Abstract
D1- and D2-types of dopamine receptors are located separately in direct and indirect pathway striatal projection neurons (dSPNs and iSPNs). In comparison, adenosine A1-type receptors are located in both neuron classes, and adenosine A2A-type receptors show a preferential expression in iSPNs. Due to their importance for neuronal excitability, Ca2+-currents have been used as final effectors to see the function of signaling cascades associated with different G protein-coupled receptors. For example, among many other actions, D1-type receptors increase, while D2-type receptors decrease neuronal excitability by either enhancing or reducing, respectively, CaV1 Ca2+-currents. These actions occur separately in dSPNs and iSPNs. In the case of purinergic signaling, the actions of A1- and A2A-receptors have not been compared observing their actions on Ca2+-channels of SPNs as final effectors. Our hypotheses are that modulation of Ca2+-currents by A1-receptors occurs in both dSPNs and iSPNs. In contrast, iSPNs would exhibit modulation by both A1- and A2A-receptors. We demonstrate that A1-type receptors reduced Ca2+-currents in all SPNs tested. However, A2A-type receptors enhanced Ca2+-currents only in half tested neurons. Intriguingly, to observe the actions of A2A-type receptors, occupation of A1-type receptors had to occur first. However, A1-receptors decreased CaV2 Ca2+-currents, while A2A-type receptors enhanced current through CaV1 channels. Because these channels have opposing actions on cell discharge, these differences explain in part why iSPNs may be more excitable than dSPNs. It is demonstrated that intrinsic voltage-gated currents expressed in SPNs are effectors of purinergic signaling that therefore play a role in excitability.
Keywords: Adenosine A1-type receptor, Adenosine A2A-type receptor, Striatal projection neurons, Excitability, Modulation of Ca2+-currents
Introduction
The neostriatum regulates motor activity, action selection and procedural memory [1]. It receives inputs from the cortex and thalamus and sends processed outputs to other basal ganglia nuclei via striatal projection neurons (SPNs) divided into direct pathway neurons (dSPNs) that facilitate movement execution and indirect pathway neurons (iSPNs) that represses movements [2, 3]. A dynamic balance between dSPNs and iSPNs is posited as essential for motor control [1].
SPNs control their excitability in part by expressing different classes of Ca2+-channels modulated by transmitters that activate G protein-coupled receptors (GPCRs). CaV1 channels increase excitability by regulating threshold and neuronal discharge, while CaV2 channels decrease excitability by activating K+-activated currents that regulate inter-spike intervals, firing frequency, and transmitter release [4–6], among other actions. For example, in addition to many other functions [7], D1-receptors increase excitability in dSPNs by enhancing CaV1-channels mediated current, while D2-receptors decrease excitability in iSPNs by reducing the same current [8, 9]. These actions occur separately in different cell classes: dSPNs and iSPNs [10].
In order to compare with other GPCRs, such as dopamine receptors, this work focus in how adenosine controls Ca2+-currents in SPNs via the two adenosine receptors: A1 and A2A [11, 12]. Notably, A1 receptors are expressed in SPNs from both pathways, while A2A receptors are mainly expressed in iSPNs [12, 13]. A1-receptors are coupled to Gi/o proteins [12, 14–23], while A2A receptors are coupled to Gs/olf proteins [11, 12, 24–31]. In many cells, the signaling cascades ignited by these two classes of G proteins have opposed actions [30–34]. Therefore, an implicit hypothesis predicts that the signaling cascades that control Ca2+-currents and excitability via A1- and A2A-receptors would have a push-pull kind of effects: One would decrease and the other would increase the activity of many final effectors in cells expressing both receptors [12, 32, 33, 35–37]. However, to our knowledge, the modulatory actions of both adenosine receptors on Ca2+-currents, expressed by the same SPNs, have not been described. A main goal of the present work is to see whether this modulation is present, as a necessary step to begin elucidating the combined actions of A1- and A2A-receptors in control and under diverse pathological conditions by using Ca2+-channels, as final intrinsic effectors of adenosine signaling. One of many examples of the importance of this signaling is that antagonists of A2A-receptors are used as coadjutant therapy for Parkinson’s disease. However, in spite of their actions in synaptic transmission, they are unable to stop the decrease in dendritic spines induced by dopamine-depletion, an action attributable to CaV1 channels [34].
Materials and methods
Studies were conducted in accordance with procedures approved by the Committee of Bioethics and Care of Experimental Animals of The Universidad Nacional Autónoma de México [UNAM], and the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals [NIH Publications No. 8023, revised 1978]. The number of animals used in the experimental samples was the minimal possible to attain statistical significance.
Acutely dissociated cells
Acutely dissociated neurons from rat brain slices were obtained as described in previous work [6, 38]. Briefly, male Wistar rats were anesthetized and decapitated. Their brains were placed in ice-cold saline (4 °C) containing [in millimolars]: 126 NaCl, 3 KCl, 26 Na2HCO3, 2 CaCl2, 1 MgCl2, 11 glucose, 0.2 thiourea, and 0.2 of ascorbic acid [25 °C; pH = 7.4 with HCl, 298 ± 5 mOsm/l with glucose; saturated with 95 % O2 and 5 % CO2]. Sagittal brain slices, 300 μm thick, were cut on a vibratome and placed in the same saline solution at room temperature for 1 h. In case the slices were programmed for current-clamp experiments, they were transferred to a recording chamber. If they were used in voltage-clamp experiments, we obtained dissociated cells from the dorsal neostriatum by enzymatic digestion—1 mg/ml of pronase E type XIV [Sigma] at 34 °C in a 10 mM HEPES saline solution for about 20 min. Then, the slices were transferred to a low calcium (0.4 mM CaCl2) saline solution to be mechanically dissociated using fire polished Pasteur pipettes. The cell suspension (2 ml) was plated into a Petri dish mounted on the stage of an inverted microscope. Neurons adhered to the bottom of the dish within 10–15 min. The dish contained 1 ml of the recording saline (in millimolars)—0.001 tetrodotoxin (TTX), 130 NaCl, 3 KCl, 5 BaCl2, 2 MgCl2, 10 HEPES, and 10 glucose (pH = 7.4 with NaOH; 298 ± 5 mOsm/l with glucose).
Voltage clamp recordings of calcium currents
Voltage-clamp recordings were performed on striatal neurons of 10–12 μm soma diameter and whole-cell capacitance of 6–7 pF (putative SPNs). Larger neurons are known to correspond to interneurons [38]. Patch pipettes of borosilicate glass (WPI, Sarasota, FL) were pulled in a Flaming-Brown puller [Sutter Instrument Corp. Novato, CA]. The internal saline contained (in millimolars)—140 N-methyl-d-glucamine, 40 HEPES, 10 EGTA, 4 MgCl2, 2 ATP, 0.4 GTP, and 0.1 leupeptin (pH = 7.2 with H2SO4; 280 ± 5 mOsm/l).
Whole-cell recordings used electrodes with resistance of 3–6 MΩ in the bath. Liquid junction potentials (<5 mV) were corrected. Recordings were obtained with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA, USA) and controlled and monitored with pClamp (version 8.2) and a 125 kHz DMA interface (Axon Ins.). The series resistance (<10MΩ) was compensated (70–80 %) and monitored.
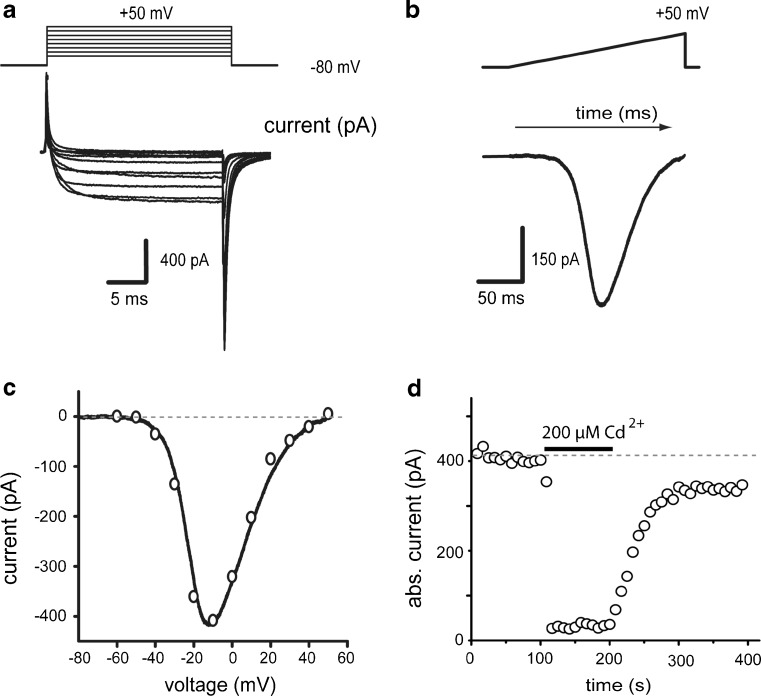
We recorded currents passing through calcium channels using Ba2+ as charge carrier. Ba2+ is a potent K+ channel blocker. Na+ channels were blocked with 1 μM TTX. In addition, intracellular potassium was replaced by 140 mM N-methyl-d-glucamine. Current-voltage relationships (I-V plots) were generated before and during drug application. Figure 1a shows a family of Ba2+ -currents evoked with 20 ms depolarizing voltage commands from −80 to 50 mV in 10 mV steps. Figure 1b shows a Ba2+-current evoked with a ramp voltage command from −80 to 50 mV (0.7 mV/ms) in the same cell. The measured current in steady state responses to step voltage commands [empty circles] and ramp voltage commands [continuous line] are plotted against voltage values in Fig. 1c. Resulting current–voltage relationships (I-V plots) coincide [4–6, 38–42], suggesting acceptable space-clamp control. For clarity, results mostly show I-V plots built from currents evoked with ramp commands. Note that this coincidence implies that amplitude of peak Ba2+-current obtained with a ramp command coincides with the minimum of the I-V plot (between −20 and 0 mV in all cases). Currents isolated in this way are completely and reversibly blocked by 200 μM Cd2+ (93 ± 1 %; n = 6; ***P < 0.001, Fig. 1d), suggesting that they flow through Ca2+-channels, and so, in the text they are named as Ca2+-currents.
Fig. 1.
Whole-cell Ca2+-currents in acutely dissociated neostriatal neurons. a Inward currents elicited by steps of depolarizing voltage commands. b Inward current elicited in the same neuron by a ramp command. c Current-voltage relationships (I-V plots) taken from data in a and b. Note close superimposition. d Time course of absolute current amplitude during application of 200 μM Cd2+
Current clamp recordings of SPNs
Current clamp recordings were performed with the patch clamp technique in the whole cell configuration in both putative dSPNs and iSPNs. The slices were visualized at 40× using infrared differential interference contrast (IR-DIC) microscopy with an upright microscope and a digital camera. For current-clamp recordings micropipettes with 2–5 MΩ D.C. resistance and filled with internal saline containing (in millimolars)—120 KMeSO4, 10 NaCl, 10 EGTA/KOH, 10 Hepes, 1 CaCl2, 0.2 Na2ATP, 0.2 Na3GTP, and 0.1 % biocytin (pH 7.3; 285 mOsm/l) were used. Recordings were carried out with Axoclamp 2A/2B (Axon Instruments, Foster City, CA). The data were acquired with acquisition software made in the laboratory using the LabView environment (National Instruments, Austin TX). Experimental drugs were stored in stock solutions to be dissolved in their final concentrations into the superfusion saline—CCPA (100 nM) and CGS (1 μM).
Drugs
Drugs were applied with a gravity-fed system that positioned a glass capillary tube 100 μm from the recording cell in the direction of superfusion flow. Solution changes used a microvalve system (Lee; Essex, CT, USA) allowing reversibly applications [41]. Substances used were: ω-conotoxin GVIA (ω-CgTx-GVIA), ω-agatoxin TK (ω-AgTx), tetrodotoxin (TTX) (from Alomone Labs; Jerusalem, Israel); nicardipine, adenosine (Sigma-Aldrich-RBI, St. Louis, MO). The A1-type receptor agonist—2-chloro-N6-cyclopentyladenosine (CCPA), the A2A-type receptor agonist: 4-(2-((6-amino-9-(N-ethyl-β-d-ribofuranuron-amidosyl)-9H-purin-2-yl)amino)ethyl)benzene propanoic acid hydrochloride (CGS 21680), the A2A-type receptor antagonist—2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo(4,3-e)(1,2,4)triazolo(1,5-c)pyrimidin-5-amine (SCH 58261) and the A1-type receptor antagonist—8-cyclopentyl-1,3-dimethylxanthine (CPT; Tocris Cookson, Ellisville, MO). Substances were dissolved in water to get stock solutions added to the superfusate to give the final concentrations. CGS 21680 was prepared in dimethylsulfoxide (DMSO, 1 %) and nicardipine was prepared in HCl (1 %); control saline also contained DMSO or HCl (0.1 %) at the same concentrations in these cases.
Data analysis
We report mean ± SEM of peak Ba2+-current changes in percentage (minimum of I-V plots, see above). When it is relevant we report medians and the 25–75 percentile range of absolute current values, since as Tukey box plots show, rarely, distribution of samples followed normality. To measure excitability, we compared firing frequency in Hertz. Statistical significance was found with free-distribution statistical tests with absolute current values or frequencies: Wilcoxon’s T test, Friedman test, or Kruskal–Wallis test with post hoc Dunn’s tests. Statistical significance was considered at P < 0.05.
Results
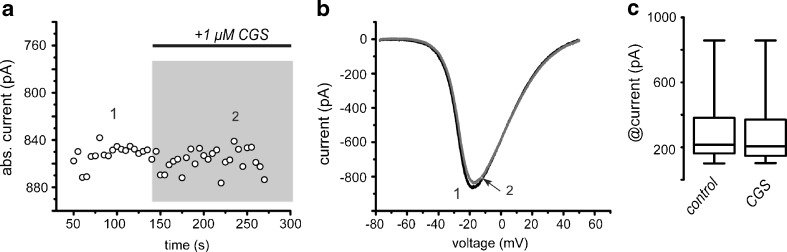
Time course in Fig. 2a shows that administration of an adenosine A2A-type receptor agonist, CGS 21680 (CGS), to the bath saline has no action by itself in Ca2+-currents amplitude. The same result was obtained using concentrations ranging from 5 nM to 10 μM, although, in some occasions, micromolar concentrations may decrease current amplitude. Figure 2b illustrates representative I-V plots taken at numbered times from A. Box plots in Fig. 2c show that there is no significant differences between Ca2+-currents maximal amplitude obtained with ramp commands (as in Fig. 1: minima of the I-V plots), with or without the A2A-agonist in all neurons tested (n = 6).
Fig. 2.
A selective A2A-type receptor agonist had no actions on Ca2+-current by itself. a The time course shows the application of an A2A-type receptor agonist: 1 µM CGS into the bath saline (horizontal bar). There is no change in current amplitude. b representative I-V plots taken at numbered times from the time course in a. c Box Tukey plots in Fig. 2c show that there is no significant differences between Ca2+-currents amplitude with or without the CGS in all neurons tested (n = 6)
Adenosine actions are biphasic and concentration-dependent
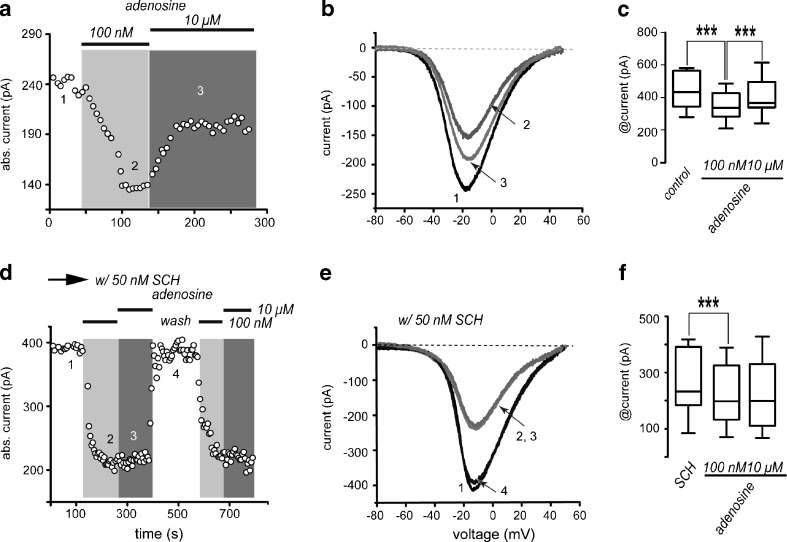
Time course in Fig. 3a shows that adenosine, when administered sequentially, at “low” (100 nM) and “high” (10 μM) concentrations, has a push-pull type of action—100 nM adenosine decreased absolute Ca2+-current amplitude by 29 ± 3 % (n = 13; ***P < 0.0001), while 10 μM adenosine increased and partially reversed the decrease induced by low concentrations: It partially “recovers”, the Ca2+-current by 21 ± 3 %. However, this latter action was only observed in 54 % of neurons tested (“responders”; n = 7/13; ***P < 0.0001; Fig. 3a). Neurons that did not exhibit an increase in current with 10 μM adenosine (“non responders”) but were able to manifest a decrease during low adenosine concentrations were considered as not capable to manifest the enhancing response and were separated as a group, because it is well known that only half of SPNs (iSPNs) express A2A-receptors [12].
Fig. 3.
Adenosine actions are biphasic and concentration-dependent. a Addition of low adenosine concentrations (100 nM) to the bath saline decreases absolute current amplitude, whereas application of higher adenosine concentrations (10 μM) partially reversed the current decrease produced by lower adenosine concentrations. b Representative I-V plots taken from the time course in a as indicated by the numbers. c Box plots summarizing a sample of experiments from neurons during both adenosine concentrations (n = 13 for left and middle boxes, n = 7 for right box). d The time course shows that reduction in current produced by low adenosine concentrations was not blocked by SCH 58261 while current enhancement mediated by micromolar adenosine was not present in any recorded cell when SCH 58261 was present. e Representative I-V plots taken from the time course in d as indicated by numbers. f Box plots summarize a sample of similar experiments (n = 13)
The action of low adenosine concentrations was reversible, present in all SPNs tested, and blocked by the A1-receptor antagonist 100 nM CPT (not shown, but see below), suggesting that it can be attributed to the activation of A1-receptors. Therefore, as expected for a GPCR associated with Gi/o protein, activation of A1-receptors decreased Ca2+-currents amplitude [12, 14–23], and in agreement with its known location, the action was observed in all SPNs tested. Representative I-V plots of currents taken at different moments during the time course are depicted in Fig. 3b (numbered in the time course, Fig. 3a).
Box-plots in Fig. 3c summarize the results from a sample of experiments: median and 25–75 percentile range in control (n = 13) was 408 (307–557) pA, reduction with 100 nM adenosine (n = 13) was to 298 (237–400) pA and reversal with 10 μM adenosine (n = 7) was to 331 (301–478) pA. All changes were significant (***P < 0.0001).
To demonstrate that high adenosine concentrations were acting on A2A-type receptors, we tested 10 μM adenosine in the presence of the A2A-type receptor antagonist SCH 58261 (50 nM SCH; Fig. 3d). Note that, in the presence of SCH, the recovery of current amplitude by high adenosine concentrations (10 μM) was not seen in any neuron (Fig. 3d), suggesting specific actions. Specificity was further supported by the fact that low adenosine concentrations (100 nM) still reduced Ca2+-currents (21 % ± 4 %; n = 13; ***P < 0.0001) in the presence of the A2A-receptor antagonist in all neurons tested. Representative I-V plots of these currents are depicted in Fig. 3e. Box plots in Fig. 3f summarize sample results. Median and 25–75 percentile range in control were 227 (176–391) pA, reduction with 100 nM adenosine was to 191 (124–322) pA. Reversal with 10 μM adenosine was not seen in any cell when SCH 58261 was present: 192 (101–327) pA. In CGS, only changes due to A1-receptor activation were significant (***P < 0.0001).
All SPNs exhibited a decrease in current after nanomolar adenosine, but only one half of SPNs exhibited the subsequent enhancement of the current after micromolar adenosine. A parsimonious hypothesis would be that this biphasic action in one half of neurons should be due to the activation of two different adenosine receptors. Therefore, we hypothesized that the enhancing action should be due to the activation of A2A-receptors (A2A-receptors activate PKA which is necessary for D1-receptors to enhance Ca2+-current) see [8].
To further support that the biphasic action of adenosine found in about one half of tested cells was due to the activation of different receptors, we tested selective agonists for both receptors in sequence.
Sequential activation of A1- and A2A-type receptors reduces and enhances Ca2+-currents, respectively
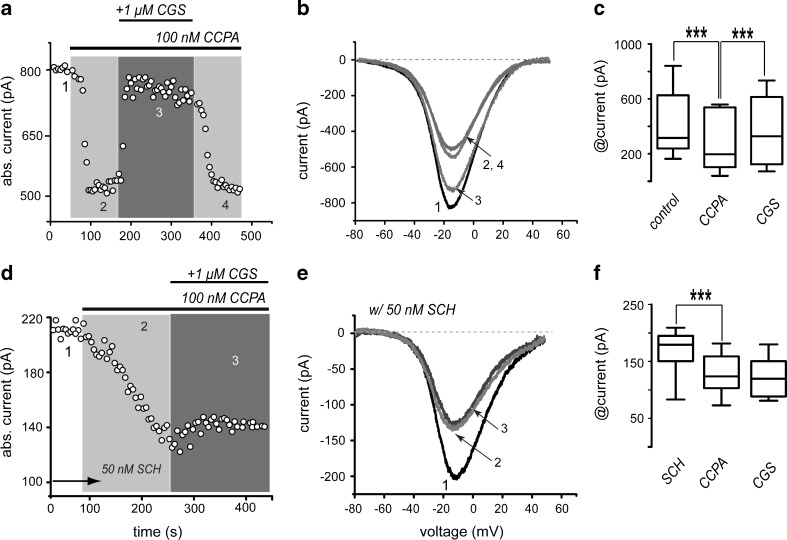
Application of 100 nM CCPA, a selective agonist of A1-receptors, decreased whole-cell Ca2+-current in all SPNs tested (n = 13). Subsequent addition of 1 μM CGS 21680, a selective agonist of A2A-receptors, had an enhancing current effect in about half of the cells (“responders”) but did not have any action in the other half (“non-responders”; Fig. 4a, b). But all cells showed the decreasing action by the A1-agonist. Interestingly, because A2A-agonists had no significant action when applied alone (Fig. 2), it appeared as though A1-receptors occupation had to precede the activation of A2A-receptors to observe their enhancing actions on Ca2+-currents [36, 37, 43–46]. The reason why A1-receptors have to be activated first, in order to see the actions of A2A-receptors, is under current research. Perhaps, many other receptors and signaling cascades also fulfill this function [33].
Fig. 4.
Sequential activation of A1- and A2A-type receptors with selective agonists reduces and enhances Ca2+ -current, respectively. a Time course of current amplitude showing that CCPA (100 nM) decreases current amplitude (1, 2). Subsequent application of 1 µM CGS 21680 (2, 3) almost completely reverses the reduction induced by CCPA in about half the neurons. b Representative I-V plots taken from the time course in a as indicated by the numbers. c Box plots summarize results from a sample of neurons with responses described in a and b (n = 13 in left and middle boxes and n = 7 in the right box). d Time course similar to that depicted in a but in the presence of 50 nM of the A2A-receptor antagonist SCH 58261. In these conditions, the action of CGS 21680 could not be observed in any neuron. e Representative I-V plots taken from the time course in d as indicated by the numbers. f Box plots summarize the experimental sample (n = 8)
In summary, the administration of A1- and A2A-receptor agonists applied in sequence (Fig. 4a, b) mimicked the biphasic actions described for adenosine at low and high concentrations, and thus, support the concept that such biphasic action is due to the activation of different adenosine receptors. CCPA decreased Ca2+-current in all striatal neurons tested (Fig. 4a1, 2) by 25 ± 4 % (n = 13; ***P < 0.0001), and the subsequent application of the A2A-receptor agonist, CGS 21680, partially restored current amplitude in 54 % of the neurons tested (Fig. 4a–c; n = 7/13). Action in only about half the neurons was expected because only iSPNs express A2A-receptors [12], supporting the hypothesis that iSPNs express both receptors. Current increase induced by CGS 21680 was 23 ± 4 % (n = 7; ***P < 0.0001). Representative I-V plots of these currents are depicted in Fig. 4b (times of recordings are numbered during the time course in Fig. 4a). CGS 21680 actions were reversible (Fig. 4a, b). Tukey box distributions in Fig. 4c summarize the results from a sample of experiments: median and 25–75 percentile range in control was 315 (239–628) pA, reduction with CCPA was to 197 (103–539) pA and reversal with CGS 21680 was to 328 (124–615) pA. All changes were significant (***P < 0.0001).
To test whether enhancement of Ca2+-current amplitude by CGS 21680 was specific, we performed experiments in the presence of SCH 58261 (50 nM), the A2A-receptors antagonist. SCH 58261 did not block the repressing action of A1-receptors, but in its presence, no neuron exhibited the A2A-receptors enhancing action (Fig. 4d). Representative I-V plots as indicated by numbers are shown in Fig. 4e. Box plots summarize results in an experimental sample: Only the action of CCPA is significant in the presence of SCH 58261 (Fig. 4d–f): a 22 ± 3 % current reduction (n = 8, ***P < 0.0001).
We conclude that biphasic actions of adenosine are due to the activation of two different receptors, A1 and A2A. A2A-type receptors are only present in half the neurons: most probably iSPNs.
A2A-receptors activation enhance CaV1 channels current
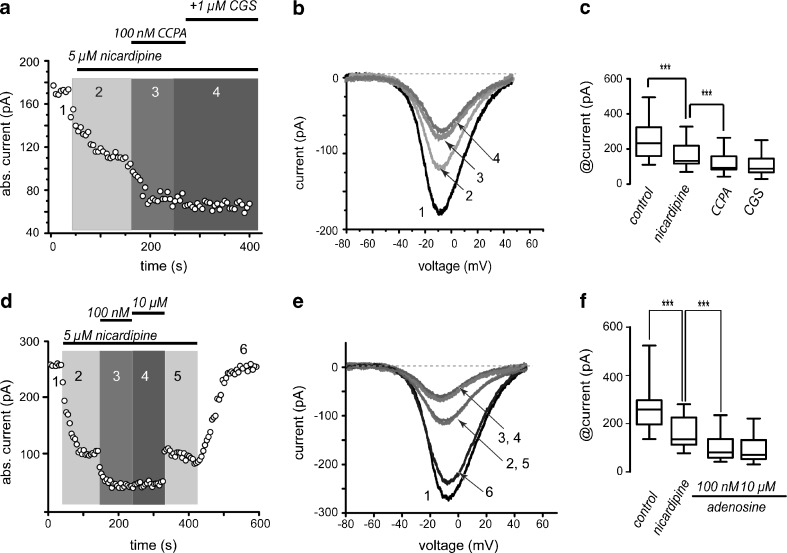
The following experiments depict a mechanism for the biphasic adenosine actions in the sense of finding out which Ca2+-currents are implicated. Figure 5a shows that 5 μM nicardipine, a CaV1-channels antagonist, decreased current amplitude; however, it did not impede the action of 100 nM CCPA that decreased current amplitude even more. However, in the presence of nicardipine, subsequent application of 1 μM CGS 21680 did not produce current enhancement in any cell tested. Representative I-V plots (numbered in the time course; Fig. 5b) and summary box plots (Fig. 5c) show that CCPA current reduction was 39 ± 10 % (n = 10; ***P < 0.0001) and CGS recovery was 32 ± 8 % (n = 6; ***P < 0.0001). Nicardipine blocked the recovery produced by CGS 21680 significantly (41 ± 4 %; n = 6; ***P < 0.0001). Control had median and percentile 25–75 range of 197 (180–243) pA; +CCPA: 130 (77–183) pA; +CGS 21680: 222 (152–238) pA; +nicardipine: 92 (81–175) pA. All changes were significant (***P < 0.0001).
Fig. 5.
A2A but not A1 receptor actions are blocked by dihydropyridines. a Time course illustrates the sequential reduction with CCPA and the reversal of current amplitude with CGS 21680. The action of the A2A-receptor agonist was blocked by 5 μM nicardipine. b Representative I-V plots taken from the time course in a as indicated by the numbers. c Box plots summarizing a sample of similar experiments (n = 10 for control; n = 6 for nicardipine, CCPA and CGS). d Time course shows that 5 μM nicardipine administered first occludes the action of high but not of low concentrations of adenosine. e Representative I-V plots taken from the time course in d as indicated by the numbers. f Box plots summarizing a sample of similar experiments (n = 12)
Similar results were obtained with the endogenous agonist (Fig. 5d–f): Adenosine (100 nM) decreased current amplitude by 20 % ± 2 %, and high concentrations (10 μM) had no effects in any cell tested if 5 μM nicardipine were present in the bath saline, suggesting that CaV1 currents are the target of A2A-receptor modulation (n = 12; ***P < 0.0001).
Activation of A1-receptors mainly reduce CaV2.2 channels current
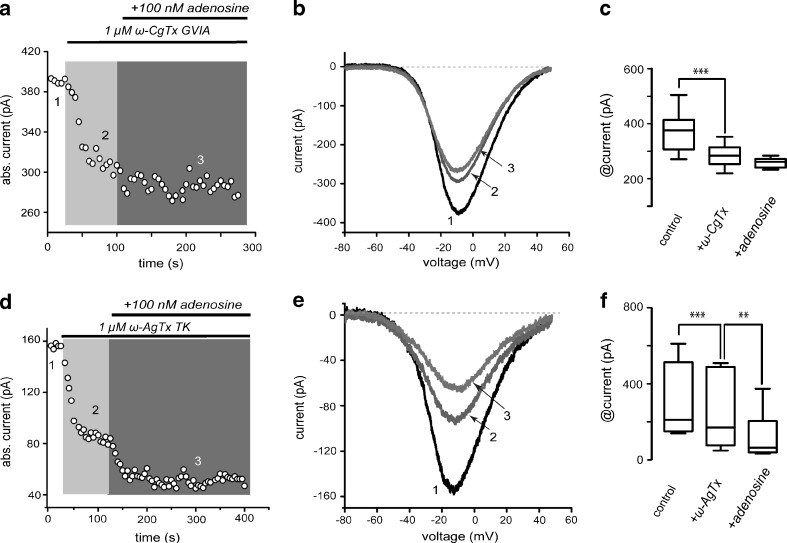
Figure 6a–c illustrates that 1 μM ω-CgTx GVIA reduces Ca2+ current in SPNs confirming the presence of Cav2.2 (N) calcium current [40, 41]. Once Cav2.2 current was blocked, low adenosine concentrations (100 nM) could no longer reduce Ca2+-current further in any cell tested, suggesting that main current component targeted by A1-receptors is CaV2.2. Current reduction by ω-CgTx was 20 % ± 3 % (n = 9, ***P < 0.0001). In contrast, 1 μM ω-AgTx could not occlude the actions of 100 nM adenosine (Fig. 6d–f; n = 6).
Fig. 6.
Activation of adenosine A1-receptor mostly reduces current through CaV2.2 (N) channels. a Time course illustrates that 1 μM ω-conotoxin GVIA almost completely occluded the action of 100 nM adenosine. b Representative I-V plots taken from the time course in a as indicated by the numbers. c Box plots summarizing a sample of similar experiments (n = 9). d Time course illustrates that 1 μM ω-agatoxin IVA did not occlude the action of 100 nM adenosine. e Representative I-V plots taken from the time course in a as indicated by the numbers. f Box plots summarizing a sample of similar experiments (n = 6)
Box plots in Fig. 6c and f summarize sample results of ω-CgTx GVIA and ω-AgTx, respectively; median and 25–75 percentile range in Fig. 6c control was 383 (311–422) pA, reduction with 1 μM ω-CgTx GVIA was to 287 (255–319) pA and with 100 nM adenosine was to 263 (241–274) pA (***P < 0.0001). When control was 158 (151–275) pA (Fig. 6f), reduction with 1 μM ω-AgTx was to 89 (53–179) pA and addition of 100 nM adenosine reduced the current even more to 64 (41–148) pA. All changes were significant (***P < 0.0001; **P < 0.025).
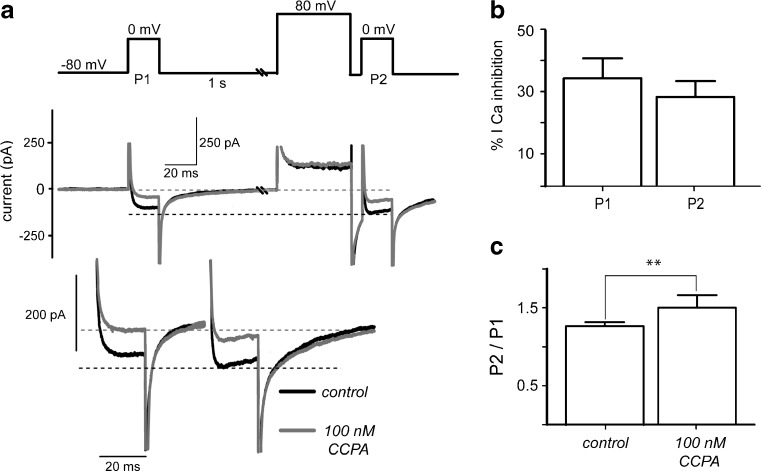
To determine whether A1-receptors modulation was voltage-dependent or independent [47], we used a standard double pulse protocol (Fig. 7). Two test voltage commands to zero millivolts evoked Ca2+-current before (P1) and after an 80 mV pre-pulse (P2) in the absence (black trace) and in the presence (gray trace) of CCPA. As expected, an increase in amplitude and a change in kinetics of control current were observed in the current evoked after the 80 mV depolarization (compare black traces before and after the pre-pulse). This is due to some constitutive G protein activity on the channels which is interrupted by the depolarization (see dashed lines for comparison). In case the predominant action of A1-receptors occurs through G proteins acting on the channel, the difference between the currents evoked before and after the pre-pulse should increase in the presence of the agonist CCPA (gray traces). This was not the case; facilitation ratio was 1.2 ± 0.05 in control, suggesting constitutive G protein action, while in the presence of CCPA it was 1.5 ± 0.16, suggesting that a very small part of the modulation was voltage-dependent (n = 10; P < 0.1). During P1, percent amplitude modulation of the current was 35 ± 6 % while it was 28 ± 5 % for current evoked by P2 (n = 10; NS), suggesting that in the present conditions, most A1-receptor modulation of the current is not voltage-dependent in SPNs.
Fig. 7.
Most adenosine A1-receptors modulation is not voltage-dependent. a Top: standard double pulse protocol. Middle: Evoked currents before (black trace) and after (gray trace) CCPA was added to the bath saline. Bottom: enhanced traces evoked with zero millivolts commands before (P1) and after the 80 mV pre-pulse (P2). Current increases in amplitude and changes its kinetics during P2 suggesting constitutive G-protein action. b However, percent amplitude modulation amplitude before and after the 80 mV pre-pulse was non-significantly different (n = 10). c Ratio of second (P2) to first response (P1) was slightly different, suggesting that most A1-receptor modulation is mainly non-voltage-dependent
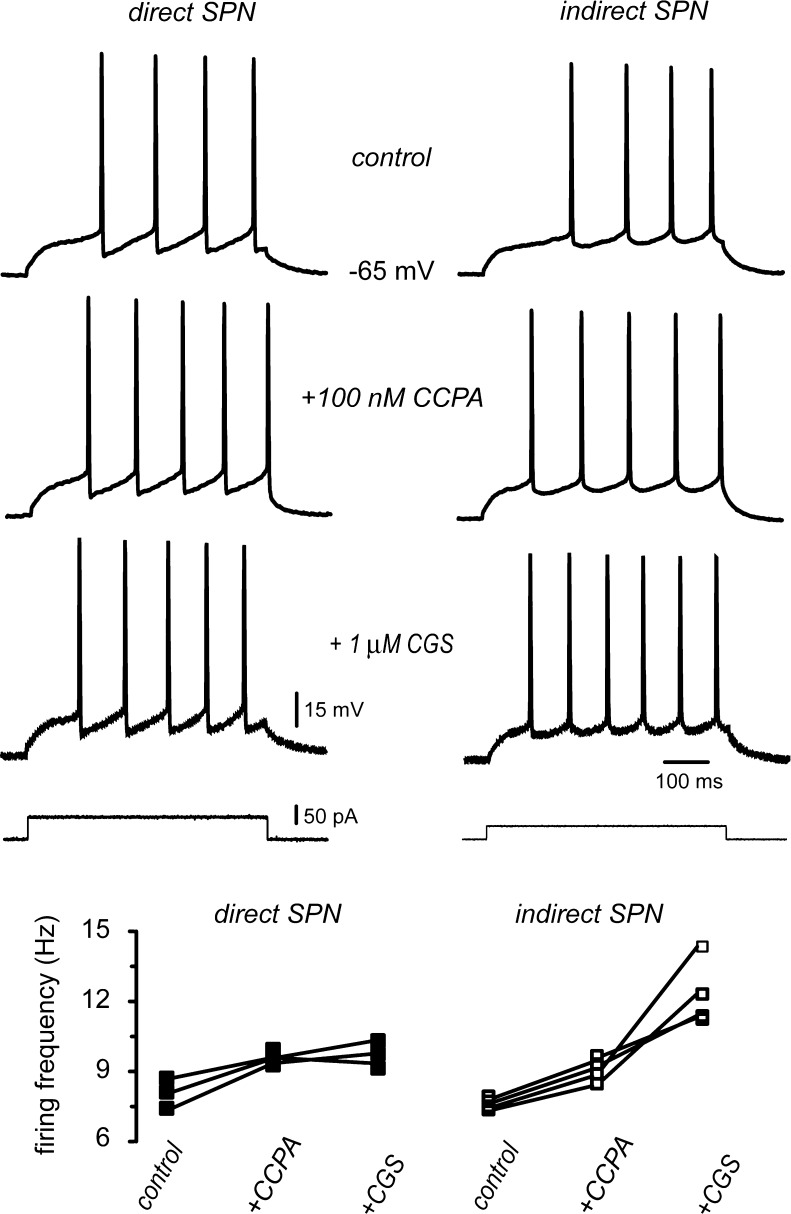
In summary, both receptors, A1 and A2A, appeared to act via their intracellular signaling cascades and not via a membrane delimited, voltage-dependent mechanism. A2A-receptors only acted in half the cells (putative iSPNs). A1-receptors activation decreased current through CaV2.2 channels while A2A-receptors acted increasing current through CaV1 channels. In the soma of SPNs, it is known that CaV2 channels are the source of Ca2+ that activates Ca2+-dependent K+-currents that make up the after hyperpolarizing potential (AHP) and thus the interspike intervals (ISIs) [4]. Therefore, suppressing this Ca2+-source would reduce ISIs and make firing frequency to increase [5]. Figure 8 shows that this inference was correct: A1-receptors increased the number of action potentials fired to the same stimulus in all SPNs tested (n = 7; P < 0.02). In addition, enhancing Ca2+ current through CaV1 channels would increase the number of action potentials fired to the same stimulus since these channels enhance the depolarization induced by the intracellular current step [5]. That is, in neurons with both A1 and A2A receptors, the actions of these receptors would be synergistic for excitability given their action on the different Ca2+ currents [6, 8, 9, 38, 40, 42, 48]. This inference was also correct, since in about half of the neurons tested (Fig. 8; n = 4/7) addition of CGS in the presence of CCPA increased, even more, the number of action potentials fired (P < 0.05). Because only iSPNs are described to express both A1 and A2A receptors, we assumed that it is in these neurons were adenosine exerts its full facilitating actions.
Fig. 8.
A1 and A2A receptors have synergistic effects on excitability. a Left and right columns illustrate the firing of a putative dSPN and an iSPN, respectively, under a stimulus strength that allowed the firing of the same number of action potentials in the control (step current at the bottom). From top to bottom, these responses were subjected to different conditions without changing the stimulus strength: First, addition of CCPA (100 nM) an agonist of A1-receptors increased the number of spikes fired in both neurons. Second, addition of CGS 21680 (1 μM) an agonists of A2A-receptors, further increased the number of spikes fired in iSPNs but not in dSPNs. Graph at the bottom shows the firing behavior of n = 7 neurons. All neurons responded to CCPA, but only four out of seven neurons responded also to CGS
Discussion
Two main sources of adenosine have been proposed [49, 50]: one caused by the release done by nucleoside transporters after ATP dephosphorylation, the other produced by ectonucleotidases following high neuronal activity. Basal extracellular levels are ca. 40–120 nM [50–55], while micromolar concentrations are reached during more intense neuronal discharge [52, 56–58]. Low [basal] adenosine concentrations stimulate A1-receptors [46, 59, 60], while high concentrations activate A2A-receptors [37, 45, 46, 60–62]. Taken together, these facts explain why the results of the sequential activation of A1-receptors on A2A-receptors activation was easily observed in dissociated cells taking Ca2+-currents as effectors (reporters). However, the reason why A1-receptors need to be occupied to observe the action of A2A-receptors actions, although has been noted before [36, 37, 46], is in need of further investigation. In fact, other receptors, e.g., dopamine D2-receptors, may also fulfill that role [33, 63, 64].
Adenosine receptors modulate Ca2+-currents as other G protein-coupled receptors
Adenosine signaling pathways are well known [11–22]; A1- and A2A-receptors have been proposed to have opposite roles by activating Gi/o and Gs/olf proteins, respectively [20–28, 46, 65, 66], similarly to dopamine D1- and D2-receptors [10] and other G protein-coupled receptors. However, while opposing dopamine receptors are located in different cell classes, different adenosine receptors are located in the same cell class: iSPNs. This fact may confer special excitable functions to iSPNs. Thus, a main goal of the present work was to disclose the source of a possible difference in the excitability of iSPNs due to purinergic mechanisms.
To see Ca2+-channels as final effectors to observe GPCRs action down their signaling cascades has a long tradition. A main reason is, precisely, the relation of these channels with neuronal excitability and transmitter release [5, 21, 23, 33, 40, 41, 46, 48, 67]. SPNs express several classes of CaV1 and CaV2 channels [5, 6, 8, 9, 38, 40, 42, 48]. For example, CaV1 current enhances excitability by decreasing firing threshold and enhancing neuronal output range. Current through CaV1 channels amplifies the stimulus. In contrast, current through CaV2 channels have opposed actions; they decrease excitability by inducing the activation of Ca2+-dependent K+-currents. These currents are in charge of the after hyperpolarizing potentials and interspike intervals that low firing frequency or control the amplitude of synaptic events [5, 33]. Adenosine signaling on Ca2+-currents has been documented in other cell classes [15–21]. Accordingly, we wanted to see whether opposing functions of these effectors, Ca2+-channels, may parallel expected opposing actions in signaling mechanisms.
As an antecedent, A1-type receptors activation decreases glutamate release while A2A-receptors increase this release [46]. But, to our knowledge, there was no reported evidence that a similar case is present in the Ca2+-currents of the same cell, iSPNs, expressing A1- and A2A-type receptors [12].
Here, we show that A1-receptors activation reduces CaV2.2 currents in all SPNs tested due to a mechanism that is mostly non-voltage-dependent [6, 16–20, 68–75]. This action would decrease AHPs and thus increase firing frequency [5] in SPNs; that is, it would favor excitation. A1-receptors could be activated either with a specific agonist [CCPA] or by low concentrations of adenosine. In addition, in half of SPNs tested, A2A-receptors enhanced CaV1 currents most probably through a phosphorylation mechanism [12, 26–28, 76]. Phosphorylation of CaV1 channels by A2A-receptors activation has been reported in conditions of high adenosine release [77]. This action would lower firing threshold and favor evoked depolarization, also increasing the number of action potentials fired. That is, opposing signaling cascades in the same cell, which induce opposing actions on Ca2+-currents, may “paradoxically” lead to a synergistic effect on excitability in iSPNs. This hypothesis was tested with current-clamp experiments and was confirmed.
To conclude, combined actions of A1 and A2A receptors on half of SPNs tested, most probably iSPNs, induces and enhancement of excitability larger than that produced by the sole activation of A1 receptors.
Only iSPNs express A2A-receptors [12]. A2A-receptors could be activated either with a specific agonist (CGS 21680) or by increasing the concentration of the endogenous transmitter [46, 60–62, 66]. In our hands, both actions were blocked by the highly selective A2A-receptor antagonist, SCH 58261, suggesting specificity. In other words, the sequential activation of receptors with opposing signaling, A1 and A2A, decrease and increase, respectively, Ca2+-currents with opposing actions on cell excitability. These actions in turn lead to an increase in excitability.
However, note that these actions were described in the somatic region, where fast sodium-dependent action potentials are generated. Another similar story has been partially built in dendritic spines where synaptic inputs arrive [33]. But further work is needed to link the known adenosine signaling cascades with excitability. The extrapolation of the present results with known channels actions [5, 41, 42] can lead to useful explanatory hypothesis: Excitability of iSPNs is more enhanced than that of dSPNs during models of Parkinson’s disease (PD). This phenomenon may in part be due to intrinsic mechanisms originated in the same iSPNs. In addition, extrinsic mechanisms may alter cortical inputs, since synaptic activity is increased [34]. Interestingly, although this increase in synaptic activity can be regulated with A2A-receptor antagonists, this regulation is not accompanied with spines recovery, thus disrupting a homeosthatic mechanism [34]. Previous work has shown that spines stability depends in part on CaV1 channels activity [76] that we demonstrate here are also enhanced by A2A receptors, making this interplay difficult to assess. In any case, the present results adds to a growing list of evidences that supports the blockade of A2A-receptors as an adjunct therapy for PD by balancing basal ganglia pathways.
Finally, we stress the notion that A2A-receptors’ action causes a division of rat striatal projections neurons in two halves: one half showing the response and the other without the response. These results were very consistent and reproducible in the different experimental samples. It is also known that striatal projection neurons are divided in halves: one half corresponds to the direct (dSPNs) and the other to the indirect (iSPNs) basal ganglia pathways. And finally, it is known that indirect pathway neurons (iSPNs) but not direct pathway neurons (dSPNs) express A2A-receptors. Taken this information together, we infer that neurons with both A1 and A2A responses are indirect pathway neurons. These neurons are precisely the neurons that increase their excitability during Parkinson’s disease, when adenosine concentrations may be very high.
Acknowledgments
We thank Antonio Laville, Gabriela X. Ayala, Adriana Hernandez, Mariana Duhne, Ernesto A. Rendon, and Dagoberto Tapia for technical support and advice and to Dr. Claudia Rivera for animal care.
Financial disclosure related to research covered in this article
This work was supported by the Consejo Nacional de Ciencia y Tecnología [CONACyT-México] grants 154131 and 98004 to JB and EG, respectively, by grants from Dirección General de Asuntos del Personal Académico. Universidad Nacional Autónoma de México (DGAPA-UNAM) to JB and EG, respectively, and by the Mexico-Germany Agreement Consejo Nacional de Ciencia y Tecnología-Deutsche Forschungsgemeinschaft (CONACyT-DFG) Grant I0110/193/10 FON.INST.-29-10 to JB. Hernandez-Gonzalez O, Hernandez-Flores T and Arias-Garcia M have CONACyT doctoral fellowships. Data in this work are part of O H-G doctoral dissertation in the Posgrado en Ciencias Biomédicas de la Universidad Nacional Autónoma de México. Prieto GA and Perez-Burgos A were graduate students in the same institution and had CONACyT doctoral fellowships. No conflicts of interest are declared.
Financial disclosures of all authors [for the preceding 12 months]
Hernandez-Gonzalez O: Graduate student with a CONACyT scholarship.
Hernandez-Flores T: Graduate student with a CONACyT scholarship.
Prieto GA: On postdoctoral residence.
Perez-Burgos A: On postdoctoral residence.
Arias-García M: Graduate student with a CONACyT scholarship
Bargas J: Universidad Nacional Autónoma de México, Professor.
Galarraga E: Universidad Nacional Autónoma de México, Professor.
Documentation of author roles
Hernandez-Gonzalez O: Design, execution of experiments, statistical analysis, and writing of the first draft of the manuscript. Hernandez-Flores T: execution of experiments. Arias-García M: execution of experiments. Prieto GA, Perez-Burgos A: execution of first experiments and assessment of viability. Bargas J: Conception, design, and organization of the research project. Galarraga E: Review, critique, and final organization of the manuscript.
References
- 1.DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord. 2009;3:S237–S240. doi: 10.1016/S1353-8020(09)70822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurons. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-K. [DOI] [PubMed] [Google Scholar]
- 3.Kravitz AV, Freeze BS, Parker PR, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilchis C, Bargas J, Ayala GX, Galván E, Galarraga E. Ca2+ channels that activate Ca2+-dependent K+ currents in neostriatal neurons. Neuroscience. 2000;95:745–752. doi: 10.1016/S0306-4522(99)00493-5. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Garci E, Bargas J, Galarraga E. The role of Ca2+ channels in the repetitive firing of striatal projection neurons. Neuroreport. 2003;14:1253–1256. doi: 10.1097/00001756-200307010-00013. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Burgos A, Perez-Rosello T, Salgado H, et al. Muscarinic M(1) modulation of N and L types of calcium channels is mediated by protein kinase C in neostriatal neurons. Neuroscience. 2008;155:1079–1097. doi: 10.1016/j.neuroscience.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-López S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-Type Ca2+ currents and excitability vía a novel PLCβ1–IP3–calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/S0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 12.Yabuuchi K, Kuroiwa M, Shuto T, et al. Role of adenosine A1 receptors in the modulation of dopamine D1 and adenosine A2A receptor signaling in the neostriatum. Neuroscience. 2006;141:19–25. doi: 10.1016/j.neuroscience.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 13.Moreau JL, Huber G. Central adenosine A(2A) receptors: an overview. Brain Res Rev. 1999;31:65–82. doi: 10.1016/S0165-0173(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 14.Linden J. Structure and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi J, Yamashita H. Adenosine inhibits voltage-dependent Ca2+ currents in rat dissociated supraoptic neurones via A1 receptors. J Physiol. 2000;526:313–326. doi: 10.1111/j.1469-7793.2000.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song WJ, Tkatch T, Surmeier DJ. Adenosine receptor expression and modulation of Ca(2+) channels in rat striatal cholinergic interneurons. J Neurophysiol. 2000;83:322–332. doi: 10.1152/jn.2000.83.1.322. [DOI] [PubMed] [Google Scholar]
- 17.McCool BA, Farroni JS. A1 adenosine receptors inhibit multiple voltage-gated Ca2+ channel subtypes in acutely isolated rat basolateral amygdala neurons. Br J Pharmacol. 2001;132:879–888. doi: 10.1038/sj.bjp.0703884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park KS, Jeong SW, Cha SK, et al. Modulation of N-type Ca2+ currents by A1-adenosine receptor activation in male rat pelvic ganglion neurons. J Pharmacol Exp Ther. 2001;299:501–508. [PubMed] [Google Scholar]
- 19.Mogul DJ, Adams ME, Fox AP. Differential activation of adenosine receptors decreases N-type but potentiates P-type Ca2+ current in hippocampal CA3 neurons. Neuron. 1993;10:327–334. doi: 10.1016/0896-6273(93)90322-I. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Ikeda SR. Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]
- 21.Umemiya M, Berger AJ. Activation of adenosine A1 and A2 receptors differentially modulates calcium channels and glycinergic synaptic transmission in rat brainstem. Neuron. 1994;13:1439–1446. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- 22.Haas HL, Selbach O. Functions of neuronal adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:375–381. doi: 10.1007/s002100000314. [DOI] [PubMed] [Google Scholar]
- 23.Ponzio TA, Hatton GI. Adenosine postsynaptically modulates supraoptic neuronal excitability. J Neurophysiol. 2005;93:535–547. doi: 10.1152/jn.01185.2003. [DOI] [PubMed] [Google Scholar]
- 24.Fredholm BB, Abbracchio MP, Burnstock G, et al. Nomenclature and classification of purinoreceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer TM, Stiles GL. Adenosine receptors. Neuropharmacology. 1995;34:683–694. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- 26.Kull B, Svenningsson P, Fredholm BB. Adenosine A2A receptors are colocalized with and activate Golf in rat striatum. Mol Pharmacol. 2000;58:771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 27.Corvol JC, Studler JM, Schonn JS, Girault JA, Hervé D. Galpha(olf) is necessary for coupling D1 and A2A receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76:1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 28.Herve D, Le Moine C, Corvol JC, et al. G(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J Neurosci. 2001;21:4390–4399. doi: 10.1523/JNEUROSCI.21-12-04390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A2A antagonists, and Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:406–413. doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Okada Y, Sakurai T, Mori M. Excitatory effects of adenosine on neurotransmission is due to increase of transmitter release in the hippocampal slices. Neurosci Lett. 1992;142:233–236. doi: 10.1016/0304-3940(92)90380-P. [DOI] [PubMed] [Google Scholar]
- 31.Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacol. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon AK, Widdowson L, Richardson PJ. Desensitisation of the adenosine A1 receptor by the A2A receptor in the rat striatum. J Neurochem. 1997;69:315–321. doi: 10.1046/j.1471-4159.1997.69010315.x. [DOI] [PubMed] [Google Scholar]
- 33.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13(8):958–66. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson JD, Goldberg JA, Surmeier DJ. Adenosine A2A receptor antagonists attenuate striatal adaptations following dopamine depletion. Neurobiol Dis. 2012;45:409–416. doi: 10.1016/j.nbd.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nörenberg W, Wirkner K, Assmann H, Richter M, Illes P. Adenosine A2A receptors inhibit the conductance of NMDA receptor channels in rat neostriatal neurons. Amino Acids. 1998;14:33–39. doi: 10.1007/BF01345239. [DOI] [PubMed] [Google Scholar]
- 36.Lopes LV, Cunha AR, Ribeiro JÁ. Cross talk between A(1) and A(2A) adenosine receptors in the hippocampus and cortex of young adult and old rats. J Neurophysiol. 1999;82:3196–3203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- 37.Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JÁ. Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition. Neurosci. 2002;112:319–329. doi: 10.1016/S0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 38.Prieto GA, Perez-Burgos A, Fiordelisio T, et al. Dopamine D(2)-class receptor supersensitivity as reflected in Ca2+ current modulation in neostriatal neurons. Neurosci. 2009;164:345–350. doi: 10.1016/j.neuroscience.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ. Cellular and molecular characterization of Ca2+ currents in acutely isolated, adult rat neostriatal neurons. J Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Rosello T, Figueroa A, Salgado H, et al. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- 42.Salgado H, Tecuapetla F, Perez-Rosello T, et al. A reconfiguration of CaV2 Ca2+ channel current and its dopaminergic D2 modulation in developing neostriatal neurons. J Neurophysiol. 2005;94:3771–3787. doi: 10.1152/jn.00455.2005. [DOI] [PubMed] [Google Scholar]
- 43.Sebastião AM, Macedo MP, Ribeiro JÁ. Tonic activation of A(2A) adenosine receptors unmasks, and of A(1) receptors prevents, a facilitatory action of calcitonin gene-related peptide in the rat hippocampus. Br J Pharmacol. 2000;129:374–380. doi: 10.1038/sj.bjp.0703048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunha-Reis D, Fontinha BM, Ribeiro JA, Sebastião AM. Tonic adenosine A1 and A2A receptor activation is required for the excitatory action of VIP on synaptic transmission in the CA1 area of the hippocampus. Neuropharmacol. 2007;52:313–320. doi: 10.1016/j.neuropharm.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Cunha-Reis D, Ribeiro JA, Sebastião AM. A1 and A2A receptor activation by endogenous adenosine is required for VIP enhancement of K+-evoked (3H)-GABA release from rat hippocampal nerve terminals. Neurosci Lett. 2008;430:207–212. doi: 10.1016/j.neulet.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 46.Ciruela F, Casadó V, Rodrigues RJ, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vivas O, Arenas I, García DE. Voltage-independent inhibition of Ca(V)2.2 channels is delimited to a specific region of the membrane potential in rat SCG neurons. Acta Biochim Biophys Sin (Shanghai) 2012;44:544–549. doi: 10.1093/abbs/gms025. [DOI] [PubMed] [Google Scholar]
- 48.Martella G, Spadoni F, Sciamanna G, Tassone A, Bernardi G, Pisani A, Bonsi P. Age-related functional changes of high-voltage-activated calcium channels in different neuronal subtypes of mouse striatum. Neurosci. 2008;152:469–476. doi: 10.1016/j.neuroscience.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 49.Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagberg H, Andersson P, Lacarewicz J, Jacobson I, Butcher S, Sandberg M. Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J Neurochem. 1987;49:227–231. doi: 10.1111/j.1471-4159.1987.tb03419.x. [DOI] [PubMed] [Google Scholar]
- 51.Pazzagli M, Corsi C, Fratti S, Pedata F, Pepeu G. Regulation of extracellular adenosine levels in the striatum of aging rats. Brain Res. 1995;684:103–106. doi: 10.1016/0006-8993(95)00471-2. [DOI] [PubMed] [Google Scholar]
- 52.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 53.Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann N Y Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- 54.Pinna A, Corsi C, Carta AR, Valentini V, Pedata F, Morelli M. Modification of adenosine extracellular levels and adenosine A(2A) receptor mRNA by dopamine denervation. Eur J Pharmacol. 2002;446:75–82. doi: 10.1016/S0014-2999(02)01818-6. [DOI] [PubMed] [Google Scholar]
- 55.Gianfriddo M, Melani A, Turchi D, Giovannini MG, Pedata F. Adenosine and glutamate extracellular concentrations and mitogen-activated protein kinases in the striatum of Huntington transgenic mice. Selective antagonism of adenosine A2A receptors reduces transmitter outflow. Neurobiol Dis. 2004;17:77–88. doi: 10.1016/j.nbd.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Sciotti VM, Park TS, Berne RM, Van Wylen DG. Changes in extracellular adenosine during chemical or electrical brain stimulation. Brain Res. 1993;613:16–20. doi: 10.1016/0006-8993(93)90448-V. [DOI] [PubMed] [Google Scholar]
- 57.Chechova S, Venton BJ. Transient adenosine efflux in the rat caudate-putamen. J Neurochem. 2008;105:1253–1263. doi: 10.1111/j.1471-4159.2008.05223.x. [DOI] [PubMed] [Google Scholar]
- 58.Cunha RA, Vizi ES, Ribeiro JA, Sebastião AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- 59.Gonçalves J, Queiroz G. Facilitatory and inhibitory modulation by endogenous adenosine of noradrenaline release in the epididymal portion of rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:367–371. doi: 10.1007/BF00171335. [DOI] [PubMed] [Google Scholar]
- 60.Correia-de-Sá P, Timóteo MA, Ribeiro JÁ. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J Neurophysiol. 1996;76:3910–3919. doi: 10.1152/jn.1996.76.6.3910. [DOI] [PubMed] [Google Scholar]
- 61.Correia-de-Sá P, Ribeiro JÁ. Adenosine uptake and deamination regulate tonic A2A receptor facilitation of evoked (3H)acetylcholine release from the rat motor nerve terminals. Neuroscience. 1996;73:85–92. doi: 10.1016/0306-4522(96)00028-0. [DOI] [PubMed] [Google Scholar]
- 62.Latini S, Pazzali M, Pepeu G, Pedata F. A2 adenosine receptors: their presence and neuromodulatory role in the central nervous system. Gen Pharmacol. 1996;27:925–933. doi: 10.1016/0306-3623(96)00044-4. [DOI] [PubMed] [Google Scholar]
- 63.Salmi P, Chergui K, Fredholm BB. Adenosine-dopamine interactions revealed in knockout mice. J Mol Neurosci. 2005;26:239–244. doi: 10.1385/JMN:26:2-3:239. [DOI] [PubMed] [Google Scholar]
- 64.Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 65.Ribeiro JA. Adenosine A2A receptor interactions with receptors for other neurotransmitters and neuromodulators. Eur J Pharmacol. 1999;375:101–113. doi: 10.1016/S0014-2999(99)00230-7. [DOI] [PubMed] [Google Scholar]
- 66.Duarte-Araújo M, Nascimento C, Alexandrina Timóteo M, Magalhães-Cardoso T, Correia-de-Sá P. Dual effects of adenosine on acetylcholine release from myenteric motoneurons are mediated by junctional facilitatory A(2A) and extrajunctional inhibitory A(1) receptors. Br J Pharmacol. 2004;141:925–934. doi: 10.1038/sj.bjp.0705697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mynlieff M, Beam KG. Adenosine acting at an A1 receptor decreases N-type calcium current in mouse motoneurons. J Neurosci. 1994;14:3628–3634. doi: 10.1523/JNEUROSCI.14-06-03628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Budd DC, Nicholls DG. Protein kinase C-mediated suppression of the presynaptic adenosine A1 receptor by a facilitatory metabotropic glutamate receptor. J Neurochem. 1995;65:615–621. doi: 10.1046/j.1471-4159.1995.65020615.x. [DOI] [PubMed] [Google Scholar]
- 69.Ambrósio AF, Malva JO, Carvalho AP, Carvalho CM. Modulation of Ca2+ channels by activation of adenosine A1 receptors in rat striatal glutamatergic nerve terminals. Neurosci Lett. 1996;220:163–166. doi: 10.1016/S0304-3940(96)13252-3. [DOI] [PubMed] [Google Scholar]
- 70.Ambrósio AF, Malva JO, Carvalho AP, Carvalho CM. Inhibition of N-P/Q- and other types of Ca2+ channels in rat hippocampal nerve terminals by the adenosine A1 receptor. Eur J Pharmacol. 1997;340:301–310. doi: 10.1016/S0014-2999(97)01451-9. [DOI] [PubMed] [Google Scholar]
- 71.Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol. 1998;8:351–356. doi: 10.1016/S0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 72.Kaneko S, Akaike A, Satoh M. Receptor-mediated of voltage-dependent Ca2+ channels via heteromeric G-proteins in neurons. Jpn J Pharmacol. 1999;81:324–331. doi: 10.1254/jjp.81.324. [DOI] [PubMed] [Google Scholar]
- 73.Chieng B, Bekkers JM. Inhibition of calcium channels by opioid- and adenosine-receptor agonists in neurons of the nucleus accumbens. Br J Pharmacol. 2001;133:337–344. doi: 10.1038/sj.bjp.0704072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, Kaneko S. Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J Neurosci. 2001;21:628–640. doi: 10.1523/JNEUROSCI.21-02-00628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- 76.Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25(5):1050–62. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oliveira L, Timóteo MA, Correia-de-Sá P. Tetanic depression is overcome by tonic adenosine A(2A) receptor facilitation of L-type Ca(2+) influx into rat motor nerve terminals. J Physiol. 2004;560:157–168. doi: 10.1113/jphysiol.2004.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]