Abstract
Magnetic Resonance Imaging (MRI) is the principal method for studying structural age-related brain changes in vivo. However, previous research has yielded inconsistent results, precluding understanding of structural changes of the aging brain. This inconsistency is due to methodological differences and/or different aging patterns across samples. To overcome these problems, we tested age effects on 17 different neuroanatomical structures and total brain volume across five samples, of which one was split to further investigate consistency (883 participants). Widespread age-related volume differences were seen consistently across samples. In four of the five samples, all structures, except the brain stem, showed age-related volume differences. The strongest and most consistent effects were found for cerebral cortex, pallidum, putamen and accumbens volume. Total brain volume, cerebral white matter, caudate, hippocampus and the ventricles consistently showed non-linear age functions. Healthy aging appears associated with more widespread and consistent age-related neuroanatomical volume differences than previously believed.
Keywords: MRI morphometry, Age, Cortex, White matter, Cerebellum, Ventricles, Hippocampus, Amygdala, Thalamus, Basal ganglia
1. Introduction
Brain changes are inevitable in aging. Still, core questions remain a matter of debate: What structures change, when do they start aging, at what rates, and are some structures spared? Many cross-sectional studies have demonstrated neuroanatomical age-related volume differences in vivo by use of magnetic resonance imaging (MRI) (Allen et al., 2005; Blatter et al., 1995; Courchesne et al., 2000; Fotenos et al., 2005; Good et al., 2001; Head et al., 2004; Head et al., 2005; Jernigan et al., 1991; Jernigan et al., 2001; Luft et al., 1999; Mu et al., 1999; Raz et al., 2004a; Raz et al., 2005a; Raz et al., 2005b; Raz and Rodrigue, 2006; Raz et al., 2004b; Raz et al., 2007; Raz et al., 2000; Salat et al., 2004; Sullivan et al., 1995; Sullivan et al., 2004; Taki et al., 2004; Tisserand et al., 2002; Walhovd et al., 2005a). Some structures are found to decline substantially, while others appear better preserved (Raz and Rodrigue, 2006). Different age trajectories have been observed, with some brain areas declining linearly from early in life, whereas others continue to increase in volume before eventually beginning to deteriorate (Allen et al., 2005; Good et al., 2001; Luft et al., 1999; Raz et al., 2004b; Walhovd et al., 2005a). Unfortunately, the results diverge much across studies, and differences in segmentation procedures and demarcation criteria complicate comparisons. Discrepant findings have been reported for most structures. Adding to this problem, in most studies only a few structures are segmented, making it difficult to assess the relative vulnerability of different structures to age.
The aim of the present paper was to overcome these problems. Data from five samples (one split-half making a total of six groups for analysis) were processed with the same segmentation tools, and the stability of age effects across samples was assessed for 16 subcortical structures as well as cortical volume and total brain volume. Three questions were asked: (1) Which structures show significant age-related volume differences across samples? (2) Which structures undergo the most prominent age-related changes, and which are relatively preserved? (3) Which structures are volumetrically changed in a linear fashion, and which show curvilinear (quadratic) age relationships?
Main findings from previous MRI studies on age-related differences in neuroanatomial volumes are summarized in the following. Further reviews can be found elsewhere (Raz and Rodrigue, 2006). It should be noted that the vast majority of studies reviewed below are of a cross-sectional nature, and unless longitudinal designs are explicitly noted, what is observed are age-differences, rather than age changes. There is consensus that gray matter (GM) volume/ thickness is smaller with higher age (Blatter et al., 1995; Courchesne et al., 2000; Fotenos et al., 2008; Good et al., 2001; Jernigan et al., 1991; Jernigan et al., 2001; Murphy et al., 1996; Pfefferbaum et al., 1994; Raz et al., 1997; Resnick et al., 2000; Salat et al., 2004; Sullivan et al., 1995; Sullivan et al., 2004; Walhovd et al., 2005a), and that this effect is seen early in life (Courchesne et al., 2000; Giedd, 2004; Giedd et al., 1999; Giedd et al., 1996; Lebel et al., 2008). Based on cross-sectional investigations, there generally appears to be somewhat greater GM loss in the cortex than in subcortical structures (Jernigan et al., 2001; Walhovd et al., 2005a). However, a longitudinal study has indicated at least as much shrinkage of the caudate and cerebellum as in the lateral frontal and orbitofrontal cortex (Raz et al., 2005a). Aging of different parts of the cortex is highly heterogeneous, and cortical volume is included in the present study mainly to allow comparisons with subcortical structures. Detailed analyses of cortical thickness are reported elsewhere (Fjell et al., submitted).
Less consistent results have been reported for the relationship between age and white matter (WM) volume. Some studies have found no age differences (Abe et al., 2008; Blatter et al., 1995; Good et al., 2001; Jernigan et al., 1991; Pfefferbaum et al., 1994; Sullivan et al., 2004), while others have found that total WM volume is negatively related to age (Allen et al., 2005; Guttmann et al., 1998; Jernigan et al., 2001; Walhovd et al., 2005a). Samples of varying ages may be a reason for the discrepant findings, and studies including the oldest participants tend to report age-effects. One study (Courchesne et al., 2000) reported white matter to be negatively related to age only from 70 years of age onwards, and this age range has not been consistently included in aging studies. Jernigan and colleagues (Jernigan et al., 2001; Jernigan and Gamst, 2005) found that despite its later onset, white matter loss was more rapid than gray matter loss, and ultimately exceeded it. In recent years, there has been increased focus on the possibly curvilinear nature of age change in WM volume (Allen et al., 2005; Jernigan and Gamst, 2005; Walhovd et al., 2005a), with gains until middle age followed by later decrease. Non-linear fits tend to significantly increase the proportion of variance in WM volume explained by age. As for gray matter, results indicate somewhat less age-related loss in deep subcortical regions than in the cerebral lobes (Jernigan et al., 2001). For instance, although some decline has also been observed in brainstem volume (Walhovd et al., 2005a), several studies have reported no effect of age on volume of the pons (Luft et al., 1999; Raz et al., 1998; Raz et al., 2001; Raz et al., 1992; Van Der Werf et al., 2001).
In the following, age effects on different subcortical brain structures from 31 cross-sectional studies are reviewed (details are presented in Table 1). All studies tested effects of age on the volume of at least one of the subcortical structures/compartments included in the present study, and a short presentation of the main results from this literature is given below:
Table 1.
Overview of studies of age-effects on subcortical brain structures The table does not necessarily encompass all studies of possible relevance. Studies were only included if they reported cross-sectional data for at least one of the structures included in the present paper, except cortical volume, white matter volume, or whole-brain volume. In several of the cases, r was not reported, and was then calculated here based on other information (e.g. R2). This may lead to slight inaccuracies due to rounding errors etc. Very different measures are used for correcting for ICV/head size/brain size/body size, and the statistical procedures used for the corrections are also often different (e.g. ratio scores, residuals from regression analyses, entered as covariate, showed to not affect the data and then left out of the final analyses). In several of the studies where normalization was not used, ICV or a proxy for ICV was calculated, but for different reasons not used (e.g. did not interact with any variables of interest), or only the results of the analyses without the correction were reported in detail. Not all studies tested for non-linear relationships, and when done, not all tested for cubic relationships. Correlations are Pearson’s r, unless stated otherwise (the type used was not stated explicitly in all studies). P ≤ .05 is regarded as significant, regardless of the chosen threshold in each study.
| Study | N | Age range |
Segmentation method |
Normalization | Age effects (Pearson’s r) |
Non-linear effects | Not age effects |
|---|---|---|---|---|---|---|---|
| Krishnan et al. (1990)(Krishnan et al., 1990) | 39 | 24–76 | Manual | None | Caudate (R = −.69) | ||
| Jernigan et al. (1991)(Jernigan et al., 1991) | 55 | 30–70 | Semi-automated | Supratentorial cranium | Caudate (−.49) | Diencephalic structures (including thalamus) | |
| Gur et al. (1991)(Gur et al., 1991) | 69 | 18–80 | Semi-automated | ICV | Ventricular CSF, Sulcal CSF (r only provided for total CSF; .76) | ||
| Cohen et al. (1992)(Cohen et al., 1992) | 54 | 20–70 | Semi-automated | None | CSF (r not provided) | ||
| Sullivan et al. (1995)(Sullivan et al., 1995)* | 72 | 21–70 | Semi-automated | ICV | Temporal lobe sulcal CSF LH (.57) and RH (.54), Lat vent LH (.33) and RH (.33), 3rd vent (.47) | Quadratic: Temporal lobe sulcal CSF LH, Lat vent LH & RH; Cubic: Temporal lobe sulcal CSF RH | Hippocampus |
| Gunning-Dixon et al. (1998)(Gunning-Dixon et al., 1998) | 148 | 18–77 | Manual | None | Caudate (−.32), Putamen (−.41) | Globus pallidus | |
| Coffey et al. (1998)(Coffey et al., 1998) | 330 | 66–96 | Manual | ICV | Lat vent, 3rd vent, sulcal CSF (men only) (r not provided) | Sulcal CSF (women only) | |
| Luft et al. (1999)(Luft et al., 1999) | 48 | 20–73 | Semi-automated | ICV | Exponential: Cerebellum | Globus pallidus | |
| Schuff et al. (1999)(Schuff et al., 1999) | 24 | 36–85 | Manual | ICV | Hippocampus (r = −.64) | ||
| Mu et al. (1999)(Mu et al., 1999) | 619 | 40–90 | Manual | ICV | Hippocampus (−.93), Amygdala (−.92) | ||
| Sullivan et al. (2000)(Sullivan et al., 2000) | 61 | 23–72 | Semi-automated | None | Cerebellum GM LH (−.39) and RH (−.45) | Cerebellum WM | |
| Xu et al. (2000)(Xu et al., 2000) | 331 | 30–79 | Manual | ICV | Thalamus (r not provided) | ||
| Good et al. (2001)(Good et al., 2001) | 465 | 18–79 | VBM | ICV/global GM loss | Global CSF | Quadratic: Global CSF (women only) | Amygdala, Hippocampus, Lat. thalamus |
| Pruessner et al. (2001)(Pruessner et al., 2001) | 80 | 18–42 | Manual | None | Hippocampus (for men only, LH r = −.47, RH r = −.44) | Hippocampus (women), Amygdala | |
| Raz et al. (2001)(Raz et al., 2001) | 190 | 18–81 | Manual | None | Cerebellar hemispheres GM (−.32), Vermian lobules (−.24 to −.32) | Vent pons | |
| Jernigan et al. (2001)(Jernigan et al., 2001)^ | 78 | 30–99 | Semi-automated | Cranial vault | Hippocampus (−.65), Caudate (−.35), Nucleus accumbens (−.33), Cortical sulcal CSF (.83), Cerebral vent CSF (.74), Cerebellar CSF (.75) | Amygdala, Thalamus, Basomesial diencephalon, Lenticular nucleus (putamen, globus pallidus), Substantia nigra | |
| Van der Werf et al. (2001)(Van Der Werf et al., 2001) | 57 | 21–82 | Manual | ICV & Brain size | Thalamus (−.71) | ||
| Scahill et al. (2003)(Scahill et al., 2003) | 39 | Semi-automated | ICV | Hippocampus, ventricles (r not provided) | |||
| Raz et al. (2003)(Raz et al., 2003) | 53 | 20–77 | Manual | ICV | Caudate (r = −.41/−.47 baseline/5 year follow-up), Putamen (r = −.46/−.47 for baseline/follow up) | Globus pallidus (significant reduction in 5 year longitudinal data) | |
| Liu et al. (2003)(Liu et al., 2003) | 90 | 14–77 | Manual/semi-automated | ICV | Cerebellum (−.37) | Hippocampus | |
| Van Petten et al. (2004)(Van Petten, 2004) | 48 | 65–85 | Manual | Cranial vault | Hippocampus | ||
| Raz et al. (2004)(Raz et al., 2004a) | 200 | 20–80 | Manual | Body height | Hippocampus (−.42) | ||
| Sullivan et al. (2004)(Sullivan et al., 2004) | 100 | 23–72 | Manual | None | Thalamus (men: −.53, women: −.59) | Pontine | |
| Sullivan et al. (2005)(Sullivan et al., 2005) | 128 | 20–85 | Manual/semi-automated | None | Hippocampus | ||
| Walhovd et al. (2005)(Walhovd et al., 2005a) | 73 | 20–88 | Automated | ICV | Hippocampus (−.40), Amygdala (−.47), Thalamus (−.78), Accumbens (−.65), Caudate (−.69), Putamen (−.47), Brainstem (−.35), Cerebellar GM (−.61), Cerebellar WM (−.56), Lat vent (−.70), Inf lat vent (.57), 3rd vent (.74) | Quadratic: Hippocampus, Pallidum, Brainstem, Cerebellar GM, Cerebellar WM, Lat vent, Inf lat vent, 3rd | Pallidum, 4th vent |
| Allen et al. (2005)(Allen et al., 2005) | 87 | 22–88 | Manual | None | Hippocampus (−.38)¤, Amygdala (−.42) | Cubic: Hippocampus | |
| Du et al. (2006)(Du et al., 2006) | 42 | 58–87 | Semi-automated | None | Hippocampus (cross-sectional, reductions at follow-up) | ||
| Lupien et al. (2007)(Lupien et al., 2007) | 177 | 18–85 | Manual | None (sex as covariate) | Hippocampus (r not known) | Quadratic: Hippocampus | |
| Nunnemann et al. (2007)(Nunnemann et al., 2007) | 133 | 29–80 | VBM | None | Putamen (men: −.6) | Putamen (women) | |
| Hasan et al. (2008)(Hasan et al., 2008) | 33 | 19–59 | Manual | ICV | Caudate (−.55) | ||
| Greenberg et al. (2008)(Greenberg et al., 2008) | 82–140 | 60–85 | Manual | None | Caudate RH (−.19) & LH (−.24), Putamen RH (−.22) & LH (−.27), Hippocampus RH (−.36) & LH (−.27), Vent CSF (.39), Nonvent CSF (.37) |
LH: Left Hemisphere
RH: Right hemisphere
CSF: Cerebrospinal Fluid
GM: Gray matter
WM: White Matter
VBM: Voxel Based Morphometry
Vent: Ventricles
Lat: Lateral
Men only
Spearman’s rho
Calculated from R2 from the cubic regression
Hippocampus
The variability among studies is high. Nine of 15 studies reviewed here found that hippocampus shrank with age (Allen et al., 2005; Greenberg et al., 2008; Jernigan et al., 2001; Lupien et al., 2007; Mu et al., 1999; Raz et al., 2004a; Scahill et al., 2003; Schuff et al., 1999; Walhovd et al., 2005a), while five found no change (Du et al., 2006; Liu et al., 2003; Sullivan et al., 1995; Sullivan et al., 2005; Van Petten, 2004). In one study, age effects on hippocampal volume were found for men but not women (Pruessner et al., 2001). In addition, age effects on hippocampal volume normalized to global GM loss were not observed in a very large study (Good et al., 2001). Notably, three of the studies found non-linear effects of age (Allen et al., 2005; Lupien et al., 2007; Walhovd et al., 2005a), and one longitudinal study reported accelerated age-related hippocampal shrinkage (Raz et al., 2005a). Part of the discrepant findings may thus stem from failure to account for nonlinearity.
Amygdala
There have been fewer studies of age effects on the amygdala, but in sum, the reports indicate smaller age effects than on the hippocampus. Three studies found smaller volume of amygdala with higher age (Allen et al., 2005; Mu et al., 1999; Walhovd et al., 2005a), while two did not (Jernigan et al., 2001; Pruessner et al., 2001), and in one age age-effects relative to global GM loss were not observed (Good et al., 2001).
Thalamus/diencephalic structures
Four studies found smaller volume with higher age (Sullivan et al., 2004; Van Der Werf et al., 2001; Walhovd et al., 2005a; Xu et al., 2000), while two did not (Jernigan et al., 1991; Jernigan et al., 2001). In addition, one study found lack of age effects on the lateral thalamus relatively to global GM loss (Good et al., 2001).
Caudate
Caudate is the only structure where all the relevant studies are in coherence, with eight studies finding linear negative relationships with age (Greenberg et al., 2008; Gunning-Dixon et al., 1998; Hasan et al., 2008; Jernigan et al., 1991; Jernigan et al., 2001; Krishnan et al., 1990; Raz et al., 2005a; Raz et al., 2005b; Raz et al., 2003; Walhovd et al., 2005a).
Putamen
Four studies found age effects (Greenberg et al., 2008; Gunning-Dixon et al., 1998; Raz et al., 2003; Walhovd et al., 2005a). Additionally, in one study, age-effects were found for men, but not women (Nunnemann et al., 2007). Age effects were not found on the lenticular nuclei in one study (Jernigan et al., 2001), but these include the globus pallidus in addition to the putamen, and the latter may explain why effects were not found.
Pallidum
None of the four studies reporting on pallidum volume in relation to age found linear negative relationships (Gunning-Dixon et al., 1998; Jernigan et al., 2001; Luft et al., 1999; Raz et al., 2003), while a quadratic relationship was found in a fifth (Walhovd et al., 2005a).
Accumbens area
Only two studies have been reported, and both found linear negative relationships with age (Jernigan et al., 2001; Walhovd et al., 2005a).
Brainstem
Smaller volume of the brainstem with higher age was found in one study (Walhovd et al., 2005a), while the ventral pons has been found to be well preserved in another (Raz et al., 2001), and no significant age-change was observed in pontine structures in a third study (Sullivan et al., 2005).
Cerebellum
Five studies have found negative age-relationships for total cerebellar volume, cerebellar GM, cerebellar WM, or other cerebellar compartments (Jernigan et al., 2001; Liu et al., 2003; Luft et al., 1999; Raz et al., 2001; Sullivan et al., 2000; Walhovd et al., 2005a). In one study no effects on cerebellar WM (Sullivan et al., 2000) were found, in contrast to a more recent study (Walhovd et al., 2005b). One study observed that the age changes were best described by an exponential fit (Luft et al., 1999). Longitudinal findings of age-related decline in cerebellar volume have been more dramatic than cross-sectional, and comparable to the declines in the association cortices and the caudate nucleus (Raz et al., 2005a)
CSF
There is agreement across studies that CSF compartments increase in volume with age (Coffey et al., 1998; Cohen et al., 1992; Good et al., 2001; Greenberg et al., 2008; Gur et al., 1991; Jernigan et al., 2001; Scahill et al., 2003; Sullivan et al., 1995; Walhovd et al., 2005a). Some studies have also found non-linear age changes (Good et al., 2001; Sullivan et al., 1995; Walhovd et al., 2005a).
The differences observed across studies may be related to sample characteristics, segmentation procedures, demarcation criteria, and procedures for intracranial volume (ICV) corrections. Based on the above findings, the following set of hypotheses could be made:
H1: Caudate nucleus, nucleus accumbens, and cerebellar volume will be negatively related to age in all samples, while CSF/ventricular volume will be positively related.
H2: Hippocampus, amygdala, putamen, thalamus volume will generally decline with age, but not consistently across all six samples.
H3: Pallidum and brainstem volume will not be consistently related to age, and age effects will be found only in a minority of the samples.
These hypotheses are strictly based on previous findings, assuming the results of the present multi-sample study would most likely to be representative of previous findings. However, to the extent that standardizing segmentation and analysis techniques has effect, such empirical hypotheses may not be confirmed, and greater consistency may be found.
2. Methods
2.1 Samples
The details of each of the samples are described in Supplementary Table 1 and Table 2, where key publications with in depth inclusion criteria are provided, including description of approvals by the relevant ethical committees. The total n of the samples was 883, with an age range of 75 years (18–93 years). All samples were screened for neurological conditions. It is likely that effects on the volume of the different brain structures largely can be attributed to the influence of normal aging.
Table 2.
Sample characteristics
| Sample | Country | N (%f) |
Age mean (range) |
Education mean (range) |
Key publications | Main screening instruments/inclusion criteria |
|---|---|---|---|---|---|---|
| 1 | Nor | 69 (57) | 51.3 (20–88) | 15 (7–20) | (Walhovd et al., 2005a) | Health interview, MMSE > 26, BDI < 16, IQ > 85, RH only |
| 2 | Nor | 208 (71) | 46.8 (19–75) | 14 (9–22) | (Espeseth et al., 2008) | Health interview, IQ > 85 |
| 3 | Swe | 106 (32) | 41.6 (19–56) | 14 (9–22) | (Jonsson et al., 2006; Nesvag et al., 2008); | Health interview, DSM-III-R, WASI vocabulary > 16a |
| 4a | USA | 155 (65) | 44.5 (18–93) | 3.5 (1–5)c | (Marcus et al., 2007) | Health interview, CDR = 0b, MMSE > 25b, RH only |
| 4b | USA | 154 (61) | 44.4 (18–94) | 3.4 (1–5)c | Similar to Sample 4a | Similar to Sample 4a |
| 5 | USA | 191 (60) | 47.3 (18–81) | 15.7 (12–21) | (Raz et al., 2004a) | Health interview, BIMCT > 30, GDQ < 15, RH only, neuroradiology, |
Nor: Norway
Swe: Sweden
f/m: the ratio of females to males
MMSE: Mini Mental Status Exam (Folstein et al., 1975)
BDI: Beck Depression Inventory (Beck, 1987)
BIMCT: Blessed Information-Memory-Concentration Test (Blessed et al., 1968)
CDR: Clinical Dementia Rating (Berg, 1984, 1988; Morris, 1993)
GDQ: Geriatric Depression Questionnaire (Auer and Reisberg, 1997)
RH: Right handed
WASI: Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
Available for 70 participants
Available for participants ≥ 60 years only
Available for all participants ≥ 60 years, and sporadically for the rest. 1: less than high school grad., 2: high school grad., 3: some college, 4: college grad., 5: beyond college
2.2 MR acquisition
All participants were scanned on 1.5T magnets, but from two different manufacturers (Siemens, Erlangen, Germany; General Electric CO. [GE], Milwaukee, WI), and four different models (Siemens Symphony Quantum, Siemens Sonata, Siemens Vision, GE Signa). With the exception of the data from Samples 4a and 4b, the separate sample data sets are from different scanners. All participants within each sample were scanned on the same scanner. The measurements were conducted on T1 weighted sequences were acquired (3D magnetization prepared gradient-echo for the Siemens scanners, and 3D spoiled gradient recalled pulse sequence for GE). Slice thickness varied between 1.5 mm (Sample 1) and 1.25 mm (Sample 4 and 5), with acquisition matrices of 256×192 (Samples 1, 3, and 5) or 256×256 (Samples 2, 4a, and 4b). In three of the samples (Samples 1, 2, 4 a, and 4b), multiple scans were acquired within the same scanning session, and averaged to increase the signal-to-noise ratio. The details of the sequences used in each are presented in Supplementary Table 2. Examples of the scan quality from each sample are presented in Supplementary Figure 1.
2.3 Volumetric analyses
The automated procedures for volumetric measures of the different brain structures were performed with FreeSurfer version 4.0.1, which can be freely downloaded (http://surfer.nmr.mgh.harvard.edu/). The segmentation for cerebrum from the average brain from sample 2 is shown in Figure 1. The procedure automatically assigns a neuroanatomical label to each voxel in an MRI volume based on probabilistic information automatically estimated from a manually labeled training set (Fischl et al., 2002). The training set included both healthy persons in the age range 18–87 yrs and a group of Alzheimer's disease patients in the age range 60–87 yrs, and the classification technique employs a registration procedure that is robust to anatomical variability, including the ventricular enlargement typically associated with aging. The technique has previously been shown to be comparable in accuracy to manual labeling (Fischl et al., 2002; Fischl et al., 2004). A newly developed atlas-based normalization procedure was used. This has been shown to increase the robustness and accuracy of the segmentations across scanner platforms (Han and Fischl, 2007). It should be mentioned that the cortical volume estimates from the whole-brain segmentation approach in FreeSurfer are probably less accurate than the estimates from the surface-based thickness calculations. Still, the results of the whole-brain procedure were used here because this approach was used for all the other structures analyzed in this paper. Estimated intracranial volume (ICV) was used to correct the volumetric data. This was calculated by use of an atlas-based normalization procedure, where the atlas scaling factor is used as a proxy for ICV, shown to correlate highly with manually derived ICV (r = .93) (Buckner et al., 2004). This procedure has recently been shown to overestimate intracranial volume with increasing atrophy in a longitudinal study of semantic dementia (Pengas et al., 2009). Although this may not be a similar problem with normal aging, ICV values were examined with a special focus on a possible problem of overestimation. No obvious outliers were detected among the ICV estimates, and ICV estimates correlated negatively, rather than positively with age (r = −.07, −.24, .01, −.25, −.20, and −.04 in samples 1, 2, 3, 4a, 4b, and 5, respectively). In sample 2, 4a and 4b, these negative correlations reached significance (p < .05), in keeping with a cohort effect (Haug, 1984), rather than possible overestimations, which could lead to a positive ICV-age correlation.
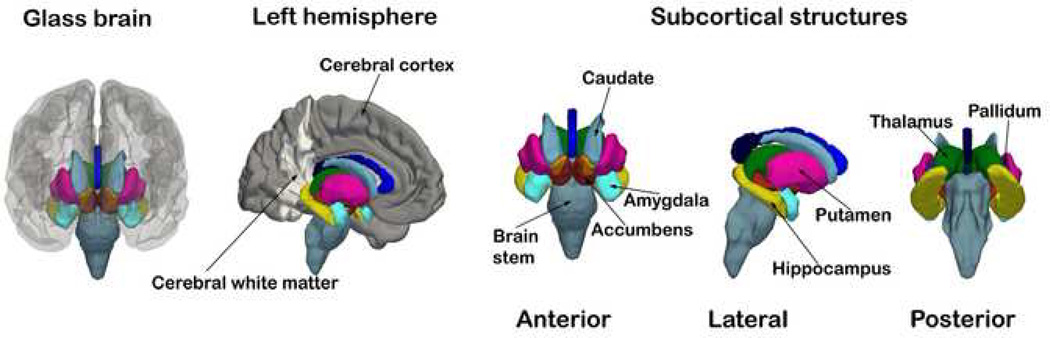
Figure 1. Whole-brain segmentation.
The figure shows the segmentation results from the average brain of sample 2. The three-dimensional renderings illustrate the shape, extension, and relative position within the brain of the different neuroanatomical structures.
2.4 Statistical analyses
Unless stated otherwise, all analyses were done separately for each sample. An ANCOVA with 13 structures × 2 hemispheres, with age as between subjects factor and with sex and sample as covariates, yielded no age × hemisphere interaction (F [75,806] = 0.88, p = .75) and no age × hemisphere × structure interaction (F [206.48,2218.99] = 1.10, p = .16). The sum of left and right hemisphere volume was thus used in the analyses. First, the average raw volumes of the total sample were calculated per decade, and percent change per decade was estimated based on these. Average percent linear change per decade was also calculated based on the ICV-corrected volumes for each sample. Multiple regression analyses with age and age2 as simultaneous predictors of the ICV-corrected volumes (the residuals after each volume was regressed on ICV) were performed to test for linear and non-linear age effects in each sample. These regression analyses were repeated for the total sample after the effects of sample were regressed out. ANOVAs with each neuroanatomical structure in turn as dependent variable was performed to test effects of sample × age interactions (sample as fixed factor).
3. Results
3.1. Relationships with age
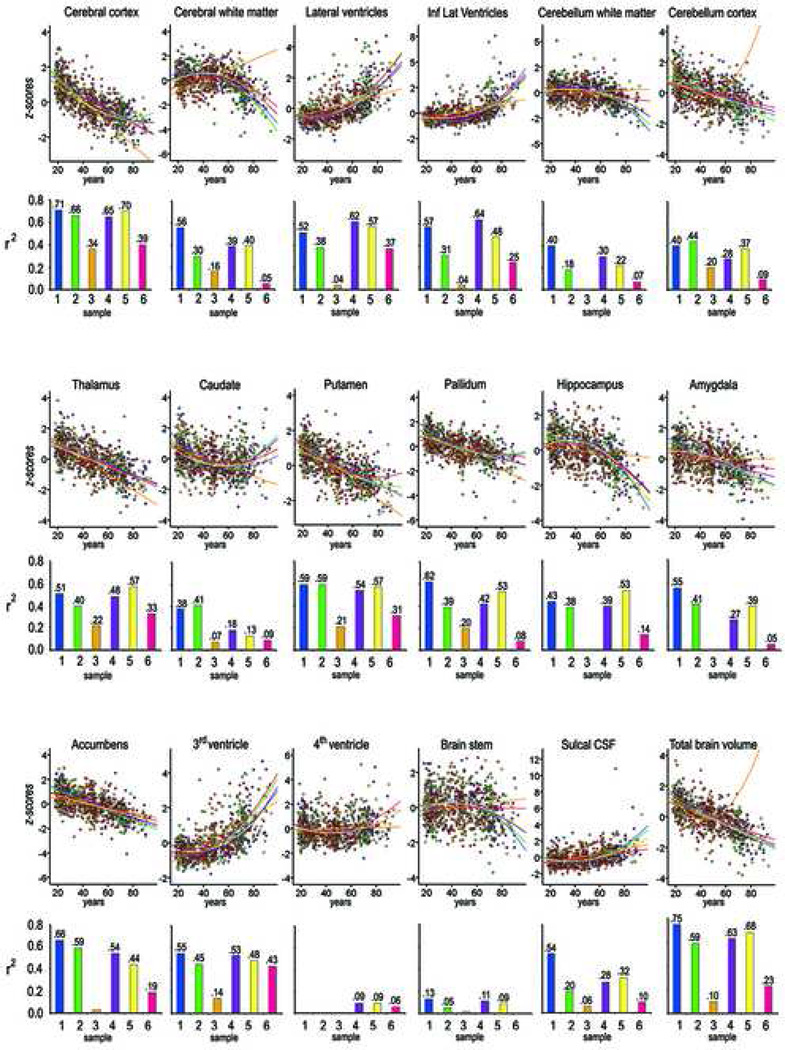
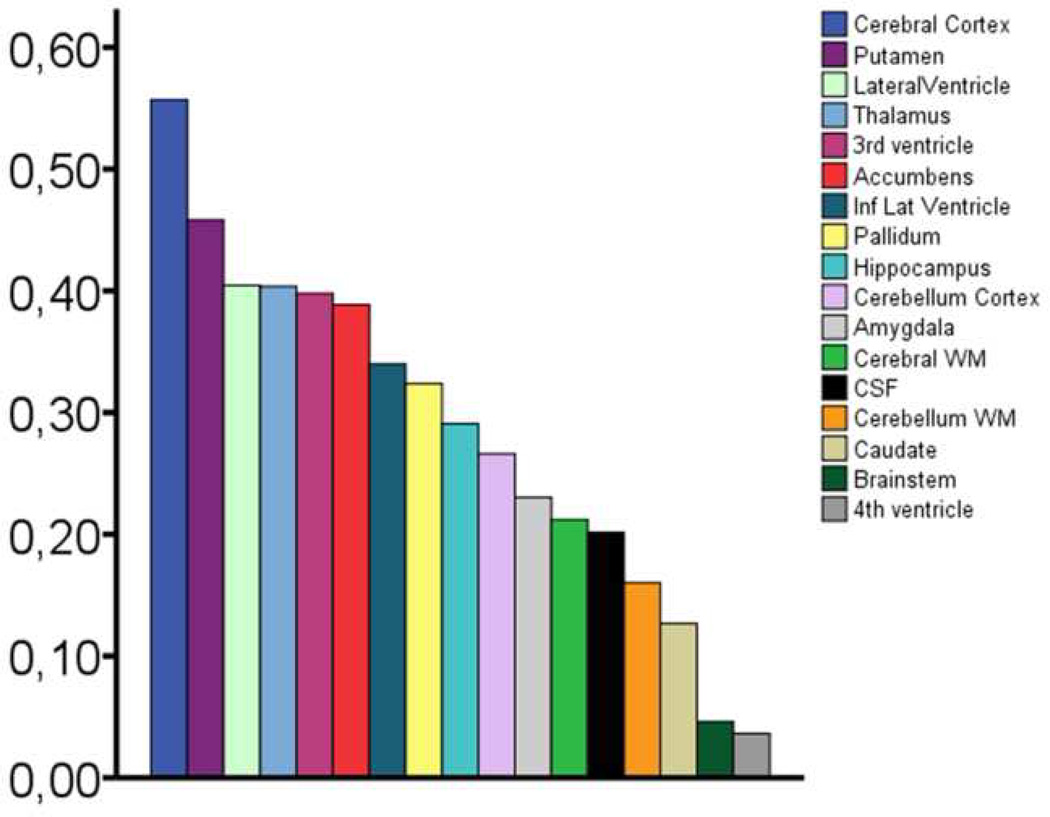
The mean volumes of the different anatomical structures are shown per decade for the total sample in Table 3. Table 4 shows the estimated percentage volumetric change in each structure per decade based on the raw volumes of the total sample. Average percent linear change per decade based on the ICV-corrected volumes for each sample is shown in Supplementary Table 3. An illustration of the age-effects on the morphometry of the three-dimensional segmentations of each structure is given in Supplementary Figure 2. Table 5 shows linear and quadratic effects of age on ICV corrected volume of each of the brain structures for each sample separately and for the total sample. Scatter plots for select structures are shown in Figure 2. All neuroanatomical volumes, with the exception of the 4th ventricle and the brain stem, were robustly related to age in at least five of the six samples, with smaller neuroanatomical structures and greater ventricular/CSF compartments in higher age. For twelve of the ICV-corrected volumes, including total brain volume, significant age relationships were found in all six samples. The strongest relationships were observed for the cerebral cortex, with the amount of variance explained by age ranging from 34 to 71 % for the linear components. There was large overlap of results across samples. Sample 3 stood out as the one in which the weakest age effects were seen. Generally, the quadratic term significantly increased the amount of explained variance. Cerebral WM, lateral ventricles, inferior lateral ventricles, 3rd ventricle, caudate, hippocampus, and total volume showed a non-linear pattern in five samples. The accumbens area, thalamus, and fourth ventricle did not show a non-linear component in any of the samples, whereas amygdala and cerebellar cortex showed a nonlinear component in one sample only. The rest of the structures displayed a mix of linear and non-linear effects across samples. Figure 4 shows the strength of the age-relationships across groups, sorted by the median explained variance, where both the linear and non-linear (where significant) contributions to the amount of explained variance are included.
Table 3.
Mean volume of the different neuroanatomical structures per decade
| Total sample (N = 883) | ||||||||||||||
| 18–29 years N = 262 |
30–39 years N = 109 |
40–49 years N = 159 |
50–59 years N = 100 |
60–69 years N = 110 |
70–79 years N = 105 |
80–95 years N = 38 |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Cerebral Cor | 517426 | 66685 | 489079 | 69076 | 484994 | 73159 | 446856 | 60459 | 419190 | 68791 | 393507 | 58007 | 389445 | 39685 |
| Cerebral WM | 448369 | 55455 | 465638 | 59881 | 473373 | 70145 | 451591 | 57500 | 427932 | 59294 | 393931 | 53765 | 360263 | 53355 |
| Lat Vent | 12659 | 6902 | 15046 | 9093 | 16152 | 9943 | 17472 | 9330 | 24566 | 12949 | 34205 | 17344 | 41336 | 17985 |
| Inf Lat Vent | 651 | 363 | 724 | 434 | 705 | 432 | 712 | 401 | 1045 | 671 | 1742 | 1130 | 2499 | 1191 |
| Cerebel WM | 28320 | 3506 | 28543 | 3444 | 28410 | 3747 | 27360 | 3587 | 25787 | 3544 | 24452 | 3198 | 22862 | 4006 |
| Cerebel Cor | 109909 | 13173 | 108925 | 12313 | 107773 | 13778 | 101211 | 13572 | 97125 | 13010 | 90332 | 13464 | 90595 | 9440 |
| Thalamus | 14002 | 1518 | 14037 | 1431 | 13624 | 1600 | 12749 | 1449 | 12241 | 1513 | 11510 | 1358 | 10931 | 1182 |
| Caudate | 7848 | 981 | 7319 | 887 | 7139 | 901 | 6939 | 850 | 6853 | 1011 | 6975 | 972 | 7285 | 1269 |
| Putamen | 12507 | 1400 | 11312 | 1360 | 10707 | 1136 | 10206 | 1127 | 9640 | 1034 | 9520 | 1158 | 9035 | 1209 |
| Pallidum | 3638 | 452 | 3395 | 481 | 3236 | 394 | 3051 | 435 | 2981 | 485 | 2889 | 336 | 2646 | 466 |
| Hippocampus | 8214 | 889 | 8319 | 941 | 8368 | 1044 | 8101 | 1027 | 7467 | 1106 | 6865 | 979 | 6201 | 730 |
| Amygdala | 3540 | 459 | 3442 | 467 | 3400 | 495 | 3216 | 477 | 3025 | 524 | 2766 | 440 | 2679 | 507 |
| Accumbens | 1492 | 266 | 1263 | 244 | 1175 | 199 | 1142 | 229 | 1060 | 178 | 1013 | 181 | 1038 | 180 |
| 3rd Vent | 1032 | 286 | 992 | 361 | 1033 | 356 | 1173 | 406 | 1419 | 526 | 1813 | 574 | 1927 | 708 |
| 4th Vent | 1979 | 576 | 1945 | 640 | 1801 | 514 | 1847 | 491 | 2016 | 666 | 2126 | 664 | 2077 | 665 |
| Brain Stem | 21456 | 2385 | 22186 | 2413 | 22206 | 2696 | 21444 | 2594 | 21277 | 2732 | 20179 | 2565 | 19086 | 2455 |
| CSF | 1195 | 241 | 1241 | 338 | 1273 | 269 | 1245 | 281 | 1412 | 330 | 1542 | 654 | 1606 | 496 |
| Total volume | 1176723 | 125178 | 1163458 | 130877 | 1164404 | 151413 | 1093865 | 126705 | 1034578 | 135408 | 963939 | 120410 | 922066 | 98074 |
| ICV | 1586302 | 161092 | 1615561 | 173216 | 1610050 | 185966 | 1552345 | 157821 | 1534473 | 159132 | 1538938 | 175808 | 1488398 | 171561 |
| Females (N = 528) | ||||||||||||||
| 18–29 years N = 154 |
30–39 years N =58 |
40–49 years N = 83 |
50–59 years N = 64 |
60–69 years N = 72 |
70–79 years N = 67 |
80–95 years N = 30 |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Cerebral Cor | 490631 | 57077 | 460475 | 53466 | 453442 | 57940 | 425692 | 52015 | 398916 | 59567 | 378820 | 49812 | 389744 | 38415 |
| Cerebral WM | 427040 | 46766 | 439966 | 43353 | 439631 | 52271 | 424193 | 40860 | 406040 | 47102 | 378277 | 50723 | 357302 | 46707 |
| Lat Vent | 11262 | 4941 | 13202 | 6490 | 15018 | 10420 | 14436 | 6368 | 23399 | 12715 | 30458 | 16178 | 38390 | 16059 |
| Inf Lat Vent | 639 | 307 | 651 | 364 | 682 | 472 | 625 | 379 | 972 | 667 | 1578 | 1066 | 2154 | 967 |
| Cerebel WM | 27375 | 2757 | 27404 | 3238 | 27310 | 3104 | 26208 | 3219 | 25006 | 2956 | 24148 | 3370 | 22853 | 3588 |
| Cerebel Cor | 104270 | 9742 | 103237 | 9566 | 101066 | 10112 | 95664 | 10922 | 93178 | 11080 | 87705 | 12021 | 89267 | 7308 |
| Thalamus | 13367 | 1257 | 13468 | 1140 | 13018 | 1411 | 12282 | 1224 | 11643 | 1136 | 11190 | 1388 | 10805 | 822 |
| Caudate | 7576 | 883 | 7072 | 709 | 6931 | 851 | 6649 | 630 | 6597 | 904 | 6830 | 863 | 7288 | 1072 |
| Putamen | 12060 | 1278 | 10832 | 1261 | 10385 | 1033 | 9882 | 1003 | 9382 | 903 | 9093 | 759 | 9134 | 1148 |
| Pallidum | 3477 | 418 | 3216 | 452 | 3075 | 381 | 2916 | 304 | 2857 | 496 | 2807 | 298 | 2624 | 398 |
| Hippocampus | 7889 | 737 | 8075 | 904 | 7954 | 870 | 7896 | 994 | 7182 | 837 | 6726 | 890 | 6166 | 688 |
| Amygdala | 3396 | 439 | 3241 | 365 | 3169 | 395 | 3039 | 379 | 2862 | 445 | 2655 | 358 | 2598 | 357 |
| Accumbens | 1461 | 246 | 1202 | 237 | 1154 | 206 | 1108 | 215 | 1030 | 186 | 1005 | 180 | 1035 | 154 |
| 3rd Vent | 978 | 231 | 912 | 293 | 978 | 343 | 1061 | 300 | 1327 | 485 | 1646 | 534 | 1708 | 482 |
| 4th Vent | 1889 | 499 | 1834 | 567 | 1706 | 500 | 1819 | 475 | 1959 | 710 | 2051 | 687 | 2033 | 704 |
| Brain Stem | 20518 | 2021 | 21265 | 2092 | 21205 | 2238 | 20505 | 2165 | 20353 | 2201 | 19394 | 2095 | 18910 | 2166 |
| CSF | 1136 | 226 | 1184 | 351 | 1186 | 255 | 1132 | 201 | 1365 | 334 | 1451 | 761 | 1480 | 369 |
| Total volume | 1119061 | 102916 | 1099454 | 87918 | 1088340 | 107907 | 1036033 | 96939 | 985044 | 108815 | 928650 | 104816 | 917726 | 85065 |
| ICV | 1510744 | 125832 | 1533802 | 131432 | 1531369 | 157510 | 1471598 | 101797 | 1465030 | 124339 | 1468898 | 140686 | 1451682 | 130944 |
| Males (N = 355) | ||||||||||||||
| 18–29 years N = 154 |
30–39 years N =58 |
40–49 years N = 83 |
50–59 years N = 64 |
60–69 years N = 72 |
70–79 years N = 67 |
80–95 years N = 30 |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Cerebral Cor | 555635 | 60678 | 521609 | 70846 | 519452 | 72801 | 484482 | 56517 | 457605 | 69402 | 419400 | 62870 | 388322 | 47000 |
| Cerebral WM | 478784 | 52778 | 494832 | 62998 | 510222 | 68864 | 500298 | 50339 | 469412 | 58311 | 421531 | 48060 | 371369 | 76358 |
| Lat Vent | 14650 | 8630 | 17144 | 11052 | 17389 | 9305 | 22870 | 11242 | 26776 | 13270 | 40812 | 17563 | 52386 | 21551 |
| Inf Lat Vent | 670 | 431 | 807 | 493 | 729 | 386 | 868 | 396 | 1184 | 666 | 2031 | 1196 | 3791 | 1097 |
| Cerebel WM | 29669 | 3999 | 29838 | 3233 | 29612 | 4030 | 29409 | 3316 | 27268 | 4098 | 24988 | 2833 | 22897 | 5611 |
| Cerebel Cor | 117948 | 13288 | 115394 | 11957 | 115097 | 13564 | 111071 | 12250 | 104604 | 13240 | 94963 | 14741 | 95572 | 14625 |
| Thalamus | 14909 | 1397 | 14684 | 1462 | 14285 | 1538 | 13580 | 1460 | 13374 | 1503 | 12074 | 1111 | 11403 | 2064 |
| Caudate | 8235 | 989 | 7599 | 988 | 7366 | 904 | 7455 | 950 | 7338 | 1036 | 7231 | 1105 | 7272 | 1938 |
| Putamen | 13145 | 1324 | 11858 | 1270 | 11059 | 1145 | 10781 | 1118 | 10129 | 1100 | 10273 | 1351 | 8664 | 1439 |
| Pallidum | 3868 | 396 | 3599 | 433 | 3411 | 330 | 3291 | 526 | 3216 | 365 | 3034 | 355 | 2727 | 693 |
| Hippocampus | 8677 | 884 | 8597 | 913 | 8821 | 1035 | 8466 | 996 | 8006 | 1343 | 7111 | 1089 | 6331 | 908 |
| Amygdala | 3744 | 407 | 3672 | 468 | 3652 | 471 | 3530 | 476 | 3336 | 526 | 2962 | 503 | 2985 | 835 |
| Accumbens | 1536 | 288 | 1333 | 235 | 1197 | 191 | 1202 | 244 | 1118 | 146 | 1027 | 184 | 1050 | 269 |
| 3rd Vent | 1108 | 337 | 1083 | 409 | 1092 | 362 | 1372 | 492 | 1593 | 562 | 2107 | 527 | 2749 | 843 |
| 4th Vent | 2108 | 651 | 2070 | 698 | 1904 | 512 | 1895 | 523 | 2124 | 569 | 2257 | 608 | 2244 | 493 |
| Brain Stem | 22794 | 2229 | 23233 | 2343 | 23299 | 2741 | 23113 | 2473 | 23029 | 2808 | 21564 | 2752 | 19747 | 3433 |
| CSF | 1279 | 239 | 1307 | 312 | 1367 | 252 | 1445 | 295 | 1503 | 305 | 1703 | 356 | 2079 | 643 |
| Total volume | 1258945 | 107088 | 1236248 | 134276 | 1247473 | 148899 | 1196677 | 107274 | 1128433 | 132181 | 1026159 | 122167 | 938339 | 143108 |
| ICV | 1694042 | 143966 | 1708541 | 168946 | 1695978 | 177193 | 1695896 | 136681 | 1666049 | 133301 | 1662430 | 164399 | 1626083 | 238950 |
Units are number of voxels (1 mm3).
Cor: Cortex
WM: White Matter
Lat: Lateral
Inf: Inferior
Vent: Ventricles
CSF: Cerebrospinal fluid in sulci
Total volume: The sum of all the other structures (CSF and ventricles not included)
Table 4.
Percentage change per decade for the total sample based on raw volumes.
| 18–29 to 30–39 |
30–39 to 40–49 |
40–49 to 50–59 |
50–59 to 60–69 |
60–69 to 70–79 |
70–79 to 80–95 |
18–29 to 80–95 |
|
|---|---|---|---|---|---|---|---|
| Cerebral Cor | −5.5 | −0.8 | −7.9 | −6.2 | −6.1 | −1.0 | −24.7 |
| Cerebral WM | 3.9 | 1.7 | −4.6 | −5.2 | −7.9 | −8.5 | −19.7 |
| Lat Vent | 18.9 | 7.4 | 8.2 | 40.6 | 39.2 | 20.8 | 226.5 |
| Inf Lat Vent | 11.2 | −2.6 | 1.0 | 46.8 | 66.7 | 43.5 | 283.9 |
| Cerebel WM | 0.8 | −0.5 | −3.7 | −5.7 | −5.2 | −6.5 | −19.3 |
| Cerebel Cor | −0.9 | −1.1 | −6.1 | −4.0 | −7.0 | 0.3 | −17.6 |
| Thalamus | 0.2 | −2.9 | −6.4 | −4.0 | −6.0 | −5.0 | −21.9 |
| Caudate | −6.7 | −2.5 | −2.8 | −1.2 | 1.8 | 4.4 | −7.2 |
| Putamen | −9.6 | −5.3 | −4.7 | −5.5 | −1.2 | −5.1 | −27.8 |
| Pallidum | −6.7 | −4.7 | −5.7 | −2.3 | −3.1 | −8.4 | −27.3 |
| Hippocampus | 1.3 | 0.6 | −3.2 | −7.8 | −8.1 | −9.7 | −24.5 |
| Amygdala | −2.8 | −1.2 | −5.4 | −5.9 | −8.6 | −3.1 | −24.3 |
| Accumbens | −15.3 | −7.0 | −2.8 | −7.2 | −4.4 | 2.5 | −30.4 |
| 3rd Vent | −3.9 | 4.1 | 13.6 | 21.0 | 27.8 | 6.3 | 86.7 |
| 4th Vent | −1.7 | −7.4 | 2.6 | 9.1 | 5.5 | −2.3 | 5.0 |
| Brain Stem | 3.4 | 0.1 | −3.4 | −0.8 | −5.2 | −5.4 | −11.0 |
| CSF | 3.8 | 2.6 | −2.2 | 13.4 | 9.2 | 4.2 | 34.4 |
| Total volume | −1.1 | 0.1 | −6.1 | −5.4 | −6.8 | −4.3 | −21.6 |
Cor: Cortex
WM: White Matter
Lat: Lateral
Inf: Inferior
Vent: Ventricles
CSF: Cerebrospinal fluid in sulci
Total volume: The sum of all the other structures (CSF and ventricles not included)
Table 5.
Effects of age on structures corrected for intracranial volume. The age relationships were all negative, with the exception of ventricular and CSF volumes, for which positive relationships were observed. For the total sample, the effects of sample were regressed out.
| Sample 1 |
Sample 2 |
Sample 3 |
Sample 4a |
Sample 4b |
Sample 5 |
Total Sample |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| age | age2 | age | age2 | age | age2 | age | age2 | age | age2 | age | age2 | age | age2 | |
| R2 | R2 | R2 | R2 | R2 | R2 | R2 | R2 | R2 | R2 | R2 | R2 | R2 | R2 | |
| Cerebral Cor | .71 | .72 | .63 | .66 | .34 | .36 | .63 | .65 | .68 | .70 | .39 | .40 | .54 | .56 |
| Cerebral WM | .48 | .56 | .21 | .30 | .16 | .17 | .27 | .39 | .20 | .40 | .00 | .05 | .12 | .21 |
| Lat Vent | .49 | .52 | .33 | .38 | .04 | .04 | .52 | .62 | .52 | .57 | .34 | .37 | .37 | .41 |
| Inf Lat Vent | .37 | .57 | .27 | .31 | .04 | .04 | .41 | .64 | .39 | .48 | .18 | .25 | .27 | .34 |
| Cerebel WM | .24 | .40 | .13 | .18 | .00 | .00 | .22 | .30 | .18 | .22 | .07 | .07 | .13 | .16 |
| Cerebel Cor | .40 | .41 | .44 | .44 | .18 | .20 | .28 | .28 | .37 | .37 | .09 | .10 | .27 | .27 |
| Thalamus | .51 | .51 | .40 | .41 | .22 | .25 | .48 | .48 | .57 | .57 | .33 | .33 | .40 | .40 |
| Caudate | .33 | .38 | .11 | .41 | .07 | .09 | .03 | .18 | .06 | .13 | .07 | .09 | .08 | .13 |
| Putamen | .59 | .60 | .58 | .59 | .21 | .21 | .54 | .55 | .57 | .57 | .29 | .31 | .45 | .46 |
| Pallidum | .59 | .62 | .38 | .39 | .20 | .21 | .42 | .42 | .53 | .54 | .08 | .08 | .32 | .32 |
| Hippocampus | .33 | .43 | .37 | .38 | .00 | .04 | .33 | .39 | .40 | .53 | .12 | .14 | .26 | .29 |
| Amygdala | .55 | .55 | .41 | .41 | .00 | .00 | .27 | .27 | .36 | .39 | .05 | .05 | .23 | .23 |
| Accumbens | .66 | .66 | .59 | .59 | .03 | .04 | .54 | .55 | .44 | .44 | .19 | .19 | .39 | .39 |
| 3rd Vent | .51 | .55 | .40 | .45 | .14 | .15 | .37 | .53 | .41 | .48 | .38 | .43 | .36 | .40 |
| 4th Vent | .00 | .01 | .00 | .02 | .00 | .01 | .01 | .09 | .03 | .09 | .02 | .06 | .01 | .04 |
| Brain Stem | .04 | .13 | .02 | .05 | .01 | .02 | .08 | .11 | .06 | .09 | .00 | .00 | .03 | .05 |
| CSF | .40 | .54 | .15 | .20 | .06 | .08 | .20 | .28 | .24 | .32 | .10 | .11 | .16 | .20 |
| Total volume | .75 | .75 | .59 | .59 | .06 | .10 | .63 | .63 | .68 | .69 | .23 | .23 | .47 | .47 |
Cor: Cortex
WM: White Matter
Lat: Lateral
Inf: Inferior
Vent: Ventricles
CSF: Cerebrospinal fluid in sulci
Total volume: The sum of all the other structures (CSF and ventricles not included)
ICV: Intra Cranial Volume
The numbers in the age2 columns indicate amount of explained variance for the model consisting of age+ age2. They are printed in bold/italic if adding a quadratic age term significantly (p < .01/.05) increased the amount of explained variance (R2), not whether the total expression is significant.
Bold: p < .01
Italic: p < .05
Figure 2. Scatter plots.
The scatter plots depict the individual data points in the relationship between age and the volume of each of the examined brain structures in each of the samples (color coded). All volumes were corrected for intracranial volume, and the standardized residual values are shown on the y-axis (z scores). Regression lines for each sample are shown. If a nonlinear (quadratic) component significantly increased the amount of explained variance, this curve is shown instead of the linear one. That does not mean that the exact quadratic fit shown depicts the true age function, and these fits should not be used to interpret the exact timing of peaks and dips in the age functions. For purpose of comparison, the age fits for each sample is calculated for the same, total age range (18−94) across samples. However, the actual age range differs across samples, and no age function should be interpreted beyond the actual age range of the sample in question. In particular, sample 3 has a relatively narrow age range extending only to 56 years of age, and the age fits should not be interpreted beyond this age. Above each scatter plot a three-dimensional rendering of the relevant Freesurfer-segmentation from the average brain from Sample 2 is shown. Below each scatter plot is a bar chart showing the amount of variance in brain structure explained by age in each sample. If the quadratic component significantly contributed, the R2 corresponds to the total contribution from the linear and non-linear components. If the quadratic component did not contribute significantly, the R2 corresponds to the contribution from the linear component only. If p ≤ .05, the coefficients are given above each bar
Figure 4. Amount of age-explained variance for each structure in the total sample.
The bars show the percentage volumetric variance explained by age in the total sample for each of the neuroanatomical structures. The effect of sample was regressed out.
3.2 Effects of sample
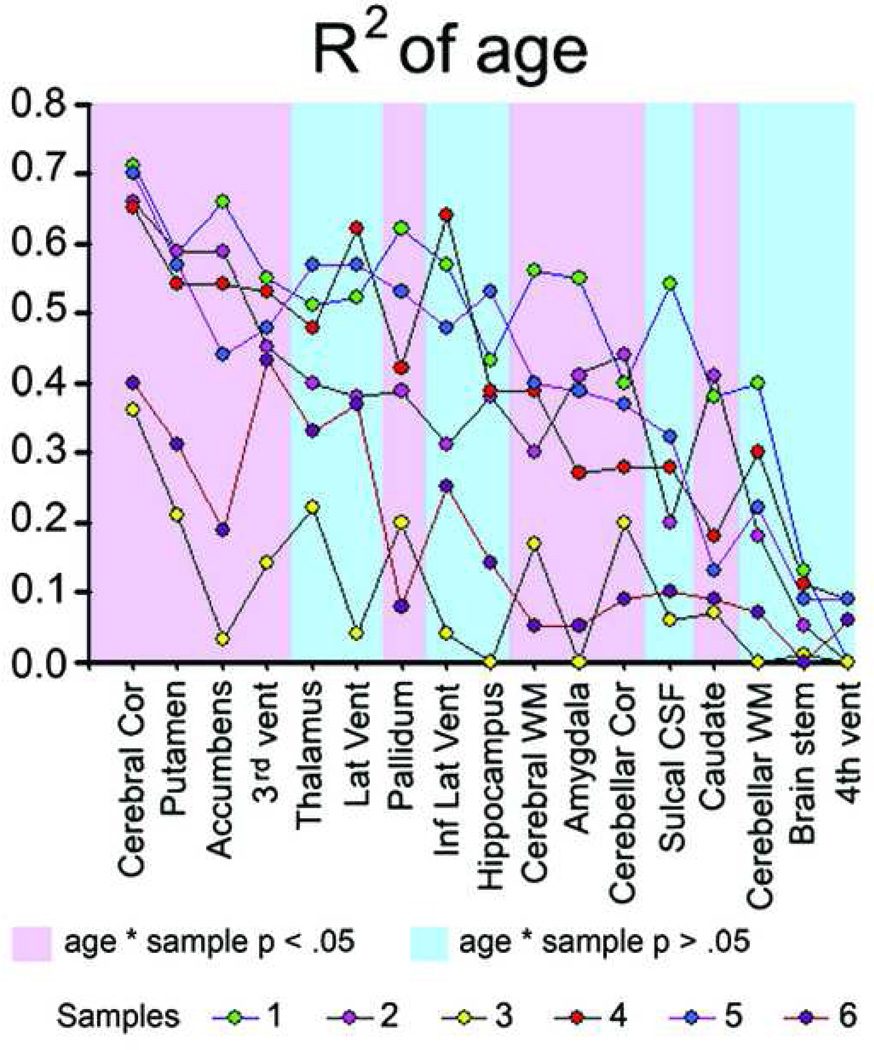
To test whether sample influenced the strength of the relationship between neuroanatomical volume and age, ANOVAs were conducted with each brain structure in turn as dependent variable, sample as fixed factor, and age as covariate (df = 5, error = 871 for all analyses). Significant interactions between age and sample were found for the cerebral cortex (F = 4.30, p < .001), cerebral WM (F = 14.09, p < 10−12), cerebellar cortex (F = 3.56, p < .01), caudate (F = 2.88, p < .05), putamen (F = 2.34, p < .05), pallidum (F = 4.18, p < .001), amygdala (f = 6.02, p < .0001), accumbens (F = 3.73, p < .005), third ventricle (F = 2.41, p < .05), and total brain volume (F = 3.60, p < .005), whereas a trend was found for hippocampus (F = 1.95, p = .084). In contrast, no sample×volume interactions were observed for the lateral ventricles (F = 0.65), inferior lateral ventricles (F = 0.11), cerebellum WM (F = 1.42), thalamus (F = 1.60), fourth ventricle (F = 0.30), brain stem (F = 1.41), and sulcal CSF (F = 0.89).
3.3 Age effects in the total sample
Regression analyses with age and age2 on residuals with the effects of sample regressed out generally confirmed the age patterns observed in the subsamples. However, largely due to increase in statistical power due to increase in the sample size (n = 883), all structures now showed significant age effects, and a quadratic age component was significant for a few additional structures, a total of 13. Reduction in the magnitude of age differences at older age suggesting age-related deceleration was observed for the cerebral cortex, caudate, putamen, pallidum, the lateral and inferior lateral ventricles, and the 3rd and 4th ventricle. On the other hand, increase in age-related differences suggesting acceleration of age-effects on volume in the latter part of the lifespan was observed for cerebral WM, cerebellar WM, hippocampus and the brainstem. The R2 for the different volumes and age in the total sample are shown in Figure 4.
4. Discussion
For most neuroanatomical volumes, age effects were observed across samples. Of the 18 neuroanatomical volumes tested, incuding total brain volume, 12 showed age effects in all six samples, while four showed effects in five of the samples. Only the 4th ventricle (related in three samples) and the brain stem (related in four of the samples) were not related to age in a consistent fashion. The first hypothesis based on previous reports was that caudate nucleus, nucleus accumbens, and cerebellum compartments would be negatively related to age in all samples, while CSF compartments would be positively related. This was mainly confirmed, in that significant age relationships were found in five of the samples for accumbens and effects on the caudate, cerebellar cortex, cerebellar WM and all CSF-measures except the 4th ventricle were found in all six samples. The second empirically based hypothesis was that hippocampus, amygdala, putamen and thalamic volume would be generally, but less consistently related to age. This was not confirmed; putamen and thalamic volume were related to age in all six samples, while the two other volumes showed age effects in five of six. Finally, we predicted that pallidum and brainstem volume would not be related to age, or related only in a minority of the samples. Pallidum volume was related to age in all samples, while volume of the brain stem showed age effects in only three. Thus, the various structures showed age effects in a more stable manner across samples than what would be expected from previous literature. In addition, all structures were significantly affected by age in the total sample analyses, indicating that when statistical power is sufficiently high, age effects are observed throughout the human brain. The present data may be useful as a reference for other researchers if they would like to see e.g. how their control group at a given age may compare to a larger sample of controls. However, we caution against using these data as a normative reference for clinical use, since this must be further validated.
The present results indicate that age affects brain structures globally, but with substantial differences in the amount of variance explained by age. Of the specific structures, the cerebral cortex showed the greatest amount of variance explained by age in all samples. Cerebral WM showed relative preservation with age, which fits with previous reports of WM increase until middle age (Allen et al., 2005; Bartzokis et al., 2003; Walhovd et al., 2005a). However, after middle age, WM volume appeared to show an accelerating decrease in volume. Hippocampus is a structure vulnerable to many cerebral insults and known to be affected at the early stages of AD. A multi-component model of brain aging (Buckner, 2004; Head et al., 2005; Raz, 2000) proposes that whereas the medial temporal lobes are affected by AD, a separate process with an anterior-to-posterior gradient affects normal aging and may underlie the executive problems often observed in late adulthood. Striatum changes have been implicated in reduced executive function in healthy aging (Rubin, 1999). The present analyses showed that hippocampal volume decreased as a function of age in five of the six samples, with a median explained variance of 38%. This result is consistent with reported longitudinal findings (Raz et al., 2005a), and indicates that hippocampus is far from spared in normal aging. However, hippocampus was not especially vulnerable to the effects of age either.
The striatal structures showed a strikingly heterogeneous aging pattern, in that pallidum and putamen volume was relatively severely affected by age (explained variance in the total sample of 46 and 32%, respectvely), while caudate seemed to be among the best preserved structures with an age-explained variance of 13%, and only 7.2 % shrinkage from the twenties to eighties/nineties, even though age-effects were identified in all striatal structures in all samples. Taken together, striatum and hippocampus seem to age at about the same speed, but there appears to be considerable heterogeneity in the aging of the striatal structures. In addition to the cerebral cortex, pallidum and accumbens volume showed relatively large age-effects. The accumbens was the structure for which the largest estimated percentage age-difference was observed (an estimated loss of 30.4% from the twenties to the eighties/nienties), and pallidum also showed large volumetric age-effects (about 5 % per decade). Thus, cross-sectional findings across the samples suggest that basal ganglia have significant vulnerability to aging. This view is consistent with longitudinal findings, although the latter suggest greater shrinkage of the caudate nucleus than the rest of the striatum (Raz et al., 2005a; Raz et al., 2005b; Raz et al., 2003).
Quadratic relationships were found for several structures consistently across samples, confirming previous reports (Allen et al., 2005; Jernigan and Gamst, 2005; Walhovd et al., 2005a). Cerebral WM, lateral ventricles, inferior lateral ventricles, 3rd ventricle, caudate, hippocampus, and total volume showed a non-linear pattern of age-related differences in five samples, while the volumes of accumbens, thalamus, and the fourth ventricle were linearly related to age. These results are interesting for several reasons. An implication is that even though age-related differences in brain morphometry seem to be global, they are heterogeneous. Different parts of the brain not only age at different rates, but also in qualitatively different ways. Some structures, such as the hippocampus and cerebral WM, showed initial increase in volume, before accelerated volume loss set in. Other structures, like the caudate, showed initial volumetric decrease followed by less prominent loss. These trends were found across five or six samples. The curvilinear nature of age relationships was also confirmed in the total sample analysis, where the majority of structures showed significant quadratic components. Diminishing effects of age in higher age ranges were observed for the cerebral cortex, caudate, putamen, and pallidum, along with a flatter rate of expanding CSF compartments (lateral, inferior lateral, 3rd and 4th ventricles). An acceleration of effects of age on neuroanatomical volumes in the latter part of the lifespan, on the other hand, was observed for cerebral WM, cerebellar WM, hippocampus and the brainstem. The significant curvilinear relationship for the cerebral cortex appeared to be due to a greater initial volume loss in the twenties rather than a true flattening late in life. However, the additional variance in cortical volume explained by the quadratic component in the total sample was on the order of 2 % only. The non-linear patterns also indicate that age-effects on brain structures should be studied as continuous processes, and only a part of the story is captured if distinct age ranges are compared. The effects of age on brain morphometry are continuous, but they are not uniform throughout the adult life-span.
The age-related differences found in the present study tended to be stronger and more consistent than most often previously reported. This is especially notable since the data were obtained from five independent projects, with five different scanners, in three different countries, thus presupposing several possible sources of variability. It is likely that the use of identical segmentation procedures for all the scans greatly reduced the inconsistency. When formally tested, sample exerted an influence on the relationship between volume and age for several structures. However, sample effects were strongest for the structures most affected by age. Hence, sample differences did generally not determine whether an effect was present, but modulated the strength of the effect. This indicates that much of the variability in previous research may be accounted for by differences in segmentation approaches and definition of ROIs. The present study used an automated segmentation technique, which has previously been shown to be comparable in accuracy to manual methods (Fischl et al., 2002; Fischl et al., 2004). The correlation between hippocampal volume and age obtained in a manual morphometry study (almost identical to sample 6) (Raz et al., 2004a) was −.42, compared to −.35 in the present study, indicating that the automated segmentation approach used did not overestimate age effects. The data from samples 3 and 6 were based on a single T1 scan, while the data from the other samples were based on multiple runs optimized for automated segmentation techniques. The larger estimated hippocampus volume in samples 3 and 6 may indicate slight problems with automatic labeling of this region in these samples. As can be seen in Figure 1, the gray/white contrast in the acquisitions from samples 1, 2, and 4/5 is different than the contrast from samples 3 and 6. This may have contributed to the lower correlations in these samples. As the atlas used for segmentation has been built from data acquired on a Siemens platform, segmentation accuracy is probably higher with Siemens scanners (sample 1,2,4a,b) than GE scanners (sample 3,5) (Han and Fischl, 2007). Still, a newly developed atlas normalization procedure was used, which has been shown to increase the robustness and accuracy of the segmentations also on data from GE scanners (Han and Fischl, 2007). Also, all segmentations were visually inspected for accuracy, ensuring no obvious segmentation errors were included.
The observed differences across samples are likely also in part due to true differences across populations, which can theoretically be due to sampling methods and criteria as well as societal differences. It is important in this regard not to conflate effect sizes such as R2 with the degree of estimated volume loss observed. For instance, sample 1, drawn from a Norwegian study, tended to show the strongest age relationships, yet not the highest percentage volume change per decade. This means that age is a strong predictor of neuroanatomical variance in the population, but not necessarily that the absolute magnitude of the age declines are great. This may for instance happen if the population is homogeneous with respect to other characteristics than age. Norway and Sweden tend to be characterized by more homogenous public health care and education than the US. One might speculate that this type of homogeneity could simultaneously make biological variables such as age a stronger predictor. However, while age was a strong predictor in Norwegian samples 1 and 2, it was a less strong predictor in Swedish sample 3. Further, volunteer participants in these kinds of studies tend to have higher education and better health than the average population, which may diminish national differences in access to health care and education. Hence, scan parameters appear a more likely factor of influence in this regard than sample characteristics.
Generally, Sample 3 showed the weakest age-relationships. This could be related to the analysis of one instead of to scans per participant, a smaller age range with an upper age limit of 56 years, or to the fact that a lower percentage of the participants were females. Some studies have found evidence for steeper age functions for males than females (Chung et al., 2006; Coffey et al., 1998; Cowell et al., 1994; Good et al., 2001; Gur et al., 2002; Murphy et al., 1996; Nunnemann et al., 2007; Pruessner et al., 2001; Raz et al., 2004a; Resnick et al., 2000; Riello et al., 2005; Sowell et al., 2007; Xu et al., 2000), though this is a controversial issue (Greenberg et al., 2008; Lemaitre et al., 2005; Resnick et al., 2003; Salat et al., 2004; Sowell et al., 2007). However, this does not seem to be the case for the present samples (Fjell et al., In press). Thus, it is hard to pinpoint the exact reason for the somewhat weaker age-effects for sample 3.
Importantly, however, the results were largely replicable across the different samples. An implication of the findings is, in line with a recent reliability study (Jovicich et al., 2009), that multi-site studies can obtain a high degree of consistency and sensitivity, in this case, to age effects. The amount of explained variance in the total sample was generally somewhat lower than the amount of variance explained in single samples. Indeed it would be surprising if this was not the case when pooling studies where no attempts have been made to standardize imaging parameters. However, all age relationships were significant in the total sample due to the increased power, so the benefits of increasing samples by including additional sites may not be substantially contradicted by the noise introduced. This will likely apply especially if efforts are made to standardize scanning parameters. The detection of age effects throughout the brain in the present study, in areas where age effects have generally not been detected, such as the brainstem, is first and foremost dependent on the large sample size. For instance, age significantly accounted for 1 % of the variance of 4th ventricle volume, corresponding to a correlation of .10. The relatively large effect sizes for some of the structures where age effects have most often previously been found, e.g. the putamen, may depend on the consistent application of a robust segmentation technique (Fischl et al., 2002; Jovicich et al., 2009). Automated methods may have some undesirable features, in that without proper quality check they may potentially allow erroneous segmentation, especially if gross anatomical anomalies that violate the assumptions inherent in the atlas used are present. However, automated methods also have several advantages over manual methods. They require minimal intervention by highly trained personnel, allow processing of many brains in a reasonable time frame and are characterized by high reliability and repeatability of measures (Fischl et al., 2002). It would be practically impossible to undertake the present study with manual segmentation, as it would require years of work.
In conclusion, the present cross-sectional study shows that age affects brain volumes globally, but the various structures are influenced in both quantitatively and qualitatively different ways.
Supplementary Material
The figure shows representative examples of image quality from the different samples. The first row is Sample 1, the second row Sample 2 etc. (Samples 4a and b are from the same scanner). F denotes female, m denotes male, and the number denotes age. The two columns to the left show T1-weighted scans from a young and an elderly participant for each sample, while the two right columns show the result of the segmentation procedure. F: Female, M: Male.
The figure displays the three-dimensional renderings of selected structures in the average brain of the participants < 40 years, from 40 to 60 years, and > 60 years. The shaded areas depict the maximum values in the x- and y-direction for the average brain of the young group, and can be used for comparing size. All structures are scaled to be similar in the x-direction for the young group. Thus, size can not be compared across structures, only across age groups. Please note that the average brains are computed from the participants’ raw volumes, and thus no corrections of scanner or intracranial volume are done. The numbers above each picture depict the mean volume expressed in mm3.
Figure 3. Amount of age-explained variance for each structure in the separate samples.
The figure shows the R2 (amount of explained variance) of age for each of the tested structures in each sample separately. Pink background: structures for which a significant (p < .05) age × sample interaction were found. Blue background: structures for which no significant (p > .05) age × sample interaction were found.
Acknowledgements
Funding: The Norwegian Research Council (177404/W50 to K.B.W., 175066/D15 to A.M.F., 154313/V50 to I.R., 177458/V50 to T.E.), University of Oslo (to K.B.W. and A.M.F.); the National Institutes of Health (R01-NS39581, R01-RR16594, P41-RR14075, R01-AG11230, and R01-RR13609); the Mental Illness and Neuroscience Discovery (MIND) Institute; The Biomedical Informatics Research Network Project (BIRN, http://www.nbirn.net, funded by the National Center for Research Resources at the National Institutes of Health (NCRR BIRN Morphometric Project BIRN002)); The Wallenberg Foundation and the Swedish Medical Research Council (K2004-21X-15078-01A 45, K2007-62X-15077-04-1, and K2007-62X-15078-04-3); Eastern Norway Health Authority (A135). We thank Vivi Agnete Larsen for assistance in processing of the data. We thank the developers of the OASIS (Open Access Series of Imaging Studies) database for access to MRI data constituting samples 4 and 5 of the present work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: Anders M. Dale is a founder and holds equity in CorTechs Labs, Inc, and also serves on the Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. All other authors state that there are no actual or potential conflicts of interest.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29:102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279–1282. [DOI] [PubMed] [Google Scholar]
- Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatr. 1997;9Suppl 1:167–171. doi: 10.1017/s1041610297004869. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Beck AaSR. Beck Depression Inventory Scoring Manual. New York: The Psychological Corporation; 1987. [Google Scholar]
- Berg L. Clinical Dementia Rating. Br J Psychiatry. 1984;145:339. [PubMed] [Google Scholar]
- Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Chung SC, Tack GR, Yi JH, Lee B, Choi MH, Lee BY, Lee SY. Effects of gender, age, and body parameters on the ventricular volume of Korean people. Neurosci Lett. 2006;395:155–158. doi: 10.1016/j.neulet.2005.10.066. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Cohen G, Andreasen NC, Alliger R, Arndt S, Kuan J, Yuh WT, Ehrhardt J. Segmentation techniques for the classification of brain tissue using magnetic resonance imaging. Psychiatry Res. 1992;45:33–51. doi: 10.1016/0925-4927(92)90012-s. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994;14:4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27:733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol Aging. 2008;29:329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat D, Greve D, Fischl B, Dale A, Walhovd KB. Minute effects of sex on the aging brain: a multi-sample MRI-study of healthy aging and Alzheimer’s disease. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.0115-09.2009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Messer DF, Payne ME, Macfall JR, Provenzale JM, Steffens DC, Krishnan RR. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiol Aging. 2008;29:290–302. doi: 10.1016/j.neurobiolaging.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Head D, McQuain J, Acker JD, Raz N. Differential aging of the human striatum: a prospective MR imaging study. AJNR Am J Neuroradiol. 1998;19:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon FM, Turetsky BI, Bilker WB, Gur RE. Brain region and sex differences in age association with brain volume: a quantitative MRI study of healthy young adults. Am J Geriatr Psychiatry. 2002;10:72–80. [PubMed] [Google Scholar]
- Gur RC, Mozley PD, Resnick SM, Gottlieb GL, Kohn M, Zimmerman R, Herman G, Atlas S, Grossman R, Berretta D, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci U S A. 1991;88:2845–2849. doi: 10.1073/pnas.88.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Han X, Fischl B. Atlas renormalization for improved brain MR image segmentation across scanner platforms. IEEE Trans Med Imaging. 2007;26:479–486. doi: 10.1109/TMI.2007.893282. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Halphen C, Boska MD, Narayana PA. Diffusion tensor metrics, T(2) relaxation, and volumetry of the naturally aging human caudate nuclei in healthy young and middle-aged adults: Possible implications for the neurobiology of human brain aging and disease. Magn Reson Med. 2008;59:7–13. doi: 10.1002/mrm.21434. [DOI] [PubMed] [Google Scholar]
- Haug H. Effect of secular acceleration on the human brain weight and its changes during aging. Gegenbaurs Morphol Jahrb. 1984;130:481–500. [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cereb Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biol Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age--consistency and interpretation of observed effects. Neurobiol Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. discussion 1275–1278. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Edman-Ahlbom B, Sillen A, Gunnar A, Kulle B, Frigessi A, Vares M, Ekholm B, Wode-Helgodt B, Schumacher J, Cichon S, Agartz I, Sedvall GC, Hall H, Terenius L. Brain-derived neurotrophic factor gene (BDNF) variants and schizophrenia: an association study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:924–933. doi: 10.1016/j.pnpbp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KR, Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB. In vivo stereological assessment of caudate volume in man: effect of normal aging. Life Sci. 1990;47:1325–1329. doi: 10.1016/0024-3205(90)90196-x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, Duncan JS. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Burk K, Klockgether T, Voight K. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cereb Cortex. 1999;9:712–721. doi: 10.1093/cercor/9.7.712. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. Neuroimage. 2007;34:479–485. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19:1498–1507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR Am J Neuroradiol. 1999;20:207–211. [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, Jonsson EG, Agartz I. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Nunnemann S, Wohlschlager AM, Ilg R, Gaser C, Etgen T, Conrad B, Zimmer C, Muhlau M. Accelerated aging of the putamen in men but not in women. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Pengas G, Pereira JM, Williams GB, Nestor PJ. Comparative reliability of total intracranial volume estimation methods and the influence of atrophy in a longitudinal semantic dementia cohort. J Neuroimaging. 2009;19:37–46. doi: 10.1111/j.1552-6569.2008.00246.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition - II. Mahwah, NJ: Earlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD. Differential effects of age and sex on the cerebellar hemispheres and the vermis: a prospective MR study. AJNR Am J Neuroradiol. 1998;19:65–71. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004a;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol. 2001;22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005a;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cereb. Cortex %R 10.1093/cercor/bhi044. 2005b;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Methodological and Conceptual Advances in the Study of Brain-Behavior Dynamics: A Multivariate Lifespan Perspective. 2006;30:730. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004b;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain Aging and Its Modifiers: Insights from in Vivo Neuromorphometry and Susceptibility Weighted Imaging. Ann NY Acad Sci %R 10.1196/annals.1379.018. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Dahle C, Head D, Acker JD. Differential age-related changes in the regional metencephalic volumes in humans: a 5- year follow-up. Neurosci Lett. 2003;349:163–166. doi: 10.1016/s0304-3940(03)00820-6. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Spencer WD, White K, Acker JD. Age-related regional differences in cerebellar vermis observed in vivo. Arch Neurol. 1992;49:412–416. doi: 10.1001/archneur.1992.00530280106030. [DOI] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Riello R, Sabattoli F, Beltramello A, Bonetti M, Bono G, Falini A, Magnani G, Minonzio G, Piovan E, Alaimo G, Ettori M, Galluzzi S, Locatelli E, Noiszewska M, Testa C, Frisoni GB. Brain volumes in healthy adults aged 40 years and over: a voxel-based morphometry study. Aging Clin Exp Res. 2005;17:329–336. doi: 10.1007/BF03324618. [DOI] [PubMed] [Google Scholar]
- Rubin DC. Frontal-striatal circuits in cognitive aging: Evidence for caudate involvement. Aging, Neuropsychology, and Cognition. 1999;6:241–259. [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999;20:279–285. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, Sato K, Ono S, Kinomura S, Nakagawa M, Sugiura M, Watanabe J, Kawashima R, Fukuda H. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging. 2004;25:455–463. doi: 10.1016/j.neurobiolaging.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PA, Vuurman E, Uylings HB, Jolles J. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysis. Brain Res Cogn Brain Res. 2001;11:377–385. doi: 10.1016/s0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005a;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275-1268. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Quinn BT, Salat D, Makris N, Fischl B. Neuroanatomical aging: Universal but not uniform. Neurobiology of Aging. 2005b;26:1279–1283. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Xu J, Kobayashi S, Yamaguchi S, Iijima K, Okada K, Yamashita K. Gender effects on age-related changes in brain structure. AJNR Am J Neuroradiol. 2000;21:112–118. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The figure shows representative examples of image quality from the different samples. The first row is Sample 1, the second row Sample 2 etc. (Samples 4a and b are from the same scanner). F denotes female, m denotes male, and the number denotes age. The two columns to the left show T1-weighted scans from a young and an elderly participant for each sample, while the two right columns show the result of the segmentation procedure. F: Female, M: Male.
The figure displays the three-dimensional renderings of selected structures in the average brain of the participants < 40 years, from 40 to 60 years, and > 60 years. The shaded areas depict the maximum values in the x- and y-direction for the average brain of the young group, and can be used for comparing size. All structures are scaled to be similar in the x-direction for the young group. Thus, size can not be compared across structures, only across age groups. Please note that the average brains are computed from the participants’ raw volumes, and thus no corrections of scanner or intracranial volume are done. The numbers above each picture depict the mean volume expressed in mm3.