Abstract
The ability of the host to respond to intestinal injury requires the regeneration of native tissue through a highly orchestrated response from the intestinal stem cells, a population of cells located within the intestinal crypts that have the capability to repopulate the entire villous. The field of intestinal stem cell biology is thus of great interest to surgeons and non-surgeons alike, given its relevance to diseases of intestinal injury and inflammation such as inflammatory bowel disease, trauma, and necrotizing enterocolitis. The field of intestinal stem cell research has been advanced recently by the identification of the putative marker, Lgr5, which has allowed for the isolation and further characterization of the intestinal stem cell. Under the control of the WNT signaling pathway, Lgr5 marks the rapidly dividing cells of the intestinal crypt, and identifies a population of cells that is capable of regenerating the entire villous. We now review the identification of Lgr5 as an intestinal stem cell marker, identify controversies in the intestinal stem cell field, and highlight the response of the intestinal stem cell to injury within the intestinal mucosa that may occur clinically.
Introduction
The intestinal mucosa is readily disrupted as a result of insults such as traumatic injury, surgical resection or diseases of inflammation such as Crohn's disease, ulcerative colitis and necrotizing enterocolitis. In response to mucosal disruption, the host initiates a healing response resulting in restoration of mucosal integrity and regeneration of the mucosal architecture. A major component of this response is the ability of the host to generate intestinal tissue de novo, a highly complex and integrated process that involves the precise function of intestinal progenitors, which have also been termed “intestinal stem cells”. It therefore stands to reason that the factors that regulate the activity of intestinal stem cells will play a dominant role in the ability of the host to respond to injury within the intestinal tract. By extension, it remains critically important for individuals working within the field of intestinal inflammation and injury to have a working understanding of this important field.
What are “stem cells”?
The term “stem cell” has been used in multiple different contexts – both lay and scientific, and means different things to different people. Beyond the scientific interest in stem cells in general, there is also significant political interest, as successive American presidents have sought involvement in the regulation of stem cell research. Given this frame of reference, it is important to clarify the definition of what constitutes a “stem cell”, which, for the purpose of the current discussion, is any cell that has three properties: the capability of being able to divide and renew itself (and therefore become another stem cell or a more specialized cell), a state of relative unspecialization, and the ability to give rise to specialized cell types under certain conditions. Each of these properties alone would represent quite a difficult task for any cell to undertake; the ability to undertake all three tasks is left to a vanishingly small minority of cells throughout the body. Stem cells can be thought of as occurring in two discrete varieties, the first of which includes the “embryonic stem cells” – cells that are derived from embryos that develop from eggs that have been fertilized in vitro, and that have the ability to give rise to all cell types of the organism from which they were harvested. The current discussion – which focuses on intestinal stem cells – will not address embryonic stem cells, despite their huge importance to the fields of regenerative medicine and surgery. The second category of stem cells includes the “somatic stem cells”, which are undifferentiated cells that reside within a particular micro-environment, or niche, within the body, and that are found among differentiated cells within a tissue. The primary roles of somatic stem cells are to maintain and repair the tissue in which they are found. As might be imagined, proving the “stemness” of such a population is not an easy task, and requires the combination of sophisticated lineage tracing and transplantation experiments. The intestinal stem cells represent one such category of somatic stem cells, in that they remain undifferentiated, yet have the ability to differentiate into each of the varying cells that exists within the intestinal epithelium, and therefore have captivated the interest of surgeons and non-surgeons as they seek to understand the ability of the host to respond to injury.
Molecular and biochemical characterization of intestinal stem cells
The recent identification of specific markers of the intestinal stem cell (ISC) has led to an explosion of interest in the biology of these previously elusive progenitors (Figure 1). In recent years, considerable attention in the field has been devoted to the role of intestinal stem cells in carcinogenesis. However, an evolving body of literature suggests that the intestinal stem cell may also play a pivotal role in response to injury and inflammation. The initial description of a multipotent region of the intestinal crypt dates back to the work of Cheng and Leblond, who, in 1974, described the derivation of the four types of intestinal epithelial cells, enteroendocrine, goblet, Paneth, and enterocytes from progenitor cells that reside in the intestinal crypts of Lieberkühn.1,2 Subsequent to these findings, the nature of the crypt based progenitors remained largely unknown until studies in the late 1990s demonstrated that cells residing within a specific position of the intestinal crypts were capable of long-term retention of labels such as bromodeoxyuridine (BRDU) or [3H] thymidine.3,4 Dubbed “label-retaining cells” or LRCs, these candidates for the intestinal stem cell were described to reside at a specific position above the Paneth cells, the so-called +4 position, indicating their location of 4 cells above the crypt base.5 Although this represented some of the most convincing work to date regarding the location of the ISC, the identification of cells interspersed with Paneth cells at the base of the crypt which were found to represent undifferentiated precursors – termed crypt-based columnar cells (CBCs)6,7 – raised questions regarding the nature and definition of the ISC. In 2007, the Clevers laboratory from the Hubrecht Institute in the Netherlands identified a putative ISC marker, Lgr5, thereby allowing for the isolation and propagation of LGR5 (+) cells. Lgr5 is a G-protein coupled receptor of unknown function, which is a target of the WNT signaling pathway. Although the function of the protein is currently unknown, it bears distinct resemblance to the receptors for TSH, FSH, and LH. In addition, LGR5 has a complex expression pattern during embryogenesis, and deficiency of LGR5 in mice leads to death shortly after birth due to anatomic malformations of the tongue and lower jaw.8 Through an elegant series of lineage tracing experiments using a tamoxifen-inducible Cre recombinase knocked-in to the Lgr5 allele, Clevers and colleagues demonstrated that Lgr5 positive cells originated in the identical position of the previously named CBCs, and could differentiate into all cell types found within the intestinal epithelium.9 In subsequent studies, Clevers and colleagues showed that Lgr5 positive cells, when cultured in vitro on a biological matrix without associated mesenchyme, were sufficient to produce a crypt-villous structure, further strengthening the argument that Lgr5 represents a putative ISC marker.10 The Lgr5 positive cells are sensitive to inactivation of the cell cycle protein CDC25, suggesting their proliferative phenotype, which, along with their lack of label retention, distinguishes them from the cells found at the +4 position.11 Thus, Lgr5 was vaulted to the top of a short list of pre-exisiting potential stem cell markers (Table 1). It is of particular interest in the stem cell field that LGR5 is thought to serve as a definitive marker of intestinal stemness. Many other adult stem cell types require multiple markers for identification, however, the lineage tracing performed by Clevers, et al, confirms the ability of Lgr5 positive cells to self-renew, actively divide, and give rise to all four epithelial subtypes, which, collectively, are properties not shared by other markers identified to date.
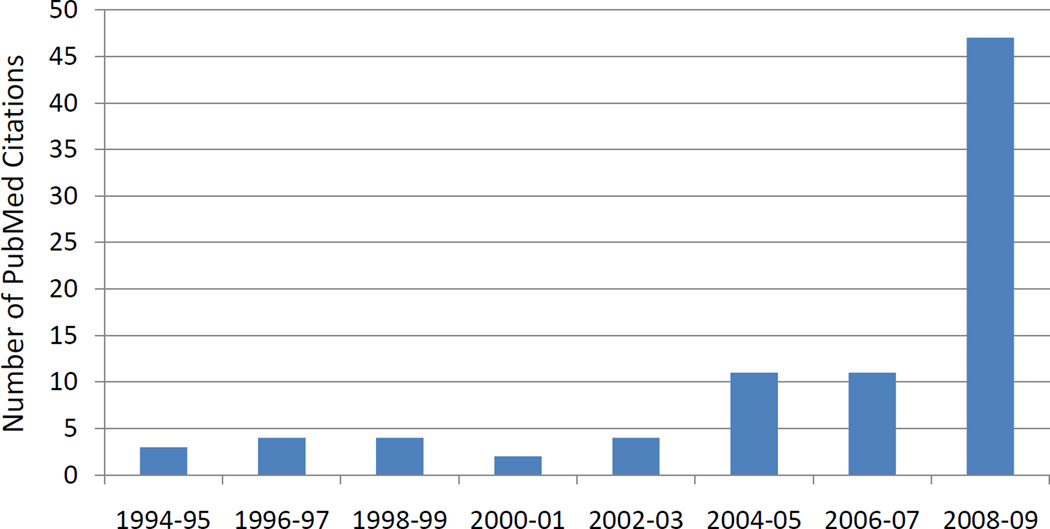
Figure 1. Frequency of literature citations for intestinal stem cells over 15 years.
A literature search was performed on PubMed for “intestinal stem cell,” which revealed 93 publications. With limitations of English language and a timeline of the last 15 years, three publications were excluded and the years of publication of the remaining 90 were charted, demonstrating a significant increase over the past five years.
Table 1.
Proposed Major Intestinal Stem Cell Markers and Location
| Marker | Location |
|---|---|
| Lgr5/GPR49 | Crypt based columnar cells |
| Bmi1 | +4 position |
| OLFM4 | Crypt based columnar cells |
| Musashi-1 | Crypt base, cell positions 1–10 |
| DCAMKL1 | Position 4–6, also some villous expression |
| BMPR1α | Label-retention cells (position 4–9) |
| Eph | receptors Position 4–6 |
Despite the enthusiasm for Lgr5 as the definitive marker of ISCs, further research reignited the controversy over the exact location of the intestinal stem cell after the discovery of an additional candidate marker. Using a similar approach to Clevers, Sangiorgi and Capecchi identified Bmi1 as a gene of interest for marking the pluripotency of intestinal stem cells.12 Bmi1 positive cells are known to be involved in self-renewal in neuronal and hematopoetic stem cells, and overexpression of the gene in tumor cells has been shown to lead to immortalization secondary to increased telomerase activity.13 Expression of Bmi1 appears critical to controlling aging and damage response, as mice deficient in Bmi1 exhibit impaired mitochondrial function, increased reactive oxygen species, abnormalities of DNA damage repair, and decreased life span.14 Similar to Lgr5, Bmi1 positive cells have been shown through lineage tracing experiments to give rise to all intestinal epithelial subtypes. These cells appear to occupy the previously described +4 position and are distinct from the CBCs.5 Whereas Lgr5 positive cells are known to be rapidly dividing, some have argued that Bmi1 positive cells are more quiescent, although this remains controversial.15 However, cells in the +4 position which express the ISC marker Musashi-1 have been shown to represent a quiescent population of multipotent cells, confirming their distinction from Lgr5 positive cells.11 These findings suggest at least two separate cell types in two distinct positions representing the intestinal stem cells, although the relationship between the two remains unknown (Figure 2, Panel A).15 Regardless of the controversies and differences between those that advocate for one position over another as the definitive stem cell compartment, at least one fact has become clear from analysis of this body of work: definitive markers identifying multipotent intestinal epithelial progenitors now reliably exist and provide a critical new tool for the study of intestinal stem cell biology.
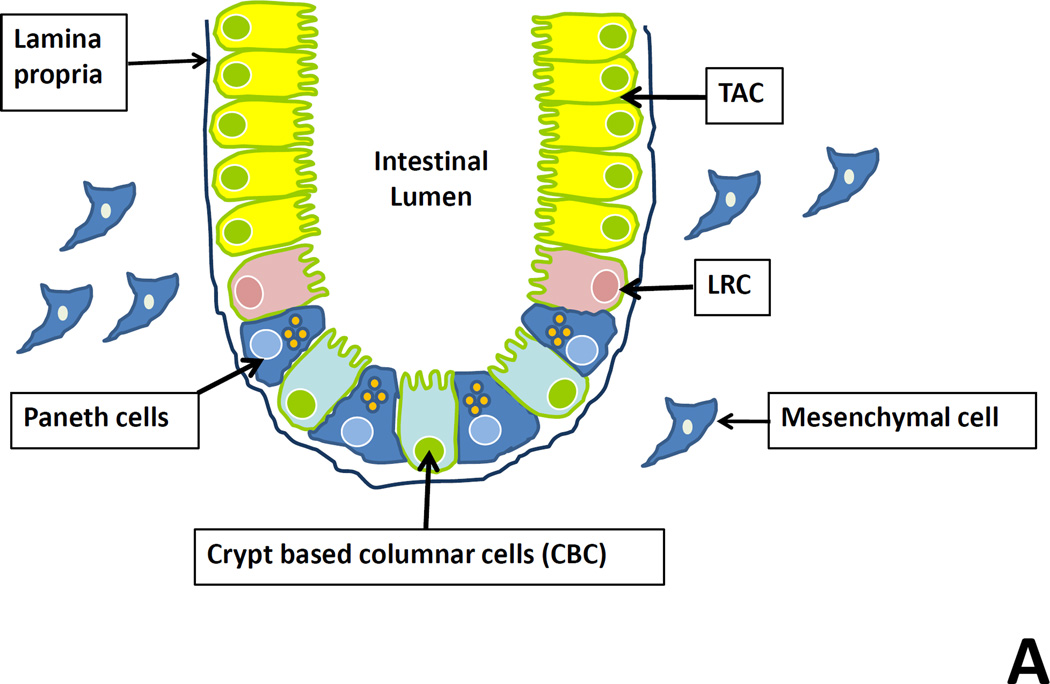
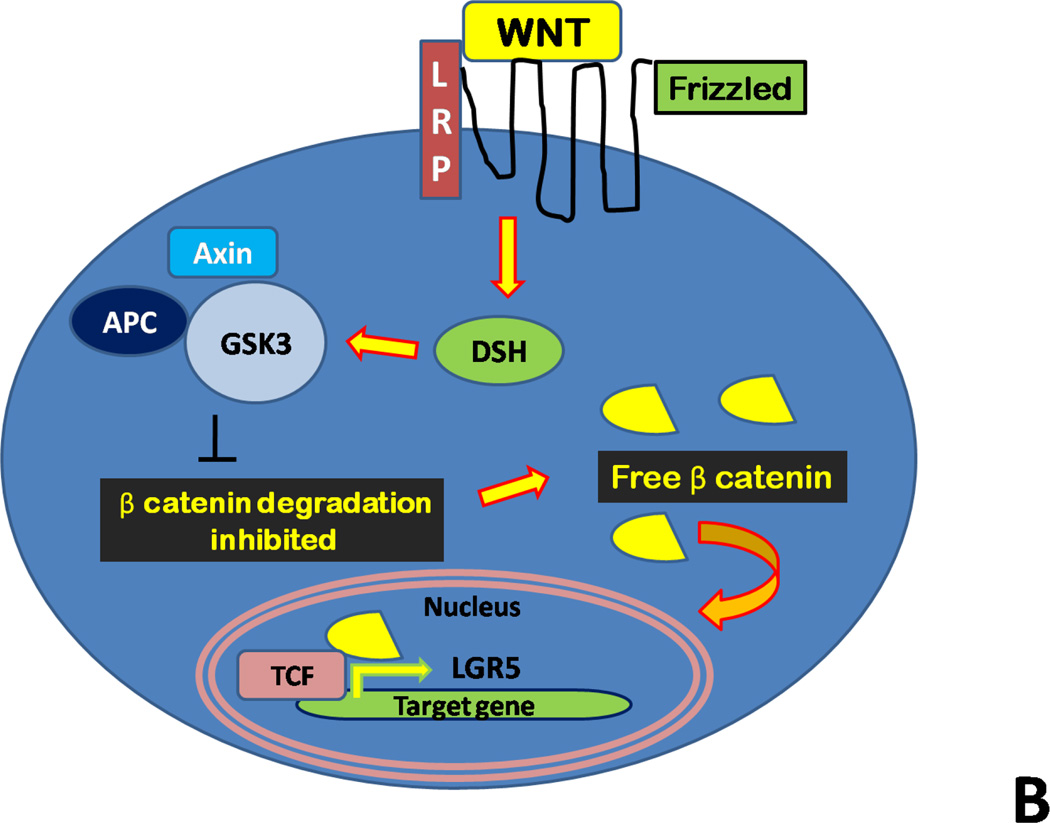
Figure 2. Schematic of the intestinal stem cell compartment and WNT signaling.
Panel A: Crypt based columnar (CBC) cells residing in interspersed between Paneth cells in the intestinal crypt express the putative stem cell marker Lgr5 and represent actively dividing cells. Cell located in the +4 position are referred to as label-retaining cells (LRC) and are thought to represent quiescent stem cells. The proliferative zone superior to these is populated by transient amplifying cells (TACs), which further differentiate into absorptive, goblet, enteroendocrine, and Paneth cells.
Panel B: WNT proteins bind to the cell surface receptor Frizzled in addition to the transmembrane receptor LRP. This binding event activates Dishevelled (DSH) family proteins, and this complex is responsible for the inhibition of a second complex of proteins includes axin, GSK-3, and the protein APC. The axin/GSK-3/APC complex controls degradation of β-catenin. After the degradation complex is inhibited, stabilized, free cytoplasmic β-catenin is able to enter the nucleus and interact with TCF transcription factors to promote specific gene expression, such as Lgr5.
Signaling pathways within the Intestinal Stem Cells
The epithelium of the small intestine is regenerated completely every 4–5 days. This brisk turnover of cells is fueled by the rapidly dividing intestinal stem cells in the intestinal crypt which give rise to transient amplifying cells (TAC) (Figure 2, Panel A), which are further responsible for differentiation into the four types of epithelial cells.2 Control of proliferation within the intestinal crypts occurs via the WNT signaling cascade, which acts as a switch between proliferation and differentiation.16 WNT signaling is highly upregulated in the intestinal crypts as opposed to the villi, indicating the proliferative nature of the crypt bottom.17 Signaling via WNT occurs in a highly orchestrated manner (Figure 2, Panel B). In brief, following activation of WNT, signaling occurs through Frizzled and low-density lipoprotein receptor– related protein (LRP) receptors, resulting in translocation of free β-catenin to the nucleus. After coupling of β-catenin to various transcription factors, the complex is responsible for transcription of target genes, including Lgr5. Inactivation of the β-catenin pathway occurs via phosphorylation by a degradation complex which involves, amongst other factors, the tumor suppressor adenomatous polyposis coli (APC), Axin, the phosphoprotein Dishevelled (DSH), casein kinase I (CKI), and the constitutively active kinases glycogen synthase kinase 3β (GSK3).18,19 While both WNT and Notch signaling are required for ISC maintenance, TGF-β and bone morphogenetic protein (BMP) have been identified as negative regulators.20 The source of these signals is hypothesized to be the stem cell niche, which is defined as the microenvironment surrounding the ISCs.21,22 Additional features regarding the WNT signaling cascade may be found in the following excellent reviews: van der Flier, Ann Rev Phys, 2009; Clevers, Cell, 2006; Logan, Ann Rev Cell Dev Biol, 2004.2,18,19
The activation of intestinal stem cells via WNT ligands has been shown to be critical for proliferation based on a number of studies demonstrating impaired proliferation in the presence of WNT inhibitors such as Dickkopf-1,23 β-catenin deletion,24 and mutation of critical WNT signaling transcription factors.25 Conversely, mutation of WNT regulators, including c-myc and APC, has been implicated in the development of colorectal cancer.2 The activation of the WNT agonist, R-spondin, has been shown to produce a phenotype of hyperproliferative crypts.26 This strengthens the association with WNT signaling as a driving force of intestinal stem cell proliferation. It has been suggested that the role of the rapidly dividing CBC stem cell population under WNT control is to provide an emergency supply of progeny in the face of injury, while the quiescent +4 cells divide infrequently to prevent errors inherent in DNA replication and preserve long-term potential.27
The Role of Intestinal Stem Cells in Injury and Repair
Having introduced the concept and reviewed a brief history of the intestinal stem cell, we will now focus on the emergence of ISCs as a critical responder to injury and inflammation. Four injury models are presented here with a review of the corresponding literature in order to gain an understanding of the intestinal stem cell response to injury and repair.
i. The Drosophila experience
Drosophila have provided an important model for assessing the intestinal stem cell and the response to injury. A finding that is unique to Drosophila as compared to mammals is that the intestinal stem cells of the Drosophila midgut are the only cells capable of undergoing mitosis and are identified using a specific marker, Delta.28 This provides a means of controlling for damage response not found in mammalian tissue where the involvement of multiple local and systemic cell types makes interpretation of response more complex. Recently, multiple groups have independently shown that intestinal stem cells in Drosophila are capable of responding to the presence of bacteria. Buchon and colleagues demonstrated that bacterial infections in Drosophila result in a significant increase in ISC proliferation and epithelial renewal as well as activation of defense mechanisms such as oxidative burst. These pathways, which are thought to include JAK-STAT and JNK, are also activated, to a lesser degree, by indigenous gut microbiota.29,30 This evidence, coupled with findings from similar studies performed by Chatterjee and Ip which showed an increase in both proliferation and differentiation in ISCs exposed to bacteria, suggests that Drosophila midgut stem cells can response to microbial injury and initiate tissue repair.28 Proliferation of the intestinal stem cell compartment triggered by bacterial infection in Drosophila has also been linked to tumorigenesis and intestinal dysplasia, indicating that interaction with bacteria may trigger a pathologically increased response by ISCs.31
ii. Radiation colitis
The response of the mouse intestinal epithelium following radiation has been the most extensively characterized model of intestinal stem cell injury and proliferation to date.32 Assays to assess for crypt cell survival following radiation injury date back over forty years.33,34 The rapidly dividing and actively proliferating cell populations of the intestinal crypt, including the CBCs and TACs, are keenly susceptible to radiation injury. Radiation injury results in crypt apoptosis and the clinical development of the gastrointestinal (GI) syndrome, which can result in severe and debilitating diarrhea. The pathogenesis of acute radiation GI syndrome is incompletely understood, however the loss of crypt stem cells has been suggested to be responsible,35 although other authors claim that loss of villous endothelial cells leads to GI syndrome.36 Regardless of the specific cell that is injured, the response of the ISC to drive reconstitution and healing has been of great interest. Early studies in the field suggested an important role for, among others factors, prostaglandins in the regulation of intestinal stem cell growth and migration after radiation injury.32,37 Cohn and colleagues, using a functional assay for quantifying stem cell survival, showed that production of prostaglandins by cyclooxygenase-1 (COX 1) was protective following ISC radiation injury.32 More recently, a number of authors have attempted to elucidate the mechanism governing intestinal progenitor cell radiosensitivity. The roles of p53 and Ataxia telangiectasia mutated (ATM) protein kinase have been studied as regulators of crypt apoptosis.38 The BH3-only, Bcl-2 family protein p53 upregulated modulator of apoptosis (PUMA) has been shown by multiple groups to play an essential role in both p53-dependent and –independent apoptosis.35,39,40 Qiu and colleagues have shown that PUMA-deficient mice exhibit blocked crypt apoptosis following radiation and that suppressing PUMA leads to radioprotection and prolonged survival in the intestinal stem cells.35 This critical role for PUMA in radiation induced intestinal stem cell injury has provided significant molecular insight into the response of intestinal stem cells to injury. Given this and the findings of others suggesting an essential role for p53 mediated growth arrest following DNA damage in ISCs, it appears that regulation of p53 activity in intestinal stem cells has emerged as a key concept in the injury response of these progenitor cells.41 Despite the strength of these observations indicating that p53 apoptosis mediates failure of ISC proliferation, evidence exists to support the ability of organisms to overcome this insult. Through a series of transgenic studies resulting in the deletion of the key p53 inhibitor, Mdm2, Valentin-Vega and colleagues have shown that although these mutants develop early intestinal abnormalities, specifically in the crypts, the organisms are able to overcome this and actually expand their stem cell population, resulting in compensation and survival.42 Fascinatingly, this expansion of ISCs occurs through WNT activation in similar fashion to intestinal neoplastic processes. However, these mice expand their ISCs without developing tumors, suggesting a molecular mechanism by which these cells can halt proliferation upon reaching homeostasis.
iii Chemical injury to the intestine
Mouse models of chemical colitis, especially dextran sodium sulfate (DSS) and 2, 4, 6-trinitrobenzenesulfonic acid (TNBS) colitis, are of particular interest to surgeons given the relevance to inflammatory bowel disease and other disorders of infectious/inflammatory colitis. Although the more rapid turnover of cells in the small intestine as compared to the large intestine leads some to hypothesize that more ISC proliferation is required in the small bowel, the mechanisms of injury repair are likely similar to the colon.43 An expansion of crypts surrounding ulcers formed in the colon44 as well as an increase in crypt cells staining positive for the proliferation marker, proliferating cell nuclear antigen (PCNA)43 have been observed following induction of chemical colitis. Although much attention has been devoted to the response of mesenchymal stem cells in models of colitis,45–47 the specific role of the intestinal stem cell in response to colitis has not been well studied (perhaps due to the prior lack of reliable markers). In fact, only one study exists in the literature specifically assessing the response of ISCs to a model of DSS colitis. Despite an observed increase in proliferation during administration of DSS, Fukui demonstrated an initial decrease in the expression of the ISC marker, Musashi-1 followed by a subsequent increase during the healing or regenerative phase of the model.43 Similarly, in a model of doxorubicin-induced intestinal injury, an initial decrease in the expression of DCAMKL1 was observed after injury, however, no significant change in Lgr5 mRNA expression was noted.48 Interestingly, both Musashi-1 and DCAMKL1 are thought to represent markers of the +4 position, while Lgr5 clearly arises from the CBC cells, suggesting a different response to injury based on stem cell position.
iv. Bacterial injury to the intestine
It has recently been shown that bacteria and bacterial by-products are important regulators of the β-catenin pathway, leading to effects on intestinal epithelial proliferation.49,50 Our lab has shown that the gram negative bacterial toxin lipopolysaccharide (LPS) inhibits β-catenin signaling via activation of GSK3β, leading to a marked inhibition in enterocyte proliferation both in vitro and in vivo, within the newborn small intestine.49 This particular finding links activation of the innate immune receptor for LPS, namely Toll like receptor 4 (TLR4), with the inhibition of enterocyte proliferation in the neonatal small bowel, and is thought to contribute to the pathogenesis of necrotizing enterocolitis, a devastating disease of premature infants that is characterized by impaired enterocyte proliferation and enhanced TLR4 signaling in the small bowel.49,51–53 Significantly, Abreu and colleagues have shown that TLR4 activation leads to increased enterocyte proliferation in models of colitis, suggesting either an effect that may be dependent upon developmental factors as well as on the location of the intestine in which the signaling occurs.54,55 These findings are supported by work from a variety of authors that show differences in proliferation in germ-free animals as well as after activation of various toll-like receptors including TLR4.56–59 Recent evidence has emerged supporting AvrA, a bacterial effector protein present in certain types of Salmonella and E. coli, as a specific regulator of β-catenin.60–61 Given the association with β-catenin, Liu and colleagues sought to address whether infection with Salmonella resulted in a change in intestinal stem cell proliferation in a mouse model. The authors found that AvrA specifically upregulated the expression of a number of WNT genes and resulted in an increase in phosphorylation of β-catenin, which led to a significant increase in crypt proliferation.62 It is of interest that this study shows a pathogenic organism (Salmonella typhimurium) induces proliferation, which is widely held to be a mechanism of injury repair.49,63 However, the authors do note in their discussion that the putative stem cell marker, Lgr5, was significantly decreased in their model,62 which again points to the need for clarity in exactly which cells of the intestinal crypt are responding to injury.
Forming a Necessary Niche: Getting Along with Your Stem Cells
In parallel with the emerging interest in the response of intestinal stem cells to injury is the recognition of the importance of the stem cell niche. The concept that the surrounding microenvironment regulates progenitor cell response has been covered thoroughly by multiple excellent reviews.64–66 One manifestation of the importance of the stem cell niche is the phenomena of crypt fission, a physiological mechanism of crypt reproduction in response to injury, which likely requires communication between adjacent progenitor cells to coordinate a response to injury. In addition to the role of the epithelial cells themselves, other cell types play a critical role in effective epithelial healing, including macrophages, endothelial cells and dendritic cells.44,52,57,63 Moreover, mesenchymal stem cells, which are multi-potent connective tissue progenitors, interact with the intestinal epithelium and progenitor cells to both maintain homeostasis and drive the response to injury.67–69 There is data emerging to suggest that these mesenchymal stem cells may play a key role in the response to injury. As an example, Sémont and colleagues have recently shown that giving bone-marrow derived mesenchymal stem cells can improve small intestinal renewal and help to drive epithelial regeneration following radiation injury.70
Conclusions and future directions
The recent identification of reliable markers for intestinal stem cells has opened the door to an array of studies that promise to shed light upon not only the location and precise description of the intestinal stem cell, but also on the role of this discrete cellular population in the response to injury to the intestinal mucosa. Future questions in the field include the understanding of the specific cells within the crypt that may be involved in the injury response, and how they interact with their surroundings. Furthermore, the exact function of the promising stem cell marker, LGR5, remains unknown. Given its homology to other ligand receptors, there is likely a “hidden” signaling role for this protein that may help to unlock the secrets of stem cell behavior embedded in the intestinal crypts. Transgenic experiments that involve conditional deletion of stem cell markers as well as overexpression may help to elucidate this question. In addition, these markers almost certainly interact with other cellular receptors and regulators of gene expression. A thorough search for associations at the cellular level with pattern recognition receptors and known molecular machinery of injury response will be key to linking intestinal stem cell response to injury. It is hoped that through the study of intestinal stem cells, both surgeons and non-surgeons alike with gain novel therapeutic insights into the manner in which the body repairs itself after injury, and in so doing, will offer renewed hope for our patients with diseases of intestinal inflammation.
Acknowledgments
Grant support: DJH is supported by R01GM078238 and RO1DK08752 from the National Institutes of Health. MDN is supported by the Wyeth Scholarship of the American College of Surgeons for the Study of Inflammation and the National Institutes of Health Loan Repayment Program. WMR is supported by the National Institutes of Health Loan Repayment Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am. J. Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 2.Van der Flier, LG and Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 3.Potten CS, Booth C, Pritchard DM. The intestinal stem cell: the mucosal governor. Int J Exp Pathol. 1997 Aug;78(4):219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 5.Batlle E. A new identity for the elusive stem cell. Nat Genet. 2008 Jul;40(7):818–819. doi: 10.1038/ng0708-818. [DOI] [PubMed] [Google Scholar]
- 6.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999 Jan;116(1):7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 7.Bjerknes M, Cheng H. Intestinal epithelial stem cells and progenitors. Methods Enzymol. 2006;419:337–383. doi: 10.1016/S0076-6879(06)19014-X. [DOI] [PubMed] [Google Scholar]
- 8.Haegebarth A, Clevers H. WNT signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009 Mar;174(3):715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker N, van Es JH, Kuipers J, Kujala P, Van Den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villous structures in vitro without a mesenchymal niche. Nature. 2009 May 14;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, White LS, Hurov KE, Stappenbeck TS, Piwnica-Worms H. Response of small intestinal epithelial cells to acute disruption of cell division through CDC25 deletion. Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4701–4706. doi: 10.1073/pnas.0900751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008 Jul;40(7):915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimri GP, Martinez JL, Jacobs JJ, Keblusek P, Itahana K, Van Lohuizen M, Campisi J, Wazer DE, Band V. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002 Aug 15;62(16):4736–4745. [PubMed] [Google Scholar]
- 14.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, Ahn BH, Kumar NG, Rovira II, Xu XL, van Lohuizen M, Motoyama N, Deng CX, Finkel T. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009 May 21;459(7245):387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010 Jan 29;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002 Oct 18;111(2):241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 17.Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H. Expression patterns of Wnt signaling components in the adult intestine. Gastroenterology. 2005 Aug;129(2):626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006 Nov 3;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 20.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004 Oct;36(10):1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 21.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001 Nov 1;414(6859):98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004 Mar 19;116(6):769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 23.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004 May;126(5):1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998 Aug;19(4):379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 26.Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, Sellers RS, Alfieri AA, Guha C. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 2009 Nov 24;4(11):e8014. doi: 10.1371/journal.pone.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Hou SX. Regulation of intestinal stem cells in mammals and Drosophila. J Cell Physiol. 2010 Jan;222(1):33–37. doi: 10.1002/jcp.21928. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee M, IP YT. Pathogenic Stimulation of Intestinal Stem Cell Response in Drosophila. J. Cell Physiol. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes and Development. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes Rde M, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009 Jul 17;325(5938):340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apidianakis Y, Pitsouli C, Perrimon N, Rahme L. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci U S A. 2009 Nov 23; doi: 10.1073/pnas.0911797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohn SM, Schloemann S, Tessner T, Seibert K, Stenson WF. Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandins synthesized through cyclooxygenase-1. JCI. 1997;99:1367–1379. doi: 10.1172/JCI119296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int. J. Radiat. Biol. 117:261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- 34.Withers HR, Elkind MM. Radiosensitivity and fractionation response of crypt cells of mouse jejunum. Radiat Res. 1969;38:598–613. [PubMed] [Google Scholar]
- 35.Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, Zhang L, Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008 Jun 5;2(6):576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 37.Hanson WR, Thomas C. 16, 16-dimethyl prostaglandin E2 increases survival of murine intestinal stem cells when given before photon radiation. Radiat Res. 1983 Nov;96(2):393–398. [PubMed] [Google Scholar]
- 38.Ch’ang HJ, Maj JG, Paris F, Xing HR, Zhang J, Truman JP, Cardon-Cardo C, Haimovitz-Friedman A, Kolesnick R, Fuks Z. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 2005;11:484–490. doi: 10.1038/nm1237. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, Zhang L, Hwang PM, Kinzler KW and Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 40.Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, Zhu L, Chittenden T. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc. Natl. Acad. Sci. USA. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.George RJ, Sturmoski MA, May R, Sureban SM, Dieckgraefe BK, Anant S, Houchen CW. Loss of p21 Waf1/Cip1/Sdi1 enhances intestinal stem cell survival following radiation injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G245–G254. doi: 10.1152/ajpgi.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valentin-Vega YA, Okano H, Lozano G. The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ. 2008 Nov;15(11):1772–1781. doi: 10.1038/cdd.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukui T, Takeda H, Shu HJ, Ishihama K, Otake S, Suzuki Y, Nishise S, Ito N, Sato T, Togashi H, Kawata S. Investigation of Musashi-1 expressing cells in the murine model of dextran sodium sulfate-induced colitis. Dig Dis Sci. 2006 Jul;51(7):1260–1268. doi: 10.1007/s10620-006-8046-3. [DOI] [PubMed] [Google Scholar]
- 44.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007 Jan;117(1):258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009 Dec 15;183(12):7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Y, Nie Y, Lai J, Wan YJ, Li Y. Comparison of the population capacity of hematopoietic and mesenchymal stem cells in experimental colitis rat model. Transplantation. 2009 Jul 15;88(1):42–48. doi: 10.1097/TP.0b013e3181a9f0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009 Mar;136(3):978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 48.Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol. 2009 Sep;297(3):G461–G470. doi: 10.1152/ajpgi.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010 Jan;138(1):185–196. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun J, Hobert ME, Rao AS, Neish AS, Madara JL. Bacterial activation of beta-catenin signaling in human epithelia. Am J Physiol Gastrointest Liver Physiol. 2004 Jul;287(1):G220–G227. doi: 10.1152/ajpgi.00498.2003. [DOI] [PubMed] [Google Scholar]
- 51.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007 Oct 1;179(7):4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 52.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006 Mar 1;176(5):3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 53.Cetin S, Ford HR, Sysko LR, Agarwal C, Wang J, Neal MD, Baty C, Apodaca G, Hackam DJ. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem. 2004 Jun 4;279(23):24592–24600. doi: 10.1074/jbc.M313620200. [DOI] [PubMed] [Google Scholar]
- 54.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006 Sep;131(3):862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005 May;288(5):G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 56.Ungaro R, Fukata M, Hsu D, Hernandez Y, Breglio K, Chen A, Xu R, Sotolongo J, Espana C, Zaias J, Elson G, Mayer L, Kosco-Vilbois M, Abreu MT. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1167–G1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005 Jan 4;102(1):99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004 Jul 23;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006 Dec;8(12):1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 60.Hardt WD, Galan JE. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc. Natl. Acad. Sci. USA. 1997;94:9887–9892. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao AP, Petrof EO, Kuppireddi S, Zhao Y, Xia Y, Claud EC, Sun J. Salmonella type III effector AvrA stabilizes cell tight junctions to inhibit inflammation in intestinal epithelial cells. PLoS One. 2008;3:e2369. doi: 10.1371/journal.pone.0002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Lu R, Wu S, Sun J. Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Letters. 2010 doi: 10.1016/j.febslet.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009 Jan 6;106(1):256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker MR, Stappenbeck TS. Deciphering the 'black box' of the intestinal stem cell niche: taking direction from other systems. Curr Opin Gastroenterol. 2008 Mar;24(2):115–120. doi: 10.1097/MOG.0b013e3282f4954f. [DOI] [PubMed] [Google Scholar]
- 65.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2(3):203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 66.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006 Mar 31;311(5769):1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 67.Sémont A, Mouiseddine M, François A, Demarquay C, Mathieu N, Chapel A, Saché A, Thierry D, Laloi P, Gourmelon P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2009 Dec 18; doi: 10.1038/cdd.2009.187. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Davies PS, Powell AE, Swain JR, Wong MH. Inflammation and proliferation act together to mediate intestinal cell fusion. PLoS One. 2009 Aug 6;4(8):e6530. doi: 10.1371/journal.pone.0006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005 Jul;48(7):1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 70.Sémont A, Mouiseddine M, François A, Demarquay C, Mathieu N, Chapel A, Saché A, Thierry D, Laloi P, Gourmelon P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2009 Dec 18; doi: 10.1038/cdd.2009.187. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]