The spleen has puzzled physicians and scientists since antiquity. Although Aristotle suggested that the spleen has no functional role, Galen (influenced by the hippocratic principles of humorism) believed that the spleen was the source of “black bile”, one of the four humors that affect behavior and health. According to these ancient humorist teachings, the spleen governs our mood causing melancholy (“melancholy” in greek literally means “black bile”, from μελας/black and χολη/bile). This notion is reflected in the use of the word “spleen” in french poetry to describe a melancholic state without an obvious cause (such as the deep existential anguish and despair expressed by Charles Baudelaire in the “Fleurs du Mal”). Modern medicine no longer considers the spleen as a source of obscure substances that govern our emotions. In human patients, the consequences of surgical removal of the spleen (splenectomy) and the findings associated with a hyperfunctional spleen (hypersplenism) have revealed important functions of the organ in iron metabolism, in storing blood, in removing senescent or injured red blood cells and in regulation of the lymphoid system. In 2009, Swirksi and co-workers 1 introduced a transformative concept in the field of inflammation, suggesting that the mouse spleen may serve as a reservoir of pro-inflammatory monocytes that, when mobilized following myocardial infarction or other tissue injury, play a critical role in regulation of inflammation. The study challenged prevailing dogma demonstrating for the first time that extramedullary populations of monocytes could be rapidly mobilized following injury to regulate inflammation. New and intriguing concepts are always tested by time. Less than five years after publication of this seminal study, are we ready to rewrite the textbooks to indicate an essential role for the spleen in inflammation?
The spleen in myocardial inflammation, cardiac remodeling and heart failure
In the current issue of Circulation Research, Ismahil and co-workers 2 provide exciting new evidence implicating the spleen in the immunoinflammatory response following myocardial infarction and suggesting that splenocytes are intricately involved in the pathogenesis of cardiac remodeling and in the development of heart failure. The authors found that chronic heart failure in mice with a large myocardial infarction is associated with intense infiltration of the myocardium with activated macrophages and with extensive alterations in the structure and cellular composition of the spleen. Mice with heart failure exhibited marked expansion of white pulp follicles and lymphoid populations, and a striking increase in the size of the marginal zone (a site important for antigen screening and processing). In contrast, pro-inflammatory monocytes in the red pulp became much less abundant following infarction, reflecting depletion of the splenic monocyte reservoir. Several lines of evidence suggested that mononuclear splenocytes mediate adverse cardiac remodeling. First, splenectomy attenuated cardiac remodeling and reduced infiltration of the infarcted myocardium with macrophages and dendritic cells. Second, splenocytes from mice with heart failure expressed inflammatory mediators and alarmins and homed to the infarcted myocardium. Third, adoptive transfer of heart failure splenocytes in mice induced systolic dysfunction and adverse dilative remodeling. The observations suggest that, in addition to its proposed role in acute inflammation following myocardial infarction 1, the spleen may also be involved in the pathogenesis of chronic heart failure by contributing to the progression of dilative remodeling. Considering the growing body of evidence suggesting that the spleen can be a source of immune cells that may modulate inflammatory and remodeling responses by homing to peripheral tissues are we ready to accept a critical role for the spleen in post-infarction heart failure?
The case for skepticism and the need for extensive validation of novel concepts
New concepts require extensive validation before they can be accepted as established knowledge. The hunger of the scientific community for “novel observations” may result in selective publication of sensationalist findings supporting improbable events, while discouraging publication of the (more likely) traditionally accepted views. Reporting a surprising, unexpected event carries a high impact and is appreciated as “novel”. This leads to a considerable publication and dissemination bias, as “surprising” low-probability findings are selectively rewarded and attract attention. For instance, at a time when the scientific community was convinced that monocytes in inflamed tissues derive from the bone marrow, publication of a well-documented study suggesting the role of an extramedullary source (such as the splenic reservoir) has high informational value and generates great interest. In contrast, at the same time, a report showing that the spleen (or any other extramedullary organ) is NOT an important source would be considered as a confirmation of an obvious fact and would have no chance of publication. It is after the surprising finding is published that a negative study refuting the new observation becomes attractive for publication. Thus, time and systematic experimentation by many different groups is needed to provide the validation necessary for acceptance of the new concept. Did the concept of the splenic monocyte reservoir in acute inflammation pass the test of time? It may be premature to answer this question; however, the early signs are positive. Time lapse analysis showed that monocytes recruited in the infarcted heart are derived first from the circulating blood then from the spleen 3. In mouse models of peritoneal inflammation 4, intestinal inflammation 5, and spinal cord injury 6, three independent groups found that the spleen is a significant source of infiltrating monocytes. On the other hand, many published studies using bone marrow chimeras have established the contribution of bone marrow-derived Ly6Chi monocytes in models of tissue injury 7, 8, 9. The splenic reservoir and the bone marrow may serve as sources of distinct monocyte subpopulations in tissue injury, or may contribute in response to different activating stimuli (Figure). Systematic experimentation dissecting the role of the bone marrow and extramedullary sources in many different models of injury is needed.
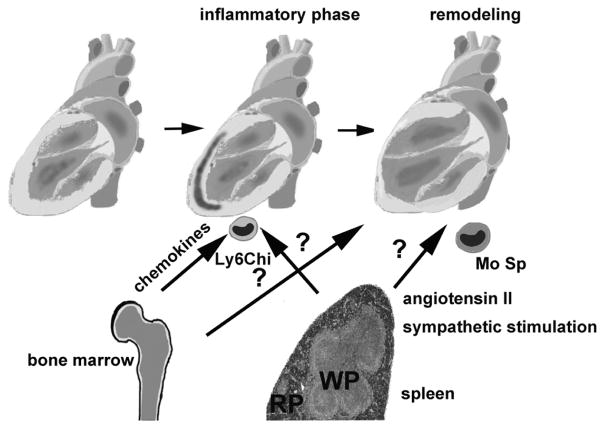
Figure.
The bone marrow and extramedullary sources contribute monocytes that regulate the inflammatory and remodeling response in the infarcted heart. Chemokine-driven pathways may be responsible for recruitment of bone marrow monocytes. A growing body of evidence suggests the involvement of the splenic monocyte reservoir in myocardial inflammation and remodeling. The spleen may contribute Ly6Chi monocytes during the inflammatory phase of infarct healing. Moreover, during the chronic remodeling phase, poorly characterized subsets of mononuclear splenocytes (Mo Sp), mobilized through neurohumoral pathways, may home to the myocardium and may be involved in the pathogenesis of heart failure (WP, white pulp; RP, red pulp).
The unique challenges of in vivo experimentation
The need for systematic study and independent confirmation is particularly important to support pathophysiologic concepts, where the dependence on animal model investigations often limits reproducibility of observations and challenges interpretation of the findings. The use of surgical splenectomy as a loss-of-function approach is a major limitation of the experimental studies investigating the role of the splenic monocyte reservoir in inflammatory processes. Splenectomy, not only removes the monocyte reservoir, but also eliminates all other splenic cells (including abundant lymphocyte populations) and has a wide range of other effects, reducing blood volume (thus affecting hemodynamic loading) and increasing red blood cell mass and platelet counts. Thus, the effects of splenectomy on cardiac remodeling and function following injury may not be necessarily due to elimination of the monocyte reservoir.
The need for exploration of mechanisms mobilizing the splenic reservoir
If the splenic reservoir serves as a major source of monocyte subpopulations in tissue injury and remodeling, which stimuli mobilize its cellular content during the reparative response? Chemokine-driven recruitment of inflammatory leukocytes from the bone marrow is activated in the early stages of the inflammatory phase of healing 10 and may be transient, as inhibitory signals rapidly suppress chemokine synthesis 11. The involvement of mononuclear splenocytes in chronic post-infarction heart failure suggests that the molecular signals involved in their mobilization may be independent of the acute inflammatory response. Neurohumoral angiotensin II-mediated signaling may be responsible for sustained mobilization of splenic monocytes following myocardial infarction 1, 12; sympathetic activation may also contribute to egress of pro-inflammatory splenocytes 4. Dissecting the pathways that govern recruitment of mononuclear cell subsets from the bone marrow and from extramedullary sources, and understanding temporal and spatial aspects of their trafficking is crucial to gain both pathophysiologic insights and therapeutically relevant information.
Of mice and men
Ultimately, the significance of murine studies suggesting a role for the splenic monocyte reservoir is dependent on whether the spleen significantly contributes to the inflammatory and remodeling responses in human patients. Because practically all available data suggesting a role for the splenic monocyte reservoir are derived from mouse models, there is a possibility that such findings may reflect species-specific effects with limited implications in human pathobiology. Although, validation of these concepts in humans represents a major challenge, there are opportunities to obtain relevant information from human samples. Autopsy material from patients with heart failure may be used to study the consequences of heart failure on splenic architecture and function. A recent clinical study showed that the number of splenic CD14+ monocytes is significantly reduced in patients dying from acute myocardial infarction 13. Moreover, investigating the inflammatory and reparative responses in splenectomized patients may provide much-needed information on the significance of the splenic reservoir in humans. Systematic experimentation in animal models and in human patients may eventually establish whether splenocytes produce a depressive “humor” that causes cardiac melancholy.
Acknowledgments
SOURCES OF FUNDING: Dr Frangogiannis’ laboratory is funded by NIH grants R01 HL76246 and R01 HL85440.
Footnotes
DISCLOSURES: None
References
- 1.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the Mononuclear Phagocyte Network Underlies Chronic Inflammation and Disease Progression in Heart Failure: Critical Importance of the Cardiosplenic Axis. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung K, Kim P, Leuschner F, Gorbatov R, Kim JK, Ueno T, Nahrendorf M, Yun SH. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res. 2013;112:891–899. doi: 10.1161/CIRCRESAHA.111.300484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeley EJ, Barry SS, Narala S, Matthay MA, Wolters PJ. Noradrenergic neurons regulate monocyte trafficking and mortality during gram-negative peritonitis in mice. J Immunol. 2013;190:4717–4724. doi: 10.4049/jimmunol.1300027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao G, van Driel B, Magelky E, O’Keeffe MS, de Waal Malefyt R, Engel P, Herzog RW, Mizoguchi E, Bhan AK, Terhorst C. Glucocorticoid-induced TNF receptor family-related protein ligand regulates the migration of monocytes to the inflamed intestine. Faseb J. 2013 doi: 10.1096/fj.13-236505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomster LV, Brennan FH, Lao HW, Harle DW, Harvey AR, Ruitenberg MJ. Mobilisation of the splenic monocyte reservoir and peripheral CX(3)CR1 deficiency adversely affects recovery from spinal cord injury. Exp Neurol. 2013;247:226–240. doi: 10.1016/j.expneurol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D, Fang P, Jiang X, Nelson J, Moore JK, Kruger WD, Berretta RM, Houser SR, Yang X, Wang H. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in LDLr/CBS-deficient mice. Circ Res. 2012;111:37–49. doi: 10.1161/CIRCRESAHA.112.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 9.Shi C, Velazquez P, Hohl TM, Leiner I, Dustin ML, Pamer EG. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J Immunol. 2010;184:6266–6274. doi: 10.4049/jimmunol.0904160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 11.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Laan AM, Ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, Lavaei M, Nahrendorf M, Horrevoets AJ, Niessen HW, Piek JJ. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]