Changing epidemiology of head and neck cancer
Incidence
Head and neck cancers represent the sixth most common cancer worldwide with approximately 630,000 new patients diagnosed annually resulting in more than 350,000 deaths every year 1. More than 90% of head and neck cancers are squamous cell carcinomas (HNSCC) that arise from the mucosal surfaces of the oral cavity (OSCC, ICD-10 code: C00-08), oropharynx (OPSCC, ICD-10 code: C09-10 and C12-14) and larynx (ICD-10 code C32-9). While in Northern America and Europe, HNSCC accounts for 5-10% of all new cancer cases, there is wide geographical variation in the incidence and anatomic distribution of HNSCC worldwide. This variation is predominately attributed to demographic differences in the habits of tobacco use and alcohol consumption which contributes to the development of almost 80% of all HNSCC diagnosed globally. In high-risk countries (i.e. India, Sri Lanka, Bangladesh and Pakistan), OSCC is the most common cancer in men and the third most common cancer in women2. Among the European countries, the highest incidence of OSCC is in France with high rates also noted in Hungary, Slovakia and Slovenia2. In the United States (U.S.), HNSCC constitutes only the eighth most common cancer among men with approximately 53,600 patients diagnosed yearly and shows a considerably lower mortality with 11,500 patient deaths annually3. The decreasing incidence of OSCC and laryngeal SCC in the U.S. and in other developed countries coincides with decline in the use of tobacco products 4. By contrast, there is a recent upsurge in the incidence of oropharyngeal squamous cell carcinoma (OPSCC) which is attributed to a change in the biologic driver of SCC in this region with an increasing frequency of an association with high-risk subtypes of human papilloma virus (HPV)4, 5. HPV associated SCC involves specific anatomic sites, specifically the oropharynx, which includes the base of the tongue (posterior 1/3 of tongue), tonsils, and the lateral surround pharyngeal walls (oropharynx) and coincides with Waldeyer’s ring of lymphoid tissue to include the nasopharynx6. Conversely, HNSCC involving the anterior 2/3 of the tongue (oral tongue), floor of the mouth, palate, buccal mucosa, sulcus, and gingiva are considered HPV-unrelated sites. Importantly, in the 1980s only 16% of carcinomas in the oropharynx in the U.S. were HPV-positive whereas now > 75% of OPSCC are HPV-positive7. Indeed, HPV-driven HNSCC is responsible for a > 25% increase in the incidence of HNSCC in the U.S. during this past decade, primarily among middle aged males6. Currently, the incidence of HPV-related HNSCC in the U.S. is 6.2 per 100,000 and 1.4 per 100,000, for males and females, respectively7. Currently, HPV-related OPSCC are recognized as a distinct subset of HNSCC because of its unique etiology, molecular pathogenesis, clinical presentation and therapeutic responses which will be discussed in detail later in this chapter.
Risk factors for HNSCC
Tobacco, alcohol, pan
The risk for developing HNSCC is associated with several factors including geographical location habits, diet, and genetic background. Among all etiologic factors, cigarette smoking and excessive consumption of alcohol represents the most important risk factors for the development of HNSCC and have a synergistic effect8. Cigar and pipe smoking also increases the risk for developing OSCC, with pipe smokers having a predilection for lower lip SCC. Reverse smoking, a habit practiced in certain areas of India and South America, in which the lighted end of the cigarette is kept inside the mouth while smoking, causes HNSCC involving the hard palate. Chewing of the “betel quid’ (also known as ‘pan’) is linked to the development of HNSCC of the buccal mucosa and the mandibular buccal sulcus. The habit of betel quid chewing is highly prevalent in countries with the highest incidence of OSCC (i.e. India, Pakistan, Bangladesh and Sri Lanka). The betel quid consists of betel leaf, areca nut and slaked lime with or without added tobacco. Tobacco and areca nut are the two important carcinogens that are linked to the devolvement of OSCC. The relative risk for OSCC was 7.74 for betel quid with tobacco whereas the relative risk reduces to 2.56 for betel quid without tobacco9. The use of smokeless tobacco in the form of loose-leaf chewing tobacco, moist or dry snuff (finely ground tobacco) or chewing tobacco, a habit prevalent in the U.S. and Scandinavia (i.e. Sweden), is linked to OSCC with predilection in the mandibular buccal sulcus and gingiva. The relative risk for OSCC associated with chewing tobacco and moist snuff is quite low, ranging from 0.6 to 1.7, whereas the use of dry snuff is associated with a higher relative risk, ranging from 4 to 1310. Although alcohol is not considered to be a carcinogen, excessive alcohol intake increases the risk of HNSCC most often acting synergistically with tobacco8, 11.
Human papilloma virus (HPV)
One fifth of HNSCC cases currently diagnosed in the U.S. are not related to cigarette smoking and/or alcohol abuse. Infection with high-risk HPV types (HPV 16, 18. 31 and 33) play a causal role in the pathogenesis of OPSCC with distinct clinical and molecular features (Table 1.) Specifically, HPV High-risk type 16 accounts for > 90% of HPV associated OPSCC in the U.S. with rare accounts of HPV type 18, 33 and others reported in the literature12. Interestingly, the shift in biology to HPV over tobacco associated SCC also accounts for the improvement in overall survival seen in HNSCC patients6. HPV is a strong prognostic factor. For SCC treated with similar therapeutic interventions (predominately radiation therapy with or without chemotherapy), HPV associated SCC showed an 82% three year survival compared to 57% survival for smokers with SCC13. This survival difference continues at 5 years. However, when a patient has both an HPV+ tumor and a strong tobacco exposure, the prognosis of these patients may not parallel HPV+ tumors exclusively. To date how HPV and smoking status should be used to potential alter therapy remains debated and under investigation in clinical trials.
Table 1.
Comparison of conventional/tobacco associated squamous cell carcinoma (SCC) and HPV associated SCC
| HPV associated SCC | SCC (Conventional/ tobacco exposure) |
|
|---|---|---|
| Age (mean yrs) | 53 | 57 |
| Sex | M>F 2.8:1 | M>F 1.5:1 |
| Location | Tonsils, base of tongue>>nasopharynx | Oral cavity, larynx |
| LN | 30-60% present at initial presentation Often cystic LN metastases Maybe bilateral |
Prognostic factor |
| Prognosis (disease specific survival) | ||
| 3 yrs | 82% | 57% |
| Morphology | Often non-keratinizing High nuclear to cytoplasmic ratio Relatively monotonous |
Usually keratinizing Surface dysplasia may be seen |
| Associated with | HPV types 16>>18>others | Tobacco exposure |
|
Molecular
alterations |
High overexpressed p16* (IHC) Blocked Rb, p53 by viral E6, E7 HPV +high risk types 16>others |
LOH in Chr3p and/or Chr 9p and others Mutated p53 |
Chr=Chromosome; F=female; IHC=immunohistochemical evaluation; LOH=loss of heterozygosity; LN=lymph node metastases; M=males; yrs=years
p16 overexpression is not always associated with HPV status particularly outside of the oropharynx.
Other contributing factors
Chronic sun exposure and associated ultraviolet light radiation is linked to the development of SCCs of the lips. Other less known risk factors for HNSCC include iatrogenic immunosuppression for solid organ or bone marrow transplant, family history of HNSCC, consuming diets deficient in antioxidants and older age2.
Diseases and syndromes associated with increased risk for HNSCC
Plummer-Vinson
Increased risk for HNSCC is seen in patients with Plummer-Vinson syndrome that is characterized by iron deficiency anemia, atrophic glossitis and esophageal webs. Plummer-Vinson syndrome frequently affects middle-aged women and is rarely encountered in the U.S.
Fanconi anemia and dyskeratosis congenita
Fanconi anemia (FA; MIM 227650) and dyskeratosis congenita (DC; MIM 30500,127550, 224230) are two hereditary cancer syndromes that predispose to HNSCC at an early age. FA is a chromosomal instability disorder inherited as an autosomal- or X-chromosomal recessive trait due to germline mutations in one of 15 FA genes involved in the DNA repair pathway resulting in increased risks for bone marrow failure, leukemia and solid malignancies14. HNSCC is the most frequently diagnosed solid cancer in FA patients. The risk of HNSCC among FA patients is 800-fold higher than in the general population and occur at a younger age (median age: 27-years) than the general population15, 16. Frequent oral screenings in FA patients for premalignant lesions is essential to try and reduce morbidity from OSCC. Similar to FA, DC is also an inherited bone marrow failure disorder that is caused by defects in telomere maintenance17. HNSCC is the most common solid malignancy seen in patients with DC. The oral cavity is the predominant site for HNSCC in both FA and DC patients, frequently occurring in the tongue18. Hence, semiannual oral cancer screenings are recommended for both FA and DC patients beginning at a very young age.
Age, Sex and Race predilection of HNSCC
Similar to other cancers, the risk of developing HNSCC also increases with age and the majority of HNSCCs occur in patients aged 50 years or over. The average age for smoking related HNSCC diagnosis is 60-years (median age: 63 years) whereas the average age for smokeless tobacco related HNSCC is 78-years19. HPV-related HNSCC is usually diagnosed at younger ages than tobacco related-HNSCC20. The median age at diagnosis of HPV-related HNSCC is 58-years for men and 61-years for women20. HNSCC is more common in men than in women and the ratios of OSCC and OPSCC by gender are currently about 1.5:1 and 2.8:1, respectively. In the U.S., African-American males have a higher incidence of conventional tobacco-related HNSCC than Caucasian males. In contrast, HPV-related HNSCC are more frequently diagnosed in Caucasian males20.
Anatomic sites of HNSCC
Tongue
Anatomic sites of HNSCC exhibit significant geographic and demographic variation due to differences in their etiology. In the U.S., oral tongue is the most common intraoral site of HNSCC, with 7,100 new cases diagnosed annually, and accounts for 25-40% of all OSCC 21,22. The incidence of OSCC of the tongue has been steadily increasing from 1975 whereas the incidence of other OSCC sites has been decreasing23-25. Furthermore, recent studies report an increased incidence of oral tongue carcinomas arising in young white females who are more likely to be never smokers and never drinkers 23, 26. Oral tongue carcinomas occurring in young patients without the traditional risk factors of tobacco and/or alcohol abuse exhibit a more aggressive clinical course characterized by higher rates of loco-regional recurrences, shorter disease free intervals and poor survival and remain without a known etiologic cause 23, 27. Carcinomas of the oral tongue is the most aggressive of all OSCC and exhibit extremely high rates of occult lymph node metastases (not detected by clinical and radiographic imaging studies) 28. Histopathologic guidelines used for the management of occult neck metastasis for early stage tongue SCC are described later.
Floor of mouth
The floor of the mouth is the second most common (15-20%) intraoral site for SCCs followed by the gingiva accounting for 10% of all OSCC. In the U.S., OSCC rarely occur in the dorsal surface of tongue, hard palate and buccal mucosa. SCCs of the lip occur in light-skinned individuals and >90% of the lip SCCs are located on the lower lip. Lip SCCs are considered distinct from intraoral carcinomas because of the differences in the etiology and pathogenesis of these tumors.
Precursor lesions of HNSCC
Similar to other solid malignancies, HNSCC development is a multistep process often preceded by precursors which are commonly known as precancerous or premalignant lesions. The expert Working Group of WHO Collaborating Center for Oral Cancer and Precancer on the terminology, definitions and classification recently recommended the use of the term “potentially malignant disorders (PMD)” that includes premalignant lesions and conditions that have increased risk for malignant transformation29.
Premalignant lesion
A morphologically altered oral mucosal lesion in which HNSCC is more likely to occur than in its normal counterpart.
Premalignant condition
A generalized state of the oral cavity, which is associated with a substantially increased risk for HNSCC.
Leukoplakia, erythroplakia and palatal lesions in reverse smokers are considered precancerous lesions, whereas actinic keratosis, oral submucous fibrosis and lichen planus are designated as precancerous conditions29. Tobacco and alcohol-related HNSCC, are often preceded by lesions that present clinically as white (leukoplakia) or red (erythroplakia) patches or plaques. Currently, there are no known precursor lesions for HPV-associated oropharyngeal cancer30.
Leukoplakia
Leukoplakia is the most common and best-known form of PMD, accounting for 85% of all oral premalignant lesions. Leukoplakia is defined as a white patch or plaque that cannot be rubbed off and cannot be characterized clinically or histopathologically to any specific disease (Figure 1). Hereditary, reactive, infectious and immune mediated disorders which present as intraoral white patches or plaques resembling leukoplakia are listed in Table 2 (Figure 2). The risk of malignant transformation of leukoplakias varies markedly and is dependent on:
Etiology (smoking and/or alcohol use versus idiopathic)
Clinical appearance
Location
Dysplasia grade on tissue biopsy
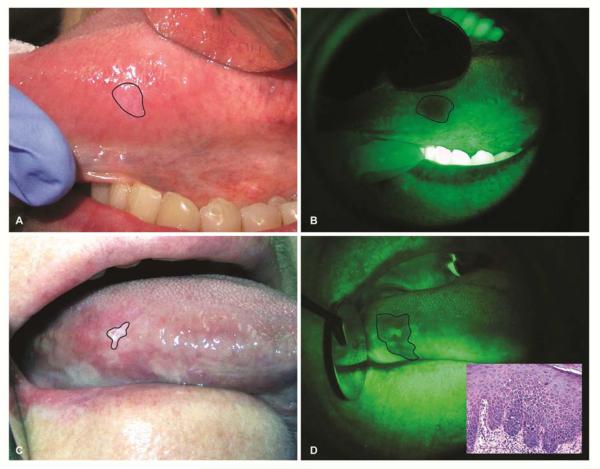
Figure 1.
Autofluorescence visualization of tongue leukoplakias. (A) A 57-year old female with a history of cigarette smoking presented with a leukoplakia that is barely visible under white light. (B) Autofluorescence visualization revealed loss of fluorescence of this leukoplakia. Excisional biopsy of this leukoplakia revealed moderate epithelial dysplasia. (C) A 65-year old female with no history of tobacco use presented with a leukoplakia in her lateral surface of the tongue. Extent of the leukoplakia involvement is markedly different when examined under white light (C) compared to autofluorescence visualization (D). Incisional biopsy of the lesion revealed moderate epithelial dysplasia (Inset).
Table 2.
Clinical differential diagnoses for intraoral white patches and plaques
| Localized reactive and infectious disorders |
Hereditary diseases | Systemic diseases |
|---|---|---|
| Frictional keratosis/ Benign alveolar ridge keratosis |
Leukoedema | Lichen planus/Lichenoid hypersensitivity reaction |
| Dentifrice-associated desquamation |
Hereditary benign intraepithelial dyskeratosis |
AIDS-Oral hairy leukoplakia |
| Nicotine stomatitis | White sponge nevus | Lupus erythematosus |
| Smokeless tobacco keratosis | Dyskeratosis congenita | |
| Submucous fibrosis | ||
| Hyperplastic candidiasis/ Candidal leukoplakia |
||
| Leukoplakia |
Figure 2.
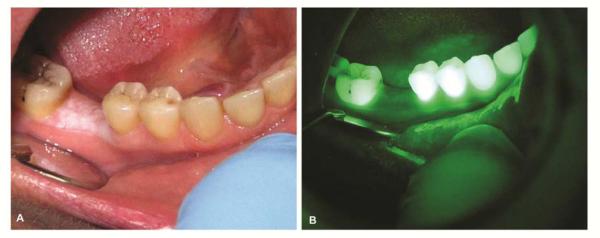
Benign alveolar ridge keratosis which resembles leukoplakia is noted in a 49-year old female (A). Autofluorescence visualization revealed no loss of fluorescence (B).
In rare cases, patients may present with leukoplakia without any known etiological factors which is designated as idiopathic leukoplakia. Idiopathic leukoplakias have a significantly increased risk of malignant transformation than leukoplakias that are associated with specific etiologic factor (i.e. tobacco use)31.
Leukoplakias most frequently occur at a single site (localized leukoplakia) and are more common in men and are associated with smoking. Localized leukoplakias presenting at a single site have two distinct clinical forms, namely homogenous and non-homogenous types, which are classified based on their surface color and appearance. Homogenous leukoplakias are uniformly white flat (patch) or slightly raised (plaque) lesions and exhibit a low malignant transformation risk. Non-homogenous leukoplakias have a verrucous/granular surface, with or without red zones (speckled leukoplakia or erythroleukoplakia), and have a higher risk for malignant transformation than homogenous leukoplakias. The intraoral site of the leukoplakia is the most important factor in determining its malignant transformation risk. In the U.S. and other Western countries, leukoplakias in the floor of the mouth, soft palate and lateral/ventral surfaces of tongue have the highest risk for malignant transformation. Overall, 9-37% of leukoplakias are expected to show either dysplasia, carcinoma in situ or invasive carcinoma at the time of biopsy.
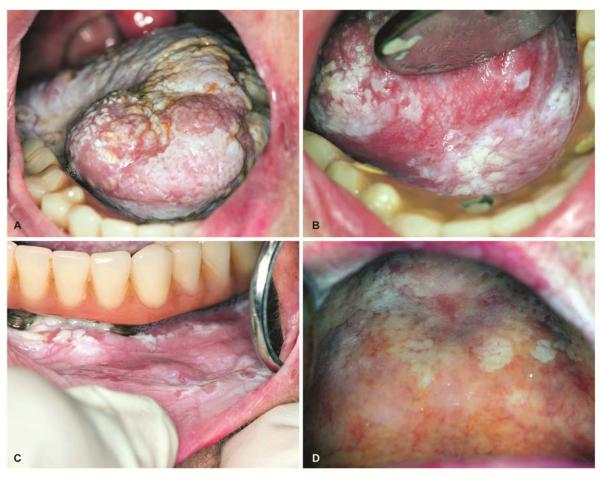
Proliferative verrucous leukoplakia
A multifocal, proliferative and progressive form of leukoplakia is recognized as proliferative verrucous leukoplakia (PVL) (Figure 3). PVL commonly begins as a simple keratosis that eventually becomes verrucous and multifocal involving large contiguous sites 32. PVL is more common in elderly women, frequently involves the gingiva and is not associated with either smoking or alcohol abuse (Figure 3). PVL tends to be persistent and frequently recurs even after surgical removal. PVL are high-risk lesions as almost 60-100% evolve into carcinoma over 10-20 years. Moreover, PVL generally lacks specific morphologic features including the classical microscopic features of epithelial dysplasia making PVL specifically a clinical diagnosis. Clinically and microscopically PVL may mimic the plaque variant of lichen planus because of its multifocal involvement and frequent presentation of lichenoid inflammation in the biopsy 33.
Figure 3.
Proliferative leukoplakia in 82-year old female with no history of tobacco use. Initial biopsy performed 10-years ago was diagnosed as lichen planus.
Erythroplakia
Erythroplakia is a less common form of a precancerous lesion or carcinoma that presents as a well-defined red, raised velvety plaque that cannot be characterized clinically as any other disease. Oral mucosal conditions that may clinically resemble erythroplakia are listed in Table 3. Erythroplakias frequently occur in older adults in the floor of the mouth, ventral tongue and soft palate. Frequently, erythroplakias are associated with adjacent leukoplakias (erythroleukoplakia). When biopsying these lesions it is important to take the biopsy from the erythroplakic areas. Erythroplakias, unlike leukoplakias, are high-risk premalignant lesions because almost all erythroplakias (100%) will exhibit microscopically either dysplasia or in situ/invasive squamous cell carcinoma at the time of biopsy. It should be emphasized that leukoplakia and erythroplakia are strictly clinical terms and are not associated with any specific histology and requires biopsy for definitive classification.
Table 3.
Clinical differential diagnoses for intraoral red patches and plaque
| Localized reactive and infectious disorders |
Neoplastic/Others | Systemic diseases |
|---|---|---|
| Erythematous (atrophic) candidiasis |
Hemangioma | Atrophic lichen planus |
| Allergic (contact) mucositis | Kaposi’s sarcoma-plaque stage |
Lupus erythematosus |
| Erythema migrans (migratory glossitis) |
Telangiectasia | Mucous membrane pemphigoid |
| Purpura |
Oral submucous fibrosis
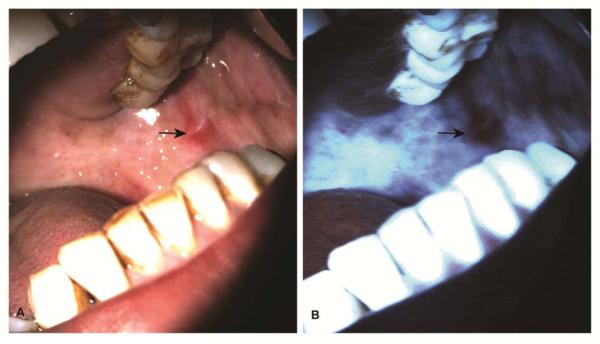
Oral submucous fibrosis is considered a premalignant condition that is more prevalent among the South Asian population and its incidence is highest in the Indian subcontinent. Oral submucous fibrosis is a chronic, progressive condition characterized by diffuse mucosal rigidity due to dense fibrosis within the lamina propria that might extend into the underlying skeletal muscle. It is caused by chewing betel quid containing areca nut. The extent and severity of this disorder is dependent on the amount of areca nut in the betel quid, duration and frequency of this habit. Oral submucous fibrosis frequently involves the buccal mucosa, tongue and soft palate. The affected mucosal surfaces appear pale, blanching marble-like with focal areas of atrophy and erythema (Figure 4). Patients commonly present with trismus, burning sensation and xerostomia; difficulties in speech, mastication and swallowing are experienced at the advanced stages. Oral submucous fibrosis is a premalignant condition with a malignant transformation rate of 8-12 % over the period of 10-15 years34.
Figure 4.
A 52-year old male with a history betel quid chewing presented with submucous fibrosis involving bilateral buccal mucosa (A). Autofluorescence visualization showed enhanced fluorescence of the affected mucosa except for the erythematous area revealing loss of fluorescence (B). An incisional biopsy taken from the area with the loss of fluorescence revealed the presence of superficially invasive squamous cell carcinoma.
Oral lichen planus
Lichen planus is the most common chronic autoimmune inflammatory disorder of oral mucosa that affects 1-2% of the adults in middle age. It is more common among females and tends to have multifocal lesions, often bilateral and symmetric in distribution. It frequently involves buccal mucosa, gingiva and tongue (Figure 5). Based on the clinical presentation, the following clinical variants of lichen planus are recognized:
Reticular variant (classic pattern): White striations and/or papules, asymptomatic, occur frequently in the buccal mucosa.
Plaque variant: Thick white plaque clinically resembling leukoplakia, asymptomatic, occurs frequently in the dorsal surface of the tongue.
Erythematous/erosive variant: Diffuse red areas with focal areas of mucosal erosions and atrophy are painful and frequently occur in the gingiva (desquamative gingivitis).
Ulcerative/bullous variant: Diffuse red and white patches with a central, chronic, non-healing ulcer are frequently seen in the lateral and ventral surfaces of the tongue and buccal mucosa.
Figure 5.
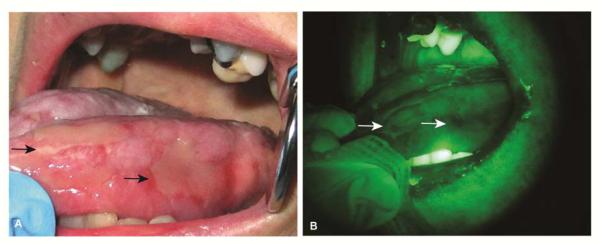
Ulcerative form of lichen planus involving the tongue of 57-year old female (A). Autofluorescence visualization demonstrates loss of fluorescence limited to the erythematous areas (arrows) due to inflammation (B).
The malignant potential of oral lichen planus has been controversial in the past, however it is now considered to have a low malignant transformation rate of 1% over a 5 year period35. Oral epithelial dysplasias (lichenoid dysplasia) may exhibit a chronic inflammatory cell infiltrate consisting of mostly lymphocytes that resembles the chronic inflammation seen in lichen planus, however has accompanying epithelial cellular alterations consistent with dysplasia as noted in Table 436, 37. Moreover, the plaque variant of lichen planus and PVL may also share similar clinical and microscopic features, leading to a misdiagnosis of lichen planus32.
Table 4.
Effects of tissue changes in autofluorescence
| Tissue Diagnosis* | Autofluorescence |
|---|---|
| Epithelial hyperplasia | No change |
| Dysplasia | Complete or partial loss |
| Invasive carcinoma | Complete loss |
| Verruciform hyperkeratosis (i.e. PVL) | No change or increase |
| Inflammation | Complete or partial loss |
| Increased vascularity or vascular tumor | Complete or partial loss |
| Extensive fibrosis (i.e. Submucous fibrosis) | Increased |
| Exogenous pigmented lesion (i.e. Amalgam tattoo) | Complete loss |
| Endogenous pigmented lesion (i.e. Focal melanosis) | Complete loss |
| Surface bacterial or fungal colonization (i.e. Hairy tongue or Candidiasis) | Altered red to orange fluorescence (porphyrin-related) |
Diagnosis as confirmed by tissue biopsy
Autofluorescence tissue imaging devices for screening of HNSCC and its precursors
Early detection by screening and subsequent diagnosis of PMD is critical to prevent the onset of HNSCC, thereby decreasing morbidity and improving survival and quality of life. The current method for screening of HNSCC and its precursors is clinical oral examination (COE) which consists of visual inspection and palpation of oral mucosa under white light. A number of studies have shown that COE has limited value in detecting and distinguishing benign oral mucosal lesions that mimic HNSCC and its precursors38 (Figure 1). Optical screening aids based on tissue reflectance and autofluorescence are increasingly used as adjuncts for COE for early detection of oral premalignancies (Box 1). Detail descriptions of the light-based screening devices for PMD and their efficacy and limitations are reviewed elsewhere39, 40.
Box 1. Screening and diagnosis of PMD.
Screening: Evaluation of an asymptomatic patient for presence of PMD
Gold standard: Clinical oral examination by an expert clinician
Adjunctive screening aids:
Transepithelial brush biopsy (i.e. OralCDx Brush Test)
Optical devices based on tissue reflectance visualization (i.e. ViziLite Plus & Microlux/DL)
Optical devices based on tissue autofluorescence visualization (i.e. VELscope, Identafi 3000 & OralID)
Diagnosis: A test performed in a symptomatic patient to determine the diagnosis and treatment
Gold standard: Scalpel biopsy for histopathologic examination
Adjunctive predictive tests*:
LOH analysis
TP53 mutational analysis
DNA aneuploidy
* Currently, these tests are not routinely done in clinical practice but used for research application only
Tissue autofluorescence imaging devices that are commercially available as adjuncts for conventional oral examination include VELScope® (LED Dental Inc., White Rock, BC, Canda), Identafi 3000 (DentalEZ group, Malvern, PA, USA) and OralID (OralID, Houston, Texas, USA). These optical devices use a special light source to illuminate oral mucosal surfaces with either blue/violet light (VELscope; 400-460 nM) or blue light (Identify and OralID; 405nM). Oral epithelium and stroma absorb high-energy photons (short wave length, 400-460 nm visible light) for excitation and emit a green (VELscope) (Figure 1) or blue (Identify and OralID) (Figure 4) fluorescence spectra at longer wave lengths. The examiner can directly view the autofluorescence emitted by the normal tissue with the use of a long-pass to block the reflected light. Epithelial fluorescence is produced by NADH, FAD and keratin whereas stromal florescence is primarily derived from collagen fibers with cross-links and elastin. Tissue autofluorescence emission can be affected by absorption and scattering of the excitation light by oxy- and deoxyhemoglobin and enlarged and crowded cellular nuclei. PMD display loss of autofluorescence due to altered metabolic activity and altered cellular and tissue architecture and appear dark-brown or black compared to adjacent healthy tissue with blue or green fluorescence (Figures 1 and 4). Tissue autofluorescence imaging is a valuable method to identify PMD with subtle mucosal changes or those that appear clinically occult under white light examination (Figure 1A-B). Fluorescence visualization is also very useful for discerning the extent of a lesions involvement, selecting optimal biopsy sites, and aiding intra-operative surgical margins (Figure 1C-D). Tissue autofluorescence imaging is more effective than conventional oral examination in finding suspicious oral mucosal lesions; however, it demonstrates a low specificity in discriminating high-risk PMD from low-risk lesions due to the higher rate of false positivity associated with benign inflammatory/ulcerative oral mucosal lesions (Figure 5). Understanding how tissue factors alter the fluorescence spectra and related limitations of this technology are critical for proper use of tissue autofluorescence imaging devices in clinical practice (Table 4).
Oral epithelial dysplasia
PMDs need to undergo a scalpel biopsy for microscopic diagnosis that will dictate their malignant transformation risk and the appropriate therapeutic management. Microscopically, these lesions may demonstrate epithelial hyperkeratosis, hyperplasia with or without dysplasia, carcinoma in situ or invasive SCC (Table 5). As oral epithelial dysplasia is a microscopic diagnosis of precancer without a specific clinical appearance this term should not be used as a clinical description. Pathologically the term “epithelial atypia” is not synonymous with oral epithelial dysplasia and use should be restricted to epithelial changes not meeting the definition of dysplasia. An example of “epithelial atypia” is reactive and regenerative epithelial changes associated with inflammation adjacent to an ulcer. Hence, the use of the term “epithelial atypia” as a microscopic diagnosis for PMD may lead to confusion and should be avoided.
Table 5.
Histopathologic grading of oral epithelial dysplasia
| Grade | Cytologic aberrations | Architectural aberrations | Level of involvement |
|---|---|---|---|
|
Hyperkeratosis &
hyperplasia |
None | Hyper-, para-, or orthokeratosis Epithelial hyperplasia |
Not applicable |
| Mild dysplasia | Increased nuclear/cytoplasmic ratio, nuclear hyperchromatism & increased number of mitotic figures |
Basal cell hyperplasia & sharply demarcated hyperkeratosis |
Lower 1/3 of the epithelium |
|
Moderate
dysplasia |
All of the above + variations in nuclear & cell size and shape, nuclear hyperchromatism, increased & abnormal mitotic figures |
All of the above + loss of polarity & stratification, increased cell density with dyscohesion, bulbous or drop-shaped rete-pegs |
Lower 1/2 of the epithelium |
| Severe dysplasia | All of the above + mitotic figures within the superficial epithelial layers, increased number and size of nucleoli & apoptotic bodies |
All of the above + dyskeratosis or keratin pearl formation |
Lower 2/3 of the epithelium |
| Carcinoma in situ | All of the above | All of the above | Full thickness epithelium |
Both cytological and architectural alterations of the oral squamous epithelium are taken into account when grading oral epithelial dysplasia (Table 5). However, microscopic evaluation of these features is subjective which leads to significant inter- and intra-observer variations in the diagnosis and grading of oral epithelial dysplasia. The malignant transformation rate of oral epithelial dysplasia varies considerably, ranging from 6% to 50% (mild dysplasia: 0-5%; moderate dysplasia: 3-15%; severe dysplasia: 7-50%)41. It should be noted a significant proportion of cases that were diagnosed as benign hyperkeratoses without dysplasia have progressed to cancer. This may be attributed to underdiagnosing clinical PVL secondary to the lack of specific cytologic features associated with dysplasia.
Although pathologic classification of oral epithelial dysplasia is not an optimal criterion for predicting the malignant transformation risk of PMD, histologic grading of oral epithelial dysplasia remains the gold standard to determine prognosis and to make treatment recommendation for these lesions. Currently, there are no reliable and reproducible molecular or genetic biomarkers that are superior to the diagnosis alone of oral epithelial dysplasia in predicting malignant transformation risks to carcinoma. Although, p53 mutations, loss of heterozygosity (LOH) and DNA ploidy analysis have been reported to predict the malignant transformation risk of PMD, neither of these techniques have been adopted in the clinical practice42. Loss of heterozygosity at chromosomes 3p and/or 9p increases the malignant transformation risk of oral epithelial dysplasias by 22.6 folds compared to dysplastic lesions with 3p and 9p retention. Oral epithelial dysplasias with additional loss of heterozygosity on chromosomes arms 4q and 17p reveal a 41.7-fold increased risk for malignant transformation43. Currently, there is no general consensus regarding the management of oral epithelial dysplasia (OED) because of its variable biologic behavior and grading of dysplasia is not the best predictor of its malignant transformation risk44. Moreover, inter- and intra-observer variability in grading of dysplasia is another confounding factor impacting treatment decisions. The conventional management of OED is based on the dysplasia grade, clinical appearance and the location of the lesion. Strategies used in the management of dysplasia include careful follow-up, surgical resection, cryotherapy, laser treatment, photodynamic therapy- and non-surgical pharmacotherapy44. Complete surgical excision is the most commonly practiced approach for treating clinically evident premalignant lesions with moderate to severe dysplasia45, 46. Mild OED can be treated with either surgery or observation depending on the location and clinical appearance of the lesion. Although surgical excision is the most effective method for preventing the recurrence and progression to invasive cancer, it is not always possible, especially in patients with OED that have widespread multifocal sites of involvement (i.e. PVL). Surgical excision of these lesions is associated with significant functional and cosmetic impairments. Patients with multifocal or widespread OED should be closely monitored and re-biopsied if there are significant changes in their clinical appearance. Laser ablation and cryotherapy are alternative methods for treating OED with widespread involvement47. The major drawback of these treatments is that the tissue biopsy cannot be procured for histologic examination. If OED is going to be treated with laser ablation, multiple representative biopsies of the OED should be taken for histopathologic diagnosis before commencing ablation. Cryosurgery with liquid nitrogen uses extreme cold to destroy dysplastic cells and is not widely used for treating OED because of higher incidence of malignant transformation in patients with OED treated with cryosurgery compared to patients treated with surgery alone48. A new therapeutic approach for treating OED is photodynamic therapy which involves the topical or systemic administration of a photosensitizing agent (i.e. Aminolevulinic acid) that when activated by light causes cytotoxic-cell death by producing reactive oxygen species49. Randomized controlled trials are required to determine the effectiveness of photodynamic therapy in treating OED. Several clinical trials have tested various therapeutic agents for treating OED and the relating data have been less impressive in preventing malignant transformation of OED. A recently published phase 1b study reported a 63% histologic response rate in OED when treated with a combination of erlotinib, an inhibitor of epithelial growth factor receptor (EGFR) and cyclooxygenase-2 (COX-2) inhibitor (Celecoxib) 50. The data of this phase1b study appears promising but needs additional clinical studies before adopting this treatment strategy in routine clinical practice. There is currently no evidenced based non-surgical pharmacotherapy for managing OED.
Prediction of prognosis, treatment response, and survival in head and neck cancer
Biopsy evaluation
Often mucosal biopsies are superficial, curl/retract on removal, and may be tangential on sectioning. On histologic review the determination of invasion may be limited or inconclusive secondary to scant underlying stroma and tissue orientation. Additionally, as a biopsy only accounts for one sampled area of the lesion, the pathologic interpretation must be correlated to the clinical findings for proper patient triage and a higher-grade process cannot be excluded within the lesion. Thus a pathology reading of SCC at least in situ requires clinical correlation regarding the degree of suspicion for an invasive tumor and this pathologic reading may represent a limitation of any superficial biopsy sample. Communication with the pathologist and possible rebiopsy should be considered when the clinical impression is discordant with the pathologic reading.
Rendering a diagnosis of SCC on a biopsy allows for further treatment planning however, the majority of prognostic factors rely on the evaluation of the resection specimen and the extent of disease. Specifically, pathologic factors in the primary tumor requiring evaluation include grade of differentiation or histologic subtypes, depth of invasion, perineural invasion, and margin status which all carry potential significance in determining the prognosis in HNSCC. Similarly, pathologic factors in regional metastasis including extracapsular spread impacts patient survival.
Histologic Subtypes of SCC
‘Conventional/keratinizing’ SCC represents the vast majority (80%) of squamous carcinomas in the head and neck outside of the oro- and nasopharynx. Conventional SCC are graded based on both the extent of keratinization and cytologic maturation, as well as, the growth pattern, into well, moderately, and poorly differentiated. This is the morphology most often associated with tobacco and/or alcohol related HNSCC.
HPV associated SCC (HPV+ OPSCC) morphologically is often more monotonous with limited keratinization compared to conventional HNSCC (Figure 6A). Terms including ‘nonkeratinizing’ and ‘basaloid’ have been confusingly used for HPV associated SCC. Since the use of these terms are not referring to the specific subtypes of SCC typically associated with these descriptors, non-keratinizing SCC of the nasopharynx and basaloid squamous cell carcinoma respectively; these terms should be avoided in this context. Interestingly, the distinct morphologic feature of HPV+ OPSCC also applies to lymph node metastases from these tumors. The lymph node metastases are often largely cystic by imaging and on histology, leading to the false association with branchial cleft cysts/carcinomas. Cystic neck masses in an adult must be fully evaluated and metastatic SCC with cystic features is the leading diagnosis. Several less frequent subtypes of SCC are compared in Table 6 and outlined below, each with their own challenges for diagnosis particularly on small biopsies.
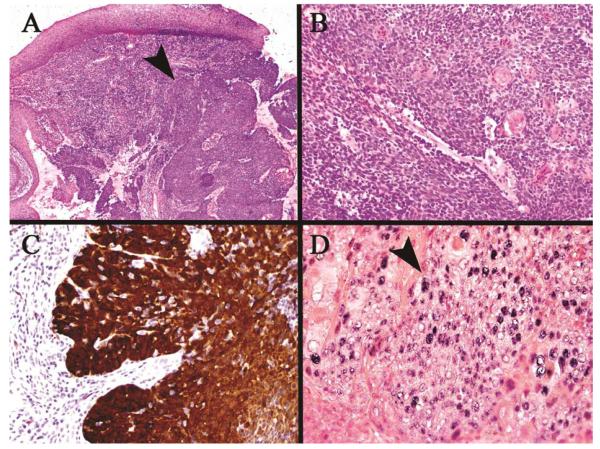
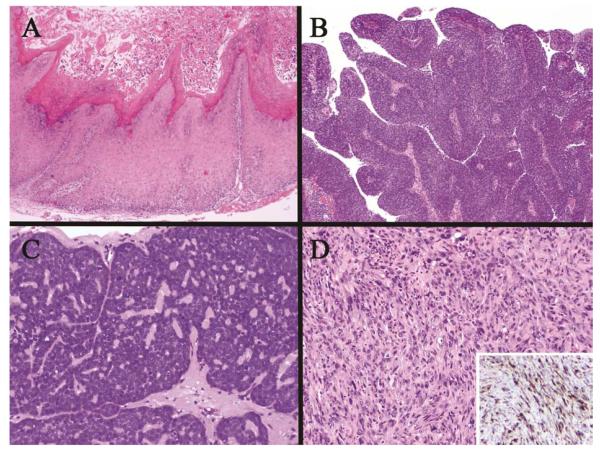
Figure 6.
HPV associated squamous cell carcinoma. (A) Low power view of the tumor arising in the tonsillar cryptic mucosa (arrow) deep to the surface mucosa, (B) Higher power of monotonous, non-keratinizing neoplastic cells typical of this phenotype, (C) Strong/over-expressed p16 immunohistochemical staining throughout the tumor, (D) HPV positive in situ hybridization staining of the tumor nuclei (arrow)
Table 6.
Comparison of histologic subtypes of squamous cell carcinoma (SCC)
| Verrucous Carcinoma |
Hybrid Verrucous/ conventional SCC |
Basaloid Squamous Carcinoma |
Papillary Squamous Carcinoma |
Sarcomatoid Carcinoma |
|
|---|---|---|---|---|---|
| Age | 7-8th decades | 7-8th decades | Older | 7th decade | |
| Sex | M>F | M>F | M>F | M (smoking) | |
| Location | Buccal, gingiva | Base of tongue, hypopharynx |
Rare oral (larynx, sinonasal) |
Larynx>oral/tongue | |
| LN mets | None (if pure) | Risk for LN METS | High risk for mets | May metastasize | May metastasize |
| Gross appearance |
Exophytic, warty, fungating |
Exophytic | Variable/ ulcerated | Exophytic- fronds, friable |
Often polypoid, ulcerated |
| Morphology |
|
|
|
|
|
| Differential diagnosis |
|
|
|
|
|
CA=carcinoma; F=females; LN=Lymph nodes; M=males; Mets=metastases; N/C=nuclear to cytoplasmic; WDSCC=well-differentiated SCC
Verrucous carcinoma is a locally aggressive carcinoma showing a broad pushing growth downward and an exophytic/warty appearance (Figure 7A). Cytologically, the cells are bland with minimal alteration (Figure 7A). Moderate dysplasia and thin angulated rete ridges should raise a concern for a hybrid or conventional SCC. This distinction is important, as pure forms of verrucous carcinomas do not metastasize, however hybrid verrucous carcinomas with conventional SCC require consideration for local and regional evaluation and treatment. Distinction on small biopsies may not be possible with the differential including verrucous hyperplasia, which only shows an exophytic component and is more localized. Complete excision of these lesions with normal adjacent mucosa allows for definitive classification and treatment of these lesions.
Figure 7.
Histologic variants of squamous cell carcinoma. (A)Verrucous carcinoma showing a broad base, minimal cytologic atypia and exophytic spire growth;(B) Papillary squamous carcinoma showing exophytic growth of fibrovascular cores covered by full thickness neoplastic cells without keratinization; (C) Basaloid squamous carcinoma with high-grade features and scant cytoplasm often lacking keratinization as in this example; (D) Sarcomatoid (spindle cell) carcinoma haphazardly growing in sheets with pleomorphism and mitoses, often retaining cytokeratin expression detected by immunohistochemical staining (inset) which aids in differentiating from true sarcomas.
Papillary squamous carcinoma is infrequently encountered in the oral cavity with greater propensity for the nasal cavity and larynx. Biopsies show long papillary fronds lined by neoplastic cells without keratinization overlying a fibrovascular core (Figure 7B). The exophytic growth pattern makes determination of invasion limited on biopsies secondary to scant underlying stroma. Thus, a pathology reading of SCC at least in situ with papillary growth requires clinical correlation regarding the degree of suspicion for an invasion tumor and often represents a limitation of a biopsy sample secondary to its superficial nature.
Basaloid squamous carcinoma is a distinct high-grade histologic variant of SCC, which morphologically overlaps with solid adenoid cystic carcinoma and neuroendocrine carcinoma requiring immunohistochemical evaluation to support final classification in the absence of abrupt keratinization (Figure 7C). Basaloid squamous cell carcinomas may be associated with HPV when this specific subtype arises in the oropharynx; however basaloid SCC arising in other anatomic sites are HPV negative51. Information is still limited regarding the clinical significance of HPV+ in basaloid SCC and if this cohort also portends an improved survival as seen in other HPV associated SCC in the oropharynx.
Sarcomatoid squamous carcinoma (Spindled cell carcinoma) is a high-grade tumor growing in sheets, often composed of pleomorphic spindled shaped cells showing frequent mitoses, and may grow as an exophytic/polypoid mass (Figure 7D). Morphologically this tumor overlaps with true sarcomas, however sarcomas represent only a small minor of primary tumors in the head and neck region. Associated dysplasia or a history of prior dysplasia is also helpful in supporting the diagnosis of sarcomatoid squamous carcinoma. Ancillary testing with cytokeratins by immunohistochemical staining often are positive supporting the mucosal origin and the diagnosis of sarcomatoid carcinoma. Biologically, the transformation from a conventional SCC to a sarcomatoid SCC is thought to represent epithelial mesenchymal transformation (EMT), with loss of adhesion molecules and gain of mesenchymal markers and invasive properties52.
Histopathology prognosticators in SCC
Histologic grading of SCC into well, moderate and poorly differentiated carcinomas is based on the degree of keratinization and cytologic maturation resembling background squamous mucosa. Histologic grade of SCC remains of limited prognostic value though shows a trend for increased lymph node metastases in higher grade tumors. In comparison, perineural invasion, as well as, close margins (<5mm) and positive margin status, have shown a direct association with increased risk of local recurrence and tumor aggressiveness that may warrant adjuvant radiation therapy to achieve optimal local control53. Additionally even early stage tumors T1, T0 with clinically/radiographically N0 necks have a risk for occult metastases54. In primary SCC of the oral tongue and floor of mouth, the best predictor of risk for regional metastases is the tumor depth of invasion when radiographically the nodes are negative (cN0). At a depth of >4 mm in an oral tongue SCC, the risk of occult metastasis has been reported as high as 40% and is considered a sufficient risk to warrant a prophylactic neck dissection in this cohort of patients moreover a recent study suggests >3 mm as a better break point in oral tongue SCC for prophylactic neck dissection55, 56. Similarly a tumor thickness of > 1.5 mm in the floor of mouth region portends a higher risk for occult nodal metastases favoring prophylactic neck dissection (TABLE 7)57. Invasion of the tumor into muscle shows similar correlation with increased risk for regional metastases58. Other histologic factors including tumor invasive patterns and associated tumor inflammation may also allow for future risk-classification and is currently undergoing prospective validation59.
Table 7.
Risk of occult metastases in oral squamous cell carcinoma considerations for elective neck dissections
| Study population | Risk | Author/Conclusion | |
|---|---|---|---|
| Stage I/II clinically N0 | Risk of Recurrence | ||
|
1/28 pN0, 1/8 pN+ 11/35 (31%) |
Yuen et al. (71 patients) Elective neck dissection identified 22% occult LN mets and reduced risk of recurrence |
|
|
| |||
| Clinically T1/T2 N0 | Tumor Depth of Invasion | Risk of Occult Metastases | |
| Tongue | < 3 mm | 1/23 (4.3%) | Zhang et al. ( 65 patients) |
| ≥ 3mm | 13/42 (31%) |
Recc neck dissection when an oral
tongue SCC has a depth of invasion ≥3 mm |
|
| Tongue | <4 mm | 0/14 (0%) | Sparano et al. (45 patients) |
| ≥4 mm | 13/18 (41%) |
Recc neck dissection when an oral
tongue SCC has a depth of invasion ≥4 mm |
|
|
| |||
| FOM | ≤1.5mm | 1/57 (2%) | Mohit-Tabatabai et al.(84 patients) |
| 1.6-3.5 mm > 3.5 mm |
4/12 (33%) 9/15 (60%) |
Favors neck dissection for floor of
mouth SCC >1.5 mm in thickness |
|
FOM=floor of mouth; Recc=recommendation
Margins
Adequacy of margins must account for multiple anatomic and tumor parameters and is not simply black and white/positive or negative. Studies evaluating treatment failure/local recurrence and margin status in OSCC have demonstrated even when a tumor is not at the margin being “close” increases risk for recurrence60. The definition of “close margin” which would warrant consideration for additional tissue resection or adjuvant therapy remains variable however the best consensus is > 5 mm from tumor for defining an adequate margin in HNSCC60. Positive margins are considered SCC in situ or invasive tumor transected at the margin tissue edge. Additional considerations when studying distance to margins has been the marked shrinkage/retraction of tissue, particularly in tongue resections from the in situ distance measured by a surgeon from tumor to tissue margin versus the ex vivo pathology measurement. Explanations for local failure with ‘close’ surgical margins include perineural invasion, lymphovascular invasion, and small tumor nests infiltrating beyond the tumor mass with intervening normal stroma. Moreover, molecular studies on histologically negative margins have shown a wide range of molecular alterations (LOH, p53, etc) including known alterations associated with malignant progression61. The observation of precancerous molecular alterations also emphasizes the idea of field effect of precancerous changes in the oral cavity associated with tobacco exposure, which increases the risk for both local recurrence and the development of second primaries and may also contribute to local recurrence60.
Lymph node metastases and extracapsular extension
In HNSCC, the most significant histologic prognostic factor for overall survival remains the presence of a positive lymph node metastasis followed by the presence of extracapsular extension (ECE) of tumor outside of a lymph node62, 63. While the majority of the data is from oral SCC, the presence of ECE is now widely recognized as an adverse feature, including in other tumor types, which warrants consideration for intensified treatment regiments64. Survival data has shown over a four- fold increase in distant metastases and in death rates compared to node negative patients. The most recent TNM tumor staging breaks extracapsular extension in lymph nodes into clinical/radiographic or macroscopic ECE versus pathologic microscopic ECE; this finding to date does not alter the overall staging of HNSCC patients.
TUMOR STAGING
Unified systems for tumor staging (TNM) have allowed for more consistent use within the oral cavity and lip, though overall staging of tumors arising in the oropharynx remains distinct 65. These sites begin with tumor size for T staging: Tis, is in situ; T1 are tumors up to 2 cm in greatest dimension; and T2 are > 2 cm but < 4 cm; T3 and T4 tumors are based on the extent of invasion into adjacent structures based on primary tumor site. N staging for the oral cavity and oropharynx is the same and is based on the metastasis size (<3, >3 to 6, and >6 cm greatest dimension), number of lymph nodes (single versus multiple) and location (bilateral, contralateral) of the lymph nodes relative to the primary. The overall staging combining the pathologic T and N score differs in oral cavity versus oropharynx primaries with allowable lymph node positivity in stage II oropharynx65. This system reflects the biological distinctions of SCC based on site of origin and the improved survival of OPSCC patients compared to OSCC patients.
Diagnostically relevant immunohistochemical stains for head and neck pathology
Immunohistochemistry
The diagnosis of SCC is based on morphology and rarely requires ancillary studies for support of mucosal origin. However, in small biopsies, particularly of high-grade tumors, confirmation of carcinoma and derivation as squamous origin is required. Immunohistochemistry utilizes antibodies specific to proteins (keratins in carcinoma, S100 and melanin in melanomas, CD45 in lymphomas, etc.) allowing for visualization of molecular expression under a light microscope. HNSCCs typically express squamous epithelial marker cytokeratins 5/6 and p63, a basal cell/stem cell-like marker, is also often diffusely positive. The work-up of high-grade basaloid tumors includes cytokeratin positivity (negative in lymphomas and melanomas), p63 (positivity in SCC, negative in solid adenoid cystic carcinomas), and neuroendocrine markers (synaptophysin, chromogranin), which would be negative in SCC and positive in neuroendocrine carcinomas, small cell carcinomas, and merkel cell carcinomas. Differentiating salivary tumors is performed primarily by their histologic pattern, however salivary tumor cells are positive for cytokeratin 7 and may show intracellular mucin (mucicarmine special stain).
Makers for infections
When evaluating tissue for infectious etiologies, special stains may be used to highlight the microorganisms. Grocott’ s methenamine silver stain (GMS) is most widely used to highlight the wall of fungal organisms, with cultures advised for speciation. Periodic-Acid-Schiff (PAS) is frequently used to detect yeast and pseudo-hyphae (i.e. candida) in tissue sections. While gram stain highlights gram positive and gram-negative bacteria, the high-level of background oral flora makes this test less useful in the oral cavity. Actinomyces may form clusters visualized on morphologic review and are also highlighted by GMS and Gram stains, though hematoxylin and eosin (H&E) identification is sufficient to report the finding.
Prognostic biomarkers for HNSCC
HPV and p16
Currently only HPV and p16 testing are routinely performed as prognostic biomarkers in HNSCCs, specifically only for the evaluation of SCCs arising in the oropharynx. Numerous methodologies exist for direct testing for HPV, however, in situ hybridization is the most universally used method allowing for screening of all known high risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66) in one test on standard paraffin tissue sections66, 67. Limited availability of this methodology for testing in the community, and concern for lower sensitivity compared to polymerase chain reaction (PCR) led to the evaluation of p16 as a surrogate marker for the presence of HPV in tumor cells. The tumor suppressor gene p16 is involved in cell cycle regulation that shows diffuse over-expression (cytoplasmic and nuclear) in tumor cells infected by HPV using standard immunohistochemical techniques. The mechanism of p16 over-expression is theorized to be secondary to viral components (E6 and E7) interfering with the function of Rb and p53 leading to compensatory up-regulation of p16. Early analysis of clinical trial tumor samples showed p16 expression in primary OPSCC tissue strongly correlated with HPV status and improved survival13. While the association between p16 expression and HPV+ is strong in the oropharynx, p16 expression in other HNSCC tissue sites may be unrelated to HPV (as confirmed by negative PCR validation in multiple studies) stressing the need to perform a concurrent direct test for HPV confirmation if testing tissue outside of the oropharynx. Currently HPV tumor status is only used as a prognostic and etiologic factor, however on-going clinical trials are looking at treatment modifications for HPV+ tumors to reduce the long-term morbidities in this younger cancer population. Additionally, while clinical trials specific to OPSCC prevention through HPV vaccination are infeasible, the implementation of the HPV vaccination in the population holds the potential for reducing the overall incidence of HPV. Vaccination is theorized to ultimately result in reducing HPV associated SCC in the head and neck in the coming decades as HPV in the oropharynx are the same high-risk types that cause cervical cancer and that the spread of HPV to the oropharynx has been linked to changes in sexual practices and increased sexual partners.
Molecular alterations in HNSCC
Tp53 and EGFR
Many HNSCCs overexpress the epidermal growth factor receptor (EGFR) a gene involved in cell proliferation, angiogenesis, migration, adhesion and invasion. This observation led to clinical trials and ultimately FDA approval of Cetuximab, a monoclonal antibody directed against EGFR, as an adjuvant treatment with radiation in HNSCC. However no biomarkers have been identified to date with regard to response or resistance to EGFR inhibitors in HNSCC.
Mutation of the TP53 tumor suppressor gene is the most common and earliest genetic alteration associated with HNSCC68. Missense mutations involving the DNA-binding domain of TP53 gene are seen in more than 50% of all conventional/tobacco-related HNSCC. Testing for TP53 mutation in biopsies of HNSCC and its precursors is labor intensive and not feasible in a clinical laboratory. Moreover, currently there are no specific treatment guidelines for HNSCC with TP53 mutations. With the development of next generation sequencing and array technologies allowing for sequencing/screening of whole genes with minimal tumor tissue, new areas of research are on-going exploring p53 association with outcomes and therapies and may lead to new personalized care for HNSCC patients69.
Key Points.
Head and neck squamous cell carcinoma is the 6th most common cancer world wide predominately associated with tobacco use
Changing etiology and increased incidence in oropharyngeal carcinomas is associated with High-Risk types of Human Papilloma Virus (HPV) and has an improved survival
Potentially malignant disorders include a range of entities that vary from low to extremely high risk of transformation to carcinoma as seen in proliferative verrucous leukoplakia.
Visual oral exam may be augmented by optical devices however their lack of specificity still warrants tissue evaluation/biopsy
Histologic factors of oral carcinomas are critical for patient management and prognostic determination including, depth of tumor invasion, perineural invasion, margin status, presence of regional lymph node metastases and presence of extracapsular extension within metastases.
Clinical biomarkers are still needed to improve early detect, predict malignant transformation and optimize therapies.
Abbreviations
- COE
Clinical oral examination
- DC
Dyskeratosis congenita
- FA
Fanconi anemia
- FAD
Flavin adenine dinucleotide
- HNSCC
Head and neck squamous cell carcinoma
- HPV
Human Papilloma Virus
- LOH
Loss of heterozygosity
- NADH
Nicotinamide adenine dinucleotide
- nM
Nanometer
- OED
Oral epithelial dysplasia
- OPSCC
Oropharyngeal squamous cell carcinoma
- OSCC
Oral squamous cell carcinoma
- PMD
Potentially malignant disorders
- PVL
Proliferative verrucous leukoplakia
- SCC
Squamous cell carcinoma
- U.S.
United States
- UV
Ultraviolet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nadarajah Vigneswaran, The University of Texas School of Dentistry at Houston Director, Oral and Maxillofacial Pathology Biopsy Service 1941 East Rd. BBSB, Rm. 5320, Houston TX.
Michelle D. Williams, UT MD Anderson Cancer Center Director, Surgical Pathology Fellowship Program Head & Neck Section, Dept. of Pathology 1515 Holcombe BLVD, Unit #085 Houston, Texas 77030.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 5.Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerging infectious diseases. 2010;16(11):1671–1677. doi: 10.3201/eid1611.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho AL, Nishimoto IN, Califano JA, et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 7.Fakhry C, D’Souza G. Discussing the diagnosis of HPV-OSCC: common questions and answers. Oral Oncol. 2013;49(9):863–871. doi: 10.1016/j.oraloncology.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282–3287. [PubMed] [Google Scholar]
- 9.Travasso C. Betel quid chewing is responsible for half of oral cancer cases in India, finds study. BMJ. 2013;347:f7536. doi: 10.1136/bmj.f7536. [DOI] [PubMed] [Google Scholar]
- 10.Rodu B, Jansson C. Smokeless tobacco and oral cancer: a review of the risks and determinants. Crit Rev Oral Biol Med. 2004;15(5):252–263. doi: 10.1177/154411130401500502. [DOI] [PubMed] [Google Scholar]
- 11.Petti S, Masood M, Messano GA, et al. Alcohol is not a risk factor for oral cancer in nonsmoking, betel quid non-chewing individuals. A meta-analysis update. Annali di igiene : medicina preventiva e di comunita. 2013;25(1):3–14. doi: 10.7416/ai.2013.1901. [DOI] [PubMed] [Google Scholar]
- 12.Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levitus M, Joenje H, de Winter JP. The Fanconi anemia pathway of genomic maintenance. Cell Oncol. 2006;28(1-2):3–29. doi: 10.1155/2006/974975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutler DI, Auerbach AD, Satagopan J, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129(1):106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 16.Masserot C, Peffault de Latour R, Rocha V, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer. 2008;113(12):3315–3322. doi: 10.1002/cncr.23954. [DOI] [PubMed] [Google Scholar]
- 17.Bessler M, Wilson DB, Mason PJ. Dyskeratosis congenita. FEBS Lett. 2010;584(17):3831–3838. doi: 10.1016/j.febslet.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheckenbach K, Wagenmann M, Freund M, et al. Squamous cell carcinomas of the head and neck in Fanconi anemia: risk, prevention, therapy, and the need for guidelines. Klin Padiatr. 2012;224(3):132–138. doi: 10.1055/s-0032-1308989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vigneswaran N, Tilashalski K, Rodu B, et al. Tobacco use and cancer. A reappraisal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80(2):178–182. doi: 10.1016/s1079-2104(05)80199-4. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease C. Prevention Human papillomavirus-associated cancers - United States, 2004-2008. MMWR Morbidity and mortality weekly report. 2012;61:258–261. [PubMed] [Google Scholar]
- 21.Chen AY, Myers JN. Cancer of the oral cavity. Dis Mon. 2001;47(7):275–361. doi: 10.1067/mcd.2001.109374. [DOI] [PubMed] [Google Scholar]
- 22.Ridge JA, Glisson BS, Lango MN, et al. Head and Neck Tumors. In: Haller DE, Wagman LD, Camphausen KA, et al., editors. Cancer Management: A Multidisciplinary Approach, Medical, Surgical & Radiation Oncology. 14th ed UBM Medica; NY, USA: 2011. pp. 1–42. [Google Scholar]
- 23.Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488–1494. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 24.Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973-1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128(3):268–274. doi: 10.1001/archotol.128.3.268. [DOI] [PubMed] [Google Scholar]
- 25.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 26.Harris SL, Kimple RJ, Hayes DN, et al. Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck. 2010;32(4):499–503. doi: 10.1002/hed.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vargas H, Pitman KT, Johnson JT, et al. More aggressive behavior of squamous cell carcinoma of the anterior tongue in young women. Laryngoscope. 2000;110(10 Pt 1):1623–1626. doi: 10.1097/00005537-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26(3-4):645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 29.Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575–580. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 30.Caporaso NE. Why precursors matter. Cancer Epidemiol Biomarkers Prev. 2013;22(4):518–520. doi: 10.1158/1055-9965.EPI-13-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37(1):1–10. doi: 10.1111/j.1600-0714.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 32.Gillenwater AM, Vigneswaran N, Fatani H, et al. Proliferative verrucous leukoplakia (PVL): Recognition and differentiation from conventional leukoplakia and mimics! Head Neck. 2013 doi: 10.1002/hed.23505. [DOI] [PubMed] [Google Scholar]
- 33.Gillenwater AM, Vigneswaran N, Fatani H, et al. Proliferative Verrucous Leukoplakia (PVL): A Review of an Elusive Pathologic Entity! Advances in anatomic pathology. 2013;20(6):416–423. doi: 10.1097/PAP.0b013e3182a92df1. [DOI] [PubMed] [Google Scholar]
- 34.Zain RB, Ikeda N, Gupta PC, et al. Oral mucosal lesions associated with betel quid, areca nut and tobacco chewing habits: consensus from a workshop held in Kuala Lumpur, Malaysia, November 25-27, 1996. J Oral Pathol Med. 1999;28(1):1–4. doi: 10.1111/j.1600-0714.1999.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 35.Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46(2):207–214. doi: 10.1067/mjd.2002.120452. [DOI] [PubMed] [Google Scholar]
- 36.Lovas JG, Harsanyi BB, ElGeneidy AK. Oral lichenoid dysplasia: a clinicopathologic analysis. Oral Surg Oral Med Oral Pathol. 1989;68(1):57–63. doi: 10.1016/0030-4220(89)90115-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Cheng X, Li Y, et al. High frequency of allelic loss in dysplastic lichenoid lesions. Lab Invest. 2000;80(2):233–237. doi: 10.1038/labinvest.3780026. [DOI] [PubMed] [Google Scholar]
- 38.Epstein JB, Guneri P, Boyacioglu H, et al. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J Am Dent Assoc. 2012;143(12):1332–1342. doi: 10.14219/jada.archive.2012.0096. [DOI] [PubMed] [Google Scholar]
- 39.Shin D, Vigneswaran N, Gillenwater A, et al. Advances in fluorescence imaging techniques to detect oral cancer and its precursors. Future Oncol. 2010;6(7):1143–1154. doi: 10.2217/fon.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lingen MW, Kalmar JR, Karrison T, et al. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44(1):10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speight PM. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007;1(1):61–66. doi: 10.1007/s12105-007-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mithani SK, Mydlarz WK, Grumbine FL, et al. Molecular genetics of premalignant oral lesions. Oral Dis. 2007;13(2):126–133. doi: 10.1111/j.1601-0825.2006.01349.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Poh CF, Williams M, et al. Loss of heterozygosity (LOH) profiles--validated risk predictors for progression to oral cancer. Cancer Prev Res (Phila) 2012;5(9):1081–1089. doi: 10.1158/1940-6207.CAPR-12-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennan M, Migliorati CA, Lockhart PB, et al. Management of oral epithelial dysplasia: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(Suppl:S19):e11–12. doi: 10.1016/j.tripleo.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Arnaoutakis D, Bishop J, Westra W, et al. Recurrence patterns and management of oral cavity premalignant lesions. Oral Oncol. 2013;49(8):814–817. doi: 10.1016/j.oraloncology.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Mehanna HM, Rattay T, Smith J, et al. Treatment and follow-up of oral dysplasia - a systematic review and meta-analysis. Head Neck. 2009;31(12):1600–1609. doi: 10.1002/hed.21131. [DOI] [PubMed] [Google Scholar]
- 47.Meltzer C. Surgical management of oral and mucosal dysplasias: The case for laser excision. J Oral Maxillofac Surg. 2007;65(2):293–295. doi: 10.1016/j.joms.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Saito T, Sugiura C, Hirai A, et al. Development of squamous cell carcinoma from pre existent oral leukoplakia: with respect to treatment modality. International journal of oral and maxillofacial surgery. 2001;30(1):49–53. doi: 10.1054/ijom.2000.0012. [DOI] [PubMed] [Google Scholar]
- 49.Wong SJ, Campbell B, Massey B, et al. A phase I trial of aminolevulinic acid-photodynamic therapy for treatment of oral leukoplakia. Oral Oncol. 2013;49(9):970–976. doi: 10.1016/j.oraloncology.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saba N, Hurwitz SJ, Kono S, et al. Chemoprevention of Head and Neck Cancer with Celecoxib and Erlotinib: Results of a Phase 1b and Pharmacokinetic Study. Cancer Prev Res (Phila) 2013 doi: 10.1158/1940-6207.CAPR-13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–92. doi: 10.1093/annonc/mdq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tai SK, Li WY, Chu PY, et al. Risks and clinical implications of perineural invasion in T1-2 oral tongue squamous cell carcinoma. Head Neck. 2012;34(7):994–1001. doi: 10.1002/hed.21846. [DOI] [PubMed] [Google Scholar]
- 54.Yuen AP, Ho CM, Chow TL, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009;31(6):765–772. doi: 10.1002/hed.21033. [DOI] [PubMed] [Google Scholar]
- 55.Sparano A, Weinstein G, Chalian A, et al. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004;131(4):472–476. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhang T, Lubek JE, Salama A, et al. Treatment of cT1N0M0 Tongue Cancer: Outcome and Prognostic Parameters. J Oral Maxillofac Surg. 2013 doi: 10.1016/j.joms.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 57.Mohit-Tabatabai MA, Sobel HJ, Rush BF, et al. Relation of thickness of floor of mouth stage I and II cancers to regional metastasis. Am J Surg. 1986;152(4):351–353. doi: 10.1016/0002-9610(86)90303-x. [DOI] [PubMed] [Google Scholar]
- 58.Chandler K, Vance C, Budnick S, et al. Muscle invasion in oral tongue squamous cell carcinoma as a predictor of nodal status and local recurrence: just as effective as depth of invasion? Head Neck Pathol. 2011;5(4):359–363. doi: 10.1007/s12105-011-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 60.Hinni ML, Ferlito A, Brandwein-Gensler MS, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35(9):1362–1370. doi: 10.1002/hed.23110. [DOI] [PubMed] [Google Scholar]
- 61.Slootweg PJ, Hordijk GJ, Schade Y, et al. Treatment failure and margin status in head and neck cancer. A critical view on the potential value of molecular pathology. Oral Oncol. 2002;38(5):500–503. doi: 10.1016/s1368-8375(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 62.Johnson JT, Myers EN, Bedetti CD, et al. Cervical lymph node metastases. Incidence and implications of extracapsular carcinoma. Archives of otolaryngology. 1985;111(8):534–537. doi: 10.1001/archotol.1985.00800100082012. [DOI] [PubMed] [Google Scholar]
- 63.Myers JN, Greenberg JS, Mo V, et al. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92(12):3030–3036. doi: 10.1002/1097-0142(20011215)92:12<3030::aid-cncr10148>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 64.Gruber G, Bonetti M, Nasi ML, et al. Prognostic value of extracapsular tumor spread for locoregional control in premenopausal patients with node-positive breast cancer treated with classical cyclophosphamide, methotrexate, and fluorouracil: long-term observations from International Breast Cancer Study Group Trial VI. J Clin Oncol. 2005;23(28):7089–7097. doi: 10.1200/JCO.2005.08.123. [DOI] [PubMed] [Google Scholar]
- 65.SE E, DR B, CC C, et al., editors. AJCC Cancer Staging Manual. 7th ed Springer; New York, NY, USA: 2009. [Google Scholar]
- 66.Schache AG, Liloglou T, Risk JM, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17(19):6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duray A, Descamps G, Arafa M, et al. High incidence of high-risk HPV in benign and malignant lesions of the larynx. Int J Oncol. 2011;39(1):51–59. doi: 10.3892/ijo.2011.1031. [DOI] [PubMed] [Google Scholar]
- 68.Rothenberg SM, Ellisen LW. The molecular pathogenesis of head and neck squamous cell carcinoma. J Clin Invest. 2012;122(6):1951–1957. doi: 10.1172/JCI59889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skinner HD, Sandulache VC, Ow TJ, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res. 2012;18(1):290–300. doi: 10.1158/1078-0432.CCR-11-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]