Abstract
Brain-derived neurotrophic factor (BDNF) signalling is critical for neuronal development and transmission. Recruitment of TrkB receptors to lipid rafts has been shown to be necessary for the activation of specific signalling pathways and modulation of neurotransmitter release by BDNF. Since TrkB receptors are known to be modulated by adenosine A2A receptor activation, we hypothesized that activation of A2A receptors could influence TrkB receptor localization among different membrane microdomains. We found that adenosine A2A receptor agonists increased the levels of TrkB receptors in the lipid raft fraction of cortical membranes and potentiated BDNF-induced augmentation of phosphorylated TrkB levels in lipid rafts. Blockade of the clathrin-mediated endocytosis with monodansyl cadaverine (100 μM) did not modify the effects of the A2A receptor agonists, but significantly impaired BDNF effects on TrkB recruitment to lipid rafts. The effect of A2A receptor activation in TrkB localization was mimicked by 5 μM forskolin, an adenylyl cyclase activator. Also, it was blocked by the PKA inhibitors Rp-cAMPs and PKI-(14-22) and by the Src-family kinase inhibitor PP2. Moreover, removal of endogenous adenosine or disruption of lipid rafts reduced BDNF stimulatory effects on glutamate release from cortical synaptosomes. Lipid raft integrity was also required for the effects of BDNF upon hippocampal long-term potentiation at CA1 synapses. Our data demonstrate, for the first time, a BDNF-independent recruitment of TrkB receptors to lipid rafts, induced by the activation of adenosine A2A receptors, with functional consequences for TrkB phosphorylation and BDNF-induced modulation of neurotransmitter release and hippocampal plasticity.
Keywords: TrkB receptors, Brain-derived neurotrophic factor, BDNF, Adenosine, A2A receptors, Lipid rafts
Introduction
The neurotrophin brain-derived neurotrophic factor (BDNF) is essential in the regulation of neuronal survival and differentiation. Abundant evidence has now established that BDNF is also involved in the modulation of synaptic transmission and plasticity [9, 44]. BDNF activates the TrkB tyrosine kinase receptor and the p75 receptor, which belongs to the tumour necrosis factor receptor family. We and others have reported that TrkB receptor function is modulated by the activation of adenosine A2A receptors [16, 20, 31, 33, 38, 54]. This TrkB/A2A receptor cross-talk has two consequences, which may operate independently: (1) facilitation of BDNF-induced actions on synaptic transmission and plasticity by A2A receptor agonists and (2) direct phosphorylation and activation of TrkB receptors, in the absence of BDNF, a process called transactivation. Notably, transactivation of TrkB receptors usually requires longer exposure to A2A agonists than facilitation of synaptic actions of BDNF.
Adenosine is an important modulator of the nervous system that acts through the activation of G protein-coupled receptors: A1, A2A, A2B and A3 [22, 48]. Adenosine receptors are distributed widely in the nervous system, where the high affinity A1 and A2A receptors are responsible for the fine tuning of neurotransmitter release and modulation of other signalling molecules [48].
Lipid rafts are cholesterol- and sphingolipid-rich membrane microdomains that concentrate specific proteins and lipids. Although protein affinity for these domains is not totally understood, it is known that palmitoylated-, myristoylated- and glycosylphosphatidylinositol-anchored proteins are enriched in these domains [42, 51, 53]. Lipid rafts have been implicated in the regulation of signal transduction in multiple cell types, including neurons, by promoting close proximity or segregation of signalling molecules [18, 35, 47]. There is now increasing evidence that lipid rafts are essential for BDNF signalling, and both TrkB and p75 receptors can be localized in these domains [28, 52, 57]. Translocation of TrkB receptors to lipid rafts is regulated by BDNF and is required for its effects on glutamate release, synaptic fatigue [52] and for activation of the phospholipase C pathway [41].
In this work, we investigated whether adenosine A2A receptor activation affects TrkB receptor localization in lipid rafts and how BDNF actions on glutamate release and long-term potentiation are affected by removal of endogenous adenosine and disruption of lipid rafts. We show that A2A receptor activation induced TrkB translocation and increased BDNF-induced phospho-TrkB (pTrkB) receptors in lipid rafts. Moreover, our results suggest that the mechanisms used by A2A receptor agonists to induce TrkB translocation are different from those used by BDNF and involve cAMP and Src-family kinase activation. Finally, lipid raft disruption abolished the potentiating effects of BDNF on glutamate release and long-term potentiation (LTP).
Materials and methods
Materials
Cell culture media, Alexa Fluor 488-coupled goat anti-rabbit antibody and Alexa Fluor 594-coupled cholera toxin subunit B, were obtained from Invitrogen (Carlsbad, CA). BDNF was a kind gift of Regeneron Pharmaceuticals (Tarrytown, NY). 4-[2-[[6-Amino-9-(N-ethyl-b-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]-benzene propanoic acid hydrochloride (CGS 21680), 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385), forskolin, 3-(4-chlorophenyl)1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP2), protein kinase inhibitor-(14-22)-amide, myristoylated (PKI 14-22) and (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801) were from Tocris Cookson (Ellisville, MO). Adenosine deaminase (ADA; EC 3.5.4.4) was from Roche (Germany). Mouse anti-TrkB antibody was from BD Biosciences (San Jose, CA). Anti-phospho-Trk (pTyr-490) was from Cell Signaling Technology (Danvers, MA). Rabbit anti-TrkB antibody was from Millipore (Bedford, MA). The antibody for Fyn and HRP-coupled anti-mouse and anti-rabbit secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). [3H] ZM 241385 and ECL plus reagent were obtained from GE Healthcare (Little Chalfont, UK). Ultra Clear centrifuge tubes were from Beckman (Palo Alto, CA). Bradford reagent was from Bio-Rad (Hercules, CA). All other reagents were purchased from Sigma (St. Louis, MO).
Neuronal cortical cultures
Cortical neurons were dissected from E18 Sprague–Dawley embryos, obtained from Harlan Interfauna Iberica, SL (Barcelona, Spain), as described previously [41]. Animals were handled according to the European Community guidelines and Portuguese law on animal care. Dissection was carried out in cold HBSS medium supplemented with 0.37 % glucose, under sterile conditions. The cortices were trypsinized for 15 min, centrifuged and resuspended in minimum essential medium (MEM) supplemented with 10 % FBS, 2 mM glutamine, 1 mM sodium pyruvate, 0.37 % glucose and 25 U/ml penicillin/streptomycin. Cells were dissociated, counted and plated in poly-l-lysine-coated dishes at a density of 105 cells/cm2. On the following day, medium was changed to Neurobasal medium supplemented with 0.5 mM glutamine, 2 % B27, 25 U/ml penicillin/streptomycin and 25 μM glutamate. On DIV 4, half the medium was replaced by the above-mentioned solution (excepting glutamate) with 5-fluoro-2-deoxy-uridine. On the day of the experiment, cells were starved for 4 h in MEM containing 0.37 % glucose, 2 mM glutamine and 10 μM MK-801. Twenty-nanomolar CGS21680 and 50 nM ZM241385 were added for 30 min, followed by the addition of 20 ng/ml BDNF for 5 or 40 min, as indicated. Inhibitors were added 15 min prior to CGS 21680 and/or BDNF incubation and remained present until cell lysis. Methyl-β-cyclodextrin (MβCD) was the only exception, being present only 15 min before incubation with CGS 21680.
Lipid raft isolation
Lipid rafts were isolated as described previously [41]. Briefly, cortical neurons (DIV 7-11) were lysed in TNE buffer (150 mM NaCl, 50 mM Tris–HCl at pH 8.0 and 5 mM EDTA) containing 0.5 % Triton X-100 and supplemented with protease and phosphatase inhibitors (2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM sodium orthovanadate, 10 mM sodium fluoride and 1 mM phenylmethylsulfonyl fluoride). After solubilization for at least 20 min at 4 °C, the lysates were combined with a 60 % Optiprep solution to yield a 35 % Optiprep mixture. This solution was placed in the bottom of the ultracentrifuge tube and overlaid with 8 ml of a 30 % Optiprep solution in lysis buffer followed by 3 ml of lysis buffer. Samples were centrifuged for 6 h at 36,400 rpm in a Beckman XL-90 ultracentrifuge, using an SW41Ti rotor, at 4 °C. After discarding the first 1 ml, eight fractions (from top to bottom) were collected and equal volumes of each fraction were applied in an 8 % sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) gel.
Immunocytochemistry
The protocol used has been previously described by Harder and colleagues [26], with minor modifications. DIV 6-7 cortical neurons were starved for 4 h prior to CGS 21680 (20 nM) treatment for 30 min. Cortical neurons were then incubated with a rabbit anti-TrkB antibody (1:500) and Alexa Fluor 594-coupled cholera toxin subunit B (2 μg/ml) for 1 h at 12 °C in MEM with 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 2 mg/ml BSA, pH 7.3. Cells were washed and incubated with the Alexa Fluor 488-coupled anti-rabbit antibody for 45 min (1:300), under the same conditions. Fixation was done with 4 % paraformaldehyde for 5 min followed by cold methanol for 5 min. Images were taken using a Zeiss (Thornwood, NY) LSM 510 confocal microscope and analysed with the help of the ImageJ software. Co-patching was quantified as the percentage of TrkB receptor clusters colocalized with GM1 patches. In each experiment, ten cells per condition were analysed.
Isolation of synaptosomes
Three- to 5-week-old Wistar rats were decapitated under halothane anaesthesia and synaptosomes were prepared as described elsewhere [8]. Briefly, the cortices were dissected in an ice-cold Krebs solution composed of (in millimolar) the following: NaCl 124; KCl 3; NaH2PO4 1.2; NaHCO3 25; MgSO4 1; CaCl2 2; and glucose 10, previously gassed with 95 % O2 and 5 % CO2, pH 7.4. The cortices were homogenized in an ice-cold isosmotic sucrose solution (0.32 M, containing 1 mM EDTA, 1 mg/ml bovine serum albumin and 10 mM HEPES, pH 7.4) and centrifuged at 3,000×g for 10 min; the supernatant was centrifuged again at 14,000×g for 12 min. The whole procedure was conducted at 4 °C. The pellet was resuspended in 45 % Percoll in KHR (in millimolar: NaCl 140, EDTA 1, HEPES 10, KCl 5 and glucose 5) and centrifuged at 14,000 rpm for 2 min. The synaptosomal fraction corresponds to the top buoyant layer and was collected from the tube. Percoll was removed by two washes with a KHR solution; synaptosomes were then kept on ice and used within 3 h.
Glutamate release from synaptosomes
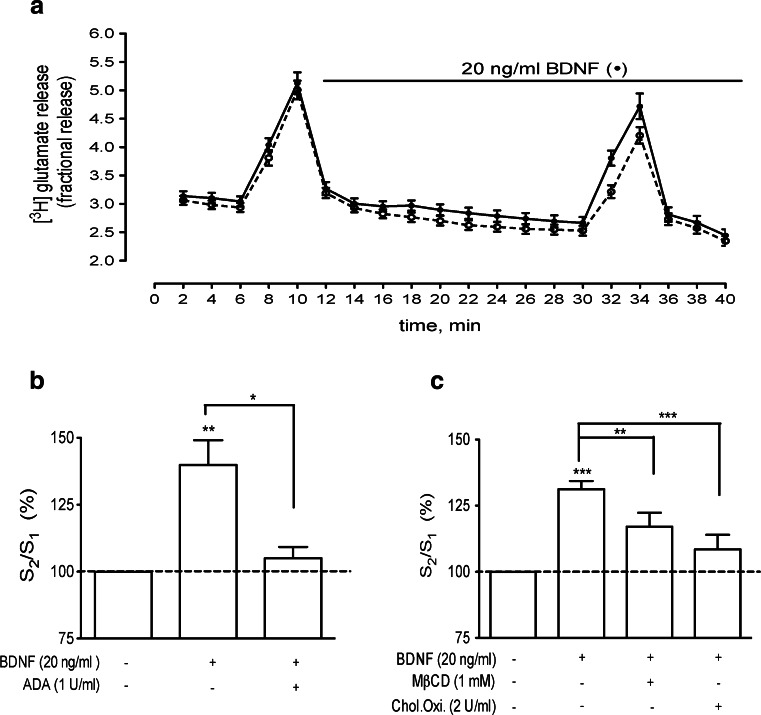
Synaptosomes were resuspended in 2 ml of oxygenated Krebs medium and allowed to equilibrate for 5 min at 37 °C. From this time onwards, all solutions applied to the synaptosomes were kept at 37 °C and continuously gassed with 95 % O2 and 5 % CO2. Synaptosomes were loaded with 0.2 μM [3H] glutamate (specific activity was 30–60 Ci/mmol) for 5 min and equally layered onto perfusion chambers over Whatman GF/C filters (flow rate 0.6 ml/min, chamber volume 90 μl). In the cholesterol oxidase experiments, 2 U/ml of the enzyme was incubated with the synaptosomes for 1 h at 37 °C in oxygenated Krebs, prior to glutamate incubation. After a 20-min washout period, samples were continuously collected for 40 min in 2-min intervals. A high-K+ solution (15 mM, isomolar substitution of Na+ by K+ in the Krebs solution) was added for 2 min in the 5th (S1) and 29th (S2) minutes to stimulate glutamate release. BDNF (20 ng/ml) was added from the ninth minute onwards, and its effect was quantified as percent changes of the S2/S1 ratio as compared with the S2/S1 ratio in the absence of BDNF in the same synaptosomal batch and under similar drug conditions. The S2/S1 ratio was calculated as the area under the curve corresponding to the amount of tritium released due to the second stimulation period (S2) over the amount of tritium released due to the first stimulation period (S1), after subtraction of basal release (averaged tritium content of the two samples before stimulation and two samples after stimulation upon returning to basal levels). To evaluate the influence of a drug upon the effect of BDNF, that drug was added 10 min after starting the washout period and remained present until the end of experiments, being therefore present during S1 and S2. In the case of exogenously added cholesterol, MβCD–cholesterol complexes were perfused only during the first 10 min of the washout period. None of the drugs affected the S2/S1 ratio, when compared with the S2/S1 ratio in the absence of any drug.
Acutely prepared hippocampal slices
Three- to 5-week-old rats were decapitated under halothane anaesthesia. Hippocampal dissection was carried out in ice-cold Krebs solution, previously gassed with 95 % O2 and 5 % CO2, as described above. Four hundred-micrometer-thick slices were cut perpendicularly to the long axis of hippocampus with a McIlwain tissue chopper and allowed to recover functionally and energetically for at least 1 h in a resting chamber, filled with oxygenated Krebs solution, at room temperature.
High-frequency stimulation of acutely prepared slices
Groups of four hippocampal slices were placed in 100 μl chambers and continuously perfused (0.5 ml/min) with oxygenated Krebs solution, at 30 °C. After 1 h, the slices were field stimulated using a high-frequency stimulation protocol. Trains of 100 Hz were applied for 50 ms, every 2 s, for 1 min (150 pulses). Thirty minutes after stimulation, the slices were homogenized in detergent-free TNE buffer containing protease and phosphatase inhibitors (as above). Triton X-100 (0.5 %) was added to the homogenate, and after 1-h incubation at 4 °C, lipid rafts were isolated in discontinuous Optiprep gradients, as described above. When 1 U/ml ADA was used, it was added to the perfusion solution 30 min prior to the high-frequency stimulation and remained present up to the end of the experiment. Using the same stimulation and perfusion conditions, it has been previously shown that considerable amounts of ATP and adenosine were released and detected in the bath after stimulation [14].
LTP
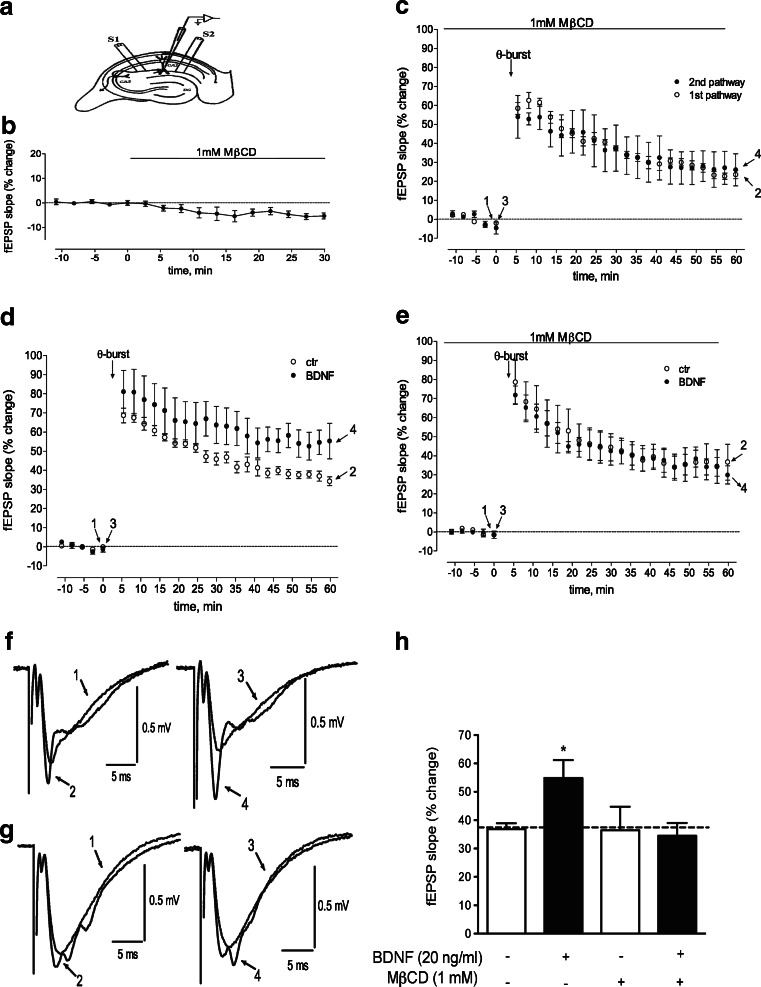
After 1 h of recovery period, as described above, one hippocampal slice was transferred to a recording chamber for submerged slices (1 + 5 ml dead volume), where it was continuously superfused at a flow rate of 1.5 ml/min with Krebs solution at 32 °C; all drugs were added to this superfusion solution. Perfusion tubes were coated with 0.1 mg/ml BSA prior to experiments to avoid adsorption of BDNF to the tubes. Evoked field excitatory postsynaptic potentials (fEPSP) were recorded extracellularly through a microelectrode filled with 4 M NaCl (2–4 MΩ resistance) placed in the stratum radiatum of the CA1 area. Two independent pathways of Schaffer collateral/commissural fibres were stimulated (rectangular pulses of 0.1 ms duration) alternately once every 20 s, by two bipolar concentric wire electrodes placed on the Schaffer fibres in the stratum radiatum, in the CA1 area (see Fig. 10a). The initial intensity of the stimulus (80–150 μA) was adjusted to obtain a sub-maximal fEPSP slope with a minimum population spike contamination, of similar magnitude in both pathways. The averages of eight consecutive fEPSP from each pathway were obtained and quantified as the slope of the initial phase of the potential. Recordings were obtained with an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA), digitized and continuously stored on a personal computer with the LTP program [1]. The independence of the two pathways was tested by evaluating paired-pulse facilitation (50 ms interval) across both pathways, less than 10 % facilitation being usually observed. When a higher facilitation was observed, the slice was discarded. LTP was induced after obtaining a stable recording of fEPSP slope in the two pathways for at least 30 min, by a θ-burst stimulation, consisting of 1 train of 15 bursts (200 ms interburst interval), with 4 pulses (100 Hz) each [20]. LTP was quantified as the percent change in the average slope of the fEPSP taken from 50 to 60 min after LTP induction in relation to the average slope of the fEPSP measured during the 10 min that have preceded the induction of LTP. One hour after LTP induction in one of the pathways, BDNF (20 ng/ml) was added to the superfusion solution and was delivered continuously to the slices. LTP was induced in the second pathway no less than 30 min after BDNF perfusion and upon stability of fEPSP slope values. The effect of BDNF upon LTP was evaluated by comparing the magnitude of LTP in the first pathway in the absence of BDNF (control pathway), with the magnitude of LTP in the second pathway in the presence of BDNF (test pathway); when BDNF was tested in the presence of 1 mM MβCD, MβCD was added at least 30 min before LTP induction in the first pathway and remained in the bathing solution until the end of the experiment. Each pathway was used as control or test in alternate days. This protocol allows the comparison between the effects of BDNF upon LTP under different experimental conditions, keeping as an internal control the magnitude of LTP under the same drug condition, but absence of BDNF in the same slice.
Fig. 10.
BDNF enhances long-term potentiation in a lipid raft-dependent manner. a Schematic representation of a transverse hippocampal slice with the electrode configuration used to record fEPSPs in the CA1 apical dendritic layer (stratum radiatum) evoked by electric stimulation of two independent pathways of the Schaffer fibres, S0 and S1. In b–e, the averaged time course changes in the fEPSP slope are shown. The small inhibition of fEPSP caused by 1 mM MβCD is illustrated in b. c–e Changes in the fEPSP slope induced by the θ-burst stimulation of slices (see “Materials and methods”), as indicated by the arrow in each panel. Zero percent corresponds to the averaged slopes recorded for 10 min before MβCD (b −0.55 ± 0.02 mV/ms, n = 7) or θ-burst stimulation (c white circle, −0.49 ± 0.02 mV/ms; black circle, −0.49 ± 0.02 mV/ms, n = 3; d, white circle, −0.52 ± 0.01 mV/ms, black circle, −0.49 ± 0.04 mV/ms, n = 6; e white circle −0.49 ± 0.01 mV/ms, black circle, −0.47 ± 0.01 mV/ms, n = 5). f, g Recordings from representative experiments, where each trace represents the average of eight consecutive responses obtained before and after LTP induction, in the absence (f, left) or presence of BDNF (f, right), or in the presence of MβCD (g, left) or MβCD + BDNF (g, right). Recordings under same conditions, but before and 60 min after LTP induction are superimposed. All recordings in f were obtained from a single slice at approximately the points indicated in d. All recordings in g were obtained from a single slice at approximately the points indicated in e. Each recording is composed by the stimulus artifact, followed by the presynaptic volley and the fEPSP. h Comparison of the effect of BDNF upon LTP in the absence or presence of MβCD, as indicated. *p < 0.05, compared to the first column
Immunoblotting
Lysates were denatured with 5× sample buffer (350 mM Tris, 30 % glycerol, 10 % SDS, 600 mM dithiothreitol and 0.012 % bromophenol blue, pH 6.8) and equal volumes were loaded into gels. Proteins were run in SDS-PAGE gels, transferred to PVDF membranes and blocked for 1 h at room temperature with 5 % non-fat milk in TBS with 0.1 % Tween-20 (TBS-T). Incubations with the primary antibodies were done overnight at 4 °C; all of them diluted in 3 % BSA in TBS-T and 0.02 % sodium azide. HRP-coupled secondary antibodies were diluted in blocking buffer and incubated for 1 h at room temperature. Detection of proteins was made with ECL plus Western blotting detection.
Radioligand binding
[3H] ZM 241385 binding was performed as described elsewhere [15] with minor modifications. Briefly, 3–4-week-old rat cortices were dissected as described above and centrifuged at 1,000×g for 10 min at 4 °C. The supernatant was centrifuged again at 14,000×g for 12 min and the pellet was resuspended in 50 mM Tris–HCl (pH 7.4) with 1 mM EDTA and 2 mM EGTA and incubated with 2 U/ml of ADA for 30 min at 37 °C. Membranes were precipitated and resuspended in 50 mM Tris–HCl (pH 7.4) with 2 mM MgCl2 and 4 U/ml ADA. Protein (110–200 μg) was incubated with 0.1–7 nM [3H] ZM 241385 for 60 min at room temperature, in a final volume of 300 μl. Specific binding was calculated by subtraction of the nonspecific binding, defined in the presence of 2 μM of xanthine amine congener. Reaction was stopped by the addition of cold incubation buffer followed by vacuum filtration through glass fibre filters (FilterMAT for receptor binding, Skatron Instruments, Lier, Norway) using a semiautomatic cell harvester from Skatron Instruments. The samples were transferred to scintillation vials, and radioactivity was measured by a liquid scintillation analyzer (TriCarb 2900TR, PerkinElmer, IL). Membrane protein content was measured using the Bio-Rad protein assay [7].
Cholesterol measurements
Cholesterol content from the gradient fractions was analysed using a colorimetric assay [50] with minor modifications. Briefly, lipids were extracted by mixing 200 μl of samples from each gradient fraction with 1 ml chloroform/methanol (2:1), transferred to a glass tube and dried under nitrogen. Reaction was started by adding 0.75 ml acetic acid (glacial) and 0.5 ml 2.5 % ferric chloride in 85 % phosphoric acid to the tube. After 30 min, absorbance was measured at 550 nm. Standard curves with cholesterol allowed direct quantification of cholesterol levels in each fraction.
Data analysis
All data are expressed as mean ± SEM from the indicated number of experiments. Statistical significance was determined using one-way analysis of variance followed by the Bonferroni or Dunnett comparison test for multiple comparisons. When only two values were directly compared, a Student’s t test analysis was performed. Values of p < 0.05 were considered to represent statistically significant differences.
Results
Adenosine A2A receptor activation induces TrkB translocation to lipid rafts and potentiates BDNF-induced phospho-TrkB levels in this membrane microdomain
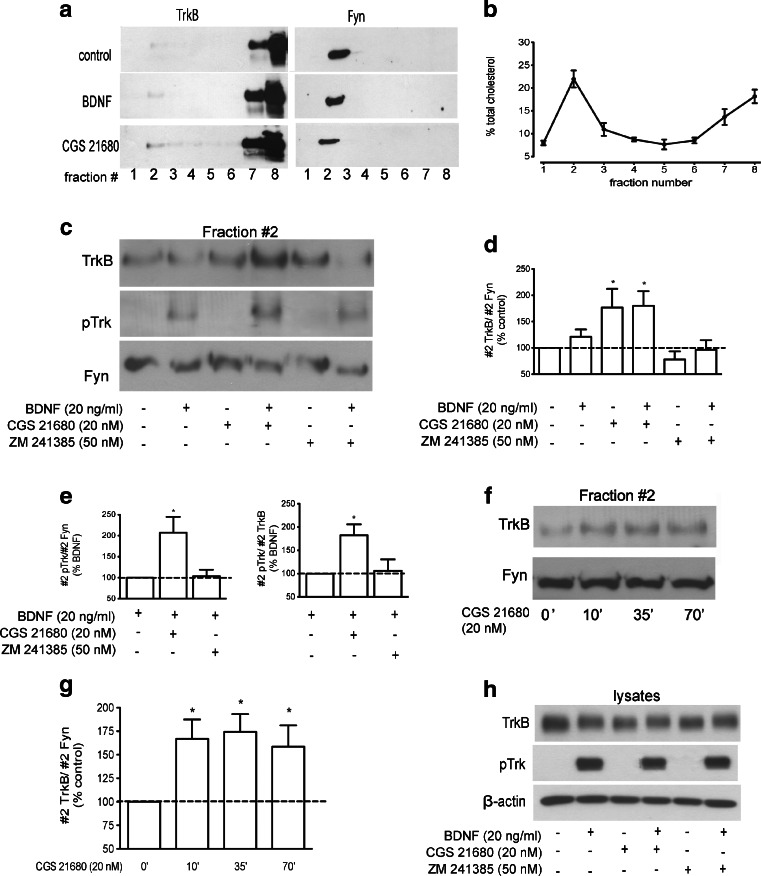
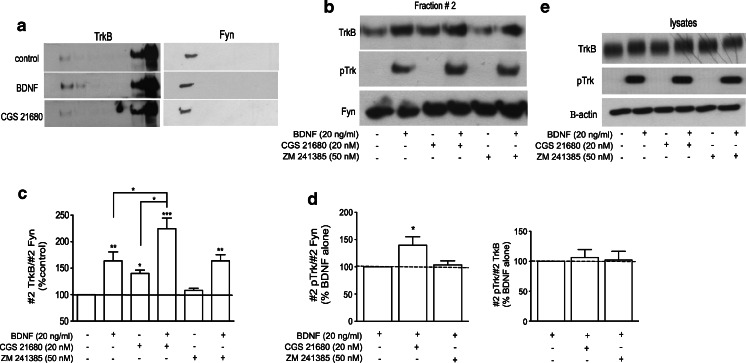
To study the role of adenosine A2A receptors in TrkB receptor distribution in different membrane domains, isolation of lipid rafts from membranes of cultured cortical neurons was performed based on their relative insolubility in non-ionic detergents (see “Materials and methods”). The low density of Triton X-100-insoluble lipid rafts allowed their subsequent separation by density gradient fractionation. Eight gradient fractions were collected and equal volumes of each fraction were analysed by Western blotting, as shown in Fig. 1a. Lipid rafts were localized in fraction #2 (from top to bottom), as demonstrated by the presence of the lipid raft marker Fyn in this fraction. The remaining membranes were found in the bottom fractions of the gradient (fractions #7 and 8, Fig. 1a). Because lipid rafts are domains enriched in cholesterol, its levels were determined for each fraction. Fraction #2 contained approximately 22 % of the total cholesterol present in the gradient, being highly enriched in this lipid as compared with its neighbouring fractions (Fig. 1b). Cholesterol was also found in the bottom fractions (13 and 18 % of total cholesterol in fractions #7 and #8, respectively; Fig. 1b), which possess the majority of cellular membranes, but no staining of the lipid raft marker Fyn (Fig. 1a).
Fig. 1.
Activation of adenosine receptors enhances the levels of TrkB-FL and potentiates BDNF-induced pTrkB localization in lipid rafts. DIV 7-11 cortical neurons were starved for 4 h and incubated with/without 20 nM CGS 21680 or 50 nM ZM 241385 for 30 min prior to 5 min-incubation with 20 ng/ml BDNF, as indicated. Lysates were prepared in 0.5 % Triton X-100-containing buffer and fractioned in a discontinuous Optiprep gradient, as described in “Materials and methods”. a Equal volumes of each gradient fraction were probed for TrkB (1:200) and Fyn (1:400). Note that Fyn, a lipid raft marker, was only detected in fraction #2, which therefore was considered the lipid raft-containing fraction. b Quantification of cholesterol content in each gradient fraction. Fraction #2 was highly enriched in cholesterol, containing approximately 22 % of total cholesterol. c Representative Western blot analysis of the lipid raft fraction (#2) obtained from the Optiprep density gradients. Antibodies used were TrkB (1:200), pTrk (pY490, 1:750) and Fyn (1:400). d Quantitative analysis of TrkB staining in fraction #2, normalized by Fyn staining in this fraction; 100 % represents staining in the absence of any drug. e Quantification analysis of BDNF-induced changes in #2 pTrk, normalized by #2 Fyn (left) or by total TrkB (right), in cells pre-incubated with CGS 21680 or ZM 241385 as indicated below each bar. One hundred percent corresponds to the staining obtained in the presence of BDNF alone. f Fraction #2 obtained from the density gradients of cells incubated with CGS 21680 for the indicated times were probed for TrkB and Fyn, which was used as a loading control. g Densitometry analysis of #2 TrkB/#2 Fyn. h Analysis of TrkB and pTrk staining in total lysates of cells treated with/without CGS 21680, ZM 241385 or 20 ng/ml BDNF, as indicated. TrkB, pTrkB, Fyn or β-actin band corresponds to approximately 150, 150, 60 and 42 kDa proteins. Results are expressed as mean ± SEM of three to seven independent experiments. *p < 0.05, compared to 100 %
The role of adenosine A2A receptors on TrkB localization was studied by treating cortical neurons with the A2A selective agonist CGS 21680 or with the A2A receptor antagonist ZM 241385. For a better comparison, we analysed in the same gel fraction #2 samples from cells incubated under different conditions, as shown in Fig. 1c. When cells were treated with CGS 21680 (20 nM) for 30 min, there was a marked increase in TrkB staining in fraction #2 (176 ± 35 % of the control, p < 0.05, n = 6, Fig. 1d). This incubation time with the A2A receptor agonist clearly induced maximal translocation of TrkB receptors to lipid rafts (Fig. 1f, g). Therefore, a 30-min pre-incubation time with CGS 21680 before addition of BDNF was used while evaluating the influence of A2A receptors upon the effect of BDNF on TrkB receptor translocation.
The A2A receptor antagonist ZM 241385 (50 nM) did not influence TrkB receptor sublocalization in lipid rafts (p > 0.05, n = 6, Fig. 1d). As expected, the effect of CGS 21680 was completely prevented by pre-incubation with ZM 241385 (78 ± 17 % of the control, p > 0.05, n = 3). We then investigated the influence of A2A agonists and antagonists on the effects of BDNF in TrkB receptor localization and phosphorylation after a short (5 min) incubation time with BDNF. Treatment with BDNF (20 ng/ml) for 5 min did not significantly change TrkB localization, but induced the phosphorylation of TrkB receptors in the lipid raft fraction (Fig. 1c). While a pre-incubation for 30 min with CGS 21680 (20 nM) alone had no effect on lipid raft TrkB phosphorylation, it resulted in an increased phosphorylation of TrkB receptors in lipid rafts in response to BDNF treatment for 5 min (Fig. 1c). This increase was observed when the pTrkB signal was normalized either to Fyn (207 ± 38 % of the BDNF condition, p < 0.05, n = 5, Fig. 1e) or to total TrkB (182 ± 24 % of the BDNF condition, p < 0.05, n = 5, Fig. 1e). This suggests that CGS 21680 facilitates BDNF-induced increase of the proportion of phosphorylated and hence active TrkB receptors in the lipid rafts. The A2A antagonist ZM 241385 (50 nM) did not modify BDNF-induced pTrkB staining in the lipid rafts (Fig. 1c).
Importantly, A2A receptor activation with CGS 21680 or blockade with ZM 241385 did not modify (112 ± 13 and 114 ± 11 % of the control, respectively, p > 0.05, n = 3) TrkB expression in total lysates or its phosphorylation (Fig. 1g), suggesting that A2A receptors act specifically as modulators of TrkB receptor localization in lipid rafts.
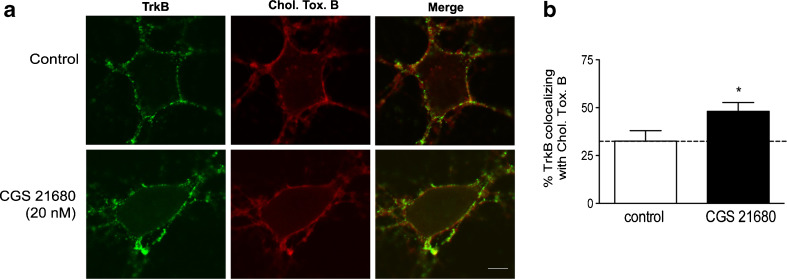
To image TrkB receptors in lipid rafts at membranes of cortical neurons, we performed co-patching experiments between TrkB receptors and the subunit B of cholera toxin. This subunit of cholera toxin specifically binds to the GM1 gangliosides present in lipid rafts, so that the coalescence of cross-linked lipid microdomains induced by this toxin allows a specific imaging of lipid rafts [24, 26]. As shown in Fig. 2, a significant proportion (33 ± 5 %, n = 5) of TrkB receptors co-patch with cholera toxin B in cortical membranes. CGS 21680 (20 nM) treatment induced a significant increase in the degree of colocalization (48 ± 5 %, p < 0.05, n = 5, Fig. 2) between TrkB receptors and the subunit B of cholera toxin.
Fig. 2.
CGS 21680 treatment increases co-patching between TrkB receptors and cholera toxin subunit B (Chol. Tox. B). a Alexa Fluor 594-coupled cholera toxin subunit B (2 μg/ml) and an anti-TrkB receptor antibody (1:500) raised against the extracellular domain of TrkB receptors were used in the co-patching experiments of cultured cortical neurons. TrkB receptor patches were labelled with an Alexa Fluor 488-coupled goat anti-rabbit antibody (1:300). Scale bar, 5 μm. b Quantification of the percentage of TrkB receptors co-patched with cholera toxin subunit B. Results are expressed as mean ± SEM of five independent experiments. *p < 0.05, compared to control
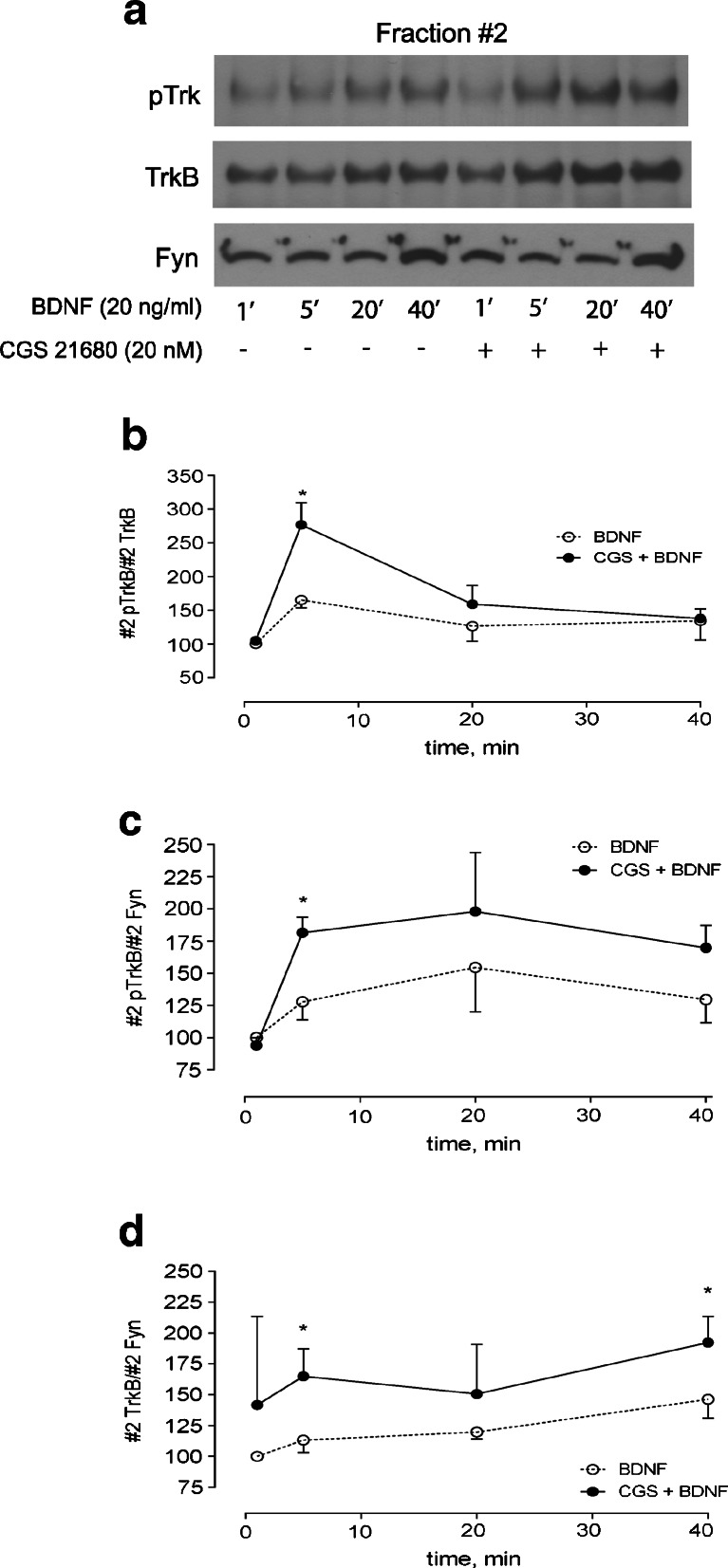
The results described above show that after a short (5 min) incubation time with BDNF, the neurotrophin itself has only a minor influence on TrkB translocation to lipid rafts. Adenosine A2A receptor activation relocates TrkB receptors in the membrane and facilitates TrkB receptor phosphorylation in lipid rafts. We next examined how CGS 21680 influences the kinetics of BDNF-mediated TrkB translocation and phosphorylation in lipid rafts. As shown in Fig. 3, the proportion of pTrkB over total TrkB was already maximal after 5 min of BDNF incubation. Facilitation of pTrkB localization in lipid rafts by adenosine A2A agonists was also maximal after a short (5 min) incubation time with BDNF. For longer incubation times, the ability of CGS 21680 to increase the pTrkB/TrkB ratio in lipid rafts markedly diminishes, being virtually null after BDNF incubation for 40 min (Fig. 3b). However, even at prolonged incubations with BDNF (40 min), the total TrkB and pTrkB staining were more pronounced in the presence of CGS 21680 (Fig. 3a, c, d).
Fig. 3.
Time course of TrkB receptor phosphorylation in lipid rafts by BDNF. a Lipid raft (fraction #2) analysis of pTrk and TrkB staining in lipid rafts from cells incubated with BDNF for 1–40 min, in the absence or presence of the A2A receptor agonist CGS 21680 (20 nM), as indicated. Fraction #2 was probed for pTrk, TrkB and Fyn. b, c Densitometry analysis of #2 pTrk staining, normalized by total #2 TrkB (b) and #2 Fyn (c). d Densitometry analysis of #2 TrkB staining, normalized by #2 Fyn. Results are expressed as mean ± SEM of two to three (1 and 20 min) or seven to ten (5 and 40 min) experiments. *p < 0.05, compared to BDNF alone
A2A receptors are not required for BDNF-induced TrkB translocation to lipid rafts
To characterize in more detail the cross-talk between adenosine A2A and TrkB receptors, in the next series of experiments, neurons were incubated with BDNF for 40 min, i.e. in conditions where BDNF per se has a marked effect on TrkB translocation to lipid rafts. Treatment of cortical neurons with BDNF (20 ng/ml) for 40 min significantly increased TrkB levels in fraction #2 (164 ± 17 % of the control, p < 0.01, n = 6, Fig. 4b, c). Activation of adenosine receptors also resulted in an increase up to 140 ± 6 % of the control in TrkB staining in lipid rafts (p < 0.05, n = 6, Fig. 4b, c). When BDNF was applied for 40 min in the presence of CGS 21680, TrkB staining was increased up to 224 ± 20 % of the control (Fig. 4c), roughly the sum of the effect of each drug alone, suggesting the existence of two different pathways involved in TrkB translocation by BDNF and CGS 21680. Furthermore, incubation with an A2A receptor antagonist, ZM 241385 (50 nM), for 30 min prior to BDNF addition did not modify (p > 0.05, n = 5, Fig. 4c) BDNF-induced TrkB translocation to lipid rafts, indicating that A2A receptors are not required for this process.
Fig. 4.
A2A receptors are not required for maximal BDNF-induced TrkB translocation to lipid rafts. DIV 7-11 cortical neurons were starved for 4 h prior to treatment with/without 20 nM CGS 21680, 50 nM ZM 241385 and/or 20 ng/ml BDNF (40 min), as indicated. a Equal volumes of each density gradient fraction were immunoblotted for TrkB and the lipid raft marker Fyn. b Staining of lipid raft fraction #2. Membranes were probed for TrkB, pTrk and Fyn. c Densitometry analysis of TrkB staining in lipid rafts (fraction #2), normalized by #2 Fyn. d Densitometric analysis of the pTrk staining in fraction #2 normalized by Fyn and total TrkB. One hundred percent corresponds to pTrk staining in the presence of BDNF alone. e Total lysates were treated as described in “Materials and methods”, lysed and probed for TrkB, pTrk and β-actin. Results are expressed as mean ± SEM of three to six independent experiments. *p < 0.05; **p < 0.01, ***p < 0.001, compared to 100 %, unless otherwise indicated
When the levels of pTrkB in lipid rafts were analysed, CGS 21680 pre-treatment resulted in a 40 % increase (Fig. 4d) in pTrkB staining in lipid rafts in response to BDNF (vs. BDNF alone). However, this reflects the augmented number of TrkB receptors in these domains induced by CGS 21680 treatment, because the fraction of pTrkB/TrkB is not modified by CGS 21680 (Figs. 3b and 4d). Blockade of A2A receptors with ZM 241385 (50 nM, 30 min) did not induce any significant change in BDNF-induced pTrkB levels in fraction #2 (Fig. 4b, d), further indicating that A2A receptors potentiate, but are not required, for BDNF-induced TrkB phosphorylation in lipid rafts.
No effects of the A2A receptor agonist CGS 21680 and antagonist ZM 241385 on TrkB expression or BDNF-induced (20 ng/ml, 40 min) TrkB phosphorylation were observed in the analysis of total lysates (p > 0.05, n = 3, Fig. 4e).
CGS 21680 and BDNF use different mechanisms to recruit TrkB receptors to lipid rafts
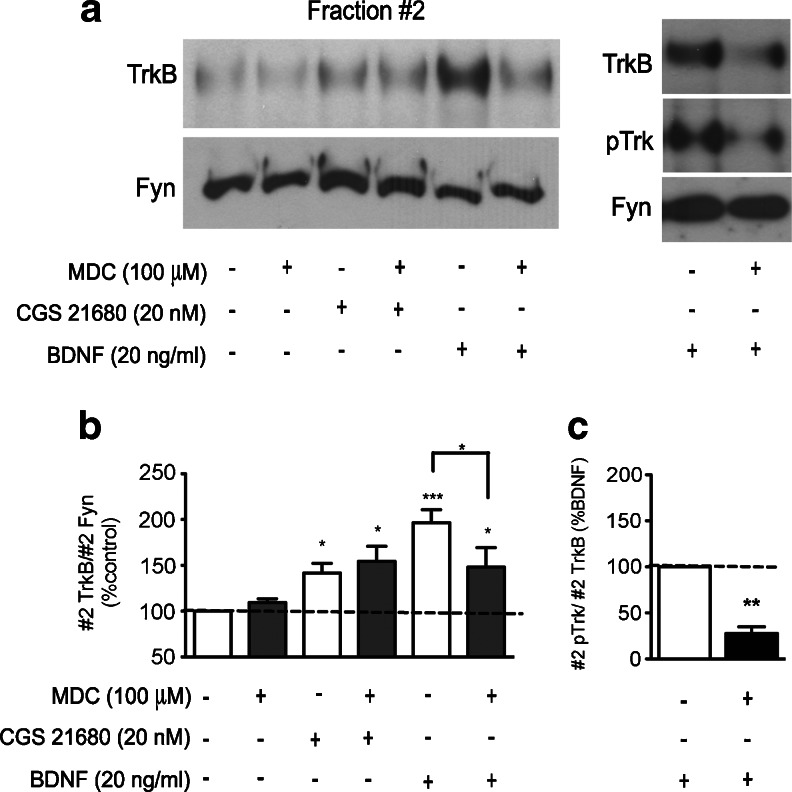
BDNF-induced TrkB translocation to lipid rafts requires TrkB receptor phosphorylation and internalization [41, 52]. It is also known that most of activated Trk receptor internalization takes place through clathrin-coated pits [17, 23, 58]. Although a 30-min incubation with CGS 21680 did not induce TrkB phosphorylation on its own (see Fig. 1c), we investigated the need of TrkB internalization for CGS 21680-induced TrkB translocation to lipid rafts, using the clathrin-dependent endocytosis inhibitor monodansyl cadaverine (MDC) [25, 29]. Alone, MDC did not significantly affect TrkB localization (Fig. 5). Interestingly, MDC did not prevent CGS 21680-induced recruitment of TrkB receptors to lipid rafts (Fig. 5), suggesting that the mechanism used by A2A receptors to translocate TrkB receptors to lipid rafts does not require internalization through clathrin-coated pits. For comparison and as a positive control, we evaluated the influence of MDC on BDNF-induced TrkB translocation. In the presence of MDC, BDNF-induced TrkB translocation to lipid rafts was significantly attenuated (Fig. 5). As expected, BDNF-induced enhancement of pTrkB levels in lipid rafts was markedly reduced in MDC-treated neurons (Fig. 5a, c).
Fig. 5.
Influence of clathrin-dependent endocytosis on CGS 21680-induced and BDNF-induced TrkB recruitment and phosphorylation to lipid rafts. DIV 7-11 cortical neurons were treated with 20 nM CGS 21680 for 30 min or 20 ng/ml BDNF for 40 min in the presence or absence of the clathrin-dependent endocytosis inhibitor monodansyl cadaverine (100 μM), where indicated. a Density gradient fraction #2 was immunoblotted and probed for TrkB, pTrk and Fyn. b Quantification of #2 TrkB/#2 Fyn. c Quantification of #2 pTrk/#2 TrkB after BDNF treatment, in the presence/absence of MDC, as indicated below each bar. Data are expressed as mean ± SEM of five to seven independent experiments. *p < 0.05; **p < 0.01, ***p < 0.001, compared to 100 %, unless otherwise indicated. Note that the clathrin-dependent endocytosis inhibitor, MDC, attenuated BDNF-induced TrkB translocation to lipid rafts, but did not influence CGS 21680-induced TrkB recruitment to these membrane domains
The effects of CGS 21680 upon TrkB translocation involve cAMP and Src-family tyrosine kinase activation
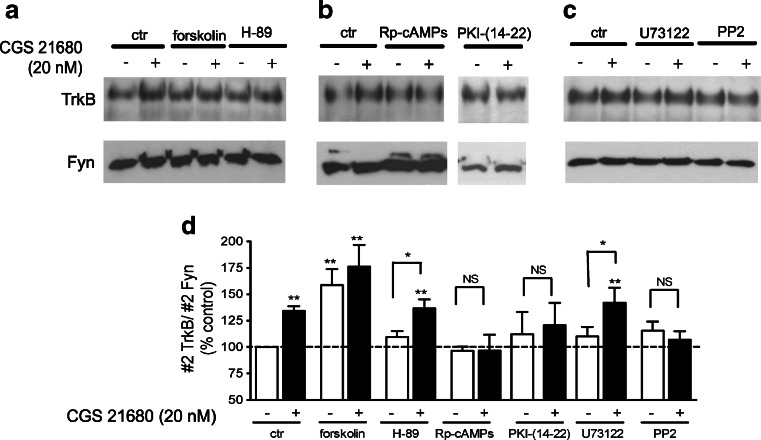
Since A2A receptor-induced translocation of TrkB receptors to lipid rafts is independent of TrkB phosphorylation and internalization (Fig. 5), occurring through a process different from that used by BDNF, we further evaluated the mechanisms involved in this process. Adenosine A2A receptors are primarily coupled to Gs proteins, and most of its actions involve activation of adenylyl cyclase. We first investigated the role of cAMP for CGS 21680-induced TrkB translocation by using forskolin, a known adenylyl cyclase activator [2]. As shown in Fig. 6 (a and d), forskolin (5 μM) mimicked the effect of CGS 21680 in inducing TrkB translocation to lipid rafts. In cells incubated in the presence of forskolin, CGS 21680 did not cause a further enhancement of TrkB staining in fraction #2 (Fig. 6a, d). We then investigated the role of protein kinase A, one of the main cAMP effectors, on CGS21680-induced TrkB translocation to lipid rafts. We used Rp-cAMPs, an inactive analogue of cAMP that inhibits activation of PKA by substrate competition [36]. In cells incubated with Rp-cAMPs (100 μM), the effect of CGS 21680 on TrkB receptor translocation to lipid rafts was fully prevented (Fig. 6b, d), supporting a role for PKA in this process. Moreover, the PKA inhibitor peptide PKI-(14-22) also prevented the effect of CGS 21680 in TrkB receptor recruitment to lipid rafts (Fig. 6b, d). Unexpectedly, incubation with H-89 (1 μM) did not block the effect of CGS21680 upon TrkB localization (Fig. 6a, d). This discrepancy might result from an H-89-induced inhibition of other kinases [34, 36, 39] that might counteract the consequences of PKA inhibition; alternatively, it may result from an inefficient inhibition of PKA by the concentration of H-89 used, since the IC50 for PKA inhibition by H-89 is highly dependent on the intracellular ATP concentration [39]. Higher concentrations of H-89 were not tested due to lack of selectivity. Neither 100 μM Rp-cAMPs, 1 μM PKI-(14-22) nor 1 μM H-89 per se influenced the levels of TrkB on lipid rafts when added in the absence of CGS 21680 (Fig. 6d).
Fig. 6.
Signalling pathways involved in CGS 21680-induced TrkB translocation to lipid rafts. Cultured cortical neurons were incubated with/without 20 nM CGS 21680 for 30 min in the presence of the adenylate cyclase activator forskolin (FSK, 5 μM), the PKA inhibitor H-89 (1 μM), the cAMP antagonist Rp-cAMPs (100 μM), the PKA inhibitor PKI-(14-22) (1 μM), the phospholipase C inhibitor U73122 (4 μM) or the Src-family kinase inhibitor PP2 (500 nM), as indicated. Cells were lysed and processed for lipid raft isolation. a–c Fraction #2 obtained from the density gradients of cells under different conditions were probed for total TrkB and Fyn, which was used as a loading control. d Densitometry analysis of TrkB/Fyn staining obtained in a–c. Data are expressed as mean ± SEM of four to nine independent experiments. *p < 0.05; **p < 0.01; NS, no statistical difference (p > 0.05), compared to 100 %, except when otherwise indicated
The possible involvement of other transduction pathways that can also be activated by A2A agonists, such as phospholipase C (PLC) (see [21]) and Src-family tyrosine kinases [45], was also investigated. Figure 6c shows the influence of the PLC inhibitor, U73122 (4 μM) [4], and the Src-family tyrosine kinase inhibitor, PP2 (0.5 μM) [3], on CGS 21680-induced TrkB translocation to lipid rafts. The PLC inhibitor did not prevent the CGS 21680 effect on TrkB staining in fraction #2. Importantly, PP2 blocked the effect of CGS 21680 on TrkB localization to lipid rafts, suggesting an involvement of Src-family tyrosine kinases (Fig. 6c, d).
Influence of membrane cholesterol content upon TrkB and A2A receptors
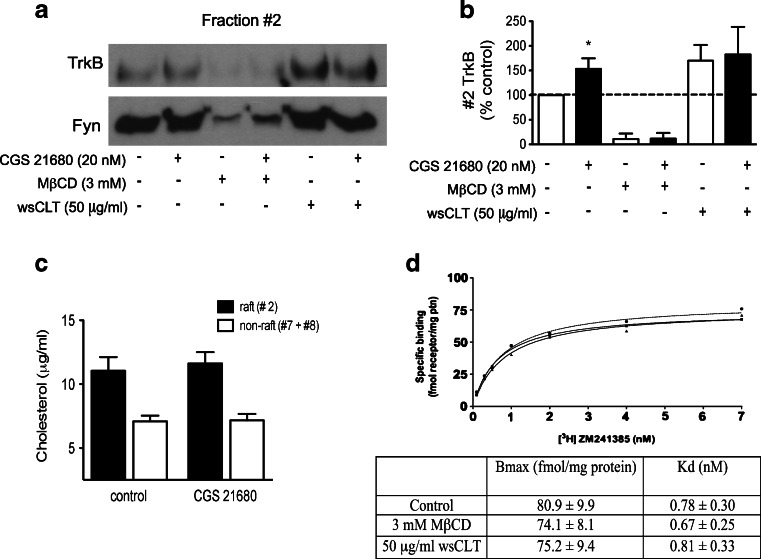
To investigate the role of cholesterol and lipid raft integrity in CGS 21680-induced TrkB translocation to lipid rafts, we used the cholesterol-sequestering agent MβCD [51]. Incubation of cortical neurons with 3 mM MβCD alone decreased TrkB levels in the lipid rafts. This reduction in TrkB staining was accompanied by a decrease of the lipid raft marker Fyn in fraction #2 (Fig. 7a). In the presence of MβCD, adenosine A2A receptor activation with CGS 21680 did not induce any detectable increase in TrkB levels in fraction #2 (Fig. 7a, b). These results show that MβCD disturbed lipid raft integrity, and that under these conditions, A2A receptor agonists were no longer able to induce TrkB translocation.
Fig. 7.
Effects of cholesterol depletion and loading on the partition of TrkB receptors to lipid rafts and on adenosine A2A receptor binding properties. Cultured cortical neurons were treated with/without 20 nM CGS 21680 for 30 min after incubation with 3 mM MβCD or MβCD–cholesterol complexes (water-soluble cholesterol, wsCLT, 50 μg/ml cholesterol). a, b Lipid raft (fraction #2) analysis of the effect of MβCD on TrkB sublocalization after CGS 21680 treatment. MβCD–cholesterol complexes were used to load cholesterol to membranes. c Cholesterol content in lipid raft (#2) and non-raft fractions (#8) [56] in control conditions and after 20 nM CGS 21680 incubation for 30 min. d Saturation curves for the specific binding of the A2A receptor antagonist [3H] ZM 241385 in cortical membranes from cells in control conditions (open circles), after incubation with MβCD (closed squares) or with wsCLT (closed triangles) treatment. Data are expressed as mean ± SEM of three to five independent experiments. *p < 0.05, compared to 100 %
Another approach was to use MβCD–cholesterol complexes (50 μg/ml cholesterol or wsCLT) to load cells with cholesterol. The MβCD/cholesterol ratio used was 6:1, which is considered optimal for cell loading [11]. This treatment led to an increased TrkB partition to lipid rafts, indicating that there is a cholesterol-dependent regulation of TrkB receptor localization in membrane subdomains (Fig. 7a, b). Interestingly, in cholesterol-loaded cells, incubation with CGS 21680 (20 nM) did not cause a further enhancement of TrkB receptor staining in the lipid raft faction (Fig. 7b), suggesting that under conditions of high cholesterol and TrkB receptor localization in lipid rafts, A2A receptors are no longer able to promote further TrkB receptor translocation.
To elucidate whether adenosine A2A receptor activation could directly influence the amount of cholesterol in lipid rafts, we measured cholesterol content in the lipid raft fraction (#2) and in the non-raft fractions (#7 and #8), after CGS 21680 treatment. No changes were observed in the cholesterol levels present in the raft fraction #2 (11.0 ± 1.1 in control and 11.6 ± 0.9 μg/ml after CGS 21680, p > 0.05, n = 5) or in the non-raft fractions #7 and #8 (7.1 ± 0.4 in control and 7.8 ± 0.5 μg/ml after CGS 21680, p > 0.05, n = 5, Fig. 7c).
To examine the influence of lipid rafts upon adenosine A2A receptors, saturation binding experiments were performed using the selective A2A antagonist [3H] ZM 241385. Membrane samples were incubated in the presence of MβCD and MβCD–cholesterol complexes. As illustrated in Fig. 7d, neither lipid raft disruption with 3 mM MβCD nor cholesterol enrichment with 50 μg/ml wsCLT resulted in changes in the parameters for [3H] ZM 241385 binding (p > 0.05, n = 3). These results do not exclude the importance of lipid rafts for optimal A2A receptor signalling [30], but strongly suggest that ligand affinity and density of this receptor are conserved while interfering with lipid raft integrity.
BDNF-induced facilitation of glutamate release depends on endogenous adenosine and lipid raft integrity
The functional relevance of A2A-induced TrkB translocation to lipid rafts was first assessed by investigating the effect of BDNF upon glutamate release after extracellular adenosine depletion and lipid raft disruption. Cortical synaptosomes were labelled with [3H] glutamate as previously [8], and neurotransmitter release was evoked twice (S1 and S2) by perfusion with 15 mM KCl for 2 min (see “Materials and methods”). In control conditions, the S2/S1 ratio was 0.79 ± 0.02 (n = 14); when BDNF (20 ng/ml) was added before S2, the ratio was increased to 1.05 ± 0.02 (p < 0.01, n = 14), corresponding to a 33 ± 3 % enhancement in evoked glutamate release (Fig. 8). To investigate how endogenous extracellular adenosine influences this effect of BDNF, synaptosomes were incubated with the adenosine-degrading enzyme ADA, which was added before S1 and remained in the perfusion up to the end of sample collection (see “Materials and methods”). As shown in Fig. 8b, the removal of endogenous adenosine with 1 U/ml ADA completely prevented the effect of BDNF upon glutamate release. The presence of ADA (1 U/ml) during S1 and S2 did not affect the S2/S1 ratio, which in the control conditions was 0.81 ± 0.04 and in the presence of ADA was 0.84 ± 0.05 (p > 0.05, n = 3).
Fig. 8.
BDNF increases glutamate release in an adenosine- and lipid raft-dependent manner. a Averaged time course of [3H] glutamate release from cortical synaptosomes. Synaptosomes were labelled with [3H] glutamate and stimulation of neurotransmitter release was induced twice, at 5–7 min (S1) and 29–31 min (S2), as described in “Materials and methods”. Samples were collected every 2 min. BDNF (20 ng/ml) was added at 9 min and remained in the perfusion solution until the end of the experiments (closed circles). Control curves in the absence of any drug, performed in parallel with the same synaptosomal batch, are represented by the open circles. b, c, S2/S1 ratios, calculated in each experiment from the time courses curves, as described in “Materials and methods”. BDNF (20 ng/ml) was tested in the presence/absence of 1 U/ml ADA, 1 mM MβCD or 2 U/ml cholesterol oxidase (Chol.Oxi.), as indicated below each bar. In each experiment, the S2/S1 ratio obtained while BDNF was present during S2 was normalized taking as 100 % the S2/S1 ratio obtained in parallel chambers under the same drug conditions but absence of BDNF. Data are represented as mean ± SEM of three to six independent experiments. *p < 0.05; **p < 0.01, ***p < 0.001, compared to 100 %, except when otherwise indicated
The relevance of lipid rafts was first studied by perfusing synaptosomes with MβCD. Per se, 1 mM MβCD treatment caused a slight increase (12 ± 3 %, n = 3) in the basal release of tritium, but did not influence S2/S1 ratios (percent change, 2.2 ± 2.5 %; n = 3) or the amount of tritium released in response to K+ stimulation (1.3 ± 4 % change, n = 3), indicating that K+-evoked glutamate release was not compromised at this concentration of MβCD. In synaptosomes incubated with MβCD, the BDNF-induced enhancement of glutamate release was significantly smaller than in control conditions (Fig. 8c), thus supporting a relevant role of lipid rafts in BDNF-induced modulation of glutamate release. However, BDNF was still able to induce a small but significant increase upon release (Fig. 8c). This could be explained either by incomplete disruption of lipid rafts with 1 mM MβCD or by involvement of lipid raft-independent mechanisms in the effect of BDNF upon glutamate release. Higher concentrations of MβCD (2–3 mM) caused a marked increase in basal release of tritium (data not shown), probably due to compromised synaptosomal integrity, precluding the possibility of testing higher concentrations of MβCD. Alternatively, we used cholesterol oxidase, an enzyme that converts cholesterol into 4-cholesten-3-one, altering the structure of rafts [27, 29]. As shown in Fig. 8c, pre-incubation of synaptosomes with 2 U/ml cholesterol oxidase abolished the effect of BDNF upon glutamate release (p < 0.01, n = 4). Taken together, these data suggest that BDNF enhances glutamate release through an adenosine- and lipid raft-dependent mechanism.
Interestingly, cholesterol addition (MβCD–cholesterol complexes, 50 μg/ml) did not modify (p > 0.05, n = 6) the facilitatory action of BDNF upon glutamate release, suggesting that either the endogenous cholesterol levels were enough to trigger the maximal effect of BDNF and/or synaptosomes were not able to efficiently incorporate exogenously added cholesterol to their membranes.
Neither the presence of MβCD (1 mM), cholesterol oxidase (2 U/ml) nor wsCLT (50 μg/ml) during S1 and S2 affected the S2/S1 ratio. In the MβCD experiments, S2/S1 ratio in the control was 0.75 ± 0.02 and in the presence of MβCD was 0.77 ± 0.02 (p > 0.05, n = 6). In the cholesterol oxidase experiments, S2/S1 ratio in the control was 0.81 ± 0.03 and in the presence of cholesterol oxidase was 0.85 ± 0.04 (p > 0.05, n = 5). In the exogenous cholesterol experiments, S2/S1 ratio in the control was 0.74 ± 0.03 and in the presence of excess cholesterol was 0.76 ± 0.03 (p > 0.05, n = 6).
High-frequency stimulation of hippocampal slices increases TrkB and pTrkB receptor localization in lipid rafts in an adenosine-dependent manner
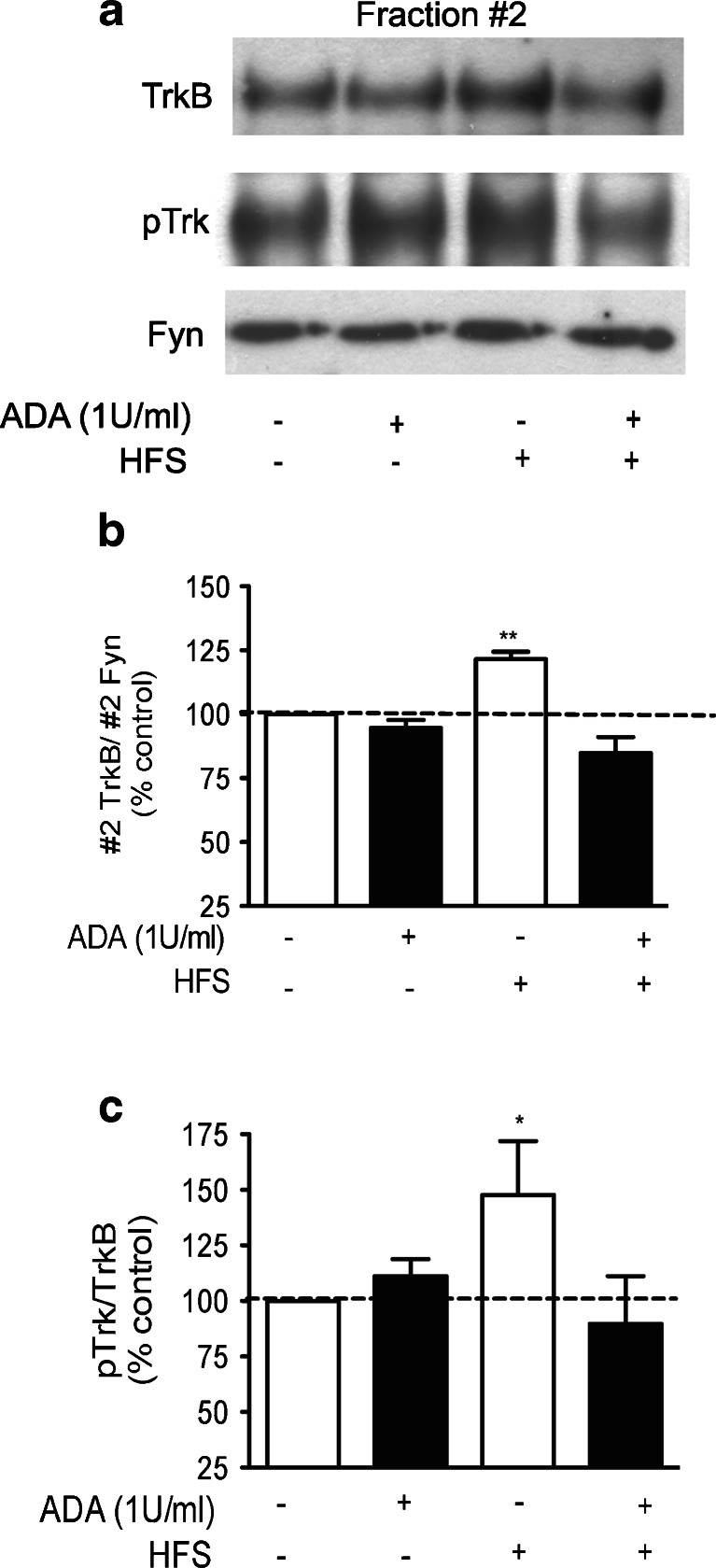
BDNF release is stimulated after increased neuronal activity (Figurov, 1996 #666), but it is still not understood how such a diffusible molecule preferentially potentiates active synapses. Neuronal activity and increased intracellular cAMP levels have been shown to gate synaptic actions of BDNF [5, 6, 40]. Adenosine concentration is also markedly increased under high-frequency neuronal firing, due to catabolism of endogenously released ATP [14, 55] and this ATP-derived adenosine preferentially activates A2A receptors [13]. We have previously demonstrated a potentiating effect of BDNF on hippocampal LTP that requires the activation of A2A receptors by endogenous adenosine [20], supporting a role for A2A-induced gating of BDNF effects upon synaptic plasticity. Therefore, we hypothesized that activity-derived activation of A2A receptors by endogenous adenosine could induce TrkB translocation to lipid rafts. To test this hypothesis, hippocampal slices were stimulated for 1 min using a high-frequency paradigm that was shown to induce the release of ATP and adenosine (see “Materials and methods”) [14]. After 30 min, the lipid rafts were isolated from slice lysates and fraction #2 was analysed by immunoblotting. As illustrated in Fig. 9, field stimulation of hippocampal slices induced TrkB translocation to lipid rafts (121.5 ± 3 % of the control, Fig. 9, p < 0.001, n = 6). Field stimulation of hippocampal slices also induced a significant increase in the pTrkB staining and the pTrkB/TrkB ratio in fraction #2 (Fig. 9, p < 0.05, n = 7). The role of endogenous adenosine in high-frequency stimulation-induced recruitment of TrkB receptors into lipid rafts was investigated by incubation of the slices with the ADA (1 U/ml) from 30 min prior to the high-frequency stimulation until the end of the experiment. Under these conditions, the effect of field stimulation upon both TrkB and pTrkB receptor localization was completely prevented (Fig. 9b), supporting the hypothesis that adenosine released during intense synaptic activity plays a role in targeting TrkB receptors to lipid rafts.
Fig. 9.
High-frequency stimulation of hippocampal slices induces TrkB translocation and increases pTrk staining in lipid rafts in an adenosine-dependent manner. Hippocampal slices were superfused with 1 U/ml ADA, where indicated, 30 min prior to the high-frequency stimulation (HFS). HFS was applied for 1 min as described in “Materials and methods”, and after 30 min, slices were homogenized and lipid rafts were isolated by discontinuous Optiprep gradients. a Fraction #2, containing lipid raft membranes, was immunoblotted for TrkB, pTrk and Fyn, which was used as a loading control. b Quantifications of #2 TrkB/#2 Fyn. c Quantifications of #2 pTrk/#2 Fyn. Data are represented as mean ± SEM of five to seven independent experiments. *p < 0.05, **p < 0.01, compared to 100 %
Lipid raft integrity is required for BDNF-induced facilitation of long-term potentiation
Since extracellular adenosine is required for BDNF-induced enhancement of LTP [20] and for high-frequency stimulation-induced TrkB translocation to lipid rafts (Fig. 9), we investigated whether lipid rafts were required for the facilitation of LTP induced by BDNF. The LTP-inducing protocol was similar to that previously used to detect facilitatory actions of endogenous or exogenous BDNF upon LTP [10, 20, 32]. To evaluate the influence of lipid raft disruption on the facilitatory effect of BDNF upon LTP, MβCD was used at a low concentration to avoid marked changes in synaptic integrity that could cause alterations in the synaptic plasticity phenomena. Hippocampal slices were incubated with MβCD (1 mM), for 30 min before and during the entire LTP experiment. At this concentration, MβCD had a very mild effect on basal synaptic transmission (−4.6 ± 0.4 %), as shown in Fig. 10b. Furthermore, in MβCD-treated slices, the magnitude of LTP was similar to what was observed in other slices in the absence of MβCD (Fig. 10h, open bars). Most importantly, the magnitude of LTP in two consecutive pathways on the same slice (see “Materials and methods”) was similar in the presence of MβCD throughout the entire LTP-inducing protocol (p > 0.05, n = 3, Fig. 10c). This allowed us to study the modulatory role of BDNF upon LTP, in slices that were in the presence of MβCD throughout the entire recording period. To evaluate the effect of BDNF upon LTP, we compared the magnitude of LTP in the first pathway (absence of BDNF) with that in the second pathway (in the presence of BDNF), in the same slice. The effect of BDNF on LTP in the absence or in the presence of MβCD was then compared.
As expected from previous reports (e.g. [20]), BDNF (20 ng/ml) enhanced (p < 0.05, n = 6, Fig. 10d, h) the magnitude of LTP in control slices (Fig. 10d). In contrast, in MβCD-treated slices, the facilitatory effect of BDNF upon LTP was not observed (p > 0.05, n = 5, Fig. 10e, h), indicating that lipid raft integrity is necessary for the facilitatory actions of BDNF upon hippocampal synaptic plasticity.
Discussion
The main finding of the present work is that adenosine A2A receptor activation increases the levels of TrkB receptors in lipid rafts and potentiates BDNF-induced TrkB phosphorylation in these membrane microdomains. Furthermore, relevant actions of BDNF at synapses, such as facilitation of glutamate release and synaptic plasticity, require both lipid raft integrity and A2A receptor activation. Altogether, our data suggest that A2A receptors contribute to the translocation of TrkB receptors towards specific membrane areas where TrkB activation and subsequent signalling occur. Noteworthy, this is the first evidence for BDNF-independent TrkB translocation to lipid rafts.
Active A2A receptors were not required for BDNF-induced recruitment of TrkB receptors, since the presence of an A2A receptor antagonist did not influence BDNF-induced translocation to lipid rafts. Furthermore, the effects of the A2A receptor agonist and of BDNF on TrkB translocation were additive. Inhibition of clathrin-dependent endocytosis with MDC did not influence the action of the A2A receptor agonist, but significantly reduced BDNF-induced TrkB translocation to lipid rafts. Taken together, these results strongly suggest that A2A agonists and BDNF act through different mechanisms to recruit TrkB receptors to lipid rafts.
We observed that incubation of cells with BDNF for 5 min did not recruit detectable amounts of TrkB receptors to lipid rafts; however, under the same conditions, we could detect pTrkB staining in these microdomains. This suggests that either BDNF phosphorylates a small amount of TrkB receptors already present in the lipid rafts and/or that BDNF-induced translocation of pTrkB was not detectable in the analysis of total TrkB receptors. Adenosine A2A receptor activation markedly increased the levels of TrkB receptors in the lipid rafts and potentiated BDNF-induced pTrkB staining in lipid rafts from cells incubated for a short period (5 min) with BDNF. It is possible that the increased concentration of TrkB receptors in lipid rafts induced by CGS 21680 leads to increased proximity and auto-phosphorylation of TrkB receptors not fully phosphorylated by a short BDNF exposure. In contrast, after BDNF treatment for 40 min, TrkB localization in lipid rafts is already high and probably maximally activated. Accordingly, a further increase in TrkB concentration in these membrane domains induced by A2A receptor activation did not change the proportion of pTrkB (as a function of total TrkB receptors) in lipid rafts. However, the total amount of pTrkB receptors was higher in cells incubated for 40 min with BDNF in the presence of the A2A agonist. Altogether, the data suggest that activation of A2A receptors per se induces translocation to lipid rafts of TrkB receptors that are prone to be phosphorylated by BDNF. Additionally, A2A receptor-induced clustering of TrkB receptors in lipid rafts may play a role in the facilitatory effects of A2A agonists on TrkB receptor function at synapses, since BDNF-induced TrkB phosphorylation in lipid rafts may be facilitated as a consequence of increased receptor proximity.
It is unlikely that the influence of A2A receptors upon TrkB translocation is due to a facilitation of endogenous BDNF actions or TrkB transactivation. This conclusion is supported by the lack of detectable amounts of pTrkB receptors in lysates or in lipid rafts isolated from cells incubated with the A2A receptor agonist (Fig. 1), whereas pTrkB receptors could be detected in lipid rafts isolated from cells incubated with BDNF. Furthermore, the inhibitor of clathrin-dependent endocytosis, MDC, differently affects BDNF-induced and CGS21680-induced TrkB translocation. Moreover, transactivation of TrkB receptors, i.e. phosphorylation of TrkB receptors in the absence of BDNF, requires prolonged (2–3 h) exposure to A2A receptor agonists and mostly involves intracellular TrkB receptors [33, 46].
Although MDC treatment significantly attenuated BDNF-induced TrkB translocation to lipid rafts, some receptors were still recruited by BDNF in the presence of MDC. This may suggest that BDNF is able to recruit some TrkB receptors to lipid rafts independently of internalization through clathrin-coated pits. Alternatively, MDC treatment may not have fully inhibited clathrin-dependent endocytosis, and/or compensatory mechanisms of receptor internalization may account for the effect of BDNF observed in the presence of MDC.
Neither BDNF nor A2A receptor activation induced translocation of the truncated TrkB receptors to lipid rafts, suggesting that A2A receptors act on the intracellular domain of TrkB receptors to induce its recruitment to lipid rafts. The tyrosine kinase Fyn is possibly one of the mediators, as inhibition of Src-family kinases prevented the influence of A2A receptors on TrkB translocation. Indeed, Fyn can be activated by A2A agonists, and it is known that Fyn is required for TrkB localization in lipid rafts [41, 45]. The requirement of cAMP for the effect of CGS 21680 on TrkB translocation suggests that A2A receptors are operating through the adenylyl cyclase/cAMP transduction pathway. Accordingly, the adenylyl cyclase activator, forskolin, mimicked the effect of the A2A receptor agonist upon TrkB translocation.
Modifications of the cholesterol content in the cells by treatment with a cholesterol-chelating compound, such as MβCD, or by addition of cholesterol fully prevented the effects of A2A receptor agonists on TrkB translocation to lipid rafts, indicating the need of optimal cholesterol levels for this process. When cells were loaded with excess cholesterol, TrkB localization in lipid rafts was increased, possibly due to alterations in size and/or properties of the lipid rafts. This may influence TrkB partition on different membrane domains, affecting the ability of A2A receptors to modulate their translocation. Excess cholesterol, however, did not increase BDNF-induced glutamate release, which further support the concept of a tightly regulated cholesterol concentration in the lipid rafts for optimal partition of proteins among these domains.
As previously observed in cultured neurons [52], the facilitatory effect of BDNF upon glutamate release from acutely isolated nerve endings was affected by lipid raft disruption. Moreover, we showed that lipid raft-disturbing drugs also prevent the facilitatory action of BDNF upon LTP. Additionally, removal of endogenous extracellular adenosine or blockade of A2A receptors prevented BDNF actions upon glutamate release (present work), synaptic transmission and LTP [15, 16, 20]. Taken together, this evidence strongly suggests that the facilitatory action of BDNF at glutamatergic synapses requires lipid raft integrity as well as the presence of extracellular adenosine and A2A receptor activation.
As occurs with A2A receptor agonists [16, 20] and cAMP [6], high-frequency neuronal firing triggers facilitatory actions of BDNF at excitatory synapses [6, 19, 37, 40]. We therefore investigated if TrkB receptors could be targeted to lipid rafts as a consequence of intense synaptic activity. We observed that high-frequency stimulation of hippocampal slices results in a higher density of both TrkB and pTrkB receptors in lipid rafts, an effect completely abolished when endogenous extracellular adenosine was removed. To our knowledge, this is the first demonstration of activity-dependent recruitment and activation of TrkB receptors in lipid rafts, and notably, this is fully dependent on the presence of extracellular adenosine.
The levels of extracellular adenosine at synapses are tightly regulated and fluctuate according to the rate of neuronal firing (for a review, see [49]). Upon high-frequency neuronal firing, the release of the adenosine precursor ATP is increased [55], A2A receptor activation is favoured [12] and adenosine inactivation systems are inhibited [43]. We demonstrated that TrkB translocation to lipid rafts is facilitated by high-frequency neuronal firing, A2A receptor activation and by adenylate cyclase/cAMP, the transducing system operated by A2A receptors. Furthermore, the actions of BDNF upon glutamate release and synaptic plasticity require both lipid raft integrity and endogenous extracellular adenosine. Altogether, the data here reported strongly suggest that A2A receptor-induced TrkB translocation to lipid rafts plays an important part in the mechanism through which enhanced neuronal activity, A2A receptor activation and cyclic AMP facilitate BDNF actions at active synapses.
Acknowledgments
This work, N.A.L., V.C.S. and D.B.P. were supported by Fundação para a Ciência e a Tecnologia (SFRH/BD/21374/2005 for N.A.L., SFRH/BD/21359/2005 for V.C.S. and SFRH/BPD/11528/2002 for D.B.P.) and by the European Union (COST B30 concerted action, NEREPLAS). We thank Regeneron Pharmaceuticals for the gift of BDNF, the Institute of Physiology of the Faculty of Medicine of Lisbon for the animal house facility, Ana Rita Costenla for help with the LTP experiments, W.W. Anderson (University of Bristol, Bristol, UK) for the gift of the electrophysiology data analysis (LTP) program, Cristina Casalou from Centro de Investigação em Patobiologia Molecular, IPO, for access to the ultracentrifuge and Moses Chao from Skirball Institute, New York University, for assistance in the initial experiments.
References
- 1.Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/S0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- 2.Awad JA, Johnson RA, Jakobs KH, Schultz G. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J Biol Chem. 1983;258:2960–2965. [PubMed] [Google Scholar]
- 3.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 5.Boulanger L, Poo MM. Gating of BDNF-induced synaptic potentiation by cAMP. Science. 1999;284:1982–1984. doi: 10.1126/science.284.5422.1982. [DOI] [PubMed] [Google Scholar]
- 6.Boulanger L, Poo MM. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Canas N, Pereira IT, Ribeiro JA, Sebastiao AM. Brain-derived neurotrophic factor facilitates glutamate and inhibits GABA release from hippocampal synaptosomes through different mechanisms. Brain Res. 2004;1016:72–78. doi: 10.1016/j.brainres.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 9.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 12.Correia-de-Sa P, Timoteo MA, Ribeiro JA. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J Neurophysiol. 1996;76:3910–3919. doi: 10.1152/jn.1996.76.6.3910. [DOI] [PubMed] [Google Scholar]
- 13.Cunha RA, Correia-de-Sa P, Sebastiao AM, Ribeiro JA. Preferential activation of excitatory adenosine receptors at rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br J Pharmacol. 1996;119:253–260. doi: 10.1111/j.1476-5381.1996.tb15979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunha RA, Vizi ES, Ribeiro JA, Sebastiao AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- 15.Diógenes MJ, Assaife-Lopes N, Pinto-Duarte A, Ribeiro JA, Sebastiao AM. Influence of age on BDNF modulation of hippocampal synaptic transmission: interplay with adenosine A2A receptors. Hippocampus. 2007;17:577–585. doi: 10.1002/hipo.20294. [DOI] [PubMed] [Google Scholar]
- 16.Diogenes MJ, Fernandes CC, Sebastiao AM, Ribeiro JA. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci. 2004;24:2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du J, Feng L, Zaitsev E, Je HS, Liu XW, Lu B. Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx. J Cell Biol. 2003;163:385–395. doi: 10.1083/jcb.200305134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fielding CJ, Fielding PE. Membrane cholesterol and the regulation of signal transduction. Biochem Soc Trans. 2004;32:65–69. doi: 10.1042/BST0320065. [DOI] [PubMed] [Google Scholar]
- 19.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 20.Fontinha BM, Diogenes MJ, Ribeiro JA, Sebastiao AM. Enhancement of long-term potentiation by brain-derived neurotrophic factor requires adenosine A2A receptor activation by endogenous adenosine. Neuropharmacology. 2008;54:924–933. doi: 10.1016/j.neuropharm.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 22.Fredholm BB, Cunha RA, Svenningsson P. Pharmacology of adenosine A2A receptors and therapeutic applications. Curr Top Med Chem. 2003;3:413–426. doi: 10.2174/1568026033392200. [DOI] [PubMed] [Google Scholar]
- 23.Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/S0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 25.Haigler HT, Maxfield FR, Willingham MC, Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980;255:1239–1241. [PubMed] [Google Scholar]
- 26.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herincs Z, Corset V, Cahuzac N, Furne C, Castellani V, Hueber AO, Mehlen P. DCC association with lipid rafts is required for netrin-1-mediated axon guidance. J Cell Sci. 2005;118:1687–1692. doi: 10.1242/jcs.02296. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi H, Yamashita T, Yoshikawa H, Tohyama M. PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. Embo J. 2003;22:1790–1800. doi: 10.1093/emboj/cdg177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008;440:15–33. doi: 10.1007/978-1-59745-178-9_2. [DOI] [PubMed] [Google Scholar]
- 30.Keuerleber S, Thurner P, Gruber CW, Zezula J, Freissmuth M. Reengineering the collision coupling and diffusion mode of the A2A-adenosine receptor: palmitoylation in helix 8 relieves confinement. J Biol Chem. 2012;287:42104–42118. doi: 10.1074/jbc.M112.393579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komaki S, Ishikawa K, Arakawa Y. Trk and cAMP-dependent survival activity of adenosine A(2A) agonist CGS21680 on rat motoneurons in culture. Neurosci Lett. 2012;522:21–24. doi: 10.1016/j.neulet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leemhuis J, Boutillier S, Schmidt G, Meyer DK. The protein kinase A inhibitor H89 acts on cell morphology by inhibiting Rho kinase. J Pharmacol Exp Ther. 2002;300:1000–1007. doi: 10.1124/jpet.300.3.1000. [DOI] [PubMed] [Google Scholar]
- 35.Lim KI, Yin J. Localization of receptors in lipid rafts can inhibit signal transduction. Biotechnol Bioeng. 2005;90:694–702. doi: 10.1002/bit.20464. [DOI] [PubMed] [Google Scholar]
- 36.Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev. 2006;24:261–274. doi: 10.1111/j.1527-3466.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto T, Numakawa T, Yokomaku D, Adachi N, Yamagishi S, Numakawa Y, Kunugi H, Taguchi T. Brain-derived neurotrophic factor-induced potentiation of glutamate and GABA release: different dependency on signaling pathways and neuronal activity. Mol Cell Neurosci. 2006;31:70–84. doi: 10.1016/j.mcn.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Mojsilovic-Petrovic J, Jeong GB, Crocker A, Arneja A, David S, Russell DS, Kalb RG. Protecting motor neurons from toxic insult by antagonism of adenosine A2a and Trk receptors. J Neurosci. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal. 2008;1:re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- 40.Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Pereira DB, Chao MV. The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts. J Neurosci. 2007;27:4859–4869. doi: 10.1523/JNEUROSCI.4587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Pinto-Duarte A, Coelho JE, Cunha RA, Ribeiro JA, Sebastiao AM. Adenosine A2A receptors control the extracellular levels of adenosine through modulation of nucleoside transporters activity in the rat hippocampus. J Neurochem. 2005;93:595–604. doi: 10.1111/j.1471-4159.2005.03071.x. [DOI] [PubMed] [Google Scholar]
- 44.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 45.Rajagopal R, Chao MV. A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol Cell Neurosci. 2006;33:36–46. doi: 10.1016/j.mcn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sebastiao AM, Colino-Oliveira M, Assaife-Lopes N, Dias RB, Ribeiro JA. Lipid rafts, synaptic transmission and plasticity: impact in age-related neurodegenerative diseases. Neuropharmacology. 2013;64:97–107. doi: 10.1016/j.neuropharm.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 48.Sebastiao AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol. 2009;193:471–534. doi: 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- 49.Sebastiao AM, Ribeiro JA. Triggering neurotrophic factor actions through adenosine A2A receptor activation: implications for neuroprotection. Br J Pharmacol. 2009;158:8. doi: 10.1111/j.1476-5381.2009.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sepulveda MR, Berrocal-Carrillo M, Gasset M, Mata AM. The plasma membrane Ca2+−ATPase isoform 4 is localized in lipid rafts of cerebellum synaptic plasma membranes. J Biol Chem. 2006;281:447–453. doi: 10.1074/jbc.M506950200. [DOI] [PubMed] [Google Scholar]
- 51.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, Mei L, Lu B, Kojima M. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol. 2004;167:1205–1215. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki T, Zhang J, Miyazawa S, Liu Q, Farzan MR, Yao WD. Association of membrane rafts and postsynaptic density: proteomics, biochemical, and ultrastructural analyses. J Neurochem. 2011;119:64–77. doi: 10.1111/j.1471-4159.2011.07404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tebano MT, Martire A, Potenza RL, Gro C, Pepponi R, Armida M, Domenici MR, Schwarzschild MA, Chen JF, Popoli P. Adenosine A(2A) receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J Neurochem. 2008;104:279–286. doi: 10.1111/j.1471-4159.2007.05046.x. [DOI] [PubMed] [Google Scholar]
- 55.Wieraszko A, Goldsmith G, Seyfried TN. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 1989;485:244–250. doi: 10.1016/0006-8993(89)90567-2. [DOI] [PubMed] [Google Scholar]
- 56.Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, Chao MV, Sendtner M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci U S A. 2007;104:17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C, Butz S, Ying Y, Anderson RG. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J Biol Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- 58.Zheng J, Shen WH, Lu TJ, Zhou Y, Chen Q, Wang Z, Xiang T, Zhu YC, Zhang C, Duan S, Xiong ZQ. Clathrin-dependent endocytosis is required for TrkB-dependent Akt-mediated neuronal protection and dendritic growth. J Biol Chem. 2008;283(19):13280–8. doi: 10.1074/jbc.M709930200. [DOI] [PubMed] [Google Scholar]