Abstract
Background
Eosinophilia is a marker of corticosteroid responsiveness and risk of exacerbation in asthma; while it has been linked to submucosal matrix deposition, its relationship to other features of airway remodelling is less clear.
Objective
The aim of this study was to investigate the relationship between airway eosinophilia and airway remodelling.
Methods
Bronchial biopsies from subjects (n=20 in each group) with mild steroid-naïve asthma, with either low (0 – 0.45 mm−2)) or high submucosal eosinophil (23.43 – 46.28 mm−2) counts and healthy controls were assessed for in vivo epithelial damage (using EGFR staining), mucin expression, airway smooth muscle (ASM) hypertrophy and inflammatory cells within ASM.
Results
The proportion of in vivo damaged epithelium was significantly greater (p=0.02) in the eosinophil-high (27.37%) than the eosinophil-low (4.14%) group. Mucin expression and goblet cell numbers were similar in the two eosinophil groups; however, MUC-2 expression was increased (p=0.002) in the eosinophil-high group compared to controls. The proportion of submucosa occupied by ASM was higher in both asthma groups (p=0.021 and p=0.046) compared to controls. In the ASM, eosinophil and T lymphocyte numbers were higher (p<0.05) in the eosinophil-high group than both the eosinophil-low group and the controls, whilst the numbers of mast cells were increased in the eosinophil-high group (p=0.01) compared to controls.
Conclusion
Submucosal eosinophilia is a marker (and possibly a cause) of epithelial damage and is related to infiltration of airway smooth muscle with eosinophils and T lymphocytes but is unrelated to mucus metaplasia or smooth muscle hypertrophy.
Keywords: Asthma, eosinophil, remodelling, epithelium, goblet cell, smooth muscle, inflammation
INTRODUCTION
Airway eosinophilia and remodelling are hallmarks of asthma [1–4] but how they are related is not well established. It is increasingly recognised that asthma has several phenotypes [5,6], including eosinophilic and non-eosinophilic, which may reflect different underlying pathologies. The eosinophilic phenotype has been linked to steroid responsiveness, asthma exacerbations and severe or fatal asthma [7–15]. Recent analysis of pooled data of the sputum counts from several clinical trials reports almost equal incidence of eosinophilic and non-eosinophilic phenotypes in mild-moderate asthma [16]. The use of cluster analysis has also identified several further clinical [17] and mixed clinical/pathological phenotypes [5,18].
Eosinophils have the potential to drive airway remodelling [19]. Reduction of eosinophil numbers in the airway mucosa with anti-IL-5 monoclonal antibody treatment, Mepolizumab, [20–23], reduces asthma exacerbations [21–23] and also the expression of extra-cellular matrix proteins in parallel with attenuated expression of the pro-fibrotic tissue transforming growth factor (TGF)-β [20]. In further support of the concept, increased thickening of the lamina reticularis has been reported in both severe [7,9] and mild-moderate asthmatics [18] with airway eosinophilia when compared to asthmatics of similar severity but without eosinophilia. Moreover, Minshall et al [24] showed a correlation between TGFβ expression, asthma severity and submucosal collagen deposition and, although their study did not show a direct relationship between eosinophil numbers and the extent of submucosal fibrosis, eosinophils were identified as the main source of TGFβ and their numbers correlated with lung function.
In this study we have focused on identifying the relationship between eosinophilia and other features of airway remodelling, including epithelial damage, goblet cell hyperplasia and smooth muscle hypertrophy. First, we sought to elucidate the relationship between eosinophils and epithelial changes. Whilst post-mortem studies have shown evidence of epithelial damage in asthma [25,26], confirmation of this in biopsies from living asthmatics has been difficult [27–30] primarily because of difficulties in distinguishing in vivo acquired damage and artefactual damage during bronchoscopic biopsy [29]. In this study we used the expression of Epidermal Growth Factor receptor (EGFR) to distinguish between epithelium damaged in vivo from that damaged during bronchoscopic sampling and/or biopsy processing. Our second hypothesis was that eosinophilia is a determinant of goblet cell hyperplasia. Whilst some studies have shown increased mucus in the airways of patients with severe or mild asthma [2–4], other studies have not [28], possibly reflecting differences in asthma severity, treatment or the degree of accompanying inflammation, but none of the studies to date have sought to link mucus hyperplasia and inflammation. Finally, we hypothesised that eosinophilic infiltration of airway smooth muscle (ASM) may be another hallmark of asthma and that this may be related to the mast cell and T lymphocyte infiltration previously reported to be increased in ASM in patients with asthma, particularly those with severe disease [31–33].
METHODS
Study design and subjects
For this current study atopic asthma subjects were randomly selected from a pool of 103 steroid naive subjects with mild-moderate asthma who had undergone full clinical evaluation and baseline bronchoscopy with collection of bronchial biopsies, as part of a previously published study [34]. For inclusion in the original study [34] the asthmatic subjects had to have a diagnosis of asthma for ≥6 months, with FEV1 between 60–90% predicted, reversibility to β-agonists ≥12% or a PC20 to methacholine <8mg/ml if they did not demonstrate reversibility. They had to be atopic and non-smoker for at least one year, with a history of less than ten pack-years. For 6 weeks prior to bronchoscopy subjects were on their “standard” asthma treatment that included no other asthma medication than a short-acting b2 agonist on demand and free from respiratory tract infections, and they should not have been hospitalised for asthma within the past 12 months.
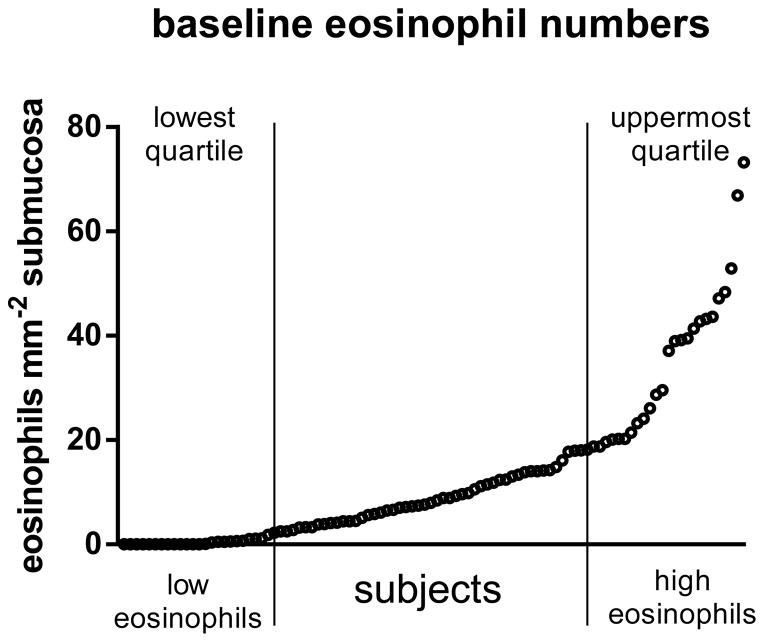
Biopsies were obtained and processed as previously described [34] and all samples were shipped for analysis at a single site. The biopsies were evaluated for eosinophil numbers by staining immunohistochemically (IHC) with the anti-eosinophil cationic antibody, EG2. As the eosinophil data were not normally distributed the subjects stratified into those with high submucosal eosinophil (eos-high, 39.04 eosinophils mm−2 submucosa) counts and those with low eosinophil (eos-low, zero eosinophils) counts using the upper and lower quartiles of the whole dataset (figure 1). Subjects were selected for analysis using a standard random selection method by a person independent from the study and blinded to the subject details. Additionally bronchial biopsies were collected from 20 healthy, non-atopic controls subjects with a PC20 metacholine greater than 16 mg/ml. The subject characteristics are summarised in table 1. Subject groups were age matched. The mean FEV1 % predicted did not differ between the eos-low (77% predicted) and eos-high (76% predicted) asthma groups but was significantly (p<0.001) lower than in control subjects (98% predicted). The bronchial biopsies were further analysed by immunohistochemistry for epithelial damage, mucin expression, and hypertrophy of and inflammatory cell influx into the ASM.
Figure 1. Eosinophil distribution in steroid naïve asthma.
Subjects were stratified based on submucosal eosinophil numbers. The range for the whole cohort was zero to 73.21 eosinophills mm−2. 20 subjects were randomly selected from the lowest quartile (0–2.21 eosinophils mm−2)(low eosinophils) and 20 from the uppermost quartile (18.41–73.21 eosinophils mm−2) (high eosinophils).
Table 1.
Subject Characteristics
| Characteristic | Healthy controls | Low Eosinophils | High Eosinophils |
|---|---|---|---|
| Number of subjects | 20 | 20 | 20 |
| Age (years) | 27.8 (19–43) | 29.2 (20–55) | 30.5 (18–57) |
| Gender: F/M | 15/5 | 14/6 | 10/10 |
| Duration of asthma: >=6 months to <1 year | not applicable | 0 | 1 |
| >=1 year to <5 years | 3 | 4 | |
| >=5 years to <10 years | 5 | 3 | |
| >=10 years to <15 years | 3 | 4 | |
| >=15 years | 9 | 8 | |
| Tobacco use: Former/never smoker | 0/20 | 6/14 | 6/14 |
| FEV1 (% predicted) | 97.9 (81.7–119.0) | 77.1 (61.9–87.7) | 75.7 (60.0–88.9) |
| Reversibility (FEV1 % change) | not applicable | 15.5 (0.6–42.3) | 15.7 (7.4–29.9) |
| PC20 methacholine (mg/ml) Submucosal eosinophils (cells mm−2) |
>16 | 1.2 (0.5–2.4)(n=2) 0 (0–0.45) |
4.1 (1.2–7.0)(n=5) 39.04 (23.43–46.28) |
| 3.58 (0–6.20) |
Clinical data are means (ranges), cell data are medians and interquartile ranges
The study was conducted in accordance with the principles of the Declaration of Helsinki. Approval was obtained from the local ethics committee at each clinical centre (see acknowledgements for approval numbers) and subjects gave written informed consent.
Assessment of epithelial Integrity
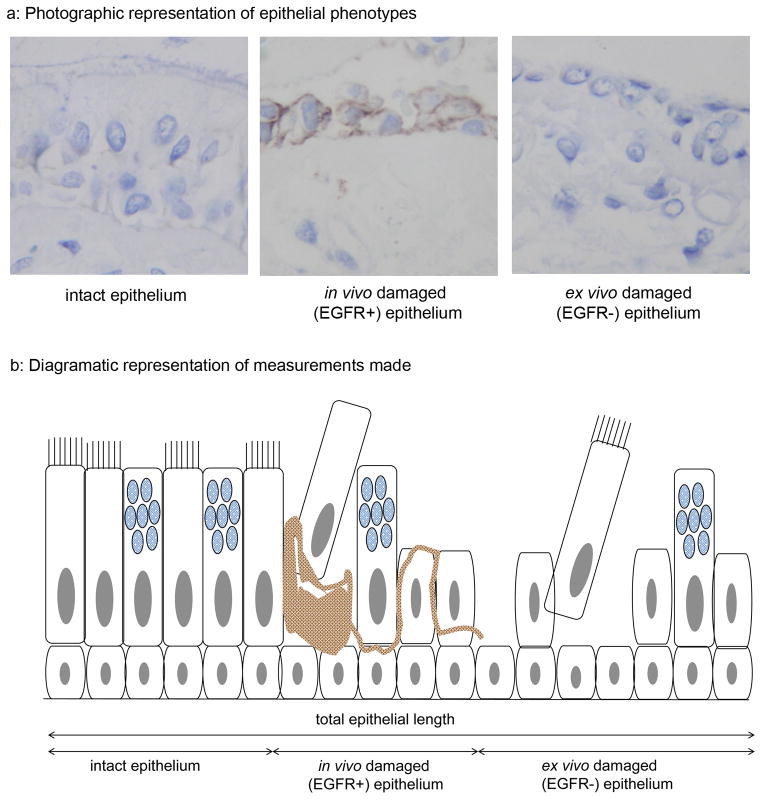
All biopsies containing epithelium were analysed to avoid selection bias. Sections were stained for epidermal growth factor receptor (EGFR) (rabbit anti-EGFR Cell Signalling, Hitchin, UK). This has previously been shown by Puddicombe et al [35] to enable distinction of epithelium with a damaged morphology acquired in vivo - consequently staining positively for EGFR - from that damaged during biopsy and/or biopsy processing and, therefore, displaying negative immunoreactivity for EGFR. Using computer assisted image analysis (Zeiss KS400, Image Associates, Bicester, UK) the following epithelial lengths were measured: total epithelial length, intact epithelium (full height well orientated with a pseudostratified appearance) in vivo damaged epithelium (EGFR+) and artefactually damaged epithelium (EGFR−)(figure 2).
Figure 2. Assessment of epithelial integrity.
Staining for EGFR was used to distinguish epithelium that had sustained damage in vivo (EGFR+)(a: middle plate) from that that had been damaged during bronchoscopy or processing (EGFR−)(a: right hand plate). Intact undamaged epithelium was EGFR negative (a: left hand plate). The total epithelial length and the lengths of intact, in vivo and ex vivo damaged epithelium were measured using computer assisted image analysis (b).
Goblet cell hyperplasia and epithelial mucin phenotype
All biopsies with intact epithelium in each of the subject groups were immunostained with monoclonal antibodies to MUC2 (BD Pharmingen, Oxford, UK), MUC4 (Zymed laboratories, Paisley, UK), MUC5AC (Chemicon, Southampton, UK) and MUC5B (Chemicon). The percentage of stained epithelium for each mucin was assessed by computerised image analysis and results expressed as area ratio, based on the red/green/blue colour composition of the DAB staining [35] within the intact epithelium. Additionally, sections were stained by the periodic acid Schiff (PAS) technique to identify all goblet cells. The numbers of PAS positive goblet cells were counted and reported as a percentage of the total number of epithelial cells.
Smooth muscle proportion and inflammatory cell infiltration
Sections from all biopsies containing smooth muscle were stained with alpha smooth muscle actin (Sigma, Poole, UK) to delineate the ASM and with antibodies to mast cells (AA1+, Abcam, Cambridge, UK), eosinophils (EG2+, Diagnostics Development, Upsala, Sweden), neutrophils (elastase+, Dako, Ely, UK) and T lymphocytes (CD3+, AbD Serotec, Kidlington, UK). A point counting grid was used on the sections stained for alpha smooth muscle actin in order to assess the muscle proportion, i.e. the volume fraction (proportion)[36] of ASM within the mucosa. The nucleated inflammatory cells within the smooth muscle were also counted and expressed relative to the area of ASM measured by computerised image analysis.
Statistical Analysis
Non-parametric statistics were used to analyse the results of Immunohistochemical analysis. The Kruskall-Wallis ANOVA test was initially used to test for differences between the 3 groups, with p <0.05 considered statistically significant. If a significant difference was observed between the three groups, the Mann Witney U test was used for further analyses.
RESULTS
The results of immunohistochemical analysis are presented in figures 3 to 5 and in table 1 in the online supplement.
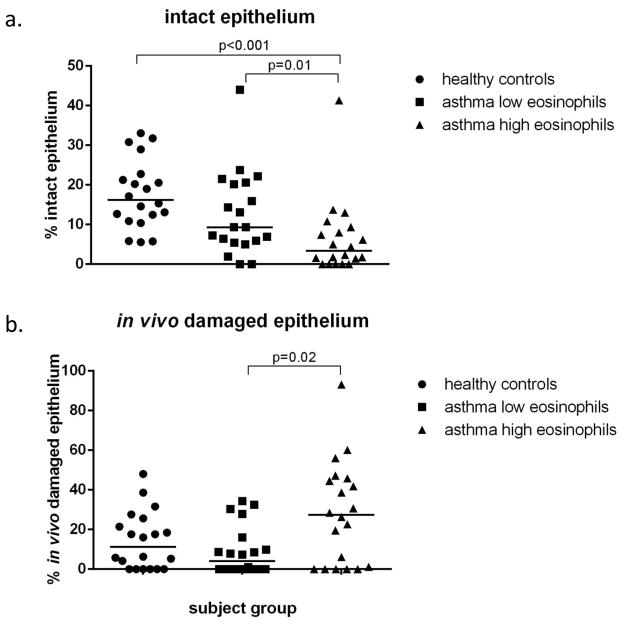
Figure 3. Integrity of the epithelium.
The percentage of intact epithelium in the high eosinophil group is decreased compared to both healthy controls and asthmatics with low eosinophil counts (a). The in vivo damaged epithelium is increased in the eosinophil high group only in comparison to the eosinophil low group (b).
Figure 5. Influx of inflammatory cells into the airway smooth muscle.
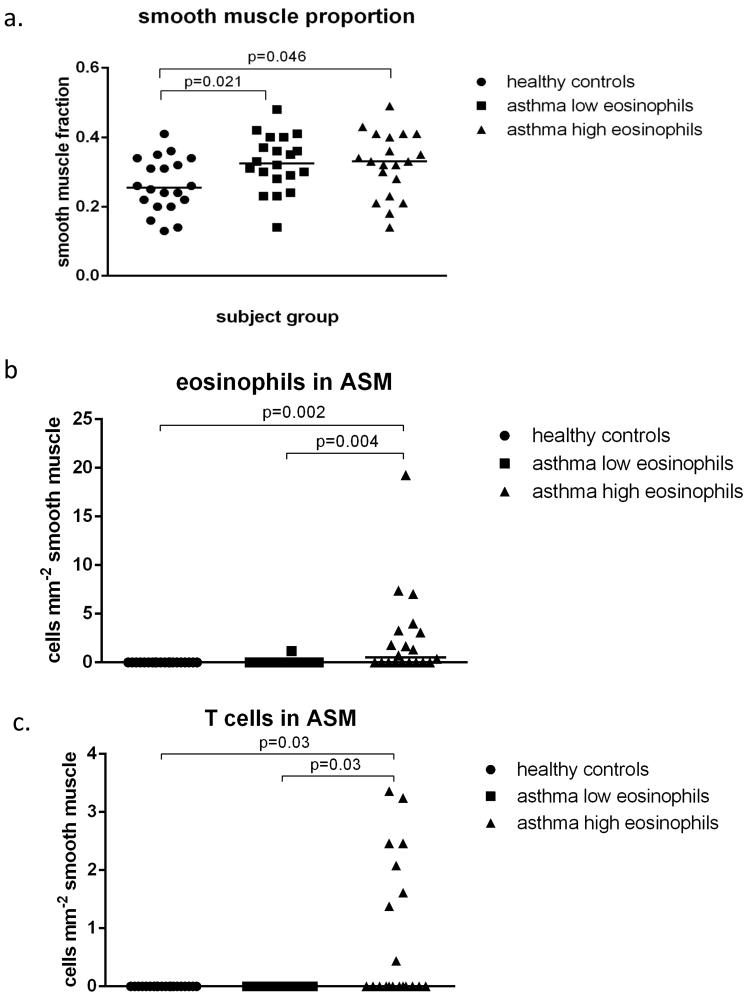
Immunohistochemical staining showing the influx of eosinophils (a), T cells (b) and mast cells (c) into the smooth muscle. Inflammatory cells are stained red and those within the ASM bundle were counted (↑). Scale bar is 50μm.
Integrity of the epithelium
Assessment of epithelial integrity was made in a mean epithelial length of 13.44 mm in a mean of 4.59 biopsies per subject. The length of intact and in vivo damaged (EGFR+) epithelium differed between the 3 groups (Kruskal Wallis p<0.001 and p=0.04 respectively), whilst the length of artefactually damaged (EGFR−) epithelium did not (p=0.256; data not shown). Further analysis showed that the length of intact epithelium with a pseudostratified appearance was significantly lower in the eos-high group (3.35%) compared to both the eos-low group (9.32%) (p=0.01) and the healthy control subjects (16.24%) (p<0.001) (Figure3a). Also there was significantly more (p=0.02) in vivo damaged (EGFR+) epithelium in the eos-high group (27.37%) when compared to the eos-low group (4.14%) but not the healthy control subjects (figure 3b).
Goblet cell hyperplasia and epithelial mucin phenotype
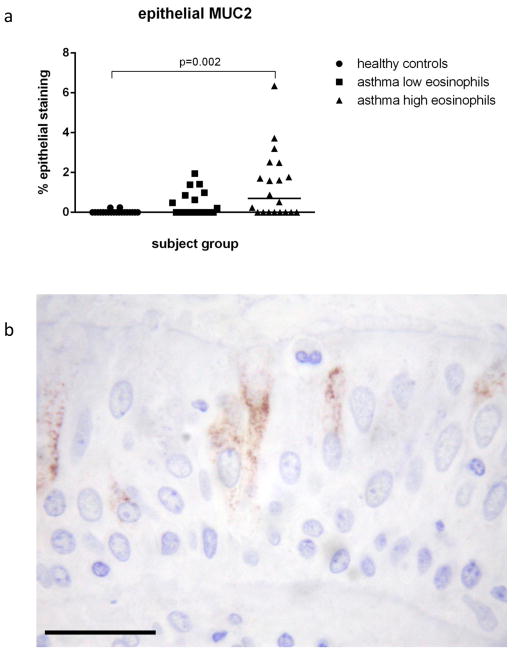
Assessment of goblet cell hyperplasia and epithelial mucin phenotype was made in a mean intact epithelial length of 1.94 mm and in a mean of 2.75 biopsies per subject. No goblet cell hyperplasia was observed in the asthmatic groups (on-line supplement table) as the percentage of PAS positive cells did not differ between the groups. No staining for MUC4 was observed in any of the biopsies. There was a significant difference (Kruskal Wallis p<0.001) in the expression of MUC2 between the three groups but no differences in MUC5AC or MUC5B. Further analysis showed higher MUC2 expression in the eos-high asthmatics (0.70%) than the control subjects (0.0%) (p=0.002) but not the eos-low asthmatics (0%) (figure 4a). A representative photograph of MUC2 staining is shown in figure 4b.
Figure 4. Epithelial mucin phenotype.
Epithelial expression of MUC2 is higher in asthmatics with high eosinophil counts compared to healthy controls but not those with low eosinophil counts (a). Immunohistochemical staining (brown) for MUC2 can be seen within the goblet cells. Scale bar is 50μm.
Smooth muscle proportion and inflammatory cells
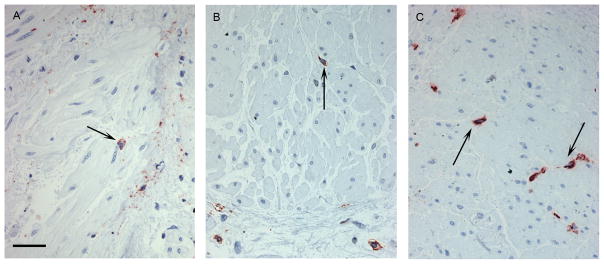
Representative photographs showing inflammatory cell influx into the ASM are shown in figure 5. A mean of 4.22 biopsies with a mean ASM area of 1.31mm2 per subject were analysed. 79% of biopsies collected contained smooth muscle. There were significant differences between the three groups in the proportion of smooth muscle (Kruskal Wallis, p=0.04) and the numbers of inflammatory cells within this layer (Kruskal Wallis, eosinophils p<0.001, mast cells p=0.023 and T lymphocytes p<0.001). Further analysis indicated that the proportion of ASM occupying the submucosa was similar in the two asthmatic groups but was significantly higher in both the eos-high (33%) (p=0.046) and eos-low (33%) (p=0.021) groups when compared to healthy control subjects (26%) (figure 6a). The number of neutrophils within the ASM did not differ between the groups, while the numbers of eosinophils within the smooth muscle were significantly higher in the eos-high group (0.54 cells mm−2) when compared both to the eos-low group (0) and the control subjects (0) (p=0.004, p=0.002) (figure 6b). The numbers of mast cells were also higher in the eos-high group (12.88 cells mm−2) compared to the control subjects (4.15 cells mm−2)(p=0.008) but not when compared to the eos-low group (7.12 cells mm−2) (figure 6d). Finally, the numbers of T cells were higher in the eos-high group compared to both the eos-low group (p=0.03) and the controls (p=0.03) (figure 6c).
Figure 6. Smooth muscle proportion and inflammatory cell influx.
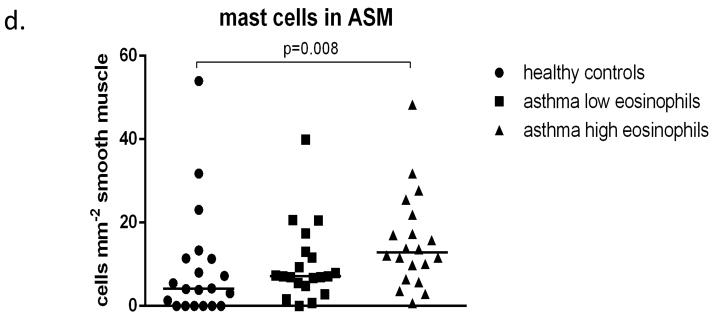
The proportion of airway smooth muscle (ASM)(a) is increased in asthmatics with low and high eosinophil counts compared to healthy controls. Positive immunostained nucleated cells were counted in the ASM. More eosinophils (b) and T cells (c) are observed in the ASM of the asthmatics with high eosinophils counts compared to those with low counts and to the healthy controls. Mast cell numbers (d) are increased in subjects with high eosinophil counts compared to healthy controls but not low eosinophil counts.
DISCUSSION
We have observed in steroid-naïve asthma, that high eosinophil numbers in the bronchial submucosa was associated with a greater degree of epithelial damage and increased numbers of eosinophils and T lymphocytes within the ASM. This extends previous studies in severe and mild-moderate asthma [6–10,15,16] by demonstrating that eosinophilic and non-eosinophilic phenotypes can also be identified in mild to moderate asthma and are associated with some features of remodelling. Our study also shows that increased epithelial MUC2 expression and increased ASM mast cells are seen mainly in asthmatic subjects with high submucosal eosinophil counts, adding to the knowledge of mast cell myositis previously described as a hall-mark of asthma [31]. Finally, ASM hypertrophy was independent of concurrent eosinophilia. These important differences in airways pathology related to the extent of eosinophilic airway inflammation add to the notion that eosinophilia is an important marker of pathological as well as clinical phenotypes of asthma.
The issue of epithelial damage in asthma has been a matter of controversy [27–29]. In order to address the concerns that the observed damage may be an artefact of biopsy or tissue processing [29], in the current study we used EGFR immunostaining as a means of identifying true, in vivo acquired, damage (figure 2). The basis for this approach was the finding by Puddicombe et al [35] that in epithelial monolayer cultures damaged physically by scrape wounding and allowed to repair spontaneously the epithelial cells were negative for EGFR immediately after wounding but exhibited positive immunoreactivity after 3 hours that was still evident at 9 hours. In the same study, intense expression of EGFR was reported in the epithelium of bronchial biopsies from asthmatic patients. Here we observed that, when compared to healthy control subjects, epithelial integrity was reduced in asthmatics with high eosinophil counts, but not in those with low counts (figure 3a). This is in concordance with the study of Benayoun et al [30] which showed decreased epithelial integrity in subjects with intermittent asthma with eosinophil counts of 25.7/mm of length of epithelial basement membrane but not in asthmatics with <10 eosinophils/m2 of mucosa. The percentage of in vivo damaged epithelium (EGFR+) correlated with eosinophilia (figure 3c) and was greater in eos-high than in eos-low asthmatics (figure 3b), in whom the damage was similar to the control group. This supports the argument that treatment that targets eosinophilic inflammation (e.g. corticosteroids) may also help in healing epithelial damage [30,37].
The association between increased eosinophil counts and decreased epithelial integrity suggests that eosinophils may damage the epithelium, probably through release of granule proteins, including major basic protein and eosinophil cationic protein, which were shown to induce epithelial cell damage in vitro [38–42]. On the other hand, eosinophils may be simply a biomarker of a more complex process involving several inflammatory cells and a susceptible epithelium.
In this study we have not seen evidence of goblet cell hyperplasia. This remodelling feature has been associated previously with severe asthma [43–45], but reports in milder asthma have been less clear. Similar to the current study, Lozewicz et al [28], who employed a point counting method, did not observe any difference in the percentage of epithelial cells that were goblet cells, whereas Ordonez et al [46] reported an increase in the number of goblet cells per unit of epithelial volume. There have been reports [18,46] of an increase in mucin volume (hypertrophy) in mild asthma, a measure that was not conducted in our study. The finding of a small but significant increase in MUC2 is, to our knowledge, novel. Previous studies have reported increased mRNA for MUC2 in the bronchial mucosa in asthma [87,46,47]; thus our study suggests that this is being translated into protein. MUC2 is reported to be a minor component of airway mucus. However, its presence changes the physical properties of mucus and makes it more difficult to remove from the airways [48] and thus may contribute to mucus accumulation and plugging which are features of asthma [2–4].
The increase in the proportion of ASM observed in both asthma groups concurs with previous reports [30,49]. The mechanism by which this hypertrophy occurs is unclear. It may be due to increased smooth muscle cell size or cell numbers. Woodruff et al [49] have reported no change in cell size but an increase in cell numbers, whereas Benayoun et al [30] have reported an increase in cell size but no evidence of smooth cell proliferation which could lead to increases cell numbers. These studies have used different methodological approaches, which may explain the discrepancies in the data. In another study Stewart et al [50] suggested that hypertrophy is due to a reduction in smooth muscle cell apoptosis which leads to an increase in cell numbers. Further studies are needed to clarify the mechanisms leading to the increase in smooth muscle.
Finally, we have found mild eosinophilic and T lymphocytic inflammation in the ASM layer. Together with observations of elevated numbers of mast cell [31–33], this would suggest that ASM inflammation is an important feature of asthma. The complex inter-play between these inflammatory cells and the ASM is beyond the scope of this paper but is likely to include a range of chemoattractants and growth factors [51,52]. Neither eosinophils nor T cells were observed in the healthy controls or the eos-low group, but there was a significant infiltration compared to both of these groups in the eos-high group, which would suggest a spill-over of the classic Th2 type inflammation into the ASM. In contrast, mast cells, although raised in asthmatics with high eosinophil counts, were also present in significant numbers in healthy subjects, suggesting that these cells also play a role in maintaining a normal function for ASM, although the nature of this interaction remains unclear. Previously, Berry et al [7] have shown mast cell infiltration into the ASM to be a feature of both non-eosinophilic and eosinophilic asthma when subjects were stratified based on sputum eosinophilia. However, in their study the number of submucosal eosinophils was considerably higher (median 4.4mm−2) than the numbers in our low-eos group (median 0mm−2). This may explain the differences in findings between our study and that of Berry and colleagues.
In summary, we have demonstrated that the proportion of in vivo damaged epithelium is increased in patients with asthma when compared to normal control subjects and that it is related to submucosal eosinophilia. We have also found that some features of airway remodelling (i.e. mucin expression, ASM proportion and goblet cell numbers) are pronounced in asthma irrespective of eosinophilia while others (i.e. MUC-2 expression) are noted in association with eosinophil infiltration. The number of eosinophils and T lymphocytes that infiltrate the ASM layer is related to submucosal eosinophil counts in asthmatics who have high eosinophil counts. These findings indicate that submucosal eosinophilia is related to and, therefore, a biomarker of epithelial damage and infiltration of eosinophils and T lymphocytes into the ASM. Further studies are required to dissect the role of eosinophils (independently or via interaction with other inflammatory cells) in the pathogenesis of airway remodelling.
Supplementary Material
Acknowledgments
The authors would like to thank ADISNET for providing archival biopsies for this study. Ethics approval numbers for each centre are given in parentheses. ADISNET members providing bronchial biopsies: Wake Forest University School of Medicine (BG01-585); University of Wisconsin School of Medicine (2001-580); University of Virginia (10570); Laval Hospital and University (994); Medical College of Georgia (01-06-271); University of Kentucky (01-0468-F1V); Meakins Christie Laboratories (MCHC#3-40); Brigham and Women’s Hospital (2002-P-00125011); University of Nebraska Medical Center (240-01-FB); University of California at Los Angeles (03-03-031-01); Cleveland Clinic Foundation (6254).
FUNDING
This work was funded by a research grant from GlaxoSmithKline
ABBREVIATIONS
- ASM
airway smooth muscle
- DAB
diamino benzidine
- EGFR
epidermal growth factor receptor
- FEV1
forced expiratory volume in 1 second
- IHC
immunohistochemistry
- mRNA
messenger ribonucleic acid
- PAS
periodic acid Schiff
- TGFb
transforming growth factor beta
Footnotes
CONFLICTS OF INTEREST
Declaration of all sources of funding:
Dr Wilson: a research grant for this study from GlaxoSmithKline
Dr Laviolette: participation in clinical trials on asthma treatment for GlaxoSmithKline, AstraZeneca, Boston Scientific and Johnson & Johnson and a research grant for this study from GlaxoSmithKline.
Dr Jarjour: a research grant for this study from GlaxoSmithKline
Professor Djukanovic: a research grant for this study from GlaxoSmithKline.
References
- 1.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 2.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122:320S–326S. doi: 10.1378/chest.122.6_suppl.320s. [DOI] [PubMed] [Google Scholar]
- 3.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4:241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Ann Rev Physiol. 2008;70:405–429. doi: 10.1146/annurev.physiol.70.113006.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halder P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 7.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, Bradding P, Wardlaw AJ, Pavord ID. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll N, Carello S, Cooke C, James A. Airway structure and inflammatory cells in fatal attacks of asthma. Eur Respir J. 1996;9:709–715. doi: 10.1183/09031936.96.09040709. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiological and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 10.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma. Proc Am Thorac Soc. 2009;6:256–259. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- 12.Pavord ID, Bigthling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353:2213–2214. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- 13.Green RH, Birightling CE, Mckenna S, hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomized controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 14.Pizzichini MMM, Pizzichini E, Clelland L, Efthimiadis A, Pavord I, Dolovoich J, Hargreave FE. Prednisone-dependant asthma: inflammatory indices in induced sputum. Eur Respir J. 1999;13:15–21. doi: 10.1183/09031936.99.13101599. [DOI] [PubMed] [Google Scholar]
- 15.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. Analayses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chincilli VM, Fahy JV. A large subgroup of mild-moderate asthma is persistently non-eosinophilic. Am J Respir Crit Care Med. 2012:185612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore WC, Meyers DA, Li H, D’Agostino R, Peters SP, Bleecker ER. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2009;179:A2522. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Arron JR, Koth LL, Fahy JV. T-helper type 2-driven inflammation defines major subtypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19:681–686. doi: 10.1016/j.coi.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subpepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacebations of refractor eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair P, Pizzichini MMM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 23.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicenter, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 24.Minshall EM, Leung DYM, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-b1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 25.Dunnill MS. The pathology of asthma with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houston JC, De Navasquez S, Trounce JR. A clinical and pathological study of fatal cases of status asthmaticus. Thorax. 1953;8:207–213. doi: 10.1136/thx.8.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985;131:599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- 28.Lozewicz S, Wells C, Gomez E, Ferguson H, Richman P, Devalia J, Davies RJ. Morphological integrity of the bronchial epithelium in mild asthma. Thorax. 1990;45:12–15. doi: 10.1136/thx.45.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ordonez C, Ferrando R, Hyde DM, Wong HH, Fahy JV. Epithelial desquamation in asthma: artifact or pathology? Am J Respir Crit Care Med. 2000;162:2324–2329. doi: 10.1164/ajrccm.162.6.2001041. [DOI] [PubMed] [Google Scholar]
- 30.Benayoun L, Druilhe A, Dombret M-C, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 31.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 32.Begueret H, Berger P, Vernejoux J-M, Dubuisson L, Marthan R, Tunon-de-Lara JM. Inflammation of bronchial smooth muscle in allergic asthma. Thorax. 2007;62:8–15. doi: 10.1136/thx.2006.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, Castro M, Gaston B, Israel E, Schwartz LB, Curran-Everett D, Moore CG, Wenzel SE. Mast cell phenotype, location, and activation in severe asthma: data from the severe asthma research program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Djukanovic R, Wilson SJ, Moore WC, Koenig SM, Laviolette M, Bleecker ER, Davis WB, Doherty DE, Olivenstein R, Israel E, Kavuru MS, Kellrup E, Reilly DS, Yancey SW, Edwards LD, Stauffer JL, Dorinksy PM, Jarjour NN. Montelukast added to fluticasone propionate does not alter inflammation or outcomes. Resp Med. 2010;104:1425–1435. doi: 10.1016/j.rmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 36.Howard CV, Reed MG. Unbiased stereology. 2. BIOS Sientific Publishers; New York: 2005. [Google Scholar]
- 37.Vignola AM, Chiappara G, Siena L, Bruno A, Gagliardo R, Merendino A-M, Polla BS, Arrigo AP, Bonsignore G, Bousquet J, Chanez P. Proliferation and activation of bronchial epithelial cells in corticosteroid- dependant asthma. J Allergy Clin Immunol. 2001;108:738–746. doi: 10.1067/mai.2001.119160. [DOI] [PubMed] [Google Scholar]
- 38.Gleich GJ, Flavahan NA, Fujisawa T, Vanhoutte PM. The eosinophil as a mediator of damage to respiratory epithelium: A model for bronchial hyperreactivity. J Allergy Clin Immunol. 1988;81:776–781. doi: 10.1016/0091-6749(88)90931-1. [DOI] [PubMed] [Google Scholar]
- 39.Hisamatsu K, Ganbo T, Nakazawa T, Murakami Y, Gleich GJ, Koyama H. Cytotoxicity of human eosinophil granule major basic protein to human nasal sinus mucosa in vitro. J Allergy Clin Immunol. 1990;86:52–63. doi: 10.1016/s0091-6749(05)80123-x. [DOI] [PubMed] [Google Scholar]
- 40.Frigas E, Motojima S, Gleich GJ. The eosinophilic injury to the mucosa of the airways in the pathogenesis of bronchial asthma. Eur Respir J. 1991;4:123s–135s. [PubMed] [Google Scholar]
- 41.Rothenberg ME. Eosinophilia. New Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 42.Trautmann A, Schmid-Grendelmeier P, Kruger K, Crameri R, Akdis M, Akkaya A, Brocker E-B, Blaser K, Akdis CA. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J Allergy Clin Immunol. 2002;109:329–337. doi: 10.1067/mai.2002.121460. [DOI] [PubMed] [Google Scholar]
- 43.Dunnill MS, Massarella GR, Anderson JA. A comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis, and in emphysema. Thorax. 1969;24:176–179. doi: 10.1136/thx.24.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimura S, Andoph Y, Haraguchi M, Shirato K. Continuity of airway goblet cells and intraluminal mucus in the airways of patients with bronchial asthma. Eur Respir J. 1996;9:1395–1401. doi: 10.1183/09031936.96.09071395. [DOI] [PubMed] [Google Scholar]
- 45.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 46.Ordonez CL, Khashaya R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 47.Laprise C, Sladek R, Ponton A, Bernier M-C, Hudson TJ, Laviolette M. Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genomics. 2004;5:21–30. doi: 10.1186/1471-2164-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies JR, Svitacheva N, Lannefors L, Kornfält, Carlstedt I. Identification of MUC5b, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis. Biochem J. 1999;344:321–330. [PMC free article] [PubMed] [Google Scholar]
- 49.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 50.Stewart AG. Emigration and immigration of mesenchymal cells: a multicultural airway wall. Eur Respir J. 2004;24:515–517. doi: 10.1183/09031936.04.00067404. [DOI] [PubMed] [Google Scholar]
- 51.Howarth PH, Knox AJ, Amrani Y, Tliba O, Paneittieri PA, Johnson M. Synthetic responses in airway smooth muscle. J Allergy Clin Immunol. 2004;114:s32–s50. doi: 10.1016/j.jaci.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 52.An SS, Bal TR, Bates JHT, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.