Abstract
The γ-tubulin complex, via its ability to organize microtubules, is critical for accurate chromosome segregation and cytokinesis in the fission yeast, Schizosaccharomyces pombe. To better understand its roles, we have purified the S. pombe γ-tubulin complex. Mass spectrometric analyses of the purified complex revealed known components and identified two novel proteins (i.e., Mbo1p and Gfh1p) with homology to γ-tubulin–associated proteins from other organisms. We show that both Mbo1p and Gfh1p localize to microtubule organizing centers. Although cells deleted for either mbo1+ or gfh1+ are viable, they exhibit a number of defects associated with altered microtubule function such as defects in cell polarity, nuclear positioning, spindle orientation, and cleavage site specification. In addition, mbo1Δ and gfh1Δ cells exhibit defects in astral microtubule formation and anchoring, suggesting that these proteins have specific roles in astral microtubule function. This study expands the known roles of γ-tubulin complex components in organizing different types of microtubule structures in S. pombe.
INTRODUCTION
The fidelity of chromosome segregation during mitosis in eukaryotic cells requires the proper assembly and functioning of the mitotic spindle. In most eukaryotic cells, the mitotic spindle is nucleated from a specific structure called the microtubule organizing center (MTOC; Urbani and Stearns, 1999). The centrosome and the spindle pole body (SPB) constitute the MTOC in animal cells and in yeast and fungi, respectively. In addition to its role in nucleation of microtubules, the MTOC also determines the polarity of the microtubule cytoskeleton by anchoring the minus ends of the microtubules (Urbani and Stearns, 1999).
The MTOC includes a multiprotein complex known as the γ-tubulin ring complex (γ-TuRC; Gunawardane et al., 2000a). γ-tubulin, a tubulin variant, participates in the nucleation of microtubules from the MTOC (Horio et al., 1991; Zheng et al., 1991; Oakley, 2000). In higher eukaryotic cells, γ-tubulin is found in two pools, a centrosomal pool and a cytosolic pool (Wiese and Zheng, 1999). The centrosomal γ-TuRC exhibits microtubule nucleating activity (Zheng et al., 1995; Oegema et al., 1999; Murphy et al., 2001) and the complex includes at least five other proteins (in human cells, hGCP2–6) in addition to γ-tubulin (Murphy et al., 1998, 2001; Gunawardane et al., 2000b). The cytosolic γ-tubulin small complex (γ-TuSC), contains just γ-tubulin and two other proteins belonging to the Spc97/98p (hGCP2/3) family and has no microtubule nucleating capacity on its own (Oegema et al., 1999; Zhang et al., 2000). The γ-TuSC is similar in size and composition to the Tub4p complex in Saccharomyces cerevisiae (Geissler et al., 1996; Knop and Schiebel, 1997; Vinh et al., 2002).
Studies on Aspergillus nidulans γ-tubulin suggested roles for it in the nucleation and organization of both mitotic spindles and cytoplasmic microtubules in interphase (Oakley et al., 1990). Consistent with the ability of γ-tubulin to function in the organization of both nuclear and cytoplasmic microtubules, distinct receptors that dock the γ-TuC to either the nuclear or the cytoplasmic side of the SPB have been identified in S. cerevisiae (Knop and Schiebel, 1998). γ-tubulin (tug1+) mutants in the fission yeast, Schizosaccharomyces pombe, exhibit pleiotropic phenotypes indicative of defects in both spindle and cytoplasmic microtubule organization (Horio et al., 1991; Paluh et al., 2000). Tug1p localizes to the SPB throughout the cell cycle and to the equatorial MTOC (EMTOC), a microtubule-organizing structure observed after anaphase in the equatorial region of fission yeast cells (Horio et al., 1991). The EMTOC is thought to be the organizer of a postanaphase array (PAA) of microtubules (Horio et al., 1991) and has recently been shown to play a role in anchoring the medial cytokinetic actin ring (CAR; Pardo and Nurse, 2003). Additional components of the S. pombe γ-tubulin complex, namely, Alp4p, Alp6p, and Alp16p, have subsequently been identified (Vardy and Toda, 2000; Fujita et al., 2002). Alp4p and Alp6p are homologous to the highly conserved Spc97p and Spc98p, respectively, and Alp16p shares regions of similarity with hGCP6 (Vardy and Toda, 2000; Fujita et al., 2002). Unlike Tug1p, Alp4p, and Alp6p, Alp16p function is dispensable for vegetative growth and deletion of alp16+ results in abnormally long cytoplasmic microtubules (Fujita et al., 2002).
A GTPase signaling cascade known as the septation initiation network (SIN) triggers cytokinesis in S. pombe and coordinates septation with nuclear division (Le Goff et al., 1999; McCollum and Gould, 2001). The defect in sin– mutants leads to multinucleate cells (Balasubramanian et al., 1998) with uneven distribution of the nuclei, suggesting that these mutants might also exhibit a nuclear migration defect (Hagan and Yanagida, 1997). Additionally, the SIN pathway has also been shown to be required for the formation and function of the EMTOC (Heitz et al., 2001; Pardo and Nurse, 2003). Sid4p and Cdc11p, two essential SIN components, are localized to the SPB throughout the cell cycle and act as anchors for other SIN components at the SPB (Chang and Gould, 2000; Krapp et al., 2001; Tomlin et al., 2002). Cdc11p is homologous to S. cerevisiae Nud1p, which has been shown to be involved in exit from mitosis and astral microtubule organization (Gruneberg et al., 2000). Cells deleted for cdc11+ also exhibit defects in astral microtubule attachment (Krapp et al., 2001) linking the SIN to astral microtubule organization.
To understand the role of SIN in organizing the EMTOC and PAA, we reasoned that it is important to identify all components of the S. pombe γ-TuC and proteins associated with it and to establish their roles in spindle and cytoplasmic microtubule organization. To that end, we targeted known S. pombe γ-TuC components for purification and identified two previously uncharacterized proteins that we establish are physically and genetically associated with them. We present here the analysis of their role in microtubule organization and γ-TuC function.
MATERIALS AND METHODS
Sequence Comparison
Sequence comparison and homology analyses were performed using ClustalW (http://www.ch.embnet.org/software/ClutalW.html) and Boxshade servers (http://www.ch.embnet.org/software/BOX_form.html).
Yeast Strains and Genetic Methods
S. pombe strains used in this study (Table 1) were grown in yeast extract (YE) or minimal medium with appropriate supplements (Moreno et al., 1991). Crosses were performed in glutamate medium and double-mutant strains were constructed by tetrad analysis. Yeast transformations were performed by electroporation method (Prentice, 1992). Regulated expression of genes from different strengths of nmt1 promoters (Basi et al., 1993; Maundrell, 1993) was achieved by shifting cells grown in media containing thiamine (promoter repressed) to media that lacks thiamine (promoter nonrepressed) after three washes with thiamine-free media.
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| KGY246 | h-leu1-32 ura4-D18 ade6-M210 | Our stock |
| KGY247 | h+leu1-32 ura4-D18 ade6-M210 | Our stock |
| KGY249 | h+leu1-32 ura4-D18 ade6-M216 | Our stock |
| KGY3315 | h-alp4-TAP::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3325 | h-alp6-TAP::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3338 | h+alp4-HA::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3335 | h+alp6-HA::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY4172 | h-gfh1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY4173 | h-alp4-HA::kanR gfh1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY4174 | h-alp6-HA::kanR gfh1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1232 | h-gfh1::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1217 | h-mad2::ura4 leu1-32 ura4-D18 ade6-M210 | S. Sazer |
| KGY2430 | h-mbo1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3312 | h-alp6-HA::kanR mbo1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3313 | h-alp4-HA::kanR mbo1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3941 | h-pcp1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY4473 | h-alp4-HA::kanR pcp1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY4474 | h-alp6-HA::kanR mbo1-myc::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3251 | h-mbo1::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY565 | h-alp16::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3339 | h+alp4-GFP::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3333 | h+alp6-GFP::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3164 | h+mbo1-GFP::kanR leu1-32 ura4-D18 ade6-M210 | This study |
| KGY713 | h-alp4-GFP::kanR alp16::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1239 | h-alp4-GFP::kanR alp6-719 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1240 | h-alp4-GFP::kanR mbo1::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1248 | h-alp4-GFP::kanR gfh1::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY714 | h-alp6-GFP::kanR alp16::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1242 | h-alp6-GFP::kanR alp4-1891 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1243 | h-alp6-GFP::kanR mbo1::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1379 | h-alp4-GFP::kanR gfh1::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1244 | h-mbo1-GFP::kanR alp4-1891 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1245 | h-mbo1-GFP::kanR alp6-719 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1247 | h-mbo1-GFP::kanR alp16::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY1249 | h-mbo1-GFP::kanR gfh1::ura4 leu1-32 ura4-D18 ade6-M210 | This study |
| KGY3187 | h-alp6-719leu1-32 ura4-D18 | T. Toda |
| KGY3188 | h-alp4-1891 | T. Toda |
Epitope tagging alp4+, alp6+, alp16+, mbo1+, and gfh1+
The genes indicated were tagged at their chromosomal loci at their 3′ ends with sequences coding for three copies of the HA epitope, or 13 copies of the Myc epitope, or green fluorescent protein (GFP), or the TAP tag (Tasto et al., 2001) by a PCR-mediated strategy as described previously (Bahler et al., 1998). Proper integration of these epitope cassettes was confirmed by PCR.
Cloning and Expression of gfh1+
The SPBC211.06 ORF was amplified from S. pombe genomic DNA by PCR. To facilitate cloning and expression of this ORF using the nmt1 promoters, a NdeI site and a BamHI site was added to the 5′ and 3′ ends of this PCR fragment. After restriction enzyme digestion, the fragment was cloned into pREP1 (Basi et al., 1993) or pREP81GFP (Maundrell, 1993) plasmids to facilitate overexpression studies and localization analyses, respectively.
Deletion of alp16+, mbo1+, and gfh1+
Deletion of alp16+ and mbo1+ ORFs was achieved by PCR-based one-step homologous recombination according to Bahler et al. (1998). ura4+ was amplified by PCR from plasmid pKG358 using a forward oligonucleotide corresponding to 80 bp upstream of the ATG codon and a reverse oligonucleotide corresponding to 80 bp downstream of the STOP codon. The amplified fragment was transformed into a diploid strain and stable integrants were selected. Deletion of one copy of either alp16+ or mbo1+ in such diploid strains was confirmed by PCR. Sporulation of the heterozygous diploids and tetrad dissection analyses revealed that these genes were dispensable for vegetative growth.
Deletion of gfh1+ was achieved in two steps. A PCR fragment containing gfh1+ and 600-bp flanking sequences on either end of gfh1+ was amplified from S. pombe genomic DNA. The PCR fragment also contained a BamHI and XbaI restriction site at the 5′ and 3′ ends, respectively. After restriction enzyme digestion, this fragment was cloned into the plasmid, pRS413 (Sikorski and Hieter, 1989) to create pKG3088. ura4+ was amplified by PCR from plasmid pKG358 using a forward oligonucleotide corresponding to 80 bp upstream of the ATG codon and a reverse oligonucleotide corresponding to 80 bp downstream of the STOP codon of gfh1+. pKG3088 was linearized with PacI and NcoI to remove a portion of gfh1+. This linear fragment of pKG3088 was transformed along with the ura4+ PCR fragment into S. cerevisiae strain PJ69-4A (James et al., 1996). Plasmids that had recombined in vivo were obtained and checked for the insertion of the ura4+ cassette. One such plasmid, pKG3089, was digested with BamHI and XbaI restriction enzymes to generate a linear fragment containing ura4+ flanked by 600 bp to facilitate integration at the gfh1+ chromosomal locus by homologous recombination. This linear fragment was transformed and the transformants were subjected to similar analyses as described earlier.
Calmodulin Overlay Assay
A portion of the C-terminal region of Mbo1p (aa residues 923-1115) was amplified by PCR from wild-type S. pombe genomic DNA. The PCR product contained EcoRI and XhoI restriction sites at the 5′ and 3′ ends, respectively. The product was cut with these restriction enzymes and cloned into similarly cut pGEX4T-1 to create a GST-Mbo1p fusion. This fusion protein along with GST and GST-Pcp1p (a generous gift of Dr. Trisha Davis) was expressed and purified as described (Tomlin et al., 2002). After purification, the proteins were resolved by SDS-PAGE on two gels run in parallel. One gel was stained with Coomassie and the other was transferred to an Immobilon membrane, followed by a calmodulin overlay assay as described (Flory et al., 2002).
Protein Methods
Total cell extracts of S. pombe were prepared in NP-40 buffer (Gould et al., 1991), and immunoprecipitations were carried out using either 12CA5 (anti-HA) or 9E10 (anti-Myc) antibodies as described (McDonald et al., 1999). Gel filtration was performed on protein lysates prepared in buffer A (20 mM Tris, pH 7.5, 20% glycerol, 0.1 mM EDTA, 1 mM β-mercaptoethanol, 5 mM ATP plus protease inhibitor cocktail [Roche, Mannheim, Germany]) using a Superose-6 column (Amersham Pharmacia Biotech, Piscataway, NJ) by FPLC. The proteins were eluted in buffer A plus 100 mM NaCl. A parallel column was run with standards consisting of dextran (2000 kDa), thyroglobulin (669 kDa), catalase (232 kDa), and aldolase (158 kDa) to calibrate molecular mass. Thirty-four 0.5-ml fractions were collected and because no protein was detected in the first 10 fractions by UV spectroscopy, fractions 11–34 were subjected to immunoblotting. For immunoblotting, proteins were resolved by SDS-PAGE on a 8% gel (except for detecting γ-tubulin-10% gel). Protein transfer, blotting, and ECL detection were performed as described (Tomlin et al., 2002). Protein complexes were obtained using TAP strategy as described (Tasto et al., 2001) with one variation: the lysates were clarified at 3000 rpm on a tabletop GS-6R centrifuge in lieu of ultracentrifugation. TAP pellets were subjected to mass spectrometric analyses as described (Yoon et al., 2002).
Microscopy Analyses
Strains producing chromosomal GFP-fusion proteins were grown in YE medium and subjected to live imaging as described (Tasto et al., 2003). Staining of DNA with DAPI was performed and analyzed as described (Tasto et al., 2003). For time-lapse experiments, cells were placed on a hanging drop glass slide containing either YE agar (for Mbo1p-GFP) or thiamine-free minimal medium agar (for Atb2-GFP) and covered with a coverslip. Time-lapse images were obtained and processed as described (Tasto et al., 2003) except for the following variations: Z-series optical sections were taken at a spacing of 1 μm at intervals of 1 or 1.5 min.
RESULTS
Purification of S. pombe γ-TuC–associated Proteins
To identify additional proteins that might be part of the S. pombe γ-TuC and/or provide a link between the γ-TuC and the SIN, we modified the alp4+ and alp6+ loci to enable the expression of C-terminally tandem affinity purification (TAP)-tagged versions of their protein products (Tasto et al., 2001). Both the alp4-TAP and alp6-TAP strains grew normally, suggesting that the epitope did not compromise the function of either protein. Tandem affinity purification steps were then carried out from both strains and the protein composition of a portion of each TAP complex was analyzed by silver staining (unpublished data) with the remainder analyzed by DALPC tandem mass spectrometry (Yoon et al., 2002). Proteins identified from both purifications at greater than 10% sequence coverage that were absent from the complex purified from untagged cells or from unrelated TAP purifications (unpublished data) are listed in Table 2. In addition to known γ-TuC components, two novel polypeptides encoded by the SPBC211.06 and SPCC417.07c ORFs of molecular weights 66.7 and 128.4 kDa, respectively, were identified. We have named these proteins as Gfh1p (for Gcp Four homolog) and Mbo1p (for microtubule organizer), respectively.
Table 2.
TAP / DALPC results
| Protein ID | Annotation | Alp4p-TAP purificationa | Alp6p-TAP purificationa |
|---|---|---|---|
| Alp4p | SPBC365.15 | 35.5 | 13 |
| Alp6p | SPBC428.20c | 32.8 | 41.8 |
| Alp16p | SPCC4G3.19 | 11.2 | 12.1 |
| Mbo1p | SPCC417.07c | 34.4 | 16.4 |
| Gfh1p | SPBC211.06 | 15.2 | 19 |
| Tug1p | SPBC32F12.04 | 42.2 | 41.9 |
Percentage coverage of each polypeptide with respect to their molecular weights.
gfh1+ Is Similar to hGCP4 and Is Required for the Proper Attachment of Astral Microtubules
As described above, Gfh1p is a novel S. pombe γ-TuC-associated protein and is encoded by ORF SPBC211.06 (Table 2 and Figure 1). Database searches with different parts of the Gfh1p sequence as query revealed that it shares sequence similarity with regions of hGCP4 and its Drosophila ortholog, dGRIP75 (Figure 1A, a blast score of 1.7 × e–07). Of particular interest is the presence of two regions in Gfh1p, from aa 87–182 and 355–503, which exhibit similarity to GRIP motifs 1 and 2 (Figure 1A), motifs that are present in most γ-TuRC members (Gunawardane et al., 2000b).
Figure 1.
Gfh1p is homologous to hGCP4/Dgrip75 and is part of the S. pombe γ-TuC. (A) Alignment of Gfh1p with human GCP4 and Drosophila melanogaster Grip75. Identical residues are indicated by black boxes and conservative substitutions are shaded in gray. (B) Protein lysates were prepared from cells expressing tagged alleles of alp4+, alp6+, gfh1+, or both alp4+ and gfh1+ and alp6+ and gfh1+. These were subjected to immunoprecipitation using either 12CA5 (IP:HA) or 9E10 (IP:MYC) antibodies. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted with either α-HA or α-Myc antibodies as indicated. (C) Protein lysates prepared from either control cells or cells expressing a MYC-tagged allele of gfh1+ were subjected to immunoprecipitation using 9E10 antibodies. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted with either anti–γ-tubulin or α-Myc antibodies as indicated. The arrow in the bottom panel indicates the band corresponding to γ-tubulin and asterisk (*) indicates IgG heavy chain. (D) Protein lysates were prepared from cells expressing tagged alleles of alp6+ and gfh1+ and were subjected to gel filtration on a Superose-6 column. Thirty-four 0.5-ml fractions were collected, and fractions 11–34 were resolved by SDS-PAGE and immunoblotted with either 12CA5 (anti-HA) or 9E10 (anti-MYC) antibodies to detect the tagged proteins as indicated. The peak positions of calibration markers are also indicated on top of the fractions.
To validate the mass spectrometry results, we modified the locus encoding Gfh1p to create a gfh1-myc strain. The tagged gfh1+ allele was combined with alp4-HA or alp6-HA alleles to create double-tagged strains. In an anti-HA immunoprecipitate from alp4-HA gfh1-myc strains but not from single-tagged strains, both Alp4p-HA and Gfh1p-myc were detected (Figure 1B, top panel, IP:HA). Similar specific complex formation was detected when the same strains were immunoprecipitated with anti-Myc antibodies (Figure 1B, top panel, IP:MYC). Identical results were obtained using double-tagged alp6-HA gfh1-myc strains but not either single-tagged strains (Figure 1B, bottom panels). To determine if Gfh1p was in a complex with γ-tubulin, we performed immunoprecipitations using anti-Myc antibodies from untagged and gfh1-myc strains.
Analysis of these immunoprepcipitates revealed that γ-tubulin specifically coimmunoprecipitated with Gfh1p-Myc (Figure 1C). To determine what percent of Gfh1p is present within the γ-tubulin complex, we performed gel filtration on protein lysates prepared from cells expressing both Alp6p-HA and Gfh1p-MYC, as was done previously for components of the S. pombe γ-tubulin complex (Vardy and Toda, 2000). Both Alp6p-HA and Gfh1p-MYC eluted in the void volume consistent with them being in large complexes that are ≥2000 kDa (Figure 1D). Although it is not possible to assess the size of complexes in the void volume, this experiment indicated that all of Gfh1p-MYC is present in a large complex. Taken together, these biochemical data indicate that Gfh1p is the likely S. pombe ortholog of hGCP4/dGRIP75p.
To determine the role of gfh1+ in vivo, we generated a deletion of one copy of the gene in a diploid and replaced it with a copy of ura4+ by homologous recombination. After sporulation, tetrads were dissected and analyzed. Such a procedure revealed that gfh1+ was not essential for vegetative growth (unpublished data and Figure 2A). Combining gfh1Δ with either alp16Δ or mbo1Δ (see below) did not reveal any synthetic genetic interactions. However, deletion of gfh1+ in alp4-1891 or alp6-719 strains reduced their restrictive temperatures from 36 to 30°C (unpublished data), suggesting that the three proteins might share some function in microtubule organization and/or nucleation.
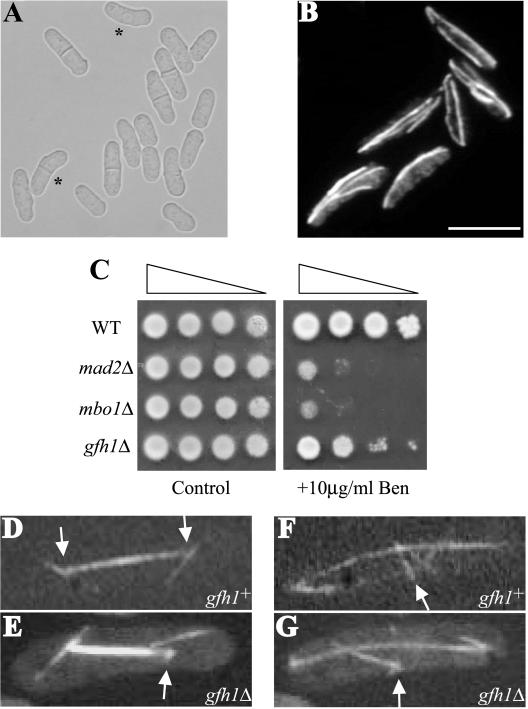
Figure 2.
gfh1Δ cells are viable and exhibit astral microtubule defects. (A) DIC image of exponentially growing gfh1Δ cells. Asterisks indicate bent cells. (B) A field of gfh1Δ cells in interphase stained with TAT-1 antibodies to visualize microtubules. Scale bar, 10 μm. (C) Serial 10-fold dilutions of the cells of the indicated genotype were spotted on YE plate (control) or YE with 10 μg/ml benomyl. (D and E) Live imaging of cells expressing GFP-α-tubulin, illustrating an example of a detached astral microtubule in gfh1Δ cells compared with that gfh1+ cells. The arrows indicate the position of the SPB. (F and G) Live imaging of cells expressing GFP-α-tubulin, illustrating an example of a disorganized EMTOC in gfh1Δ cells compared with that of gfh1+ cells. Arrows indicate the position of the EMTOC ring.
A subset of exponentially growing gfh1Δ cells (7%) exhibited bent or banana-shaped morphology that is indicative of defects in microtubule function (Figure 2A). Mutants that are defective in microtubule function are often sensitive to microtubule-depolymerizing agents such as benomyl (Vardy et al., 2002; Vardy and Toda, 2000). Indeed, gfh1Δ cells were benomyl sensitive, although less so than cells lacking Mad2p, a known component of the spindle assembly checkpoint (He et al., 1997; Figure 2C). Furthermore, staining of gfh1Δ cells with antibodies to detect microtubules did not reveal any major defects in microtubule structures in interphase cells (Figure 2B). However, live confocal imaging of gfh1Δ cells expressing GFP-α-tubulin revealed a reproducible defect in the microtubule organization of mitotic cells. In all 10 mitotic gfh1Δ cells examined by time-lapse videomicroscopy, astral microtubules were observed to detach from the SPBs and float away (see Figure 2E and Video 1). This was never observed in wild-type mitotic cells (0 of 19 cells examined; Figure 2D and Video 2) indicating that gfh1+ function is required for proper anchoring of astral microtubules at SPBs. Despite this defect, spindles of gfh1Δ cells elongated at the same rate as wild-type cells during anaphase B, presumably because new astral microtubules were produced from the SPBs (Video 1). Another microtubule defect observed in all 10 mitotic gfh1Δ cells examined was that a compact EMTOC ring did not form (Figure 2G and Video 1) as it did in wild-type cells (Figure 2F and Video 2), and the PAA in these cells appeared somewhat disorganized and fragmented (unpublished data).
Gfh1p Overexpression Results in Polarity Defects and a G2 Arrest
To gain additional insight into the role of Gfh1p in microtubule function, we overexpressed it from the strong thiamine-repressible nmt1 promoter in wild-type cells. Although microcolonies were able to form, such colonies contained highly elongated and bent cells (Figure 3A). Similarly, in liquid cultures of wild-type cells overproducing Gfh1p, most cells were elongated and some were bent (Figure 3B). FACS analysis revealed that the cells arrested with a 2N DNA content consistent with a G2 arrest (unpublished data). Staining with DAPI and antibodies to tubulin revealed that the cells were uninucleate and contained an interphase array of microtubules. However, the microtubules were fewer in number, if present at all, than in wild-type cells and often curved around the tips of the cell (Figure 3B), suggesting that high levels Gfh1p affected the organization of the interphase microtubule array. Gfh1p overexpression generated the same phenotype in either alp16Δ or mbo1Δ (see below) cells (unpublished data), indicating its role is independent of these genes.
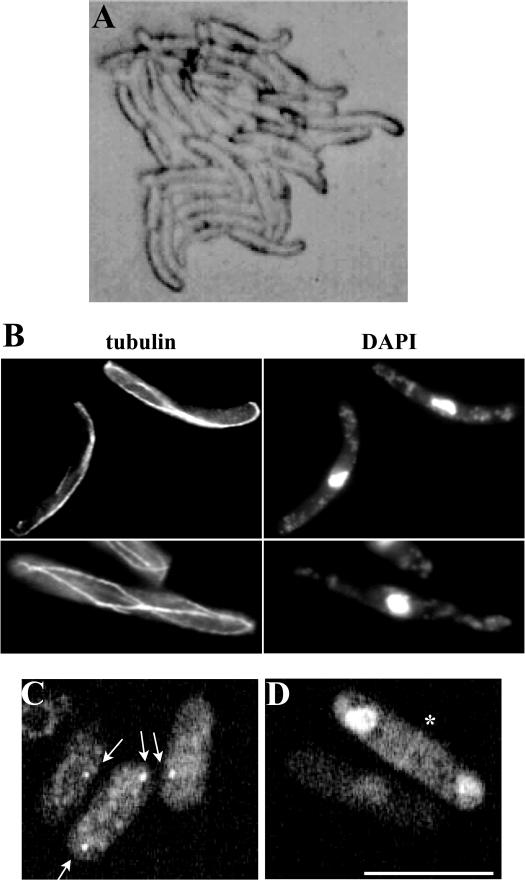
Figure 3.
Gfh1p localizes to the SPB and the EMTOC and its overexpression results in polarity and microtubule defects. (A) Cells of a microcolony overexpressing gfh1+. (B) Cells overexpressing gfh1+ were fixed and stained with either TAT-1 antibodies to visualize microtubules (tubulin) or DAPI to visualize DNA. (C and D) Cells expressing Gfh1p-GFP were subjected to live imaging using a GFP filter set. The localization of the fusion protein to SPBs is indicated by arrows (C) and to the medial EMTOC ring structure in a binucleate cell is indicated by an asterisk (D). Scale bar, 10 μm.
Gfh1p Localizes to the SPB and the Medial EMTOC Ring
To determine the localization of Gfh1p, its cDNA was cloned in frame with GFP downstream of the low-strength thiamine-repressible nmt81 promoter. Cells expressing this fusion protein did not exhibit any detectable phenotypes, suggesting that expression at this level was not toxic. As expected for a γ-TuC component, GFP-Gfh1p localized to either one or two dots juxtaposed to the nucleus (Figure 3C), indicative of SPB localization, and also to a faint ring in the medial region of the cell that is likely the EMTOC (Figure 3D). Additionally, however, GFP-Gfh1p was detected in the nucleus under these circumstances (Figure 3D). Interestingly, the SPB localization pattern of Gfh1p was unperturbed in alp16Δ or mbo1Δ (see below) cells at 27°C or in alp4-1891 and alp6-719 cells shifted to 36°C for 4 h (see Figure 7C). This suggests that the SPB docking of Gfh1p is independent of the presence of core γ-TuC components.
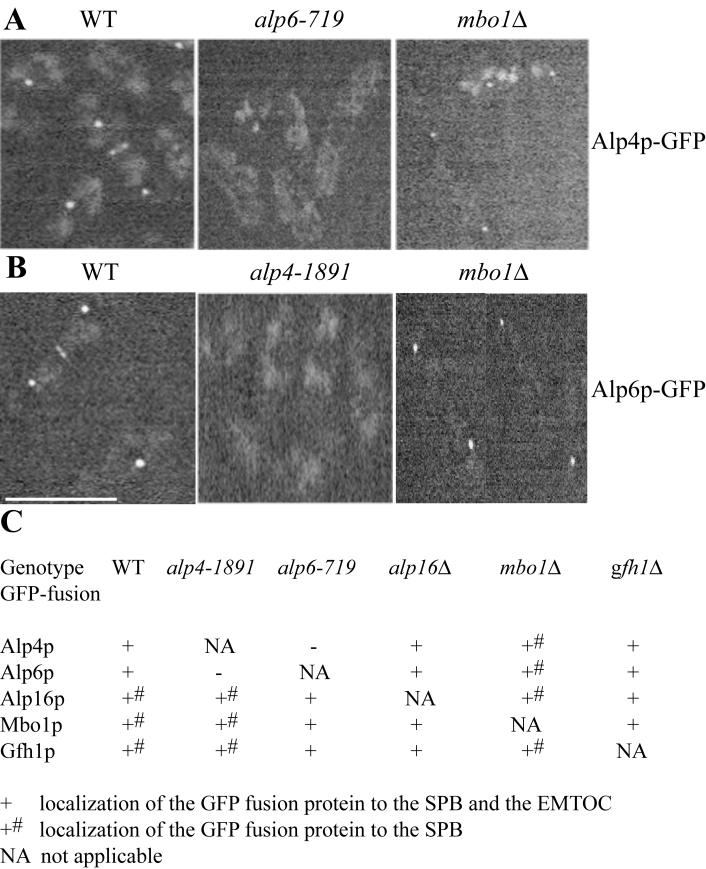
Figure 7.
Localization of γ-TuC components, associated proteins, and their interdependence. (A) Cells expressing Alp4p-GFP were subjected to live imaging using a GFP filter set. The localization of the fusion protein was analyzed in wild-type and alp6-719 cells at 36°C and in mbo1Δ cells was analyzed at 27°C. (B) Cells expressing Alp6p-GFP were subjected to live imaging using a GFP filter set. The localization of the fusion protein was analyzed in wild-type and alp4-1891 cells at 36°C and in mbo1Δ cells was analyzed at 27°C. Scale bar, 10 μm. (C) Summary of the localization studies. The strains of the indicated genotype expressing different GFP-fusion proteins were subjected to live imaging using a GFP filter set. Localization studies in wild-type, alp4-1891, and alp6-719 strains were carried out at 36°C, whereas that in alp16Δ, mbo1Δ, and gfh1Δ cells were carried out at 27°C.
mbo1+ Is Similar to pcp1+ and SPC110 and Is Dispensable for Vegetative Growth
Searches of the S. pombe and S. cerevisiae genome databases with Mbo1p, the protein encoded by the ORF SPCC417.07c, revealed that it shares significant sequence similarity to two known SPB components: S. pombe Pcp1p, a protein homologous to S. cerevisiae Spc110p (Flory et al., 2002), and S. cerevisiae Spc110p, which interacts with and anchors the γ-TuC to the nuclear face of the S. cerevisiae SPB (Knop and Schiebel, 1998; Vinh et al., 2002). Like the aforementioned proteins, Mbo1p contains predicted coiled-coil regions and a region at its extreme C-terminus related to the C-termini of Pcp1p and Spc110p (Figure 4A) that includes their calmodulin-binding sites. Despite some sequence conservation between Mbo1p and its relatives in this region, Mbo1p lacks certain conserved residues that have been implicated in calmodulin binding (Flory et al., 2000), and we therefore sought to determine if this region bound calmodulin. To this end, we produced a GST-Mbo1p fusion protein containing the C-terminus of Mbo1p. A calmodulin overlay assay (Flory et al., 2002) showed that calmodulin specifically bound to the GST-Mbo1p fusion protein as well as to GST-Pcp1p (Figure 4D), suggesting that this region of Mbo1p does contain a calmodulin-binding site. However calmodulin binding to GST-Mbo1p appears to be less efficient than to GST-Pcp1p.
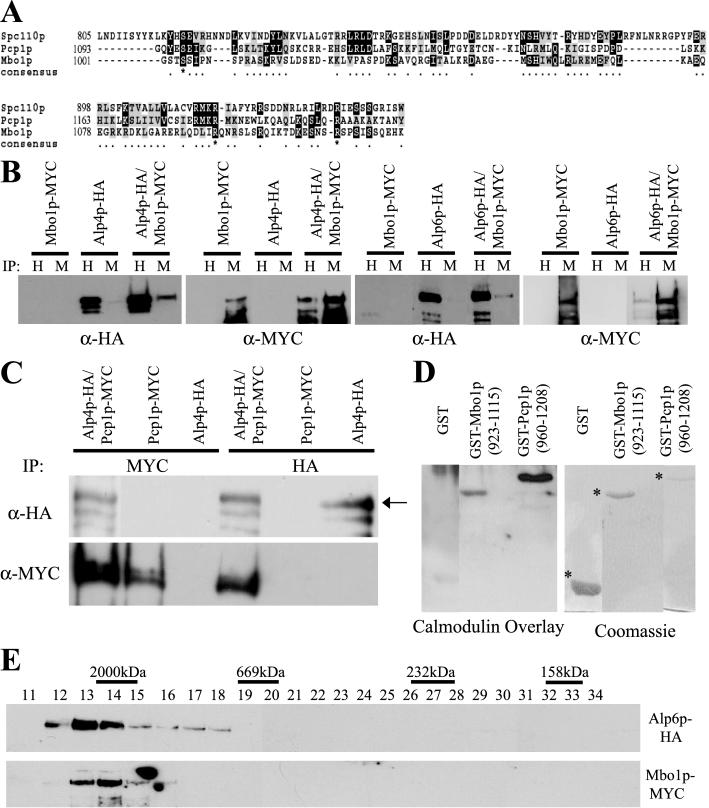
Figure 4.
Mbo1p is related to ScSpc110p and SpPcp1p and both S. pombe proteins associate with the γ-TuC. (A) Alignment of the C-terminal region of Mbo1p, SpPcp1p, and ScSpc110p. Identical residues are shaded in black and conserved residues are shaded in gray. The regions used for this alignment correspond to aa1001–1115 of Mbo1p, aa1093–1208 of Pcp1p, and aa805–944 of Spc110p. (B) Protein lysates prepared from cells expressing tagged alleles of alp4+, alp6+, mbo1+, or both alp4+ and mbo1+ and alp6+ and mbo1+ were subjected to immunoprecipitation using either 12CA5 (IP:H) or 9E10 (IP:M) antibodies. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted with either α-HA or α-Myc antibodies as indicated. (C) Protein lysates prepared from cells expressing tagged alleles of alp4+, pcp1+, or both alp4+ and pcp1+ were subjected to immunoprecipitation using either 12CA5 (IP:HA) or 9E10 (IP:MYC) antibodies. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted with either α-HA or α-Myc antibodies as indicated. The arrow in the top panel indicates the band corresponding to the migration of Alp4p-HA. (D) The C-terminal regions (as indicated) of Mbo1p and Pcp1p were fused to GST and expressed in bacteria were purified along with GST alone. These proteins were resolved by SDS-PAGE and transferred to Immobilon, and the blot was subjected to a Calmodulin overlay assay as described in MATERIALS AND METHODS. A parallel gel was run and stained with Coomassie to visualize the recombinant proteins. The asterisks indicate the position of GST-containing proteins. (E) Protein lysates were prepared from cells expressing tagged alleles of alp6+ and mbo1+ and were subjected to gel filtration on a Superose 6 column. Thirty-four 0.5-ml fractions were collected, and fractions 11–34 were resolved by SDS-PAGE and immunoblotted with either 12CA5 (anti-HA) or 9E10 (anti-MYC) antibodies to detect the tagged proteins as indicated. The peak positions of calibration markers are also indicated on top of the fractions.
To validate the mass spectrometry analyses identifying Mbo1p, the locus encoding Mbo1p was modified to produce MYC- and GFP-tagged variants and the mbo1-myc allele was combined with alp4-HA and alp6-HA alleles. Both Alp4p-HA and Mbo1p-Myc were detected in anti-HA and anti-Myc immunoprecipitates from double-tagged strains but not from either single-tagged control strains (Figure 4B, top panels). Identical specific complex formation was observed between Alp6p-HA and Mbo1p-myc (Figure 4B, bottom panels).
Given that Mbo1p is homologous to Pcp1p and that we detected Pcp1p in our purifications, although at low sequence coverage (1.2%), we wanted to ascertain whether Pcp1p was indeed in a complex with the γ-TuC and whether that complex also contained Mbo1p. First, we constructed strains that expressed both Pcp1p-MYC and Alp4p-HA from their endogenous loci. Immunoprecipitation experiments showed that both Alp4p-HA and Pcp1p-MYC were detected in both anti-HA and anti-MYC immunoprecipitations from double-tagged strains but not control single-tagged strains (Figure 4C). These biochemical data confirm that Pcp1p is in a complex with the γ-TuC, consistent with its localization to the SPB (Flory et al., 2002) and its homology to Spc110p. Interestingly, we did not detect a complex between Mbo1p-MYC and Pcp1p-HA in S. pombe lysates (unpublished data), suggesting that these proteins may be present in different γ-tubulin complexes. To ascertain what percentage of Mbo1p exists within a complex, we performed gel filtration on protein lysates prepared from cells expressing both Alp6p-HA and Mbo1p-MYC. Both Alp6p-HA and Mbo1p-MYC eluted in the void volume, indicating that they were present in complexes ≥2000 kDa (Figure 4E). Mbo1p-MYC existed solely in a large complex because none of the protein was detected in the lower-molecular-weight fractions.
To gain insight into the function of mbo1+, we deleted one copy of this gene in a diploid strain and replaced it with a copy of ura4+ by homologous recombination. After sporulation, tetrads were dissected, and such analyses revealed that mbo1+ was not essential for vegetative growth (unpublished data and Figure 5A). However, an exponentially growing population of mbo1Δ cells exhibited several abnormal phenotypes, some indicative of microtubule defects. First, about one-fourth of the cells (26%) exhibited bent or “banana-shaped” morphology (Figure 5A, *). A second notable phenotype was the varied division length. Although some cells appeared to divide at a normal cell length, others were considerably shorter or longer (Figure 5A, cells 1–3). A quantification of this phenotype revealed the heterogeneity in division length in mbo1Δ cells (Figure 5D). A third striking phenotype was the misplacement of septa in dividing cells; frequently (39% of cells) the septum was not formed in the middle of the cell with respect to its longitudinal axis (Figure 5A, arrows). Finally, mbo1Δ cells were hypersensitive to benomyl, similar to the extent of mad2Δ cells (Figure 2C). Three different combinations of double mutants (i.e., mbo1Δalp16Δ, mbo1Δgfh1Δ, alp16Δgfh1Δ) did not reveal any synthetic genetic interactions (unpublished data), however, suggesting that other factor(s) contribute to microtubule-related functions in the absence of these gene products.
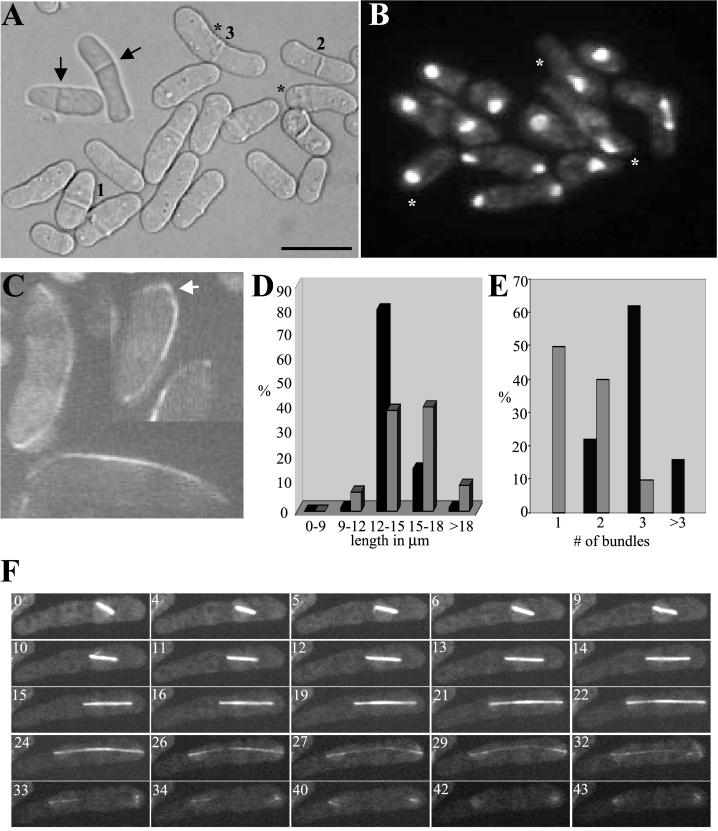
Figure 5.
mbo1Δ cells are viable but exhibit polarity and microtubule and nuclear positioning defects. (A) DIC image of exponentially growing mbo1Δ cells. The numbers 1, 2, and 3 indicate three cells dividing at different cell lengths, and the asterisks indicate bent cells. The inset illustrates two cells with mispositioned septa indicated by arrows. Scale bar, 10 μm. (B) DAPI image of exponentially growing mbo1Δ cells. Asterisks indicate cells in which the nucleus is not positioned in the middle of the cell. (C) Live imaging of interphase mbo1Δ cells expressing GFP-α-tubulin. The arrow indicates a cell in which the microtubule bundle is curved around the tip of the cell. (D) Quantification of cell length at division for wild-type cells (dark bars) and mbo1Δ cells (light bars). (E) Quantification of the cytoplasmic microtubule bundles in wild-type cells (dark bars) and mbo1Δ cells (light bars). (F) Time-lapse confocal microscopy of live mbo1Δ cells expressing GFP-tubulin. The numbers on each panel correspond to the time (in min) elapsed since the capturing of the first frame.
Because the position of the nucleus determines the cleavage plane (Paoletti and Chang, 2000), we stained an exponentially growing population of mbo1Δ cells with DAPI to visualize the position of nuclei. In uninucleate mbo1Δ cells, the nucleus was uncentered in 37% of the cases (Figure 5B, *). This phenotype was observed in only 3% of isogenic wild-type cells (unpublished data).
mbo1Δ Cells Lack Astral Microtubules and Fail to Form an EMTOC
To explore the defects in microtubules, live cell confocal imaging of mbo1Δ cells expressing a GFP-α-tubulin fusion protein was performed. Almost all of the interphase mbo1Δ cells contained 2 or fewer microtubule bundles (Figure 5, C and E). Also, some cells exhibited microtubules that extended beyond the cell tip and curved around the cell end (Figure 5C). Mitotic cells had a pronounced microtubule defect in that they all lacked visible astral microtubules (Figure 5F and Video 3). As mentioned above, the nucleus was frequently not in the middle of the cell when anaphase began and therefore spindle elongation resulted in one of the two daughter nuclei never reaching the end of the cell upon completion of anaphase B (Figure 5F, min 40–43).
Both the onset and rate of spindle elongation were delayed in mbo1Δ cells. Apparently, because of the lack of astral microtubules and the consequent inability to rotate the spindle to orient it along the longitudinal axis of the cells, mbo1Δ cells were frequently stalled at the metaphase-anaphase transition. Illustrating this point is the difference in the timing of anaphase onset between two mitotic mbo1Δ cells (Video 4). These two cells (bottom left and top right, referred to as BL and TR, respectively) possessed short metaphase spindles at the start of observation. Despite the lack of astral microtubules, anaphase ensued in the TR cell and the spindle elongated to reach cell ends. However, the spindle within the BL cell did not align along the longitudinal axis and spindle elongation was not initiated during the course of the observation (28 min). Once spindle elongation began, the average rate in mbo1Δ cells was 0.28 μm/min. This number was derived from the seven mitotic cells examined by time-lapse videomicroscopy. In wild-type cells, the average rate was 0.86 μm/min (n = 7 mitotic cells examined), indicating that spindle elongation in mbo1Δ cells took more than three times longer.
Another abnormality observed in all mbo1Δ cells was the breakdown of the spindle without the formation of an EM-TOC (Figure 5F, 40–43 min). In wild-type cells, the EMTOC is initially seen about the time the spindle reaches its maximum length and helps nucleate microtubules called the PAA (Hagan and Petersen, 2000; see Video 2). Thus, Mbo1p function is required for EMTOC formation.
Mbo1p Localizes to the Spindle Pole Bodies and the EMTOC
Given that Mbo1p copurifies with known γ-TuC components and plays a role in microtubule related processes, we wanted to determine its intracellular localization. To that end, we created a strain in which a GFP tag was fused to the 3′end of the mbo1+ locus to produce Mbo1p-GFP from its endogenous promoter. The mbo1-GFP strain exhibited normal morphology, indicating that the addition of the GFP tag did not affect Mbo1p function (Figure 6A). In an asynchronous population of cells, we observed three different Mbo1p-GFP localization patterns. One or two dots of Mbo1p-GFP juxtaposed to the nucleus were observed in all cells (Figure 6A, arrows). The position of these dots, their dynamics during the cell cycle, and their colocalization with the known SPB protein, Sid4p-CFP (unpublished data), suggests that this dot corresponds to SPBs. Mbo1p-GFP was also detected as a discontinuous ring in binucleate cells, often observed as two cortical dots at the opposite ends of a medial ring (Figure 6A, left panel) or a discrete dot in the medial region of binucleate cells (Figure 6A, right panel). This pattern of Mbo1p-GFP localization is similar to that of other γ-TuC components and is indicative of the EMTOC (Vardy and Toda, 2000; Heitz et al., 2001; Fujita et al., 2002). Mbo1p recruitment to the EMTOC was also visualized by confocal imaging of live cells. As cells progressed through anaphase, Mbo1p-GFP appeared at the medial region of the cell as a ring (Figure 6B). The Mbo1p-GFP signal at the SPBs did not diminish upon the appearance of the ring, suggesting that the ring is formed de novo and not at the expense of Mbo1p-GFP at the SPBs. At even later stages of mitosis, the Mbo1p-GFP ring appeared to constrict (Figure 6B, compare the ring at 11 and 24 min and Video 5). Indeed, the Mbo1p-GFP ring was observed as discrete strands (Figure 6D, 0–6 min and Video 6) that coalesced into a tight ring and further on to a medial spot (Figure 6D, 8–15 min). To gain details into the Mbo1p medial ring structure, we deconvolved these images and rotated them along their vertical axis. One example of such a rotated image illustrates that the Mbo1p-GFP ring is not a continuous structure and that the maximal GFP localization is found at the diametrically opposite cortical spots (Figure 6C). These analyses clearly demonstrate that Mbo1p-GFP is an integral component of the EMTOC and that its association with this ring structure is largely restricted to the cortical region, similar to that of γ-tubulin itself (Heitz et al., 2001).
Figure 6.
Localization of Mbo1p-GFP. (A) Cells expressing Mbo1p-GFP were subjected to live imaging using a GFP filter set. The localization of the fusion protein to SPBs juxtaposed to nuclei and to that of the medial EMTOC is indicated by arrowheads. Scale bar, 10 μm. (B) Time-lapse live imaging of cells expressing Mbo1p-GFP released from a cdc25-22 block to follow synchronous mitosis. Arrows indicate SPB localization, and asterisks indicate the medial ring structure. The numbers indicate the time (in min) elapsed from the capturing of the first frame. (C) 3D-deconvolution image of the medial ring structure that Mbo1p-GFP decorates. The arrowheads indicate the circumferential Mbo1p-GFP ring (D) Time-lapse live imaging of cells expressing Mbo1p-GFP released from a cdc25-22 block to follow synchronous mitosis. The arrow indicates the medial ring structure, and the numbers in each panel indicates the time (in min) elapsed since the capturing of the first frame.
Given that mbo1Δ cells do not form an EMTOC and that Mbo1p localizes to this structure during mitosis, we reasoned that Mbo1p might act as a progenitor in the assembly of the γ-TuC at the medial region. To address this possibility, we analyzed the localization of other γ-TuC components in mbo1Δ cells. Although mbo1Δ cells displayed normal SPB localization of the γ-TuC components tested (Figure 7), these proteins were absent from the medial region of late mitotic cells (for example, see Figure 7). These data are consistent with the absence of the EMTOC structure in mbo1Δ cells and suggest that Mbo1p functions upstream of other γ-TuC members in EMTOC formation.
The SPB Localization of Gfh1p, Mbo1p, and Alp16p Is Independent of Alp4-Alp6p
Temperature-sensitive alp4+ and alp6+ mutants lose the capacity to nucleate microtubules from the SPB at their restrictive temperatures (Vardy and Toda, 2000). To determine whether this correlates with the loss of γ-TuC components from the SPB, we examined the interdependencies of Alp4p, Alp6p, Mbo1p, Alp16p, and Gfh1p for localization. In the alp4-1891 mutant, the localization of Alp16p, Mbo1p, and Gfh1p appeared normal although Alp6p-GFP was lost from the SPB (Figure 7B). Similarly, in the alp6-719 mutant, the only component whose localization was affected was Alp4p-GFP (Figure 7A). In the absence of Alp16p, Mbo1p, and Gfh1p function, Alp4p and Alp6p localized normally to the SPB. Also, Alp16p, Mbo1p, and Gfh1p localized correctly in the absence of the other two components (Figure 7C). These localization patterns are consistent with the distinct loss of function phenotypes associated with these proteins and indicate that Alp16p, Mbo1p, and Gfh1p are anchored to the SPB independent of the core γ-TuC.
DISCUSSION
The γ-TuC is essential for the formation and function of a mitotic spindle, organization of cytoplasmic and astral microtubules, and cytokinesis. However, it is not clear how the complex performs these related processes and whether there are specific roles for its various components at different stages of a cell division cycle. In this article, we describe the functions of two proteins that copurify with known S. pombe γ-TuC components. One (Gfh1p) is the homolog of a known γ-TuRC component from higher eukaryotes, whereas the other (Mbo1p) is similar in structural organization to known calmodulin-binding proteins that associate with the γ-TuC. Our analysis of the S. pombe γ-TuC indicates that it is more similar in composition to the complex found in higher eukaryotes such as Drosophila and humans than in S. cerevisiae. Furthermore, we have identified factors associated with the γ-TuC that specifically influence astral microtubule formation and function.
Gfh1p, a New Addition to the S. pombe γ-TuC
Based on sequence alignments and functional characterization, Gfh1p appears to be the S. pombe homolog of a γ-TuRC component present in higher eukaryotic cells, hGCP4/Dgrip75. An apparent homolog of this protein is not present in S. cerevisiae. Immunodepletion and add-back experiments have suggested that 76p (the Xenopus ortholog of hGCP4) is required for the nucleation of asters from Xenopus sperm basal bodies in vitro (Fava et al., 1999). However, adding 76p back to γ-tubulin–immunodepleted extracts did not restore aster formation, suggesting that 76p is not a limiting factor for microtubule nucleation and that it does not have microtubule-nucleating capacity on its own (Fava et al., 1999). Interestingly, we did not detect any protein in our purifications, nor is one evident in the S. pombe protein database, similar to Dgp71WD, a recent addition to the Drosophila γ-TuRC (Gunawardane et al., 2003).
Our analyses of gfh1Δ cells indicate that Gfh1p does not play a role in the formation of a spindle or an interphase microtubule array but rather in astral microtubule attachment to the SPBs during anaphase in vivo. Unlike the check-point mediated delay in anaphase onset brought about by the absence of astral microtubules in the mia1Δ (Oliferenko and Balasubramanian, 2002), we did not detect a delay in the rate of anaphase spindle elongation in gfh1Δ cells, suggesting that a checkpoint is not invoked by the detachment of astral microtubules during anaphase. Interestingly, there was a rapid replacement of the detached microtubules in gfh1Δ cells and it will be interesting to determine the mechanism by which the cell responds to detached microtubules and proceeds to replenish them.
Given that Gfh1p does not appear to have a role in microtubule nucleation, what role might it play in γ-TuC function? Electron microscopic imaging of the Drosophila γ-TuRC shows that it consists of a ring-like structure formed by the repeated arrangement of γ-tubulin and dGRIPs 84 and 91(homologues of S. cerevisiae Spc97p and Spc98p, respectively). This structure has been speculated to contact the ends of microtubules, whereas an asymmetric cap structure including the other γ-TuRC components (dGRIPs 75, 128, and 163) has been proposed to anchor the whole complex to the centrosome and/or modify the function of the γ-TuRC (Moritz et al., 2000). If we extend this model to the S. pombe γ-TuC, it is conceivable that Gfh1p lies adjacent to a γ-tubulin-Alp4p-Alp6p ring structure as part of the cap and helps to tether microtubules to the cytoplasmic SPBs (Figure 8A). This model predicts that Gfh1p would bind directly to Alp4p/Alp6p and probably indirectly to microtubules, a model that can be tested in future experiments.
Figure 8.
Models for Gfh1p and Mbo1p functions. (A) Gfh1p might play a specialized role as part of a γ-TuC cap structure at the cytoplasmic face of the SPB. This aids proper anchoring of astral microtubules to the SPB (top cell). In the absence of Gfh1p function (bottom cell), the cap would be altered resulting in the detachment of astral microtubules from the SPB. (B) During mitosis, Mbo1p localizes to a discontinuous ring at the medial region (top cell) and recruits other members of the γ-TuC (middle cell). The assembled complex might help facilitate the coalescence of the ring to an EMTOC and nucleate the PAA (bottom cell).
Overexpression of Gfh1p results in reduced numbers of microtubules, a bent morphology and a G2 arrest. Interestingly, certain conditional mutant alleles of tug1+ exhibit similar phenotype under restrictive conditions (Paluh et al., 2000), suggesting that overexpression of Gfh1p (and ablation of Mbo1p function) could interfere with the normal function of γ-tubulin in S. pombe. The curving of the microtubules around the cell ends was reminiscent of the phenotype associated with alp16Δ cells (Fujita et al., 2002), suggesting that these microtubules lost their ability to shrink once they reached cell ends. Such a phenotype could result from increased bundling of microtubules during interphase and/or changes in the loading of microtubule plus-end binding factors and consequent changes to the properties of plus ends. Alternatively, such a phenotype could result from the titration of important proteins normally present at the sites of microtubule nucleation caused by the presence of extramolecules of Gfh1p. The G2 arrest caused by Gfh1p overexpression could similarly be due to its ability to titrate an important factor(s) from microtubule nucleation sites and/or result from activation of a checkpoint that detects a microtubule defect under these conditions such as the cell morphogenesis checkpoint (Rupes et al., 2001) that monitors for proper cell growth and couples it to cell division. Future experiments should reveal Gfh1p-interacting factors that may regulate microtubule dynamics.
Mbo1p and Astral Microtubule Function
Mbo1p, a 128-kDa protein, is similar in primary structure to that of ScSpc110p and SpPcp1p. Interestingly, however, Mbo1p and Pcp1p appear to perform distinct functions as members of separate γ-TuC complexes. Pcp1p function is essential for cell viability (Flory et al., 2002; our unpublished results) and it is likely to be important during mitosis and meiosis for spindle formation and function, whereas Mbo1p is not. Rather, deletion of mbo1+ results in defects associated primarily with astral microtubules and the EMTOC. Hence, we propose that Pcp1p tethers the γ-TuC in the nuclear side of the S. pombe SPB, whereas Mbo1p does so at the cytoplasmic face. Distinguishing these localization patterns will require ultrastructural studies. This model is analogous to that in S. cerevisiae, where Spc72p and Spc110p have been proposed to act as receptors for the γ-TuC at the cytoplasmic and nuclear faces, respectively (Knop and Schiebel, 1998).
The C-termini of both Mbo1p and Pcp1p contain calmodulin-binding regions, although the C-terminal region of Mbo1p does not exhibit high-sequence conservation with the PACT domain, as defined by Gillingham and Munro (2000). S. pombe Cam1p-GFP localizes to the sites of polarized growth and to the SPB. Phenotypic characterization of a conditional calmodulin mutant revealed essential roles for it in spindle formation and function (Moser et al., 1997). It will be interesting to determine its role, if any, in the astral microtubule organization and function and if it exerts such an effect through the regulation of Mbo1p. Because calmodulin appeared to bind Mbo1p less efficiently than Pcp1p in our assay (Figure 4D), it is also conceivable that Mbo1p binds to a different EF-hand protein in vivo, and we point out that the sequence of the S. pombe genome predicts the existence of a second uncharacterized calmodulin-like protein.
Analyses of mia1Δ cells have revealed important roles for astral microtubules in the orientation of the mitotic spindle along the long axis of an S. pombe cell (Oliferenko and Balasubramanian, 2002). mia1Δ cells delay at the metaphase-anaphase transition and recent reports highlight the roles of the spindle orientation and spindle assembly checkpoints in mediating such a delay (Oliferenko and Balasubramanian, 2003; Sato et al., 2003). Similar to mia1Δ cells, mbo1Δ cells display a significant delay in the initiation of anaphase that appears to correspond to the absence of proper spindle alignment. Analyses of spindle dynamics in mbo1Δ cells in combination with either atf1Δ (part of the spindle orientation checkpoint) or components of the spindle assembly check-point will help clarify the roles of these checkpoint proteins in the delay. Unlike the situation with mia1Δ cells, mbo1Δ spindles elongate more slowly than wild-type cells and it will be of interest to determine the cause of this. It is possible, for example, that the interaction and properties of microtubule motors are altered in mbo1Δ cells, leading to slower rate of spindle elongation.
Mbo1p and the EMTOC Function
The EMTOC is formed during late anaphase in S. pombe cells and establishes a postanaphase array of MTs (Hagan and Petersen, 2000). The role of this structure is not clear, although it has recently been proposed to be analogous to the metazoan midbody structure and to play a role in tethering the actomyosin ring between the divided DNA masses during cell division (Pardo and Nurse, 2003). Although septation initiation mutants (sin–) lack an EMTOC, they also fail at cytokinesis and die. Therefore, it has not been possible previously to determine the role of the EMTOC in cell division. Because mbo1Δ cells lack an EMTOC structure but are viable, we can conclude that the EMTOC is not essential for normal S. pombe cell division, a finding consistent with the possibility it plays a restricted role late in anaphase that is uncovered only if cells are delayed at that stage. A phenotype shared between sin– and mbo1Δ mutants is a nuclear positioning defect (Heitz et al., 2001; Pardo and Nurse, 2003). Given this correlation, it is tempting to speculate that it is the lack of EMTOC formation that leads to this defect. In any case, mbo1Δ cells provide a unique opportunity to study the role of EMTOC in the S. pombe cell cycle. Given that Mbo1p is required for the EMTOC localization of other γ-TuC members, Mbo1p may act as a progenitor of the medial microtubule ring (Figure 8B), and it will be interesting to determine the role Mbo1p plays in assembling this structure.
The localization analyses presented in this report suggest that Alp4p and Alp6p (along with γ-tubulin) function together as a complex since mutations in either alp4+ or alp6+ abolish SPB localization of the other and result in more severe microtubule and mitotic defects than loss of gfh1+ or alp16+. This proposal is consistent with observations in S. cerevisiae, demonstrating that ScSpc97p and ScSpc98p directly associate (Knop et al., 1997; Knop and Schiebel, 1997). The other members of the γ-TuC and associated proteins are likely to play important roles as specificity factors for the different aspects of the complex's function. Studies of the S. pombe γ-TuC will help define the distinct roles of these different components in microtubule nucleation, organization, and attachment.
Supplementary Material
Acknowledgments
We thank Takashi Toda and Shelley Sazer for strains, Trisha Davis for providing the GST-Pcp1p construct and calmodulin for the calmodulin overlay assay, Tim Stearns for anti–gamma-tubulin polyclonal antibodies, Craig Vander Kooi for helping with Superose 6 FPLC, and Sally McFall and Rick Morimoto for sharing unpublished results. This work was supported by the Howard Hughes Medical Institute of which K.L.G. is an investigator.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–10–0728. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–10–0728.
Abbreviations used: EMTOC, equatorial microtubule organizing center; MTOC, microtubule organizing center; PAA, postanaphase array; SPB, spindle pole body.
Online version of this article contains supporting material.
Online version is available at www.molbiolcell.org.
References
- Bahler, J., Wu, J.Q., Longtine, M.S., Shah, N.G., McKenzie, A., 3rd, Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, M.K., McCollum, D., Chang, L., Wong, K.C.Y., Naqvi, N.I., He, X., Sazer, S., and Gould, K.L. (1998). Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149, 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi, G., Schmid, E., and Maundrell, K. (1993). TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123, 131–136. [DOI] [PubMed] [Google Scholar]
- Chang, L., and Gould, K.L. (2000). Sid4p is required to localize components of the septation initiation pathway to the spindle pole body in fission yeast. Proc. Natl. Acad. Sci. USA 97, 5249–5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava, F., Raynaud-Messina, B., Leung-Tack, J., Mazzolini, L., Li, M., Guillemot, J.C., Cachot, D., Tollon, Y., Ferrara, P., and Wright, M. (1999). Human 76p: a new member of the gamma-tubulin-associated protein family. J. Cell Biol. 147, 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory, M.R., Morphew, M., Joseph, J.D., Means, A.R., and Davis, T.N. (2002). Pcp1p, an Spc110p-related calmodulin target at the centrosome of the fission yeast Schizosaccharomyces pombe. Cell Growth Differ. 13, 47–58. [PubMed] [Google Scholar]
- Flory, M.R., Moser, M.J., Monnat, R.J., Jr., and Davis, T.N. (2000). Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc. Natl. Acad. Sci. USA 97, 5919–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, A., Vardy, L., Garcia, M.A., and Toda, T. (2002). A fourth component of the fission yeast gamma-tubulin complex, Alp16, is required for cytoplasmic microtubule integrity and becomes indispensable when gamma-tubulin function is compromised. Mol. Biol. Cell 13, 2360–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler, S., Pereira, G., Spang, A., Knop, M., Soues, S., Kilmartin, J., and Schiebel, E. (1996). The spindle pole body component Spc98p interacts with the gamma-tubulin-like Tub4p of Saccharomyces cerevisiae at the sites of microtubule attachment. EMBO J. 15, 3899–3911. [PMC free article] [PubMed] [Google Scholar]
- Gillingham, A.K., and Munro, S. (2000). The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 1, 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, K.L., Moreno, S., Owen, D.J., Sazer, S., and Nurse, P. (1991). Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. Eur. J. Mol. Biol. 10, 3297–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg, U., Campbell, K., Simpson, C., Grindlay, J., and Schiebel, E. (2000). Nud1p links astral microtubule organization and the control of exit from mitosis. Eur. J. Mol. Biol. 19, 6475–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R.N., Lizarraga, S.B., Wiese, C., Wilde, A., and Zheng, Y. (2000a). gamma-tubulin complexes and their role in microtubule nucleation. Curr. Top. Dev. Biol. 49, 55–73. [DOI] [PubMed] [Google Scholar]
- Gunawardane, R.N., Martin, O.C., Cao, K., Zhang, L., Dej, K., Iwamatsu, A., and Zheng, Y. (2000b). Characterization and reconstitution of Drosophila gamma-tubulin ring complex subunits. J. Cell Biol. 151, 1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan, I.M., and Petersen, J. (2000). The microtubule organizing centers of Schizosaccharomyces pombe. Curr. Top. Dev. Biol. 49, 133–159. [DOI] [PubMed] [Google Scholar]
- Hagan, I.M., and Yanagida, N. (1997). Evidence for cell cycle-specific, spindle pole body-mediated nuclear positioning in the fission yeast Schizosaccharomyces pombe. J. Cell Sc i. 110, 1851–1866, [DOI] [PubMed] [Google Scholar]
- He, X., Patterson, T.E., and Sazer, S. (1997). The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA 94, 7965–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz, M.J., Petersen, J., Valovin, S., and Hagan, I.M. (2001). MTOC formation during mitotic exit in fission yeast. J. Cell Sci. 114, 4521–4532. [DOI] [PubMed] [Google Scholar]
- Horio, T., Uzawa, S., Jung, M.K., Oakley, B.R., Tanaka, K., and Yanagida, M. (1991). The fission yeast gamma-tubulin is essential for mitosis and is localized at microtubule organizing centers. J. Cell Sci. 99(Pt 4), 693–700. [DOI] [PubMed] [Google Scholar]
- James, P., Halladay, J., and Craig, E.A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., Pereira, G., Geissler, S., Grein, K., and Schiebel, E. (1997). The spindle pole body component Spc97p interacts with the gamma-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J. 16, 1550–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., and Schiebel, E. (1997). Spc98p and Spc97p of the yeast gamma-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 16, 6985–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., and Schiebel, E. (1998). Receptors determine the cellular localization of a gamma-tubulin complex and thereby the site of microtubule formation. EMBO J. 17, 3952–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp, A., Schmidt, S., Cano, E., and Simanis, V. (2001). S. pombe cdc11p, together with sid4p, provides an anchor for septation initiation network proteins on the spindle pole body. Curr. Biol. 11, 1559–1568. [DOI] [PubMed] [Google Scholar]
- Le Goff, X., Utzig, S., and Simanis, V. (1999). Controlling septation in fission yeast: finding the middle, and timing it right. Curr. Genet. 35, 571–584. [DOI] [PubMed] [Google Scholar]
- Maundrell, K. (1993). Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127–130. [DOI] [PubMed] [Google Scholar]
- McCollum, D., and Gould, K.L. (2001). Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 11, 89–95. [DOI] [PubMed] [Google Scholar]
- McDonald, W.H., Ohi, R., Smelkova, N., Frendewey, D., and Gould, K.L. (1999). Myb-related fission yeast cdc5p is a component of a 40S snRNP-containing complex and is essential for pre-mRNA splicing. Mol. Cell. Biol. 19, 5352–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Moritz, M., Braunfeld, M.B., Guenebaut, V., Heuser, J., and Agard, D.A. (2000). Structure of the gamma-tubulin ring complex: a template for microtubule nucleation. Nat. Cell Biol. 2, 365–370. [DOI] [PubMed] [Google Scholar]
- Moser, M.J., Flory, M.R., and Davis, T.N. (1997). Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J. Cell Sci. 110, 1805–1812. [DOI] [PubMed] [Google Scholar]
- Murphy, S.M., Preble, A.M., Patel, U.K., O'Connell, K.L., Dias, D.P., Moritz, M., Agard, D., Stults, J.T., and Stearns, T. (2001). GCP5 and GCP 6, two new members of the human gamma-tubulin complex. Mol. Biol. Cell 12, 3340–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S.M., Urbani, L., and Stearns, T. (1998). The mammalian gamma-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J. Cell Biol. 141, 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley, B.R. (2000). gamma-Tubulin. Curr. Top. Dev. Biol. 49, 27–54. [DOI] [PubMed] [Google Scholar]
- Oakley, B.R., Oakley, C.E., Yoon, Y., and Jung, M.K. (1990). Gamma-tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell 61, 1289–1301. [DOI] [PubMed] [Google Scholar]
- Oegema, K., Wiese, C., Martin, O.C., Milligan, R.A., Iwamatsu, A., Mitchison, T.J., and Zheng, Y. (1999). Characterization of two related Drosophila gamma-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliferenko, S., and Balasubramanian, M.K. (2002). Astral microtubules monitor metaphase spindle alignment in fission yeast. Nat. Cell Biol. 4, 816–820. [DOI] [PubMed] [Google Scholar]
- Oliferenko, S., and Balasubramanian, M.K. (2003). Reply. Deletion of Mia1/Alp7 activates Mad2-dependent spindle assembly checkpoint in fission yeast. Nat. Cell Biol. 5, 766. [DOI] [PubMed] [Google Scholar]
- Paluh, J.L., Nogales, E., Oakley, B.R., McDonald, K., Pidoux, A.L., and Cande, W.Z. (2000). A mutation in gamma-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein pkl1p. Mol. Biol. Cell 11, 1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti A., and Chang, F. (2000). Analysis of Mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. Biol. Cell 11, 2757–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo, M., and Nurse, P. (2003). Equatorial retention of the contractile actin ring by microtubules during cytokinesis. Science 300, 1569–1574. [DOI] [PubMed] [Google Scholar]
- Prentice, H.L. (1992). High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 20, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupes, I., Webb, B.A., Mak, A., and Young, P.G. (2001). G2/M arrest caused by actin disruption is a manifestation of the cell size checkpoint in fission yeast. Mol. Biol. Cell 12, 3892–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, M., Koonrugsa, N., Toda, T., Vardy, L., Tournier, S., and Millar, J.B. (2003). Deletion of Mia1/Alp7 activates Mad2-dependent spindle assembly checkpoint in fission yeast. Nat. Cell Biol. 5, 764–766; author reply, 766. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasto, J.J., Carnahan, R.H., McDonald, W.H., and Gould, K.L. (2001). Vectors and gene targeting modules for tandem affinity purification in Schizosaccharomyces pombe. Yeast 18, 657–662. [DOI] [PubMed] [Google Scholar]
- Tasto, J.J., Morrell, J.L., and Gould, K.L. (2003). An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin, G.C., Morrell, J.L., and Gould, K.L. (2002). The spindle pole body protein cdc11p links sid4p to the fission yeast septation initiation network. Mol. Biol. Cell 13, 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani, L., and Stearns, T. (1999). The centrosome. Curr. Biol. 9, R315–R317. [DOI] [PubMed] [Google Scholar]
- Vardy, L., Fujita, A., and Toda, T. (2002). The gamma-tubulin complex protein Alp4 provides a link between the metaphase checkpoint and cytokinesis in fission yeast. Genes Cells 7, 365–373. [DOI] [PubMed] [Google Scholar]
- Vardy, L., and Toda, T. (2000). The fission yeast gamma-tubulin complex is required in G(1) phase and is a component of the spindle assembly check-point. Eur. J. Mol. Biol. 19, 6098–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh, D.B., Kern, J.W., Hancock, W.O., Howard, J., and Davis, T.N. (2002). Reconstitution and characterization of budding yeast gamma-tubulin complex. Mol. Biol. Cell 13, 1144–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, C., and Zheng, Y. (1999). Gamma-tubulin complexes and their interaction with microtubule-organizing centers. Curr. Opin. Struct. Biol. 9, 250–259. [DOI] [PubMed] [Google Scholar]
- Yoon, H.J., Feoktistova, A., Wolfe, B.A., Jennings, J.L., Link, A.J., and Gould, K.L. (2002). Proteomics analysis identifies new components of the fission and budding yeast anaphase-promoting complexes. Curr. Biol. 12, 2048–2054. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Keating, T.J., Wilde, A., Borisy, G.G., and Zheng, Y. (2000). The role of Xgrip210 in gamma-tubulin ring complex assembly and centrosome recruitment. J. Cell Biol. 151, 1525–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., Jung, M.K., and Oakley, B.R. (1991). Gamma-tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell 65, 817–823. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Wong, M.L., Alberts, B., and Mitchison, T. (1995). Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature 378, 578–583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.