Abstract
Using a biochemically complex cytoplasmic extract to reconstitute actin-based motility of Listeria monocytogenes and polystyrene beads coated with the bacterial protein ActA, we have systematically varied a series of biophysical parameters and examined their effects on initiation of motility, particle speed, speed variability, and path trajectory. Bead size had a profound effect on all aspects of motility, with increasing size causing slower, straighter movement and inhibiting symmetry-breaking. Speed also was reduced by extract dilution, by addition of methylcellulose, and paradoxically by addition of excess skeletal muscle actin, but it was enhanced by addition of nonmuscle (platelet) actin. Large, persistent individual variations in speed were observed for all conditions and their relative magnitude increased with extract dilution, indicating that persistent alterations in particle surface properties may be responsible for intrinsic speed variations. Trajectory curvature was increased for smaller beads and also for particles moving in the presence of methylcellulose or excess skeletal muscle actin. Symmetry breaking and movement initiation occurred by two distinct modes: either stochastic amplification of local variation for small beads in concentrated extracts, or gradual accumulation of strain in the actin gel for large beads in dilute extracts. Neither mode was sufficient to enable spherical particles to break symmetry in the cytoplasm of living cells.
INTRODUCTION
The actin-based motility of intracellular bacterial pathogens such as Listeria monocytogenes, in which moving bacteria leave in their wake a characteristic comet-like tail of cross-linked actin filaments (Tilney and Portnoy, 1989), has served as a useful model for understanding some aspects of the dynamics of actin assembly at the leading edge of motile cells (Cameron et al., 2000; Pollard et al., 2000; Pantaloni et al., 2001). Biochemical analysis of this form of actin-based motility has been greatly facilitated by the ability of L. monocytogenes to move normally in cell-free cytoplasmic extracts from eggs (Theriot et al., 1994), platelets (Welch et al., 1998), or brain tissue (May et al., 1999). Identification of the molecules involved in this process has culminated in the reconstitution of bacterial actin-based motility by using purified proteins (Loisel et al., 1999). Only one bacterial factor, ActA, is required for L. monocytogenes to perform actin-based motility in infected cells (Kocks et al., 1992), cytoplasmic extracts (Kocks et al., 1995; Smith et al., 1995), or an appropriate mixture of purified proteins (Loisel et al., 1999; Samarin et al., 2003). In addition to actin and ATP, just three host cell proteins are absolutely required for comet tail formation and movement; the Arp2/3 complex for dendritic actin nucleation, capping protein to limit filament elongation, and ADF/cofilin to accelerate filament depolymerization (Loisel et al., 1999). Three additional proteins enhance the speed or stability of movement; VASP, profilin, and α-actinin (Loisel et al., 1999).
After the biochemical characterization of the bacterial and host cell factors necessary and sufficient for this form of actin-based movement, attention has turned toward understanding the biophysics of motility driven by actin polymerization by using artificial particles such as polystyrene beads or phospholipid vesicles as replacements for the bacterial cargo (Cameron et al., 1999; van Oudenaarden and Theriot, 1999; Bernheim-Groswasser et al., 2002; Giardini et al., 2003; Upadhyaya et al., 2003). Polystyrene beads coated with a variety of proteins that activate actin nucleation by the Arp2/3 complex are capable of generating superficially similar actin comet tails and moving unidirectionally in several kinds of cytoplasmic extracts (Cameron et al., 1999; Yarar et al., 1999) and mixtures of purified proteins (Bernheim-Groswasser et al., 2002; Samarin et al., 2003; Wiesner et al., 2003). There are important quantitative and qualitative differences among the various reconstituted motility systems with respect to the identities of the proteins that can support actin comet tail formation, the size of particles capable of initiating movement, the structural organization of the actin comet tail, and the biophysical parameters governing movement. For example, very large beads (up to 10 μm in diameter) are capable of breaking symmetry and initiating movement in a mixture of purified proteins (Bernheim-Groswasser et al., 2002), whereas symmetry-breaking is observed only for submicron particles in concentrated cytoplasmic extracts (Cameron et al., 1999). Addition of methylcellulose to cytoplasmic extracts to increase their effective viscosity significantly decreases bacterial speed (McGrath et al., 2003), but similar addition of methylcellulose to a mixture of purified proteins has only modest effects on the speed of N-WASP–coated polystyrene beads (Wiesner et al., 2003). Beads coated with the VCA domain of N-WASP cannot initiate movement in brain cytoplasmic extracts (Suetsugu et al., 2001), although they can in a mixture of purified proteins (Bernheim-Groswasser et al., 2002). It is currently unknown whether these types of differences are primarily due to the reduced biochemical complexity of the purified protein system or to general physical differences between the two types of systems such as overall protein concentration or viscosity.
Two recent articles have examined the effects of systematic variation of biophysical parameters, including particle size and surface protein density on various quantitative parameters of actin-based motility by using polystyrene beads coated with N-WASP or the VCA domain of N-WASP in a mixture of purified proteins (Bernheim-Groswasser et al., 2002; Wiesner et al., 2003). In this report, we have systematically and quantitatively compared the motility of live bacteria expressing ActA and of ActA-coated polystyrene beads in biochemically complex cytoplasmic extracts while varying a series of biophysical parameters, including particle size, ActA surface density, extract dilution, actin concentration, and viscosity. We find a continuum of changes in particle behavior, indicating that biochemically similar mechanisms for generation of actin-based motility can produce quantitatively and qualitatively distinct behaviors in different biophysical regimes.
MATERIALS AND METHODS
ActA-coated Beads
Protein purification and bead coating was performed as described previously (Cameron et al., 1999). A hexa-histidine–tagged form of ActA was purified from strain DP-L2723 of L. monocytogenes (a gift from Daniel Portnoy, University of California, Berkeley, CA) expressing a truncated actA gene encoding amino acids 1–613 (Welch et al., 1998). Carboxylated polystyrene beads (Polysciences, Warrington, PA) of size 0.2–2 μm were incubated in protein solution containing the stated percentage of ActA-His and supplemented with ovalbumin to 2 mg/ml final protein concentration. In all cases, conditions were such that the amount of protein was saturating. For example, 2 μl of 0.5 μm beads (2.5% solids) were added to 10 μl of protein solution; the volume of beads was adjusted to account for changes in surface area for beads of different sizes. ActA binding to the surface of the beads (over the range of surface densities) was confirmed by SDS-PAGE.
Motility Assays
Bead motility assays were performed as described previously (Cameron et al., 1999) by adding ActA-His–coated beads to Xenopus laevis egg cytoplasmic extract supplemented with tetramethylrhodamine iodoacetamide-labeled actin and ATP-regenerating mix. The assay mixture was incubated on ice briefly before making a sample slide for as long as 1 h, depending on parameters in the particular experiment. Then, a 1.2-μl sample was removed and squashed between a microscope slide and 22-mm2 glass coverslip and sealed with Vaseline:lanolin:paraffin at 1:1:1. The preparation was incubated at room temperature for between 15 min and 1 h in the dark before observing on the microscope, depending on the experiment. Most observations were performed on an Axioplan2 microscope (Carl Zeiss, Thornwood, NY) equipped with phase contrast and epifluorescence optics. Time-lapse microscopy was achieved with a digital cooled charge-coupled device (CCD) camera (Micro-MAX:512BFT, Princeton Instruments, Roper Scientific, Trenton, NJ) specialized for low light level and high frequency imaging, by using MetaMorph (Universal Imaging, Media, PA) software. Every 10 s, phase contrast (with 50-ms exposure) and fluorescence (400-ms exposures) image pairs were recorded. Some observations were performed on a Nikon Diaphot-300 inverted microscope equipped with phase contrast and epifluorescence optics with an attached intensified CCD camera (Dage-MTI GenIISys/CCD-c72) for time-lapse videomicroscopy. Video images were captured, digitized, and analyzed using MetaMorph software. Phase contrast and fluorescence image pairs were recorded every 10 s with eight video frames averaged at each time point. Bead trajectories were tracked by hand using the MetaMorph software Track Points function to denote the coordinates of the center of each bead in the phase contrast image at the pixel level. Bacteria (L. monocytogenes strain SLCC-5764) were tracked similarly, but coordinates corresponded to the end of the bacteria associated with the actin tail.
Extract Alterations
Crude egg extract was diluted from full strength with XB (100 mM KCl, 0.1 mM CaCl2, 2 mM MgCl2, 5 mM EGTA, 10 mM K-HEPES, pH 7.7) to a range of extract concentrations denoted as the percentage of full-strength extract. In addition to diluting extracts with XB, some crude extract samples were diluted with methylcellulose 4000 (M-0512; Sigma-Aldrich, St. Louis, MO) to a final concentration of 0.2%. The methylcellulose equivalent of crude extract viscosity is 0.2% (for M-0512; by falling ball assay); thus, dilution with this concentration of an unreactive macromolecular viscosity agent keeps the viscosity of the diluted extract the same while diluting the protein concentration. Measurements were performed as described above for fraction of beads that form tails and average speed of moving beads. For some experiments, purified monomeric actin from rabbit skeletal muscle (Pardee and Spudich, 1982) or human platelets (Schaier, 1992) was added to X. laevis egg extract. The concentration of actin in X. laevis egg extract was determined to be 7 μM by the DNase I inhibition assay (Nefsky and Bretscher, 1992). To prevent unintentional actin filament formation the components were mixed in the following order: X. laevis egg extract, G-actin, XB salts, and then beads. G-buffer was substituted for XB in some experiments to test whether the salt concentration in XB was affecting actin filament formation. No difference in filament formation (assayed by fluorescence microscopy) was detected between XB and G-buffer, so XB was used as the dilution buffer in actin add back experiments.
Speed and Motility Initiation Analysis
Average speed was measured using MetaMorph software tracking function to measure distance moved per time point for beads moving in at least 10 and up to 90 consecutive frames. For the measurement of fraction of beads with tails, the number of clouds and tails in three to six different fields was counted using a standard cell counter or by capturing single frames of different fields of view and then counting number of clouds and tails. At least 150 and up to 1000 beads were counted for each sample parameter.
Curvature Analysis
The X-Y coordinates obtained from the Track Points function of MetaMorph software were analyzed for angular deviation by plotting the average cosine of the angle formed by all possible pairs of segments in a trajectory separated by x μm (Auerbuch et al., 2003). Averages for which <30 independent measurements could be made were excluded.
Particle Attachment
Small fluorescent beads were attached to the surface of L. monocytogenes as described previously (Robbins and Theriot, 2003). L. monocytogenes hyperhemolytic strain SLCC-5764 were grown overnight in brain-heart infusion at 37°C with shaking. Bacteria were then washed and resuspended in ∼1:10 volumes of XB buffer and then incubated in a 1:1 ratio with 0.05-μm-diameter YG fluorescent carboxylated microspheres (Polysciences), which had been diluted to 0.0025% solids. Incubation times varied from 1 to 10 min. Assays were performed at a final X. laevis extract concentration of 50%. At least three trajectories were tracked for each bacterium using the MetaMorph Track Points function: one representing the bacterial end associated with the comet tail, one for the opposite end and one for each bead. The former two were tracked using phase contrast images and the latter from fluorescent images separated by a fixed amount of time (∼0.3 s). Bead displacement was calculated by orthogonal projection of the bead coordinate onto the line formed through the two bacterial end coordinates.
Delivery of Beads into Cells
Madin-Darby canine kidney (MDCK) cells expressing green fluorescent protein (GFP)-actin were cultured on coverslips as described previously (Robbins et al., 1999). For delivery of beads by using the gene gun, plastic tubing was cut into 0.5-inch segments to serve as “bullets.” Droplets of buffer containing 0.2-μm-diameter ActA-coated beads were placed on the interior sides of the tubes, and the bullets were chilled overnight at 4°C for drying. Dried beads retained normal motility in X. laevis egg extracts after resuspension. Bullets were loaded into the chamber of a Helios gene gun (Bio-Rad, Hercules, CA). Media were aspirated from monolayers of MDCK cells expressing GFP-actin, and the gun was fired from 2–4 inches away at 250–300 psi. For delivery of beads by internalization, MBP-invasin and His-tagged listeriolysin O were purified as described previously (Leong et al., 1990; Gedde et al., 2000). ActA-coated beads were incubated for 30 min in MBP-invasin, rinsed twice in 20% phosphate-buffered saline (PBS), incubated for 15 min at room temperature in 30 mM nickel sulfate, rinsed twice in 20% PBS, resuspended in full-strength PBS, and incubated in His-LLO as described previously (Monack and Theriot, 2001). Triple-coated beads also retained normal motility in X. laevis egg extracts. Protein-coated beads were spun onto MDCK cells expressing GFP-actin, which were plated on glass coverslips held in six-well plates in a clinical centrifuge.
RESULTS
Large ActA-coated Particles Move in Diluted Cellular Extracts
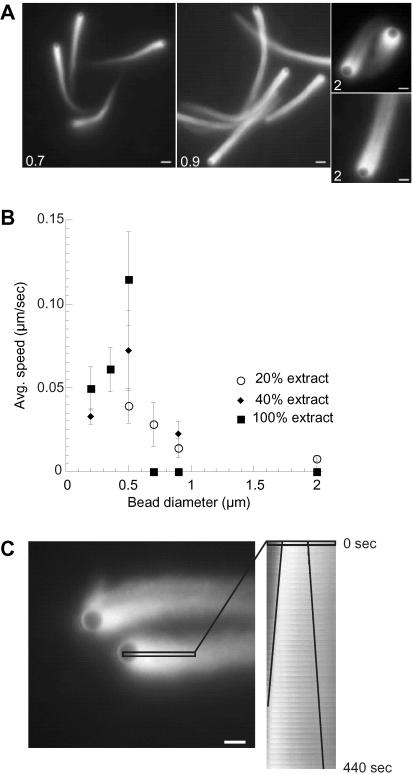
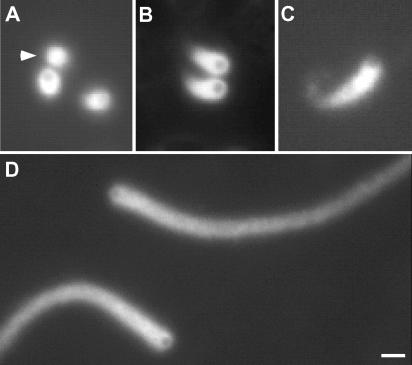
Actin-based motility of ActA-coated polysytrene beads in cell-free cytoplasmic extract of X. laevis eggs has previously been shown to be size dependent, where particles >0.5 μm in diameter fail to initiate movement (Cameron et al., 1999). The most dramatic difference between motility in cytoplasmic extracts and in mixtures of purified proteins is the ability of the latter to support the movement initiation of very large (10-μm) beads (Bernheim-Groswasser et al., 2002). To test whether larger beads could move in cytoplasmic extracts where protein concentration and viscosity were lower, more closely approximating the purified protein systems, we added large ActA-coated beads (0.7–2 μm in diameter) to X. laevis egg extract, which was diluted (by volume) from full-strength crude extract with isotonic buffer. We found that if the extract was diluted to 40% of the original crude extract concentration, larger beads (up to 0.9 μm in diameter) formed actin tails and moved (Figure 1B). Beads as large as 2 μm in diameter exhibited symmetry-breaking and actin-based motility in extract diluted to 20% of its initial concentration (Figure 1A).
Figure 1.
Large beads move in diluted extracts. Beads were coated with 37.5% ActA and added to diluted extract, which was 20% of full-strength crude extract. (A) Beads 0.7 μm (left), 0.9 μm (middle), and 2 μm (top and bottom right) in diameter all form tails in diluted extracts. Micrometer diameter of the bead noted in the bottom left of each image. (B) Average speeds of beads (0.2–2 μm in diameter (x-axis) added to crude (100%, filled squares), 40% extract (filled diamonds), or 20% extract (open circles) were calculated from tracked position. Error bars are SD. (C) Retrograde flux of actin tail on 2-μm bead. A rectangular region containing bead and fluorescence in tail (black box in left image) is monitored at each 10-s time interval. The position of the region is shown in the first time point in the image on the left, and the region at each consecutive 10-s time point is presented from 0 to 440 s as a stacked image on the right (“kymograph” function in MetaMorph). A line denoting the position of the bead is shown on the left and a line denoting the fluorescence intensity shift to the right is shown on the right in the kymograph. The slope is calculated and based on the time and the distance, the rate of movement of the bead position and the fluorescence intensity of a spot were calculated. The bead is moving to the left at 0.0052 μm/s, and the tail is moving to the right at 0.004 μm/s. The sum of these rates equals approximately the same rate as 2-μm beads moving without retrograde flux. Bars, 2 μm.
To determine the effect of dilution on quantitative parameters of motility, a range of bead sizes (0.2–2 μm in diameter) were coated with ActA protein and added to crude X. laevis egg extract that was diluted with various amounts of isotonic buffer and the average speed of each moving particle was measured. Small beads that move in crude extract (0.2–0.5 μm in diameter) moved significantly more slowly in extracts diluted with isotonic buffer (by rank-sum test for all data sets with n ≥ 10; Figure 1B and Table 1), confirming previous observations (Cameron et al., 2001). Larger beads (0.7–2 μm), which do not move in crude undiluted extracts (Cameron et al., 1999), were able to form tails and move in extract diluted to 20%, but more slowly than all smaller beads tested (Figure 1B). The decrease in speed (in 20% extract) as the diameter increases suggests that average speed is dependent on bead size in this regime. Small beads (0.2 and 0.35 μm in diameter) moved slower than 0.5-μm beads in both 100 and 40% extract, indicating that 0.5 μm is the optimal size for efficient motility.
Table 1.
Measurement of speed of objects moving via actin-based motility
| Bead diameter (μm) | % ActA | % Extract | n | Average speed ± SEM (μm/s) | Average SD (μm/s) |
|---|---|---|---|---|---|
| 0.2 | 3 | 100 | 9 | 0.071 ± 0.004 | 0.011 |
| 0.2 | 3 | 40 | 5 | 0.046 ± 0.009 | 0.020 |
| 0.2 | 12.5 | 100 | 21 | 0.063 ± 0.003 | 0.012 |
| 0.2 | 12.5 | 40 | 6 | 0.058 ± 0.007 | 0.018 |
| 0.2 | 37.5 | 100 | 137 | 0.057 ± 0.002 | 0.023 |
| 0.2 | 37.5 | 40 | 10 | 0.033 ± 0.002 | 0.005 |
| 0.35 | 37.5 | 100 | 56 | 0.063 ± 0.002 | 0.018 |
| 0.5 | 12.5 | 100 | 3 | 0.079 ± 0.014 | 0.024 |
| 0.5 | 25 | 100 | 18 | 0.134 ± 0.006 | 0.026 |
| 0.5 | 37.5 | 100 | 29 | 0.129 ± 0.005 | 0.027 |
| 0.5 | 50 | 100 | 18 | 0.124 ± 0.006 | 0.025 |
| 0.5 | 75 | 100 | 9 | 0.112 ± 0.005 | 0.016 |
| 0.5 | 100 | 100 | 17 | 0.103 ± 0.004 | 0.018 |
| 0.5 | 37.5 | 20 | 20 | 0.057 ± 0.007 | 0.033 |
| 0.5 | 37.5 | 40 | 35 | 0.071 ± 0.004 | 0.023 |
| 0.5 | 37.5 | 40+MCa | 26 | 0.055 ± 0.004 | 0.018 |
| 0.5 | 37.5 | 40+2.4sa | 11 | 0.058 ± 0.003 | 0.011 |
| 0.5 | 37.5 | 40+6.4s | 16 | 0.041 ± 0.002 | 0.013 |
| 0.5 | 37.5 | 40ca | 16 | 0.078 ± 0.007 | 0.023 |
| 0.5 | 37.5 | 40c+2.4pa | 14 | 0.098 ± 0.006 | 0.027 |
| 0.5 | 37.5 | 40c+2.4s | 14 | 0.052 ± 0.004 | 0.018 |
| 0.7 | 37.5 | 20 | 14 | 0.028 ± 0.003 | 0.012 |
| 0.9 | 37.5 | 20 | 18 | 0.014 ± 0.001 | 0.006 |
| 0.9 | 37.5 | 40 | 10 | 0.023 ± 0.002 | 0.007 |
| 2 | 37.5 | 20 | 13 | 0.007 ± 0.0006 | 0.002 |
| Lmb | 100 | 49 | 0.104 ± 0.005 | 0.034 | |
| Lm | 100ba | 38 | 0.145 ± 0.009 | 0.055 | |
| Lm | 50b | 32 | 0.088 ± 0.005 | 0.028 | |
| Lm | 25b | 19 | 0.048 ± 0.003 | 0.015 |
+MC, Methylcellulose; +2.4 and +6.4 indicate the addition of 2.4 or 6.4 μM actin; p and s indicate platelet and skeletal muscle actin respectively; and b and c designate two additional different preparations of X. laevis egg extract. All bead motility experiments were performed in the “a” extract unless indicated.
Lm, L. monocytogenes bacteria.
Polymerization-dependent Rearward Movement of Actin Tails Attached to a Subset of Large Beads
Most large beads (0.7–0.9 μm) added to diluted extract moved in a similar manner to small beads (0.2–0.5 μm). This was confirmed by a combination of phase and fluorescence microscopy, which demonstrated that the addition of new actin subunits at the interface of the bead and the actin tail propelled the large beads forward in diluted extracts, whereas the tail remained stationary, as seen previously for small beads in undiluted crude extract (Cameron et al., 1999) and as originally demonstrated for ActA-directed actin-based motility by bacteria in infected cells (Sanger et al., 1992; Theriot et al., 1992).
However, observations of the motility of 2-μm beads in 20% extract revealed that a subset (∼30%) of these large beads moved at a slower rate, and the fluorescence in the actin tail was not stationary but seemed to be moving away from the surface of the bead, in contrast to the normal situation where the actin comet tail is stationary and the bead moves forward. We analyzed the rearward flow of actin in the tail over time by monitoring the fluorescence intensity of a region of interest containing both the bead and a section of the tail. A kymograph (stacking the series of 10-s time points of the fluorescence intensity map of a boxed region in a column) allowed us to measure both the forward movement of the bead and rearward movement of the tail as the slope drawn through two constant points (the bead and a point in the tail) in that column (Figure 1C). Interestingly, these two rates added together equaled the approximate rate of motility of 2-μm beads with normal tails that were not exhibiting rearward flux, indicating that the net rate of actin gel growth at the surface remained unchanged and the force due to actin polymerization was being partitioned between forward movement of the bead and retrograde movement of the actin tail. Retrograde flow of actin filaments has been previously demonstrated to be a myosin-dependent process in growth cones (Lin et al., 1996), but theoretically it could be driven by the free energy of actin polymerization (Hill and Kirschner, 1982).
Average Speed Is Determined by the Surrounding Environment
The finding that larger beads move in diluted extract enabled us to analyze a much larger matrix of variables and their effects on actin-based motility than had been previously experimentally accessible. These variables included particle size, environment (both viscosity and host protein concentration), and ActA surface density on beads (Table 1). Several interesting trends emerged.
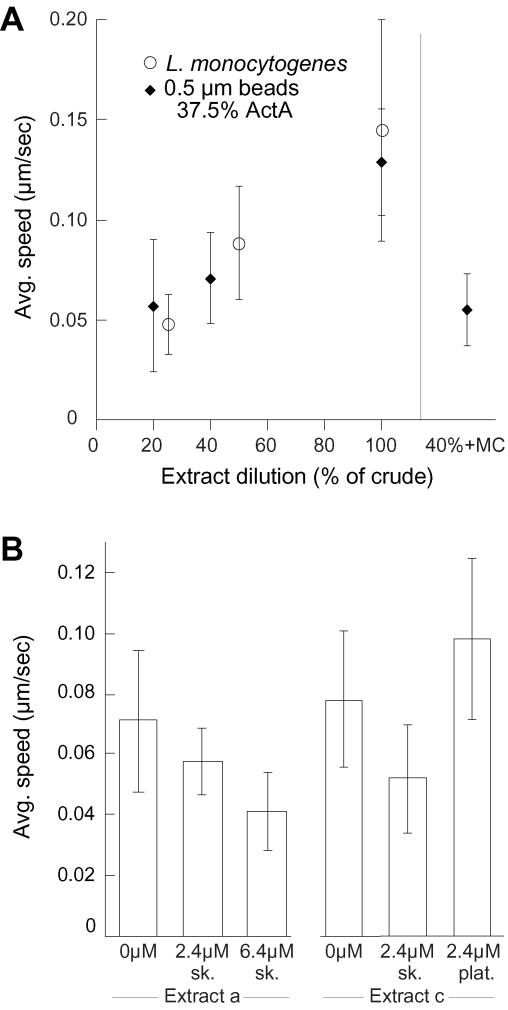
As described above, ActA-coated beads moved significantly more slowly in extracts diluted with an isotonic buffer than in crude extract (for all bead sizes and ActA surface densities by rank-sum test for all data sets with n ≥ 10) (Figures 1B and 2A and Table 1). L. monocytogenes also exhibited this decrease in speed in diluted extracts, demonstrating that the effect was not unique to microspheres, but a genuine property of the surrounding extract environment (Figure 2A and Table 1). Dilution of crude X. laevis egg extract has two main effects: decreased viscosity of the extract and dilution of protein components in the extract that may be required for motility. To determine whether the decrease in speed of moving beads in diluted extracts was due to a decrease in viscosity, we increased the viscosity of 40% extract by adding methylcellulose, an inert macroviscosity agent, until the viscosity of crude extract was restored. Diluting the extract from full-strength crude extract to 40% of crude extract decreased the viscosity from ∼2.4 centipoise to 1.4 centipoise; 0.2% methylcellulose restored the viscosity of 40% extract to that of crude extract as confirmed by falling ball assay. In this way, we can directly compare the motility of beads in 40% extract, which has both decreased extract viscosity and decreased protein concentration, to motility in 40% extract with higher viscosity (0.2% methylcellulose; M-0512; Sigma-Aldrich). Beads in the presence of 0.2% methylcellulose moved at a significantly lower speed (by both rank-sum and Student's t-test, p < 0.05) than beads in diluted extract the absence of methylcellulose (Figure 2A), consistent with similar observations on addition of methylcellulose (M-0387; Sigma-Aldrich) to bovine brain cytoplasmic extracts (McGrath et al., 2003). This decrease in speed in the presence of methylcellulose is probably not due to a decrease in the rate of actin polymerization, because it has been shown that even higher levels of methylcellulose do not alter actin polymerization (McGrath et al., 2003). In contrast, addition of various length methylcellulose polymer at concentrations up to 4% (20-fold higher than used in this study; M-0512, M-0262, and M-7140; Sigma-Aldrich) to a purified protein system was reported to have highly variable but not reproducibly significant effects on the speed of 2-μm beads coated with N-WASP (Wiesner et al., 2003). Whether the variation in the effects of methylcellulose is due purely to its effects on viscosity or on other aspects of the system such as actin filament bundling (Wiesner et al., 2003) or elongation rates (Drenckhahn and Pollard, 1986) that may differ between extracts and a purified protein system remains to be determined. In infected cells, effective cytoplasmic viscosity for large particles is very high compared with extracts or purified protein mixtures, but increasing the mobility of bacteria by removing intermediate filaments has no effect on speed (Giardini and Theriot, 2001), suggesting that under normal biological conditions environmental viscosity is only a minor determinant of average speed.
Figure 2.
Decreasing viscosity via extract dilution and altering actin concentration changes average speed. (A) The 0.5-μm beads coated with 37.5% ActA (filled diamond) or L. monocytogenes strain SLCC-5764 (open circle) were added to various extract dilutions and to 40% extract diluted with methylcellulose to 0.2% final viscosity. Average speeds in different conditions were calculated and plotted versus extract condition. (B) Average speed of 0.5-μm beads coated with 37.5% ActA in 40% extract with purified G-actin added. Error bars are SD. In two different extract preparations (designated a and c), addition of 2.4 or 6.4 μM rabbit skeletal muscle actin (sk.) significantly decreased speed. In contrast, addition of 2.4 μM human platelet actin (plat.) significantly increased speed.
If the slower rate of bead motility in diluted extracts is not due to a decrease in viscosity caused by dilution with isotonic buffer, it might be due to the reduction in the concentration of protein components in the extract. We chose to test specifically the effect of altered monomeric actin concentration, which for technical reasons has not been examined in the purified protein system (Loisel et al., 1999). The concentration of actin in the crude X. laevis egg extract used in this work was measured by DNase I assay to be 7 μM, whereas 40% diluted extract contains ∼2.8 μM actin. By adding back monomeric actin to extract diluted to 40%, we tested whether the increase in actin concentration restores the average speed of moving beads. Surprisingly, 0.5-μm beads in extract diluted with isotonic buffer and with additional rabbit skeletal muscle actin moved slower than beads in diluted extract without any additional actin, and the speed decreased further as more actin was added. However, beads in 40% extract with nonmuscle human platelet actin added moved significantly faster than beads in diluted extract without added actin (Figure 2B). These findings are consistent with the simple hypothesis that actin filament elongation can be rate limiting for movement and raise the concern that skeletal muscle actin may behave anomalously in cell-free reconstituted systems for actin-based motility.
Individual Speed Is Persistent and Determined by Features of the Individual
Extract dilution not only affects the speed of bead motility but also alters the variability in movement from one time point to the next. Calculating the SD around the mean of the instantaneous velocities for each individual bead reveals the degree of speed variation of the bead within each measured track. We calculated this and normalized the variability to average speed (Table 2). We also examined the distribution of instantaneous velocities across the entire population for each condition and found that the variation from one bead to another was always greater than the variation in speed within a single bead track, consistent with findings for bacteria in cells (Kuo and McGrath, 2000; Giardini and Theriot, 2001). This result suggests that, although the local environment determines the range of speeds available to an individual, specific features of the individual and its recent history determine the exact average “set-point” speed it selects. The individual is then able to maintain that speed with a high degree of persistence over time scales of at least several minutes. Interestingly, the relative amount of individual-to-individual variation increased in diluted extracts, whereas the relative amount of internal speed variation remained essentially constant and was independent of viscosity (Table 2). This observation suggests that the set-point differences among individuals may be due in part to a persistent biochemical alteration of the particle surface, for example, the recruitment to the surface of soluble factor that binds cooperatively and has a relatively slow off-rate. Possible candidates include members of the Ena/VASP protein family (Auerbuch et al., 2003), although unknown factors may also contribute.
Table 2.
Comparison of the standard deviation of the instantaneous velocities of individual beads versus the entire population
| Bead diameter (μm) | % ActA | % Extract | n | Population SD | Average individual SD | Normalized population SD | Normalized individual SD |
|---|---|---|---|---|---|---|---|
| 0.5 | 37.5 | 20 | 20 | 0.030 | 0.012 | 0.53 | 0.21 |
| 0.5 | 37.5 | 40 | 35 | 0.023 | 0.011 | 0.33 | 0.16 |
| 0.5 | 37.5 | 40+MC | 26 | 0.020 | 0.011 | 0.36 | 0.20 |
| 0.5 | 37.5 | 100 | 29 | 0.043 | 0.032 | 0.33 | 0.25 |
Path Curvature Varies According to Object Size and Shape
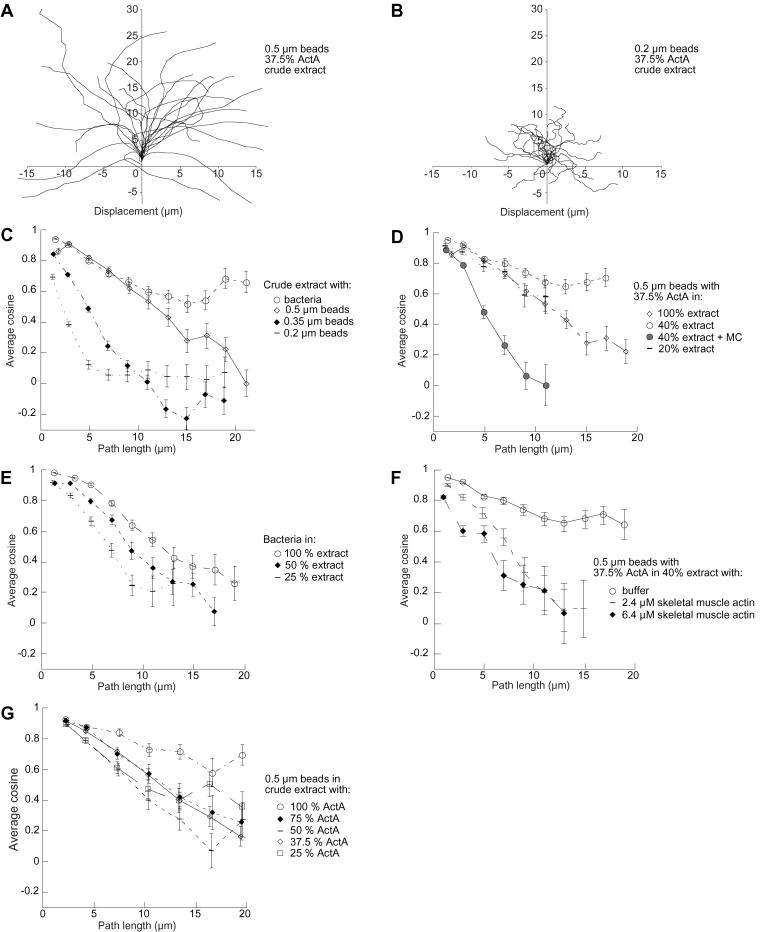
We noticed that the behavior of individual beads and bacteria varied not only with respect to speed but also with respect to the path that the bead or bacterium followed. For example, a plot of the x and y coordinates for each 10-s time point of several individual 0.5-μm-diameter beads moving in crude extract shows that the beads tend to follow smooth paths, curving only gradually (Figure 3A). In contrast, the paths followed by 0.2-μm-diameter beads were more erratic than those of larger beads (and bacteria), exhibiting more frequent and tighter turns (Figure 3B).
Figure 3.
Path curvature is influenced by several variables. (A) The trajectory of 0.5-μm-diameter beads during actin-based movement tends to be relatively straight with gradual turns. Tracks of 24 randomly selected individual bacteria paths are shown during movement for 150 to 200 s. (B) The 0.2-μm beads curve and change direction frequently during actin-based movement. Tracks of 24 randomly selected individual bead paths are shown (150–200 s). (C) Comparison of path curvature shows L. monocytogenes (open circle) and 0.5-μm (open diamond) beads curve less than 0.2 μm (dash) and 0.35-μm (filled diamond) beads. (D) Extract viscosity influences curvature: 0.5-μm beads coated with 37.5% ActA moving in 20% extract (dash), 40% extract (open circle), 40% extract with methyl cellulose (filled circle), or 100% extract (open diamond). (E) L. monocytogenes make sharper turns in more dilute extract: bacteria moving in 25% extract (dash), 50% extract (filled diamond), or 100% extract (open circle). (F) An increase in actin concentration in 40% extract results in greater curvature of 0.5 μm beads coated with 37.5% ActA: 40% (open circle) extract with no additional actin, 40% extract with additional 2.4 μM actin (filled diamond); 40% extract with additional 6.4 μM actin (dash). (G) Greater ActA surface density on 0.5 μm beads results in an increase in the curvature of the path of the moving bead: 25% ActA (open square), 37.5% ActA (open diamond), 50% ActA (dash), 75% ActA (filled diamond), and 100% ActA (open circle).
We can quantitate the path curvature, or the bead's propensity to turn, by calculating the average cosine of the angle formed by the coordinates of the bead position in pairs of track segments separated by progressively increasing distances (Figure 3C). Thus, a bead that has perfect path persistence, i.e., one with a trajectory that does not curve, would have a 0° angle separating any pair of track segments regardless of the distance between segments. Its cosine as a function of path length would be a straight line at 1. The plot of a trajectory with a random, erratic path would fall quickly to 0. Figure 3C includes such plots for the data in both Figure 3A (0.5-μm beads, open diamonds) and 3B (0.2-μm beads, bars) within it. Comparison of the path curvature of 0.2-μm beads with that of 0.35- and 0.5-μm beads in crude extract demonstrates that size does influence path trajectory and that curvature increases monotonically with decreasing size (Figure 3C).
The path curvatures of bacteria and 0.5-μm beads were very similar in the short term (over ∼10 μm), which is not surprising because they have similar diameters. But over the long term, bacteria had a greater persistence, presumably due to their ellipsoidal shape and/or the sheath of actin that wraps around them. The high degree of curvature found in the paths of small beads suggests that they are more subject either to the whims of the actin filaments guiding them or to heterogeneity in the environment than are larger beads.
Increased Viscosity or Addition of Skeletal Muscle Actin Increases Propensity to Turn
Dilution of extract affects path curvature as well, but only slightly: over short distances, beads moving in 20, 40, or 100% extract have very similar path curvatures, although over longer distances 0.5-μm beads in extract diluted to 40% follow straighter paths than beads in 100% extract (Figure 3D). The path curvature of bacteria slightly increased with dilution of the extract (Figure 3E).
To determine whether the differences in path curvature with respect to extract dilution results from altered viscosity or altered host cell protein concentrations, we analyzed path curvature of 0.5-μm beads moving in diluted extract with 0.2% methylcellulose (which generates an extract viscosity equivalent to crude extract). One might expect that path curvature in diluted extracts with 0.2% methylcellulose would be similar to that in 100% extract. However, adding methylcellulose to increase viscosity resulted in a massive increase in curvature of bead trajectories (Figure 3D). Surprisingly, adding back monomeric rabbit skeletal muscle actin to diluted extract also caused a decrease in path persistence and more turns (Figure 3F). However, adding up to 2.4 μM additional human platelet actin does not alter curvature. Thus, the effects on path curvature due to extract dilution cannot be due directly to a predictable decrease in viscosity, and skeletal muscle actin has an anomalous deleterious effect on path persistence just as it did with speed. In contrast to all other biophysical parameters characterized, average ActA surface density changes had only modest effects on path curvature (Figure 3G).
Particle Surface Heterogeneities Do Not Influence Path Curvature
One possible explanation for path curvature is that a there is a persistent, asymmetric heterogeneity on the particle surface (such as varied ActA surface density) that causes more force to be produced on one side of the particle than on the other and thus a persistent, slightly faster speed for one side, resulting in a curved path (Rutenberg and Grant, 2001). If the particle rotates around its long axis during forward movement, an S-shaped or random-walk trajectory might result from this persistent surface heterogeneity. To test this hypothesis, we coupled very small (50 nm in diameter), fluorescent marker beads to the L. monocytogenes surface and examined the relationship between the marker bead position and local bacterial path curvature. The marker bead acts as a fixed reference point on the cell surface. If the surface of one side of the bacterium is more likely to promote turning, then one would expect to find turns consistently associated with a similar position of the marker bead with respect to the bacterial long axis. For example, a marker bead that always seems on the “outside” of a curved path as a bacterium's path changes curvature from clockwise to counter-clockwise would be associated with a position on the bacterial surface that causes persistently faster actin filament growth than the position on the opposite side. We calculated the shortest distance (orthogonal projection) from the marker bead to the longitudinal axis of the bacterium. This was done for each 10-s interval of 27 L. monocytogenes trajectories, all at least 500 s in length. We then examined whether each marker bead was consistently on the outside or inside of the curve. Any bacterium whose marker bead was consistently in the same region when it turned in a particular direction would exhibit a probability significantly greater or less than 0.50. None met this criterion (population average = 0.51, SD = 0.08). As a further test for a statistically significant correlation between marker bead position and path curvature, we compared these 27 trajectories to a randomized null data set where the measured magnitudes of the orthogonal projections had been randomly reordered with respect to the curvature at that point (Giardini and Theriot, 2001). The actual data were not significantly different from the randomized trajectories (average = 0.51, SD = 0.07, by Student's t-test, p > 0.50). Furthermore, careful examination of the length plotted as a function of curvature showed that for all tracks the bead position associated with instantaneous curvatures of the greatest magnitudes could always be revisited during curvatures of the opposite sign. This analysis demonstrates that persistent heterogeneities in the bacterial surface cannot explain curvature variations in L. monocytogenes trajectories.
Symmetry-Breaking Increases with Decreased Viscosity
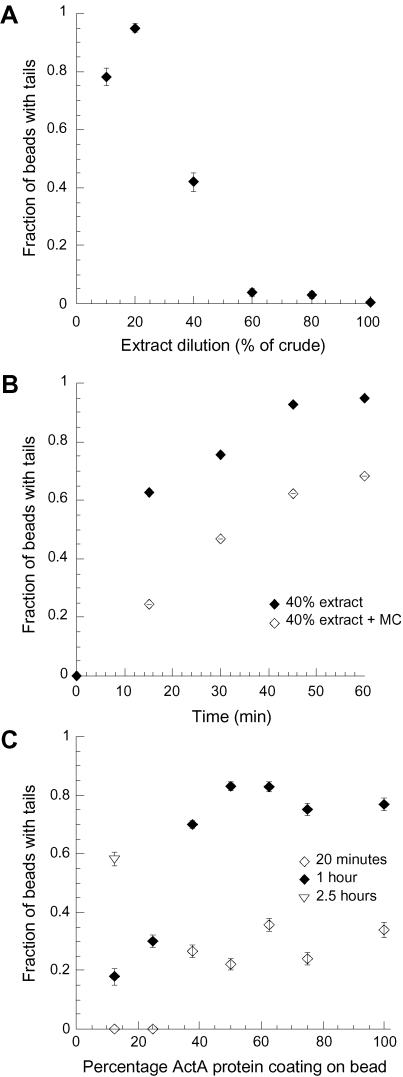
Bacteria and beads in cytoplasmic extracts are initially surrounded by a uniform, symmetric actin cloud that must break symmetry to form the polarized comet tail (Cameron et al., 1999; van Oudenaarden and Theriot, 1999), and a similar cloud-to-tail transition is required for movement initiation for bacteria in infected cells (Tilney and Portnoy, 1989). Both the likelihood and the timing of symmetry-breaking are strongly influenced by variations in the biophysical parameters explored in this study. Although the rate of movement of beads in diluted extracts was slower, the total fraction of particles that broke symmetry and formed tails after 20 min increased as the extract became more dilute (Figure 4A). Almost all 0.5-μm beads formed tails within 20 min in 20% extract, with a monotonically decreasing likelihood of symmetry-breaking in more concentrated extracts (Figure 4A). Similar results were found for a range of smaller bead sizes (0.2–0.7 μm) (Cameron et al., 2001). The addition of methylcellulose to a final concentration of 0.2% in 40% extract to increase extract viscosity and dampen Brownian motion reduced the efficiency of symmetry breaking approximately one-third (Figure 4B), indicating that at least part of the improvement of symmetry-breaking efficiency in dilute extracts is simply due to the decrease in viscosity.
Figure 4.
Fraction of beads that form tails increases in diluted extract. (A) A larger fraction of 0.5-μm beads coated with 37.5% ActA forms tails in extract diluted from full-strength crude with isotonic buffer (data collected 20 min after slides were made). (B) The fraction of 0.5-μm beads coated with 37.5% ActA that form tails in 40% extract in the absence (filled diamond) or presence (open diamonds) of methylcellulose at a final viscosity of 0.2% increases over time. (C) The fraction of 0.5-μm beads, which form tails in 40% extract increases as a function of percentage of ActA used to coat the beads over time, 20 min (open diamonds), 1 h (filled diamonds), and 2.5 h (open triangle). Error bars are SE of binomial distribution.
For smaller beads, the transition from symmetric actin cloud to asymmetric comet tail seemed to be stochastic, and the net likelihood of movement initiation increased as a function of time (Figure 4, B and C). Twenty minutes after being mixed with diluted extract, less than one-half of the 0.5-μm beads formed tails over a range of ActA surface densities, but after 1 h, many more beads initiated motility (∼80% for beads with a coating of ≥37.5% ActA). Beads in 40% extract initiated tails more often than beads in full-strength crude extract regardless of the ActA surface density (Cameron et al., 1999 for crude extract data). At high levels of ActA-coating on beads, surface density had very little effect on motility initiation, but beads with lower surface densities of ActA (<25%) started moving much less frequently in 40% extract (Figure 4C). However, if given enough time (2.5 h), a large fraction of beads initiated motility, even for the lowest ActA surface densities (Figure 4C, open triangle). This suggests that any apparent ActA surface density dependence on efficiency of tail formation was actually a time delay in symmetry-breaking.
Stepwise Symmetry-Breaking for Large (0.9- and 2-μm) Particles
The mechanism of symmetry breaking and tail formation for large particles (0.9 and 2 μm in diameter) in diluted extracts was observed to be very different from the stochastic mechanism that has been described here and previously for small particles (≤0.5 μm in diameter). Instead of approaching steady-state over time until a certain fraction of beads formed tails, 0.9-μm (and 2-μm) beads all gradually went through four morphological transitions: 1) symmetric actin cloud, 2) asymmetric actin cloud, 3) short actin tail, and 4) long actin tail generating force to push the bead forward (Figure 5). The first stage, symmetric cloud, was complete by 10 min with the asymmetric clouds forming around approximately one-half of the beads by 15 min after the start of the experiment. The process of forming short actin tails occurred for the majority of beads over a period of 30–60 min. By 2 h, >95% of the beads were in their final end state with long actin tails. Although virtually 100% of the large beads went through all four stages of actin tail organization, they were not completely synchronized. At early time points as many as three stages were present with varying percentages depending on the particular experiment. Although motility initiation was slower for large particles, the transition from symmetric cloud to asymmetric cloud did not involve as many excursions as are seen with smaller particles (Cameron et al., 1999; van Oudenaarden and Theriot, 1999). Instead, a cloud on a large bead gradually built asymmetry until the bead began moving away from it in a unidirectional manner. These observations are not consistent with a stochastic model for symmetry-breaking (van Oudenaarden and Theriot, 1999) and are more consistent with a model based on gradual accumulation of strain in the growing actin gel (Noireaux et al., 2000). Because small beads seem to undergo a stochastic form of symmetry-breaking even in diluted extracts (Figure 4), and large beads cannot break symmetry at all in concentrated extracts (Figure 1 and Cameron et al., 1999), we propose that these two different modes of symmetry-breaking represent extremes on a continuum of behaviors that is governed by particle geometry and protein concentration such that strain accumulation can only serve to break symmetry for large objects in dilute protein solutions, where actin filament depolymerization (which would normally serve to relieve strain in the gel) is particularly slow.
Figure 5.
Cloud-to-tail transition occurs in distinct steps for 0.9-μm beads. All 0.9-μm beads form actin tails from actin clouds through a series of steps that seem more distinct than the steps between cloud and tail for smaller beads. (A) The actin clouds of three beads are detected using fluorescence microscopy of rhodamine-actin. Arrowhead denotes bead with symmetric actin cloud. Two other beads show formation of asymmetric actin clouds. (B) After asymmetric clouds form, short little tails form, as seen here. (C) Then, slightly longer tails form. (D) After 2 h, all beads have formed long actin tails. Bar, 2 μm.
Uniformly Coated Beads Do Not Break Symmetry in Cells
The observation that different modes of motility initiation may exist depending on prevailing biophysical parameters raises the question of which case is more similar to conditions inside a living cell. To address the factors governing symmetry-breaking in living cell cytoplasm, we devised two independent methods for introducing spherical ActA-coated beads into epithelial MDCK cells expressing GFP-actin (Robbins et al., 1999). The first method used a combination of bacterial virulence factors to mimic natural modes of invasion. ActA-coated beads were further derivatized with invasin from Yersinia pseudotuberculosis, which induces particle uptake by tight attachment to β1 integrins (Leong et al., 1990) and with listeriolysin O, an L. monocytogenes gene product that disrupts phagosomal membranes (Gedde et al., 2000). The combination of these two bacterial virulence factors has been previously shown to efficiently deliver nonpathogenic bacteria into the host cell cytoplasmic compartment (Monack and Theriot, 2001). In the other method, ActA-coated beads were shot into cells by using an air-pressure–driven gene gun. The 0.2-μm beads delivered into cell cytoplasm by either method were able to recruit GFP-actin and form clouds with high efficiency (Figure 6). However, under no circumstances were any actin tails ever seen associated with intracytoplasmic beads, and directed movement was never observed. Because 0.2-μm-diameter beads form comet tails with very high efficiency under all extract conditions, this observation indicates that the viscous, structured environment of living cell cytoplasm, together with the very high turnover rate of actin filaments in vivo, preclude either in vitro mode of symmetry-breaking from taking place and impose an absolute requirement for bacterial surface asymmetry in actin-based motility generated during the course of natural infections.
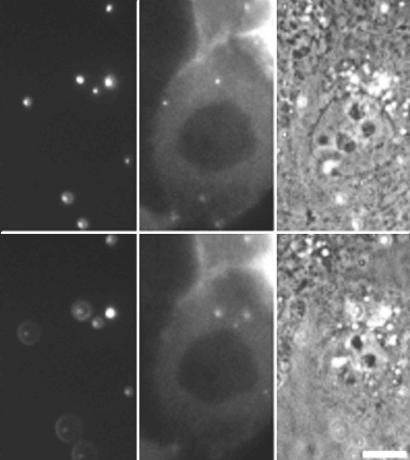
Figure 6.
The 0.2-μm spherical beads fail to break symmetry in living cells. MDCK cells expressing GFP-actin were “infected” with polystyrene beads coated with ActA, invasin, and listeriolysin O. Left, fluorescence of beads at two focal planes within the cell separated by ∼1 μm. Middle, GFP-actin signal. Some but not all beads have accumulated actin clouds. Right, phase contrast. At no time after bead “infection” were actin comet tails or directed motility ever observed.
DISCUSSION
Polystyrene beads coated with a variety of proteins that activate actin nucleation by the Arp2/3 complex have become widely used as biophysically manipulable artificial cargo for the study of force generation by actin polymerization. In this work, we have chosen a motility medium of biochemically complex cytoplasmic extract and performed a series of systematic comparisons, including altering particle size, surface density of protein coating, extract dilution, and extract viscosity to explore a range of parameters and quantitatively analyze biophysical aspects of actin-based motility. We have found some areas of agreement with several recent reports describing similar systematic studies on movement of polystyrene beads coated with various Arp2/3 activators in a mixture of purified proteins (Bernheim-Groswasser et al., 2002; Wiesner et al., 2003; Samarin et al., 2003), but several areas of striking dissimilarity as detailed below.
Factors Governing Speed
No reconstituted motility system is yet capable of replicating the extremely rapid speed of bacteria moving inside infected cells (up to 1.4 μm/s; Dabiri et al., 1990), although generally bacterial speeds in cytoplasmic extracts (∼0.14 μm/s; Table 1) are faster than speeds in purified protein systems (0.04 μm/s; Loisel et al., 1999). Changes in particle size have a major effect on speed for ActA-coated beads in cytoplasmic extract (Figure 1B). The optimal size for ActA-coated bead movement in X. laevis egg extract is 0.5 μm in diameter, as speed decreases for larger and smaller beads (diameter range 0.2–2 μm), and speeds of 0.5-μm beads closely approximate the speeds of live bacteria under several conditions (Table 1 and Figure 2A). A reduced rate of motility with increasing bead diameter was also found for VCA-coated beads (1–10 μm) moving in the purified protein system (Bernheim-Groswasser et al., 2002). Both the amount and size dependence of the speed decrease is consistent for the two different systems. Because changes in hydrodynamic drag are calculated to have a negligible effect, it is likely that the effect of particle size on speed is due either to curvature-dependent changes in the rate of accumulation of strain in the actin gel growing at the bead surface (Noireaux et al., 2000) or to the increased retarding force due to attachment of actin filaments over a larger surface area (Mogilner and Oster, 2003). But, experiments using full length N-WASP on beads in the purified protein system showed no significant effect on speed due to varying bead diameter (0.2–3 μm; Wiesner et al., 2003); it is not clear why N-WASP–coated beads should behave abnormally with respect to this fundamental biophysical parameter. Why beads <0.5 μm in diameter move slower is also unclear; perhaps a smaller number of working filaments interacting with less overall surface area of a smaller bead leads to slower movement (Mogilner and Oster, 2003).
The rate of movement of ActA-coated beads also decreases when the crude X. laevis egg extract is diluted (Figure 2A). It is likely that dilution decreases speed simply by decreasing the concentration of proteins essential to motility, because lowering the concentration any of several specific factors can decrease speed in a purified protein system (Loisel et al., 1999), and speed is not restored (but instead further decreased) by reconstituting the extract viscosity by addition of methylcellulose (Figure 2A). Addition of rabbit skeletal muscle actin to diluted extracts had the paradoxical effect of slowing movement further; however, addition of nonmuscle platelet actin caused an increase in speed (Figure 2B). The differences observed between platelet and skeletal muscle actin are significant and need to be considered in future work, particularly in light of the fact that skeletal muscle actin is commonly used in purified protein systems for reconstitution of this form of actin-based motility (Loisel et al., 1999; Bernheim-Groswasser et al., 2002; Samarin et al., 2003; Wiesner et al., 2003). In contrast to the significant effects of changing concentrations of soluble factors, changing the surface density of the protein attached to the polystyrene bead has no effect (ActA) or only a modest effect (N-WASP) on particle speed (Cameron et al., 1999; Wiesner et al., 2003). In this respect, both the purified protein system and cytoplasmic extracts seem to faithfully reflect the relative importance of concentrations of both bacterial surface factors and host cell factors on speed determination in infected cells.
The large variation of average speed among individuals is a striking characteristic of bacterial movement in infected host cells (Sanger et al., 1992; Theriot et al., 1992; Geese et al., 2000; Kuo and McGrath, 2000; Lacayo and Theriot, 2004) and in cytoplasmic extracts (Theriot et al., 1994; McGrath et al., 2003), whereas individual variability is reported to be significantly suppressed in mixtures of purified proteins (Loisel et al., 1999; Bernheim-Groswasser et al., 2002). In this study, we have found that in all cases the variation of speed within the trajectory of any individual particle is much less than the variation from particle to particle, suggesting that a persistent alteration of the particle surface is responsible for varying the speed set-point. The biochemical factors responsible for set-point determination are apparently not represented in the purified protein systems and remain to be identified.
Factors Governing Symmetry-Breaking
Polystyrene beads uniformly coated with an Arp2/3 activating protein initially assemble a uniform spherical actin cloud in both cytoplasmic extracts (Cameron et al., 1999) and purified protein systems (Bernheim-Groswasser et al., 2002; Samarin et al., 2003; Wiesner et al., 2003), and this initial symmetry must be broken for a motile comet tail to form. Two models have been proposed for the mechanism of symmetry-breaking, cooperative amplification of small local variations in actin filament growth (van Oudenaarden and Theriot, 1999) or gradual accumulation of strain in the growing actin gel followed by a rupture event (Noireaux et al., 2000; Bernheim-Groswasser et al., 2002). The strain accumulation model is well supported by observations in the purified protein system (Bernheim-Groswasser et al., 2002) but cannot be reconciled with observations in concentrated cytoplasmic extracts (Cameron et al., 1999, 2001). For example, nearly 100% of beads in a purified protein system form tails, and movement initiation occurs at approximately the same time for all particles under a particular condition, as expected under strain accumulation (Bernheim-Groswasser et al., 2002). In contrast, the fraction of moving beads in concentrated extracts increases slowly over time, and the fraction of moving beads approaches >90% only for the most favorable conditions, with other conditions reaching a lower steady state, both of which are more consistent with a stochastic model for symmetry-breaking. Very large beads (up to 10 μm in diameter) can break symmetry in the purified protein system, whereas only small (<0.5-μm beads) can do so in concentrated extracts.
Here, we have found that extract diluted to 20% is in some respects more similar to the purified protein system than to crude extract; for example all 2-μm beads in extract diluted to 20% eventually formed tails. It seems likely that the system undergoes a transition from a stochastic mode of symmetry breaking, which depends on thermal motion and actin cooperativity (van Oudenaarden and Theriot, 1999), to a strain accumulation mode, in which an actin gel slowly develops increased stress (Noireaux et al., 2000; Bernheim-Groswasser et al., 2002), by increasing the bead size and diluting the extract. The most important variable governing the transition from a stochastic mode to a strain accumulation mode is probably the turnover rate of the actin filaments. In crude extracts, the average half-life for actin filaments in the tail is only ∼42 s (Theriot et al., 1994), precluding any significant strain accumulation in the actin cloud, whereas depolymerization is severely compromised in the purified protein systems (Samarin et al., 2003), and we also noticed a slower depolymerization rate in 20% extracts, because motile beads produced longer tails even though they were moving more slowly (Figure 5). Strikingly, actin filament turnover in cells is even faster than in concentrated cytoplasmic extracts (Theriot et al., 1992), and even very small (0.2-μm-diameter beads) introduced into live cells cannot break symmetry at all (Figure 6). In this respect, the purified protein system and very dilute extracts are not at all representative of physiological conditions within a living cell, although the observations of strain accumulation and rupture do reveal interesting physical capabilities of the actin gel system.
Factors Governing Path Curvature
In contrast to speed and symmetry-breaking, the physical and biochemical factors determining curvature of the particle trajectories have received relatively little experimental or theoretical attention (Auerbuch et al., 2003). We have found that the curvature of the trajectory that a bead follows is strongly influenced by its size and by the surrounding environment. Of the various biophysical parameters that we have tested, particle size has the strongest effect on trajectory curvature, with smaller particles following more sharply curved paths. Similar results were found for VCA-coated beads moving in a mixture of purified proteins (Bernheim-Groswasser et al., 2002). In most cases, dilution of extract resulted in greater path curvature. But, surprisingly, diluted extract that was restored to the viscosity of crude extract with 0.2% methylcellulose displayed the greatest curvature of any extract concentration. Bacteria also made more frequent turns as extract was diluted, similar to results from studies in cells where bacteria are more likely to turn as viscosity decreases due to removal of intermediate filaments (Giardini and Theriot, 2001). The bacterial surface or the density of ActA at the surface of a bead does not have much (if any) effect on the tendency of the trajectory to curve.
The physical basis of trajectory curvature remains to be elucidated. It has been suggested that curvature might result from one side of a particle consistently generating actin gel growth at a slightly faster rate than the other side (Rutenberg and Grant, 2001). We have ruled out this explanation by marking specific sites on the surface of the bacteria and determining that curvature is uncorrelated with location on the surface. Instead, we favor the possibility that persistent curvature is due to the autocatalytic nature of actin gel growth (Carlsson, 2001, 2003; Rutenberg and Grant, 2001), such that local variations in actin gel growth rate from the outside of the curve to the inside of the curve are correlated with recent history of that part of the gel but are not correlated with the properties of the particle surface. The unexpected effect of methylcellulose on path curvature may be due to a form of autocatalytic path determination, where the tendency of methylcellulose to cause the collapse of actin filaments into bundles (Wiesner et al., 2003) may locally alter tail structure in a persistent manner.
Concentrated cytoplasmic extracts represent a system with biological complexity intermediate between completely defined mixtures of purified proteins and intact living cells. Although actin-based movement of bacteria (Loisel et al., 1999), ActA coated beads (Boujemaa-Paterski et al., 2001; Samarin et al., 2003), and N-WASP (or its Arp2/3 activator domain VCA)-coated beads (Bernheim-Groswasser et al., 2002; Wiesner et al., 2003) can be reconstituted with purified proteins, there are several significant differences in motility from that in cells and cytoplasmic extracts. This suggests that there are other cellular factors not included that are important for certain aspects of actin-based motility, most notably enhanced speed, persistent individual variation in speed set-point, and a nonstrain mode of symmetry-breaking. Complete understanding of the physical factors that influence this form of actin-based motility under realistic biological conditions will require further systematic, quantitative experiments in living cells.
Acknowledgments
We thank Paula Giardini for some measurements of bacterial motility in extracts, Ann Goldstein for assistance with the gene gun, and Peter Jackson for housing X. laevis. We are also grateful to Dan Portnoy for the gift of L. monocytogenes strain DP-L2723 for expressing ActA-His and DP-L2161 for expressing His-LLO and to Ralph Isberg (Tufts University) for the gift of pRI283, which encodes MBP-invasin. This work was supported by National Institutes of Health grant AI-36929 and a Fellowship from the David and Lucile Packard Foundation (to J.A.T.). J.R.R. was supported by a Stanford Graduate Fellowship.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–12–0913. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–12–0913.
References
- Auerbuch, V., Loureiro, J.J., Gertler, F.B., Theriot, J.A., and Portnoy, D.A. (2003). Ena/VASP proteins contribute to Listeria monocytogenes pathogenesis by controlling temporal and spatial persistence of bacterial actin-based motility. Mol. Microbiol. 49, 1361–1375. [DOI] [PubMed] [Google Scholar]
- Bernheim-Groswasser, A., Wiesner, S., Golsteyn, R.M., Carlier, M.F., and Sykes, C. (2002). The dynamics of actin-based motility depend on surface parameters. Nature 417, 308–311. [DOI] [PubMed] [Google Scholar]
- Boujemaa-Paterski, R., et al. (2001). Listeria protein ActA mimics WASP family proteins: it activates filament barbed end branching by Arp2/3 complex. Biochemistry 40, 11390–11404. [DOI] [PubMed] [Google Scholar]
- Cameron, L.A., Footer, M.J., van Oudenaarden, A., and Theriot, J.A. (1999). Motility of ActA protein-coated microspheres driven by actin polymerization. Proc. Natl. Acad. Sci. USA 96, 4908–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, L.A., Giardini, P.A., Soo, F.S., and Theriot, J.A. (2000). Secrets of actin-based motility revealed by a bacterial pathogen. Nat. Rev. Mol. Cell. Biol. 1, 110–119. [DOI] [PubMed] [Google Scholar]
- Cameron, L.A., Svitkina, T.M., Vignjevic, D., Theriot, J.A., and Borisy, G.G. (2001). Dendritic organization of actin comet tails. Curr. Biol. 11, 130–135. [DOI] [PubMed] [Google Scholar]
- Carlsson, A. (2001). Growth of branched actin networks against obstacles. Biophys. J. 81, 1907–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson, A. (2003). Growth velocities of branched actin networks. Biophys. J. 84, 2907–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri, G.A., Sanger, J.M., Portnoy, D.A., and Southwick, F.S. (1990). Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc. Natl. Acad. Sci. USA 87, 6068–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn, D., and Pollard, T.D. (1986). Elongation of actin filaments is a diffusion-limited reaction at the barbed end and is accelerated by inert macromolecules. J. Biol. Chem. 261, 12754–12758. [PubMed] [Google Scholar]
- Gedde, M.M., Higgins, D.E., Tilney, L.G., and Portnoy, D.A. (2000). Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect. Immun. 68, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geese, M., Schluter, K., Rothkegel, M., Jockusch, B.M., Wehland, J., and Sechi, A.S. (2000). Accumulation of profilin II at the surface of Listeria is concomitant with the onset of motility and correlates with bacterial speed. J. Cell Sci. 113, 1415–1426. [DOI] [PubMed] [Google Scholar]
- Giardini, P.A., Fletcher, D.A., and Theriot, J.A. (2003). Compression forces generated by actin comet tails on lipid vesicles. Proc. Natl. Acad. Sci. USA 100, 6493–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardini, P.A., and Theriot, J.A. (2001). Effects of intermediate filaments on actin-based motility of Listeria monocytogenes. Biophys. J. 81, 3193–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, T.L., and Kirschner, M.W. (1982). Bioenergetics and kinetics of microtubule and actin filament assembly-disassembly. Int. Rev. Cytol. 78, 1–125. [PubMed] [Google Scholar]
- Kocks, C., Gouin, E., Tabouret, M., Berche, P., Ohayon, H., and Cossart, P. (1992). L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68, 521–531. [DOI] [PubMed] [Google Scholar]
- Kocks, C., Marchand, J.B., Gouin, E., d'Hauteville, H., Sansonetti, P.J., Carlier, M.F., and Cossart, P. (1995). The unrelated surface proteins ActA of Listeria monocytogenes and IcsA of Shigella flexneri are sufficient to confer actin-based motility on Listeria innocua and Escherichia coli respectively. Mol. Microbiol. 18, 413–423. [DOI] [PubMed] [Google Scholar]
- Kuo, S.C., and McGrath, J.L. (2000). Steps and fluctuations of Listeria monocytogenes during actin-based motility. Nature 407, 1026–1029. [DOI] [PubMed] [Google Scholar]
- Lacayo, C., and Theriot, J.A. (2004). Listeria monocytogenes motility varies depending on subcellular location: a kinematic probe of cytoarchitecture. Mol. Biol. Cell 15, 2164–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong, J.M., Fournier, R.S., and Isberg, R.R. (1990). Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 9, 1979–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.H., Espreafico, E.M., Mooseker, M.S., and Forscher, P. (1996). Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron 16, 769–782. [DOI] [PubMed] [Google Scholar]
- Loisel, T.P., Boujemaa, R., Pantaloni, D., and Carlier, M.F. (1999). Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613–616. [DOI] [PubMed] [Google Scholar]
- May, R.C., Hall, M.E., Higgs, H.N., Pollard, T.D., Chakraborty, T., Wehland, J., Machesky, L.M., and Sechi, A.S. (1999). The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes. Curr. Biol. 9, 759–762. [DOI] [PubMed] [Google Scholar]
- McGrath, J.L., Eungdamrong, N.J., Fisher, C.I., Peng, F., Mahadevan, L., Mitchison, T.J., and Kuo, S.C. (2003). The force-velocity relationship for the actin-based motility of Listeria monocytogenes. Curr. Biol. 13, 329–332. [DOI] [PubMed] [Google Scholar]
- Mogilner, A., and Oster, G. (2003). Force generation by actin polymerization II: the elastic ratchet and tethered filaments. Biophys. J. 84, 1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack, D.M., and Theriot, J.A. (2001). Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell Microbiol. 3, 633–647. [DOI] [PubMed] [Google Scholar]
- Nefsky, B., and Bretscher, A. (1992). Yeast actin is relatively well behaved. Eur. J. Biochem. 206, 949–955. [DOI] [PubMed] [Google Scholar]
- Noireaux, V., Golsteyn, R.M., Friederich, E., Prost, J., Antony, C., Louvard, D., and Sykes, C. (2000). Growing an actin gel on spherical surfaces. Biophys. J. 78, 1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni, D., Le Clainche, C., and Carlier, M.F. (2001). Mechanism of actin-based motility. Science 292, 1502–1506. [DOI] [PubMed] [Google Scholar]
- Pardee, J.D., and Spudich, J.A. (1982). Purification of muscle actin. Methods Enzymol. 85, 164–181. [DOI] [PubMed] [Google Scholar]
- Pollard, T.D., Blanchoin, L., and Mullins, R.D. (2000). Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29, 545–576. [DOI] [PubMed] [Google Scholar]
- Robbins, J.R., Barth, A.I., Marquis, H., de Hostos, E.L., Nelson, W.J., and Theriot, J.A. (1999). Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J. Cell Biol. 146, 1333–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, J.R., and Theriot, J.A. (2003). Listeria monocytogenes rotates around its long axis during actin-based motility. Curr. Biol. 13, R754–R756. [DOI] [PubMed] [Google Scholar]
- Rutenberg, A.D., and Grant, M. (2001). Curved tails in polymerization-based bacterial motility. Phys Rev E Stat Nonlin Soft Matter Phys 64, 021904. [DOI] [PubMed] [Google Scholar]
- Samarin, S., Romero, S., Kocks, C., Didry, D., Pantaloni, D., and Carlier, M.F. (2003). How VASP enhances actin-based motility. J. Cell Biol. 163, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger, J.M., Sanger, J.W., and Southwick, F.S. (1992). Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect. Immun. 60, 3609–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaier, S.R. (1992). Purification and characterization of platelet actin, actin-binding protein, and alpha-actinin. Methods Enzymol. 215, 58–77. [DOI] [PubMed] [Google Scholar]
- Smith, G.A., Portnoy, D.A., and Theriot, J.A. (1995). Asymmetric distribution of the Listeria monocytogenes ActA protein is required and sufficient to direct actin-based motility. Mol. Microbiol. 17, 945–951. [DOI] [PubMed] [Google Scholar]
- Suetsugu, S., Miki, H., Yamaguchi, H., and Takenawa, T. (2001). Requirement of the basic region of N-WASP/WAVE2 for actin-based motility. Biochem. Biophys. Res. Commun. 282, 739–744. [DOI] [PubMed] [Google Scholar]
- Theriot, J.A., Mitchison, T.J., Tilney, L.G., and Portnoy, D.A. (1992). The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature 357, 257–260. [DOI] [PubMed] [Google Scholar]
- Theriot, J.A., Rosenblatt, J., Portnoy, D.A., Goldschmidt-Clermont, P.J., and Mitchison, T.J. (1994). Involvement of profilin in the actin-based motility of L. monocytogenes in cells and in cell-free extracts. Cell 76, 505–517. [DOI] [PubMed] [Google Scholar]
- Tilney, L.G., and Portnoy, D.A. (1989). Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109, 1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya, A., Chabot, J.R., Andreeva, A., Samadani, A., and van Oudenaarden, A. (2003). Probing polymerization forces by using actin-propelled lipid vesicles. Proc. Natl. Acad. Sci. USA 100, 4521–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oudenaarden, A., and Theriot, J.A. (1999). Cooperative symmetry-breaking by actin polymerization in a model for cell motility. Nat. Cell Biol. 1, 493–499. [DOI] [PubMed] [Google Scholar]
- Welch, M.D., Rosenblatt, J., Skoble, J., Portnoy, D.A., and Mitchison, T.J. (1998). Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science 281, 105–108. [DOI] [PubMed] [Google Scholar]
- Wiesner, S., Helfer, E., Didry, D., Ducouret, G., Lafuma, F., Carlier, M.F., and Pantaloni, D. (2003). A biomimetic motility assay provides insight into the mechanism of actin-based motility. J. Cell Biol. 160, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarar, D., To, W., Abo, A., and Welch, M.D. (1999). The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr. Biol. 9, 555–558. [DOI] [PubMed] [Google Scholar]