Abstract
Purpose
This study investigated factors associated with declines in emotional well-being (EWB) over time in breast cancer survivors.
Methods
Women with breast cancer (Stages I-III) residing in Los Angeles, CA or Detroit, MI and reported to the Surveillance, Epidemiology, and End Results registries between June 2005-February 2007 completed surveys at 9 months and 4 years after diagnosis. EWB was measured by the Functional Assessment of Cancer Treatment-Breast. Using a stress coping framework, logistic regression models assessed associations between personal, social, and clinical correlates, appraisal (e.g., worry about recurrence) and coping factors (e.g., emotional support) to EWB declines.
Results
Among eligible women who completed primary breast cancer treatment, 772 completed both surveys and 192 (24.9%) experienced EWB declines over time. Women with past or current depression were more likely to report EWB decline (p<.01). Survivors who perceived they did not receive enough information about risk of breast cancer recurrence during primary treatment were more likely to have EWB decline (OR 0.53, 95% CI 0.32-0.87). Greater perceived likelihood of recurrence (OR 1.95, 95% CI 1.01-5.29) and increased worry about recurrence (OR 1.38, 95% CI 1.10-1.72) were associated with EWB decline. Higher spirituality beliefs and practices were associated with EWB decline.
Conclusions
A considerable number of breast cancer patients report emotional well-being declines over time. Early identification of women who are vulnerable, such as women with past depression, is crucial to improve quality of care. Women would benefit from education about cancer recurrence and tailored strategies to manage worry about recurrence over time.
Implication for Cancer Survivors
Understanding actual risk of recurrence and managing worry about recurrence is important for cancer survivors. Emotional concerns are common for individuals with cancer so survivors should feel free to reach out and discuss such concerns with providers well into the survivorship period.
Introduction
Improvements in the diagnosis and treatment of breast cancer have resulted in greater numbers of women living in post-treatment survivorship [1]. The proliferation of breast cancer survivorship research over the last decade has been associated with a subsequent increase in the number of studies about long term quality of life (QOL) in disease-free women [2-4]. Quality of life is conceptualized as a multi-dimensional construct that includes physical, social, functional and emotional well-being. Most longitudinal studies to date suggest that women generally experience improvements in QOL as they move further away from the diagnosis and primary treatment experience 5-9]. However, there is a subset of women who experience declines in QOL over time [7, 10, 11]. Despite this mixed evidence on the trajectory of QOL over time, few studies have identified which women are at risk for worse outcomes [3, 8, 12, 13] and/or what factors are associated with long-term adjustment [8, 13]. Among the important dimensions of QOL, one that deserves further attention in survivorship research is the longer term impact of a breast cancer diagnosis on emotional well-being (EWB).
Many studies that have examined QOL have used cross-sectional designs and have sampled participants with wide variation since time of breast cancer diagnosis. Most of these studies report that the QOL of breast cancer survivors varies across sociodemographic factors [3, 9, 14]. In general, women who are younger [15], have lower levels of education [16], and those who are unmarried/not partnered at diagnosis [17] report lower QOL during the survivorship period. Some studies suggest racial/ethnic variation in QOL among survivors, with Latina women reporting more physical limitations [18] and African American (AA) women reporting both poorer physical and social well-being but less emotional distress compared with white women [19]. Latinas with lower levels of acculturation have been found to have lower overall QOL as assessed by the Functional Assessment of Cancer Treatment – Breast (FACT-B), and specifically lower functional well-being scores [3]. More advanced cancer stage [20], the presence of other comorbidities at the time of the cancer diagnosis [21] and the receipt of chemotherapy [9, 15, 22, 23] have been associated with lower QOL. Additionally, prior depression is known to put women who are newly diagnosed with breast cancer at risk for greater emotional distress during survivorship [24, 25].
The studies that have reported sociodemographic differences specifically as it relates to EWB suggest that compared to their older counterparts, younger women report lower EWB [18, 26-28]. Regarding racial/ethnic differences, Latinas have reported lower EWB compared to African American [18] and white women [13, 21]. A few studies, including our own work [3], have found that Latina women with limited English proficiency are particularly vulnerable to lower EWB [13]. Low income and less education have also been associated with lower EWB in breast cancer survivors [13]. Most of these studies to date are cross-sectional with wide ranges of time since breast cancer diagnosis.
Other factors that may contribute to our understanding of women at risk for declines in EWB include a growing body of research suggesting that worry about recurrence consistently ranks among the most pressing concerns identified by cancer survivors [15, 16, 29-39]. In addition, identifying women with unmet information needs [40-43] or those who experience decision regret may provide further understanding of those at risk for emotional distress [44]. Coping factors used by women in survivorship related to EWB deserve further attention. Previous studies have found a positive correlation between social support and EWB, however, most have examined the relationship early in survivorship [8, 45, 46]. The various sources of support (e.g., family, provider) as well as types of support (informational, decisional, and emotional) may contribute to our understanding of survivor needs over time [47, 48]. The role of spirituality on emotional well-being has not been fully explored, especially in longer term survivorship.
To improve the psychosocial care delivered to survivors of breast cancer, there remains a need to better understand what factors are associated with declines in EWB over time. Improved understanding of these factors can lead to earlier identification of women most vulnerable to declines as well as to the development of interventions to address these factors. While prior research has linked demographic and treatment-related factors to quality of life outcomes, many of these findings (a) have been limited by cross-sectional designs, (b) do not focus specifically on emotional well-being, (c) have relatively small samples drawn from a single institution, or (d) have samples with limited racial/ethnic diversity. This study addressed the current gaps in the literature by examining the following questions in a population-based multi-ethnic longitudinal study of women who had completed primary treatment for breast cancer:
What are the personal, social, and clinical correlates of decline in EWB over time?
Are women's appraisal factors such as their risk of/worry about cancer recurrence, unmet information needs or decisional regret, and women's coping factors such as emotional support and spirituality associated with clinically significant declines in EWB and do such appraisal factors and coping factors mediate the relationship between EWB and personal, social and clinical factors?
Methods
Conceptual Model
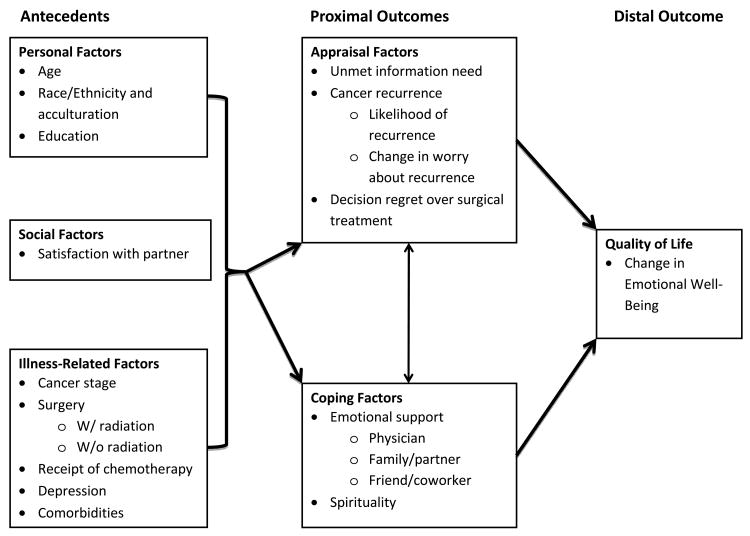
A modified stress/appraisal theoretical framework adapted from Lazarus [49, 50] and used by Northouse et al. [51] guided our study (See Figure 1). The stress appraisal framework includes antecedent variables (personal factors, social factors, and illness-related factors) and proximal outcomes (e.g., appraisal factors and coping factors) that directly or indirectly affect the distal outcome of QOL, defined in this study as change in EWB between the original and follow up survey. Antecedent variables exert an influence on appraisal factors (e.g., unmet information needs, appraisal of cancer recurrence, decision regret) and coping factors (e.g., emotional support and spirituality). In turn these proximal outcomes influence the distal outcome.
Figure 1. Modified Stress-Coping Conceptual Model.
Study population and data collection
Eligible participants for the overall study were women in the metropolitan area of Los Angeles, California, and Detroit, Michigan, aged 20 to 79 years and diagnosed with invasive breast cancer (stages 0 through III) between June 2005 and February 2007, reported to the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program registries. To ensure sufficient representation of racial/ethnic minorities, Latina (in Los Angeles) and African American (in both Los Angeles and Detroit) patients were oversampled. Patients were identified via rapid case ascertainment as they were reported monthly to the collaborating SEER registries, yielding a representative sample of the two metropolitan areas. We notified physicians of our intent to contact patients, followed by the patient mailing which included a letter, survey materials, risk and benefit consent sheet, a return envelope, and a $10 cash gift. All materials were sent in English and Spanish to those with Spanish surnames. The study protocol was approved by the institutional review boards of the University of Michigan, University of Southern California, and Wayne State University and endorsed implicit patient consent based on the provision of appropriate information about the study and completion of the survey.
For the larger study, women were initially recruited and surveyed at an average time of nine months after diagnosis (completion window: mean=9, min=5, max=14 months). Hereafter, the initial survey timepoint is referred to as “Time 1”. At the Time 1 survey, the majority of women had completed primary treatment for their breast cancer although approximately 28% were in the final phases of either radiation or chemotherapy. The details of the data collection protocol have been published elsewhere [3, 52]. We then conducted a follow-up study and surveyed these women again approximately four years later (completion window in months: Mean=50, SD=4.4, min=36, max=65). Hereafter, this follow-up survey is referred to as “Time 2”. To encourage survey response, a modified Dillman survey method was used for both surveys that includes (1) an initial mailing; (2) a reminder contact; (3) a second survey to nonrespondents; and (4) follow-up calls to assess final status.
Our case ascertainment methods identified 3,252 women. We excluded 119 women for the following reasons: 59 too ill to participate, 23 denied a cancer diagnosis, the patient's physician refused contact for 20 patients, and 17 spoke a language other than English or Spanish. Another 432 women could not be reached and 411 women were contacted but did not respond. The baseline survey was completed by 2,290 women, for a response rate of 73%. At the four year follow up survey, 1,536 women who met eligibility criteria for these analyses completed the baseline survey also completed the follow up survey at four years, for an overall response rate of 67 percent. Ten patients who were missing EWB information were excluded from the analysis. Given our interest in the impact of EWB in breast cancer survivors who had invasive disease, for this study we excluded 405 women who were diagnosed with Stage 0 (ductal carcinoma in situ). We further excluded 349 women who had not completed primary treatment at the time of the first survey, resulting in an analytic sample of 772.
Survey Measures
The Time 1 and Time 2 survey measures were based on our conceptual model and prior work with the population [3, 52, 53]. Pilot tests confirmed measure validity and response reliability in both English-and Spanish-speaking women. To assure congruence between English and Spanish versions of the survey, forward and back translation techniques were performed. We examined reliability by computing internal consistency tests. Measures were collected at either the Time 1 survey, Time 2 survey, or at both Time 1 and Time 2 as specified below.
Dependent Variable
Our distal outcome was decline in EWB, as assessed by decline in the EWB scale score of the Functional Assessment of Cancer Therapy-Breast (FACT-B) [53] from Time 1 to Time 2. Women were asked to indicate “how true” 6 items were in describing their experience over the past seven days (e.g., I feel sad, I am satisfied with how I am coping with my illness, and I feel nervous) on a 5-point Likert scale from “not at all” to “very much”. Consistent with previous work establishing a clinically meaningful difference in the EWB scale [55] we computed the difference between total EWB scores between Time 1 and Time 2, and considered a negative change (or drop) of 2.0 points or greater to represent a meaningful decline in EWB. For analysis purposes, we dichotomized the EWB dependent variable into two categories: (1) no decline in EWB (less than a 2-point drop in EWB from Time 1 to Time 2) versus (2) decline in EWB (greater than or equal to a 2-point drop in EWB from Time 1 to Time 2).
Independent Variables
Antecedent Factors
Personal factors were obtained at Time 1 unless noted otherwise. They included age (continuous), and education (some high school, high school graduate, some college or more). Race was first determined as (white, African American and Latina, and then Latinas were further specified as high or low acculturated). The Short Acculturation Scale for Hispanics (SASH Manual) determined language preference for Latina women. This measure is an efficient, reliable, and valid measure to identify Latinas with low or higher acculturation [56]. The four items in SASH indicate preference for English or Spanish in different contexts (usually read/speak, think, use at home, use with friends) on a 5-point scale (from “English only” to “Spanish only”). We aggregated across the four items to calculate a mean score. Latina women who scored ≤ 4 on the 5-point scale strongly preferred Spanish across contexts, and thus were considered less acculturated. Race/ethnicity was thus divided into four categories (White, African American, Latinas-higher acculturation [Latinas-high], and Latinas-lower acculturation [Latinas-low]).
Social factors were measured as “Satisfaction with partner” at Time 2. This measure was assessed with a 6-item scale modified from Heyman et al. [57], and was scored on a five-point scale to reflect satisfaction with communication, conflict resolution, sexual satisfaction, sharing household duties, intimacy, and overall satisfaction (from “very unsatisfied” to “very satisfied”). For modeling purposes, we combined this measure with marital status to form a categorical variable as follows: 1) respondent did not report a partner; 2) respondent is slightly satisfied or very satisfied with partner relationship, or; 3) respondent is neutral, slightly or very unsatisfied with their partner.
Illness-related factors were obtained from both the patient survey and data abstracted by the SEER. Cancer stage at diagnosis (I, II, or III) was obtained at Time 1 from the participating cancer registries. Patient surveys at Time 1 captured type of breast operation (mastectomy or lumpectomy), receipt of radiation therapy (yes/no) and, receipt of chemotherapy (yes/no). Given our focus on EWB, at Time 2 we specifically measured depression history (no history, history of depression without current symptoms, history of depression with current symptoms). We also inquired about the presence (i.e., have you ever been told by a doctor that you have the following condition, yes/no) of nine other comorbid conditions on the Charlson comorbidity index [58].
Proximal Outcomes
Proximal outcomes were categorized into appraisal factors and coping factors. Within appraisal factors, women were asked at Time 1 to indicate whether they had received enough information from their doctors or the staff about risk of breast cancer recurrence (yes/no). In addition, women were assessed at Time 2 regarding their perceived likelihood of breast cancer recurrence and their worry about recurrence at Time 1 and 2. Perceived likelihood of recurrence was measured by asking women how likely it is that their cancer will recur (”not at all likely” to “very likely to recur”). Worry about recurrence was the mean score across three items (each on a five-point Likert scale): concern the cancer would recur in the same breast, the other breast, or to another part of the body. In this study, the worry about recurrence variable considered is the change from Time 1 to Time 2 scores. Third, we used an existing measure to assess decision regret with their treatment [44], categorized as low, medium, or high for descriptive analyses. In final regression analyses, 1= a lot of decision regret versus 0 = none or some decision regret.
Coping factors included the perceived strength of emotional support and the respondent's reported spirituality. At Time 2, we asked women to assess the degree to which they received support from 1) health care providers 2) family members, and 3) friends/co-workers/members of their religious community on a scale from 1-5. The strength of emotional support was dichotomized (1= quite a bit/a lot and 0 = none/a little/some). At Time 2, we measured spirituality using a modified version of the System of Beliefs Inventory – 15 revised (SBI-15R) [59]. The modified measure included four items from the Beliefs and Practices subscale (e.g., “Religion is important in my day-to-day life”, “Prayer has helped me cope during times of serious illness”) and four items from the Social Support subscale (e.g., “I enjoy attending religious functions held by my religious or spiritual group”, “I know someone in my religious or spiritual community that I can turn to”). Items from each subscale were averaged for all participants, with a range of values from 1 (strongly disagree) to 5 (strongly agree). The mean score was used analytically with higher scores on both subscales indicating stronger/higher beliefs and practices or social support.
Analyses
We first compared key variables of the responders who completed both Time 1 and Time 2 surveys with those of non-responders based on information from SEER registry on the sampled patients. We then calculated summary statistics on our overall analytical sample and compared the distributions of antecedent and proximal factors between patients who experienced emotional decline and those who did not. For these two-group comparisons, we used Chi-squared tests for categorical variables and Wilcoxon signed-rank tests for continuous variables.
We fitted separate logistic regression models to examine the association between decline in EWB (adjusting for Time 1 EWB) and each of the proximal outcomes while adjusting for antecedent variables (age, race, education, satisfaction with relationship, cancer stage, primary treatment, depression diagnosis, comorbidities) and geographic location. We then reported the covariate-adjusted probabilities of emotional decline by each of the proximal factors.
To examine whether proximal outcomes mediate the associations between antecedent variables and decline in emotional well-being, we fitted two logistic regressions. Both models consist of antecedent factors, Time 1 EWB levels and geographic locations. Model 2 includes proximal factors while Model 1 does include such factors in the Model. We examined the changes in the magnitudes of associations between antecedents and the emotional decline with and without the inclusion of proximal outcomes.
All analyses described previously were weighted to account for differential probabilities of sample selection and unit non-response and to allow our statistical inference more representative of the targeted population [60]. We calculated the design weight to compensate for disproportionate sampling across sites and race. . We also calculated the unit nonresponse weight to account for the fact that people with certain characteristics are less likely to respond in both waves of surveys. The final weight is the multiplication of design weight and unit nonresponse weight. We incorporated the weight using proper survey procedures (e.g., PROC SURVEYLOGISTIC) in all analyses using SAS software, Version 9.2 [61].
Results
An analysis of sampled patients comparing non-respondents with respondents who completed both the Time 1 and Time 2 surveys showed that there were no significant differences by age at diagnosis. However, compared with respondents, non-respondents were more likely to be African American (35.2% versus 26.7%; p < 0.001) or Latina (17.2% versus 13.3%; p=0.002), more likely to have stage II or stage III disease (54.9% versus 37.8%; p < 0.001), and more likely to have received mastectomy (37.5% versus 30.8%; p < 0.001).
For this study, a change of 2 points or more on the EWB subscale of the FACT-B from Time 1 to Time 2 was defined as clinically meaningful. From the initial survey (Time 1) until the follow-up (Time 2), across the sample, 192 women (24.9%) experienced a decline in EWB, 303 women (39.2%) had no change, and 277 women (35.9%) experienced an improvement in EWB over time. Given our interest in women who declined, we combined those who improved with those who remained the same for all further analyses. Table 1 displays the sample characteristics for the entire sample and for those women who had a 2 point decline in their EWB scores. In terms of personal factors, the mean age was 59.1 (SD 11.3), and the racial ethnic breakdown was as follows: white (47.3%), black (16.8%), Latina, higher acculturation (21.3%) and Latina, lower acculturation (15.6%). About 58.1% of the women completed some college while 21.0% reported less than a high school education. Approximately 45.0% were not married/partnered and 22.7% were married/partnered but not satisfied with their relationship.
Table 1. Description of Study Sample overall and by decline in Emotional Well-Being over time (N= 772).
| N | % | % EWB Decline | P value* | ||||
|---|---|---|---|---|---|---|---|
| ANTECEDENT | |||||||
| Personal Factors | |||||||
| Age, mean (sd) | 59.1 | (11.3) | 0.714 | ||||
| Race/Ethnicity | 0.303 | ||||||
| White | 431 | 47.3 | 22.0 | ||||
| Black | 167 | 16.8 | 23.0 | ||||
| Latina: Higher Acculturation | 99 | 21.3 | 20.2 | ||||
| Latina: Lower Acculturation | 75 | 15.6 | 30.4 | ||||
| Education | 0.140 | ||||||
| Not H.S. Graduate | 118 | 21.0 | 26.7 | ||||
| H.S. Graduate | 161 | 20.9 | 21.5 | ||||
| Some College/More | 485 | 58.1 | 21.9 | ||||
| Social Factors | |||||||
| Satisfaction w/ Partner | 0.241 | ||||||
| No Partner | 329 | 45.0 | 24.7 | ||||
| Not Satisfied w/Partner | 174 | 22.7 | 27.7 | ||||
| Satisfied w/ Partner | 269 | 32.3 | 18.5 | ||||
| Clinical Factors | |||||||
| Cancer Stage | 0.221 | ||||||
| I | 453 | 55.3 | 21.0 | ||||
| II | 247 | 36.0 | 21.7 | ||||
| III | 66 | 8.7 | 39.3 | ||||
| Surgery | 0.458 | ||||||
| Mastectomy | 276 | 40.7 | 22.5 | ||||
| Lumpectomy | 482 | 57.8 | 22.3 | ||||
| None | 5 | 1.5 | 70.0 | ||||
| Radiation | 0.957 | ||||||
| No | 219 | 35.5 | 22.7 | ||||
| Yes | 518 | 64.5 | 22.5 | ||||
| Chemotherapy | 0.313 | ||||||
| No | 403 | 54.8 | 24.6 | ||||
| Yes | 342 | 45.2 | 22.0 | ||||
| Depression | <0.001 | ||||||
| None | 578 | 73.3 | 17.7 | ||||
| Past | 128 | 18.0 | 35.1 | ||||
| Current | 66 | 8.7 | 43.7 | ||||
| Comorbidities | 0.028 | ||||||
| None | 152 | 20.8 | 17.9 | ||||
| ≥1 | 620 | 79.2 | 24.5 | ||||
| PROXIMAL | |||||||
| Appraisal Factors | |||||||
| Sufficient Information on Recurrence | 0.035 | ||||||
| No | 244 | 33.4 | 21.1 | ||||
| Yes | 512 | 66.6 | 26.7 | ||||
| Likelihood of Recurrence | 0.001 | ||||||
| Not at all/A little | 507 | 70.6 | 18.9 | ||||
| Somewhat | 161 | 23.2 | 28.2 | ||||
| Quite/Very | 46 | 6.2 | 36.9 | ||||
| Change in Worry Score, mean (sd) | -0.6 | (1.2) | <.001 | ||||
| Decision Regret, mean (sd) | 2.0 | (0.9) | 0.037 | ||||
| COPING FACTORS | |||||||
| Emotional Support | |||||||
| Physician | 0.270 | ||||||
| Low Support | 368 | 47.0 | 25.5 | ||||
| High Support | 392 | 53.0 | 21.1 | ||||
| Family/Partner | 0.691 | ||||||
| Low Support | 181 | 23.4 | 27.2 | ||||
| High Support | 591 | 76.6 | 21.9 | ||||
| Friend/Coworker | 0.889 | ||||||
| Low Support | 272 | 37.1 | 23.1 | ||||
| High Support | 500 | 63.0 | 23.1 | ||||
| Spirituality | |||||||
| Beliefs and Practices, mean (sd) | 3.9 | (1.1) | 0.017 | ||||
| Social Support, mean (sd) | 3.2 | (1.2) | 0.038 | ||||
P values were obtained by comparing the distributions of listed factors between the patients who had emotional well being decline and those who did not.
Bivariate analyses were conducted to examine the association between each of the personal, social, illness-related factors and EWB decline. Among the factors examined, women with a history of or current depression and those who had at least one comorbid condition were more likely to report a significant decline in their EWB over time. Among all the proximal measures (appraisal and coping factors), women who perceived they did not receive enough information about risk of breast cancer recurrence during primary treatment, those who expressed higher likelihoods for breast cancer recurrence, women with increased worry about recurrence between baseline and follow up surveys, and women who expressed regret over their treatment decision reported more EWB decline. With regard to spirituality, women reporting higher beliefs and practices and higher social support as measured by the SBI-15R were more likely to report declines in EWB.
We then assessed EWB decline with the four appraisal factors (sufficient information about disease recurrence, perceived likelihood of recurrence, change in worry of recurrence, and decision regret) adjusting for antecedent variables (age, race/ethnicity, satisfaction with partner, surgery, radiation, and chemotherapy), baseline EWB score, and geographic site and incorporating weights. Each appraisal factor was significantly associated with decline in EWB. Nearly one-third (29.2%) of women who reported not receiving enough information on recurrence experienced a decline in EWB, compared to 19.7% who reported sufficient information (p< .01). Over 31% of women who perceived the likelihood of having a recurrence to be quite/highly likely reported a decline in EWB versus 28.5% who perceived recurrence as somewhat likely and 20.0% of women who reported recurrence was not at all or a little likely (p=.01). Of the 85 women who experienced an increase in their worry about recurrence scores over time, 42% reported a decline in EWB. Conversely, of the 331 women who experienced a decrease in their worry about recurrence, 14% reported a decline in EWB. Of the 282 women who reported high treatment decision regret, 27% reported EWB declines. Of the 232 women who reported low treatment decision regret, 21% reported EWB declines.
Next, we examined the relationship between EWB decline and the two coping factors: emotional support and spirituality adjusting for antecedent variables, baseline EWB level, geographic site and incorporating weights. More women with EWB declines reported low support from health care providers, compared with women who reported high support (26% versus 21%, respectively, p <.05). Similar trends were observed when family/partner and friends/coworker support was assessed, but these differences were not significant. Declines in EWB were most often reported by women with high spiritual beliefs and practices (26.5%), followed by medium (26.1%), and finally low (15.7%) spiritual beliefs and practices (p < .05). After adjustment for antecedent variables, the social support subscale of the SBI-15R was no longer significant (p=.12), so the subscale was dropped from further analyses.
The first multivariable model (Table 2, Model 1) shows the relationship between antecedent variables on decline in EWB. These models are also adjusted for Time 1 EWB levels, geographic location and incorporated weights. Variables significantly associated with declines in EWB included age, stage, race/ethnicity and depression diagnosis. Compared with whites, lower-acculturated Latinas (OR 2.55, 95% CI 1.15-5.63) were significantly more likely to report EWB decline. Compared with women without a history of depression, women with a history of depression (OR 2.99, 95% CI 1.70-5.26) and women with current depression (OR 6.53, 95% CI 3.24-13.14) were significantly more likely to report EWB declines. No significant differences were observed by age, education, satisfaction with partner relationship, cancer stage, type of surgery, receipt of radiation or receipt of chemotherapy, or comorbid conditions.
Table 2. Logistic Regressions: Decline in Emotional Well-Being.
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| ANTECEDENTS | O.R. | CI | O.R. | CI | |||
| Person Factors | |||||||
| Age | 0.98 | 0.95 - 1.01 | 0.97 | 0.95 - 1.01 | |||
| Race/Ethnicity | White (ref) | 1 | - | * | 1 | - | |
| Black | 1.10 | 0.66 - 1.86 | 0.77 | 0.44 - 1.37 | |||
| Latina, Higher Acculturation | 1.89 | 0.98 - 3.63 | 1.41 | 0.69 - 2.86 | |||
| Latina, Lower Acculturation | 2.55 | 1.15 - 5.63 | 1.51 | 0.64 - 3.6 | |||
| Education | Not HS Grad (ref) | 1 | - | 1 | - | ||
| HS Grad | 0.96 | 0.47 - 1.97 | 0.80 | 0.35 - 1.79 | |||
| Some College or More | 0.80 | 0.41 - 1.56 | 0.69 | 0.33 - 1.46 | |||
| Social Factors | |||||||
| Satisfaction w/ Partner | Not Satisfied (ref) | 1 | - | 1 | - | ||
| No partner | 0.88 | 0.49 - 1.58 | 0.84 | 0.45 - 1.57 | |||
| Satisfied | 0.79 | 0.44 - 1.39 | 0.90 | 0.49 - 1.65 | |||
| Clinical Factors | |||||||
| Cancer Stage | I (ref) | 1 | - | 1 | - | ||
| II | 1.48 | 0.85 - 2.59 | 1.38 | 0.78 - 2.47 | |||
| III | 1.04 | 0.45 - 2.45 | 0.98 | 0.4 - 2.41 | |||
| Surgery | Mastectomy(ref) | 1 | - | 1 | - | ||
| Lumpectomy | 1.52 | 0.74 - 3.13 | 1.65 | 0.75 - 3.64 | |||
| Radiation | No (ref) | 1 | - | 1 | - | ||
| Yes | 0.99 | 0.44 - 2.22 | 1.10 | 0.47 - 2.62 | |||
| Chemotherapy | No (ref) | 1 | - | 1 | - | ||
| Yes | 1.03 | 0.59 - 1.79 | 1.12 | 0.61 - 2.06 | |||
| Depression | None (ref) | 1 | - | ** | 1 | - | ** |
| Past | 2.99 | 1.7 - 5.26 | 3.32 | 1.78 - 6.2 | |||
| Current | 6.53 | 3.24 - 13.14 | 6.99 | 3.32 - 14.74 | |||
| Comorbidities | None (ref) | 1 | - | 1 | - | ||
| ≥1 | 1.09 | 0.6 - 1.99 | 1.03 | 0.56 - 1.9 | |||
| PROXIMAL OUTCOMES | |||||||
| Appraisal Factors | |||||||
| Model 2 | |||||||
| Sufficient Information on Recurrence | No (ref) | 1 | - | * | |||
| Yes | 0.53 | 0.32 - 0.87 | |||||
| Likelihood of Recurrence | Not at all/A little/Somewhat | 1 | - | * | |||
| Quite/Very Likely | 1.95 | 1.01 - 5.29 | |||||
| Change in Worry Score | 1.38 | 1.1 - 1.72 | ** | ||||
| Decision Regret | Low (ref) | 1 | - | ||||
| High | 1.59 | 0.98 - 2.56 | |||||
| Coping Factors | |||||||
| Emotional Support | |||||||
| Physician | Low Support (ref) | 1 | - | ||||
| High Support | 0.85 | 0.51 - 1.43 | |||||
| Family/Partner | Low Support (ref) | 1 | - | ||||
| High Support | 0.85 | 0.45 - 1.58 | |||||
| Friend/Coworker | Low Support (ref) | 1 | - | ||||
| High Support | 1.11 | 0.54 - 2.25 | |||||
| Spirituality | |||||||
| Beliefs and Practices | 1.29 | 1.03 - 1.62 | * | ||||
p<0.05
p<0.01. Note that these p values are based on the overall significance tests for the factor.
Confidence intervals not including ones are bolded and indicate significant differences between the case groups and their reference groups.
Note. All models adjusted for geographic location, baseline EWB levels and weighted to account for sample design and non-response.
The second multivariable model (Table 2, model 2) incorporates the antecedent variables, geographic locations, and Time 1 EWB levels, is weighted to account for sample design and non-response, and adds the proximal outcomes, which include the appraisal and coping factors. Depression diagnosis remained significantly associated with EWB declines. Race/ethnicity was no longer significant. Women who indicated they did not receive enough information from their doctors or the staff about risk for breast cancer recurrence were significantly more likely to report EWB decline (OR 0.53, 95% CI 0.32-0.87). Women who reported their likelihood of breast cancer recurrence as quite/very likely compared with less than likely had a higher likelihood to report EWB declines (OR 1.95 95% CI 1.01-5.29). A higher score on the worry about recurrence scale from Time 1 survey (approximately nine months post diagnosis) to the Time 2 survey (four-years later) was associated with a higher likelihood of EWB decline (OR 1.38, 95% CI 1.10-1.72). A higher mean score on the four items from the beliefs and practices subscale of Spiritual Beliefs Inventory – 15R was associated with EWB decline (OR 1.29, 95% CO 1.03-1.62). No significant differences were observed by decision regret, or emotional support.
Discussion
For the majority of breast cancer survivors, QOL including the dimension of emotional well-being, improves as time since diagnosis elapses [5, 15, 47, 62, 63]. However, because this is not the case for all women, there is a need to better understand what defines those women who experience decline in EWB. The aim of this diverse, longitudinal, population-based study was to identify and examine the antecedent (personal, social and clinical) and proximal (appraisal and coping) factors associated with declines in EWB over time. A substantial number of women who completed breast cancer treatment (almost 25%) reported declines in EWB from nine months to four years after breast cancer diagnosis. This is consistent with a previous study of older women with breast cancer that found between 20-30% reported an EWB decline over five years [64]. In a ten year follow-up, one study reported slight EWB improvements in the first five years post-diagnosis, followed by a slight declines between years five and ten [65]. Overall, we found the appraisal factors examined in this study (e.g., perceptions about the risk of recurrence and worry about recurrence) had a stronger association with decline in EWB than the coping factors measured (e.g., emotional support from others). Those with past or current depression were particularly vulnerable to declines in EWB over time. Several of the factors found to be associated with declines in EWB are amenable to educational and psychosocial interventions, for example, providing more information about the likelihood of breast cancer recurrence and ways to alleviate worry of recurrence.
We did not find younger age to be significantly associated with decline in EWB. A number of prior studies have reported younger women were more likely to experience a decline in EWB than older women [5, 13, 15, 16, 18, 22, 34, 47, 66]. The fact that all women in this study had to have completed primary treatment at the Time 1 survey may have contributed to our finding. A greater number of younger women were excluded from the analyses when this criterion was used. Previous studies have suggested that younger women are likely to receive aggressive treatment regimens that could extend beyond our mean of 9 months from diagnosis.
In the current study, race/ethnicity was not associated with declines in EWB over time after all other factors were considered. Consistent with some of our previous work [3], in this study Latinas with low acculturation were found to experience more declines in EWB than whites until we included the appraisal and coping factors. Other studies have found that Latinas report worse mental health and EWB than AAs and more physical symptoms than women in any of the other ethnic categories surveyed [13, 18, 21. A number of previous studies on early survivorship have found that whites reported worse EWB than AAs, despite reporting better physical and social well-being [18, 19, 53]. Our study findings suggest that racial/ethnic differences in EWB may become less significant by four years and/or may be linked to cultural variation in appraisal and coping of the breast cancer experience. This speaks to the need for educational interventions to provide appropriate information for women to understand their cancer diagnosis and risk of recurrence. These needs extend beyond the initial period of diagnosis and treatment.
When considering clinical factors, cancer stage and treatment were not found to be significant in the multivariable models. While the number of comorbidities was not significant, a depression diagnosis was a highly significant factor associated with decline in EWB. Women with either a current or previous diagnosis of depression had more EWB decline than women without a depression diagnosis. Previous studies have indicated that women with a history of depression are at increased risk for depressive symptomatology [15, 67] and initial levels of depression are strong predictors of successive depression, delayed cancer recovery, and reduced quality of life [68]. Our survey does not allow us to determine if the diagnosis of depression came before or after the cancer diagnosis. Breast cancer patients with a history of resolved major depressive disorder are still at increased risk for declines in physical functioning during chemotherapy relative to patients with no history of depression [69]. Oncology providers should assess for both past and current depression, recognize the relationship between depression and overall emotional well-being, and refer patients to appropriate supportive services when necessary.
Those women who perceived they did not receive sufficient information about risk of recurrence early in the survivorship period (i.e., Time 1) were more likely to report a decline in EWB over time. Previous studies have suggested that women desire information about their risk of recurrence [53, 70, 71] and that some women desire more information than is being presented during the treatment phase [53]. We also found that women who reported a higher perceived likelihood of experiencing a cancer recurrence were more likely to have a decline in EWB. These findings reinforce the need for clinicians to provide education to patients about their risk for recurrence and to evaluate the efficacy of tools in assisting women to better understand their individual recurrence risk.
The affective measure of worry about recurrence was measured at Time 1 and Time 2. When worry about recurrence increased over time, women were more likely to report a decline in EWB. Many studies have reported that worry about recurrence is an ongoing concern of for women during survivorship [15, 31, 35, 38, 43, 72, 73] and that high levels of worry about recurrence are associated with decline in EWB over time [74]. Even though one would expect a gradual lessening of worry as the years of survivorship increase, Liu et al. [73] reported 29% of women with DCIS and early invasive breast cancer reported moderate to high levels of worry at 2 years. In this study some women reported greater worry at four years than they did shortly after primary treatment was completed. Routine assessment of worry about recurrence in the clinic setting may help identify women with excessive worry who are at risk for decline in EWB over time.
Among the coping factors assessed, we did not find emotional support provided by others over the cancer period to be a significant factor when assessed at four years follow-up. This finding was somewhat surprising given the well-established link between social support and EWB in the early treatment and survivorship period [8, 45, 46, 48, 75]. However, previous studies also have noted that the substantial amount of informational, decisional, and emotional support offered following diagnosis, often drops considerably within the first year [47, 48]. It is possible that emotional support is a more critical ingredient to emotional well-being earlier, rather than later in the survivorship period.
Our findings on the association between spirituality and declines in EWB over time were mixed. Two dimensions of spirituality were assessed. There was no association found between EWB decline and the social support dimension of spirituality when controlling for other factors, however, higher reported spiritual beliefs and practices was found to be associated with declines in EWB. While a positive correlation between spirituality and QOL has been previously reported (76, 77, 78), further studies are needed that examine the relationship of spirituality and emotional well-being longitudinally and explore the different domains of the construct of spirituality as they relate to emotional well-being. A review by Visser [78] found that among the 27 studies investigating spirituality and well-being, using primarily cross-sectional designs, 14 found that the feeling of connectedness to self and others was more important to improved well-being than having a belief in and connection to a higher power. Purnell et al. [79] found that spirituality was significantly associated with QOL whereas there was no such correlation between religious practice and QOL. Our measure of spirituality was only included in the Time 2 survey so we are unable to establish fully a temporal relationship between EWB and spirituality. In addition our measure was restricted to four items chosen from the beliefs and practices subscale of the Systems of Beliefs Inventory – 15R, so may not have captured the entire domain of spiritual beliefs and practices.
The strengths of this study include a large, multiethnic, population based sample with a longitudinal design to assess EWB over time. The study was guided by a conceptual model of stress and coping, and measured EWB using the scale from a validated measure of QOL (FACT-B). In addition, we defined decline in EWB by using an established clinically meaningful difference rather than relying on a statistical significance between Time 1 and Time 2. Furthermore, we included appraisal and coping factors (e.g., worry about recurrence, emotional support, and spirituality) that have not been extensively explored as they relate to declines in EWB over time. Study limitations include that the measures were self-reported and may be subject to recall bias. In addition, the Time 1 survey was administered approximately nine months after the cancer diagnosis so we do not have baseline/pre-diagnosis information on measures such as social support, spirituality, or other attitudes and beliefs. The findings can only be generalized to the two large metropolitan areas from which our study sample was drawn.
Implications and Conclusions
In addition to implications for clinical practice, our findings provide direction for future research and intervention development. Specifically, our findings suggest that patients would benefit from receiving both more information about recurrence risk as well as additional support and strategies to alleviate and/or manage their fear about recurrence. These needs extend beyond the immediate diagnostic and treatment phase. Additional studies are needed to determine whether, from whom, and under what circumstances, cancer patients are receiving risk information.
Women with a history of depression are particularly vulnerable to declines in EWB. Identifying these women early in their diagnostic and treatment phase could ameliorate these declines as they enter the recovery period. Any interventions developed must be culturally sensitive, and tailored to differences in communication style, social support and coping strategies. We need to understand providers' perceptions about their roles and responsibilities about assessing EWB over time and in managing women who are vulnerable to and/or experience decline in EWB over time. Future research efforts must recognize the multifaceted nature of women's emotional reactions to a breast cancer diagnosis, including racial/ethnic variation and cultural differences.
Acknowledgments
Ethical standards: This study was approved by the University of Michigan Institutional Review Board. All persons gave their informed consent prior to their inclusion in the study.
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
Contributor Information
Nancy K. Janz, University of Michigan, Department of Health Behavior and Health Education, School of Public Health, 1415 Washington Heights, 2830-SPH1, Ann Arbor, Michigan 48109-2029.
Christopher R. Friese, University of Michigan, School of Nursing Division III, 4162 SNB, Ann Arbor, Michigan, 48109-5482.
Yun Li, University of Michigan, Biostatistics, M4015 SPH II, 2029 Ann Arbor, Michigan.
John J. Graff, Cancer Institute of New Jersey, Robert Wood Johnson Medical School, 195 Little Albany Street, New Brunswick, New Jersey, 08901.
Ann S. Hamilton, University of Southern California Norris Center, Preventive Medicine, 1441 Eastlake Avenue, MC 9175, Los Angeles, California 90089-9175.
Sarah T. Hawley, University of Michigan Internal Medicine-General Medicine, NCRC 2800, Plymouth Road Building, 16-40GE, Ann Arbor, Michigan Breast Cancer, Survivorship, Emotional Well-Being, Worry about recurrence.
References
- 1.Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Wildes KA, Miller AR, de Majors SS, Otto PM, Ramirez AG. The satisfaction of Latina breast cancer surviviors with their healthcare and health-related quality of life. J Womens Health. 2011;20(7):1065–74. doi: 10.1089/jwh.2010.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janz NK, Mujahid MS, Hawley ST, et al. Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv. 2009;3(4):212–22. doi: 10.1007/s11764-009-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom JR, Stewart SL, Oakley-Girvan I, et al. Quality of life of younger breast cancer survivors: persistence of problems and sense of well-being. Psychooncology. 2012;21(6):655–65. doi: 10.1002/pon.1965. [DOI] [PubMed] [Google Scholar]
- 6.Carver CS, Smith RG, Antoni MH, et al. Optimistic personality and psychosocial well-being during treatment predict psychosocial wellbeing among long-term survivors of breast cancer. Health Psychology. 2005;24:508–16. doi: 10.1037/0278-6133.24.5.508. [DOI] [PubMed] [Google Scholar]
- 7.Deimling, Sterns S, Bowman KF, Kahana B. The health of older-adult, long-term cancer survivors. Cancer Nursing. 2004;28(6) doi: 10.1097/00002820-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychology. 2004;23(1) doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- 9.Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. Journal of the National Cancer Institue. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 10.Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: a systematic review of the literature. Journal of Cancer Survivorship. 2011;5(2):191–207. doi: 10.1007/s11764-011-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trentham-Dietz A, Sprague BL, Klein BE, et al. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Research and Treatment. 2008;109(2) doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knobf M. Clinical update: psychosocial responses in breast cancer survivors. Semin Oncol Nurs. 2011;27:e1–e14. doi: 10.1016/j.soncn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Ashing-Giwa KT, Lim JW. Examining emotional outcomes among a multiethnic cohort of breast cancer survivors. Oncol Nurs Forum. 2011;38(3):279–288. doi: 10.1188/11.ONF.279-288. [DOI] [PubMed] [Google Scholar]
- 14.Garofalo JP, Hamann HA, Ashworth K, Baum A. Stress and quality of life in African American cancer survivors. Ethnicity & Disease. 2006;16(3):732–8. [PubMed] [Google Scholar]
- 15.Costanzo ES, Lutgendorf SK, Mattes ML, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J of Cancer. 2007;97:1625–31. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: how are they faring? Cancer. 2005;104(S11):2565–2576. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 17.Peuckmann V, Ekholm O, Rasmussen NK, Møller S, et al. Health-related quality of life in long-term breast cancer survivors: nationwide survey in Denmark. Breast cancer research and treatment. 2007;104(1):39–46. doi: 10.1007/s10549-006-9386-6. [DOI] [PubMed] [Google Scholar]
- 18.Giedzinska AS, Meyerowitz BE, Ganz PA, Rowland JH. Health-related quality of life in a multiethnic sample of breast cancer survivors. Ann Behav Med. 2004;28(39):39–51. doi: 10.1207/s15324796abm2801_6. [DOI] [PubMed] [Google Scholar]
- 19.Rao D, Debb S, Blitz D, et al. Racial/ethnic differences in the health-related quality of life of cancer patients. Journal of pain and symptom management. 2008;36(5):488–496. doi: 10.1016/j.jpainsymman.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain. Cancer. 2010;117(9):1994–2003. doi: 10.1002/cncr.25761. [DOI] [PubMed] [Google Scholar]
- 21.Ashing-Giwa KT, Tejero JS, Kim J, et al. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. 2007;16(3):413–28. doi: 10.1007/s11136-006-9138-4. [DOI] [PubMed] [Google Scholar]
- 22.Janz N, Mujahid M, Lantz PM, et al. Population-based study of the relationship of treatment and sociodemographics on quality of life for early stage breast cancer. Quality of Life Research. 2005;16(6):1467–79. doi: 10.1007/s11136-005-0288-6. [DOI] [PubMed] [Google Scholar]
- 23.Ahles TA, Titus-Ernstoff L, Skalla K, et al. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. Journal of Clinical Oncology. 2005;23:4399–4405. doi: 10.1200/JCO.2005.03.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ell K, Sanchez K, Vourlekis B, et al. Depression, correlates of depression, and receipt of depression care among low-income women with breast or gynecologic cancer. Journal of Clinical Oncology. 2005;23(13):3052–3060. doi: 10.1200/JCO.2005.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potash M, Breibart W. Affective disoders in advanced cancer. Hematol Oncol Clin North Am. 2002;16(3):671–700. doi: 10.1016/s0889-8588(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 26.Ganz PA, Guadagnoli E, Landrum MB, et al. Breast cancer in older women: quality of life and psychosocial adjustment in the 15 months after diagnosis. Journal of Clinical Oncology. 2003;1(21):4027–33. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 27.Ganz PA, Greendale GA, Peterson L, et al. Breast cancer in younger women: reproductive and late health effects of treatment. Journal of Clinical Oncology. 2003;21(22):4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 28.Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with braest cancer. Psycho-oncology. 2006;15(1):9–19. doi: 10.1002/pon.907. [DOI] [PubMed] [Google Scholar]
- 29.Figueiredo JC, Ennis M, Knight JA, et al. Influence of young age at diagnosis and family history of breast or ovarian cancer on breast cancer outcomes in a population-based cohort study. Breast Cancer Research and Treatment. 2007;105(1):69–80. doi: 10.1007/s10549-006-9433-3. [DOI] [PubMed] [Google Scholar]
- 30.Chantler M, Podbilewicz-Schuller Y, Mortimer J. Change in need for psychosocial support for women with early stage breast cancer. Journal of Psychosocial Oncology. 2006;23(2-3):65–77. doi: 10.1300/j077v23n02_05. [DOI] [PubMed] [Google Scholar]
- 31.Jiwa M, Thompson J, Coleman R, Reed M. Breast cancer follow-up: could primary care be the right venue? Curr Med Res Opin. 2006;22:625–30. doi: 10.1185/030079906X96407. [DOI] [PubMed] [Google Scholar]
- 32.Lee-Jones C, Humphris G, Dixon R, Hatcher M. Fear of cancer recurrence --A literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psychooncology. 1997;6(95):95–105. doi: 10.1002/(SICI)1099-1611(199706)6:2<95::AID-PON250>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Stephens PA, Osowski M, Fidale MS, Spagnoli C. Identifying the educational needs and concerns of newly diagnosed patients with breast cancer after surgery. Clinical Journal of Oncology Nursing. 2008;12(2):253–258. doi: 10.1188/08.CJON.253-258. [DOI] [PubMed] [Google Scholar]
- 34.Spencer SM, L J, Wynings C, Arena P, Carver CS, Antoni MH, et al. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychology. 1999;18:158–168. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- 35.van den Beuken-van Everdingen MH, Peters ML, de Rijke JM, Schouten HC, van Kleef M, Patijn J. Concerns of former breast cancer patients about disease recurrence: a validation and prevalence study. Psycho-oncology. 2008;17(11):1137–1145. doi: 10.1002/pon.1340. [DOI] [PubMed] [Google Scholar]
- 36.Vickberg SM. The concerns about recurrence scale (CARS): A systematic measure of women's fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25:16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- 37.Ganz PA, Coscarelli A, Fred C, et al. Breast cancer survivors: psychosocial concerns and quality of life. Breast cancer research and treatment. 1996;38(2):183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 38.Lebel S, Rosberger Z, Edgar L, Devins GM. Emotional distress impacts fear of the future among breast cancer survivors not the reverse. Journal of Cancer Survivorship. 2009;3:117–127. doi: 10.1007/s11764-009-0082-5. [DOI] [PubMed] [Google Scholar]
- 39.Schmid Büchi S, Halfens RJ, Dassen T, Van Den Borne B. A review of psychosocial needs of breast-cancer patients and their relatives. Journal of Clinical Nursing. 2008;17(21):2895–2909. doi: 10.1111/j.1365-2702.2008.02490.x. [DOI] [PubMed] [Google Scholar]
- 40.Janz NK, Mujahid MS, Hawley ST, et al. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5) doi: 10.1002/cncr.23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arora NK, Johnson P, Gustafson DH, et al. Barriers to information access, perceived health competence, and psychosocial health outcomes: test of a mediation model in a breast cancer sample. Patient Education and Counseling. 2002;47(1):37–46. doi: 10.1016/s0738-3991(01)00170-7. [DOI] [PubMed] [Google Scholar]
- 42.Davies NJ, Kinman G, Thomas RJ, Bailey T. Information satisfaction in breast and prostate cancer patients: implications for quality of life. Psycho-oncology. 2008;17(10):1048–1052. doi: 10.1002/pon.1305. [DOI] [PubMed] [Google Scholar]
- 43.Clayton MF, Mishel MH, Belyea M. Testing a model of symptoms, communication, uncertainty, and well-being, in older breast cancer survivors. Res Nurs Health. 2006;29:18–39. doi: 10.1002/nur.20108. [DOI] [PubMed] [Google Scholar]
- 44.Hawley ST, Janz NK, Hamilton A, et al. Latina patient perspectives about informed treatment decision making for breast cancer. Patient Education and Counseling. 2008;73(2):363–370. doi: 10.1016/j.pec.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carver CS, Smith RG, Petronis VM, Antoni MH. Quality of life among long-term survivors of breast cancer: different types of antecedences predict different classes of outcomes. Psychooncology. 2006;15(9):749–58. doi: 10.1002/pon.1006. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Han JY, Shaw B, et al. The roles of social support and coping strategies in predicting breast cancer patients' emotional well-being. J Health Psychol. 2010;15(4):543–552. doi: 10.1177/1359105309355338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psycho-oncology. 2004;13(3):147–60. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- 48.Arora NK, Finney Rutten LJ, Gustafson DH, et al. Perceived helpfulness and impact of social support provided by family, friends, and health care providers to women newly diagnosed with breast cancer. Psycho-oncology. 2007;16:474–86. doi: 10.1002/pon.1084. [DOI] [PubMed] [Google Scholar]
- 49.Lazarus RS, Folkman S. Evolution of a model of stress, coping, and discrete emotions. In: VH R, editor. Handbook of Stress, Coping, and Health. Sage; Thousand Oaks, CA: 2000. p. 195222. [Google Scholar]
- 50.Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- 51.Northouse LL, Mood D, Kershaw T, et al. Quality of life of women with recurrent breast cancer and their family members. Journal of Clinical Oncology. 2002;20(19):4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- 52.Hamilton AS, Hofer TP, Hawley ST, Morrell D, Leventhal M, Deapen D. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiology Biomarkers & Prevention. 2009;18(7):2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janz NK, Hawley ST, Mujahid MS, et al. Correlates of worry about recurrence in a multiethnic population-based sample of women with breast cancer. Cancer. 2011;117(9):1827–36. doi: 10.1002/cncr.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy (FACT) scale: Development and validation of the general version. Journal of Clinical Oncology. 1993;11(3):5709. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 55.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;16(1):79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marin G, Sabogal F, Marin BV, et al. Development of a short acculturation scale for Hispanics. Hispanic Journal of Behavioral Sciences. 1987;9(2):183–205. [Google Scholar]
- 57.Heyman RE, Sayers SL, Bellack AS. Global marital satisfaction versus marital adjustment: An empirical comparison of three measures. Journal of Family Psychology. 1994;8(4):432–446. [Google Scholar]
- 58.Mary Charlson, Szatroski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 59.Holland JC, Kash KM, Passik S, et al. A brief spiritual beliefs inventory for use in quality of life research in life-threatening illness. Psycho-oncology. 1998;7:460–469. doi: 10.1002/(SICI)1099-1611(199811/12)7:6<460::AID-PON328>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 60.Grovers RM, F FJ, Couper MP, Lepkoswski JM, Singer E, Tourangean R. Survey Methodology. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 61.SAS Software v. 9.2. SAS Insitute; Cary, NC: [Google Scholar]
- 62.Stanton AL. Psychosocial concerns and interventions for cancer survivors. Journal of Clinical Oncology. 2006;24(32):5132–37. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 63.Taira N, Shimozuma K, Shiroiwa T, Ohsumi S, Kuroi K, Saji S, et al. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast cancer research and treatment. 2011;128(3):735–747. doi: 10.1007/s10549-011-1631-y. [DOI] [PubMed] [Google Scholar]
- 64.Clough-Gorr KM, Ganz PA, Silliman RA. Older breast cancer survivors: factors associated with change in emotional well-being. Journal of clinical oncology. 2007;25(11):1334–1340. doi: 10.1200/JCO.2006.09.8665. [DOI] [PubMed] [Google Scholar]
- 65.Koch L, Jansen L, Herrmann A, Stegmaier C, Holleczek B, Singer S, et al. Quality of life in long-term breast cancer survivors-a 10-year longitudinal population-based study. Acta Oncologica. 2013;(0):1–10. doi: 10.3109/0284186X.2013.774461. [DOI] [PubMed] [Google Scholar]
- 66.Friedman LC, Kalidas M, Elledge R, et al. Optimism, social support and psychosocial functioning among women with breast cancer. Psycho-oncology. 2006;15:595–603. doi: 10.1002/pon.992. [DOI] [PubMed] [Google Scholar]
- 67.Reyes-Gibby CC, Anderson KO, Morrow PK, et al. Depressive symptoms and health-related quality of life in breast cancer survivors. J Womens Health. 2012;21(3):311–8. doi: 10.1089/jwh.2011.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Badger TA, Braden CJ, Mishel MH, Longman A. Depression burden, psychological adjustment, and quality of life in women with breast cancer: patterns over time. Res Nurs Health. 2004;27:19–28. doi: 10.1002/nur.20002. [DOI] [PubMed] [Google Scholar]
- 69.Jim HS, Small BJ, Minton S, et al. History of major depressive disorder prospectively predicts worse quality of life in women with breast cancer. Annals of Behavioral Medicine. 2012;43(3):17. doi: 10.1007/s12160-011-9333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raupach JC, Hiller JE. Information and support for women following the primary treatment of breast cancer. Health Expectations. 2002;5(4):289–301. doi: 10.1046/j.1369-6513.2002.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gray RE, Fitch M, Greenberg M, Hampson A, Doherty M, Labrecque M. The needs of well, longer-term survivors of breast cancer. Patient Educ Couns. 1998;33:245–255. doi: 10.1016/s0738-3991(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 72.Bower JE, Meyerowitz BE, Desmond KA, et al. Perceptions of positive meaning and vulnerability following breast cancer: predictors and outcomes among long-term breast cancer survivors. Ann Behav Med. 2005;29:236–45. doi: 10.1207/s15324796abm2903_10. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Pérez M, Schootman M, et al. Correlates of fear of cancer recurrence in women with ductal carcinoma in situ and early invasive breast cancer. Breast cancer research and treatment. 2011;130(1):165–173. doi: 10.1007/s10549-011-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waters EA, Liu Y, Schootman M, Jeffe DB. Worry About Cancer Progression and Low Perceived Social Support: Implications for Quality of Life Among Early-Stage Breast Cancer Patients. Annals of Behavioral Medicine. 2013;45:57–68. doi: 10.1007/s12160-012-9406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salonen P, Tarkka MT, Kellokumpu-Lehtinen PL, et al. Effect of social support on changes in quality of life in early breast cancer patients: a longitudinal study. Scand J Caring Sci. 2012 doi: 10.1111/j.1471-6712.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 76.Cotton SP, Levine EG, Fitzpatrick CM, et al. Exploring the relationships of spiritual well-being, quality of life, and psychological adjustment in women with breast cancer. Patient Education and Counseling. 2000;8(5):429–38. doi: 10.1002/(sici)1099-1611(199909/10)8:5<429::aid-pon420>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 77.Shapiro SL, Lopez AM, Schwartz GE, et al. Quality of life and breast cancer: relationship to psychosocial variables. Journal of Clinical Psychology. 2001;57(4):501–19. doi: 10.1002/jclp.1026. [DOI] [PubMed] [Google Scholar]
- 78.Visser A, Garssen B, Vingerhoets A. Spirituality and well-being in cancer patients: a review. Psycho-oncology. 2010;19:565–72. doi: 10.1002/pon.1626. [DOI] [PubMed] [Google Scholar]
- 79.Purnell JQ, Andersen BL, Wilmot JP. Religious Practice and Spirituality in the Psychological Adjustment of Survivors of Breast Cancer. Couns Values. 2009;53(3):165. doi: 10.1002/j.2161-007x.2009.tb00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]