Abstract
This study investigated whether slow-releasing organic hydrogen sulfide donors act through the same mechanisms as those of inorganic donors to protect neurons from oxidative stress. By inducing oxidative stress in a neuronal cell line HT22 with glutamate, we investigated the protective mechanisms of the organic donors: ADT-OH [5-(4-hydroxyphenyl)-3H-1, 2-dithiole-3-thione], the most widely used moiety for synthesizing slow-releasing hydrogen sulfide donors, and ADT, a methyl derivative of ADT-OH. The organic donors were more potent than the inorganic donor sodium hydrogensulfide (NaHS) in protecting HT22 cells against glutamate toxicity. Consistent with previous publications, NaHS partially restored glutamate-depleted glutathione (GSH) levels, protected HT22 from direct free radical damage induced by hydrogen peroxide (H2O2), and NaHS protection was abolished by a KATP channel blocker glibenclamide. However, neither ADT nor ADT-OH enhanced glutamate-depleted GSH levels or protected HT22 from H2O2-induced oxidative stress. Glibenclamide, which abolished NaHS neuroprotection against oxidative stress, did not block ADT and ADT-OH neuroprotection against glutamate-induced oxidative stress. Unexpectedly, we found that glutamate induced AMPK activation and that compound C, a well-established AMPK inhibitor, remarkably protected HT22 from glutamate-induced oxidative stress, suggesting that AMPK activation contributed to oxidative glutamate toxicity. Interestingly, all hydrogen sulfide donors, including NaHS, remarkably attenuated glutamate-induced AMPK activation. However, under oxidative glutamate toxicity, compound C only increased the viability of HT22 cells treated with NaHS, but did not further increase ADT and ADT-OH neuroprotection. Thus, suppressing AMPK activation likely contributed to ADT and ADT-OH neuroprotection. In conclusion, hydrogen sulfide donors acted through differential mechanisms to confer neuroprotection against oxidative toxicity and suppressing AMPK activation was a possible mechanism underlying neuroprotection of organic hydrogen sulfide donors against oxidative toxicity.
Keywords: hydrogen sulfide donors, neuroprotection, AMPK, oxidative stress
Hydrogen sulfide, well known as a toxic gas, is increasingly recognized as the third gaseous signaling molecular in addition to nitric oxide and carbon monoxide (Wang, 2002). Hydrogen sulfide is endogenously synthesized from cysteine by several enzymes, and the production of hydrogen sulfide is high in the brain. Deficiency in hydrogen sulfide leads to many neurological diseases (Abe and Kimura, 1996). In addition to functioning as an endogenous signaling gas, hydrogen sulfide protects neurons from oxidative stress. Indeed, the inorganic hydrogen sulfide donor NaHS has been extensively studied as a neuroprotant in a variety of neuronal oxidative stress models (Kimura and Kimura, 2004; Kimura et al., 2006; Tay et al., 2010). Since neurons are particular vulnerable to oxidative stress and oxidative stress is an important feature of various neurological diseases such as stroke, Alzheimer's and Parkinson's disease (Lin and Beal, 2006; Lo et al., 2005), there is a growing interest in developing hydrogen sulfide donors as neuroprotants in treating neurological diseases. However, excessive hydrogen sulfide instantaneously released from inorganic donors may exacerbate pathogenesis of neurological diseases in that hydrogen sulfide at supra-physiological concentrations has been shown to be cytotoxic (Predmore et al., 2012; Whiteman and Winyard, 2011). Thus, various organic molecules that are capable to release hydrogen sulfide over extended periods of time have been developed (Gong et al., 2011; Gu and Zhu, 2011; Martelli et al., 2010; Osborne et al., 2012; Predmore et al., 2012).
So far, the neuroprotective mechanisms of hydrogen sulfide against oxidative stress are almost exclusively obtained from studies using inorganic donors. However, it has been suggested that the biological effects as well as the mechanisms by which slow-releasing donors induce the biological effects are different from those of inorganic donors (Li et al., 2008; Whiteman and Winyard, 2011). The neuroprotection of the inorganic donor NaHS against oxidative stress has been attributed to three mechanisms: restoring cellular levels of glutathione (GSH), an essential component of the cell defense system against oxidative stress; activating ATP-sensitive K + (KATP) channels and scanvenging free radicals directly (Hu et al., 2011). However, it currently remains unclear whether these mechanisms also contribute to neuroprotective effects of slow-releasing organic donors against oxidative stress. Moreover, as indicated by studies on the other two signaling gases, research on gaseous mediator signaling can be greatly facilitated by using organic compounds that release gaseous mediators slowly.
Thus, in this study we investigated the neuroprotective mechanisms against oxidative toxicity underlying two slow-releasing organic hydrogen sulfide donors: [5-(4-hydroxyphenyl)-3H-1,2-dithiocyclopentene-3-thione] (ADT-OH), the most widely used moiety for synthesizing slow-releasing organic hydrogen sulfide donors, and ADT, a methyl derivative of ADT-OH (Martelli et al., 2010). It has been reported that ADT-OH as well as its derivatives are enzymatically metabolized to release hydrogen sulfide by the mitochondria of cultured cells (Lee et al., 2010). Unlike excessive hydrogen sulfide instantaneously released from NaHS (Li et al., 2008), hydrogen sulfide generated from ADT-OH and its derivatives slowly increased intracellularly over 48 hours (Lee et al., 2010).
In this study, we mainly used a glutamate-induced neurotoxicity model in HT22 hippocampal neuronal cells to investigate the neuroprotective mechanisms underlying hydrogen sulfide donors. HT22 cells lack functional glutamate receptors and thereby glutamate-induced neurotoxicity in HT22 cells has been exclusively attributed to oxidative stress (van Leyen et al., 2005). Thus, glutamate-induce HT22 cell death represents a unique cellular model that has been used widely for investigating neuroprotective mechanisms against oxidative stress (Fukui et al., 2009; Kimura et al., 2006; Pallast et al., 2009; van Leyen et al., 2005; Xu et al., 2007).
Material and methods
Cell cultures and glutamate cytotoxicity assay
Mouse hippocampal neuronal HT22 cells were cultured in Dulbecco's modified Eagle's Medium (DMEM, Invitrogen Corporation, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco Laboratories, Grand Island, NY, USA) and 100 μg/ml penicillin-streptomycin at 37°C with 5% CO2. The medium was changed every two days. For glutamate toxicity assay, cells were trypsinized and seeded onto 24-well plates at the density of 2×104 cells per well. After 24 hours incubation, cells were exposed to 5 mM glutamate in the presence or absence of hydrogen sulfide donors. At 24 hours after glutamate exposure, cell viability was measured with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as described previously with modification (Liu et al., 2011). Briefly, 50 μL of MTT (5 mg/ml) was added to each well and then cells were incubated at 37°C with 5% CO2. After 2 hours incubation, medium was aspirated and 500 μL DMSO was added to cells to dissolve dark blue crystals of formazan, a MTT metabolite formed only in live cells but not in dead cells. The absorbance was measured at 570 nm using the Microplate Reader (Infinite M200 PRO, Tecan, Switzerland). Relative cell viabilities were calculated and presented as the percentages of controls without glutamate treatment.
H2O2-induced Free radical damage model
To investigate whether hydrogen sulfide donors protected against oxidative toxicity by directly scanvenging reactive oxygen species (ROS), we used the free radical H2O2 to induced oxidative toxicity in HT22 cells as described previously (Xu et al., 2007). Briefly, HT22 cells were seeded onto 24-well plates at the density of 2×104 cells per well. After 24 hours incubation, cells were exposed to 100 μM H2O2 in the presence or absence of hydrogen sulfide donors. At 24 hours later, cell viability was measured with MTT method as described above.
Inhibitor studies
To investigate if hydrogen sulfide donors protected HT22 cells from oxidative stress by activating K(ATP) channels, cells were pretreated with the K(ATP) inhibitor glibenclamide at a final concentration of 50 μM at 30 minutes before glutamate exposure. Then, cells were treated with glutamate in the presence or absence of hydrogen sulfide donors. At 24 hours after glutamate treatment, cell viability was measured with MTT method as described above.
To investigate if glutamate induces oxidative toxicity in part via activating AMPK and if AMPK activation contributes to ADT-OH and ADT neuroprotection against oxidative stress, HT22 cells were seeded on 24-well plates at the density of 2×104 cells per well for 24 hours and then were co-treated with AMPK inhibitor compound C (10 μM) and/or glutamate in the presence or absence of hydrogen sulfide donors. At 24 hours after treatment, the cell viability was measured with MTT method as described above.
Total intracellular GSH assay
HT22 cells were seeded on 6-well plates at the density of 1×105 cells per well for 24 hours. Then, cells were exposed to glutamate in the presence or absence of hydrogen sulfide donors. At 6-hour after exposure, cells were washed with ice-cold phosphate-buffered saline (PBS) for three times and then lysed with RIPA buffer. Lysates were left on ice for 30 minutes and then centrifuged at 13200 rpm/min for 20 minutes. After centrifugation, supernatants were collected for the measurement of total GSH and determination of protein concentration with the commercial BCA kit (Pierce, Thermo Fisher Scientific, Rockford, IL, USA). Total GSH was assayed using the commercial Kit per the manual provided by the manufacturer (Beyotime Institute of Biotechnology, Nantong, China). Briefly, 10 μL GSH standard or supernatants were added to 150 μL reaction solution containing 0.044 mg/ml DTNB (5,5′-Dithiobis(2-nitrobenzoic acid) and 1×glutathione reductase. Reactions lasted for 5 minutes and then 50 μL NADPH (0.16 mg/ml) was added. The absorbance at 412 nm was monitored with Microplate Reader at 5-minute intervals. GSH concentrations were calculated form GSH standard curves, and final results were presented as percentages of GSH contents in control cells without glutamate treatments.
Western blot analysis of total and phosphorylated AMPK
HT22 cells seeded on 6-well plates at the density of 10×104 cells per well were treated with glutamate in the presence or absence of hydrogen sulfide donors. At 6 hours after treatment, cells was rinsed with PBS for 3 times and then lysed with 50 μL RIPA per well. Cell lysates were centrifuged at 13200 rpm/min for 20 min. Supernatants were collected and protein concentrations were determined with commercial BCA kit. After denatured at 95 °C for 5 min, the proteins (30 μg/sample) were electrophoresed with on 10% SDS–PAGE gels and then transferred to polyvinylidene membranes (PVDF, Millipore, Billerica, MA, USA). The membranes were blocked with 5% nonfat milk for 2 hours and then incubated with primary antibodies against total AMPK (rabbit polyclonal, 1:1000, Cell Signaling, Beverly, MA, USA) or phosphorylated AMPK (rabbit polyclonal, 1:1000, Cell Signaling, Beverly, MA, USA) for 24 h at 4°C. After washing three times, the membranes were probed with secondary antibodies (anti-rabbit IgG, HRP-linked, 1:2000, Cell signaling, Beverly, Beverly, MA, USA) for 2 hours at room temperature. Protein bands were visualized with image Lab system using a SuperSignal West Pico chemiluminescence kit (Pierce, Rockford, IL, USA). β-actin was used as a loading control. The optical densities of protein bands were semi-quantified with Image J software, and final results were expressed as the ratios of phosphorylated AMPK to total AMPK.
Statistics
Statistical analysis was performed using a standard software package (SPSS 17.0). The results were expressed as mean ± SEM. Comparison was performed using one-way ANOVA models, followed by Turkey post-hoc tests. For all analyses, the null-hypothesis was rejected at the 0.05 level.
Results
Slow-releasing organic hydrogen sulfide donors ADT and ADT-OH were more potent in protecting HT22 cells against oxidative glutamate toxicity
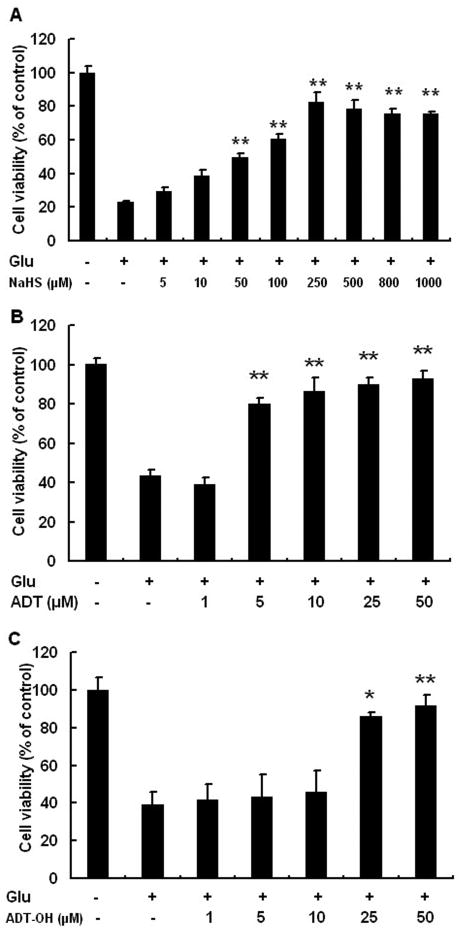
As indicated by MTT results, the viability of HT22 was significantly decreased after glutamate treatment. Both inorganic and slow-releasing organic hydrogen sulfide donors protected HT22 from glutamate-induced oxidative stress. NaHS at 50-1000 μM reversed cell death induced by glutamate in a concentration-dependent manner. The lowest concentration at which NaHS conferred maximum neuroprotection against glutamate oxidative stress was 250 μM (Figure 1A). The result was similar to previous finding that NaHS provides maximum protection against glutamate oxidative toxicity in HT22 cells at 300 μM (Kimura et al., 2006). Compared to NaHS, the organic sulfide hydrogen donors displayed maximum protective effects at much lower concentrations. The maximum protective effects were observed when ADT was at 10-50 μM and ADT-OH at 50 μM (Figure 1B and 1C). We could not assess the neuroprotective effects of ADT-OH and ADT at the concentrations beyond 50 μM due to the poor solubility of ADT-OH and ADT in DMEM medium. In the following experiments, neuroprotective mechanisms were investigated at the concentrations of 250 μM for NaHS, 10 μM for ADT and 50 μM for ADT-OH unless otherwise stated.
Figure 1.
Slow-releasing organic hydrogen sulfide donors ADT and ADT-OH were more potent than the inorganic hydrogen sulfide donor NaHS in protecting HT22 neuronal cells from oxidative glutamate toxicity. A: NaHS conferred concentration-dependent neuroprotection in HT22 cells treated with glutamate and maximum protection was observed at 250 μM. B: The organic donor ADT increased HT22 cell survival following glutamate exposure and maximum protection was observed at 10 μM. C: The organic donor ADT-OH increased HT22 cell survival following glutamate exposure and maximum protection was observed at 50 μM. ** p<0.01 and * p<0.05 compared with HT22 cells treated with glutamate alone, n = 4 for each group.
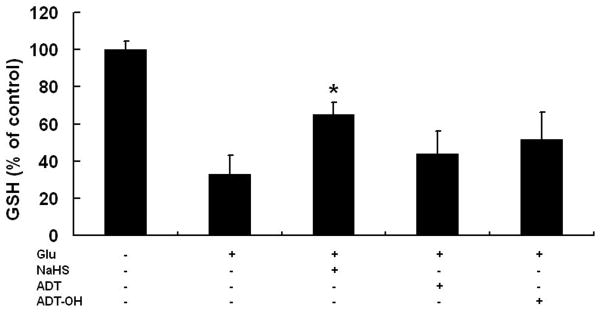
Slow-releasing organic hydrogen sulfide donors did not restore glutamate-induced depletion of GSH
NaHS is reported to protect neurons from glutamate oxidative toxicity in part by restoring cellular GSH levels depleted by glutamate (Kimura and Kimura, 2004; Kimura et al., 2006). As expected, we observed that glutamate-depleted cellular GSH levels were partly restored by 250 μM NaHS at 6 hours after glutamate treatment (Figure 2). However, neither ADT at 10 μM nor ADT-OH at 50 μM enhanced glutamate-depleted cellular GSH levels (Figure 2).
Figure 2.
Effects of hydrogen sulfide donors on cellular GSH levels following glutamate exposure. GSH levels were measured at 6 hours after glutamate exposure. Inorganic donor NaHS, but not organic donors ADT and ADT-OH increased glutamate-depleted GSH. * p<0.05 compared with the glutamate-treated cells (n = 4)
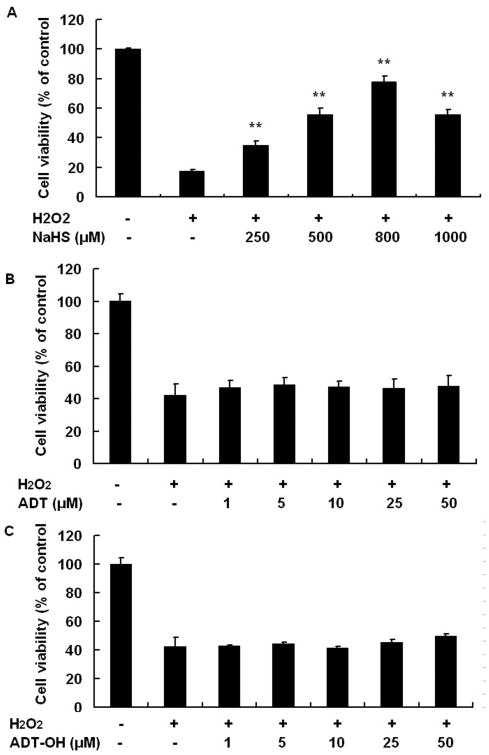
NaHS, but not slow-releasing organic donors, protected HT22 from free radical damage induced by H2O2
Under physiological conditions, hydrogen sulfide dissociates to yield hydrosulfide anion, which directly quenches ROS (Olson, 2009). To explore whether hydrogen sulfide donors confer protection against oxidative stress via acting as direct ROS scanvengers, we used the H2O2-induced free radical damage model, as described previously (Xu et al., 2007). NaHS at 250 μM remarkably protected, and at 800 μM almost completely recued HT22 from H2O2-induced cell death (Figure 3). In contrast, the organic donors ADT-OH and ADT did not display any neuroprotective effects in the H2O2 model at the concentrations tested (1-50 μM, Figure 3B and 3C). These results suggested that the free radical scanvenging ability in part contributed to neuroprotection of the inorganic donor NaHS, but did not contribute to neuroprotection of slow-releasing organic donors.
Figure 3.
Effects of the inorganic donors on HT22 cell death induced by the free radical H2O2. A: NaHS concentration-dependently increased survival of HT22 cells following H2O2 treatment. B: ADT did not protect HT22 cells following H2O2 treatment. C: ADT-OH did not protect HT22 cells following H2O2 treatment. ** p<0.01 compared with the H2O2-treated cells (n = 4).
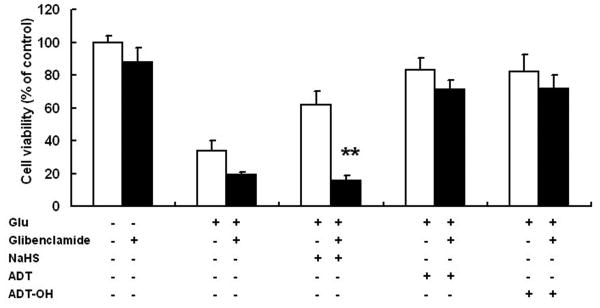
The KATP channel blocker glibenclamide abolished the neuroprotective effects of NaHS, but not those of ADT-OH and ADT
The inorganic donor NaHS has been shown to protect both HT22 neuronal cells and primary neurons from oxidative stress in part via activating KATP channels (Kimura et al., 2006). Glibenclamide, a well-established KATP channel blocker, did not affect the survival of HT22 cells either with or without glutamate treatment. As expected, glibenclamide abolished neuroprotective effects of NaHS on glutamate-induced HT22 cell death (Figure 4). In contrast, glibenclamide did not affect neuroprotection conferred by ADT-OH and ADT.
Figure 4.
Effects of the KATP channel blocker glibenclamide on neuroprotection conferred by hydrogen sulfide donors. Glibenclamide abolished the neuroprotective effects of NaHS but not those of ADT and ADT-OH in HT22 cells treated with glutamate. ** p<0.01 compared with HT22 cells treated with glutamate and NaHS (n = 4).
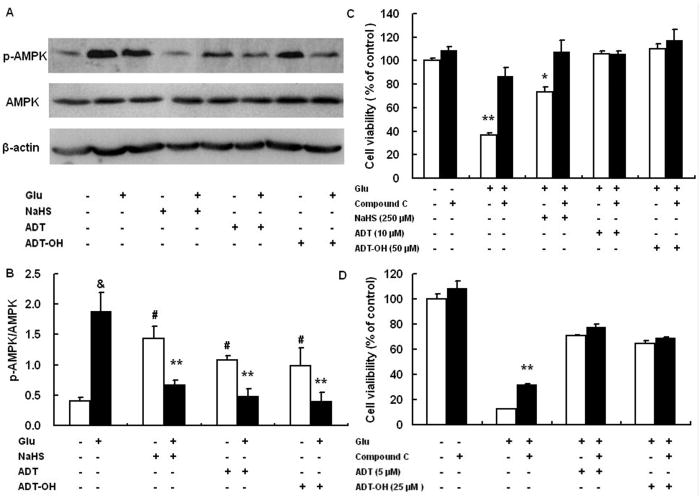
Glutamate-induced oxidative neuronal death involved AMPK activation, through which ADT-OH and ADT conferred neuroprotection against oxidative toxicity
AMPK, a critical sensor of cellular energy, is increasingly recognized to play an essential role in determining neuronal survival (Weisova et al., 2011). We investigated if AMPK played a role in neuronal death induced by glutamate oxidative toxicity and if ADT-OH and ADT acted through AMPK signaling to confer neuroprotection under glutamate oxidative toxicity. Western blot analysis showed that HT22 exhibited increased activation (phosphorylation) of AMPK at 6 hours after exposure to glutamate (Figure 5A and B). Compound C, a well-established AMPK inhibitor, robustly decreased HT22 death induced by glutamate oxidative stress although it had no effect on baseline survival of HT22 cells (Figure 5C). These results suggested that AMPK activation contributed to glutamate oxidative stress-induced neuronal death. Surprisingly, although all hydrogen sulfide donors, including inorganic donors NaHS, increased AMPK activation in the absence of glutamate, these hydrogen sulfide donors remarkably decreased glutamate-induced AMPK activation in HT22 cells under glutamate oxidative stress (Figure 5A). Furthermore, under glutamate oxidative stress, while the AMPK inhibitor compound C increased survival of HT22 cells treated with 250μM NaHS, it did not further increased survival of HT22 treated with 50 μM ADT-OH and 10 μM ADT (Figure 5C).
Figure 5.
AMPK activation contributed to cell death induced by glutamate and suppressing AMPK activation was a mechanism accounting for ADT and ADT-OH neuroprotection against oxidative glutamate toxicity. A: a representative image of western blot (upper panel) showed that, at 6 hours after glutamate exposure, HT22 cells exhibited increased phosphorylation (activation) of AMPK and that all hydrogen sulfide donors NaHS (250 μM), ADT-OH (50 μM) and ADT (10 μM) remarkably decreased glutamate-induced AMPK phosphorylation. B: The bar graph of western blot results. ** p<0.01 compared to cells treated with glutamate alone (n = 4); # p<0.05 compared to control cells without glutamate and hydrogen sulfide donor treatment; & p<0.01 compared to control cells without glutamate and hydrogen sulfide donor treatment. C: the AMPK inhibitor compound C robustly decreased HT22 death induced by glutamate oxidative stress and conferred additional neuroprotection in NaHS (250 μM) -treated HT22 cells following glutamate exposure, while it did not increased survival of HT22 cells treated with 50 μM ADT and 10 μM ADT-OH following glutamate exposure. ** p<0.01 and * p<0.05 compared with the corresponding black bars (cells treated with glutamate alone or cells with glutamate plus NaHS, n = 4). D: Compound C did not display synergetic effects on protecting HT22 cells from glutamate toxicity even when ADT and ADT-OH at the concentrations provided suboptimal protection (5 μM for ADT and 25 μM for ADT-OH, n=4). ** p<0.01 compared with glutamate-treated cells.
Since ADT-OH at 50 μM and ADT at 10 μM almost completely protected HT22 from glutamate oxidative toxicity, we further investigated whether compound C displays a synergetic effect on protecting HT22 from glutamate -toxicity when ADT and ADT-OH at the concentrations provide suboptimal protection. Following glutamate exposure, ADT at 5 μM and ADT-OH at 25 μM provided protection comparable to that conferred by NaHS at 250 μM (Figure 5D). However, compound C did not increase survival of HT22 treated with 5 μM ADT and 25 μM ADT-OH (Figure 5D). Collectively, these results suggested that, unlike NaHS, ADT and ADT-OH acted through suppressing AMPK activation to protect against glutamate oxidative stress.
Discussion
This study presents three major findings. First, our study showed that hydrogen sulfide donors acted through differential mechanisms to confer neuroprotection against oxidative stress. Second, we provided evidence suggesting that AMPK activation contributed to neuronal death induced by glutamate oxidative toxicity. Third, our data suggested that ADT-OH, the most widely used moiety for synthesizing slow-releasing organic hydrogen sulfide donors (Martelli et al., 2010), and its derivative ADT, protected neurons from oxidative toxicity by suppressing AMPK activation. Our findings are of significance for developing novel hydrogen sulfide donors as well as advance our understanding of the mechanisms underlying hydrogen sulfide neuroprotection against oxidative stress.
Our current understanding about the mechanisms underlying neuroprotection of hydrogen sulfide against oxidative stress is almost exclusively obtained from studies using the inorganic donor NaHS. The mechanisms responsible for NaHS neuroprotection against oxidative stress include: increasing cellular GSH levels, activating KATP channels and scanvenging free radicals directly (Hu et al., 2011; Kimura and Kimura, 2004; Kimura et al., 2006; Kimura et al., 2010; Olson, 2009; Whiteman et al., 2004). Consistent with these published data, we observed that 1) NaHS at the protection concentration partially restored intracellular GSH levels; 2) NaHS directly protected HT22 cells from H2O2-induced oxidative stress; 3) NaHS neuroprotection was blocked by the well-established KATP channel blocker, glibenclamide.
Depletion of GSH by glutamate is thought as a major contributor to glutamate-induced cell death in HT22 cells (Maher and Davis, 1996). Strikingly, although ADT-OH and its derivative ADT were more potent in protecting HT22 cells from oxidative toxicity (Figure 1), neither of them prevented glutamate-induced GSH depletion. Nevertheless, our finding was consistent with the previous publication showing that the neuroprotant melatonin protects HT22 cells from glutamate oxidative stress without preventing GSH depletion (Herrera et al., 2007). Furthermore, ADT and ADT-OH did not protect against H2O2-induced free radical damage, suggesting that they did not act as direct free radical scanvengers to protect neurons against oxidative stress. Glibenclamide, the KATP channel blocker that almost completely abolished neuroprotection of NaHS, had no effect on ADT and ADT-OH neuroprotection against oxidative stress. ADT-OH is the most widely used moiety for synthesizing slow-releasing organic hydrogen sulfide donors (Martelli et al., 2010). However, our results clearly showed that none of the currently known mechanisms underlying NaHS neuroprotection against oxidative stress contributed to neuroprotective effects of ADT-OH and ADT.
AMPK is a critical sensor of cellular energy balance. More recently, AMPK is found to play an essential role in regulating neuronal apoptosis (Li et al., 2010; Weisova et al., 2011). For instance, AMPK activation contributes to neuronal death under pathological conditions, including stroke and Alzheimer's disease (Kwon et al., 2010; Li et al., 2007; McCullough et al., 2005; Venna et al., 2012). However, it remains unclear whether AMPK is also involved in cell death induced by oxidative glutamate toxicity, which is increasingly recognized to represent a new form of programmed cell death different from apoptosis. Glutamate-induced HT22 cell death neither resembles typical apoptosis morphologically (Tan et al., 1998; Tan et al., 2001) nor be blocked by pan-caspase inhibitors (van Leyen et al., 2005). Here we showed that glutamate induced AMPK activation in HT22 cells and that the AMPK inhibitor compound C remarkably reduced glutamate-induced oxidative cell death. Together, these data suggested a contributing role of AMPK activation in cell death induced by glutamate oxidative stress.
To further investigate mechanisms underlying ADT and ADT-OH neuroprotection against oxidative stress, we performed western blot to examine if ADT and ADT-OH conferred neuroprotection by reducing AMPK activation. Surprisingly, although all hydrogen sulfide donors, including NaHS, increased basal AMPK activation in HT22 cells, they remarkably reduced glutamate-induced AMPK activation at 4 hours after glutamate exposure. Interestingly, under glutamate oxidative stress, the AMPK inhibitor compound C further increased survival of NaHS-treated HT22 cells, but had no effect on survival of the cells treated with ADT and ADT-OH. Together, these results suggested that suppressing AMPK activation contributed to neurorprotection of ADT and ADT-OH against oxidative stress, but not that of NaHS.
Our results suggested that glutamate-induced AMPK activation was indispensible to glutamate-induced cell death under oxidative stress since AMPK inhibition remarkably recued the HT22 neuronal cells from glutamate toxicity. Of note, hydrogen sulfide donors also elevated basal AMPK activation levels, while did not display neurotoxicity effects under basal conditions. Indeed, it is well-accepted that AMPK activation plays a dual role in determining neuronal fates, either driving cell death or survival. Thus, other modulators or interactors couple with AMPK activation to determine neuronal cell death or survival (Cardaci et al., 2012). The modulators or interactors, which predominate under glutamate oxidative toxicity to couple with AMPK activation to induce neuronal death but are absent under basal condition, remain to be identified.
In conclusion, by inducing the oxidative glutamate toxicity in HT22 neuronal cells, we showed that the neuroprotective mechanisms against oxidative stress underlying ADT-OH, the most widely used moiety for synthesizing slow-releasing organic hydrogen sulfide donors, and its methyl derivative ADT, were different from those of the inorganic donor NaHS. Furthermore, for the first time, our study suggested that AMPK activation contributed to glutamate oxidative toxicity and that suppressing AMPK activation was a possible mechanism underlying neuroprotection of organic hydrogen sulfide donors against oxidative toxicity. Our findings are of significance for developing novel hydrogen sulfide donors and advance our understanding of the neuroprotective mechanisms of hydrogen sulfide.
Acknowledgments
Source of funding: The project is supported by the grants from National Science Foundation of China (81171246, 81100918, 81070959 and 81130023), National Basic Research Plan (2009CB522000, 2011CB5C4403), and Priority Academic Program Development of Jiangsu Higher Education Institutes (PAPD).
Abbreviations
- ADT-OH
5-(4-hydroxyphenyl)-3H-1, 2-dithiole-3-thione
- ADT
5-(4-methoxyphenyl) -3H-1, 2-dithiole-3-thione
- AMPK
AMP-activated protein kinase
- GSH
glutathione
- H2O2
hydrogen peroxide
- KATP channel
ATP-sensitive potassium channel
- NaHS
sodium hydrogensulfide
- ROS
reactive oxygen species
Footnotes
Disclosures: A patent application on therapeutic effects of hydrogen sulfide donors in stroke has been filed to China Intellectual Property Office.
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–71. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J Cell Sci. 2012;125:2115–25. doi: 10.1242/jcs.095216. [DOI] [PubMed] [Google Scholar]
- Fukui M, Song JH, Choi J, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Gong QH, Wang Q, Pan LL, Liu XH, Xin H, Zhu YZ. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-kappaB pathway in rats. Brain Behav Immun. 2011;25:110–9. doi: 10.1016/j.bbi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Gu X, Zhu YZ. Therapeutic applications of organosulfur compounds as novel hydrogen sulfide donors and/or mediators. Expert Rev Clin Pharmacol. 2011;4:123–33. doi: 10.1586/ecp.10.129. [DOI] [PubMed] [Google Scholar]
- Herrera F, Martin V, Garcia-Santos G, Rodriguez-Blanco J, Antolin I, Rodriguez C. Melatonin prevents glutamate-induced oxytosis in the HT22 mouse hippocampal cell line through an antioxidant effect specifically targeting mitochondria. J Neurochem. 2007;100:736–46. doi: 10.1111/j.1471-4159.2006.04228.x. [DOI] [PubMed] [Google Scholar]
- Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011;15:405–19. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–7. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–70. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- Kwon KJ, Kim HJ, Shin CY, Han SH. Melatonin Potentiates the Neuroprotective Properties of Resveratrol Against Beta-Amyloid-Induced Neurodegeneration by Modulating AMP-Activated Protein Kinase Pathways. J Clin Neurol. 2010;6:127–37. doi: 10.3988/jcn.2010.6.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Tazzari V, Giustarini D, Rossi R, Sparatore A, Del Soldato P, McGeer E, McGeer PL. Effects of hydrogen sulfide-releasing L-DOPA derivatives on glial activation: potential for treating Parkinson disease. J Biol Chem. 2010;285:17318–28. doi: 10.1074/jbc.M110.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–9. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Benashski SE, Venna VR, McCullough LD. Effects of metformin in experimental stroke. Stroke. 2010;41:2645–52. doi: 10.1161/STROKEAHA.110.589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–60. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu YY, Sparatore A, Del Soldato P, Bian JS. ACS84, a novel hydrogen sulfide-releasing compound, protects against amyloid beta-induced cell cytotoxicity. Neurochem Int. 2011;58:591–8. doi: 10.1016/j.neuint.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–92. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996;16:6394–401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli A, Testai L, Breschi MC, Blandizzi C, Virdis A, Taddei S, Calderone V. Hydrogen sulphide: novel opportunity for drug discovery. Med Res Rev. 2010 doi: 10.1002/med.20234. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta. 2009;1787:856–63. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Ji D, Majid AS, Del Soldata P, Sparatore A. Glutamate oxidative injury to RGC-5 cells in culture is necrostatin sensitive and blunted by a hydrogen sulfide (H2S)-releasing derivative of aspirin (ACS14) Neurochem Int. 2012;60:365–78. doi: 10.1016/j.neuint.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem. 2009;111:882–9. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predmore BL, Lefer DJ, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 2012;17:119–40. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- Tan S, Schubert D, Maher P. Oxytosis: A novel form of programmed cell death. Curr Top Med Chem. 2001;1:497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- Tay AS, Hu LF, Lu M, Wong PT, Bian JS. Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience. 2010;167:277–86. doi: 10.1016/j.neuroscience.2010.02.006. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Siddiq A, Ratan RR, Lo EH. Proteasome inhibition protects HT22 neuronal cells from oxidative glutamate toxicity. J Neurochem. 2005;92:824–30. doi: 10.1111/j.1471-4159.2004.02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venna VR, Li J, Benashski SE, Tarabishy S, McCullough LD. Preconditioning induces sustained neuroprotection by downregulation of adenosine 5′-monophosphate-activated protein kinase. Neuroscience. 2012;201:280–7. doi: 10.1016/j.neuroscience.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–8. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Weisova P, Davila D, Tuffy LP, Ward MW, Concannon CG, Prehn JH. Role of 5′-adenosine monophosphate-activated protein kinase in cell survival and death responses in neurons. Antioxid Redox Signal. 2011;14:1863–76. doi: 10.1089/ars.2010.3544. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–8. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- Xu X, Chua CC, Kong J, Kostrzewa RM, Kumaraguru U, Hamdy RC, Chua BH. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–14. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]