Abstract
Osteocytes reside as a cellular network throughout the mineralised matrix of bone and are considered the primary mechanosensors of this tissue. They sense mechanical stimulation such as fluid flow and are able to regulate osteoblast and osteoclast functions on the bone surface. Previously, we found that ATP is released load-dependently from osteocytes from the onset of mechanical stimulation. Therefore, the aim of the present study was to investigate whether and how ATP release can be evoked in osteocytes via purinergic receptor activation. ATP release was quantified by real-time determination using the luciferin-luciferase assay and the release pathway was investigated using pharmacological inhibition. The P2Y receptor profile was analysed using gene expression analysis by reverse transcription polymerase chain reaction, while functional testing was performed using measurements of intracellular calcium responses to P2 receptor agonists. These investigations demonstrated that MLO-Y4 osteocytes express functional P2Y2, P2Y4, P2Y12 and P2Y13 receptors in addition to the previously reported P2X receptors. Further, we found that osteocytes respond to nucleotides such as ATP, UTP and ADP by increasing the intracellular calcium concentration and that they release ATP dose-dependently upon stimulation with 1–10 μM UTP. In addition to this, osteocytes release large amounts of ATP upon cell rupture, which might also be a source for other nucleotides, such as UTP. These findings indicate that mechanically induced ATP signals may be propagated by P2 receptor activation and further ATP release in the osteocyte network and implicate purinergic signalling as a central signalling pathway in osteocyte mechanotransduction.
Keywords: Osteocyte, ATP release, UTP, P2 receptors, Vesicles, Mechanotransduction
Introduction
During physical activity such as walking or running, mechanical loading causes repetitive pressure gradients, which lead to flow of extracellular fluid through the lacunocanalicular network of the mineralised bone matrix [1–3]. Osteocytes are situated in these lacunae and connected by numerous dendritic processes through the canaliculi to both neighbouring osteocytes inside the bone and to the osteoblasts on the bone surface [4, 5]. Osteocytes are highly sensitive to fluid shear stress and they seem to constitute an essential mechanosensitive signal transducing network through the bone [2, 6–8]. Osteocyte mechanotransduction is defined as a sequence of mechanosensation, translation of the mechanical signal into a biochemical signal and finally the expression or release of molecules, which regulate osteoblasts and osteoclasts [9–11]. A number of mechanosensors, such as primary cilia and integrins, have been suggested to sense mechanical loading in osteocytes. They are reported to translate mechanical stimulation into intracellular signals such as increased intracellular calcium concentration ([Ca2+]i) and decreased cyclic adenosine monophosphate (cAMP) levels [12, 13]. These intracellular events lead to the regulated expression of downstream signalling molecules, which affect bone formation and resorption, such as prostaglandin E2 (PGE2), etc [10, 11].
A growing body of data demonstrates that both bone-forming osteoblasts and bone-resorbing osteoclasts express a variety of P2 receptors and apply these in mechanically induced intercellular communication [14–19]. Furthermore, it is observed that both osteoblast and osteoclast formation and activity are regulated by nano- to micromolar concentrations of nucleotides such as ATP, ADP, UTP and UDP [20–25]. In particular, the P2Y2 receptor seems to play an important role in bone. It has been shown to mediate intercellular communication between osteoblasts [14] and low concentrations of UTP have been observed to inhibit osteoblastic bone formation [25, 26]. Furthermore, P2Y2 receptor knockout in mice is associated with increased bone formation [27]. Therefore, P2Y2 receptors have been characterised as an “off-switch” in bone formation. The P2X7 receptor has been suggested to play an important role in bone mechanotransduction. P2X7 receptors are expressed in MLO-Y4 osteocytes and knockout of the P2X7 receptor in mice is associated with a reduced anabolic response to mechanical loading [28]. Hence, nucleotide signalling through P2 receptors plays an important role in the regulation of bone remodelling.
P2Y receptors are G-protein-coupled receptors, which are primarily activated by nucleotides such as UTP, UDP or ADP [29, 30]. The majority of P2Y receptors (P2Y1–11) couples to Gq/11 proteins, which leads to the generation of inositol triphosphate, calcium release from intracellular stores and the activation of protein kinase C (PKC). PKC then phosphorylates target proteins such as c-Fos [30, 31], which is also observed to be up-regulated in osteocyte mechanotransduction [32, 33]. In contrast, P2Y12–14 receptors generally couples to Gi/o, which leads to the inhibition of adenylyl cyclase and a decrease in cAMP levels [30, 31].
Vesicular exocytosis, connexin 43 (Cx43) hemichannels and pannexin 1 (Panx1) channels have been shown to mediate ATP release, which can be initiated by, e.g. elevated [Ca2+]i [34–39]. Mechanically induced ATP release is reported to occur by vesicular exocytosis from osteoblasts and via Cx43 from osteocytes and associates with the release of PGE2 [36, 37]. In osteocytes, fluid flow stimulation induces not only intracellular calcium signalling, which can be significantly reduced when P2 receptors are antagonized, intracellular calcium stores are depleted or in calcium-free medium [40], but also hemichannel-mediated ATP release [37]. Furthermore, recent investigations in our laboratory demonstrated that osteocytes in fact emit acute and load-dependent ATP signals from the onset of fluid flow stimulation [41] and that they express P2X2 and P2X7 receptors, which may propagate mechanically initiated nucleotide signals in the osteocyte network. Moreover, we have shown that the acute phase of mechanically induced ATP release is mediated by vesicular exocytosis and possibly membrane channels [41].
Therefore, the aim of the present study was to investigate whether ATP release can be evoked in osteocytes by purinergic receptor activation and how it is released. Since the P2Y2 receptor seems to play an important role in bone remodelling, we chose to study UTP-induced ATP release.
Methods
Cell culturing
MLO-Y4 osteocytes were cultured according to a previously published protocol [42]. In brief, cells were cultured in plates or flasks coated with 0.15 mg/ml rat tail collagen type I (BD Biosciences, Bedford, MA, USA) and all experiments were done using 80 % confluent cells seeded 2 days before with medium change the day before the experiment.
Reverse transcription PCR
MLO-Y4 derived total RNA was isolated using the RNeasy minikit and the RNase-free DNase Set (Qiagen, Copenhagen, Denmark). RNA was reverse transcribed into cDNA using the Omniscript Reverse Transcriptase Kit (Qiagen). cDNA was amplified using HotStarTaq DNA Polymerase Kit (Qiagen) and P2X receptor specific primers (Table 1). MgCl2 was added to PCR reactions amplifying P2Y1 and P2Y12 receptors. PCR reactions were carried out on a thermal cycler (Eppendorf, Hamburg, Germany or Applied Biosystems (Life Technologies) Paisley, UK), according to the following PCR protocol (40 cycles): initial activation at 95 °C for 15 min, denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 1 min and final extension at 72 °C for 10 min. Annealing temperature differed for P2Y4 (56 °C), P2Y6 (55 °C) and P2Y13 (53 °C) receptors. Amplified DNA was separated by agarose gel electrophoresis at 140 V for 30–35 min and visualized by UV using either the Syngene System and GeneSnap (v. 4) or Fujifilm LAS-4000 (P2X2 and P2X3 receptors). Sterile water represented the negative control, whereas positive control RNA was obtained from BALB/cJ mouse brain, spleen and femoral bone marrow. The tissue was ground in liquid nitrogen or cut in small pieces in RNAlater using a pair of scissors and total RNA was isolated as described for MLO-Y4 samples.
Table 1.
Primers used for RT-PCR amplification of P2Y receptors
| Receptor | Forward primer (5′→3′) | Reverse primer (5′→3′) | Product length (bp) |
|---|---|---|---|
| P2Y1 | aaa aac aaa act gtc acc tgc t | tgt cca ggt cat tgt aaa tca a | 166 |
| P2Y2 | ggg gag agt agt gta gct gat g | gaa cca gtt gtt gga tag gtg t | 161 |
| P2Y4 | ctc gtg ccc aac ctc ttc tt | agc agc acc atg att gtg ga | 125 |
| P2Y6 | ggc tat gaa ggg cag caa ga | tca gac agt gag atg aag gcg | 739 |
| P2Y12 | tat tcc cgg aga cac tca tat c | tac tgc gga tct gaa aga aaa t | 231 |
| P2Y13 | ggc cac tag atg tca cct ttt c | gat ggt ggg gtg gta act aga a | 105 |
bp base pairs
Calcium imaging
Calcium imaging experiments were conducted as described previously [15]. In brief, cells were cultured on 25 mm glass coverslips for 2 to 3 days, then loaded with 5 μM fura-2/AM (Invitrogen Molecular Probes, Eugene, OR) dissolved in experiment medium (2.5 mM probenecid, 20 mM NaHCO3 in α-modified MEM, pH 7.35–7.45) and experiments were carried out at 37 °C in 11 % CO2 superfusion on a Zeiss Axiovert 35 microscope (Carl Zeiss Inc., Thornwood, NY). Cells were exposed to 0.1, 1, 10, 100 or 1,000 μM of the following nucleotide analogues: ADP (Roche, Mannheim, Germany), 2-methyl-thio-ATP (2-meSATP, Sigma, St. Louis, MO), UTP (Roche) and UDP (Sigma). Ratios of light emission were calibrated into calcium concentrations using the fura-2 Calcium Imaging Calibration Kit (Invitrogen). Calcium responses were calculated as the average calcium increase in five individual cells per experiment.
ATP release measurements
ATP release was measured using luciferin-luciferase reagent from the ATP Bioluminescence kit HS II (Roche) on a NOVOstar microplate reader (BMG Labtech, Ortenberg, Germany) according to an earlier described method [43]. In short, cells were cultured in white, clear bottom 96-well plates for 2 days. First, 50 μl sterile HCO3−-free buffer (÷BIC) (140 mM NaCl, 1 mM MgCl2·6H2O, 10 mM Hepes, 1.6 mM K2HPO4·3H2O, 0.4 mM KH2PO4, 1.5 mM CaCl2·2H2O, 5 mM Glucose·H2O, pH 7.4) was added to the wells, and cells rested at 37 °C and 5 % CO2 for 10 min. Then, 50 μl luciferin-luciferase reagent dissolved in ÷BIC was added and cells rested 30 min in the NOVOstar before starting the experiment. The ATP release pathway was investigated using pharmacological inhibitors. Membrane channels were blocked by carbenoxolone (CBX, Sigma), Gap26 (Cx43) mimetic peptide (VCYNKSFPISHVR) (ANASpec, Fremont, CA), 10panx1 mimetic peptide (WRQAAFVDSY) (ANASpec) and probenecid (Sigma). Vesicular exocytosis was challenged by bafilomycin A1 (Calbiochem (EMD Millipore) Billerica, MA), which blocks vesicular ATP loading [44]. All pharmacological inhibitors were allowed to pre-incubate for approximately 40 min before experiment start except for bafilomycin A1, which was pre-incubated for 2 h. Since UTP induced a slow kinetic response, ATP release was measured in plate mode: 1 s/cycle, for a total of 55 cycles with a cycle time of 10 s. This allowed us to measure up to six wells/cycle and resulted in a total measurement time of 9 min. Baseline luminescence was measured for ten cycles followed by the addition of UTP using the lowest speed, 100 μl/s. UTP and vehicle solutions were kept on ice in the NOVOstar throughout the experiment, but had room temperature when they were injected. Vehicle (sterile H2O or DMSO) in working solutions were ≤1 % and did not affect cells or the assay. Each experiment represents the mean of triplicate measurements each calibrated using ATP standard curves produced at the end of the day. For the inhibitor-based assays, ATP standards were made for both vehicle and inhibitor solutions. Final reaction volume was 150 μl and ATP release was normalized to 106 cells by post-experimental cell counting.
To account for potential impurities of the UTP solution with ATP that might affect the luciferin-luciferase assay directly, all UTP solutions and experiment mixtures of UTP and the different inhibitors used were tested for activity in the assay. The luminescence of the experimental mix was used as background and subtracted from the relevant measurement on the wells containing cells. If unacceptably high luminescence from the UTP solution or the UTP + inhibitor solution was detected, the solution was discarded. After each experiment, cell viability was checked by visual inspection using light microscopy. Cells were checked for morphology, attachment to the bottom of the culture dish, cell numbers and other signs of viability. If signs of cell death were observed, the experiment was excluded from data analysis.
Statistical analysis
Results are presented as individual data points and means except for ATP release curves, which are presented as mean ± SEM. Significant differences between groups were determined by Kruskal–Wallis and Mann–Whitney statistical analyses using IBM SPSS statistical analysis software, version 19 (IBM, Armonk, NY). Values of p ≤ 0.05 was considered significant, and the following notations were used in the graphs: *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
Results
Osteocytes express P2Y receptors
RT-PCR analysis was conducted to investigate the P2 receptor mRNA profile of MLO-Y4 osteocytes and demonstrated the expression of P2Y2, P2Y4, P2Y6, P2Y12 and P2Y13 receptor mRNA, whereas it was not possible to demonstrate the expression of P2Y1 receptor mRNA (Fig. 1 and Table 2).
Fig. 1.
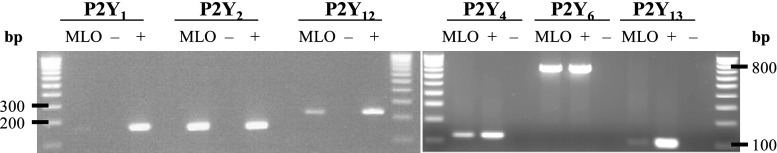
P2Y receptor mRNA expression in MLO-Y4. Agarose gel electrophoresis visualizing RT-PCR amplified P2 receptor cDNA from reverse-transcribed MLO-Y4-derived RNA. The gel demonstrates the expression of P2Y2, P2Y4, P2Y12 and P2Y13 receptor mRNA in MLO-Y4 osteocytes. P2Y1 receptor mRNA could not be identified. DNA marker, 100 bp DNA ladder. Positive control (+), cDNA obtained from BALB mouse bone marrow, brain and spleen. Negative control (−), sterile water. bp base pairs
Table 2.
The P2Y receptor profile of MLO-Y4 osteocytes determined as the expression of mRNAs and functional receptors (agonist response)
| Receptor | mRNA | Agonist response | Selective agonist |
|---|---|---|---|
| P2Y1 | − | − | ADP, 2meSATP |
| P2Y2 | + | + | ATP, UTP |
| P2Y4 | + | + | ATP, UTP |
| P2Y6 | + | − | UTP, UDP |
| P2Y12 | + | + | ADP |
| P2Y13 | + | + | ADP |
The outcome of individual endpoints is indicated as positive (+) or negative (−)
Osteocytes respond to P2Y receptor agonists
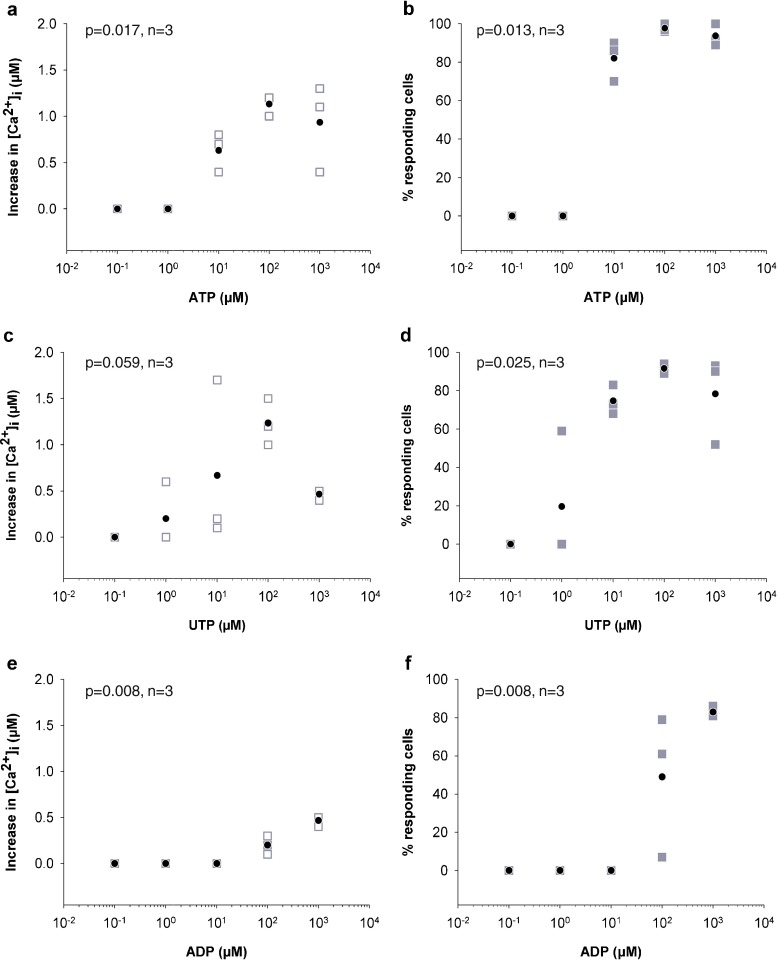
The expression of functional P2Y receptors on the osteocyte cell surface was determined by measuring the intracellular calcium response to different nucleotide analogues. First, cells were stimulated with ATP and demonstrated an increase in [Ca2+]i when stimulated with concentrations of 10 μM and above (Fig. 2a, b and Table 2), which indicate the expression of P2X1-7, P2Y2 and P2Y4 receptors. To further explore the expression of P2Y2, P2Y4 and P2Y6 receptors, cells were exposed to UTP and responded with an increase in [Ca2+]i when stimulated with concentration from 1 μM UTP and up (Fig. 2c, d). Finally, cells were exposed to P2Y1, P2Y12 and P2Y13 receptor-selective ADP and responded to 100 and 1,000 μM (Fig. 2e, f). In contrast, cells did not respond to the equally potent P2Y1 receptor-selective 2-meSATP or P2Y6 receptor-selective UDP (data not shown). Vehicle did not induce any calcium responses (n = 5).
Fig. 2.
Osteocytes respond to P2 receptor agonists. Nucleotide-induced intracellular calcium responses were measured in fura-2 loaded MLO-Y4 osteocytes by exposing them to 0.1, 1, 10, 100 or 1,000 μM nucleotide. MLO-Y4 responded to ATP (a, b), UTP (c, d) and ADP (e, f) by increasing [Ca2+]i indicating the functional expression of P2Y2 and/or P2Y4, and P2Y12 and/or P2Y13 receptors. Empty grey box represents the average calcium response in each experiment, filled grey box represents the percent of cells responding in each individual experiment and filled black circle represents the mean (n = 3)
Cell rupture- and UTP-induced ATP release
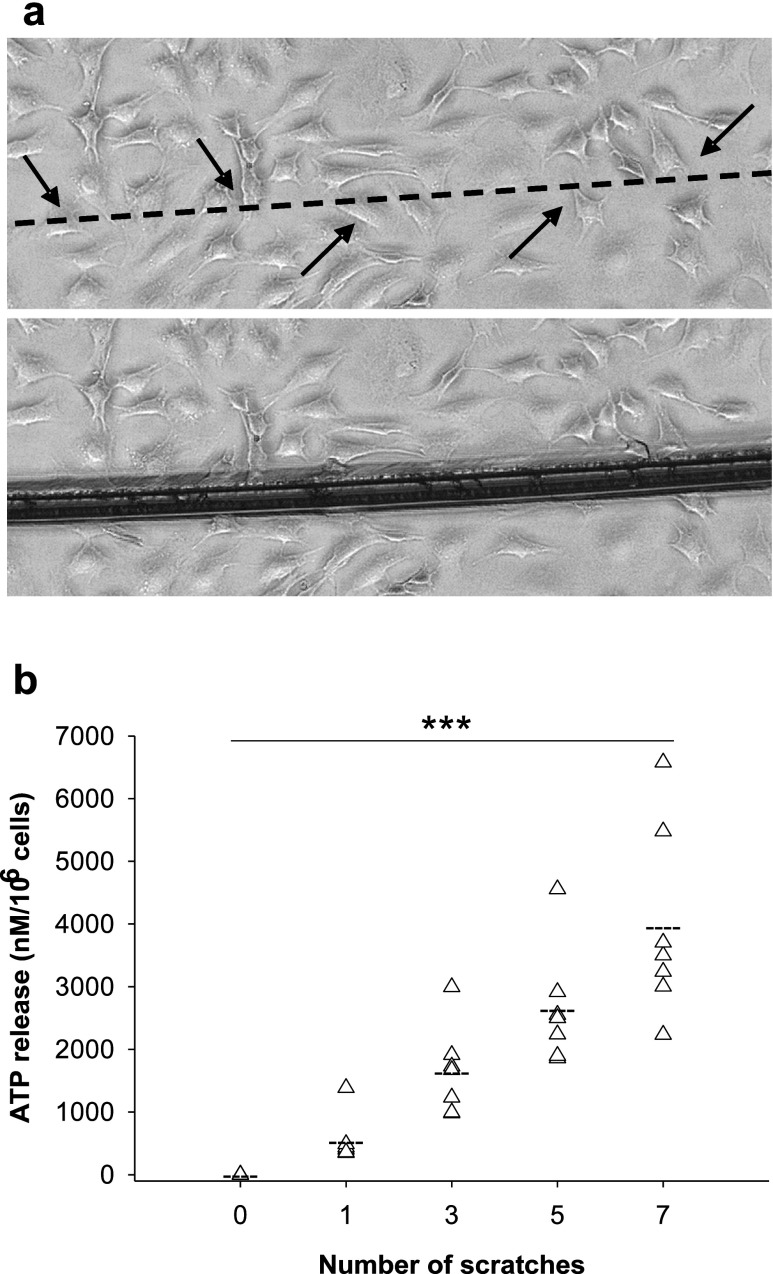
Next, we wanted to know whether nucleotides can be released by cell rupture and nucleotide stimulation. Cell rupture-associated ATP release was studied by scratching the cells with a needle zero, one, three, five or seven times and cells demonstrated the release of large amounts of ATP depending on the number of scratches applied (n = 7, p < 0.001) (Fig. 3a, b).
Fig. 3.
Cell rupture-related ATP release. a Scratching the cells with a needle resulted in rupture of both cell bodies and dendrites. Upper panel, before scratch; lower panel, after scratch. b Cells were scratched with a needle zero, one, three, five and seven times, which led to release of ATP depending on the number of scratches (n = 7). Arrow indicates ruptured dendrites or cell bodies, triangle represents ATP release in each experiment, broken line represents mean ATP release and three asterisks represent p ≤ 0.001
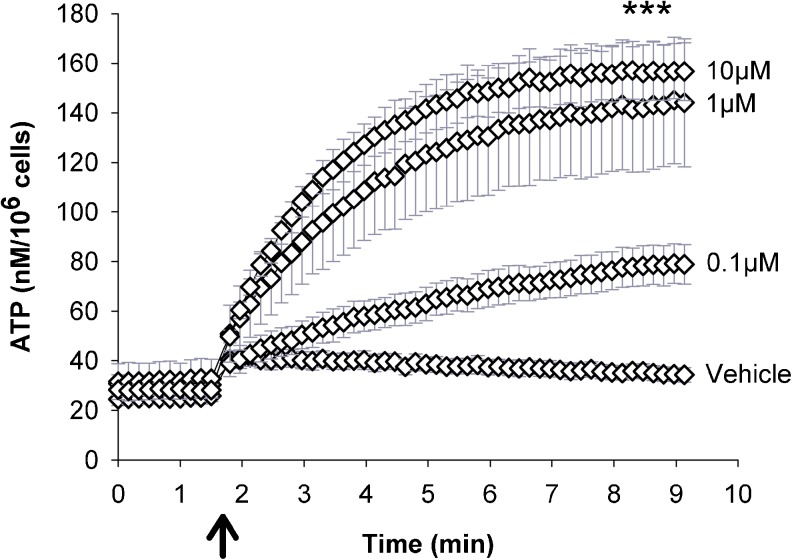
Since, the above data indicate the expression of P2Y2 and P2Y4 receptors, UTP was chosen to study the nucleotide-induced ATP release potential. Cells were exposed to 0.1, 1 and 10 μM UTP, which led to slowly increasing ATP release with a calculated initial release rate of 0.5 nM/s (0.1 μM UTP), 1.0 nM/s (1 μM) and 1.2 nM/s (10 μM) (Fig. 4a). ATP release peaked after 7–8 min with a dose-dependent peak ATP release of 45 nM (0.1 μM UTP), 102 nM (1 μM) and 119 nM (10 μM), (n = 5, p = 0.001 between groups, Fig. 4). ATP release associated with vehicle injection corresponded to the mechanically induced ATP release at 100 μl/s, which has been observed earlier [41].
Fig. 4.
UTP induced ATP release. After ten baseline measurements, UTP (0.1, 1 and 10 μM) was injected in the wells leading to slowly increasing and dose-dependent ATP release for the subsequent 7–8 min. Vehicle induced the release of a low amount of ATP, which reflect mechanically induced ATP release at the applied injection speed (n = 5). ATP release induced by UTP was quantified and normalised to 106cells. Data are given as mean ± SEM; arrow indicates UTP addition, three asterisks represent p ≤ 0.001
UTP-induced ATP release pathway
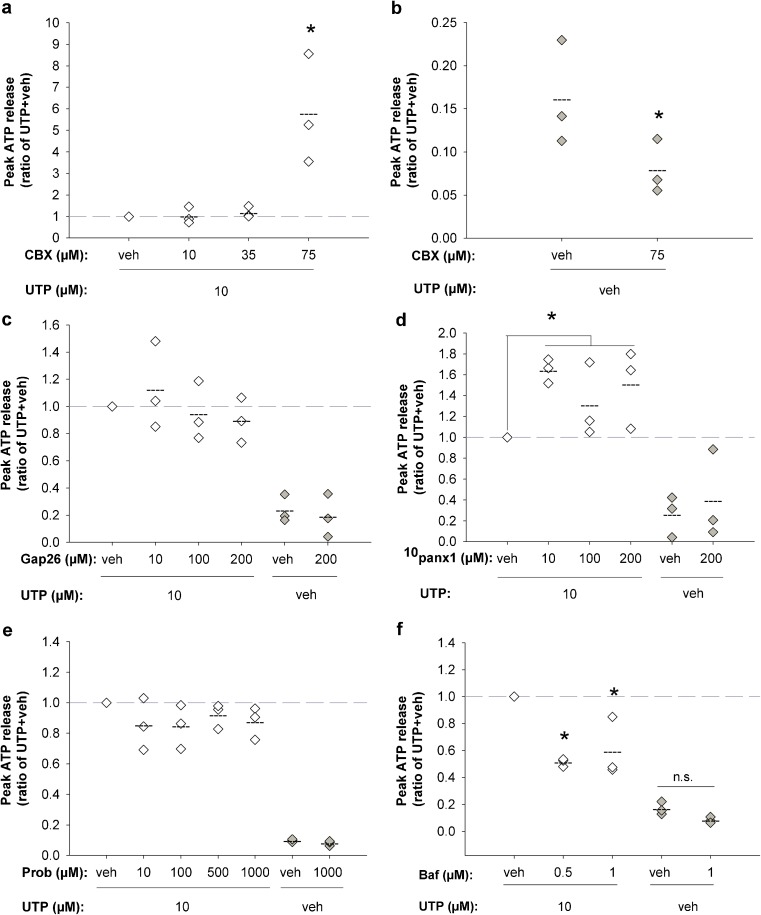
To study the general involvement of gap junctions/membrane channels in UTP-induced ATP release, cells were treated with CBX. 10–35 μM CBX had no significant effect on ATP release, but 75 μM CBX led to an increase in ATP release of more than fivefold (n = 3, p = 0.05, Fig. 5a). Control experiments in this setup demonstrated that injection of vehicle instead of UTP induced the release of a small amount of ATP, which corresponds to approximately 15 % of the UTP-induced response (Fig. 5b) and equals the mechanically induced ATP release observed at this injection speed. Addition of 75 μM CBX to this setup significantly reduced ATP release by approximately 50 % (n = 3, p = 0.05, Fig. 5b), as observed earlier for mechanically induced ATP release [41]. Gap26 (10–200 μM) had no effect on UTP-induced ATP release, indicating no involvement of Cx43 (n = 3, Fig. 5c).
Fig. 5.
Analysis of the UTP-induced ATP release pathway. ATP release induced by 10 μM UTP (100 μl/s) was challenged by pharmacological inhibitors. a 10–35 μM CBX had no effect on ATP release, whereas 75 μM CBX resulted in a dramatic increase in ATP release of >5-fold. b The addition of vehicle instead of UTP induced the release of a small amount of ATP from the cells corresponding to approximately 15 % of the UTP-induced ATP release. When 75 μM CBX was added to this setup, ATP release was reduced by approximately 50 %. c Gap26-mediated inhibition of Cx43 hemichannels did not affect ATP release. d 10panx1-mediated inhibition of Panx1 channels was associated with significantly increased ATP release, when the treated group was compared to the vehicle group. e Probenecid (Prob)-mediated inhibition of Panx1 channels did not affect ATP release. f Bafilomycin A1 (Baf) treatment resulted in up to 49 % reduction of UTP induced ATP release. Diamond represents ATP release in ratios of the control group (UTP + vehicle of the inhibitor), broken line represents mean ATP release in ratio of vehicle and asterisk represents p ≤ 0.05
To further study the involvement of Panx1 channels, cells were treated with 10panx-1 (10–200 μM), which resulted in an average increase in ATP release of 49 %, when comparing the treated group with the vehicle group (n = 3, p = 0.042, Fig. 5d). Treatment with 10–1,000 μM probenecid (n = 3), a proposed Panx1 inhibitor [45], had no effect on ATP release (Fig. 5e).
UTP-induced ATP release was then challenged by bafilomycin A1. This treatment reduced ATP release by 49 % (0.5 μM, p = 0.05) and 44 % (1 μM, p = 0.05) (n = 3, Fig. 5f), which indicates vesicular exocytosis of ATP.
After each individual experiment, cells were checked for viability by visual inspection. No signs of decreased viability/cell death were detected.
Discussion
We have previously shown that osteocytes release ATP immediately as mechanical stimulation occurs and that they express functional ATP-sensing P2X receptors. This indicates that P2 receptor-mediated ATP release may be an early signal in osteocyte mechanotransduction. In the present study, the aim therefore was to study whether osteocytes are able to respond to nucleotide stimulation by P2 receptor activation and ATP release.
First, we analysed the expression of P2Y receptors in order to find the P2 receptor profile of osteocytes. RT-PCR revealed the expression of P2Y2, P2Y4, P2Y6, P2Y12 and P2Y13 receptor mRNA, whereas P2Y1 receptor mRNA expression could not be identified. P2Y2/P2Y4 and P2Y12/P2Y13 functional receptor expression was demonstrated by UTP- and ADP-induced calcium responses, respectively. The availability of P2Y2 and P2Y4 receptor-selective agonists and antagonist was highly limited, which made further pharmacological distinction between these receptors unfeasible. On the human P2Y4 receptor, UTP is observed to be more potent than ATP. However, on the mouse P2Y4 receptor, they are equipotent [30] and pharmacological discrimination by analysing the different potencies of UTP and ATP was therefore not possible.
P2Y2 receptors have been implicated in osteoblast intercellular communication and characterized as an “off-switch” in bone remodelling [14, 25, 26]. Its expression on osteocytes may also implicate it in osteocyte signalling pathways, which we investigated further by studying UTP-induced ATP release.
Discrimination between P2Y12 and P2Y13 receptors was similarly limited by the lack of specific agonists. P2Y13 receptor mRNA was identified by a weak band and receptor expression may thereby be low. The ADP-induced calcium response may thereby indicate P2Y12 receptor expression. However, quantitative analysis of P2 mRNA expression should be performed to further clarify this issue and is highly relevant. Based on the current data, P2Y13 receptor expression cannot be excluded. In contrast, P2Y1 receptor mRNA was not identified and 2-meSATP did not induce an intracellular calcium response, which indicate the complete absence of P2Y1 receptors on osteocytes. Taken together, these results indicate the functional expression of P2Y2 and/or P2Y4, and P2Y12 and/or P2Y13 receptors.
The P2Y2 receptor is characterised as an important P2 receptor in osteoblast communication and function [14, 25, 26]. P2Y2, P2Y4 and P2Y6 receptors are activated by UTP, but since cells did not respond to UDP, functional P2Y6 receptors seems to be absent. Thus, the induction of ATP release by UTP occurs via the activation of P2Y2 and/or P2Y4 receptors. Various sources exist for UTP in the extracellular milieu. Constitutive as well as mechanically induced UTP release has been observed in a number of cell types [46, 47]. Moreover, UTP can be generated extracellularly from the nucleoside diphosphokinase-catalysed transphosphorylation of UDP by ATP [48]. However, another potential source of high extracellular nucleotide concentrations is cell death or cell rupture and tissue damage. In bone, microfractures occur frequently throughout the skeleton and one of the functions of the remodelling process is repair these to maintain a strong and durable skeleton. This process involves the initiation of osteoclastic bone resorption and subsequently osteoblastic bone formation at the bone multicellular unit. A microfracture involves rupture of osteocytes that are embedded in the bone matrix. Our data from the scratch-wound assay showed that cell rupture of the osteocytic cells resulted in significant amounts of ATP being released. The implications of this could be that not only mechanical stimulation of the cells could induce nucleotide-induced ATP release but also cell rupture occurring in relation to microfractures in the bone tissue could initiate ATP release from neighboring osteocytes and potentially propagation of the signal to the bone surface where the ATP could activate osteoclasts and initiate the remodelling process.
Using functional studies, we showed that UTP in concentrations ≥1 μM induces intracellular calcium responses and initiates ATP release from osteocytes. Moreover, when UTP concentration was increased, ATP release increased dose-dependently. This indicates that osteocytes can translate UTP signals into ATP release and that they are able to communicate the strength of the initial UTP signal to neighbouring cells by graded ATP release. Thus, osteocytes seem to be able to communicate fine-tuned nucleotide messages in the network.
However, since ATP release was quantified 7–8 min after stimulation, it must be considered a mixed response to first UTP stimulation and then nucleotide autocrine stimulation from released ATP (and possibly other nucleotides). Therefore, the entire response may be a result of more general P2 receptor activation initiated by UTP and potentially reflects the propagation and/or potentiation of ATP signals by auto/paracrine stimulation in the culture. The fact that UTP-stimulated P2Y receptor activation induces nucleotide release and subsequent positive feedback activation of other P2 receptors implies that the system, at least theoretically, may function as an on–off switch that can keep the system in either the on or off position. This way activation of P2Y receptors in one area of the osteocyte network can be activated, for example by microfractures with release of nucleotides from dying osteocytes or by mechanical loading on the bone and initiate an ATP-mediated P2 purinergic signalling cascade through the osteocyte canalicular network reinforcing the signal along the osteocyte dendrites until the signal reaches the bone surface. As the peridendritic space is narrow and thus the layer of extracellular fluid thin, high concentrations of ATP can be obtained around the osteocytes making this a highly efficient signalling system within the bone tissue.
The involvement of specific P2 receptor subtypes in UTP-induced ATP release may be further investigated by selective antagonists or by using cells from knockout animals. UTP release and its extracellular generation have not been investigated in osteocytes, but based on the present results, these investigations are highly relevant. The involvement of P2Y2 and P2Y4 receptors in the mediation of nucleotide signals in the osteocyte network is novel information, which can be applied in the analysis of bone phenotypes of P2 receptor knockout animals. P2Y2 receptors are involved in osteoblast intercellular communication and its activation has been observed to reduce bone formation, thereby functioning as an off-switch in bone remodelling [14, 25, 26]. P2Y2 receptor knockout animals are observed to have increased bone mass possibly because of blocked inhibition of bone formation. The present data indicate that P2Y2 receptors are also involved in the mediation of nucleotide signals in the osteocyte network; thus, increased bone mass in P2Y2 receptor knockout mice might also be caused by inhibited osteocyte “off-signalling” to osteoblasts. Nonetheless, it is highly relevant to study P2Y2 receptor signalling in osteocytes further and how it affects bone remodelling.
Since we also found the expression of functional P2Y12 receptors on osteocytes, they may also be involved in osteocyte intercellular communication. In fact, decreased intracellular cAMP levels have been observed as a response to mechanical stimulation of osteocytes and the same intracellular event occurs in P2Y12 receptor-expressing cells upon P2Y12 receptor activation [13, 29, 30]. In osteoblasts and osteoclasts, P2Y12 receptor antagonism inhibits their formation and viability and additionally decrease bone formation and resorption in vitro. However, despite the fact that both bone formation and resorption is affected, the overall effect of P2Y12 receptor antagonism in vivo is decreased bone mineral density and trabecular bone volume and number [49]. If P2Y12 receptors are additionally involved in osteocyte signalling, this provides a third dimension to the analysis of P2Y12 receptor antagonism in vivo and could explain why the overall bone phenotype is negatively affected. Flow-induced mechanosensing via primary cilia in osteocytes associates with an immediate decrease in cAMP levels and later compensatory increase in cAMP levels in addition to increased COX-2 expression [13], of which the latter is involved in the synthesis of the bone anabolic PGE2. The P2Y12 receptor-associated decrease in cAMP levels may thereby also lead to the release of bone formation-promoting signals such as PGE2 from the osteocyte, which would be blocked by P2Y12 receptor antagonism. Thus, P2Y12 receptor antagonism in vivo may result in an overall negatively affected bone homeostasis due to blocked P2Y12 receptor signalling in both osteocytes and osteoblasts.
Next, we challenged ATP release by pharmacological inhibitors to identify the involved release pathway. We previously found that mechanically induced ATP release could be inhibited by bafilomycin A1 [41] and here we find that 0.5 μM bafilomycin inhibits UTP-induced ATP release by 49 %. ATP release could not be further inhibited by increasing concentration to 1 μM, indicating full inhibition of the vesicular H+-pump and supposedly the accumulation of nucleotides in intracellular vesicles. Thus, alternative ATP release pathways to vesicular exocytosis may exist in osteocytes and the release pathway was further challenged by targeting membrane channels.
Previously, we found that 35 μM CBX significantly inhibited mechanically induced ATP release [41]. In the present study, it did not affect UTP-induced ATP release. Since both Cx43 hemichannels and Panx1 channels are blocked by low micromolar concentrations of CBX [50, 51], membrane channels are not likely to be involved in UTP-induced ATP release.
Most unexpectedly, however, when CBX concentration was increased to 75 μM, ATP release was observed to increase dramatically. Since this effect was not observed in control measurements of vehicle addition, it seems to be UTP/P2Y2/4 receptor-dependent. UTP-induced enhanced ATP release was also observed in the presence of 10panx1, but not in the presence of the less potent Panx1 inhibitor, probenecid. CBX- and 10panx1-based experiments thus indicate that blockade of Panx1 channels during UTP stimulation enhances ATP release from osteocytes. The mechanism is difficult to explain but the following observations may provide an indication. Panx1 channels have been proposed to associate with both P2Y2 and P2X7 receptor subtypes. Osteocytes express both P2Y2 and P2X7 receptors (Fig. 1, Table 2) [28]. Panx1 currents have been observed to be potently inhibited by CBX and high concentrations of both ATP and UTP, whereas P2X7 receptor currents are observed to be increased 5–10 % by CBX (50 μM) [50]. UTP leads to ATP release (Fig. 4) possibly via P2Y2 receptor activation and prolonged ATP stimulation of P2X7 receptors leads to pore-opening and release of large quantities of ATP. Panx1 has been suggested as the P2X7 receptor-associated pore, but the P2X7 receptor channel itself can also be dilated to mediate the passage of larger molecules such as fluorescent dyes [52, 53]. Thus, if we assume that Panx1 channels are inhibited by CBX and 10panx1 in our experiments, enhanced UTP induced ATP release via P2Y2 receptor activation could be due to release via P2X7 receptors or a Panx1 cross talk effect on the P2X7 receptor channel. Alternatively, the enhanced ATP release could be a compensatory response to blocked Panx1 channels by the up-regulation of, e.g. exocytotic ATP release. However, as the blockers used are “dirty” and thus inhibits not only the channels intended but also a range of other channels, more specific inhibition of channels with either siRNA or the use of osteocytes from mice with targeted deletion of the individual channels should be done in order to more specifically address the mechanism for the increased release of ATP.
In conclusion, previous and present data indicate that osteocytes express P2X2, P2X7, P2Y2 and/or P2Y4 and P2Y12 and/or P2Y13 receptors, and since they all lead to intracellular calcium signalling upon stimulation, they are possible candidates in osteocyte intercellular signalling. Present data further indicate that, in addition to mechanical stimulation, osteocytes are able to release ATP in response to two other stimuli, cell rupture and nucleotide stimulation. Moreover, they can translate UTP stimulation into ATP release via P2Y2 and/or P2Y4 receptor activation. UTP-induced ATP release was found to be mediated by vesicular exocytosis and was graded according to the initial level of nucleotide stimulation, indicating that osteocytes can transduce fine-tuned nucleotide signals in the network. Since previous data further demonstrated that mechanical sensitivity of osteocytes is linked to acute ATP release, we hypothesise that P2 receptor-mediated nucleotide signalling is a central pathway in osteocyte mechanotransduction.
Based on previous and present data, we propose the following model for P2 receptor-mediated nucleotide signalling in osteocytes: (a) In mechanotransduction, the mechanical stimulation such as intra-lacunocanalicular fluid flow activates mechanosensors on the osteocyte surface, leading to load-dependent release of nucleotides. (b) Microfractures involving osteocytic cell rupture releases large amounts of ATP locally within the bone. Under both circumstances, the released nucleotides may be pushed through the lacunocanalicular network by pressure gradients and associated fluid flow and may activate P2 receptors expressed on neighbouring osteocytes. P2 receptor activation results in intracellular calcium signalling and dose-dependent vesicular ATP release, which serves to propagate the nucleotide signal through the bone. Eventually, this cascade of P2 receptor-mediated nucleotide signalling may reach the osteoblasts and osteoclasts on the bone surface, potentially leading to bone remodelling. These findings support the very intriguing hypotheses put forward independently by the groups of Timothy Skerry [54] and Charles H. Turner [55] that osteocytes may resemble a “neural network” in bone as excitatory agents usually found in the central nervous system also have roles in osteogenesis coupled to mechanical loading. We hypothesize that this network mediates nucleotide messages from the interior of bone telling that mechanical stimulation or structural damage has occurred and the magnitude of it.
Acknowledgments
We thank Dr. Lynda F. Bonewald (University of Missouri, Kansas City School of Dentistry, Kansas City, MO, USA) for kindly providing us with the MLO-Y4 osteocytes and Ole Vang (Roskilde University, Roskilde, Denmark) for academic discussion of study design and results.
The work was kindly supported by the European Commission under the 7th Framework Programme (proposal #202231) performed as a collaborative project among the members of the ATPBone Consortium (Copenhagen University, University College London, University of Maastricht, University of Ferrara, University of Liverpool, University of Sheffield, and Université Libre de Bruxelles) and is a substudy under the main study “Fighting osteoporosis by blocking nucleotides: purinergic signalling in bone formation and homeostasis”. The work was also supported by The Faculty of Health Sciences, Copenhagen University (grant# 211-0457/08-3012), The Danish Council for Independent Research, Medical Sciences (grant# 271080789) and Natural Sciences (10-085217) and The Toyota Foundation (grant# OH/BG-6360).
References
- 1.Price C, Zhou X, Li W, Wang L. Real-time measurement of solute transport within the lacunar–canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J Bone Miner Res. 2011;26:277–285. doi: 10.1002/jbmr.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 3.Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36:1427–1437. doi: 10.1016/S0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 4.Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 5.Kamioka H, Ishihara Y, Ris H, Murshid SA, Sugawara Y, Takano-Yamamoto T, Lim SS. Primary cultures of chick osteocytes retain functional gap junctions between osteocytes and between osteocytes and osteoblasts. Microsc Microanal. 2007;13:108–117. doi: 10.1017/S143192760707016X. [DOI] [PubMed] [Google Scholar]
- 6.Adachi T, Aonuma Y, Tanaka M, Hojo M, Takano-Yamamoto T, Kamioka H. Calcium response in single osteocytes to locally applied mechanical stimulus: differences in cell process and cell body. J Biomech. 2009;42:1989–1995. doi: 10.1016/j.jbiomech.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 8.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Neve A, Corrado A, Cantatore FP. Osteocytes: central conductors of bone biology in normal and pathological conditions. Acta Physiol (Oxf) 2012;204:317–330. doi: 10.1111/j.1748-1716.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 10.Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litzenberger JB, Kim JB, Tummala P, Jacobs CR. Beta1 integrins mediate mechanosensitive signaling pathways in osteocytes. Calcif Tissue Int. 2010;86:325–332. doi: 10.1007/s00223-010-9343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, Yoshimoto Y, Ishikawa H, Chihara K, Takano-Yamamoto T, Fujita T, Mikuni-Takagaki Y. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner Metab. 2006;24:498–504. doi: 10.1007/s00774-006-0716-x. [DOI] [PubMed] [Google Scholar]
- 13.Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010;24:2859–2868. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen NR, Henriksen Z, Brot C, Eriksen EF, Sorensen OH, Civitelli R, Steinberg TH. Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. J Bone Miner Res. 2000;15:1024–1032. doi: 10.1359/jbmr.2000.15.6.1024. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen NR, Henriksen Z, Sorensen OH, Eriksen EF, Civitelli R, Steinberg TH. Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J Biol Chem. 2002;277:7574–7580. doi: 10.1074/jbc.M104608200. [DOI] [PubMed] [Google Scholar]
- 17.Orriss IR, Burnstock G, Arnett TR. Expression of multiple P2 receptor subtypes by osteoblasts and osteoclasts. Bone. 2009;44(Supplement 2):S304. doi: 10.1016/j.bone.2009.03.559. [DOI] [Google Scholar]
- 18.Reyes JP, Sims SM, Dixon SJ. P2 receptor expression, signaling and function in osteoclasts. Front Biosci (Schol Ed) 2011;3:1101–1118. doi: 10.2741/214. [DOI] [PubMed] [Google Scholar]
- 19.Gartland A, Orriss IR, Rumney RM, Bond AP, Arnett T, Gallagher JA. Purinergic signalling in osteoblasts. Front Biosci. 2012;17:16–29. doi: 10.2741/3912. [DOI] [PubMed] [Google Scholar]
- 20.Hoebertz A, Meghji S, Burnstock G, Arnett TR. Extracellular ADP is a powerful osteolytic agent: evidence for signaling through the P2Y (1) receptor on bone cells. FASEB J. 2001;15:1139–1148. doi: 10.1096/fj.00-0395com. [DOI] [PubMed] [Google Scholar]
- 21.Buckley KA, Hipskind RA, Gartland A, Bowler WB, Gallagher JA. Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-[kappa] B ligand. Bone. 2002;31:582–590. doi: 10.1016/S8756-3282(02)00877-3. [DOI] [PubMed] [Google Scholar]
- 22.Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ. Extracellular nucleotides act through P2X7 receptors to activate NF-kappaB in osteoclasts. J Bone Miner Res. 2004;19:642–651. doi: 10.1359/JBMR.040108. [DOI] [PubMed] [Google Scholar]
- 23.Orriss IR, Knight GE, Ranasinghe S, Burnstock G, Arnett TR. Osteoblast responses to nucleotides increase during differentiation. Bone. 2006;39:300–309. doi: 10.1016/j.bone.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 24.Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, Dixon SJ. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol. 2008;181:859–871. doi: 10.1083/jcb.200708037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoebertz A, Mahendran S, Burnstock G, Arnett TR. ATP and UTP at low concentrations strongly inhibit bone formation by osteoblasts: a novel role for the P2Y2 receptor in bone remodeling. J Cell Biochem. 2002;86:413–419. doi: 10.1002/jcb.10236. [DOI] [PubMed] [Google Scholar]
- 26.Orriss IR, Utting JC, Brandao-Burch A, Colston K, Grubb BR, Burnstock G, Arnett TR. Extracellular nucleotides block bone mineralization in vitro: evidence for dual inhibitory mechanisms involving both P2Y2 receptors and pyrophosphate. Endocrinology. 2007;148:4208–4216. doi: 10.1210/en.2007-0066. [DOI] [PubMed] [Google Scholar]
- 27.Orriss IR, Evans HR, Gartland A, Arnett TR. MicroCT analysis of P2Y1 and P2Y2 receptor knockout mice demonstrates significant changes in bone phenotype. Calcif Tissue Int. 2008;83:2–3. [Google Scholar]
- 28.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 29.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahner BN, Shankar H, Murugappan S, Prasad GL, Kunapuli SP. Nucleotide receptor signaling in platelets. J Thromb Haemost. 2006;4:2317–2326. doi: 10.1111/j.1538-7836.2006.02192.x. [DOI] [PubMed] [Google Scholar]
- 32.Lean JM, Mackay AG, Chow JW, Chambers TJ. Osteocytic expression of mRNA for c-fos and IGF-I: an immediate early gene response to an osteogenic stimulus. Am J Physiol. 1996;270:E937–E945. doi: 10.1152/ajpendo.1996.270.6.E937. [DOI] [PubMed] [Google Scholar]
- 33.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276:32925–32932. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- 35.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genetos DC, Kephart CJ, ZHANG Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N, De BM, Antoons G, Gadicherla AK, Bol M, Decrock E, Evans WH, Sipido KR, Bukauskas FF, Leybaert L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res Cardiol. 2012;107:304. doi: 10.1007/s00395-012-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Lu XL, Huo B, Park M, Guo XE. Calcium response in osteocytic networks under steady and oscillatory fluid flow. Bone. 2012;51:466–473. doi: 10.1016/j.bone.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kringelbach TM, Novak I, Schwarz P, Jorgensen NR (2013) Nucleotide and mechanically induced ATP release pathways in osteocytes. Bone Abstracts (1), PP237
- 42.Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12:2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 43.Yegutkin GG, Samburski SS, Jalkanen S, Novak I. ATP-consuming and ATP-generating enzymes secreted by pancreas. J Biol Chem. 2006;281:29441–29447. doi: 10.1074/jbc.M602480200. [DOI] [PubMed] [Google Scholar]
- 44.Haanes KA, Novak I. ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J. 2010;429:303–311. doi: 10.1042/BJ20091337. [DOI] [PubMed] [Google Scholar]
- 45.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarowski ER, Homolya L, Boucher RC, Harden TK. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem. 1997;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- 47.Lazarowski ER, Harden TK. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br J Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 49.Syberg S, Brandao-Burch A, Patel JJ, Hajjawi M, Arnett TR, Schwarz P, Jorgensen NR, Orriss IR. Clopidogrel (Plavix), a P2Y12 receptor antagonist, inhibits bone cell function in vitro and decreases trabecular bone in vivo. J Bone Miner Res. 2012;27:2373–2386. doi: 10.1002/jbmr.1690. [DOI] [PubMed] [Google Scholar]
- 50.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Browne LE, Compan V, Bragg L, North RA. P2X7 receptor channels allow direct permeation of nanometer-sized dyes. J Neurosci. 2013;33:3557–3566. doi: 10.1523/JNEUROSCI.2235-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mason DJ, Suva LJ, Genever PG, Patton AJ, Steuckle S, Hillam RA, Skerry TM. Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone. 1997;20:199–205. doi: 10.1016/S8756-3282(96)00386-9. [DOI] [PubMed] [Google Scholar]
- 55.Warden SJ, Bliziotes MM, Wiren KM, Eshleman AJ, Turner CH. Neural regulation of bone and the skeletal effects of serotonin (5-hydroxytryptamine) Mol Cell Endocrinol. 2005;242:1–9. doi: 10.1016/j.mce.2005.06.005. [DOI] [PubMed] [Google Scholar]