Summary
Fine-control of Wnt signaling is essential for various cellular and developmental decision making processes. However, deregulation of Wnt signaling leads to pathological consequences including cancer. Here, we identify a novel function of PAF, a component of translesion DNA synthesis, in modulating Wnt signaling. PAF is specifically overexpressed in colon cancer cells and intestinal stem cells, and required for colon cancer cell proliferation. In Xenopus laevis, ventrovegetal expression of PAF hyperactivates Wnt signaling, developing secondary axis with β-catenin target gene upregulation. Upon Wnt signaling activation, PAF is dissociated from PCNA, and directly binds to β-catenin. Then, PAF recruits EZH2 to β-catenin transcriptional complex, and specifically enhances Wnt target gene transactivation, independently of EZH2's methyltransferase activity. In mouse, conditional expression of PAF induces intestinal neoplasia via Wnt signaling hyperactivation. Our studies reveal an unexpected role of PAF in regulating Wnt signaling, and propose a novel regulatory mechanism of Wnt signaling during tumorigenesis.
Keywords: Wnt, β-catenin, PAF, KIAA0101, EZH2
Introduction
Strict regulation of stem cell proliferation and differentiation is required for mammalian tissue homeostasis, and its repair in the setting of tissue damage. These processes are precisely orchestrated by various developmental signaling pathways, with dysregulation contributing to disease and genetic disorders, including cancer (Beachy et al., 2004). Cancer is initiated by the inactivation of tumor suppressor genes and activation of oncogenes. For instance, colon cancer cells harbor genetic mutations in Wnt/β-catenin pathway constituents such as adenomatous polyposis coli (APC), Axin, and β-catenin (Polakis, 2007). In mouse models, inactivation of APC or activation of β-catenin results in the development of intestinal hyperplasia and adenocarcinoma (Moser et al., 1990), indicating that hyperactivation of Wnt signaling promotes intestinal tumorigenesis.
In canonical Wnt signaling, Wnt ligand induces stabilization of β-catenin protein via inhibition of the protein destruction complex (glycogen synthase kinase 3, APC, casein kinase I, and Axin). Then, activated β-catenin is translocated into the nucleus and binds to its nuclear interacting partners, TCF/LEF. Finally, β-catenin-TCF/LEF transactivates the expression of its target genes (Clevers and Nusse, 2012).
Although various Wnt/β-catenin modulators have been identified (Wnt homepage; wnt.stanford.edu), the pathological relevance of these modulators to tumorigenesis remains elusive. Also, many reports have suggested that mutation-driven Wnt signaling activation can be enhanced further (Goentoro and Kirschner, 2009; He et al., 2005; Suzuki et al., 2004; Vermeulen et al., 2010), which implies the presence of an additional layer of Wnt-signaling regulation in cancer beyond genetic mutations in APC or β-catenin. Here, we unraveled a novel function of the DNA repair gene, PAF (PCNA-associated factor) /KIAA0101). PAF was shown to be involved in translesion DNA synthesis (TLS), an error-prone DNA repair process that permits DNA replication machinery to replicate DNA lesions with specialized translesion DNA polymerase (Emanuele et al., 2011; Povlsen et al., 2012; Sale et al., 2012). Our comprehensive approaches uncover that cancer-specifically expressed PAF hyperactivates Wnt/β-catenin signaling and induces intestinal tumorigenesis.
Results
Mitogenic role of PAF via Wnt signaling
To identify colon cancer-specific Wnt signaling regulators, we analyzed multiple sets of human colon cancer tissue samples using the publicly available database (www.oncomine.org), and selected genes that are highly expressed in colon cancer cells (fold change > 2; P < 0.0001; top 10% ranked). Among several genes, we investigated the biological role of PAF, based on its significant overexpression in human colon adenocarcinoma with correlated expression of Axin2, a well-established specific target gene of β-catenin (Jho et al., 2002; Lustig et al., 2002)(Figure 1A). To validate our in silico analysis, we performed immunostaining of colon cancer tissue microarray, and confirmed that PAF was highly expressed in colon cancer cells, whereas its expression was barely detectable in normal intestine (Figure 1B). Consistently, PAF was strongly expressed and mainly localized in the nucleus of colon cancer cell lines (Figure 1C). Additionally, we found that PAF was not expressed in non-transformed cells such as NIH3T3, mouse embryonic fibroblasts, and mammary epithelial cells (data not shown). Next, to assess the relevance of PAF upregulation in colon cancer cell proliferation, we depleted endogenous PAF using short hairpin RNAs (shRNAs) in these cell lines. Intriguingly, PAF knockdown (sh-PAF) inhibited colon cancer cell proliferation (Figures 1D and 1E). Given that PAF was shown to interact with PCNA via PIP box (Yu et al., 2001), we also examined whether PAF-PCNA interaction is required for mitogenic effects of PAF. In reconstitution experiments, sh-PAF-induced cell growth inhibition was rescued by ectopic expression of both shRNA non-targetable wild-type PAF (nt-PAF) and PIP mutant PAF (mutPIP-PAF) (Figure 1F), indicating that the PAF-PCNA interaction is not necessary for PAF-mediated colon cancer cell proliferation. Interestingly, PAF knockdown downregulated cell proliferation–related genes (Cyclin D1 and c-Myc) (Figure 1G). Given that Cyclin D1 and c-Myc are β-catenin direct target genes (He et al., 1998; Tetsu and McCormick, 1999), PAF likely participates in regulating Wnt/β-catenin signaling. Interestingly, PAF depletion-induced downregulation of Cyclin D1 and c-Myc was only observed in SW620 colon cancer cells, but not in Panc-1 and MDA-MB-231 cells (Figure 1G), indicating the specific effects of PAF on Cyclin D1 and c-Myc expression in colon cancer cells. We also assessed the effects of PAF knockdown on Axin2. Indeed, PAF knockdown suppressed Axin2 transcription in colon cancer cells (Figure 1H). Moreover, as nt-PAF did, β-catenin ectopic expression reverted sh-PAF–induced cell growth arrest (Figure 1I), implying that PAF might be functionally associated with Wnt/β-catenin. We also examined whether other mitogenic signaling pathways mediate PAF's mitogenic role. Of note, except HT29, other colon cancer cell lines (SW620, HCT116, HCC2998, and HCT15) harbor oncogenic mutations in K-Ras gene. Nonetheless, PAF depletion induced the suppression of cell growth on all five colon cancer cells (Figure 1D), indicating that PAF's mitogenic function is independent of Ras/MAPK signaling activation. Additionally, overexpression of wild-type Akt or constitutively active form of Akt (myristoylated form of Akt [Myr-Akt]) did not rescue sh-PAF-induced inhibition of cell proliferation (Figure 1I). Moreover, β-catenin activation did not revert cell proliferation suppression resulted from MAPK or PI3K inhibition (Figure 1J), indicating that β-catenin-mediated mitogenic function is independent of MAPK and PI3K signaling pathways. These results suggest that PAF contributes to colon cancer cell proliferation, possibly via Wnt/β-catenin signaling.
Figure 1. Mitogenic role of PAF in colon cancer cells.
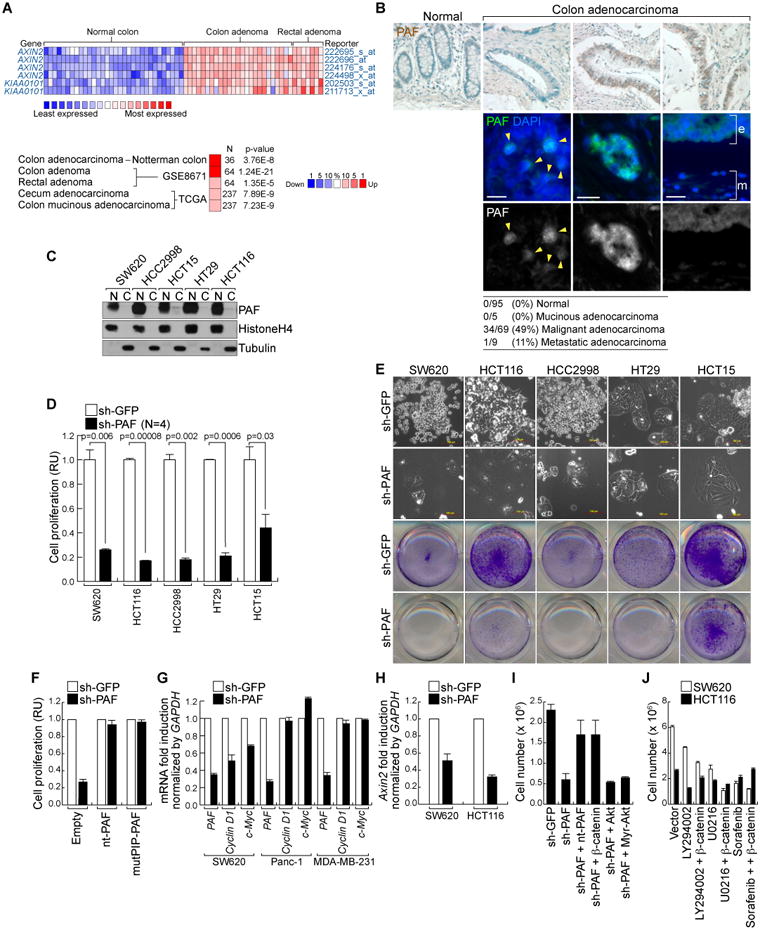
(A) Expression of PAF in human colon cancer cells.
Oncomine analysis of PAF gene expression in human colon cancer cells. Comparison of Axin2 and PAF expression (GSE8671; fold change > 2;P value < 0.0001; gene rank < top 10 %) (upper panel). PAF expression in colon cancer tissues compared to normal tissues (fold change > 4; average P value = 9.34 × 10-6; gene rank < top 10%; N = sample number) (lower panel).
(B) Upregulation of PAF in human colon cancer tissues.
Human colon cancer tissue microarray samples were analyzed for immunohistochemistry (DAB [brown]: PAF; hematoxylin [blue]: nuclei) and immunofluorescent staining (arrowheads: nuclear PAF; e: epithelial cells; m: mesenchymal cells). Scale bar = 20 μm
(C) Expression of PAF in human colon cancer cell lines.
Colon cancer cell lines were fractionated into nuclear (N) and cytosolic (C) fractions for immunoblotting (IB). Fractionation controls: Histone H4 (nucleus) and tubulin (cytosol).
(D and E) PAF Depletion inhibits cell proliferation.
Each cell lines stably expressing shRNAs (sh-GFP [control] and sh-PAF) were analyzed using cell counting (D) and crystal violet staining (E); RU: relative units.
(F) PCNA interaction-independent mitogenic role of PAF.
SW620 (sh-GFP and sh-PAF) were stably transfected with nt-PAF or mutPIP-PAF for cell proliferation analysis (cell counting).
(G) Downregulation of Cyclin D1 and c-Myc by PAF knockdown.
SW620, Panc-1, and MDA-MB-231 cells stably expressing sh-GFP or sh-PAF were analyzed using quantitative reverse transcriptase PCR (qRT-PCR).
(H) Axin2 downregulation by PAF depletion.
SW620 and HCT116 cells were analyzed using qRT-PCR.
(I) β-catenin rescues PAF depletion-induced cell growth inhibition.
SW620 (sh-GFP and sh-PAF) cells were transfected with each plasmid, and analyzed by cell counting. N = 3.
(J) β-catenin is not involved in activating MAPK and PI3K signalings.
SW620 and HCT116 cells stably expressing β-catenin or empty vector were treated with LY294002 (PI3K inhibitor; 10 μm), U0216 (MEK1/2 inhibitor; 10 μm), or Sorafenib (Raf inhibitor; 10 μm). After 3 days, cells were counted.
All error bars indicate standard deviation.
PAF positively modulates Wnt signaling
Given that many cancers develop as a result of deregulation of developmental signalings (Beachy et al., 2004), analyzing PAF expression during development may provide insights into the mechanisms of PAF-mediated signaling regulation. Whole mount immunostaining of mouse embryos, showed that PAF was specifically enriched in the apical ectodermal ridge (AER) of the limb bud, midbrain, hindbrain, and somites (Figure 2A and data not shown). During limb development, AER induction is specifically coordinated by active Wnt signaling (Figure 2B)(Kengaku et al., 1998). Using, Axin2-LacZ, a β-catenin reporter (Lustig et al., 2002), mouse embryos, we confirmed the specific activation of Wnt signaling in AER (Figure 2C). Intriguingly, Wnt signaling activity as exhibited in the AER, overlapped with the pattern of PAF expression (Figures 2A and 2C). Given that (1) Wnt signaling is deregulated in most colon cancer, (2) PAF is highly overexpressed in colon cancer cells, (3) PAF is required for colon cancer cell proliferation (Figure 1D), and (4) PAF is enriched in AER where Wnt signaling is active (Figure 2A), we hypothesized that PAF modulates the Wnt signaling pathway. To test this, we first examined the impact of PAF on β-catenin transcriptional activity using TOPFLASH reporter assays. In HeLa cells, PAF knockdown decreased β-catenin reporter activation by 6-bromoindirubin-3′-oxime, a GSK3 inhibitor (Figure 2D). Similarly, Wnt3A-induced transcriptional activation of Axin2 was also inhibited by PAF depletion (Figure 2E). These data suggest that PAF might be required for Wnt target gene expression.
Figure 2. Activation of Wnt signaling by PAF.
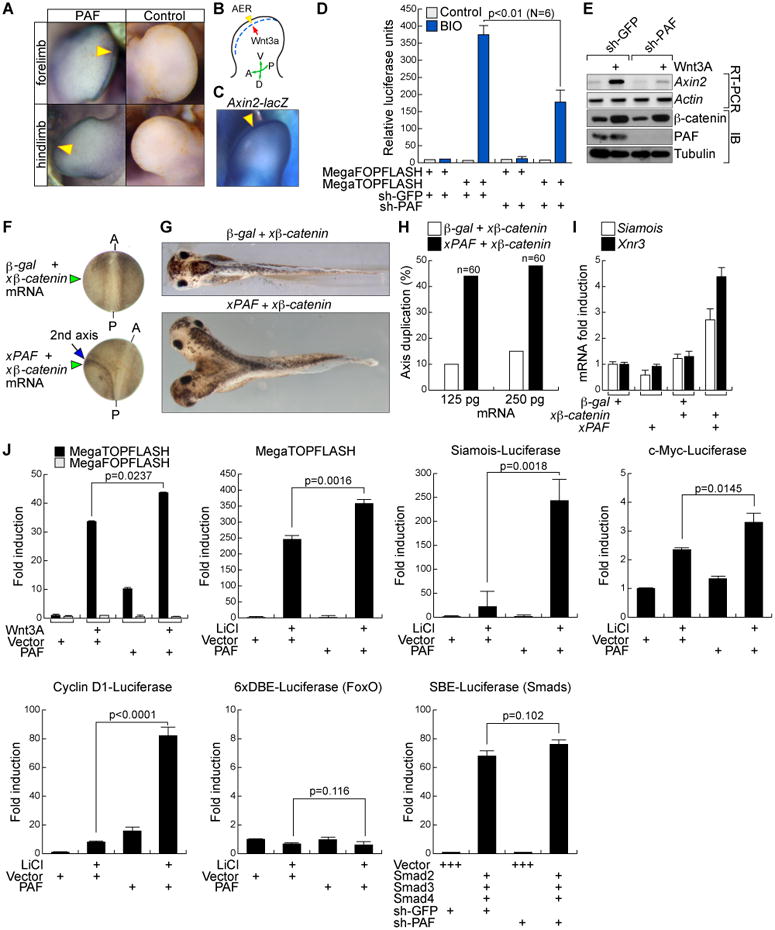
(A) PAF expression in AER.
Whole-mount immunostaining for PAF in mouse embryos at embryonic day 11. Arrowheads: PAF.
(B) Schematic diagram of AER in the limb bud.
AER; dotted line; A, anterior; P, posterior; D, dorsal; V, ventral.
(C) Active Wnt/β-catenin signaling in the AER.
Axin2-LacZ mouse embryos at embryonic day 12 were stained with 5-bromo-4-chloro-indolyl-β-D-galactopyranoside (X-gal: arrowhead).
(D and E) PAF knockdown suppresses β-catenin transcriptional activity.
HeLa (sh-GFP or –PAF) were transiently transfected with β-catenin reporter and renilla plasmids. 24 h after transfection, cells were treated with 0.5 μM BIO. After 6 h, luciferase activity was measured (D). 293T cells stably transduced with sh-GFP or sh-PAF lentivirus were treated with Wnt3A (100 ng/ml, 6 h) for RT-PCR (E).
(F-H) Axis duplication by xPAF-induced β-catenin hyperactivation.
xPAF or β-galactosidase mRNA with β-catenin mRNA was injected into the ventrovegetal blastomeres of X. laevis embryos. The secondary axis was examined from neurulation (F) to tail bud stages (G). The xβ-catenin mRNA concentration was titrated to prevent induction of axis duplication per se. A: anterior; P: posterior. (H) Two different doses of xPAF mRNA with β-catenin mRNA were injected into frog embryos for axis duplication analysis.
(I) β-catenin target gene upregulation by xPAF. X. laevis embryos injected with each mRNA at two-cell stage were collected at the gastrulation stage for qRT-PCR. Ornithine decarboxylase (ODC): an internal control.
(J) PAF-induced hyperactivation of Wnt/β-catenin target gene reporters. 293T cells transfected with each plasmid were treated with Wnt3A (100 ng/ml, 24 h) or LiCl (25 mM, 24 h), and analyzed for luciferase assays. N = 3.
All error bars indicate standard deviation.
To gain better insight of PAF's role in Wnt signaling regulation, we utilized Xenopus laevis embryos for axis duplication assays (Funayama et al., 1995), as previously performed (Park et al., 2009). Because of Wnt signaling's pivotal role in vertebrate anterior-posterior axis development, the effects of Xenopus PAF (xPAF) on Wnt signaling can be monitored and quantified on the basis of secondary axis formation following injection of in vitro transcribed mRNAs. xβ-catenin mRNA, titrated to a subphenotypic level when expressed in isolation, was co-injected with xPAF mRNA into ventrovegetal blastomeres. Unlike the controls (β-catenin and β-galactosidase mRNA), the experimental group (β-catenin and xPAF mRNA) displayed axis-duplications (Figures 2F-H). Of note, the ventrovegetal injection of xPAF mRNA alone failed to induce secondary axes (data not shown), showing that PAF hyperactivates Wnt/β-catenin signaling only in the presence of active β-catenin. Consistent with the results of axis duplication assays, qRT-PCR assays showed that xPAF expression upregulated expression of Siamois and Xnr3, β-catenin targets in frogs (Figure 2I). Furthermore, we examined the specificity of PAF on Wnt/β-catenin signaling activity, using various luciferase assays. Ectopic expression of PAF hyperactivates Wnt3A or LiCl, a GSK3 inhibitor, -induced activation of β-catenin target gene reporter activity (MegaTOPFLASH, Siamois, c-Myc, and Cyclin D1). Of note, BMP/Smad pathway also plays an essential role in the developing limb AER (Ahn et al., 2001). However, PAF knockdown or overexpression did not affect BMP/Smad or FoxO signalings, respectively, (Figure 2J) indicating the specificity of PAF in regulating Wnt signaling. These results suggest that PAF positively modulates Wnt/β-catenin signaling in vitro and in vivo.
PAF-EZH2-β-catenin transcriptional complex formation
Next, we investigated the molecular mechanism underlying PAF hyperactivation of Wnt signaling. Given that stabilization of β-catenin protein is a key process in transducing Wnt signaling, we asked whether PAF affects β-catenin protein level. However, we found that the level of β-catenin protein was not altered by PAF knockdown or overexpression (Figures 2E and 3A), leading us to test whether PAF controls the β-catenin/TCF transcriptional complex activity. Owing to the nuclear specific localization of PAF in colon cancer cells (Figure 1C), we tested whether PAF interacts with β-catenin transcriptional complex. Using a glutathione S-transferase (GST) pull-down assay, we found that PAF bound to β-catenin and TCF proteins (Figure 3B). Also, endogenous PAF interacted with β-catenin and TCF3 in SW620 cells that display constitutive hyperactivation of Wnt signaling by APC mutation (Figure 3C). Moreover, binding domain mapping assays showed that the Armadillo repeat domain of β-catenin was essential for its interaction with PAF (Figure 3D). Although PAF is a cell cycle-regulated anaphase-promoting complex substrate (Emanuele et al., 2011), PAF-β-catenin interaction was not affected (Figure S1). These data suggest that PAF directly binds to β-catenin transcriptional complex and this interaction is independent of cell cycle. Next, due to interaction of PAF with β-catenin and TCF, we tested whether PAF is also associated with Wnt/β-catenin target genes. First, we analyzed the subnuclear localization of PAF by chromatin fractionation. We found that PAF was only detected in the chromatin fraction of HCT116 cells (Figure 3E). Additionally, chromatin immunoprecipitation (ChIP) assays showed that both ectopically expressed and endogenous PAF occupied the TCF-binding element (TBE)-containing proximal promoter of the β-catenin targets (c-Myc and Cyclin D1) in HCT116 cells (Figures 3F and 3G). These data show that PAF is specifically associated with the promoters of Wnt/β-catenin target genes.
Figure 3. PAF-EZH2-β-catenin transcriptional complex at target gene promoters.
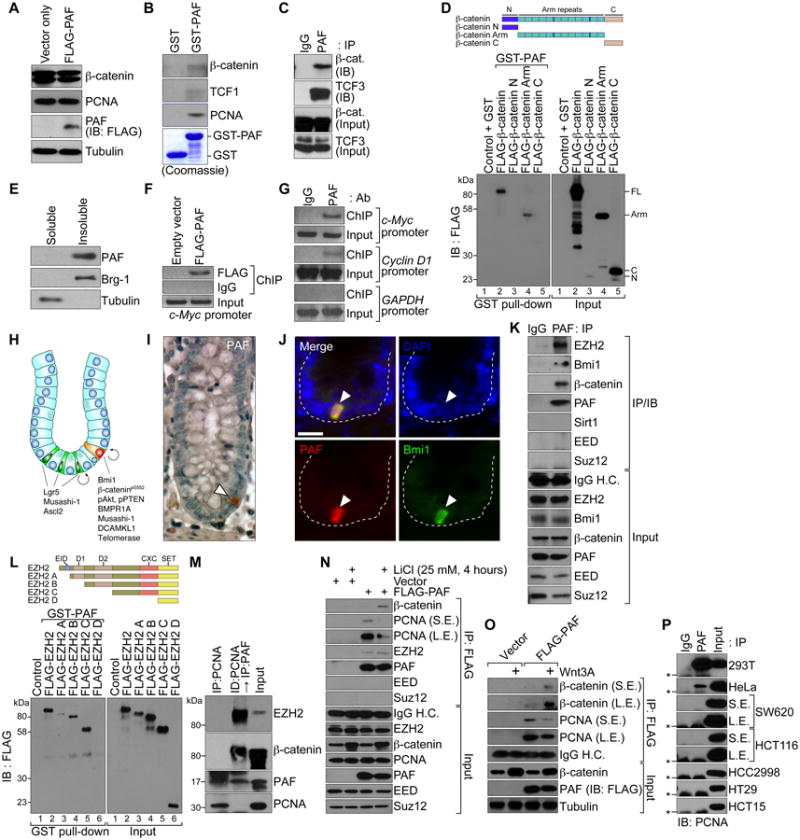
(A) No effect of PAF on β-catenin protein stability.
HeLa cells were transiently transfected with FLAG-PAF-pcDNA for IB.
(B and C) Interaction of PAF with β-catenin and TCF/LEFs.
GST-PAF was used for pull-down with SW620 cell lysates and IB (B). SW620 cells were analyzed for co-immunoprecipitation (co-IP) and IB (C).
(D) PAF-β-catenin interaction via the armadillo repeat domain.
GST-PAF was incubated with in vitro transcribed and translated FLAG-tagged β-catenin deletion mutants (N, N-term; Arm, Armadillo repeat domain; C, C-term) and analyzed using GST pull-down and IB.
(E) PAF is a chromatin-associated protein.
A chromatin-associated lysate (insoluble) and a soluble fraction of HCT116 were analyzed for IB. Brg-1: a chromatin fraction control.
(F and G) PAF occupies TBEs.
HCT116 stably expressing FLAG-PAF (F) and parental cells (G) were analyzed using ChIP. GAPDH promoter: a negative control.
(H) Lgr5 positive and Bmi1 positive ISCs are located in the crypts. ISCs divide into transit-amplifying (TA) cells and differentiate into IECs. Wnt/β-catenin signaling is highly active in crypts containing ISCs and TA cells.
(I) PAF expression in IECs of crypts.
A small intestine tissue was immunostained for PAF (arrowhead); hematoxylin: blue.
(J) PAF expression in Bmi1 positive ISCs.
Immunofluorescent staining of murine colon tissue samples for PAF and Bmi1. Scale bars = 20 μm.
(K) PAF interaction with EZH2.
Co-IP and IB assays of SW620. H.C.; heavy chain.
(L) PAF-EZH2 interaction via the CXC region.
GST-PAF was incubated with HeLa cell lysates expressing each FLAG-EZH2 deletion mutant (A-D), and analyzed for GST pull-down and IB. EID, EED interaction domain; D1 and D2, homologous domains 1 and 2; CXC, cysteine-rich domain; SET, SU(var)3-9, E(z), and Trithorax histone methyltransferase domain.
(M) Interaction of PAF with EZH2 and β-catenin, independently of PCNA. Co-IP of HCT116. A PCNA-immunodepleted (ID) supernatant was used for IP and IB.
(N and O) Wnt-dependent PAF-β-catenin interaction.
HeLa (vector or FLAG-PAF) were treated with LiCl (25 mM, 4 h) (N) or Wnt3A (100 ng/ml, 4 h) (O), and analyzed for co-IP and IB. L.E.: long exposure; S.E.: short exposure.
(P) PAF-PCNA interaction in colon cancer cells.
Co-IP and IB assays. Asterisks: IgG light chain.
In intestine, Wnt/β-catenin signaling constitutively activates intestinal stem cells (ISCs) to give rise to progenitor cells, which replenishes intestinal epithelium (Figure 3H). Given the involvement of PAF on Wnt/β-catenin signaling regulation (Figure 2), we analyzed the spatial expression of PAF in intestinal epithelium. Immunostaining showed that PAF was specifically expressed in B lymphoma Mo-MLV insertion region 1 homolog (Bmi1) positive intestinal stem cells (ISCs)(Figures 3I and 3J). Bmi1 and its associated components in Polycomb-repressive complex 1 (PRC1) and 2 (PRC2) are shown to epigenetically regulate gene expression (Sparmann and van Lohuizen, 2006). Due to (1) specific association of PAF with TBEs of β-catenin target promoters (Figures 3F and 3G) and (2) co-localization with Bmi1 positive ISCs (Figure 3J), we asked whether PAF is associated with components of PRC1 and PRC2, using co-immunoprecipitation (co-IP) assays. Intriguingly, PAF interacted with both Bmi1 and enhancer of zeste homolog 2 (EZH2) in SW620 cells (Figure 3K), which led us to test whether either Bmi1 or EZH2 is functionally associated with PAF-mediated Wnt signaling hyperactivation. To do this, we assessed the effects of Bmi1 and EZH2 on β-catenin transcriptional activity, using β-catenin reporter assays. We observed that ectopic expression of EZH2 upregulated β-catenin transcriptional activity, but Bmi1 overexpression did not (data not shown), implying that EZH2 might be associated with Wnt signaling activation. Binding domain mapping analysis showed that EZH2 bound to PAF via the middle region of EZH2 including the CXC cysteine-rich domain (Figure 3L). In conjunction with the Bmi1-containing PRC1, EZH2-containing PRC2 catalyzes histone H3 lysine 27 trimethylation (H3K27me3) via histone methyltransferase domain. Despite the crucial role of EZH2 in H3K27me3-meidated gene regulation, we found that other core components of PRC2, EED, and Suz12 were not associated with PAF (Figure 3K). Moreover, although EZH2 overexpression in cancer induces PRC4 formation in association with the NAD+-dependent histone deacetylase Sirt1 (Kuzmichev et al., 2005), the PAF-EZH2 complex did not contain Sirt1 (Figure 3K). These data indicate that PAF-EZH2 complex is distinct from the conventional PRCs in cancer cells. Also, we questioned whether PCNA is required for PAF's interaction with either PAF or β-catenin. Interestingly, β-catenin-PAF and EZH2-PAF complexes existed only in PCNA-free fractions (Figure 3M, compare lanes 1 and 2), which is consistent with PCNA-independent mitogenic role of PAF in colon cancer cell proliferation (Figure 1I). Due to exclusive interaction of PAF with either PCNA or β-catenin, we asked whether Wnt signaling activation affects either PAF-β-catenin or PAF-PCNA interaction. Co-IP assays showed that, in HeLa cells, PAF-β-catenin interaction was only detected upon LiCl treatment, while PAF-EZH2 interaction remained constant. Moreover, PAF-PCNA association was decreased by LiCl or Wnt3A treatment (Figures 3N and 3O, compare lanes 3 and 4). These data suggest that Wnt signaling activation is required for PAF-β-catenin interaction. Due to absence of endogenous Wnt signaling activity in HeLa cells, we also assessed the effects of active Wnt/β-catenin signaling on PAF-PCNA binding in colon cancer cell lines that exhibit hyperactivation of Wnt signaling by genetic mutations in APC or β-catenin alleles. Surprisingly, PAF-PCNA interaction was barely detectable in colon cancer cell lines, whereas 293T and HeLa cells displayed strong PAF-PCNA association (Figure 3P), implying that active β-catenin may sequester PAF from PCNA. In binding domain mapping analysis, we also found that N-terminal and PIP regions are required for β-catenin interaction (Figure S2), suggesting that β-catenin competes with PCNA for PAF interaction. These results suggest that, upon Wnt signaling activation, PAF is conditionally associated with β-catenin transcriptional complex.
PAF activates β-catenin target genes by recruiting EZH2 to promoters
Previous studies showed that EZH2 interacts with β-catenin (Li et al., 2009; Shi et al., 2007). Also, we found that PAF is physically associated with EZH2, independently of PRC2 complex (Figure 3). These evidences prompted us to ask whether EZH2 mediates PAF-induced Wnt signaling hyperactivation. Given PAF-EZH2-β-catenin complex formation, we tested whether EZH2 is also associated with the promoters of β-catenin target genes. Intriguingly, PAF, EZH2, and β-catenin steadily co-occupied the promoters of c-Myc, Cyclin D1, and Axin2 in HCT116 cells carrying β-catenin mutation, whereas PCNA, EED, and Suz12 did not (Figure 4A), which recapitulates PRC2 complex-independent association of EZH2 with PAF (see Figures 3K and 3N). Next, we asked whether PAF, EZH2, and β-catenin are recruited to β-catenin target gene promoter upon Wnt signaling activation, as PAF-β-catenin interaction was dependent of Wnt signaling activation (Figure 3N). In HeLa cells, we found that PAF, EZH2, and β-catenin conditionally bound to TBEs in the c-Myc and Axin2 promoters, only upon LiCl treatment (Figure 4B), indicating that Wnt signaling activation is a prerequisite for PAF-β-catenin-EZH2's promoter association. To further confirm the specificity of PAF-EZH2-β-catenin's recruitment to β-catenin target promoters, we performed ChIP promoter scanning of 10 kb of the c-Myc promoter, and found that PAF, EZH2, and β-catenin specifically co-occupied the proximal promoter containing TBEs of the c-Myc gene (at -1037 and -459 bp) (He et al., 1998) in HCT116 cells (Figure 4C). Also, the analysis of EZH2 ChIP-sequencing data from mouse embryonic stem cells showed that EZH2 was specifically enriched in the proximal promoters of β-catenin targets (Lef1, Lgr5, c-Myc, and Axin2) (Figure 4D).
Figure 4. PAF promotes EZH2-β-catenin interaction.
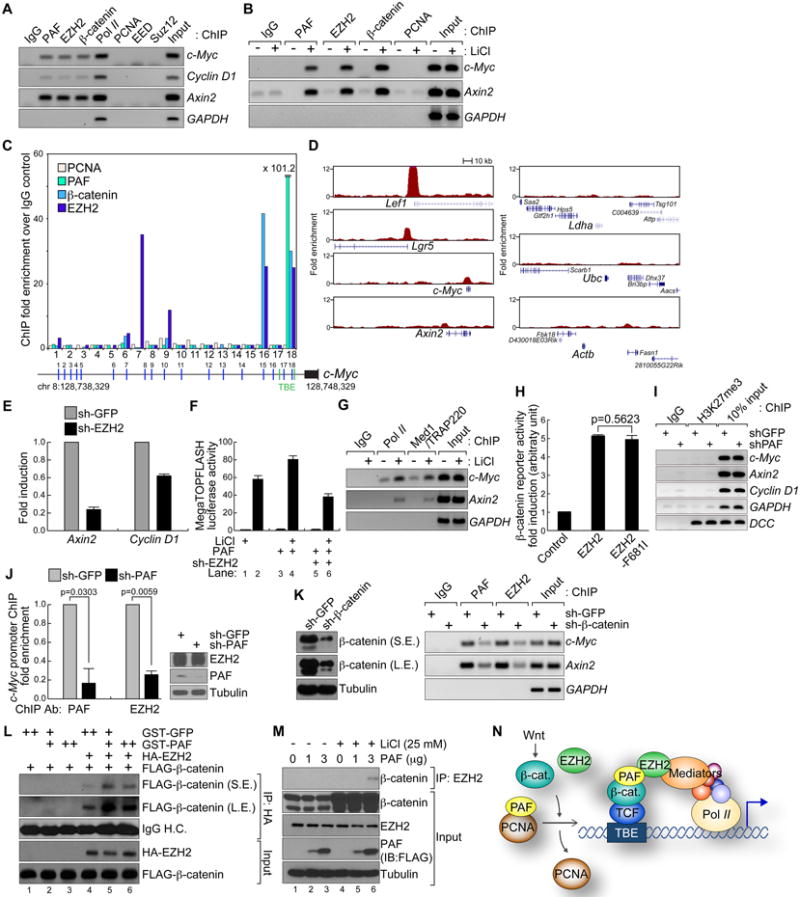
(A) Constant association of EZH2, PAF, and β-catenin with the β-catenin target promoters.
ChIP assays using HCT116 nuclear lysates. RNA Pol II: positive controls for the proximal promoter.
(B) β-catenin-induced recruitment of EZH2 and PAF to promoters.
HeLa cells (control and LiCl-treated; 25 mM, 4 h) were used for ChIP analysis. IgG and GAPDH served as negative controls for IP and ChIP-PCR, respectively.
(C) ChIP promoter scanning of c-Myc promoter.
HCT116 cells were analyzed by ChIP assays on the c-Myc promoter with 18 pairs of PCR primers. Average size of amplicons = 150 bp; green vertical bars: TBEs.
(D) EZH2 association with β-catenin target promoters.
EZH2 ChIP-seq data from mouse embryonic stem cells (GSE13084) were analyzed for EZH2 association in the promoters (200 kb) of β-catenin targets (Lef1, Lgr5, c-Myc, and Axin2). Ldha (lactate dehydrogenase A), Ubc (ubiquitin C), and Actb (β-actin): negative controls.
(E) Downregulation of β-catenin targets by EZH2 depletion.
HCT116 (sh-GFP or -EZH2) were analyzed by qRT-PCR.
(F) EZH2 depletion inhibits PAF-mediated hyperactivation of β-catenin reporter activity.
293T cells (sh-GFP or -EZH2) were transfected with PAF and pMegaTOPFLASH plasmids, and treated with LiCl (25 mM, 24 h) for luciferase assays.
(G) Recruitment of Pol II and Med1/TRAP220 to promoters upon β-catenin activation.
ChIP assays using HeLa (control or LiCl [25 mM, 4 h]).
(H) 293T cells transfected with EZH2, EZH2-F681I and pMegaTOPFLASH were analyzed for luciferase assays (N = 4).
(I) ChIP assays of HCT116 sh-GFP or-PAF using semi-quantitative PCR. DCC promoter: a positive control for H3K27me3 (Derks et al., 2009).
(J) PAF depletion impairs EZH2 recruitment to the c-Myc promoter.
ChIP assays of HCT116 sh-GFP or –PAF using qPCR. Of note, PAF knockdown did not downregulate EZH2 (right IB panel).
(K) β-catenin depletion decreases recruitment of PAF and EZH2 to promoters. ChIP assays of HCT116 (sh-GFP or sh-β-catenin).
(L) PAF enhances EZH2-β-catenin interaction in vitro.
GST-PAF pull-down of EZH2 and β-catenin protein mixture. EZH2-β-catenin interaction was then analyzed by co-IP and IB.
(M) PAF increases β-catenin-EZH2 binding in vivo.
HeLa (vector or PAF) treated with LiCl (25 mM, 4 h) analyzed for co-IP and IB.
(N) Illustration of PAF-induced hyperactivation of the β-catenin transcriptional complex. In the setting of Wnt signaling activation, stabilized β-catenin sequesters PAF from PCNA. As a molecular adaptor, PAF facilitates interaction between β-catenin and EZH2. The EZH2/Mediator complex recruits RNA Pol II-associated transcriptional machinery to TBEs, and transactivates β-catenin target genes.
All error bars indicate standard deviation.
Next, we asked whether EZH2 promoter recruitment is necessary for activation of β-catenin target gene transcription. Previously, depletion of EZH2 was shown to inhibit c-Myc expression in DLD-1 colon cancer cells (Fussbroich et al., 2011). Consistently, EZH2 knockdown downregulated β-catenin target genes, Axin2 and Cyclin D1 in HCT116 cells (Figure 4E), and decreased LiCl-induced β-catenin reporter activation (Figure 4F), suggesting that EZH2 is required for PAF-mediated Wnt target gene hyperactivation. These results are also supported by previous finding that EZH2 enhances β-catenin transcriptional activity by connecting β-catenin with the Med1/RNA polymerase II (Pol II) complex (Shi et al., 2007). Indeed, Med1/TRAAP220 and Pol II conditionally binds to c-Myc and Axin2 promoters in LiCl-treated HeLa cells (Figure 4G). Given that PRC2-indepednent interaction between EZH2 and PAF (Figures 3K and 3N), we asked whether EZH2's histone methyltransferase activity is dispensable in β-catenin regulation. We utilized an EZH2 point mutant (F681I) that disrupts the contact between the EZH2 hydrophobic pocket and histone lysine residue H3K27 (Joshi et al., 2008). Ectopic expression of either EZH2 or EZH2-F681I enhanced β-catenin reporter activity (Figure 4H). Also, PAF knockdown did not change the H3K27 methylation status (H3K27me3) of proximal promoters of c-Myc, Axin2, Cyclin D1, and DCC in HCT116 cells (Figure 4I). These results indicate a methyltransferase-independent role of EZH2 in transactivating β-catenin targets.
Due to PAF's (1) small size (111 amino acids, one α-helix), (2) lack of a specific catalytic domain, and (3) binding to both β-catenin and EZH2, PAF may facilitate the interaction between EZH2 and β-catenin through recruiting EZH2 to the promoter. We tested this using ChIP assays for EZH2 in the setting of PAF depletion. Indeed, PAF-depleted HCT116 cells displayed decreased EZH2-association at the c-Myc promoter (Figure 4J), suggesting that PAF assists or is needed to recruit EZH2 to β-catenin transcriptional complex. Also, β-catenin knockdown decreased recruitment of PAF and EZH2 to promoters (Figure 4K), showing that PAF and EZH2 occupy target promoters via β-catenin. We then asked whether PAF promotes β-catenin-EZH2 binding. In vitro binding assays showed that the addition of GST-PAF protein increased EZH2-β-catenin association (Figure 4L). Moreover, ectopic expression of PAF promoted the EZH2-β-catenin interaction in HeLa cells treated with LiCl (Figure 4M). Additionally, we tested whether Wnt signaling-induced post-translational modification of either β-catenin or PAF is required for EZH2 interaction. However, in GST pull-down assays, we found that bacterially expressed either GST-β-catenin or –PAF bound to EZH2 (Figure S3). Due to the lack of post-translational modification in GST protein expression system, these data indicate that post-translation modification of either β-catenin or PAF is not necessary for EZH2 interaction. Together, these results suggest that PAF acts as a molecular adaptor to facilitate EZH2-β-catenin complex, and subsequently enhances the transcriptional activity of the β-catenin transcriptional complex at Wnt target promoters (Figure 4N).
Intestinal tumorigenesis following PAF conditional expression
Having determined that PAF is overexpressed in colon cancer cells and hyperactivates Wnt/β-catenin signaling, we aimed to determine whether mimicking PAF overexpression drives intestinal tumorigenesis, using genetically engineered mouse models. To conditionally express PAF, we generated doxycycline (doxy)-inducible PAF transgenic mice (TetO-PAF-IRES-emGFP [iPAF]). For intestine-specific expression of PAF, we used iPAF:Villin-Cre:Rosa26-LSL-rtTA mouse strains. Villin-Cre is specifically expressed in intestinal epithelial cells (IECs), including ISCs and progenitor cells. Cre removes a floxed stop cassette (loxP-STOP-loxP [LSL]) from the Rosa26 allele and induces rtTA expression. Upon doxy treatment, rtTA drives the transcriptional activation of the tetracycline-responsive element promoter, resulting in conditional transactivation of PAF selectively in IECs. We also utilized the Rosa26-rtTA strain for ubiquitous expression of PAF (Figure 5A and Figure S4). First, we examined the effects of PAF induction on IEC proliferation using a crypt organoid culture system (Figure S5A). Intriguingly, PAF conditional expression (2 weeks) induced expansion of the crypt organoids (Figures 5B and 5C), which recapitulates the mitogenic function of PAF (Figure 1). In mouse, IEC-specific PAF expression (iPAF:Villin-Cre:Rosa26-LSL-rtTA; 2 months) developed adenoma in both small intestine and colon (Figure 5D). Also, microscopic analysis using hematoxylin and eosin (H&E) staining showed aberrant IEC growth and crypt foci formation (Figures 5E and 5F), with disorganized epithelial cell arrangements (Figure S5B). Consistently, PAF-induced IEC hyperproliferation was manifested by increased Ki67 expression, a mitotic marker (Figure 5G). Importantly, these lesions exhibited the upregulation of CD44, a β-catenin target gene, whereas CD44 was expressed strictly in the crypts of normal intestine (Figure 5H). Next, we examined whether PAF directly hyperactivates Wnt/β-catenin in vivo using BAT-gal, a β-catenin reporter transgenic mouse carrying multiple TBEs followed by a LacZ reporter. To quantify the early effects of PAF on β-catenin activity, we treated mice with doxy for 1 week, and found that short-term induction of PAF increased β-catenin transcriptional activity as represented by enhanced X-gal staining (Figure 5I). Moreover, conditional PAF expression upregulated the β-catenin target genes, Axin2, Lgr5, CD44, Cyclin D1, and c-Myc in crypt organoids (Figure 5J). Additionally, mice ubiquitously expressing PAF exhibited intestinal hypertrophy (Figure S5C), which is similar to that induced by R-Spondin1, a secreted Wnt agonist (Kim et al., 2005). These data strongly suggest that PAF expression is sufficient to initiate intestinal tumorigenesis via Wnt signaling hyperactivation
Figure 5. Induction of intestinal neoplasia by PAF expression.
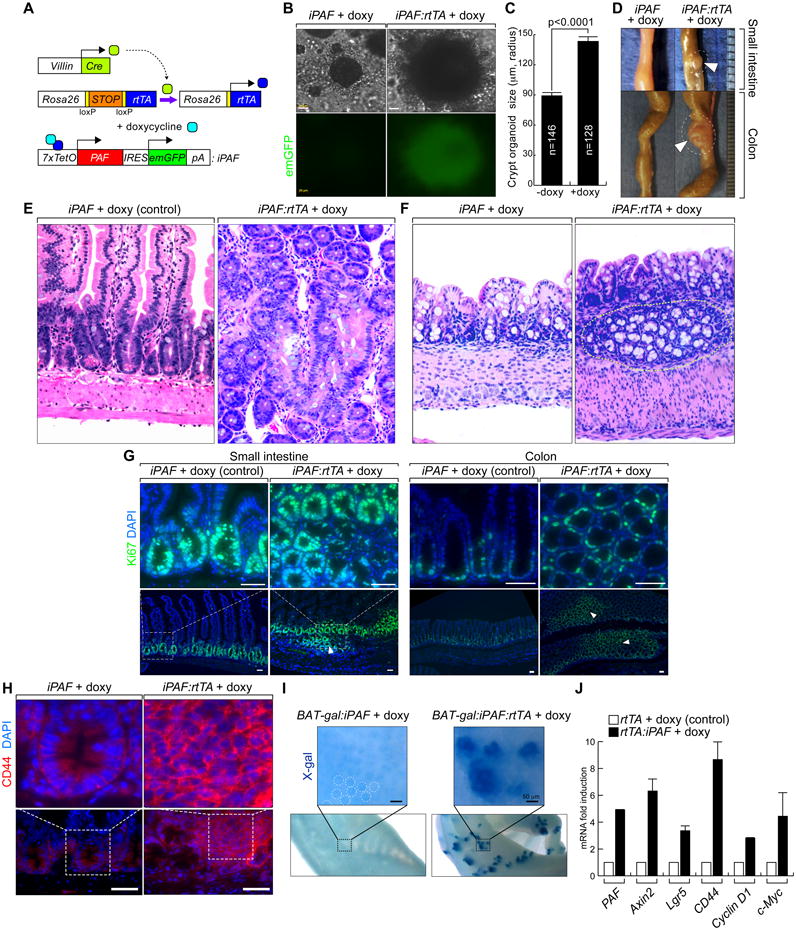
(A) PAF conditional inducible mouse models.
(B and C) Colonic crypts were isolated from Rosa26-rtTA:iPAF mice and maintained with or without doxy treatment (2 weeks). Phase-contrast images (B); quantification of the size of crypt organoids (C).
(D-F) Induction of PAF expression develops intestinal microadenoma.
Villin-Cre:Rosa26-LSL-rtTA:iPAF mice (experimental group) and iPAF mice (control) were given doxy (2 mg/ml in drinking water; small intestine [2 months] and colon [4 months]). Arrowheads: adenomas (D). H&E staining of small intestine (E) and colon (F). Dotted circles: aberrant crypt foci.
(G) IEC hyperproliferation by PAF. Villin-Cre:Rosa26-LSL-rtTA:iPAF and iPAF mice were given doxy (2 months). Ki67 immunostaining; arrowheads: hyperplastic lesions. Scale bars = 50 μm.
(H) Upregulation of CD44 by PAF.
CD44 immunostaining of small intestine specimens from control and PAF-induced (4 months) mice. Scale bars = 500 μm.
(I) PAF hyperactivates β-catenin reporter activity.
X-gal staining of iPAF:BAT-gal and Rosa26-rtTA:iPAF:BAT-gal mice (doxy [7 days]). Dotted circles: endogenous Wnt signaling activity in crypts.
(J) Upregulation of β-catenin target genes by PAF.
Crypts isolated from Rosa26-rtTA:iPAF mice were treated with doxy (1 μg/ml, 36 h) for qRT-PCR.
All error bars indicate standard deviation.
Discussion
Herein we reveal the unexpected role of PAF in modulating Wnt/β-catenin signaling. PAF enhances the transcription of Wnt targets by recruiting EZH2 to the β-catenin transcriptional complex. This is similar to the mechanism by which Lgl/BCL9 binds to β-catenin and thereby recruits the PHD-finger protein Pygopus, to bridge the β-catenin/TCF complex to Med12 and Med13 (Carrera et al., 2008). Importantly, due to specific overexpression of PAF in cancer cells, our studies identified an additional layer of the regulatory mechanism of β-catenin target gene transactivation.
In cancer cells, the upregulation of EZH2 contributes to tumorigenesis through the epigenetic repression of various genes including tumor suppressor genes, Wnt antagonists, and DNA repair genes (Chang et al., 2011; Cheng et al., 2011; Kondo et al., 2008). Our results propose a noncanonical function of EZH2 in activating β-catenin target genes in conjunction with PAF. Consistently, recent study also suggests methyltransferase activity-independent function of EZH2 in gene activation (Xu et al., 2012). Moreover, this non-canonical role of EZH2 is supported by several lines of evidence: (a) EZH2 transactivates β-catenin target genes (Li et al., 2009; Shi et al., 2007) (Figures 4E and 4F); (b) EZH2 overexpression in murine mammary epithelium induces ductal hyperplasia (Li et al., 2009), which phenocopies that in a ∆Nβ-catenin (constitutively active form of β-catenin) mouse model (Imbert et al., 2001); (c) EZH2 occupies β-catenin target promoters (Figures 4A-D); and (d) EZH2's methyltransferase activity is dispensable for β-catenin target activation (Figures 4H and 4I). Moreover, similar to PAF expression in the AER (Figure 2A), EZH2 is also specifically expressed there to maintain of Hox cluster gene transcription (Wyngaarden et al., 2011). Thus, it is plausible that EZH2 and PAF cooperatively control Hox gene activation in the developing limb. Interestingly, despite the presence of a physical and functional connection between Bmi1 and EZH2 in H3K27me3-mediated gene repression, EZH2 is expressed only in crypt IECs including ISCs (Figure S6), whereas Bmi1 is expressed in ISCs at position 4 (Figure 3J), implying a Bmi1-independent role for EZH2 in gene regulation. These results demonstrate the novel function of EZH2 in β-catenin target gene activation, independent of the histone methyltransferase activity of EZH2.
Previously, we found that TERT, a catalytic subunit of telomerase, positively modulates Wnt signaling (Park et al., 2009), elucidating a non-telomeric function of telomerase in development and cancer. Here our results propose that one component of DNA damage bypass process also functions in regulating Wnt signaling, dependent of context. In cancer, PAF overexpression may play a dual role in inducing (a) cell hyperproliferation (via Wnt signaling hyperactivation) and (b) the accumulation of mutations arising from DNA lesion bypass (by PAF-mediated TLS) (Povlsen et al., 2012). Importantly, PAF is only expressed in cancer cells, but not in normal epithelial cells. Thus, upon DNA damage, instead of cell growth arrest to permit high-fidelity DNA repair, the PAF overexpression in cancer cells is likely to induce DNA lesion bypass by facilitating TLS. However, in the setting of Wnt signaling deregulation, nuclear β-catenin sequesters PAF from PCNA and utilize PAF as a co-factor of transcriptional complex, which induces Wnt signaling hyperactivation and possibly lead to increased mutagenesis.
We observed that PAF marked the stemness of ISCs and mouse embryonic stem cells (Figure S7), implicating its roles in stem cell regulation under physiological conditions. In a previous study, a PAF germline knockout mouse model displayed defects in hematopoietic stem cell self-renewal (Amrani et al., 2011), suggesting a crucial role of PAF in stem cell maintenance and activation. In the intestine, β-catenin activation in Lgr5-positive or Bmi1-positive cells is sufficient to develop intestinal adenoma (Barker et al., 2009; Sangiorgi and Capecchi, 2008), suggesting an essential role of tissue stem cells in tumor initiation. Considering PAF expression in Bmi1-positive ISCs, PAF upregulation in ISCs likely hyperactivates the Wnt/β-catenin signaling and contributes to intestinal tumor initiation.
Despite the critical role of Wnt signaling in early vertebrate development, PAF germline knockout mice are viable (Amrani et al., 2011). It is noteworthy that, whereas deletion of any core component in the Wnt signaling pathway causes embryonic lethality, mice with germline knockout of Wnt signaling modulators, including Nkd1/2, Pygo1/2, and BCL9/9-2, exhibit no lethal phenotypes (Deka et al., 2010; Schwab et al., 2007; Zhang et al., 2007). This may result from the robustness of Wnt signaling during embryogenesis because of functional compensation not only via the presence of multiple Wnt signaling regulators per se but also via other types of signaling crosstalk. Therefore, as described previously in pRb studies (Sage et al., 2003), acute deletion of PAF in a conditional knockout mouse model may disrupt the developmental balance or tissue homeostasis, and then reveal the full spectrum of the physiological and pathological roles of PAF in tumorigenesis. Taken together, our findings reveal unexpected function of PAF and EZH2 in modulating Wnt signaling, and highlight the impacts of PAF-induced Wnt signaling deregulation on tumorigenesis.
Experimental Procedures
Oncomine database analysis
cDNA microarray datasets of colon adenocarcinoma and normal tissue samples from Oncomine (www.oncomine.org; September 2012 release) were analyzed to identify genes that are specifically expressed in colon cancer cells, (fold change > 2; P < 0.0001; top 10% ranked).
Immunohistochemistry
Tissue samples were collected and fixed in 10% formalin and processed for paraffin embedding. Sectioned samples were immunostained according to standard protocols. Information for antibodies is available in Supplemental Information.
Mammalian cell culture
Cell lines were maintained in Dulbecco's modified Eagle medium containing 10% fetal bovine serum. Cell fractionation was performed using NE-PER nuclear and cytoplasmic extraction reagents (Pierce). For gene depletion, at least 5 different shRNA lentiviruses (sh-PAF, sh-EZH2, sh- β-catenin) (Sigma; MISSION shRNA) were stably transduced into target cells using puromycin selection (1 to 2 μg/ml). LY294002, U0216, and Sorafenib were purchased from Sigma.
GST pull-down assay
GST, GST-GFP, PAF, and β-catenin proteins were purified from an Escherichia coli BL21 strain using a standard procedure. Each protein (0.1 µg) was incubated with HeLa cell lysates expressing binding proteins for 1 h and precipitated using glutathione sepharose 4B (GE Healthcare), and analyzed by immunoblotting.
Transgenic animals
A TetO minimal promoter-mPAF-IRES-EmGFP-BGHpA DNA fragment was injected into the pronucleus of the zygotes to generate transgenic iPAF mice. iPAF pups from three independent founder strains were utilized for analysis. PAF transgene expression was induced by doxy administration in the late generations crossed with C57BL/6 mice. All mice were maintained according to institutional guidelines and Association for Assessment and Accreditation of Laboratory Animal Care International standards.
Constructs
All constructs were generated from cDNA or open reading frame sources via PCR. Mutants were constructed using PCR-based mutagenesis.
Immunoblotting and immunoprecipitation
Whole cell lysates of mammalian cells were prepared and analyzed for IB and IP, as previously performed (Jung et al., 2013). Information for antibodies is available in Supplemental Information.
Axis duplication assays
X. laevis embryos were microinjected with in vitro transcribed mRNAs into ventrovegetal regions in four-cell-stage embryos, as previously performed (Park et al., 2009).
Statistical analysis
The Student t-test was used for comparisons of two samples. Calculation of average was performed using at least three biological replicas. P values less than 0.05 were considered significant. Error bars indicate standard deviation.
Full Experimental Procedures are available in Supplemental Information.
Supplementary Material
Highlights.
PAF is required for colon cancer cell proliferation via Wnt signaling activation
PAF-EZH2-β-catenin complex hyperactivates Wnt signaling
PAF facilitates EZH2-β-catenin association on β-catenin target promoters
PAF ectopic expression induces intestinal neoplasia.
Acknowledgments
We thank S. Cho, J. Chen, and L. Li for helpful comments on the manuscript; J. Chen, S. Cho, S. Kim, M. Lee, and A. Brunet for providing reagents; and W. Wang and I. Park for technical assistance. This work was supported by the Duncan Family Institute Research Program Grant, the University Cancer Foundation (IRG-08-061-01), American Association for Cancer Research - Pancreatic Cancer Action Network (11-20-25-PARK), Center for Stem Cell and Developmental Biology Transformative Pilot Grant (MD Anderson), Institutional Research Grant (MD Anderson), New Faculty Award (MD Anderson Cancer Center Support Grant), Metastasis Research Center Grant (MD Anderson), SPORE in Ovarian cancer (MD Anderson), and Mike Hogg Research Grant. Genetically Engineered Mouse Facility and DNA Analysis Core Facility were supported by the National Institutes of Health through MD Anderson's Cancer Center Support Grant, CA016672.
Footnotes
Author Contributions: J.-I.P. conceived the experiments; H.-Y.J., S.J., H.-C.K., M.L., X.W., H.J., and J.-I.P. performed the experiments; J.-I.P. and P.D.M. analyzed the results; and J.-I.P. wrote the manuscript.
References
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., 3rd BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Amrani YM, Gill J, Matevossian A, Alonzo ES, Yang C, Shieh JH, Moore MA, Park CY, Sant'Angelo DB, Denzin LK. The Paf oncogene is essential for hematopoietic stem cell function and development. J Exp Med. 2011;208:1757–1765. doi: 10.1084/jem.20102170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- Carrera I, Janody F, Leeds N, Duveau F, Treisman JE. Pygopus activates Wingless target gene transcription through the mediator complex subunits Med12 and Med13. Proc Natl Acad Sci U S A. 2008;105:6644–6649. doi: 10.1073/pnas.0709749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Yang JY, Xia W, Chen CT, Xie X, Chao CH, Woodward WA, Hsu JM, Hortobagyi GN, Hung MC. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell. 2011;19:86–100. doi: 10.1016/j.ccr.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AS, Lau SS, Chen Y, Kondo Y, Li MS, Feng H, Ching AK, Cheung KF, Wong HK, Tong JH, et al. EZH2-mediated concordant repression of Wnt antagonists promotes beta-catenin-dependent hepatocarcinogenesis. Cancer Res. 2011;71:4028–4039. doi: 10.1158/0008-5472.CAN-10-3342. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Deka J, Wiedemann N, Anderle P, Murphy-Seiler F, Bultinck J, Eyckerman S, Stehle JC, Andre S, Vilain N, Zilian O, et al. Bcl9/Bcl9l are critical for Wnt-mediated regulation of stem cell traits in colon epithelium and adenocarcinomas. Cancer Res. 2010;70:6619–6628. doi: 10.1158/0008-5472.CAN-10-0148. [DOI] [PubMed] [Google Scholar]
- Derks S, Bosch LJ, Niessen HE, Moerkerk PT, van den Bosch SM, Carvalho B, Mongera S, Voncken JW, Meijer GA, de Bruine AP, et al. Promoter CpG island hypermethylation- and H3K9me3 and H3K27me3-mediated epigenetic silencing targets the deleted in colon cancer (DCC) gene in colorectal carcinogenesis without affecting neighboring genes on chromosomal region 18q21. Carcinogenesis. 2009;30:1041–1048. doi: 10.1093/carcin/bgp073. [DOI] [PubMed] [Google Scholar]
- Emanuele MJ, Ciccia A, Elia AE, Elledge SJ. Proliferating cell nuclear antigen (PCNA)-associated KIAA0101/PAF15 protein is a cell cycle-regulated anaphase-promoting complex/cyclosome substrate. Proc Natl Acad Sci U S A. 2011;108:9845–9850. doi: 10.1073/pnas.1106136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussbroich B, Wagener N, Macher-Goeppinger S, Benner A, Falth M, Sultmann H, Holzer A, Hoppe-Seyler K, Hoppe-Seyler F. EZH2 depletion blocks the proliferation of colon cancer cells. PLoS One. 2011;6:e21651. doi: 10.1371/journal.pone.0021651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goentoro L, Kirschner MW. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol Cell. 2009;36:872–884. doi: 10.1016/j.molcel.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–3058. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001;153:555–568. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and cellular biology. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P, Carrington EA, Wang L, Ketel CS, Miller EL, Jones RS, Simon JA. Dominant alleles identify SET domain residues required for histone methyltransferase of Polycomb repressive complex 2. J Biol Chem. 2008;283:27757–27766. doi: 10.1074/jbc.M804442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HY, Wang X, Jun S, Park JI. Dyrk2-associated EDD-DDB1-VprBP E3 ligase inhibits telomerase by TERT degradation. J Biol Chem. 2013;288:7252–7262. doi: 10.1074/jbc.M112.416792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengaku M, Capdevila J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Izpisua Belmonte JC, Tabin CJ. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, Yamochi T, Urano T, Furukawa K, Kwabi-Addo B, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- Kuzmichev A, Margueron R, Vaquero A, Preissner TS, Scher M, Kirmizis A, Ouyang X, Brockdorff N, Abate-Shen C, Farnham P, et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci U S A. 2005;102:1859–1864. doi: 10.1073/pnas.0409875102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gonzalez ME, Toy K, Filzen T, Merajver SD, Kleer CG. Targeted overexpression of EZH2 in the mammary gland disrupts ductal morphogenesis and causes epithelial hyperplasia. Am J Pathol. 2009;175:1246–1254. doi: 10.2353/ajpath.2009.090042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Molecular and cellular biology. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Povlsen LK, Beli P, Wagner SA, Poulsen SL, Sylvestersen KB, Poulsen JW, Nielsen ML, Bekker-Jensen S, Mailand N, Choudhary C. Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass. Nat Cell Biol. 2012;14:1089–1098. doi: 10.1038/ncb2579. [DOI] [PubMed] [Google Scholar]
- Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol. 2012;13:141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab KR, Patterson LT, Hartman HA, Song N, Lang RA, Lin X, Potter SS. Pygo1 and Pygo2 roles in Wnt signaling in mammalian kidney development. BMC Biol. 2007;5:15. doi: 10.1186/1741-7007-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B, Liang J, Yang X, Wang Y, Zhao Y, Wu H, Sun L, Zhang Y, Chen Y, Li R, et al. Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells. Mol Cell Biol. 2007;27:5105–5119. doi: 10.1128/MCB.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nature reviews Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Wyngaarden LA, Delgado-Olguin P, Su IH, Bruneau BG, Hopyan S. Ezh2 regulates anteroposterior axis specification and proximodistal axis elongation in the developing limb. Development. 2011;138:3759–3767. doi: 10.1242/dev.063180. [DOI] [PubMed] [Google Scholar]
- Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, Wu X, Stack EC, Loda M, Liu T, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Huang B, Shen M, Lau C, Chan E, Michel J, Xiong Y, Payan DG, Luo Y. p15(PAF), a novel PCNA associated factor with increased expression in tumor tissues. Oncogene. 2001;20:484–489. doi: 10.1038/sj.onc.1204113. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cagatay T, Amanai M, Zhang M, Kline J, Castrillon DH, Ashfaq R, Oz OK, Wharton KA., Jr Viable mice with compound mutations in the Wnt/Dvl pathway antagonists nkd1 and nkd2. Mol Cell Biol. 2007;27:4454–4464. doi: 10.1128/MCB.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


