Abstract
Beta-lactamase producing strains of Pseudomonas aeruginosa are important etiological agents of hospital infections. Carbapenems are among the most effective antibiotics used against Pseudomonas infections, but they can be rendered infective by group B β-lactamase, commonly called metallo-beta lactamase. In this study, the antimicrobial sensitivity patterns of P. aeruginosa strains isolated from 9 different hospitals in Tehran, Iran, as well as the prevalence of MBLs genes (bla-VIM and bla-IMP) were determined. A total of 212 strains of P. aeruginosa recovered from patients in hospitals in Tehran were confirmed by both biochemical methods and PCR. Their antimicrobial sensitivity patterns were determined by Kirby-Bauer disk diffusion method. Following MIC determination, imipenem resistant strains were selected by DDST method which was followed by PCR tests for determination of MBLs genes: bla-IMP and bla-VIM. The results indicated that, in the DDST phenotypic method, among the 100 imipenem resistant isolates, 75 strains were MBLs positive. The PCR test indicated that 70 strains (33%) carried bla-VIM gene and 20 strains (9%) harbored bla-IMP. The results indicated that the extent of antibiotic resistance among Pseudomonas aeruginosa is on the rise. This may be due to production of MBLs enzymes. Therefore, determination of antibiotic sensitivity patterns and MBLs production by these bacteria, can be important in control of clinical Pseudomonas infection.
1. Introduction
Pseudomonas aeruginosa is one of the commonest causes of infection in burn patients and an important agent for hospital acquired infections and death in immunocompromised such as cystic fibrosis and cancer patients [1]. This bacterium is often resistant to many antimicrobial agents. The cause of resistance can be efflux pumps, decreased outer membrane permeability, and secretion of beta-lactamase enzymes [2]. Several kinds of beta-lactamase enzymes have been recognized. These enzymes were initially seen in Gram-negative bacteria which were detected in periplasmic space [3]. Metallo-beta lactamases are classified in group B of Ambler classification. This group is divided into three subclasses: BI, BII, and BIII. The BI subclass is divided into four categories according to their molecular structures: the IMP, VIM, GIM, and SPM types [4]. The first MBLs enzymes were IMP-1 which was initially found in S. marcescens in Japan (1991), VIM-1 originally detected in Italy (1997), SPM-1 first detected in Brazil (1997), and finally GIM detected in Germany (2002) [5, 6]. Carbapenems are effective antibiotics against Pseudomonas infections. But because the genes for MBLs are often carried on plasmids and class I integron, they can rapidly spread among different species of this bacterium and other bacteria [7, 8]. MBLs can potently hydrolyze all beta-lactam antibiotics except azetreonam. These enzymes require zinc ion as cofactor [9]. Hence, their activity is inhibited by chelators like ethylenediaminetetraacetic acid (EDTA), sodium mercaptoacetic acid (SMA), 2-mercaptopropionic acid (MPA), and dipicolinic acid (DPA). Sulbactam, tazobactam, and clavulanic acid which are often used to inhibit beta-lactamase enzymes are not effective against MBLs [2, 10]. Several phenotypic methods are available for detection of MBLs producing bacteria. All these methods are based on the ability of metal chelators such as EDTA to inhibit the activity of MBLs. The double disk synergy test method was employed in this investigation [9]. The goal of this study was to determine the antibiotic resistance pattern in P. aeruginosa species isolated from nine hospitals in Tehran, Iran, and evaluate the prevalence of MBLs genes, bla- VIM and bla- IMP, in imipenem resistance strains.
2. Materials and Methods
2.1. Collection of Strains
A total of 212 strains of P. aeruginosa were collected during six-month period from October 2011 to March 2012 from Motahari, Shariati, Hashemi Nejad, Kasra, Hazrat Rasoul, Milad, Mehr, Tebbi Kodakan, and Baghiatallah hospitals in Tehran, Iran. These strains were isolated from wound, blood, urine, trachea, sputum, pleural fluid, eye, catheter, and larynges samples. 148 isolates were obtained from male patients and 64 isolates from female patients. These isolates were subcultured on Brucella agar and their identification was performed by both biochemical methods such as oxidase test, catalase test, OF test, growth at 42°C, and PCR using specific primers for oprL gene (oprL is a constitutively produced peptidoglycan-associated lipoprotein which contains covalently bound fatty acyl chains) [11]. Bacterial strains were preserved in Trypticase soy broth.
2.2. Antibiotic Susceptibility Tests
Antimicrobial sensitivity tests were performed on Mueller-Hinton agar (Biolab-Hungary) by Kirby-Bauer disk diffusion method [12] and interpreted according to CLSI (Clinical and Laboratory Standards Institute) standard tables. Pseudomonas aeruginosa ATCC27853 was used as control for the susceptibility tests. The antibiotic disks used were Imipenem (10 μg), Ciprofloxacin (5 μg), Gentamicin (10 μg), Tetracyclin (30 μg), Ceftazidime (30 μg), Cefotaxime (30 μg), Azithromycin (15 μg), Tobramycin (10 μg), Ticarcylin (75 μg), and Piperacillin (100 μg) (Padtan Teb, Iran). At first the bacteria were cultured into TSB and incubated at 35°C for 24 hours. After 24 hours, microbial suspension was prepared equivalent to the turbidity of 0.5 McFarland standard. With sterile swabs they were plated on MH agar. The antibiotic disks were placed on the plate and incubated at 35°C for 24 hours. Following incubation, the diameters of the zone of inhibition were measured.
2.3. Minimum Inhibitory Concentration (MIC)
Determination of MIC was performed for imipenem resistant strains by agar dilution method according to CLSI standards. Isolates with MIC value of ≥16 μg/mL were screened as MBLs producing strains. Pseudomonas aeruginosa ATCC27853 was used as a control strain for the susceptibility testing.
2.4. Detection of MBLs Producing Isolates by Double Disk Synergy Test (DDST) Method
Imipenem resistance isolates were investigated for MBLs producing strains by DDST method. The bacterial suspension with turbidity equivalent to 0.5 McFarland standard was prepared and cultured on Mueller-Hinton agar [13]. For preparation of IMP-EDTA disk, 750 μg of EDTA solution was added to 10 μg imipenem disk and dried in an incubator [13]. At first, the bacterial suspension with turbidity equivalent to 0.5 McFarland was prepared and cultured with sterile swab on MH agar. Then, two 10 μg imipenem and imipenem-EDTA disks were placed on the agar surface. After 18 hours of incubation at 35°C, the inhibition zone of imipenem disk and IMP-EDTA were measured. An increase of seven mm or more in the zone diameter for IMP-EDTA disk in comparison with imipenem disk alone was considered as a MBLs producing isolate [14].
2.5. DNA Extraction
DNA from P. aeruginosa isolates was extracted by boiling method. In this method, a number of bacterial colonies were inoculated in 10 mL of LB broth and incubation at 37°C for 16 hours. 1.5 mL of the LB broth culture was centrifuged at 13,000 ×g at room temperature for 10 min. The bacterial pellet was suspended in 300 μL sterile water. The cells in the suspension were lysed by heating at 100°C for 10 min and the leftover cells were removed by centrifugation at 13,000 ×g at room temperature for 10 min. The supernatant was transferred into new tubes and used as template DNA for PCR reactions. For purity assurance, the template DNA was electrophoresed on agarose gel [10].
2.6. PCR Reaction for Confirmation of Pseudomonas aeruginosa Strains (oprL Gene)
PCR reaction for identification of P. aeruginosa strains (oprL gene) was performed in a final volume of 25 μL: PCR Buffer (10x) 2.5 μL, MgCl2 (50 mM) 0.75 μL, dNTPs (10 mM) 1 μL, forward (5′ATG-GAA-ATG-CTG-AAA-TTC-GG-<C>3′) and reverse (5′CTT-CTT-CAG-CTC-GAC-GCG-AC-<G>3′) primers [11] 500 bp (10 pmol/μL) 1 μL + 1 μL, Taq DNA polymerase (5 U/μL) 1 μL, distilled water 16.75 μL, and Template DNA 1 μL. The thermocycler program for oprL gene consisted of 3 min initial denaturation at 94°C, 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 5 min.
2.7. PCR Assays for Detection of MBLs Genes
2.7.1. Primers
For design of primers, the nucleotide sequences of bla- VIM and bla- IMP genes in P. aeruginosa were obtained from Gene bank and aligned with Clastalw2 software (alignment program). After identification of commonality region, Gene Runner program was used for primer design. Finally for confirmation of primer specificity, they were subjected to BLAST program.
PCR reactions for bla- IMP and bla- VIM genes were performed in a final volume of 25 μL containing the following.
bla- IMP. PCR Buffer (10x) 2.5 μL, MgCl2 (50 mM) 1 μL, dNTPs (10 mM) 1 μL, forward (5′GTTTGAAGAAGTTAACGGGTGG3′) and reverse (5′ATAATTTGGCGGACTTTGGC3′) primers (designed) 459 bp (10 pmol/μL) 1 + 1 μL, Taq DNA polymerase (5 U/μL) 1 μL, template DNA 3 μL, and distilled water 14.5 μL. The thermocycler program for bla- IMP gene consisted of 4 min initial denaturation at 94°C, 35 cycles of denaturation at 94°C for 1 min, annealing at 61°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 5 min.
bla- VIM. PCR Buffer (10x) 2.5 μL, MgCl2 (50 mM) 1 μL, dNTPs (10 mM) 1 μL, forward (5′TGGTGTTTGGTCGCATATCG3′) and reverse (5′GAGCAAGTCTAGACCGCCCG3′) primers (designed) 595 bp (10 pmol/μL) 1 + 1 μL, Taq DNA polymerase (50 U/μL) 1 μL, template DNA 2 μL, and distilled water 15.5 μL. Scheduled program for bla- VIM gene by thermocycler was 4 min initial denaturation at 94°C, 35 cycles of denaturation at 94°C for 1 min, annealing at 62°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 10 min.
Water was used as negative control and P. aeruginosa strains producing MBLs genes (bla- VIM and bla- IMP) (provided from Pasteur Institute, Iran) were used as positive controls for MBL detection.
The PCR products were confirmed by gel electrophoresis in 1% (w/v) agarose gel (HT bioscience, UK) in TBE buffer and visualized with ethidium bromide staining and photographed with UV waves through Gel Documentation (Technogen, Iran) (Figures 2, 3, and 4).
Figure 2.
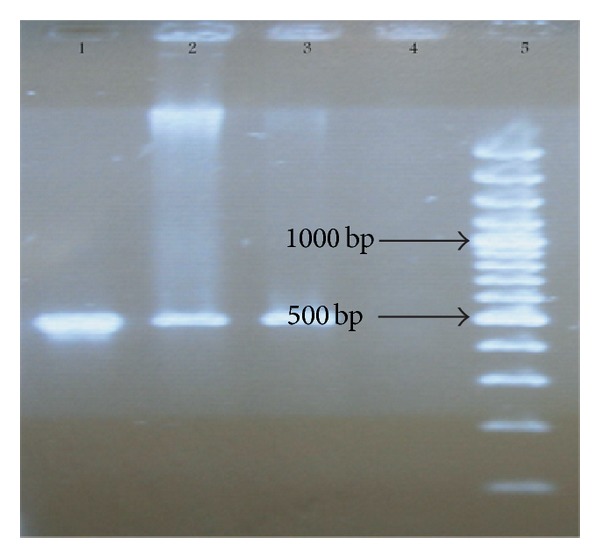
Electrophoresis of oprL (500 bp) PCR products on agarose gel. Line 1 shows the positive control. Lines 2 and 3 show P. aeruginosa strains. Line 4 shows the negative control. Line 5 shows 100–1000 bp ladder.
Figure 3.
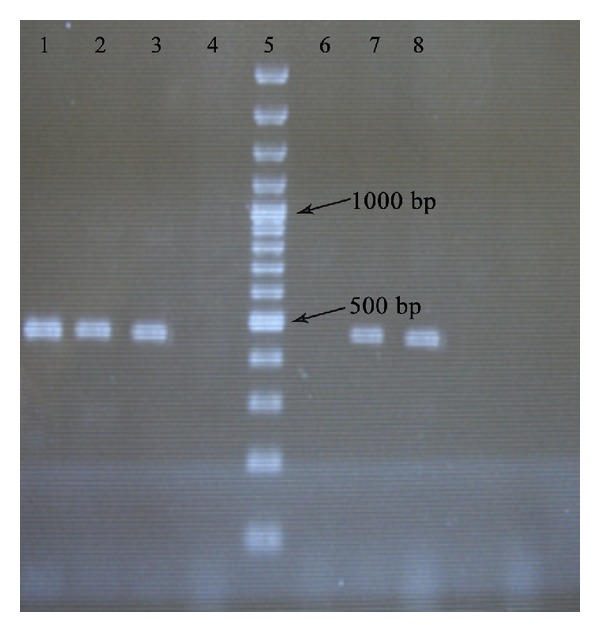
Electrophoresis of bla- IMP (459 bp) PCR products on agarose gel. Line 1 is the positive control. Lines 2 and 3 show isolates positive for IMP. Line 4 is negative in PCR products. Line 5 shows 100–1000 bp ladder. Line 6 is negative control. Lines 7 and 8 show isolates positive for IMP.
Figure 4.
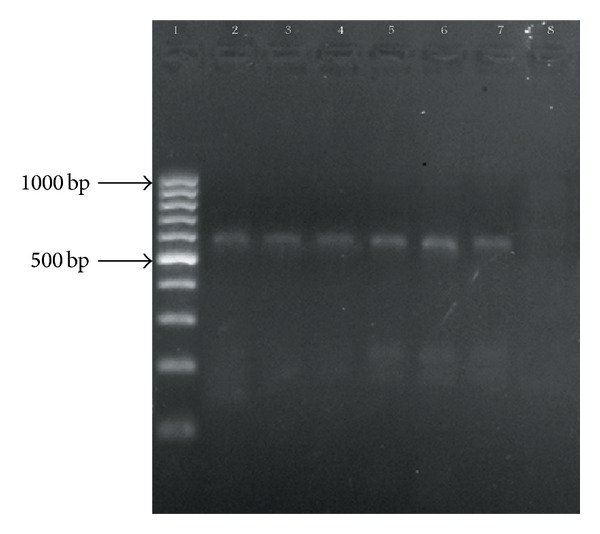
Agarose gel electrophoresis of bla- VIM (595 bp) PCR products. Line 1 is 100–1000 bp ladder. Line 2 is positive control. Lines 3, 4, 5, 6, and 7 show isolates positive for VIM. Line 8 is negative control.
3. Results
In total, 212 P. aeruginosa isolates were collected. After performing initial bacteriological tests, they were confirmed to be P. aeruginosa by PCR assay. They were obtained from clinical specimens such as wound (n = 78), urine (n = 62), blood (n = 39), trachea (n = 16), sputum (n = 7), pleural fluid (n = 5), eye (n = 2), catheter (n = 2), and larynges (n = 1). The majority were from patients in burn unit (n = 58) and the least were from patients in cardiac unit (n = 1).
3.1. Antibiotic Susceptibility
Antibiotic susceptibility of the 212 isolates in the initial disk diffusion method against 10 antibiotics is presented in Figure 1. The isolates showed high resistance to tetracycline (86%) and the most effective antibiotic was ciprofloxacin (44%).
Figure 1.
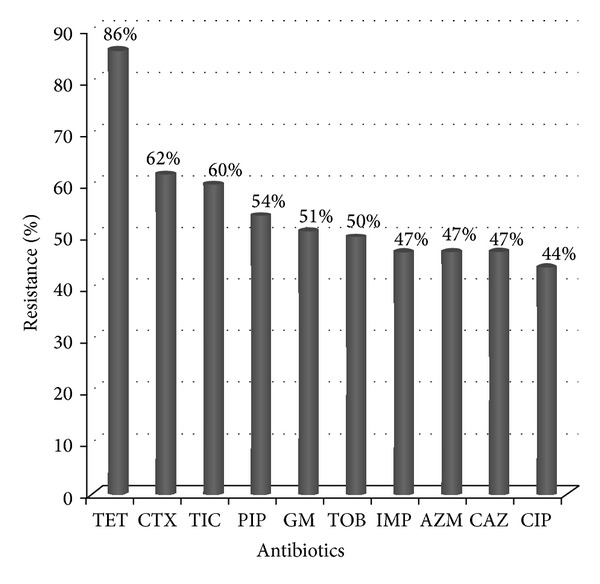
Antibiotic resistance among isolates of P. aeruginosa.
3.2. MIC
Determination of MIC for imipenem by agar dilution method indicated that 47.16% (n = 100) of the strains were resistant to imipenem (MIC ≥ 16 μg/mL).
3.3. Detection of MBLs Producing Isolates by Double Disk Method
In the double disk method performed on the 100 imipenem resistance isolates, 70 strains were shown to be positive by this phenotypic method.
3.4. Molecular Analysis
The PCR assays indicated that 20 (9%) of these strains contained the IMP gene, whereas 70 (33%) of them harbored the VIM gene.
4. Discussion
Pseudomonas aeruginosa is an opportunistic human pathogen [15, 16]. Different antibiotics are commonly used for the treatment of Pseudomonas infections, such as aminoglycosides, beta-lactamases, and quinolones [16–18]. Carbapenems are potent beta-lactam antibiotics against MBLs producing and multidrug resistance P. aeruginosa [19]. There have been many recent reports that clinical isolates of P. aeruginosa and Gram-negative bacilli are becoming resistant to carbapenems in many countries [19]. In recent years, MBLs have been identified from clinical isolates with increasing frequency across the world and strains that produce these enzymes have been responsible for prolonged treatment and acute infections [20]. A study from Japan showed that patients infected with MBLs producing P. aeruginosa needed to receive multiple antibiotics and infections leading to death due to IMP producing P. aeruginosa were more common than those with bla- IMP negative P. aeruginosa [21]. MBLs producing P. aeruginosa is a serious intimidation in hospital locations especially in burn units. These strains can create significant problem in treatment and spread of resistance among other bacteria [22]. Resistance to carbapenems via acquirement of MBLs genes among P. aeruginosa strains have increased rapidly in Asia, Europe, and South America. This has led to a drastic change in the pattern of antibiotics usage against multidrug resistant P. aeruginosa [23]. Detection of MBLs producing strains can be effective for optimal treatment of patients particularly in burned and hospitalized patients and control the spread of resistance [6]. Resistance to imipenem is increasing in Iran in recent years [9, 22, 24–27]. The differences in the reported values between the present study and those reported earlier may be due to the difference in geographical regions, difference in kind of infections, the enormous usage of antibiotics, or difference in antibiotic therapy regimens in the selective hospitals in this study than those in other studies. Among the 100 isolates which were resistant to imipenem, 70 (70%) were found to be MBLs producers. In the other strains which were resistant to imipenem but were MBLs negative, resistance to imipenem may be due to efflux systems, decreased outer membrane permeability, or production of Ampc enzymes. For confirmation of MBLs producing strains, PCR is an important and accurate method [10]. In this study all isolates were screened for VIM and IMP genes by PCR. 20 isolates had IMP gene and 70 isolates had VIM gene. Of the 11 isolates that were negative with phenotypic method, 4 harbored IMP gene and 7 isolates had VIM gene as detected by PCR. Also, 3 isolates that were positive with DDST method were negative with PCR. This shows that there may be genes other than VIM and IMP responsible for MBLs trait.
Yazdi et al. isolated 126 P. aeruginosa strains from nonburn patients in Iran in 2007. Production of MBL in these isolates was determined by E-test, followed by PCR to detect bla- IMP and bla- VIM. Among 70 imipenem resistant P. aeruginosa strains, 8 strains produced MBLs by E-test all of which carried bla- VIM. None of them were carriers of bla- IMP gene [24]. In another study in Iran during 2008, Shahcheraghi et al. collected 243 P. aeruginosa strains from nonburn patients. 22 strains were MBLs positive; 15 of them had bla- VIM and none was bla- IMP positive [25]. In a study carried out in India between 2005 and 2007, among 61 P. aeruginosa strains collected, 20 strains produced MBLs. Of the 20 MBLs confirmed strain by E-test, 17 strains were subjected to PCR testing. 15 of these strains were bla- VIM positive and two isolates were negative for both bla- VIM and bla- IMP and all were negative for bla- IMP [28]. In 2008, Khosravi and Mihani collected 100 P. aeruginosa in Iran. Production of MBLs was determined both by E-test and PCR method. Among 41 imipenem resistant P. aeruginosa, 8 strains were shown to be MBLs producer by E-test and all of these 8 strains carried bla- VIM and none of them had bla- IMP [22]. In another study in Turkey, 100 P. aeruginosa strains were collected from patients in a Turkish university hospital. One (1%) isolate was found to carry bla- VIM gene, whereas 9 (9%) carried bla- IMP gene. Among 9 isolates that carried bla- IMP gene, only 4 isolates were shown to be MBL producer by E-test [29]. In our study, the percent of strains that carried bla- VIM and bla- IMP genes was higher than those reported in previous studies. The reasons maybe an overall increase in the extent of acquirement of MBLs genes among P. aeruginosa. More MBLs genes are found to be located on the class I integron and can therefore easily transfer between P. aeruginosa strains [7]. In the majority of studies in Iran and other countries vim-type MBL was the most prevalent gene reported [30–32].
5. Conclusion
This study illustrated that the majority of P. aeruginosa strains were resistant to various antibiotics. The high rate of antibiotic resistanceamong P. aeruginosa strains is very alarming and can be responsible for serious infections. So identification of MBLs producing strains and taking efforts to reduce the rate of transfer between different strains are important goal for treatment of P. aeruginosa infections.
Acknowledgments
The authors thank the Microbiology Department staff of the studied hospitals and Pasteur Institute of Iran for their assistance.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Neu HC. The role of Pseudomonas aeruginosa in infections. Journal of Antimicrobial Chemotherapy. 1983;11:1–13. doi: 10.1093/jac/11.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa Y, Shibata N, Shibayama K, et al. Convenient test for screening metallo-β-lactamase-producing gram- negative bacteria by using thiol compounds. Journal of Clinical Microbiology. 2000;38(1):40–43. doi: 10.1128/jcm.38.1.40-43.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senda K, Arakawa Y, Ichiyama S, et al. PCR detection of metallo-β-lactamase gene (bla(IMP)) in gram-negative rods resistant to broad-spectrum β-lactams. Journal of Clinical Microbiology. 1996;34(12):2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrobial Agents and Chemotherapy. 1995;39(6):1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirel L, Magalhaes M, Lopes M, Nordmann P. Molecular analysis of metallo-beta-lactamase gene bla (SPM-1) surrounding sequences from disseminated Pseudomonas aeruginosa isolates in Recife, Brazil. Antimicrobial Agents and Chemotherapy. 2004;48(4):1406–1409. doi: 10.1128/AAC.48.4.1406-1409.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordmann P, Poirel L. Emerging carbapenemases in Gram-negative aerobes. Clinical Microbiology and Infection. 2002;8(6):321–331. doi: 10.1046/j.1469-0691.2002.00401.x. [DOI] [PubMed] [Google Scholar]
- 7.Cornaglia G, Mazzariol A, Lauretti L, Rossolini GM, Fontana R. Hospital outbreak of carbapenem-resistant Pseudomonas aeruginosa producing VIM-1, a novel transferable metallo-β-lactamase. Clinical Infectious Diseases. 2000;31(5):1119–1125. doi: 10.1086/317448. [DOI] [PubMed] [Google Scholar]
- 8.Yatsuyanagi J, Saito S, Harata S, et al. Class 1 integron containing metallo-β-lactamase gene bla VIM-2 in Pseudomonas aeruginosa clinical strains isolated in Japan. Antimicrobial Agents and Chemotherapy. 2004;48(2):626–628. doi: 10.1128/AAC.48.2.626-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saderi H, Karimi Z, Owlia P, Bahar A, Akhavi Rad SMB. Phenotypic detection of metallo-beta-lactamase producing Pseudomonas aeruginosa strain isolated from burned patients. Iranian Journal of Pathology. 2008;3(1):20–24. [Google Scholar]
- 10.Pitout JDD, Gregson DB, Poirel L, McClure J-A, Le P, Church DL. Detection of Pseudomonas aeruginosa producing metallo-β-lactamases in a large centralized laboratory. Journal of Clinical Microbiology. 2005;43(7):3129–3135. doi: 10.1128/JCM.43.7.3129-3135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahabir E, Bulian D, Bensch S, Schmidt J. Elimination of P. aeruginosa in mice by treatment with chlorine, and the use of microbiological and PCR analyses. Scandinavian Journal of Laboratory Animal Science. 2009;36(4):355–361. [Google Scholar]
- 12.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 13.Hemalatha V, Sekar U, Kamat V. Detection of metallo betalactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian Journal of Medical Research. 2005;122(2):148–152. [PubMed] [Google Scholar]
- 14.Irfan S, Zafar A, Guhar D, Ahsan T, Hasan R. Metallo-β-lactamase-producing clinical isolates of Acinetobacter species and Pseudomonas aeruginosa from intensive care unit patients of a tertiary care hospital. Indian Journal of Medical Microbiology. 2008;26(3):243–245. doi: 10.4103/0255-0857.42035. [DOI] [PubMed] [Google Scholar]
- 15.Lari AR, Alaghehbandan R. Nosocomial infections in an Iranian burn care center. Burns. 2000;26(8):737–740. doi: 10.1016/s0305-4179(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 16.Hanberger H, Garcia-Rodriguez J-A, Gobernado M, Goossens H, Nilsson LE, Struelens MJ. Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. Journal of the American Medical Association. 1999;281(1):67–71. doi: 10.1001/jama.281.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Altoparlak U, Erol S, Akcay MN, Celebi F, Kadanali A. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns. 2004;30(7):660–664. doi: 10.1016/j.burns.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Rastegar Lari A, Bahrami Honar H, Alaghehbandan R. Pseudomonas infections in Tohid Burn Center, Iran. Burns. 1998;24(7):637–641. doi: 10.1016/s0305-4179(98)00090-4. [DOI] [PubMed] [Google Scholar]
- 19.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clinical Microbiology Reviews. 2006;19(2):403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitout JDD, Revathi G, Chow BL, et al. Metallo-β-lactamase-producing Pseudomonas aeruginosa isolated from a large tertiary centre in Kenya. Clinical Microbiology and Infection. 2008;14(8):755–759. doi: 10.1111/j.1469-0691.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 21.Mirsalehian A, Feizabadi M, Nakhjavani FA, Jabalameli F, Goli H, Kalantari N. Detection of VEB-1, OXA-10 and PER-1 genotypes in extended-spectrum β-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients. Burns. 2010;36(1):70–74. doi: 10.1016/j.burns.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Khosravi AD, Mihani F. Detection of metallo-β-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagnostic Microbiology and Infectious Disease. 2008;60(1):125–128. doi: 10.1016/j.diagmicrobio.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Griswold JA. White blood cell response to burn injury. Seminars in Nephrology. 1993;13(4):409–415. [PubMed] [Google Scholar]
- 24.Yazdi HR, Nejad GB, Peerayeh SN, Mostafaei M. Prevalence and detection of metallo-β-lactamase (MBL)-producing Pseudomonas aeruginosa strains from clinical isolates in Iran. Annals of Microbiology. 2007;57(2):293–296. [Google Scholar]
- 25.Shahcheraghi F, Nikbin VS, Shooraj F, Bagheri A, Shafiee M, Arabestani MR. PCR detection of VIM-1, VIM-2 and IMP-1 metallo-beta-lactamases in clinically multi-drug resistant P. aeruginosa isolated in Tehran. Iranian Journal of Clinical Infectious Diseases. 2008;12:p. 118. [Google Scholar]
- 26.Sepehriseresht S, Boroumand MA, Pourgholi L, Sotoudeh Anvari M, Habibi E, Sattarzadeh Tabrizi M. Detection of vim- and ipm-type metallobeta-lactamases in Pseudomonas aeruginosa clinical isolates. Archives of Iranian Medicine. 2012;15(11):670–673. [PubMed] [Google Scholar]
- 27.Fazeli H, Moslehi Takantape Z, Irajian GH, Salehi M. Determination of antibiotic resistance pattern and identification of bla-VIM gene in P. aeruginosa isolates from Imam Mosa Kazem Burns Hospital, Isfahan, Iran. Iranian Journal of Medical Microbiology. 2009;3(4):1–8. [Google Scholar]
- 28.Manoharan A, Chatterjee S, Mathai D. Detection and characterization of metallo beta lactamases producing Pseudomonas aeruginosa . Indian Journal of Medical Microbiology. 2010;28(3):241–244. doi: 10.4103/0255-0857.66486. [DOI] [PubMed] [Google Scholar]
- 29.Ozgumus OB, Caylan R, Tosun I, Sandalli C, Aydin K, Koksal I. Molecular epidemiology of clinical Pseudomonas aeruginosa isolates carrying IMP-1 metallo-β-lactamase gene in a university hospital in Turkey. Microbial Drug Resistance. 2007;13(3):191–198. doi: 10.1089/mdr.2007.748. [DOI] [PubMed] [Google Scholar]
- 30.Luzzaro F, Endimiani A, Docquier J-D, et al. Prevalence and characterization of metallo-β-lactamases in clinical isolates of Pseudomonas aeruginosa . Diagnostic Microbiology and Infectious Disease. 2004;48(2):131–135. doi: 10.1016/j.diagmicrobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Oh E-J, Lee S, Park Y-J, et al. Prevalence of metallo-β-lactamase among Pseudomonas aeruginosa and Acinetobacter baumannii in a Korean University hospital and comparison of screening methods for detecting metallo-β-lactamase. Journal of Microbiological Methods. 2003;54(3):411–418. doi: 10.1016/s0167-7012(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 32.Kim I-S, Lee NY, Ki C-S, Oh WS, Peck KR, Song J-H. Increasing prevalence of imipenem-resistant Pseudomonas aeruginosa and molecular typing of metallo-β-lactamase producers in a Korean hospital. Microbial Drug Resistance. 2005;11(4):355–359. doi: 10.1089/mdr.2005.11.355. [DOI] [PubMed] [Google Scholar]


