Abstract
Prompt removal of apoptotic cells by phagocytes is important for maintaining tissue homeostasis. The molecular and cellular events that underpin apoptotic cell recognition and uptake, and the subsequent biological responses are increasingly better defined. The detection and disposal of apoptotic cells generally promote an anti-inflammatory response at the tissue level, as well as immunological tolerance. Consequently, defects in apoptotic cell clearance have been linked with a variety of inflammatory diseases and autoimmunity. Conversely, under certain conditions such as killing tumour cells by specific cell death inducers, the recognition of apoptotic tumour cells can promote an immunogenic response and anti-tumour immunity. Here, we review the current understanding of the complex process of apoptotic cell clearance in physiology and pathology, and discuss how this knowledge could be harnessed for new therapeutic strategies.
Apoptosis or programmed cell death (in contrast to necrosis – see Box 1) occurs throughout life in essentially all tissues as part of normal development, homeostasis, and pathogenic processes. Despite the constant turnover of cells through apoptosis, apoptotic cells are rarely seen under physiological conditions, even in tissues with high rates of apoptosis. For example, about 80% of developing thymocytes eventually undergo apoptosis, yet free apoptotic cells are rarely observed in the thymus. This suggests that in the steady state, the rate of apoptotic cell removal is high, and this seems to be a pre-requisite for the continued clearance of the estimated one million cells that undergo apoptosis in various tissues every second in adult humans1. Dying cells are removed either by tissue-resident professional phagocytes (such as macrophages and immature dendritic cells (DCs)) or by neighbouring non-professional phagocytes.
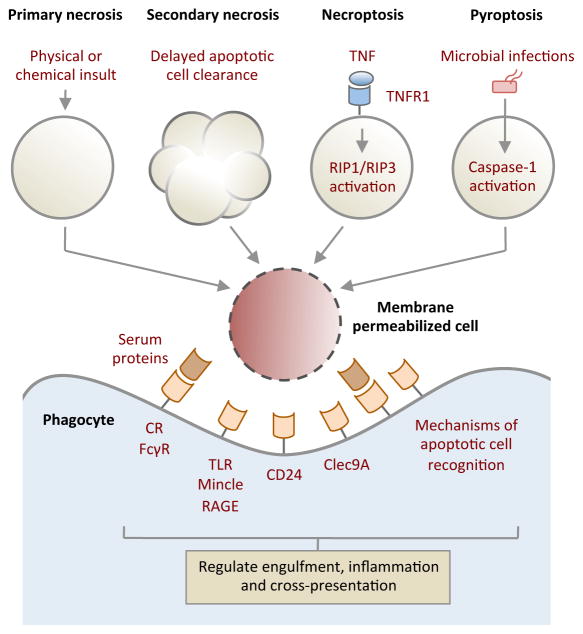
Box 1. Immune recognition of membrane-permeabilized (necrotic) cells.
The plasma membrane can be become permeable in response to physical and chemical insult (primary necrosis) or when uncleared apoptotic cells begin to lose membrane integrity (secondary necrosis). Membrane lysis can also occur through an active mechanism, when tumour necrosis factor receptor 1 (TNFR1) signalling is activated by TNF along with caspase 8 inhibition, a process known as necroptosis or programmed necrosis. Initiation of necroptosis depends on the activation of receptor-interacting protein 1 (RIP1) and RIP3 kinases148. Activation of caspase 1 by pathological stimuli such as microbial infection can also trigger membrane permeabilization by a form of cell death known as pyroptosis149. Furthermore, neutrophils and eosinophils can undergo another form of programmed cell death with release of extracellular traps (termed NETs) in response to pathogens and in response to sterile inflammatory mediators150,151 with potential antimicrobial but pro-inflammatory consequences.
A key feature of membrane lysis is the display and/or release of intracellular molecules that are otherwise hidden from the extracellular environment. Exposure of certain intracellular molecules can trigger inflammation and signal ‘danger’152 to the immune system. Such endogenous molecules (also known as damage-associated molecular patterns (DAMPs)) include: high-mobility group box 1 (HMGB1), SAP130, heat shock protein 90 (HSP90), DNA, uric acid and monosodium urate crystals, and IL-33. These endogenous molecules can be recognized variably by Toll-like receptors (TLRs), the C-type lectin Mincle, receptor for advanced glycation end-products (RAGE) and ST2153,154. Interestingly, interaction of HMGB1 and HSP90 with CD24 on responding cells may dampen their immunostimulatory properties to fine-tune the immune response155. Membrane permeabilized cells may also expose molecules that are similar to intact apoptotic cells (such as PtdSer), so the recognition mechanisms that are used to mediate apoptotic and necrotic cell removal may overlap. Notably, in addition to direct recognition by phagocytes, many serum opsonins have been found to preferentially aid the clearance of membrane permeabilized cells156. Furthermore, selective detection of membrane-damaged cells by receptors such as Clec9A may have an important role in regulating antigen cross-presentation by CD8α+ DCs157,158.
Box 1.
Handling membrane permeabilizwd (necrotic) cells
In contrast to phagocytosis of bacteria and other ‘danger-associated’ particles, clearance of apoptotic cells is immunologically quiescent under physiological circumstances, and does not involve influx of inflammatory cells into the healthy tissues or a breakdown in immune tolerance against self-antigens. Recently, there has been a significant accumulation of knowledge on the molecular details of the apoptotic cell clearance process and on its functional relevance to disease. Such knowledge has created an exciting stage to further explore the potential therapeutic benefits of targeting the apoptotic cell clearance machinery in a variety of diseases ranging from autoimmunity to cancer.
In this Review, we introduce the key molecular features of the apoptotic cell clearance process, and then discuss the relevance of apoptotic cell clearance process to infection, inflammatory disease, autoimmunity, transplantation, and cancer. Finally, we examine how targeting this clearance machinery could provide therapeutic benefits.
Molecular steps in apoptotic cell removal
Prior to their recognition by phagocytes, apoptotic cells undergo a number of distinct morphologic changes. These changes may in turn facilitate an apoptotic cell to be recognized and cleared. An intriguing issue with respect to morphologic changes during apoptosis is whether phagocytes engulf the apoptotic cells ‘in whole’ or in smaller ‘bite-size’ fragments. There is evidence for both. In most instances, the professional phagocytes appear to phagocytose the targets in their entirety – this is particularly apparent in the case of macrophages or DCs that engulf apoptotic thymocytes or neutrophils2,3. Even fibroblasts and epithelial cells appear to engulf similarly sized dying brethren in tissues and ex vivo4,5. However, there are other instances where a phagocyte simply cannot engulf the dying target in its entirety, possibly due to sheer size difference between the phagocyte and the target. For example, in inflamed adipose tissue, dying adipocytes appear to be engulfed by multiple macrophages that form ‘crown-like structures’ around a single adipocyte and ingest smaller fragments of the dying cell6. This has also been observed during clearance of dying cells by fibroblasts in the absence of macrophages2. In fact, the formation of plasma membrane blebs (a common morphologic feature of apoptosis) is required for the generation of smaller apoptotic cell fragments (known as apoptotic bodies). In multiple cell types, activation of Rho-associated kinase ROCK I by caspase 3-mediated cleavage enhances phosphorylation of myosin light chain, which in turn promotes actomyosin contraction, membrane blebbing and the formation of apoptotic bodies7,8 (Figure 1). Although some of the extensive membrane blebbing that has been observed in cultured cancer cells following apoptosis induction may be due to lack of neighbouring phagocytes or represent late stages of cell death, apoptotic blebs have been observed on apoptotic cells in native tissues9. What fraction of the apoptotic cells that might be cleared through the formation of such ‘bite-size’ fragments in vivo, and whether the ‘rest’ of the corpse gets ingested as a larger target remains to be determined. Thus, in different tissues, depending on the relative sizes of the phagocyte and the target being ingested, the corpses may be taken either in whole, or in smaller fragments. However, it is notable that in the case of substantial and excessive apoptosis, uncleared apoptotic cells and fragments may lose their membrane integrity (undergoing secondary necrosis) and are likely removed via other phagocytic mechanisms (Box 1).
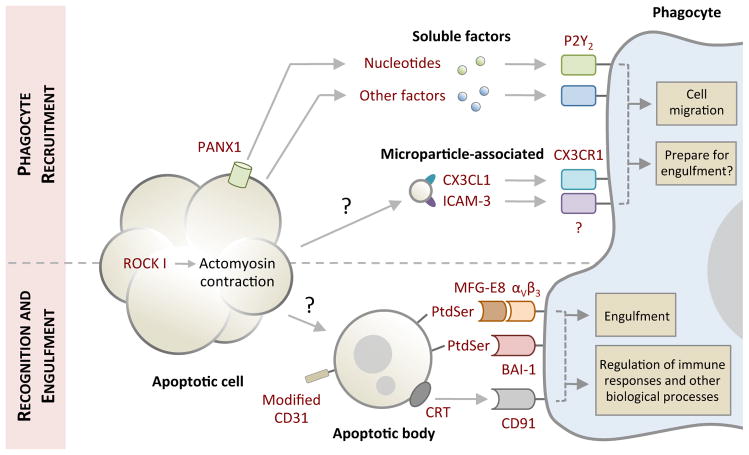
Figure 1. Phases of apoptotic cell clearance.
Cells undergoing apoptosis often exhibit morphological changes (for example membrane blebbing and cellular shrinkage) to facilitate cell detachment and organelle fragmentation. Prior to or during the onset of apoptotic morphology, apoptotic cells also release ‘find-me’ signals in the form of soluble factors (for example nucleotides) or microparticle-associated molecules (including CX3C chemokine ligand 1 (CX3CL1) and intercellular adhesion molecule 3 (ICAM3)) to recruit phagocytes for cell clearance. Nucleotides are released from apoptotic cells via caspase-activated pannexin 1 (PANX1) membrane channels. Whether detection of ‘find-me’ signals by phagocytes can prepare molecular machinery necessary for engulfment in addition to cell migration warrants further investigation. Exposure of ‘eat-me’ signals (such as phosphatidylserine (PtdSer) and calreticulin (CRT)) accompanied by modification of ‘don’t eat-me’ signals (CD31) on apoptotic cells or fragments of apoptotic cell (also referred to as apoptotic bodies) mediate their recognition by phagocytes. Phagocytes can engage ‘eat-me’ signals directly via cell surface receptors (such as brain-specific angiogenesis inhibitor (BAI-1) and CD91) or indirectly through bridging molecules (such as milk fat globule-EGF factor 8 (MFG-E8)) that are in turn detected by membrane receptors (αVβ3). Subsequent downstream signalling initiates engulfment and engulfment-associated responses from phagocytes. The mechanism underpinning the formation of apoptotic bodies and microparticles is not fully defined. P2Y2, purinergic receptor P2Y2; ROCK I, Rho-associated coiled-coil containing protein kinase I.
Another interesting question with respect to morphology of dying cells is how apoptotic cells that are part of an epithelial sheet, such as epithelial cells in the gut or airways, can be removed, and how the integrity of the epithelial barrier is maintained. This is not a trivial issue when one considers that in the gut of an adult human, an epithelial surface area roughly equivalent to a tennis court is replaced every 4–7 days. For cells held by attachment to the extracellular matrix, to neighbouring cells or to synthetic surfaces, detachment from the surrounding environment can be induced by caspase-mediated cleavage of components regulating focal adhesion10,11 and adherens junction components, such as E-cadherin, P-cadherin and β-catenin12,13. Viable neighbouring cells might replace such ‘loosened’ dying epithelial cells while the corpse is being removed. Alternatively, cell extrusion into the organ lumen or another tissue space14 might allow for subsequent apoptotic cell removal by luminal phagocytes such as alveolar macrophages. It remains to be determined whether phagocytosis of apoptotic epithelial cells in different tissues involves mainly cell extrusion or other mechanisms.
Recruiting the ‘right’ phagocyte to prevent inflammation
It is now becoming increasingly clear that apoptotic cells at the earliest stages of death ‘advertise’ their presence to facilitate their own removal by recruiting phagocytes. The latter are usually motile tissue-resident phagocytes, although in model systems recruitment directly from the circulation can also occur15. Apoptotic cells can attract phagocytes through the release of chemotactic factors, which are known as ‘find-me’ signals. These find-me signals can be soluble, or signal via submicron membrane vesicles (Figure 1 and Table 1).
Table 1.
Molecular machinery of apoptotic cell recognition
| SIGNAL | RELEASE/EXPOSURE MECHANISM | RECOGNITION MECHANISM | DETAILS/COMMENTS | REFERENCE |
|---|---|---|---|---|
| ‘Find-me’ | ||||
| Nucleotides | PANX1 | P2Y2 | Release of ATP/UTP from apoptotic cells promotes monocyte and macrophage migration in vitro and in vivo. Other P2Y family members may also facilitate detection of nucleotides by phagocytes. | 15,16 |
| LPC | ? | G2A | Caspase 3-mediated activation of iPLA2 is also necessary to generate LPC during apoptosis. LPC augments monocyte migration in vitro. | 18 |
| SIP | ? | ? | Purified SIP enhances monocyte and macrophage migration in vitro. | 19 |
| ICAM-3 | Microparticles | ? | ICAM-3 localizes to apoptotic blebs and microparticles during apoptosis. ICAM-3 may also facilitate tethering of apoptotic B cells to macrophages. | 21,184 |
| CX3CL1 | Microparticles | CX3CR1 | CX3CL1/CX3CR1 participates in the recruitment of macrophages to lymphoid follicles undergoing germinal center reactions. | 22 |
| EMAP II | ? | ? | Generation and release of mature EMAP II occurs during apoptosis. The ability of apoptotic cell-derived EMAP II to promote phagocyte migration has not been examined directly. | 185 |
| Annexin A1 | Membrane lysis | ? | Release and proteolytic processing of annexin A1 by ADAM10 during secondary necrosis promotes migration of monocytes. Annexin A1 may also participate in the engulfment step of apoptotic cell clearance. | 186,187 |
| ‘Keep-out’ | ||||
| Lactoferrin | ? | ? | Release from apoptotic cells to inhibit neutrophil migration. | 23 |
| ‘Eat-me’ | ||||
| PtdSer | Phospholipid scramblase/amino-phospholipid translocase? | BAI1 | BAI1 functions upstream of the ELMO1-Dock180-Rac module to mediate apoptotic cell recognition and engulfment. BAI1 interacts with PtdSer though its thrombospondin type 1 repeats. | 30 |
| TIM1/3/4 | Metal-ion-dependent ligand binding site located in the IgV domain of TIM-4 mediates PtdSer binding. TIM-3 may also play an important role in regulating cross-presentation of apoptotic cell-associated antigens by CD8+DCs. | 33–35 | ||
| Stabilin-2 | Stabilin-2 functions upstream of GULP and thymosin β4 to aid apoptotic cell clearance. Stabilin-2 binds PtdSer via its epidermal growth factor-like domain repeats. | 31,32,36,37 | ||
| MFG-E8 – αVβ3 | MFG-E8 is secreted by ‘activated’ macrophage and immature DCs to promote apoptotic cell engulfment. MFG-E8 interacts with PtdSer and αVβ3 via its factor-VIII-homologous domains and RGD motif, respectively. | 40 | ||
| Protein S/Gas6 – Tryo-3/Axl/Mer | Protein S and Gas6 interact with PtdSer and TAM receptors via their Gla domains and sex hormone binding globulin domains, respectively. Usage of different TAM receptors is dependent on phagocyte and organ type. | 39,41,188,189 | ||
| RAGE | RAGE is thought to function upstream of Rac1 to aid apoptotic cell recognition and uptake by alveolar macrophages. | 190 | ||
| CRT | Exocytic? | CD91 | Conditions that can induce both apoptosis and ER stress can facilitate pre-apoptotic exposure of CRT. | 42,44,191 |
| ‘Don’t eat-me’ | ||||
| CD31 | N/A | CD31 | Homophilic interaction of CD31 on leukocytes and macrophages promotes cell detachment. How signalling-disabled CD31 are generated on apoptotic leukocytes is unknown. | 192 |
| CD47 | N/A | SIRP α | Besides functioning as a ‘don’t eat-me signal’, evidence also suggest that CD47 on apoptotic lymphocytes could aid apoptotic cell binding to macrophages. | 42,193 |
Nucleotides such as ATP and UTP have been identified as key mediators of phagocyte recruitment towards apoptotic cells in vitro and in vivo. This process requires caspase-mediated activation of pannexin 1 (PANX1) channels to release nucleotides from apoptotic cells16 and subsequent nucleotide detection by purinergic receptors (such as P2Y2 and possibility others) on monocytes and macrophages15. It is notable that nucleotides like ATP can also be secreted from cells via other active mechanisms (for example through exocytosis, autophagy-dependent and independent processes) and passive mechanisms (for example through membrane permeabilization). The release of ATP into the extracellular milieu can further modulate inflammation in a complex manner depending on its concentration, and how rapidly it is being degraded into immunosuppressive adenosine (discussed extensively in a recent review17). Lysophosphatidylcholine and sphingosine 1-phosphate have also been linked with monocyte recruitment to the proximity of apoptotic cells, but their in vivo relevance remains to be further defined18,19.
Small membrane vesicles released from apoptotic germinal centre B cells have been reported to enhance monocyte migration20. Consistent with this observation, certain molecules including intercellular adhesion molecule 3 (ICAM3) and a proteolytically processed form of CX3C-chemokine ligand 1 (CX3CL1; also known as fractalkine) were found to associate with apoptotic cell-derived microparticles and promote macrophage chemotaxis21,22. It is worth noting that phagocyte recruitment via microparticle-associated molecules could be a mechanism restricted to certain cell types that generate microparticles during apoptosis (for example, Burkitt lymphoma cells) and this area remains to be better explored.
In addition to attracting certain phagocytes, apoptotic cells are thought to release factors, referred to as ‘keep-out’ signals, to exclude inflammatory cells such as neutrophils (Table 1). Lactoferrin, a multifunctional glycoprotein, is the only keep-out signal discovered to date23, and its expression is upregulated in various cell types following induction of apoptosis. Lactoferrin is released by apoptotic cells and purified lactoferrin can inhibit neutrophil chemotaxis in vitro and in vivo, possibly by dampening neutrophil activation. Importantly, although it has also been shown to limit eosinophil recruitment24, lactoferrin had no effect on monocyte or macrophage migration towards the chemoattractant complement component C5a, demonstrating selectivity in inhibiting neutrophil and eosinophil migration23,24. It is important to note that there is limited information regarding the repertoire of find-me and keep-out signals released by different cell types during apoptosis. In fact, whether various find-me signals can function synergistically or additively to recruit phagocytes is not well defined. Nevertheless, it is intriguing to consider the possibility that apoptotic cells can release a unique combination of factors to control the recruitment of appropriate phagocytes for cell clearance and limit inflammation.
Specific recognition of apoptotic cells by phagocytes
Phagocytes identify apoptotic cells among healthy viable cells based on a unique combination of signals on the surface of apoptotic cells (Figure 1 and Table 1). Increased surface exposure of the inner-membrane lipid phosphatidylserine (PtdSer) is a common (but not exclusive) feature of apoptotic cells and functions as a key ‘eat-me’ signal to trigger phagocytic uptake25. The precise machinery that controls surface exposure of PtdSer during apoptosis is still being defined. However, recent studies suggest that both the calcium-dependent and calcium-independent activities of phospholipid scramblase disrupt the aminophospholipid asymmetry of the plasma membrane and promote PtdSer exposure during apoptosis26–28. It is worth noting that a substantial amount of PtdSer exposure is necessary for detection by phagocytes29.
Several PtdSer recognition mechanisms have been identified recently (Table 1). PtdSer can be detected directly via membrane receptors, such as brain-specific angiogenesis inhibitor 1 (BAI1)30, stabilin-231,32 and members of T cell immunoglobulin mucin domain (TIM) protein family (including TIM1, TIM3 and TIM4)33–35. Recognition of PtdSer by the seven span transmembrane protein BAI1 can signal through the evolutionarily conserved ELMO1–DOCK180–RAC complex to facilitate cytoskeletal rearrangement for engulfment (Figure 1)30. Similarly, stabilin-2 may interact with the adaptor protein GULP and thymosin β4 to initiate apoptotic cell uptake following PtdSer binding36,37. TIM4 seems to function primarily as a tethering protein for PtdSer and to signal through its associated proteins to promote engulfment38. In addition to these bona fide PtdSer receptors, bridging molecules, including milk fat globule-EGF factor 8 (MFG-E8), Protein S and Gas6, can bind PtdSer and are in turn recognized by their cell surface receptors on phagocytes, such as the integrin αVβ3 and the Tryo3–Axl–Mer (TAM) family of receptors39–41 (Table 1). It is intriguing that multiple PtdSer recognition mechanisms have been described for apoptotic cell clearance. It remains to be defined in mammals whether a particular mode of PtdSer recognition may be required only under specific conditions (for example, tissue development, homeostatic cell turnover, and inflammation) or whether these multiple mechanisms provide a degree of redundancy. Nevertheless, the growing availability of mice deficient in PtdSer recognition receptors and bridging molecules and in vivo models to assess the functional consequences of apoptotic cell removal are expected to help understand the need for such array of PtdSer sensing pathways.
In addition to PtdSer, surface exposure of calreticulin (CRT) on apoptotic cells may function as another eat-me signal. Induction of cancer cells to undergo apoptosis through mechanisms that also promote endoplasmic reticulum (ER) stress (such as anthracyclin treatment and photodynamic therapy) seems to result in rapid translocation of CRT from the ER to the plasma membrane42–45. Exposed CRT can subsequently be detected by CD91 (which is also known as low density lipoprotein (LDL)-receptor related protein) on phagocytes to stimulate engulfment42 (Figure 1). Notably, CRT exposure seems to trigger an immunogenic response against apoptotic cell-derived antigens, rather than inducing immunological tolerance43–45.
It is apparent that displaying certain eat-me signals alone may not be sufficient to trigger apoptotic cell engulfment42,46. For example, constitutive PtdSer exposure on viable lymphoma cells that express a mutant form of the scramblase TMEM16F (also known as Anoctamin 6) did not promote their uptake by peritoneal macrophages or CD8+ splenic DCs46. These observations support the idea that healthy viable cells, which can also expose PtdSer under physiological circumstances, might actively suppress phagocytic uptake by displaying ‘don’t eat-me’ signals such as CD31, CD47 and CD46 (Table 1). Engagement of CD47 (also known as integrin-associated protein) on viable cells with signal regulatory protein-α (SIRPα) on the macrophages can negatively regulate engulfment42,47,48, whereas redistribution or loss of CD47 during apoptosis may promote cell clearance42. Furthermore, loss of complement regulatory protein CD46 on various cell types during apoptosis can lead to complement opsonization49, a process that may aid phagocyte recognition. In addition, it remains to be fully investigated whether the exact configuration of PtdSer on the cell surface of live and apoptotic cells mediates differing signals. Collectively, exposure of a sufficient amount of eat-me signals and loss of don’t eat-me signals on the surface of apoptotic cells are necessary to trigger their removal by phagocytes.
Translating the final message
As cell death can arise under a variety of physiological and pathological conditions, including tissue development, homeostatic cell turnover, tissue injury, inflammation, tumorigenesis and infection, apoptotic cells might carry important and complex information for the regulation of the downstream immune response in a context-dependent manner. So, how do apoptotic cells convey such a diverse array of immunological information? Answering this question requires the consideration of all potential variables that occur with cell death.
The first parameter to consider is the quality of apoptotic cells, which determines what type of eat-me signals are being exposed to the immune system. Factors that can determine the quality of apoptotic cells include cell type, cause of cell death, and activation status of the dying cells. As discussed above, induction of apoptosis via certain anti-cancer drugs can render apoptotic tumour cells pro-immunogenic, whereas apoptosis during developmental or ‘homeostatic’ processes is largely anti-inflammatory and immunologically silent.
In addition, the quantity of apoptotic cells may determine the magnitude of signals. Although cell death in steady state tissues is easily and efficiently handled without inducing an immune response, large numbers of apoptotic cells such as those observed during infection or induced by anti-tumour therapies may overwhelm the engulfment capacity locally, and the uncleared cells or other components of these dying cells could induce a pro-immunogenic response. Another key parameter to consider is the apoptotic cell microenvironment, which determines what type of phagocyte is available to mediate clearance and regulate the subsequence immune response. This is particularly relevant for immune-privileged tissues such as the brain, eye and testes.
Finally, the timing of cell death and duration of apoptotic cell-derived signals may also contribute to the final immunological outcome. Thus, depending on the specific experimental condition, apoptotic cells may promote immunity or tolerance. It should also be noted that in certain instances, apoptotic cells can have a beneficial effect in tissue development and repair, as observed in myoblast fusion9 and wound healing50. A better characterization of the parameters of cell death and apoptotic cell clearance that influence immune activation might help us to understand certain disease states and to develop apoptotic cell-based or cell clearance-targeting therapeutic approaches.
Targeting apoptotic cell clearance for therapy
Apoptotic cells are rarely detected under physiological conditions, and the presence of uncleared apoptotic cells has been linked to several different diseases that involve inflection, inflammation, autoimmunity and cancer. In this section, we review the evidence that links defective cell clearance with the initiation and progression of pathology and discuss potential therapeutic implications (Figure 2 and Box 2).
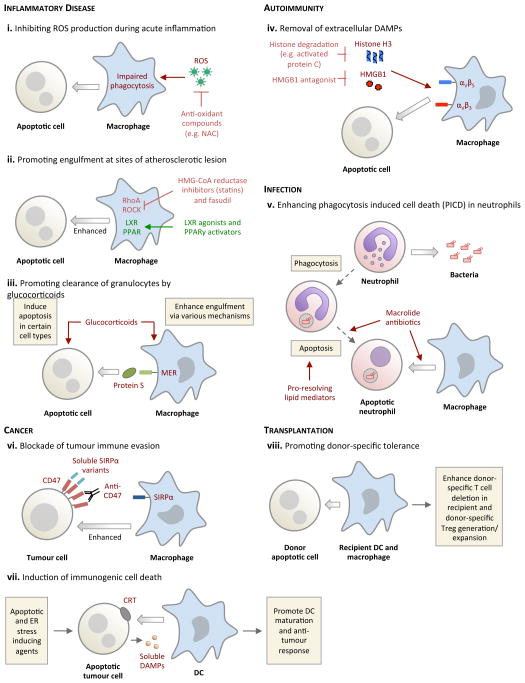
Figure 2. Potential approaches for targeting the apoptotic cell clearance process for therapeutic benefits.
a. Bacterial infection. Following phagocytosis of invading bacteria, neutrophils frequently undergo a form of ‘phagocytosis induced cell death’ (PICD) with subsequent engulfment by surrounding phagocytes, providing a second round of pathogen destruction. The engulfing phagocytes also increase production of pro-resolving lipid mediator release (for example RvD1) with enhanced host-directed bacterial killing. b. Acute inflammation. Reactive oxygen species (ROS) that are produced at sites of acute inflammation impair phagocytosis through the activation of RhoA within phagocytes. Scavenging ROS (for example by using N-acetylcystein; NAC) enhances apoptotic cell clearance. c. Glucocorticoids can potentially augment eosinophil clearance by promoting both eosinophil apoptosis and cell clearance via a protein S–Mer-dependent pathway. d. Atherosclerosis. Impaired engulfment in atherosclerosis is, in part, mediated by increased activity of RhoA and its downstream mediator ROCK, both of which are negative regulators of apoptotic cell engulfment. RhoA inhibition by HMG-CoA reductase inhibitors (statins) or ROCK inhibition seems to have a beneficial effect in atherosclerosis, possibly by regulating engulfment. e. Rheumatoid arthritis. At sites of inflammation, extracellular damage-associated molecular patterns (DAMPs) such as histones and HMGB1 negatively regulate apoptotic cell engulfment by binding to integrins on the surface of phagocytes. Strategies to degrade DAMPs (for example through the degradation of histone H3 by antigen presenting cells (APCs)) can improve apoptotic cell clearance. f. Recognition and uptake of donor apoptotic cells by recipient DCs and macrophages can promote donor-specific tolerance in the recipients and limit allograft rejection. g. Induction of tumour cell death accompanied with CRT and DAMPs exposure can promote DC-mediated engulfment and DC maturation to initiate an anti-tumour immune response. h. Targeting tumour cells with anti-CD47 blocking antibodies or soluble SIRPα variants inhibits CD47-SIRPα interaction and facilitates tumour cell removal by macrophages.
Box 2. Endogenous controllers of apoptotic cell clearance.
During the spontaneous resolution of an episode of inflammation, locally produced molecules modulate apoptotic cell clearance. These include the specialised pro-resolving lipid mediators lipoxins (which are generated from arachidonic acid), resolvins and protectins (which are generated from omega-3 fatty acids)159. Although these different classes of pro-resolving lipids are distinct in both their production and biological effects, they all reduce neutrophil recruitment and enhance clearance of apoptotic cells.
Apoptotic neutrophils enhance the production of pro-resolving lipid mediators by macrophages during their engulfment160. The benefits of these molecules have been shown in animal models of inflammation including asthma161, lung injury62,162 and colitis163. Furthermore, emerging evidence has demonstrated that they also contribute to anti-microbial defence during bacterial infection164. There is also evidence that pro-resolving lipid production may be deficient in human inflammatory disease165 although this is an area requiring further study. Despite the locally acting and short-lived nature of these lipid mediators, structural analogues with longer half-lives have been developed, and pro-resolving lipid mediators are undergoing early clinical trials in humans (ClinicalTrials.gov; NCT01675570 and NCT01639846).
Lysophosphatidylserine (lysoPS), a lipid exposed on apoptotic cells (particularly neutrophils) in a NADPH oxidase-dependent manner has been shown to enhance macrophage engulfment166,167. This may partly explain why patients with chronic granulomatous disease (CGD) that lack a functional NADPH oxidase have defects in apoptotic cell clearance and a hyper-inflammatory phenotype with a propensity for autoimmune disease168.
Tissue resident macrophages express 12/15-lipoxygenase that can generate oxidised phosphatidylethanolamine (oxPE) on their cell membranes169. oxPE has been shown to bind soluble apoptotic cell-bridging molecule MFG-E8 and thus prevent uptake of apoptotic cells by recruited inflammatory monocytes169. This binding and sequestering of MFG-E8 by oxPE does not inhibit the engulfment of apoptotic cells by tissue-resident macrophages, which predominantly recognize apoptotic cells through the PtdSer receptor T cell immunoglobulin domain and mucin domain protein 4 (TIM4). Lack of 12/15-lipoxygenase results in apoptotic cell clearance by inflammatory monocytes or macrophages, with the subsequent presentation of apoptotic cell-derived intracellular antigens and development of autoimmunity with glomerulonephritis169. Whether defects in lysoPS production or in the control of 12/15-lipoxygenase activity are defective in human disease is currently unknown, but the targeting and mimicking of endogenous controllers of apoptotic cell clearance is an attractive therapeutic avenue.
Box 2.
Apoptotic cells as a potential therapeutic intervention
Infection
In response to an acute episode of infection or tissue injury, tissue-resident cells (both immune and parenchymal) detect pathogen-associated molecular patterns (PAMPs), including bacterial endotoxin and viral nucleic acids, as well as damage-associated molecular patterns (DAMPs), the latter being mainly intracellular proteins and substrates released upon cell death51. As a consequence, leukocytes are recruited to the site of inflammation, with innate immune cells such as neutrophils often being the first cells to appear and accumulation of mononuclear cells and macrophages occurring at a later stage52. This initial robust immune response is a beneficial one, designed to contain and destroy invading pathogens and enhance repair of tissue function53,54. Once the initial threat has been eliminated, leukocyte recruitment ceases and the already recruited cells are disposed of to restore homeostasis. Although recruited neutrophils can be cleared through transepithelial migration into the airway lumen in the context of lung inflammation55, or through migration via lymphatic vessels56, it appears that a main clearance route is by local neutrophil apoptosis and subsequent phagocytic clearance57,58. Both tissue resident and recruited macrophages, as well as local epithelial cells, can ingest apoptotic leukocytes59.
Following neutrophil recruitment into infected tissue, exposure to bacterial-derived products initially enhances the lifespan of neutrophils. However, the phagocytosis of pathogens, such as Escherichia coli or Staphylococcus aureus, promotes a form of apoptotic cell death of neutrophils that is termed phagocytosis-induced cell death (PICD)60 (Figure 2). This response is believed to be primarily protective for the host, allowing a second round of destruction of pathogens that might remain within the engulfed apoptotic neutrophils. Incidentally, pharmacological acceleration of neutrophil apoptosis is protective in pneumococcal meningitis with accelerated recovery and reduced brain haemorrhage61. The production of small, locally acting, endogenous lipid-derived autacoids that promote the removal of apoptotic cells (termed pro-resolving lipids) are also involved in limiting infection-associated inflammation (Box 2). The pro-resolving lipid Resolvin E1 has recently been demonstrated to promote PICD and thereby enhance the resolution of bacterial infection in mice62.
However, pathogens can also utilise engulfment machinery for their benefit: phagocytosis of apoptotic neutrophils infected with the intracellular pathogen Chlamydia pneumoniae has been shown to result in the subsequent infection of macrophages63. This ‘Trojan horse’ strategy adopted by C. pneumoniae increases its virulence and replication when compared to direct infection of macrophages. Moreover, bacteria can enter a cell using cytoplasmic proteins that are also involved in engulfment of apoptotic cells; for example, IpgB1 of Shigella flexneri induces membrane ruffling via ELMO1 activation, promoting bacterial invasion of epithelial cells64. Therefore, enhancing the activity of the engulfment machinery or neutrophil apoptosis in specific infections to mediate pathogen clearance is an exciting possibility, but more investigation is needed.
Lung inflammation
Impaired or defective clearance of dying neutrophils during inflammation can lead to a prolonged inflammatory response. Although the best evidence for this has come from animal studies, such a phenomenon has also been observed in human disease, including chronic obstructive pulmonary disease (COPD)65, pulmonary fibrosis66 and cystic fibrosis67. The mechanism underlying impaired phagocytosis in inflammation involves, in part, the production of reactive oxygen species (ROS) by neutrophils (Figure 2). ROS activate the GTPase RhoA (a negative regulator of efferocytosis; a term that has also been used to describe the phagocytic clearance of apoptotic cells) in surrounding phagocytes, thereby reducing apoptotic cell engulfment by neighbouring cells68–70. Interestingly, antioxidants such as the thiol compound N-acetylcysteine (NAC) promote clearance of apoptotic cells by macrophages during lipopolysaccharide (LPS)-mediated lung inflammation in mice by inhibiting both ROS production and RhoA activity and NAC can also enhance transforming growth factor-β (TGFβ) production71. However, anti-oxidant therapy in humans with acute lung injury and acute respiratory distress syndrome has thus far provided no convincing evidence of efficacy72. Perhaps the drugs fail to reach the relevant phagocytes, or such therapies need to be targeted to specific subgroups of patients with this disorder.
Although alveolar macrophages from adult patients with asthma of mild to moderate severity have normal phagocytic capacity, those from patients with severe asthma are defective in clearing apoptotic cells73. Similarly, alveolar macrophages from children with poorly controlled asthma have defective phagocytosis74. The molecular events causing defective phagocytosis in patients with severe asthma are not yet understood, but it is relevant to note that corticosteroids, the mainstay of treatment in asthma, not only induce eosinophil apoptosis75 but also enhance eosinophil engulfment by monocyte-derived macrophages in vitro76. This corticosteroid-induced enhanced clearance depends on the binding of protein S to apoptotic cells and the upregulation of Mer tyrosine kinase (a member of the TAM family) on the surface of macrophages77 (Figure 2). Furthermore, enhanced clearance of apoptotic eosinophils by macrophages has been observed in asthmatic humans after steroid therapy78. Steroid treatment appears less effective in neutrophil-dominated lung inflammatory disorders and the ability of steroids to induce neutrophil apoptosis seems to be context dependent79,80. In addition to alveolar macrophages and lung-associated DCs, airway epithelial cells have been recently reported to engulf neighbouring apoptotic cells, and a defect in this process increases the production of pro-inflammatory mediators and exacerbates airway inflammation5. With this increased evidence of defective apoptotic cell clearance in lung inflammatory diseases, and the mechanisms behind the therapeutic benefits of commonly used anti-inflammatory medications such as corticosteroids, it is hoped that novel approaches for targeting inflammatory diseases (within the lung and in other tissues) are on the horizon.
Atherosclerosis
Atherosclerosis is one of the leading causes of death in western societies, and its pathogenesis involves chronic inflammation of the vascular wall, predominantly as a result of the accumulation of mononuclear immune cells81. Monocytes and macrophages have a crucial role in the initiation and progression of atherosclerosis. Although there are resident macrophages in the arterial wall, recruitment of Ly6C+ inflammatory monocytes and Ly6C− ‘patrolling’ monocytes, and their differentiation into macrophages and DCs are thought to critically influence atherosclerosis82. After taking up various oxidized lipids in the intima, lipid-laden macrophages undergo apoptosis and can be engulfed by surrounding macrophages82. In the early stages of atherosclerosis, apoptosis in the vascular walls seems to be counterbalanced by rapid and efficient engulfment82. However, in mature atherosclerotic lesions (known as plaques), there is reduced clearance of apoptotic cells and progression to secondary necrosis (Box 1), which coincides with plaque lesion expansion and an increased risk of rupture83. Such plaque rupture leads directly to acute coronary syndromes and stroke in humans.
This reduction in apoptotic cell clearance observed in mature plaques appears central to the pathological process of plaque progression84, as defects in the phagocytic components such as Mer, MFG-E8, or the complement component C1q results in the accumulation of apoptotic debris within plaques and accelerates atherosclerosis85–88. Conversely, induction of apoptosis within plaques by a physiological stimulus (for example through TNF-related apoptosis-inducing ligand (TRAIL)), has been shown to be beneficial and atheroprotective89, which is thought to be due to increased anti-inflammatory signalling within this microenvironment. Therefore, defective macrophage engulfment, and in turn, a more pro-inflammatory state, seems to drive accelerated atherosclerosis. Importantly, healthy phagocytes release anti-inflammatory cytokines that dampen inflammation following apoptotic cell clearance82 and may help control atherosclerosis progression.
But, why do human macrophages in situ lose the ability to rapidly clear dead cells? Although the reasons for this are incompletely understood and likely multifactorial, several possible mechanisms have been suggested. Oxidised lipoproteins, which are present in plaques in vivo, inhibit efferocytosis in vitro by binding to CD1490. In addition, the activity of Rho kinase is reported to be increased in atherosclerotic lesions91. Interestingly, the widely used HMG-CoA reductase inhibitors (also known as statins), which are principally used as cholesterol lowering agents in atherosclerosis and vascular disease, have long been thought to have additional anti-inflammatory effects partly due to enhancement of the phagocytic activity of macrophages92. The mechanism underlying enhanced phagocytosis induced by statins involves RhoA inhibition93 (Figure 2). RhoA activates the Rho-associated protein kinases (ROCKs), which have an inhibitory effect on engulfment. Interestingly, the ROCK inhibitor Fasudil inhibits both the early development and late progression of atherosclerosis in mice94, although these beneficial effects have not yet been linked to enhanced efferocytosis. Statins and ROCK inhibitors also display anti-inflammatory effects in other non-vascular pre-clinical models of inflammation including acute lung injury95, bleomycin-induced pulmonary fibrosis96 and inflammatory arthritis97, although a direct link between these effects and phagocytosis of dying cells is not yet established.
Uptake of apoB-containing lipoproteins by macrophages that accumulate within the vascular walls, which leads to foam cell formation, is a key early event in atherosclerosis98,99. Improved cholesterol efflux from foam cells can revert this stage of atherosclerosis leading to macrophage egress and a reduction in lesion size100. It was previously shown that when macrophages engage apoptotic cells (but not necrotic cells), cholesterol efflux is stimulated from the engulfing macrophages101. The enhanced cholesterol efflux by macrophages occurs through upregulation of mRNA and protein for the cholesterol transporter ABCA1101. ABCA1 is an important molecule in macrophage cholesterol efflux and transports free cholesterol from within the cells to lipid-poor apoA1 that is then modified in the plasma for transport to the liver and excretion102,103. Loss of ABCA1 promotes atherogenesis, whereas overexpression of ABCA1 reverses the disease104,105. Recent reports suggest that ABCA1 upregulation and signalling downstream of ABCA1 (after binding to apoA1) can also dampen macrophage inflammatory responses81,106,107. Thus, ‘foamy’ macrophages undergoing necrosis in late-stage lesions might fail to upregulate ABCA1 expression, thereby preventing the cholesterol efflux and ABCA1-mediated immuno-suppressive effects81,106,107. The precise phagocytic receptor(s), which upon apoptotic cell recognition would induce the upregulation of ABCA1, and in turn the cholesterol efflux from the phagocytes are not yet defined.
Other downstream modulators of the phagocyte response to ingested apoptotic cells include activation of the nuclear receptors liver X receptors (LXRs) and peroxisome proliferator-activated receptors (PPARs), which can act as positive regulators of engulfment by upregulating Mer expression108 (Figure 2). LXR activation by synthetic agonists has been demonstrated to have beneficial effects in animal models of atherosclerosis109. In addition, activators of PPARs have already been approved for clinical use in the treatment of diabetes, as they have been shown to enhance murine macrophage efferocytosis110 and reduce progression of atherosclerosis in humans in a glucose homeostasis-independent manner111. Therefore such treatments may have additional beneficial effects via promotion of apoptotic cell clearance.
Autoimmunity
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disorder with a variable clinical presentation commonly affecting skin, lungs, kidneys and the central nervous system. It is characterised by the presence of auto-antibodies that are specific for nuclear components and, frequently, with the detection of DNA and nucleosomes in the circulation112. In patients with SLE, there is increased spontaneous appearance of apoptotic cells within lymph nodes and blood, and accumulation of apoptotic cells within the skin following UV exposure113,114. This increase in apoptotic cells observed in SLE is thought to reflect an impaired ability of SLE phagocytes to engulf dead cells, rather than an intrinsic alteration in the apoptotic programme115. In mice lacking MFG-E8, an accumulation of apoptotic lymphocytes within lymph nodes and development of an SLE-like disease that involves autoantibody formation, splenomegaly and glomerulonephritis are observed116. Genetic polymorphisms and aberrant splicing of MFG-E8 have been reported in a small subsets of patients with SLE, suggesting that this pathway of apoptotic cell clearance might be dysregulated in a subset of human SLE patients117,118. Such impaired clearance of apoptotic cells eventually leads to secondary necrosis, which allows intracellular antigens, normally compartmentalised within an apoptotic cell, to gain access to the extracellular environment. This presumably increases the risk of such antigens being recognised as non-self with production of auto-antibodies and consequent autoimmunity. Complement proteins also have a key role in apoptotic cell clearance and development of autoimmunity, and deficiencies in complement components, especially C1q, are highly associated with SLE119,120.
It is notable that not all defects in apoptotic cell clearance seem to result in autoimmunity: the absence of CD14 or mannose binding lectin (MBL) in mice leads to defective apoptotic cell engulfment and their accumulation in tissues, but does not have any pro-inflammatory or autoimmune consequences121,122. Whether apoptotic cells in these deficient mice can still provide a downstream immunomodulatory signal to prevent autoimmunity, despite not being engulfed, requires further investigation. If apoptotic cell engagement alone by a phagocyte can be beneficial in ameliorating autoimmunity, harnessing those features could offer a therapeutic modality even in the context of continued defective apoptotic cell clearance.
Rheumatoid arthritis is a chronic systemic inflammatory disease associated with progressive joint destruction. It is a systemic autoimmune disease with most individuals having circulating autoantibodies against citrullinated peptides. Although there is little direct evidence that human inflammatory arthritis is caused by defects in cell clearance123,124, extracellular debris present at sites of inflammation such as oxidised lipids and intracellular components including HMGB1, histone H3 and histone H4 are negative regulators of efferocytosis125. Histone H3 binds to macrophages, most likely by the binding to αvβ5 integrins, which decreases uptake in vitro and in vivo. This reduced engulfment by histones can be reversed by administration of activated protein C which causes degradation of histones125 (Figure 2). Similarly, HMGB1 reduces phagocytosis both by the binding to and masking of PtdSer on apoptotic neutrophils as well as binding the αvβ3 integrin on phagocytes126 (Figure 2). Furthermore, increasing the levels of TAM receptor-agonists protein S and Gas-6127, LXR agonists128 and PPARγ activators129 have shown therapeutic benefits in mouse models of inflammatory arthritis. Whether such agents have benefits in human disease requires further work, with a recently concluded human trial of PPARγ agonists in rheumatoid arthritis awaiting reporting (ClinicalTrials.gov; NCT00554853).
Transplantation
Prescription of immunosuppressive drugs to patients following transplantation is often necessary to delay or prevent allograft rejection. However, such immunosuppressive medications exhibit numerous side effects and may not be effective for long-term allograft survival. Apoptotic cells carry self-antigens and actively dampen immunity and apoptotic cell-based therapy has been developed to limit allograft rejection by promoting immunological tolerance towards donor organs, tissues or cells130 (Box 3). Inoculation of apoptotic cells from the donor in transplant recipients, prior to or during transfusion or transplantation, seems to improve donor cell engraftment and solid allograft survival in a variety of murine models131–134 (Figure 2). Depending on the experimental system, it was shown that recipient DCs and/or macrophages are necessary to mediate the apoptotic cell-induced tolerogenic effects on engraftment134,135. Masking the PtdSer on apoptotic cells failed to provide the same benefit, suggesting that recognition and uptake of donor apoptotic cells is necessary induce allograft tolerance132. Mechanistically, uptake of donor apoptotic cells by splenic DCs was found to promote the generation/expansion of CD4+ FOXP3+ regulatory T cells and deletion of alloreactive CD4+ T cells, representing a potential mechanism to reduce the risk of transplant rejection133. The potential use of apoptotic cell-based therapy to induce immune tolerance towards certain antigens has implications beyond transplantation, in particular the treatment of autoimmune diseases (Box 3).
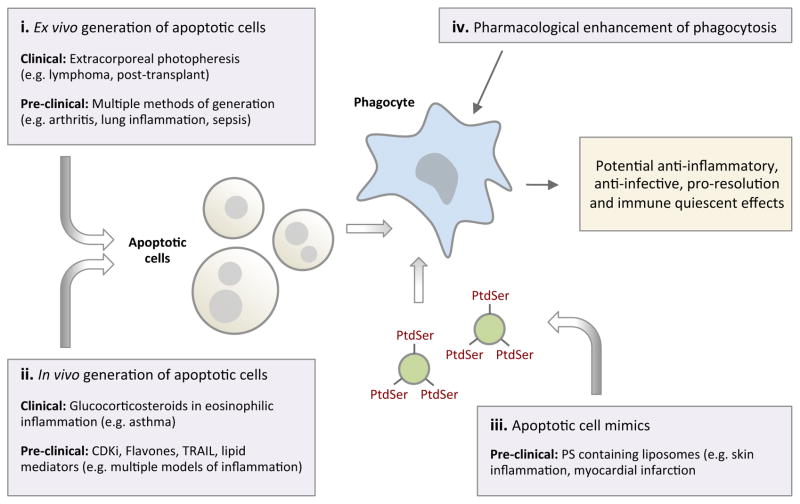
Box 3. Apoptotic cells as a potential therapeutic intervention.
Apoptotic cells or apoptotic cell mimics have immunomodulatory functions and their administration could be used as a potential therapeutic intervention. Extracorporeal photopheresis, where leukocytes are made apoptotic ex vivo prior to systemic re-administration, is already an accepted treatment for cutaneous T cell lymphoma in humans and has shown benefits in transplant rejection, graft-versus-host disease and autoimmune disorders170.
Although the molecular events underlying the potential immune regulating function of apoptotic cells are less clear, transforming growth factor-β (TGFβ)-dependent expansion of regulatory T cells as well as changes in macrophage phenotype have been implicated in apoptotic cell-mediated immune modulation134,171. Administration of cells made apoptotic ex vivo has been shown to reduce both the acute and chronic phases of inflammatory arthritis in rodents172 by reducing the levels of tumour necrosis factor (TNF) (which negatively regulates apoptotic cell clearance)173 and enhancing the production of TGFβ and the generation of regulatory T cells172. Local administration of apoptotic cells has also been used to attenuate both bleomycin- and lipopolysaccharide (LPS)-induced lung inflammation, with reduced neutrophil recruitment into the lung, enhanced phagocytosis by alveolar macrophages, reduced pro-inflammatory cytokine production and increased TGFβ production after apoptotic cell delivery174,175. Infusion of apoptotic cells 24 hour post initiation of sepsis has also been shown to protect against lethality in a mouse model of sepsis with reduced pro-inflammatory cytokines and neutrophil recruitment into organs176. At least part of the beneficial effect in the sepsis model was mediated by the direct binding of LPS by apoptotic cells, which led to the recognition and clearance of LPS-covered apoptotic cells by macrophages in an anti-inflammatory manner176.
However the therapeutic use of apoptotic cells needs to be carefully considered in cases where the capacity for apoptotic cell engulfment is reduced in vivo, as administered cells may progress into secondary necrosis, which could exacerbate inflammation or autoimmunity. Notably, macrophages that ingest necrotic cells cause increased T cell proliferation177. Moreover, the repeated administration of apoptotic cells can lead to autoimmunity178. Whether apoptotic cell mimics, such as PtdSer-containing liposomes, can deliver the benefits of apoptotic cells without risking autoimmunity awaits further investigation, but this strategy has already been used to improve skin oedema and post-myocardial infarct repair in mice179,180. In addition, strategies that generate apoptotic cells in situ in models of inflammation have shown potential: therapeutic agents with cyclin-dependent kinase inhibitors181, flavones182 and the death receptor ligand TRAIL183 have all demonstrated benefits in models of inflammation. Furthermore, combined delivery of apoptotic cells with enhancers of phagocytosis may be required for full therapeutic efficacy to prevent secondary necrosis of apoptotic cells.
Cancer
High levels of cell death can occur within a tumour milieu, and the mechanisms through which dying tumour cells are cleared can profoundly influence tumour-specific immunity. Thus, manipulation of the immunological context of dying cell removal has a great potential for the control of tumour progression and generation of an anti-tumour response136. One possible approach to promote anti-tumour immunity is by counteracting the immunosuppressive properties of apoptotic cells. Supporting this notion, interfering with PtdSer-mediated recognition of dying cells by masking PtdSer with annexin V favours an anti-tumour response, possibly by delaying apoptotic cell clearance and bias the type of phagocyte (for example DCs) that mediates cell clearance137. However, it is important to note that blocking apoptotic cell engulfment may promote sterile inflammation through the release and exposure of DAMPs by uncleared secondary necrotic cells (Box 1). Chronic inflammation that results from this approach might conversely favour tumour growth138,139, as well as autoimmunity115, and needs to be considered carefully.
An alternative approach to promote anti-tumour immunity is by triggering an immunogenic form of tumour cell death through specific cell death inducers. The ability of certain chemotherapeutic drugs such as doxorubicin (an anthracycline) to augment anti-tumour immunity via a caspase-, DC- and CD8+ T cell-dependent mechanism140, was found to depend on the molecular machinery for dying cell clearance44,45. Anthracyclines seem to promote exposure of the eat-me signal CRT on tumour cells, and blockade of CRT exposure on anthracycline-treated tumour cells can markedly reduce DC-mediated cell clearance and anti-tumour immunity44 (Figure 2). Importantly, supplying exogenous CRT to dying tumour cells that cannot display endogenous CRT enhanced their phagocytosis by DCs and their immunogenicity44. In addition to CRT, other DAMPs that are released by dying tumour cells, such as HSP90 and HMGB1, can promote anti-tumour immunity through a DC-dependent process141,142.
Cancer cells often hijack a variety of normal cellular processes to enable survival and expansion in an organism. Recently, the ability of tumour cells to upregulate the don’t eat-me signal CD47 to evade recognition and engulfment by phagocytes was found in many mouse myeloid leukemias, as well as in patients with myeloid proliferative diseases (including acute myeloid leukemia and myeloid blast crisis phase chronic myeloid leukemia)143. Importantly, higher expression of CD47 in patients with acute myeloid leukemia, non-Hodgkin lymphoma, ovarian cancer, glioma and glioblastoma correlated with poor prognosis143–145, indicating a link between CD47 upregulation and tumorigenicity. Consistent with the function of CD47 as a don’t eat-me signal through interaction with macrophage SIRPα, ectopic expression of CD47 in a CD47low acute myeloid leukemia cell line was reported to increase tumour cell survival by limiting their engulfment by macrophages143. Importantly, CD47 blockade using a CD47-specific antibody in mice that had received CD47high tumour cells from human patients reduced tumour engraftment, growth and metastasis, thereby indicating therapeutic potential for CD47-targeting in cancer144–147 (Figure 2). Recently, combination therapy using tumour-specific monoclonal antibodies (for example rituximab and trastuzumab) and soluble SIRPα variants that can antagonize CD47 function exhibit synergistic effect in promoting engulfment of tumour cells by macrophages and regression of tumour growth in mouse models147.
Taken together, the evidence suggests that manipulation of the apoptotic cell clearance process by delaying apoptotic cell recognition and removal, inducing immunogenic cell death, and targeting cell clearance machinery hijacked by tumour cells, can effectively promote anti-tumour immunity. However, challenges remain in validating these novel therapeutic approaches clinically. Moreover, whether other aspects of the apoptotic cell clearance process, such as phagocyte recruitment and formation of apoptotic bodies and microparticles, can be modulated to control tumour progression warrants further investigation.
Concluding remarks
It has been suggested that prompt and efficient clearance of apoptotic cells is the ultimate goal of the apoptotic program, as well as a key process that can prevent inflammation and maintain self-tolerance under physiological conditions. Accumulating evidence suggests that clearance of apoptotic cells is impaired in multiple human disease processes, with mounting evidence indicating that the defect in apoptotic cell clearance is directly involved in driving disease pathogenesis in several different contexts. Furthermore, over the past several years, a number of exciting discoveries have been made on the molecules and mechanisms regulating apoptotic cell clearance from model organisms to humans.
What is particularly revealing is that several steps within the process can be beneficial: the molecules exposed on the apoptotic cells themselves appear to provide important differentiation signals; the phagocytic recognition step induces several anti-inflammatory mediators that dampen the immune response; the actual corpse internalization process appears to help limit certain infections; depending on the type of apoptosis induction and the type of phagocyte, the engulfment process can also be made pro-immunogenic in specific conditions. This combined knowledge opens new avenues in therapeutic intervention for both dampening inflammation in specific autoimmune or inflammatory disease states and promoting effective immune responses against tumour-derived antigens. The next challenge for the field is in harnessing the benefits of apoptotic cell clearance process for human therapies.
Glossary
- Professional and non-professional phagocytes
Professional phagocytes such as macrophages and immature dendritic cells can efficiently detect and engulf pathogens and dying cells. Non-professional phagocytes including fibroblasts, epithelial cells, and endothelial cells can also engulf a variety of particles including their dying brethren, but their primary function is not phagocytosis
- Plasma membrane bleb
A globular protrusion seen at the plasma membrane. Membrane blebs are dynamic and can occur during cell migration, cytokinesis, and apoptosis
- Apoptotic bodies
Subcellular fragments released from apoptotic cells that are approximately 1–5 μm in size. Apoptotic bodies are non-uniform membrane-bound particles that contain portions of cytoplasm and fragmented organelles
- Focal adhesion
A macromolecular complex that functions as a structural link between the cell and the extracellular matrix. Components of focal adhesion are also important for regulating intracellular signalling
- Adherens junction
An inter-cellular macromolecular complex that mediates cell-cell adhesion. Cadherin and catenin are key components of adherens junction
- Germinal centre
A lymphoid structure that arises within follicles after immunization with, or exposure to, a T-cell-dependent antigen. It is specialized for facilitating the development of high-affinity, long-lived plasma cells and memory B cells
- Apoptotic cell-derived microparticles
Another category of subcellular fragments released from apoptotic cells that are approximately 0.1–1 μm in size. Apoptotic cell-derived microparticles and apoptotic bodies represent a spectrum of membrane-bound apoptotic vesicles characterized mainly by size and density
- Aminophospholipid asymmetry of the plasma membrane
Distribution of aminophospholipids (such as PtdSer, phosphatidylethanolamine and phosphatidylcholine) between the outer and inner leaflet of the plasma membrane is often asymmetrical, and may differ depending on the cell type, activation status, and viability. This asymmetry is actively maintained by ATP-dependent processes, and compromised by activation of phospholipid scramblases
- Endoplasmic reticulum stress (ER stress)
A response by the ER that results in the disruption of protein folding and in the accumulation of unfolded proteins in the ER
- Photodynamic therapy
A treatment that uses a combination of a specific wavelength of light and a photosensitizing agent to induce the production of reactive oxygen species and cause lethal damage to the cells
- Pathogen-associated molecular patterns (PAMPs)
Molecular signatures that are found in pathogens but not in mammalian cells. Examples include terminally mannosylated and polymannosylated compounds (which bind the mannose receptor) and various microbial components, such as bacterial lipopolysaccharide, hypomethylated DNA, flagellin and double-stranded RNA (all of which bind Toll-like receptors)
- Damage-associated molecular patterns (DAMPs)
As a result of cellular stress, cellular damage and non-physiological cell death, DAMPs are released from the degraded stroma (for example, hyaluronate), from nucleus (for example, high-mobility group box 1 protein, HMGB1), from the cytosol (for example, ATP, uric acid, S100 calcium-binding proteins and heat-shock proteins) and from mitochondria (formylated peptides and mitochondrial DNA). Such DAMPs are thought to elicit both local and systemic inflammatory reactions
- Chronic obstructive pulmonary disease (COPD)
A group of diseases characterized by the pathological limitation of airflow in the airway, including chronic obstructive bronchitis and emphysema. Is most often caused by tobacco smoking, but can also be caused by other airborne irritants, such as coal dust, and occasionally by genetic abnormalities, such as α1-antitrypsin deficiency
- Pulmonary fibrosis
A heterogenous group of disorders characterised by diffuse abnormalities of the pulmonary interstitium, with increased and variable inflammation and fibrosis. Frequently of unknown aetiology, pulmonary fibrosis can also be related to autoimmune disease and secondary to medications
- Cystic fibrosis
An autosomal recessive genetic condition secondary to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR; a chloride channel). This leads to a multisystem disorder with lung, gastrointestinal, endocrine and fertility complications. Chronic infection of the lungs ensues leading to significant morbidity and mortality
- Intima
The innermost layer of an artery, which consists of loose connective tissue and is covered by a monolayer if endothelium. Atherosclerotic plaques form in the intima
- C1q
A complement protein and component of the classical complement pathway. Involved in diverse functions including immune function, autoimmunity and clearance of apoptotic cells
- Statins
A family of inhibitors targeting hydroxymethylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyses the conversion of HMG-CoA to L-mevalonate. These molecules are mainly used as cholesterol-lowering drugs, but they also have immunoregulatory and anti-inflammatory properties. L-Mevalonate and its metabolites are implicated in cholesterol synthesis and other intracellular pathways
- Foam cell
A macrophage in the arterial wall that ingests oxidized low-density lipoprotein and assumes a foamy appearance. These cells secrete various substances contributing to plaque growth
- Cross-presentation
A process that describes the ability of antigen-presenting cells to display a peptide fragment from exogenous antigen, via MHC class I molecules, to CD8+ T cells
- Organelle fragmentation
A process during apoptosis that aids disassembly of organelles into smaller portions. Organelle fragmentation is driven by caspase-mediated cleavage of certain proteins and actomyosin contraction
References
- 1.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–17. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood W, et al. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127:5245–52. doi: 10.1242/dev.127.24.5245. [DOI] [PubMed] [Google Scholar]
- 3.Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10:857–60. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]
- 4.Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod. 2008;78:586–94. doi: 10.1095/biolreprod.107.065045. [DOI] [PubMed] [Google Scholar]
- 5.Juncadella IJ, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493:547–51. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Sebbagh M, et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–52. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 8.Coleman ML, et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–45. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 9.Hochreiter-Hufford AE, et al. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–7. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levkau B, Herren B, Koyama H, Ross R, Raines EW. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J Exp Med. 1998;187:579–86. doi: 10.1084/jem.187.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kook S, et al. Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol Biol Cell. 2000;11:929–39. doi: 10.1091/mbc.11.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmeiser K, Grand RJ. The fate of E- and P-cadherin during the early stages of apoptosis. Cell Death Differ. 1999;6:377–86. doi: 10.1038/sj.cdd.4400504. [DOI] [PubMed] [Google Scholar]
- 13.Brancolini C, Lazarevic D, Rodriguez J, Schneider C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J Cell Biol. 1997;139:759–71. doi: 10.1083/jcb.139.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–57. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 15.Elliott MR, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chekeni FB, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–7. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krysko DV, et al. Immunogenic cell death and DAMPs in cancer therapy. Nature reviews. Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 18.Lauber K, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 19.Gude DR, et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 2008;22:2629–38. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segundo C, et al. Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: role of apoptotic blebs in monocyte chemotaxis. Blood. 1999;94:1012–20. [PubMed] [Google Scholar]
- 21.Torr EE, et al. Apoptotic cell-derived ICAM-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death Differ. 2012;19:671–9. doi: 10.1038/cdd.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truman LA, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–36. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 23.Bournazou I, et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bournazou I, Mackenzie KJ, Duffin R, Rossi AG, Gregory CD. Inhibition of eosinophil migration by lactoferrin. Immunology and cell biology. 2010;88:220–3. doi: 10.1038/icb.2009.86. [DOI] [PubMed] [Google Scholar]
- 25.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 26.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bratton DL, et al. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272:26159–65. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341:403–6. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- 29.Borisenko GG, et al. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells--existence of a threshold. Arch Biochem Biophys. 2003;413:41–52. doi: 10.1016/s0003-9861(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 30.Park D, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–4. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 32.Park SY, Kim SY, Jung MY, Bae DJ, Kim IS. Epidermal growth factor-like domain repeat of stabilin-2 recognizes phosphatidylserine during cell corpse clearance. Mol Cell Biol. 2008;28:5288–98. doi: 10.1128/MCB.01993-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi N, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–40. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–9. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama M, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–30. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, So IS, Park SY, Kim IS. Thymosin beta4 is involved in stabilin-2-mediated apoptotic cell engulfment. FEBS Lett. 2008;582:2161–6. doi: 10.1016/j.febslet.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 37.Park SY, et al. Requirement of adaptor protein GULP during stabilin-2-mediated cell corpse engulfment. J Biol Chem. 2008;283:10593–600. doi: 10.1074/jbc.M709105200. [DOI] [PubMed] [Google Scholar]
- 38.Toda S, Hanayama R, Nagata S. Two-step engulfment of apoptotic cells. Mol Cell Biol. 2012;32:118–25. doi: 10.1128/MCB.05993-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson HA, et al. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003;4:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- 40.Hanayama R, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 41.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem. 2000;127:411–7. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- 42.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Garg AD, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–79. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 45.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual review of immunology. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 46.Segawa K, Suzuki J, Nagata S. Constitutive exposure of phosphatidylserine on viable cells. Proc Natl Acad Sci U S A. 2011;108:19246–51. doi: 10.1073/pnas.1114799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 48.Okazawa H, et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174:2004–11. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 49.Elward K, et al. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J Biol Chem. 2005;280:36342–54. doi: 10.1074/jbc.M506579200. [DOI] [PubMed] [Google Scholar]
- 50.Li F, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 52.Serhan CN, et al. Resolution of inflammation: state of the art, definitions and terms. Faseb J. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zemans RL, et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:15990–5. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farnworth SL, et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Persson CG, Uller L. Resolution of cell-mediated airways diseases. Respir Res. 2010;11:75. doi: 10.1186/1465-9921-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beauvillain C, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood. 2011;117:1196–204. doi: 10.1182/blood-2009-11-254490. [DOI] [PubMed] [Google Scholar]
- 57.Savill JS, et al. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. The Journal of clinical investigation. 1989;83:865–75. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. American journal of respiratory and critical care medicine. 1999;160:S5–11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 59.Sexton DW, Al-Rabia M, Blaylock MG, Walsh GM. Phagocytosis of apoptotic eosinophils but not neutrophils by bronchial epithelial cells. Clin Exp Allergy. 2004;34:1514–24. doi: 10.1111/j.1365-2222.2004.02054.x. [DOI] [PubMed] [Google Scholar]
- 60.Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–92. [PubMed] [Google Scholar]
- 61.Koedel U, et al. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 2009;5:e1000461. doi: 10.1371/journal.ppat.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:14983–8. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rupp J, et al. Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages. PLoS One. 2009;4:e6020. doi: 10.1371/journal.pone.0006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Handa Y, et al. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nat Cell Biol. 2007;9:121–8. doi: 10.1038/ncb1526. [DOI] [PubMed] [Google Scholar]
- 65.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol. 2003;81:289–96. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 66.Morimoto K, Janssen WJ, Terada M. Defective efferocytosis by alveolar macrophages in IPF patients. Respir Med. 2012;106:1800–3. doi: 10.1016/j.rmed.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vandivier RW, et al. Impaired clearance of apoptotic cells from cystic fibrosis airways. Chest. 2002;121:89S. doi: 10.1378/chest.121.3_suppl.89s. [DOI] [PubMed] [Google Scholar]
- 68.McPhillips K, et al. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol. 2007;178:8117–26. doi: 10.4049/jimmunol.178.12.8117. [DOI] [PubMed] [Google Scholar]
- 69.Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. The Journal of biological chemistry. 2006;281:8836–42. doi: 10.1074/jbc.M510972200. [DOI] [PubMed] [Google Scholar]
- 70.Tosello-Trampont AC, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J Biol Chem. 2003;278:49911–9. doi: 10.1074/jbc.M306079200. [DOI] [PubMed] [Google Scholar]
- 71.Moon C, Lee YJ, Park HJ, Chong YH, Kang JL. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. Am J Respir Crit Care Med. 2009;181:374–87. doi: 10.1164/rccm.200907-1061OC. [DOI] [PubMed] [Google Scholar]
- 72.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med. 2006;21:119–43. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huynh ML, et al. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172:972–9. doi: 10.1164/rccm.200501-035OC. [DOI] [PubMed] [Google Scholar]
- 74.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol. 2008;121:1372–8. 1378, e1–3. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–8. [PubMed] [Google Scholar]
- 76.Liu Y, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J Immunol. 1999;162:3639–46. [PubMed] [Google Scholar]
- 77.McColl A, et al. Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages. J Immunol. 2009;183:2167–75. doi: 10.4049/jimmunol.0803503. [DOI] [PubMed] [Google Scholar]
- 78.Woolley KL, et al. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:237–43. doi: 10.1164/ajrccm.154.1.8680686. [DOI] [PubMed] [Google Scholar]
- 79.Vago JP, et al. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. Journal of leukocyte biology. 2012;92:249–58. doi: 10.1189/jlb.0112008. [DOI] [PubMed] [Google Scholar]
- 80.Marwick JA, et al. Oxygen levels determine the ability of glucocorticoids to influence neutrophil survival in inflammatory environments. Journal of leukocyte biology. 2013 doi: 10.1189/jlb.0912462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1506–16. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–64. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 83.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–61. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 84.Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–80. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Ait-Oufella H, et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–77. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 86.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature reviews. Immunology. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1421–8. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhatia VK, et al. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–26. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deftereos S, et al. Association of soluble tumour necrosis factor-related apoptosis-inducing ligand levels with coronary plaque burden and composition. Heart. 2012;98:214–8. doi: 10.1136/heartjnl-2011-300339. [DOI] [PubMed] [Google Scholar]
- 90.Miller YI, et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–8. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 91.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–34. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 92.Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–7. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 93.Morimoto K, et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176:7657–65. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 94.Wu DJ, et al. Effects of fasudil on early atherosclerotic plaque formation and established lesion progression in apolipoprotein E-knockout mice. Atherosclerosis. 2009;207:68–73. doi: 10.1016/j.atherosclerosis.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 95.Grommes J, et al. Simvastatin reduces endotoxin-induced acute lung injury by decreasing neutrophil recruitment and radical formation. PLoS One. 2012;7:e38917. doi: 10.1371/journal.pone.0038917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang C, et al. Fasudil, a rho-kinase inhibitor, attenuates bleomycin-induced pulmonary fibrosis in mice. Int J Mol Sci. 2012;13:8293–307. doi: 10.3390/ijms13078293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leung BP, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–30. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 98.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 99.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nature medicine. 2002;8:1235–42. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 100.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–55. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 101.Kiss RS, Elliott MR, Ma Z, Marcel YL, Ravichandran KS. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Current biology: CB. 2006;16:2252–8. doi: 10.1016/j.cub.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 102.Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Current opinion in lipidology. 2005;16:19–25. doi: 10.1097/00041433-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 103.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiological reviews. 2005;85:1343–72. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 104.Van Eck M, et al. Macrophage ATP-binding cassette transporter A1 overexpression inhibits atherosclerotic lesion progression in low-density lipoprotein receptor knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:929–34. doi: 10.1161/01.ATV.0000208364.22732.16. [DOI] [PubMed] [Google Scholar]
- 105.Aiello RJ, et al. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:630–7. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 106.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. The Journal of biological chemistry. 2009;284:32336–43. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu X, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. Journal of lipid research. 2010;51:3196–206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.A-Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–58. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vucic E, et al. Regression of inflammation in atherosclerosis by the LXR agonist R211945: a noninvasive assessment and comparison with atorvastatin. JACC Cardiovasc Imaging. 2012;5:819–28. doi: 10.1016/j.jcmg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandez-Boyanapalli R, et al. PPARgamma activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood. 2010;116:4512–22. doi: 10.1182/blood-2010-02-272005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nissen SE, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. Jama. 2008;299:1561–73. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 112.Rumore PM, Steinman CR. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J Clin Invest. 1990;86:69–74. doi: 10.1172/JCI114716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perniok A, Wedekind F, Herrmann M, Specker C, Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus. 1998;7:113–8. doi: 10.1191/096120398678919804. [DOI] [PubMed] [Google Scholar]
- 114.Baumann I, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 115.Herrmann M, et al. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–50. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 116.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 117.Hu CY, et al. Genetic polymorphism in milk fat globule-EGF factor 8 (MFG-E8) is associated with systemic lupus erythematosus in human. Lupus. 2009;18:676–81. doi: 10.1177/0961203309103027. [DOI] [PubMed] [Google Scholar]
- 118.Yamaguchi H, et al. Aberrant splicing of the milk fat globule-EGF factor 8 (MFG-E8) gene in human systemic lupus erythematosus. Eur J Immunol. 2010;40:1778–85. doi: 10.1002/eji.200940096. [DOI] [PubMed] [Google Scholar]
- 119.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- 120.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 121.Devitt A, et al. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14−/− mice. J Cell Biol. 2004;167:1161–70. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 123.Kenyon KD, et al. IgG autoantibodies against deposited C3 inhibit macrophage-mediated apoptotic cell engulfment in systemic autoimmunity. J Immunol. 2011;187:2101–11. doi: 10.4049/jimmunol.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hurst NP, Nuki G, Wallington T. Functional defects of monocyte C3b receptor-mediated phagocytosis in rheumatoid arthritis (RA): evidence for an association with the appearance of a circulating population of non-specific esterase-negative mononuclear phagocytes. Ann Rheum Dis. 1983;42:487–93. doi: 10.1136/ard.42.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Friggeri A, et al. Extracellular histones inhibit efferocytosis. Mol Med. 2012;18:825–33. doi: 10.2119/molmed.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friggeri A, et al. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am J Physiol Cell Physiol. 2010;299:C1267–76. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van den Brand BT, et al. Therapeutic efficacy of Tyro3, Axl, and Mer tyrosine kinase agonists in collagen-induced arthritis. Arthritis Rheum. 2013;65:671–80. doi: 10.1002/art.37786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park MC, Kwon YJ, Chung SJ, Park YB, Lee SK. Liver X receptor agonist prevents the evolution of collagen-induced arthritis in mice. Rheumatology (Oxford) 2010;49:882–90. doi: 10.1093/rheumatology/keq007. [DOI] [PubMed] [Google Scholar]
- 129.Tomita T, Kakiuchi Y, Tsao PS. THR0921, a novel peroxisome proliferator-activated receptor gamma agonist, reduces the severity of collagen-induced arthritis. Arthritis Res Ther. 2006;8:R7. doi: 10.1186/ar1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morelli AE, Larregina AT. Apoptotic cell-based therapies against transplant rejection: role of recipient’s dendritic cells. Apoptosis. 2010;15:1083–97. doi: 10.1007/s10495-010-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bittencourt MC, et al. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood. 2001;98:224–30. doi: 10.1182/blood.v98.1.224. [DOI] [PubMed] [Google Scholar]
- 132.Sun E, et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 2004;11:1258–64. doi: 10.1038/sj.cdd.4401500. [DOI] [PubMed] [Google Scholar]
- 133.Wang Z, et al. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am J Transplant. 2006;6:1297–311. doi: 10.1111/j.1600-6143.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 134.Kleinclauss F, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Z, et al. In situ-targeting of dendritic cells with donor-derived apoptotic cells restrains indirect allorecognition and ameliorates allograft vasculopathy. PLoS One. 2009;4:e4940. doi: 10.1371/journal.pone.0004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol. 2011;223:177–94. doi: 10.1002/path.2792. [DOI] [PubMed] [Google Scholar]
- 137.Bondanza A, et al. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J Exp Med. 2004;200:1157–65. doi: 10.1084/jem.20040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 140.Casares N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spisek R, et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–45. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 143.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–85. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Willingham SB, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tseng D, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110:11103–8. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weiskopf K, et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 149.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nature reviews. Microbiology. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 151.Ueki S, et al. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–83. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matzinger P. Tolerance, danger, and the extended family. Annual review of immunology. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 153.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bonilla WV, et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–9. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 155.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–5. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010;17:381–97. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 157.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang JG, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity. 2012;36:646–57. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 159.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–91. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levy BD, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–23. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 162.El Kebir D, et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–9. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Arita M, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–6. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chiang N, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fredman G, et al. Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PLoS One. 2011;6:e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Frasch SC, et al. Neutrophils regulate tissue neutrophilia in inflammation via the oxidant modified lipid lysophosphatidylserine. J Biol Chem. 2013;5:5. doi: 10.1074/jbc.M112.438507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Frasch SC, Bratton DL. Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog Lipid Res. 2012;51:199–207. doi: 10.1016/j.plipres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fernandez-Boyanapalli RF, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–55. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Uderhardt S, et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36:834–46. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 170.Szodoray P, Papp G, Nakken B, Harangi M, Zeher M. The molecular and clinical rationale of extracorporeal photochemotherapy in autoimmune diseases, malignancies and transplantation. Autoimmun Rev. 2010;9:459–64. doi: 10.1016/j.autrev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 171.Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front Immunol. 2011;2:57. doi: 10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perruche S, Saas P, Chen W. Apoptotic cell-mediated suppression of streptococcal cell wall-induced arthritis is associated with alteration of macrophage function and local regulatory T-cell increase: a potential cell-based therapy? Arthritis Res Ther. 2009;11:R104. doi: 10.1186/ar2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23:844–54. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 174.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee YJ, et al. Apoptotic cell instillation after bleomycin attenuates lung injury through hepatocyte growth factor induction. Eur Respir J. 2012;40:424–35. doi: 10.1183/09031936.00096711. [DOI] [PubMed] [Google Scholar]
- 176.Ren Y, et al. Apoptotic cells protect mice against lipopolysaccharide-induced shock. J Immunol. 2008;180:4978–85. doi: 10.4049/jimmunol.180.7.4978. [DOI] [PubMed] [Google Scholar]
- 177.Barker RN, et al. Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clin Exp Immunol. 2002;127:220–5. doi: 10.1046/j.1365-2249.2002.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ramos GC, et al. Apoptotic mimicry: phosphatidylserine liposomes reduce inflammation through activation of peroxisome proliferator-activated receptors (PPARs) in vivo. Br J Pharmacol. 2007;151:844–50. doi: 10.1038/sj.bjp.0707302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Harel-Adar T, et al. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011;108:1827–32. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rossi AG, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nature medicine. 2006;12:1056–64. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 182.Lucas CD, et al. Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013;27:1084–94. doi: 10.1096/fj.12-218990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McGrath EE, et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. Journal of leukocyte biology. 2011;90:855–65. doi: 10.1189/jlb.0211062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Moffatt OD, Devitt A, Bell ED, Simmons DL, Gregory CD. Macrophage recognition of ICAM-3 on apoptotic leukocytes. Journal of immunology. 1999;162:6800–10. [PubMed] [Google Scholar]
- 185.Knies UE, et al. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc Natl Acad Sci U S A. 1998;95:12322–7. doi: 10.1073/pnas.95.21.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Blume KE, et al. Cleavage of annexin A1 by ADAM10 during secondary necrosis generates a monocytic “find-me” signal. J Immunol. 2012;188:135–45. doi: 10.4049/jimmunol.1004073. [DOI] [PubMed] [Google Scholar]
- 187.Arur S, et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–98. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 188.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 189.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. Journal of immunology. 2007;178:5635–42. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 190.He M, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12:358–64. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Panaretakis T, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. The EMBO journal. 2009;28:578–90. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brown S, et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–3. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 193.Nilsson A, Oldenborg PA. CD47 promotes both phosphatidylserine-independent and phosphatidylserine-dependent phagocytosis of apoptotic murine thymocytes by non-activated macrophages. Biochemical and biophysical research communications. 2009;387:58–63. doi: 10.1016/j.bbrc.2009.06.121. [DOI] [PubMed] [Google Scholar]