Abstract
Objectives
The purpose of this study was to provide clinical evidence of the use of contrast-enhanced sonography in detecting and quantifying changes in intraneural vascularity due to median mononeuropathy.
Methods
Five Macaca fascicularis monkeys were exposed to 20 weeks of repetitive work to increase their risk of developing median mononeuropathy. Contrast-enhanced sonograms were obtained in 30-second increments for 7 minutes while a contrast agent was being delivered. Data were collected immediately at the conclusion of the 20-week work exposure and then again during a recovery phase approximately 3 months after the completion of work. Quantitative analysis and trend graphs were used to analyze median nerve perfusion intensity. This study also compared the use of both manual counting of pixels and semiautomatic measurement using specialized software.
Results
Based on the average data, maximum intensity values were identified as the best indicators of nerve hyperemia. Paired t tests demonstrated significantly higher maximum intensities in the working stage for 4 of the 5 subjects (P < .01).
Conclusions
This study provides preliminary evidence that (1) in a controlled exposure model, a change in intraneural vascularity of the median nerve between working and recovery can be observed; (2) this vascular change can be measured using an objective technique that quantifies the intensity of vascularity; and (3) contrast-enhanced sonography may improve the ability to reliably capture and measure low-flow microvascularity.
Keywords: carpal tunnel syndrome, contrast-enhanced sonography, median mononeuropathy, musculoskeletal ultrasound
Mononeuropathy can be caused by trauma, compression, or infection of a peripheral nerve. In the case of the median nerve, mononeuropathy manifests itself with pain, pares-thesia, and numbness in the thumb and first two digits of the hand, and in advanced stages, it is clinically diagnosed as carpal tunnel syndrome.1 In acute stages of median mononeuropathy, an entrapped or compressed nerve responds by swelling at the next proximal node of Ranvier and dispensing Nissl substance.2 This process of repair results in an increase in metabolic activity and corresponding inflammation of that area of the peripheral nerve.
Sonographically, median mononeuropathy has been described as nerve swelling in the proximal portion of the carpal tunnel and flattening in the distal tunnel.3 This combination of swelling and compression has been described as a “notch sign” and is used to support an imaging diagnosis of median mononeuropathy.3,4 The grayscale sonographic pattern has been described as a uniformly hypoechoic pattern proximal to the site of injury, and disturbance in the microvasculature has been noted.5 This increase in vascularity represents a pathogenic repair process of the nerve and can be appreciated with color and power Doppler imaging.5
Although there are a plethora of human studies to support these sonographic diagnostic criteria,6-10 numerous gaps continue to exist. Since the median nerve is classified as a large peripheral nerve, evaluation of the pathophysiologic mechanism of acute repair of the nerve and associated intraneural vascular activity should be easy to document with high-resolution sonography. Unfortunately, varied results across numerous studies that image hyperemia within the nerve continue to highlight the difficulty in quantifying this Doppler measure. Additionally, most previous studies have been relegated to imaging median mononeuropathy in its chronic state, which may limit detection of changes due to the pathophysiologic mechanism of the acute injury.
To this end, we became interested in the use of contrast-enhanced sonographic techniques to document the metabolic activity and inflammation in an acutely injured median nerve. We hypothesized that amplifying the microvasculature would enhance the ability to detect and record increased metabolic activity. Additionally, we were interested in determining the ability of sonography to document the longitudinal development of the median mononeuropathy and associated intraneural vascular disturbance.
We report a study of 5 Macaca fascicularis that had a 20-week controlled exposure to a repetitive thumb and finger pinching task. Contrast-enhanced sonography was used to show intraneural vascularity associated with median mononeuropathy. A multi-incremental sampling set of images was retrospectively analyzed to identify markers of physiologic repair in the early development of this compressive disorder of the median nerve.
Materials and Methods
This study was designed to gather preclinical safety information and determine the utility of contrast-enhanced sonography as a means for amplifying median nerve vascularity. An abbreviated review of materials and methods specific to this study is presented; detailed methods have been previously reported as indicated below.
Subjects
Five young adult female monkeys (M fascicularis) were trained to complete a repetitive pinching task with the left thumb and fingers.11 The subjects received 1 month of training and worked for 20 weeks, followed by a recovery period during which time no work was performed. The subjects were managed in accordance with the protocols approved by The Ohio State University’s Institutional Animal Care and Use Committee.
Equipment
A LOGIQ i ultrasound system (GE Healthcare, Milwaukee, WI) with a 12.0-MHz linear broadband transducer was used. The output power was reduced to 4% to preserve the contrast activity. Throughout the series of experiments, quality control was verified via weekly checks by imaging tissue-mimicking and flow phantoms.
Contrast Agent Dosing
Definity (Lantheus Medical Imaging, Billerica, MA) was used as the contrast agent for this study because it possesses the smallest microspheres available (1.1–1.3 μm, stability of <10 minutes, and resonance at 4 MHz).12 These unique features of Definity made it ideal for this experimental application. The dosing protocol was developed in consultation with the manufacturer and with cardiac sonographers who had experience using the product for human studies. A nurse practitioner graduate student managed the preparation of the doses, designed to increase visualization of selective anatomic structures. The contrast agent was activated according to the instructions provided by the manufacturer and was vigorously agitated in the syringes immediately before injection.13 Each subject was sedated, and a 22-gauge catheter was placed in a hind leg vein for direct injection. The cumulative dose of the contrast agent injections included 0.04 mL of Definity and 0.96 mL of saline delivered over 4 minutes 15 seconds.
Contrast-Enhanced Sonogram Acquisition and Analysis
Contrast-enhanced sonography was used to collect images from the subjects after working for up to 20 weeks and during recovery after working had ceased for approximately 3 months. The ultrasound transducer was placed and held stationary over the median nerve in the longitudinal plane at the wrist crease. A 7-minute power Doppler cine clip was obtained, beginning at the time of initial contrast agent injection. Power Doppler region of interest (ROI) dimensions were kept consistent across subjects to ensure standardization. For analysis, imaging samples were captured from each cine clip beginning at baseline (time 0) and at every 30-second increment for a total of 15 image samples.
Power Doppler pixels were manually counted on each of the incremental images for all subjects at both phases of the study according to the method of Klauser et al14 for counting power Doppler pixels with the ROI. A second semiautomatic measurement method used PixelFlux Scientific software (Chameleon Software GmbH, Münster, Germany) to objectively quantify vascularity within the median nerve (Figure 1). First, an ROI was carefully drawn outlining the median nerve in the longitudinal plane. After this step was completed, the software calculated the perfusion intensity of the power Doppler pixels within the manually identified ROI surrounding the median nerve.15 The maximum intensity of power Doppler pixels within the ROI and an average intensity across all power Doppler pixels were identified for each of the 15 image samples for each subject at completion of work exposure and in the recovery phase.
Figure 1.
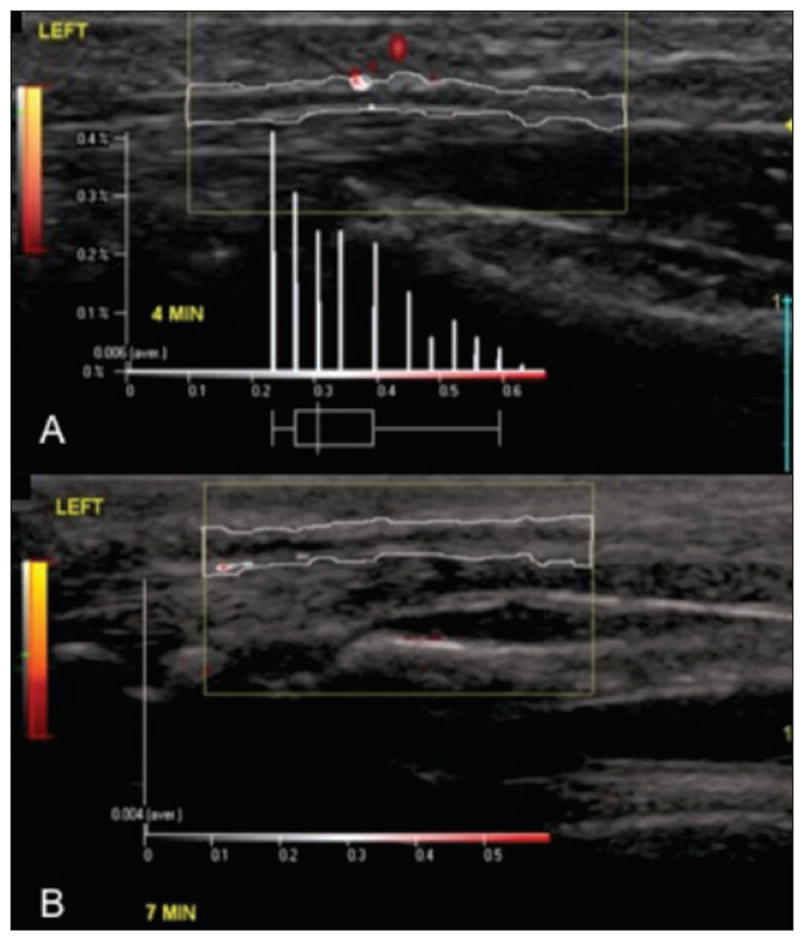
Semiautomatic method for analysis of contrast-enhanced sonographic intensity using the PixelFlux Scientific software for one frame capture in a multi-incremental manner. Subject X (A) represents a positive case study and subject W (B) a negative case study for amplification of median nerve vascularity.
Statistical Analysis
Manual pixel counts and semiautomatic intensity measurements were averaged across the 15 incremental image samples for both the working and recovery phases. This process was done by subject to identify the measurement with the greatest potential for detection of differences in vascularity. Paired t tests were used to compare differences across the 15 time points between working and recovery phases for each subject. Trend graphs were created to illustrate differences between the two phases and determine potential longitudinal effects of the contrast for amplifying the measurement of vascularity surrounding each subject’s median nerve across 7 minutes from the time of injection. Significance of P < .05 in this small-cohort study was interpreted as a trend in the data requiring further investigation.
Results
Five subjects (S, U, W, X, and Y) were injected during the two study phases. All of the subjects maintained their original weight throughout the study, with the minimum overall weight for subject U, weighing 4.00 kg, and the maximum for subject S, weighting 5.70 kg. Manual grading and semiautomatic measurements were averaged across the 15 incremental time samples in each phase by subject (Table 1). Based on these average data, maximum intensity values were identified as the best indicators of hyperemia within the nerve tissue due to the objectivity of the measurement and having the largest distribution of the resulting data. Paired t tests were conducted to determine differences in maximum intensities across all 15 sampled time points between working and recovery phases for each subject. Significantly higher maximum intensities were noted during the working phase for all subjects with the exception of subject W (Table 2).
Table 1.
Individual Subject Average Measurements (SD) for 15 Sampled Contrast-Enhanced Sonograms in Each Phase
| Subject | Dot Counta | Sonographic Gradinga | Maximum Intensityb | Average Intensityb |
|---|---|---|---|---|
| Working | ||||
| S | 13.13 | 2.73 | 3.20 (1.44) | 0.84 (0.63) |
| U | 2.22 | 1.00 | 1.36 (0.38) | 0.59 (0.31) |
| W | 7.60 | 1.93 | 2.93 (1.46) | 0.87 (0.72) |
| X | 23.27 | 3.00 | 4.44 (0.88) | 2.28 (1.19) |
| Y | 26.67 | 3.00 | 4.73 (0.37) | 0.88 (0.80) |
| Recovery | ||||
| S | 1.26 | 0.73 | 0.52 (0.45) | 0.28 (0.10) |
| U | 1.53 | 0.73 | 0.31 (0.25) | 0.21 (0.04) |
| W | 7.93 | 1.87 | 2.22 (1.46) | 0.71 (0.45) |
| X | 1.73 | 0.87 | 0.45 (0.24) | 0.26 (0.06) |
| Y | 0.80 | 0.73 | 0.44 (0.53) | 0.26 (0.07) |
Klauser method.
PixelFlux.
Table 2.
Paired t Tests for Maximum Intensity Across the 15 Time Samples Between Working and Recovery for Each Subject
| Subject | Mean | SD | t | df | P |
|---|---|---|---|---|---|
| S | 2.67 | 1.28 | 8.11 | 14 | <.01 |
| U | 1.06 | 0.44 | 9.28 | 14 | <.01 |
| W | 0.71 | 1.42 | 1.93 | 14 | .07 |
| X | 3.99 | 0.94 | 16.34 | 14 | <.01 |
| Y | 4.29 | 0.64 | 25.89 | 14 | <.01 |
Trend graphs were created to illustrate changes in maximum contrast intensity across the 7-minute contrast-enhanced sonographic cycle collected in the two phases for each of the subjects (Figure 2). Increased maximum intensities during the working phase compared to recovery are clearly depicted for all subjects with the exception of subject W, consistent with the t test results. Longitudinal assessment of the effects of contrast during the 7 minutes of image acquisition indicated a slightly elevated trend in the first 5 minutes of the cycle with somewhat reduced/varied intensities in the final 2 minutes.
Figure 2.
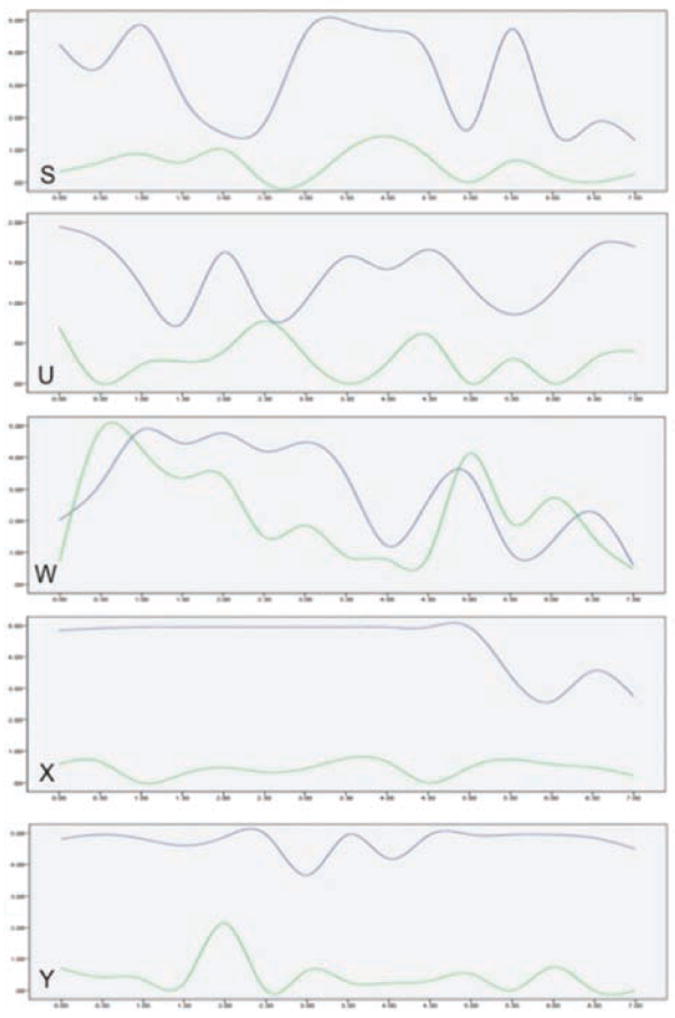
Trend graphs of maximum power Doppler intensities across the 7-minute contrast-enhanced sonographic sample for each subject, obtained in the work phase (blue lines) and during recovery (green lines).
Discussion
Based on our review of the literature, this is the first pre-clinical study of the use of contrast-enhanced sonography to detect intraneural vascular flow associated with the median nerve. As has been previously stated, chronic external pressure caused by repetitive stress or repetitive activities can cause neuropathy involving superficial or deep branches of the nerve.3 Therefore, this controlled model was designed to simulate risk factors that contribute to median mononeuropathy. Additionally, we have identified an objective measure of vascular intensity and a time series evaluation method that may be useful in future studies attempting to document vascularity that could be associated with neuropathy of the median nerve. This small cohort helps in formulating a hypothesis that contrast-enhanced sonography could help amplify the intraneural vascular activity associated with the development of median mononeuropathy.
The first objective of this preclinical experiment was to demonstrate the safety of Definity for use in musculoskeletal imaging of intraneural vascular flow. The cumulative dose provided to each subject included 0.04 mL of Definity and 0.96 mL of saline, injected over 4 minutes 15 seconds. This dose was well below amounts indicated in toxicology reports of sonographic contrast agent injections in monkeys.16,17 During the working phase, all subjects were intubated during contrast agent injections for fear of contrast agent reactions. However, all subjects tolerated the injections well and had a quick recovery after sedation; therefore, intubation was not used for contrast-enhanced sonography in the recovery phase. The lack of any negative effects due to the injection of the contrast agent at this dose for musculoskeletal imaging provides promising evidence for translational applications of contrast-enhanced sonography in human imaging that is currently not approved in the United States.
Next, our work attempted to document changes in vascularity in the early development and repair of median mononeuropathy. The injections corresponded uniformly across the subjects after extended work exposure and after a similar period of recovery from the working task. Quantitative data and trending indicate elevated vascularity within the median nerve for the working phase compared to recovery. Although increased numbers were noted across all measurement techniques, maximum intensity measurements using PixelFlux software seemed to provide the best measure. The Klauser method of counting pixels seemed rather subjective, and we had difficulty in obtaining a consensus among the researchers in our laboratory (Figure 3). This scoring system, although providing information about the incidence of vascularity within the nerve, does not necessarily provide enough latitude to detect intensity differences. For example, a nerve that has color in 25 very small pixel regions may not, in fact, have more intense overall vascularity than a nerve with color appearing in 10 very large pixel regions. In contrast, measuring the intensity of vascularity within the ROI using PixelFlux provided more latitude for measuring this concept. The use of this method for analyzing contrast-enhanced sonograms is an improvement over non–contrast-enhanced Doppler measurements in previous work with a larger sample.18,19
Figure 3.
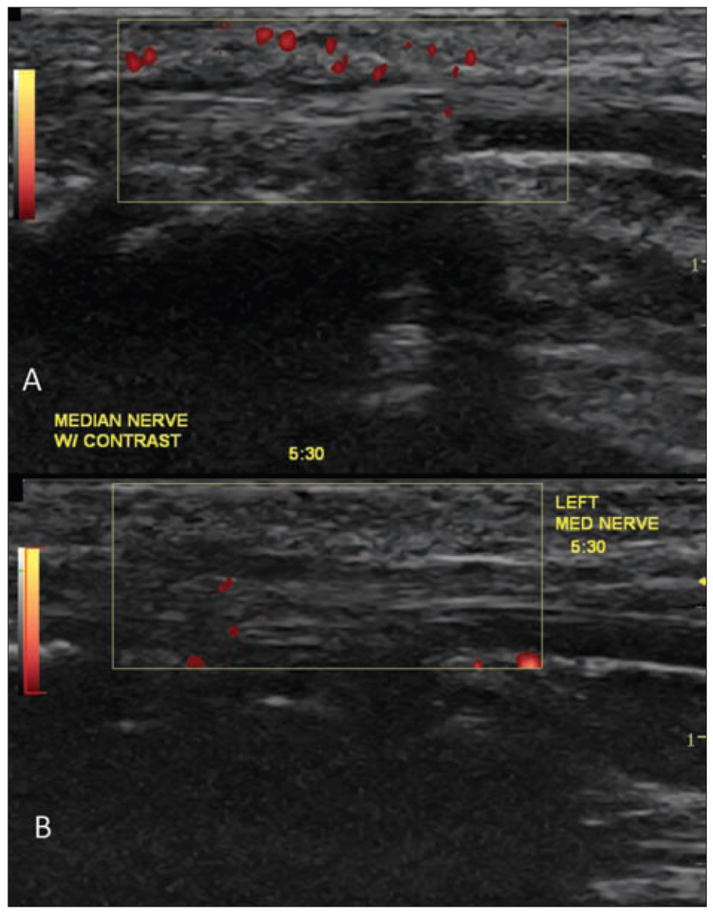
Contrast-enhanced sonograms captured in a multi-incremental manner and evaluated manually by counting pixels according to the Klauser method for assessing contrast amplification. Subject X (A) represents a case study with multiple amplified pixels, compared to subject W (B), a case study showing low amplification of median nerve vascularity.
The trend graphs provided a time series review of the behavior of the intensity of the contrast-enhanced blood flow around the nerve. This set of contrast-enhanced sonographic data collected during recovery showed a lower level of enhancement for subjects S, U, X, and Y. The recovery data collected for subject W did not differ in a statistically significant manner from the working data. Correspondingly, the trend graph also did not show a remarkable change. The fact that subject W represented a negative study (low amplification of median nerve vascularity) may underscore the variability among subjects and could explain the lower sensitivity and specificity demonstrated in human studies.20 This result is important to consider, given that even in a fairly well-controlled study, not all subjects have markedly increased intraneural vascularity during the course of developing median mononeuropathy. To achieve increased sensitivity and reliable reproducibility when translating this process to human studies, further investigation and refinement of instrumentation settings and contrast agent dosing are needed.
In addition to differences based on the study phase, we also observed a trend among the subjects across the 7-minute cycle. Qualitatively, when watching the cine clips, we noted a decrease in contrast perfusion at approximately 5 minutes after the initial injection. We saw a similar downward trend in the quantitative maximum intensity trend graphs. This downward trend coincides with the last contrast agent booster injection, which was given at 4 minutes 15 seconds. This decrease in contrast perfusion and decrease in the qualitative measure of intensity in the final 2 minutes may be preliminary evidence that contrast agent injections do enhance the ability to detect low-flow microvascularity of the vessels within the median nerve that could not otherwise be reliably captured with non–contrast-enhanced Doppler evaluation.
Further development and validation of contrast-enhanced sonographic methods to study kinetics of vessels within the median nerve are necessary. Enhancing the visualization and measurement of vascularity will have an important translational impact on distinguishing the behavior of vascularity within the normal nerve to document inflammation associated with median mononeuropathy. The use of kinetics coupled with our work on the cross-sectional area of the layers of the nerve21 may increase the sensitivity for the stages of development of median mononeuropathy. Together, documenting the inflammation process and identifying median mononeuropathy staging have the potential to lead to the development of specific biomarkers for early identification of median mononeuropathy in the acute stages before diagnosis as advanced carpal tunnel syndrome.
Limitations
This work was a preclinical study used primarily to demonstrate safety and determine the feasibility of contrast-enhanced sonographic measures as a means of describing progressive median mononeuropathy due to controlled exposure. Because this study design was pre-experimental, the threats to internal and external validity do not allow for the results to be generalized. These results were also linked to the equipment used and contrast agent that provided these experimental results (eg, size of the Definity microspheres). This cohort was part of a larger study, and because of a shortage of the contrast product, our work was sufficiently delayed, which made the collection of baseline data impossible. Therefore, any discussion of the results in this study is only limited to changes in vascularity from a point after exposure to a recovery state. The utility of this study is that it (1) provides feasibility and trend data for various contrast-enhanced sonographic acquisition and analysis methods for use in future studies and (2) gives impetus for developing higher levels of evidence in the area of median nerve vascularity.
Conclusions
This study provides preliminary evidence that (1) in a controlled exposure model, a change in intraneural vascularity of the median nerve between working and recovery can be observed; (2) this vascular change can be measured using an objective technique that quantifies the intensity of vascularity, not merely the incidence, as in the Klauser method; and (3) contrast-enhanced sonography may enhance the ability to reliably capture and measure low-flow microvascularity. However, accurate velocity measurements need to be taken, which would allow for a more accurate correlation with risk factors and symptoms associated with compressive neuropathies.
Acknowledgments
Supported by the American Institute of Ultrasound in Medicine Endowment for Education and Research
This research was made possible by grants from the American Institute of Ultrasound in Medicine Endowment for Education and Research, the National Institute of Occupational Safety and Health (grant 5R21OH009907-02), and the National Center for Research Resources (grant UL1RR025755). Dr Roll was funded by the National Institutes of Health Rehabilitation Research Career Development Program (grant K12HD055929) at the time this manuscript was prepared. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Center for Research Resources.
Abbreviations
- ROI
region of interest
References
- 1.Porth CM. Essentials of Pathophysiology: Concepts of Altered Health States. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. Disorders of neuromuscular function; pp. 787–821. [Google Scholar]
- 2.Sugerman RA. Structure and function of the neurologic system. In: Huenther SE, McCane KL, editors. Understanding Pathophysiology. 4. St Louis, MO: Elsevier/Mosby; 2008. pp. 273–304. [Google Scholar]
- 3.Bianchi S, Martinoli C. Wrist. In: Bianchi S, Martinoli C, editors. Ultrasound of the Musculoskeletal System. Berlin, Germany: Springer-Verlag; 2007. pp. 425–494. [Google Scholar]
- 4.Lee D, van Holsbeeck MT, Janevski PK, Ganos DL, Ditmars DM, Darian VB. Diagnosis of carpal tunnel syndrome: ultrasound versus electromyography. Radiol Clin North Am. 1999;37:859–872. doi: 10.1016/s0033-8389(05)70132-9. [DOI] [PubMed] [Google Scholar]
- 5.Valle M, Zamorani MP. Nerve and blood vessels. In: Bianchi S, Martinoli C, editors. Ultrasound of the Musculoskeletal System. Berlin, Germany: Springer-Verlag; 2007. pp. 97–136. [Google Scholar]
- 6.Evans KD, Roll SC, Volz KR, Friemer M. Relationship between intra-neural vascular flow measured with sonography and carpal tunnel syndrome diagnosis based on electro diagnostic testing. J Ultrasound Med. 2012;31:729–736. doi: 10.7863/jum.2012.31.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghasemi-Esfe AR, Khalilzadeh O, Vaziri-Bozorg SM, et al. Color and power Doppler US for diagnosing carpal tunnel syndrome and determining its severity: a quantitative image processing method. Radiology. 2011;261:499–506. doi: 10.1148/radiol.11110150. [DOI] [PubMed] [Google Scholar]
- 8.Joy V, Therimadasamy AK, Chan YC, Wilder-Smith EP. Combined Doppler and B-mode sonography in carpal tunnel syndrome. J Neurol Sci. 2011;308:16–20. doi: 10.1016/j.jns.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Akcar N, Ozkan S, Mehmetoglu O, Calisir C, Adapinar B. Value of power Doppler and gray- scale us in the diagnosis of carpal tunnel syndrome: contribution of cross-sectional area just before the tunnel inlet as compared with the cross-sectional area at the tunnel. Korean J Radiol. 2010;11:632–639. doi: 10.3348/kjr.2010.11.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallouhi A, Pultzl A, Trieb T, Piza H, Bodner G. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2006;186:1240–1245. doi: 10.2214/AJR.04.1715. [DOI] [PubMed] [Google Scholar]
- 11.Sommerich CM, Lavender SA, Buford JA, Banks JJ, Korkmaz SV, Pease WS. Toward development of a nonhuman primate model of carpal tunnel syndrome: performance of a voluntary repetitive pinching task induces median mononeuropathy in Macaca fascicularis. J Orthop Res. 2007;25:713–724. doi: 10.1002/jor.20363. [DOI] [PubMed] [Google Scholar]
- 12.Hedrick WR, Hykes DL, Starchman DE. Contrast agents. In: Hedrick WR, Hykes DL, Starchman DE, editors. Ultrasound Physics and Instrumentation. St Louis, MO: Elsevier/Mosby; 2005. pp. 265–271. [Google Scholar]
- 13.Definity (perflutren lipid microsphere) North Billerica, MA: Lantheus Medical Imaging; 2011. package insert. [Google Scholar]
- 14.Klauser A, Frauscher F, Schirmer M, et al. The value of contrast-enhanced color Doppler ultrasound in the detection of vascularization of finger joints in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:647–653. doi: 10.1002/art.10136. [DOI] [PubMed] [Google Scholar]
- 15.Wieczorek AP, Woźniak MM, Stankiewicz A, et al. Quantitative assessment of urethral vascularity in nulliparious females using high-frequency endovaginal ultrasonography. World J Urol. 2011;29:625–632. doi: 10.1007/s00345-011-0732-x. [DOI] [PubMed] [Google Scholar]
- 16.Greener Y, Killam AL, Cornell ST, Osheroff MR, Wolford ST. Nonclinical safety assessment of intravenous Optison®: a perfluoropropane (PFP)-filled albumin microsphere contrast agent for ultrasonography. Int J Toxicol. 1998;17:631–662. [Google Scholar]
- 17.Forsberg F, Liu JB, Patel M, et al. Preclinical acute toxicology study of surfactant-stabilized ultrasound contrast agents in adult rats. Int J Toxicol. 2010;29:32–39. doi: 10.1177/1091581809354342. [DOI] [PubMed] [Google Scholar]
- 18.Evans KD, Volz KR, Hutmire C, Roll SC. Morphologic characterization of intraneural flow associated with median nerve pathology. J Diagn Med Sonography. 2012;28:11–19. doi: 10.1177/8756479311426777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghasemi-Esfe AR, Khalilzadeh O, Mazloumi M, et al. Combination of high-resolution and color Doppler ultrasound in diagnosis of carpal tunnel syndrome. Acta Radiol. 2011;52:191–197. doi: 10.1258/ar.2010.100299. [DOI] [PubMed] [Google Scholar]
- 20.Rahmani M, Ghasemi-Esfe AR, Bozorg SM, Mazloumi M, Khalilzadeh O, Kahnouji H. The ultrasonographic correlates of carpal tunnel syndrome in patients with normal electrodiagnostic tests. Musculoskelet Radiol. 2011;116:489–496. doi: 10.1007/s11547-011-0632-6. [DOI] [PubMed] [Google Scholar]
- 21.Volz KR, Evans KD, Fout LT, Hutmire C, Sommerich CM, Buford JA. Utilization of sonography compared with magnetic resonance imaging in determining the cross-sectional area of the median nerve in a sample of working Macaca fascicularis: a preclinical study. J Diagn Med Sonography. 2012;28:279–288. [Google Scholar]


