysis.
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, Lisette Johnson-Hill, Michael W. Plankey, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Maurice O'Gorman, David Ostrow, Frank Palella, Ann Ragin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Ross D. Cranston, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; and the Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Alvaro Munoz (Co-PI), Alison Abraham, Keri Althoff, Christopher Cox, Jennifer Deal, Gypsyamber D'Souza, Priya Duggal, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR000424 (JHU CTSA). Website located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
We also wish to thank the following individuals and their teams for providing genotyping data: by Jacques Fellay, M.D., Stephen O'Brien, Ph.D., and James I. Mullins, Ph.D.
Sandra Reynolds does not have any financial relationship with the sponsor of this research.
Christopher Cox does not have any financial relationship with the sponsor of this research.
Janet S. Sinsheimer does not have any financial relationship with the sponsor of this research.
James T. Becker does not have any financial relationship with the sponsor of this research.
Eileen Martin does not have any financial relationship with the sponsor of this research.
Ned Sacktor does not have any financial relationship with the sponsor of this research.
Footnotes
CONFLICT OF INTEREST This study was funded through a grant awarded to Andrew J. Levine by NIDA.
Dr. Miller is the author of the CalCAP Reaction Time Program and has a financial interest in this software. His work with the Multicenter AIDS Cohort Study is funded by NIAID. He has no financial relationship with NIDA or the UCLA AIDS Institute.
REFERENCES
- Ahmed F, Tessarollo L, Thiele C, Mocchetti I. Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain. Brain Res. 2008;1227:1–11. doi: 10.1016/j.brainres.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27:217–28. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson AC. Plots, Transformations and Regression. Clarendon Press; Oxford: 1985. [Google Scholar]
- Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Bales RA, et al. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol. 2004;10:223–32. doi: 10.1080/13550280490463532. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, et al. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43:2099–104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Effects of past noninjection drug abuse upon executive function and working memory in HIV infection. J Clin Exp Neuropsychol. 2003;25:893–903. doi: 10.1076/jcen.25.7.893.16489. [DOI] [PubMed] [Google Scholar]
- Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40:122–31. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Atkinson JH, Heaton RK, Grant I, et al. COMT Val158Met Polymorphism, Executive Dysfunction, and Sexual Risk Behavior in the Context of HIV Infection and Methamphetamine Dependence. Interdiscip Perspect Infect Dis. 2010;2010:678648. doi: 10.1155/2010/678648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. Aids. 2004;18(Suppl 1):S75–8. [PubMed] [Google Scholar]
- Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A. 2008;105:8718–23. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10:185–90. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280:105–13. [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 2003;984:133–42. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- Chang L, Andres M, Sadino J, Jiang CS, Nakama H, et al. Impact of apolipoprotein E epsilon4 and HIV on cognition and brain atrophy: Antagonistic pleiotropy and premature brain aging. NeuroImage. 2011;58:1017–27. doi: 10.1016/j.neuroimage.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Hadi AS. Sensitivity Analysis in Linear Regression. John Wiley & Sons; New York: 1988. [Google Scholar]
- Comalli PE, Jr., Wapner S, Werner H. Interfernce effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- Corder EH, Robertson K, Lannfelt L, Bogdanovic N, Eggertsen G, et al. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4:1182–4. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ, Halman M, Catalan J, Sacktor N, et al. Undetectable cerebrospinal fluid HIV RNA and beta-2 microglobulin do not indicate inactive AIDS dementia complex in highly active antiretroviral therapy-treated patients. J Acquir Immune Defic Syndr. 2005;39:426–9. doi: 10.1097/01.qai.0000165799.59322.f5. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–7. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Dal Pan GJ, McArthur JH, Aylward E, Selnes OA, Nance-Sproson TE, et al. Patterns of cerebral atrophy in HIV-1-infected individuals: results of a quantitative MRI analysis. Neurology. 1992;42:2125–30. doi: 10.1212/wnl.42.11.2125. [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4:192–6. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- Durvasula RS, Myers HF, Satz P, Miller EN, Morgenstern H, et al. HIV-1, cocaine, and neuropsychological performance in African American men. J Int Neuropsychol Soc. 2000;6:322–35. doi: 10.1017/s1355617700633076. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ferrando S, Goggin K, Sewell M, Evans S, Fishman B, Rabkin J. Substance use disorders in gay/bisexual men with HIV and AIDS. Am J Addict. 1998;7:51–60. [PubMed] [Google Scholar]
- Ferrando SJ, Batki SL. Substance abuse and HIV infection. New Dir Ment Health Serv. 2000:57–67. [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp Neurol. 2003;179:60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–61. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175:1148–59. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–96. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Dhanda R, Bamshad M, Mummidi S, Geevarghese R, et al. Global survey of genetic variation in CCR5, RANTES, and MIP-1alpha: impact on the epidemiology of the HIV-1 pandemic. Proc Natl Acad Sci U S A. 2001;98:5199–204. doi: 10.1073/pnas.091056898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A. 2002;99:13795–800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–9. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Gupta S, Bousman CA, Chana G, Cherner M, Heaton RK, et al. Dopamine receptor D3 genetic polymorphism (rs6280TC) is associated with rates of cognitive impairment in methamphetamine-dependent men with HIV: preliminary findings. J Neurovirol. 2011 doi: 10.1007/s13365-011-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol (Berl) 2000;99:376–84. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–6. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kim CH, Zabetian CP, Cubells JF, Cho S, Biaggioni I, et al. Mutations in the dopamine beta-hydroxylase gene are associated with human norepinephrine deficiency. Am J Med Genet. 2002;108:140–7. [PubMed] [Google Scholar]
- Klinkenberg WD, Sacks S. Mental disorders and drug abuse in persons living with HIV/AIDS. AIDS Care. 2004;16(Suppl 1):S22–42. doi: 10.1080/09540120412331315303. [DOI] [PubMed] [Google Scholar]
- Klove H, Matthews CG. Psychometric and adaptive abilities in epilepsy with differential etiology. Epilepsia. 1966;7:330–8. doi: 10.1111/j.1528-1157.1966.tb03812.x. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64:133–45. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Langford D, Sanders VJ, Mallory M, Kaul M, Masliah E. Expression of stromal cell-derived factor 1alpha protein in HIV encephalitis. J Neuroimmunol. 2002;127:115–26. doi: 10.1016/s0165-5728(02)00068-1. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Hardy DJ, Miller E, Castellon SA, Longshore D, Hinkin CH. The effect of recent stimulant use on sustained attention in HIV-infected adults. J Clin Exp Neuropsychol. 2006;28:29–42. doi: 10.1080/13803390490918066. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Service S, Miller EN, Reynolds SM, Singer EJ, et al. Genome-wide association study of neurocognitive impairment and dementia in HIV-infected adults. Am J Med Genet B Neuropsychiatr Genet. 2012a doi: 10.1002/ajmg.b.32071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Singer EJ, Sinsheimer JS, Hinkin CH, Papp J, et al. CCL3 genotype and current depression increase risk of HIV-associated dementia. Neurobehav HIV Med. 2009;1:1–7. doi: 10.2147/nbhiv.s6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Singer EJ, Sinsheimer JS, Hinkin CH, Papp J, Dandekar S, Giovanelli A, Shapshak P. CCL3 genotype and current depression increase risk of HIV-associated dementia. Neurobehavioral HIV Medicine. 2009;1:1–7. doi: 10.2147/nbhiv.s6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Sinsheimer JS, Bilder R, Shapshak P, Singer EJ. Functional polymorphisms in dopamine-related genes: effect on neurocognitive functioning in HIV+ adults. J Clin Exp Neuropsychol. 2012b;34:78–91. doi: 10.1080/13803395.2011.623118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–34. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Zhang L, Desmond T, Frey KA, Dalack GW, Cassin BJ. Striatal dopaminergic abnormalities in human cocaine users. Am J Psychiatry. 1999;156:238–45. doi: 10.1176/ajp.156.2.238. [DOI] [PubMed] [Google Scholar]
- Liu H, Chao D, Nakayama EE, Taguchi H, Goto M, et al. Polymorphism in RANTES chemokine promoter affects HIV-1 disease progression. Proc Natl Acad Sci U S A. 1999;96:4581–5. doi: 10.1073/pnas.96.8.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W, Potenza MN, Cosgrove KP, Mazure CM. Sex differences in vulnerability to stimulant abuse. In: BS Brady KT, Cosgrove KP, Greenfield SF, editors. Women and Addiction: A Comprehensive Handbook. Guilford; New York: 2009. [Google Scholar]
- Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychol Rev. 2009;19:215–31. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, et al. Cognitive impulsivity and HIV serostatus in substance dependent males. J Int Neuropsychol Soc. 2004;10:931–8. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Mathis KL, Dozois EJ, Larson DW, Cima RR, Sarmiento JM, et al. Ileal pouch-anal anastomosis and liver transplantation for ulcerative colitis complicated by primary sclerosing cholangitis. Br J Surg. 2008;95:882–6. doi: 10.1002/bjs.6210. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–55. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–21. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. Chapman and Hall; London: 1989. [Google Scholar]
- McDermott DH, Beecroft MJ, Kleeberger CA, Al-Sharif FM, Ollier WE, et al. Chemokine RANTES promoter polymorphism affects risk of both HIV infection and disease progression in the Multicenter AIDS Cohort Study. Aids. 2000;14:2671–8. doi: 10.1097/00002030-200012010-00006. [DOI] [PubMed] [Google Scholar]
- Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, et al. HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr. 2013;63:67–76. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. California Computerized Assessment Package. Norland Software; Los Angeles: 1990. [Google Scholar]
- Miller EN, Selnes OA, McArthur JC, Satz P, Becker JT, et al. Neuropsychological performance in HIV-1-infected homosexual men: The Multicenter AIDS Cohort Study (MACS) Neurology. 1990;40:197–203. doi: 10.1212/wnl.40.2.197. [DOI] [PubMed] [Google Scholar]
- Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, et al. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27:326–35. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Mossner R, Daniel S, Albert D, Heils A, Okladnova O, et al. Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF) Neurochem Int. 2000;36:197–202. doi: 10.1016/s0197-0186(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–9. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–5. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Ahmed F, Yakovlev A, Meyer EM, Ren K, et al. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–84. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Mocchetti I, Bachis A. Brain-derived neurotrophic factor as a prototype neuroprotective factor against HIV-1-associated neuronal degeneration. Neurotox Res. 2005;8:187–98. doi: 10.1007/BF03033829. [DOI] [PubMed] [Google Scholar]
- Osterrieth P. Le test de copie d'une figure complexe. Archives Psychologie. 1944;30:206. [Google Scholar]
- Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, et al. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–29. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Kong PA, Crawford TO, Wesselingh S, Glass JD, et al. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol. 1993;34:339–50. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- Quasney MW, Zhang Q, Sargent S, Mynatt M, Glass J, McArthur J. Increased frequency of the tumor necrosis factor-alpha-308 A allele in adults with human immunodeficiency virus dementia. Ann Neurol. 2001;50:157–62. [PubMed] [Google Scholar]
- Rabkin JG, Ferrando SJ, Jacobsberg LB, Fishman B. Prevalence of axis I disorders in an AIDS cohort: a cross-sectional, controlled study. Compr Psychiatry. 1997;38:146–54. doi: 10.1016/s0010-440x(97)90067-5. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, Ferrando SJ. Mood and substance use disorders in older adults with HIV/AIDS: methodological issues and preliminary evidence. Aids. 2004;18(Suppl 1):S43–8. [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reitan R. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271. [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Arch Psychologie. 1941;28:286. [Google Scholar]
- Rey A. L'examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Roman T, Schmitz M, Polanczyk GV, Eizirik M, Rohde LA, Hutz MH. Further evidence for the association between attention-deficit/hyperactivity disorder and the dopamine-beta-hydroxylase gene. Am J Med Genet. 2002;114:154–8. doi: 10.1002/ajmg.10194. [DOI] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Lau E, Tamamoto A, Uniatowski J, Troelstrup D. Feasibility assessment of cerebrospinal fluid from HIV-1-infected children for HIV proviral DNA and monocyte chemoattractant protein 1 alleles. J Investig Med. 2006;54:468–72. doi: 10.2310/6650.2006.06007. [DOI] [PubMed] [Google Scholar]
- Silverstein PS, Shah A, Gupte R, Liu X, Piepho RW, et al. Methamphetamine toxicity and its implications during HIV-1 infection. J. Neurovirol. 2011 doi: 10.1007/s13365-011-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Barroga CF, Hughes MD, Chen J, Raskino C, et al. Genetic influence of CCR5, CCR2, and SDF1 variants on human immunodeficiency virus 1 (HIV-1)-related disease progression and neurological impairment, in children with symptomatic HIV-1 infection. J Infect Dis. 2003;188:1461–72. doi: 10.1086/379038. [DOI] [PubMed] [Google Scholar]
- Singh KK, Ellis RJ, Marquie-Beck J, Letendre S, Heaton RK, et al. CCR2 polymorphisms affect neuropsychological impairment in HIV-1-infected adults. J Neuroimmunol. 2004;157:185–92. doi: 10.1016/j.jneuroim.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol digit modalities test. Western Psychological Service; Los Angeles: 1982. [Google Scholar]
- Smith MW, Dean M, Carrington M, Winkler C, Huttley GA, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997;277:959–65. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012 doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector SA, Singh KK, Gupta S, Cystique LA, Jin H, et al. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS. 2010;24:1471–9. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector SA, Singh KK, Gupta S, Cystique LA, Jin H, et al. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS (London, England) 24:1471–9. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall R, Paul JP, Greenwood G, Pollack LM, Bein E, et al. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the Urban Men's Health Study. Addiction. 2001;96:1589–601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Nath A, Maragos WF. Progress in understanding basal ganglia dysfunction as a common target for methamphetamine abuse and HIV-1 neurodegeneration. Curr HIV Res. 2007;5:301–13. doi: 10.2174/157016207780636515. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Chang L, de Castro Caparelli E, Telang F, Ernst T. The human immunodeficiency virus reduces network capacity: acoustic noise effect. Ann Neurol. 2006;59:419–23. doi: 10.1002/ana.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004;157:197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Wei J, Xu HM, Ramchand CN, Hemmings GP. Is the polymorphic microsatellite repeat of the dopamine beta-hydroxylase gene associated with biochemical variability of the catecholamine pathway in schizophrenia? Biol Psychiatry. 1997;41:762–7. doi: 10.1016/S0006-3223(96)00218-1. [DOI] [PubMed] [Google Scholar]
- Winkler C, Modi W, Smith MW, Nelson GW, Wu X, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC) Science. 1998;279:389–93. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet. 2001;68:515–22. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, et al. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer's disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141:387–93. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Looney D, Taub D, Chang SL, Way D, et al. Cocaine opens the blood-brain barrier to HIV-1 invasion. J Neurovirol. 1998;4:619–26. doi: 10.3109/13550289809114228. [DOI] [PubMed] [Google Scholar]