Abstract
The existence and function of extranuclear steroid receptors (SR) to rapidly modulate signal transduction is now acknowledged as present in cells and organs throughout the body. Work over the past 15 years has defined key mechanisms that are required for sex steroid receptors to traffic to the plasma membrane, but mechanisms of localization in other cell organelles such as mitochondria is still unclear. Signaling by membrane-localized SR has now been reported to impact many aspects of adult organ functions, while the roles in organ development are under investigation. In hormone-responsive cancers, both extranuclear and nuclear sex steroid receptors appear to collaborate in the regulation of some key genes that promote malignancy. Here, I review what is understood about the impact of extranuclear steroid receptor signaling to mitigate or promote disease processes.
Keywords: Estrogen Receptor, Androgen Receptor, Steroid Receptor, Rapid Signaling, Nuclear Estrogen Receptor
Introduction
Steroid receptors outside the nucleus have now been identified in many organs and cells including various hormone-responsive malignancies [1]. These receptors transduce multiple rapid signals that impact the functional biology of target tissues. Rapid signaling occurs in concert with steroid hormone action in the nucleus and is sometimes required for regulation of specific gene transcription [2]. However, membrane initiated steroid signaling (MISS) can also affect important functions in a non-genomic fashion. The latter most often occurs from plasma membrane-localized receptors signaling to the posttranslational modification of existing proteins such as enzymes [3]. In this way, the body can rapidly adapt to environmental or other stresses, impacting fundamental processes for cardiovascular regulation, bone health, reproductive tract functions, and cell survival. Importantly, we previously showed that it is the membrane and not the nuclear estrogen receptor (ER) that is responsible for the ability of the sex steroid to activate rapid signal transduction [4, 5]. In this review, I provide examples of how MISS affects in vivo animal models of disease and human functions.
Hormone-Responsive Cancers
Breast
The breast cancer field has focused on how tyrosine kinase growth factor receptors for insulin, insulin-like growth factor I (IGF1), epidermal growth factor (EGF), and other ErbB receptor family members signal to the pathogenesis and progression of this malignancy [6–8]. Important pathways downstream of these membrane receptors include PI3K-AKT-mTOR and MEK-ERK signaling that contribute through innumerable ways to breast cancer ontogenesis. Such signaling also underlies some forms of resistance to endocrine therapies in ER/progesterone receptor (PR) positive tumors [9, 10]. Interestingly, the ability of membrane ER or PR to signal through the same multiple pathways in breast cancer epithelial cells partially occurs when membrane-localized sex steroid receptors form complexes with the oncogenic tyrosine kinase Src and are subsequently activated by either estrogen or progesterone [11, 12]. Rapid signaling trans activates the EGFR, and IGFR1 proteins [13, 14], resulting in activation of multiple downstream kinase cascades that are essential to the proliferation and survival of malignant cells.
Recently, Poulard et al. reported that in ER/PR + human breast cancer tissues, ∼55 % of 175 samples show ER/PR in complex with Src just under/at the plasma membrane [15]. High amounts of complexes seen in the tissue sections correlated to multiple clinical factors of poor prognosis, including lymph node involvement, HER2 overexpression and higher tumor grade, and decreased disease-free survival [15]. Key oncogenes in this malignancy such as cyclin D1 and c-myc [12, 16–18] are upregulated at the mRNA/protein levels by sex steroids in part from various rapid signals that include the Wnt pathway [19]. Cyclin D1 also acts as a nuclear co-activator, collaborating with PR in breast cancer cells to regulate genes that promote proliferation and may contribute to endocrine therapy resistance [20]. In addition, tumor suppressors such as p53 are functionally inhibited from posttranslational protein modifications that result from MISS [21].
Identical rapid signaling from membrane ER, PR, or growth factor tyrosine kinase receptors modifies transcription factor abundance and recruitment to DNA [2], stimulates co-activator phosphorylation that promotes recruitment to gene promoters [20, 22], and modulates the epigenome. An example of the latter is that estradiol (E2) activates PI3K-AKT in breast cancer cells, phosphorylating the EZH2 histone methyltransferase at an inhibitory site, serine 21. As a result, the repressive histone 3 lysine 27 tri-methyl (H3K27me3) mark is lifted at the promoter of key genes such as PR [23, 24]. This allows for chromatin remodeling that results in E2 and AKT-dependent transactivation of the PR gene, a target of great importance for the ability of hormone replacement after menopause to cause an increased incidence of breast cancer [25]. Bredfelt et al. also showed this epigenetic modification occurs in response to diethylstilbestrol, an estrogenic compound given to women to stabilize their pregnancy that unfortunately caused a high incidence of a previously rare vaginal cancer in the female offspring of the women who used this drug [23]. This suggests that xenoestrogens and perhaps environmental steroid hormone mimetics/disruptors could activate similar pathways to produce toxic effects.
Although it is not yet clear as to how pervasively important MISS is to gene transcription, work from Miguel Beato and his colleagues suggests there is strong impact. His group reported that membrane PR signaling through extracellular signal-regulated kinase (ERK) and the MSK1 kinase results in important chromatin modifications that promote gene transcription by nuclear PR in MCF7 cells [26]. These investigators showed that 28 % of genes that were upregulated by progesterone in these cells specifically required ERK signaling [26] and ERK/MSK-1 inhibition prevented E2 or progesterone-induced proliferation of breast tumor xenografts [27]. The multiple mechanisms by which MISS contributes to transcription suggests that many important genes will ultimately be shown to require this input for regulation, working in conjunction with nuclear steroid receptor functions.
In addition, rapid signaling by estrogen may impact tumor metabolism by non-genomic mechanisms. Depending on glucose substrate, membrane ER signaling through AKT promotes glycolysis in breast cancer cells, or shifts the cells into the Kreb’s cycle and oxidative phosphorylation when glucose is reduced [3]. E2/ER promotes the latter compensation as a survival mechanism, stimulating AMP kinase-induced phosphorylation and activation of the pyruvate dehydrogenase enzyme, thereby allowing utilization of the glucose metabolite pyruvate for entry into the Kreb’s cycle. Lowering glucose and inhibiting this compensatory pathway greatly enhanced γ-radiation-induced apoptosis of the tumor cells [3].
Prostate
Compared to breast cancer, there has been comparatively little work on the importance of membrane-localized androgen receptors (AR) in prostate cancer. Peterziel et al. described MEK-ERK, PI3K, and protein kinase C activation by androgens in prostate cancer cells [28]. Although it was unclear whether this action originated from extranuclear AR, such signaling was described to correlate to relative androgen independence in aggressive prostate cancer cells [29]. Similar to ER and PR, AR acts as a G protein-coupled receptor to initiate such signaling [30]. Recent work in both cell lines [31] and xenograft models [32] indicates that the paxilin protein is phosphorylated by either androgen or EGF-induced ERK signaling, facilitating signal transduction and resulting in paxillin translocation to the nucleus. In the nucleus, paxillin serves as a co-activator for AR-induced upregulation of various oncogenes such as CFOS and CCND1, linking MISS and nuclear AR actions [32].
Osteoporosis
From utilization of membrane ER-engaging estrogenic compounds, strong evidence indicates that signaling from this pool of ERα may be sufficient to prevent osteoclast development, mitigating osteoporosis in mouse models [33]. Such prevention may specifically involve kinase-mediated signaling by estrogens to the upregulation of expression and phosphorylation of important transcription factors [34]. Membrane ERα signaling also impacts survival of osteoblast progenitor cells and stimulates osteoclast apoptosis in cortical bone [35].
Mitigation of Cardiac Hypertrophy and Progression
Abundant data now exists from many in vivo and in vitro models that rapid signaling by membrane ERβ prevents hypertension, resulting cardiac hypertrophy, fibrosis, and progression to heart failure [36–38]. These actions occur from the membrane ERβ pool blocking the actions of hypertrophic stimuli that stimulate ERK and AKT activity, and protein phosphatase 2B activation. As a result, hypertrophic transcription factors (TFs) are retained in cytoplasm (e.g., NFATs), or are kept inactive in the nucleus by inhibitory phosphorylation (e.g., GATA4) [39, 40]. Recently, estrogen acting at membrane ERβ was shown to repress pro-hypertrophic histone deacetylase (HDAC) expression and activity, while also simulating the production and nuclear localization of anti-hypertrophic HDACs. This resulted in prevention of hypertrophic gene expression [41]. E2 and ERβ also suppress cardiac fibrosis; this occurs when membrane ERβ blocks TGFβ production, the transition of fibroblasts to myofibroblasts, and TGFβ signaling to pro-fibrotic gene expression [42]. Using an ERβ-specific agonist that avoids the uterine and breast proliferation induced by E2, strong benefit was shown in all these aspects in wild-type but not ERβ-deleted mice [42]. These findings also extend to ERβ preventing or even reversing pulmonary hypertension and resulting right ventricular hypertrophy in rat models [43]. Several types of acute vascular endothelial injury in mice have also been shown to be prevented by administration of an estrogen-dendrimeric compound that only binds to membrane and not nuclear ER in blood vessel cells [44].
Mitochondrial Estrogen Receptors
Although steroid action in mitochondria was originally identified many years ago, the nature of the proteins that mediated steroid action was poorly defined. Recent work has identified classical glucocorticoid and estrogen receptors in mitochondria of various cells. Regarding ER, both alpha and beta isoforms have been identified in mitochondria of cardiomyocytes, neurons, and breast cancer [45, 46]. Interestingly, ERα and ERβ in breast cancer cells have been shown by electron microscopy to be localized primarily to the mitochondrial matrix (interior) [47]. Some data suggests that mitochondrial gene regulation occurs in part from E2 binding to ER in this organelle in breast cancer cell lines [47]. Other data indicates that mitochondrial ERβ mediates cyto-protection of MCF7 breast cancer cells upon estrogen binding this receptor pool as an agonist [48]. In response to radiation of such cells, strong oxidant stress triggers the intrinsic apoptotic program, leading to cell death. Estrogen mitigates ROS formation by upregulating manganese superoxide dismutase (MnSOD) activity, preventing superoxide-induced apoptosis. Thus, conceptually, women with this malignancy that are receiving adjuvant therapies and are undergoing symptoms of estrogen deprivation can’t take hormone replacement because it opposes the fundamental mechanism of adjuvant therapy action.
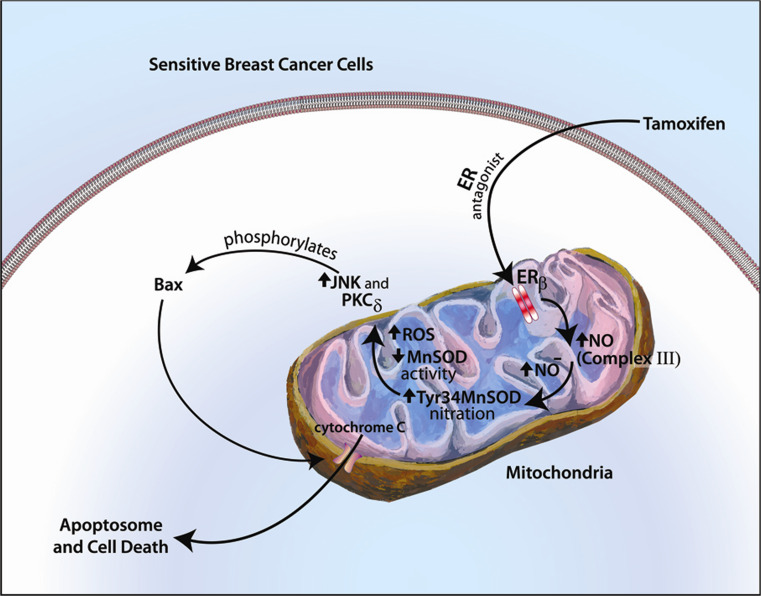
In addition, endocrine therapy sensitivity or resistance in breast cancer might be governed by mitochondrial ERβ acting as an ROS rheostat [49]. From in vitro and xenograft mouse models of tamoxifen (TAM) resistance, the SERM is an effective cytotoxic therapy when it generates large amounts of superoxide. In part, this is due to TAM initiating nitric oxide (NO) generation from binding ERβ in mitochondria of breast cancer cells. Nitric oxide causes nitrosylation of tyrosine 34 of MnSOD thereby inactivating this enzyme. As a result, superoxide levels are very high and subsequently activate apoptosis (Fig. 1). In contrast, TAM-resistant tumors that grow in the presence of a TAM pellet under the skin of mouse xenograft models show high amounts of MnSOD activity due to lack of NO generation. Targeting the in vivo tumors with lenti-virus shRNA to MnSOD completely restored the cytotoxic response to TAM, causing massive apoptosis and tumor involution in 3 weeks. Current strategies include using nanosphere-siRNA to MnSOD for expression in TAM-resistant breast tumors in vivo and appear promising [50]. Additional studies in lung cancer implicate mitochondrial ERβ as stimulating Bcl2 gene expression and moderating oxidant stress, thus promoting the survival of this malignancy [51].
Fig. 1.
Cytotoxic effects of tamoxifen in responsive breast cancer cells and tumors. Tamoxifen binds mitochondrial ERβ as an antagonist, stimulating nitric oxide generation from complex III of the electron transport chain, nitrosylating MnSOD to reduce its activity that results in high amounts of superoxide. Oxidative stress induces the intrinsic apoptotic program. In tamoxifen-resistant cells, this selective estrogen receptor modulator binds ERβ as an agonist and does not reduce MnSOD activity, thus promoting tumor survival
Therapeutic Targets Arise from Understanding Membrane Steroid Receptor Signaling
The realization that rapid but often sustained signaling occurs when steroid ligands bind to receptor isoforms outside the nucleus suggests that selective engagement of these receptors could either prevent or treat existing diseases. Engagement of membrane ERα alone prevents osteoporosis [33] or acute vascular damage [44] in female mouse models. Membrane ERα also signals through AMPK to the suppression of all lipid synthesis in the in vivo liver. These actions do not involve nuclear ER and potentially impact cholesterol homeostasis and related disorders [5]. Thus, compounds such as EDC that do not enter the cell and only bind to membrane ER point to an approach that could prove fruitful, avoiding toxicities that also require engagement of nuclear ER.
In some cells, steroid receptor isoforms such as ERβ exist mainly outside the nucleus, and agonists for this receptor isoform could prevent or treat multiple aspects of cardiovascular disease [40]. ER antagonists that only bind to membrane receptors could also prove therapeutically useful, limiting toxicities that arise from nuclear ER binding. In the first regard, favorable brain remodeling from rapid signaling by estrogen/membrane ERα to MEK-ERK involves dendritic spine and synapse formation [52, 53]. This mechanism along with reduction of oxidant stress may underlie the ability of estrogen to prevent the death of dopamine-secreting neurons that underlies the development of Parkinson’s disease [54]. MEK-ERK activation by E2 in the brain cortex limits the extent of stroke, although the latter benefit is largely confined to younger mammals as supported by animal models [55]. From understanding the key signals generated to mediate the effects of extranuclear steroid receptors, these pathways can also be targeted by existing kinase, calcium, or other directed inhibitors. Many therapies for disease require a multifactorial approach, such as in hormone-responsive cancers. Novel extranuclear steroid receptor agonists or antagonist molecules that specifically affect extranuclear steroid receptor functions could prove valuable in these regards.
Acknowledgments
The studies were supported by a Merit Review Award from the Department of Veterans Affairs and grant 2RO1CA100366 from the National Institutes of Health.
Conflicts of Interest
Ellis Levin discloses no apparent conflicts.
References
- 1.Hammes S, Levin ER. Recent advances in extra-nuclear steroid receptor actions. Endocrinology. 2011;152:4489–4495. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 3.O’Mahony F, Pedram A, Razandi M, Levin ER. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol Endocrinol. 2012;26:2058–2070. doi: 10.1210/me.2012-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedram A, Razandi M, Kim JK, O’Mahony F, Lee E, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor α (MOER) mouse. J Biol Chem. 2009;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedram A, Razandi M, O’Mahony F, Harvey H, Harvey BJ, Levin ER. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci Signal. 2013;6:RA36. doi: 10.1126/scisignal.2004013. [DOI] [PubMed] [Google Scholar]
- 6.Werner H. Tumor suppressors govern insulin-like growth factor signaling pathways: implications in metabolism and cancer. Oncogene. 2012;31:2703–2714. doi: 10.1038/onc.2011.447. [DOI] [PubMed] [Google Scholar]
- 7.Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat. 2012;136:331–345. doi: 10.1007/s10549-012-2289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen DL, Kümler I, Palshof JA, Andersson M. Efficacy of HER2-targeted therapy in metastatic breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Breast. 2013;22:1–12. doi: 10.1016/j.breast.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456:663–666. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 12.Skildum A, Faivre E, Lange CA. Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol Endocrinol. 2005;19:327–339. doi: 10.1210/me.2004-0306. [DOI] [PubMed] [Google Scholar]
- 13.Razandi M, Pedram A, Parks ST, Levin ER. Proximal events in ER signaling from the plasma membrane. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 14.Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–4101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulard C, Treilleux I, Lavergne E, Bouchekioua-Bouzaghou K, Goddard-Leon S, Chabaud S, Tredan O, Corbo L, Le Romancer M. Activation of rapid estrogen signaling in aggressive human breast cancers. EMBO Mol Med. 2012;4:1200–1213. doi: 10.1002/emmm.201201615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razandi M, Pedram A, Rosen EM, Levin ER. BRCA1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. Mol Cell Biol. 2004;24:5900–5913. doi: 10.1128/MCB.24.13.5900-5913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marino M, Acconcia F, Bresciani F, Weisz A, Trentalance A. Distinct nongenomic signal transduction pathways controlled by 17β-estradiol regulate DNA synthesis and cyclin D1 gene transcription in HepG2 cells. Mol Biol Cell. 2002;13:3720–3729. doi: 10.1091/mbc.E02-03-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H, Abd ZY, Elmageed JJ, Naura AS, Abdel-Mageed AB, Varughese S, Paul D, Alahari S, Catling A, Kim JG, Hamid Boulares A. PDZK1 is a novel factor in breast cancer that is indirectly regulated by estrogen through IGF-1R and promotes estrogen-mediated growth. Mol Med. 2013;19:253–262. doi: 10.2119/molmed.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedram A, Razandi M, Evinger AJ, Lee E, Levin ER. Estrogen inhibits ATR signaling to cell cycle checkpoints and DNA repair. Mol Biol Cell. 2009;20:3374–3389. doi: 10.1091/mbc.E09-01-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dressing, Gwen E, Todd P Knutson, Matthew J Schiewer, Andrea R Daniel, Christy R Hagan, Caroline H Diep, Karen E Knudsen, Carol A Lange (2014) Progesterone receptor-cyclin D1 complexes induce cell cycle-dependent transcriptional programs in breast cancer cells. Mol Endocrinol Feb 25:me20131196 [DOI] [PMC free article] [PubMed]
- 22.York B, Chundong Y, Sagen JV, Liu Z, Nikolai BC, Ray-Chang W, Finegold M, Jianming X, O’Malley BW. Reprogramming the posttranslational code of SRC-3 confers a switch in mammalian systems biology. Proc Natl Acad Sci. 2010;107:11122–11127. doi: 10.1073/pnas.1005262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bredfeldt TG, Leigh Greathouse K, Safe SH, Hung M-C, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedram A, Razandi M, Deschenes R, Levin ER. DHHC 7 and 21 are palmitoylacyltranferases for sex steroid receptors. Mol Biol Cell. 2012;23:188–199. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrocich H, McTiernan A, Investigators WHI. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 26.Vicent GP, Cecilia Ballaré A, Nacht S, Clausell J, Subtil-Rodriguez A, Quiles I, Jordan A, Beato M. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Reyes D, Carlos B, Giuliano C, Daniel S, Bagó JR, Jose B, Miguel B. Activation of mitogen- and stress-activated kinase 1 is required for proliferation of breast cancer cells in response to estrogens or progestins. Oncogene. 2013 doi: 10.1038/onc.2013.95. [DOI] [PubMed] [Google Scholar]
- 28.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signaling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- 29.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 30.Sen A, Prizant H, Hammes SR. Understanding extranuclear (nongenomic) androgen signaling: what a frog oocyte can tell us about human biology. Steroids. 2011;76:822–828. doi: 10.1016/j.steroids.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen A, O’Malley K, Wang Z, Raj GV, Defranco DB, Hammes SR. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem. 2010;285:28787–28795. doi: 10.1074/jbc.M110.134064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen A, De Castro I, Defranco DB, Deng F-M, Melamed J, Kapur P, Raj GV, Rossi R, Hammes SR. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Invest. 2012;122:2469–2481. doi: 10.1172/JCI62044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O’Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 34.Kousteni S, Han L, Chen J-R, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–1664. doi: 10.1172/JCI200317261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartell SM, Han L, Kim H-n, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS, Chambliss KL, Shaul PW, Roberson PK, Weinstein RS, Jilka RL, Almeida M, Manolagas SC. Non-nuclear-initiated actions of the estrogen receptor protect cortical bone mass. Mol Endocrinol. 2013;27:649–656. doi: 10.1210/me.2012-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavasin MA, Sankey SS, Ai-Li Y, Menon S, Yang X-P. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1560–H1569. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 37.Satoh M, Matter CM, Ogita H, Takeshita K, Wang C-Y, Dorn GW, II, Liao JK. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007;115:3197–3204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jazbutyte V, Arias-Loza PA, Kai H, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier K-H, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. Ligand-dependent activation of ER lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized SHR. Cardiovasc Res. 2008;77:774–781. doi: 10.1093/cvr/cvm081. [DOI] [PubMed] [Google Scholar]
- 39.Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor beta to inhibit calcineurin. Endocrinology. 2008;149:3361–3369. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedram A, Razandi M, Korach K, Narayanan R, Dalton J, Levin ER. ERβ selective agonist inhibits angiotensin-induced cardiovascular pathology in female mice. Endocrinology. 2013;154:4352–4364. doi: 10.1210/en.2013-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedram A, Razandi M, Narayanan R, Dalton J, McKinsey TA, Levin ER. Estrogen regulates histone deacetylases to prevent cardiac hypertrophy. Mol Biol Cell. 2013;24:3805–3818. doi: 10.1091/mbc.E13-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER. Estrogen receptor beta prevents cardiac fibrosis. Mol Endocrinol. 2010;24:2152–2165. doi: 10.1210/me.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadadur RD, Umar S, Wong G, Eghbali M, Iorga A, Matori H, Partow-Navid R, Eghbali M. Reverse right ventricular structural and extracellular matrix remodeling by estrogen in severe pulmonary hypertension. J Appl Physiol. 2012;113:149–158. doi: 10.1152/japplphysiol.01349.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambliss KL, Qian W, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor a signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang S-H, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel A-M, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J-Q, Delannoy M, Cooke C, Yager JD. Mitochondrial localization of ERα and ERβ in human MCF-7 cells. Am J Physiol Endocrinol Metab. 2004;28:E1011–E1022. doi: 10.1152/ajpendo.00508.2003. [DOI] [PubMed] [Google Scholar]
- 47.Chen J-Q, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta. 2005;1746:1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell. 2006;17:2125–2137. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Razandi M, Ali Pedram V, Jordan C, Fuqua S, Levin ER. Tamoxifen regulates cell fate through mitochondrial estrogen receptor beta in breast cancer. Oncogene. 2013;32:3274–3285. doi: 10.1038/onc.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho SK, Pedram A, Levin ER, Kwon YJ. Acid-degradable core-shell nanoparticles for reversed Tamoxifen-resistance in breast cancer by silencing manganese superoxide dismutase (MnSOD) Biomaterials. 2013;34:10228–10237. doi: 10.1016/j.biomaterials.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang GF, Yanamala N, Lathrop KL, Zhang L, Klein-Seetharaman J, Srinivas H. Ligand-independent antiapoptotic function of estrogen receptor-beta in lung cancer cells. Mol Endocrinol. 2010;24:1737–1747. doi: 10.1210/me.2010-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabatadze N, Smejkalova T, Woolley CS. Distribution and posttranslational modification of synaptic ERα in the adult female rat hippocampus. Endocrinology. 2013;154:819–830. doi: 10.1210/en.2012-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Perez AI, Dominguez-Meijide A, Lanciego JL, Guerra MJ, Labandeira-Garcia JL. Inhibition of Rho kinase mediates the neuroprotective effects of estrogen in the MPTP model of Parkinson’s disease. Neurobiol Dis. 2013;58:209–219. doi: 10.1016/j.nbd.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res. 2013;4:390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]