Abstract
Contaminant accumulation analysis is important in the study of sentinels. This research determined cadmium accumulation and bioconcentration factors of whole organism, liver, kidney, ovary and testis of Fejervarya limnocharis exposed to different environmental cadmium levels. Frogs from contaminated sites had significantly higher hepatic (1.939 mg/kg), renal (7.253 mg/kg) and testicular (1.462 mg/kg) cadmium than those from the reference sites (0.205, 0.783 and 0.379 mg/kg, respectively). Cadmium accumulation was the highest during the late dry and early rainy seasons. If this species is used as a sentinel for cadmium accumulation, the utilization of its whole organism, liver, kidney and testis is appropriate.
Keywords: Cadmium, Environment, Fejervarya limnocharis, Sentinel species
Cadmium in the environment has the ability to accumulate in the body. Bervoets et al. (2001) reported that levels of accumulated metals in tissue were related to metal levels in sediment, water and food. This is further supported by Bervoets and Blust (2003) saying that it is more likely that tissue levels reflect environmental levels because metal concentrations in tissue follow concentrations in the environment. Francis et al. (1984) reported that Carrasius auratus, Rana pipiens and Micropterus salmoides showed strong correlations between cadmium concentrations in water and tissue, and sediment and tissue. Different tissues have the ability to accumulate metals differently (Loumbourdis and Wray 1998; Bervoets et al. 2001; Loumbourdis and Vogiatzis 2002; Bervoets and Blust 2003 and Burger et al. 2007). Loumbourdis and Vogiatzis (2002) reported that the liver is one of the main target organs of cadmium accumulation in Rana ridibunda. In another study, Loumbourdis and Wray (1998) found that Rana ridibunda liver has higher cadmium concentrations than carcass. A study by Loumbourdis et al. (2007) found that upon exposure, cadmium started to deposit in the liver, kidney and the gut of Rana sp. However, the kidney is found to be the main site of accumulation. Flament et al. (2003) reported that cadmium is also readily incorporated in the kidneys and reproductive tissues. Lee (1983) supported this notion by saying that cadmium directly targets testis. In addition, adult Chrysemys picta from impacted sites has higher cadmium concentration in liver, kidneys and gonads (Rie 2000).
In this study, the rice frog, Fejervarya limnocharis has been chosen to be a representative sentinel species for cadmium accumulation. This species is chosen based on a few criteria. The first and the most important reason for choosing the species is because it is categorized under the “Least Concern” or LC category by the IUCN (IUCN, Conservation International and NatureServe 2006; AmphibiaWeb 2008). The status is given to the species because of its wide distribution, its tolerance of a broad range of habitats and its large and stable population. Therefore, using this species will not put it under undue extinction pressure. The rice frog is also of a suitable size. It is not too large that it may cause logistic problem and yet it is also not too small that sampling and analysis are greatly hampered. This species also has a wide range of distribution, covering Southeast Asia, South Asia and parts of East Asia. Hence it is a suitable candidate as the region's sentinel species. F. limnocharis is also utilized as human food in some countries (IUCN, Conservation International and NatureServe 2006), including Thailand. Therefore, the species plays an important role as a link between environmental cadmium and bioavailable cadmium through the food chain. However, data on the use of F. limnocharis, either from wild populations or lab raised populations, for cadmium monitoring is non-existent. Therefore, this research aimed to determine the accumulation of cadmium in wild F. limnocharis that are naturally exposed to different cadmium levels. Accumulation is investigated in selected tissues (liver, kidney, ovary and testis) as well as in whole organisms.
Materials and Methods
Frog samples were collected on monthly basis during November 2007 and October 2008 from several rice fields in Mae Tao and Mae Pa in Mae Sot District, Tak Province. The contaminated site, Mae Tao, was located at 16°45′13″N; 98°35′25″E. This area is irrigated by the Mae Tao Creek. Simmons et al. (2005) reported that there were elevated cadmium levels in the paddy soils and rice grain in the vicinity of Mae Tao Creek downstream of a zinc mining area. The reference site, Mae Pa, was located 8.4 km north of the contaminated site at 16°40′43″N; 98°35′36″E. The area is irrigated by Huay Luek Creek and not on the path of the potential contaminated plume. Preliminary analysis showed that the cadmium concentrations from the contaminated site ranged from 0.0019 to 0.0021 mg/L in water samples, and 2.9260 to 3.2888 mg/kg in sediment samples. The concentration ranges at the reference site were 0.0018 to 0.0020 mg/L (water) and 0.1013 to 0.2206 mg/kg (sediment).
Frogs were individually subjected to cold anesthesia before killed by double-pith at brain and spinal cord. The liver, kidney and gonad were removed and weighed. Tissue and whole organism samples were dried in an oven at 80°C to a constant weight. The samples were then subjected to a microwave digestion procedure with concentrated nitric acid followed by cadmium determination using Graphite Furnace Atomic Absorption Spectrometer (AAS ZEEnit 700 by Analytik Jena). The standard curve range used was 0–10 μg/L and the detection limit of this instrument is 0.02 μg/L.
All data were statistically analyzed with two-way ANOVA and Student-Newman Keuls test using the SigmaStat 2.0 program.
Results and Discussion
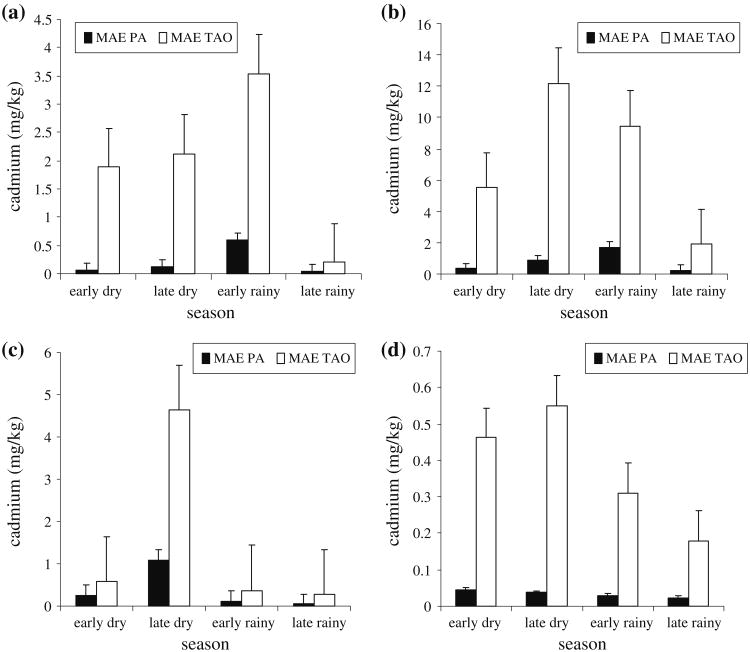
Figure 1a shows the quarterly average hepatic cadmium in F. limnocharis caught from both sites. Hepatic cadmium in F. limnocharis caught from Mae Pa ranges from 0.044 to 0.592 mg/kg. On the other hand, in F. limnocharis caught from Mae Tao, the range is from 0.199 to 3.543 mg/kg. Further analysis showed that hepatic cadmium concentration in Mae Tao frogs are between 4.5 and 32.2 times higher than Mae Pa frogs. Frogs in both sites show similar fluctuation of hepatic cadmium when compared seasonally. In both sites, hepatic cadmium concentration is the highest during the early rainy season (April, May and June).
Fig. 1.
Quarterly average a hepatic, b renal, c testicular and d whole organismal cadmium concentration in F. limnocharis caught from Mae Sot, Tak. All mean differences betweens stations are statistically significant (P < 0.05)
For cadmium in kidney, the results are shown in Fig. 1b. It is found that renal cadmium in F. limnocharis caught from Mae Pa ranges from 0.239 to 1.715 mg/kg. In F. limnocharis caught from Mae Tao, the range is from 1.890 to 12.175 mg/kg. The result showed that Mae Tao frogs had renal cadmium concentration of between 5.5 to 16.2 times higher than Mae Pa frogs. Seasonal fluctuationwise, renal cadmium concentration is the highest during the early rainy season for Mae Pa frogs and during the late dry season for Mae Tao frog.
Site-wise comparison of ovarian cadmium revealed that the differences in ovarian cadmium are not statistically significant. When season-wise comparisons are made, ovarian cadmium concentrations in both sites are the highest during the late dry season.
Figure 1c shows the results for quarterly testicular cadmium in F. limnocharis caught from Mae Pa and Mae Tao. The graph showed that testicular cadmium in F. limnocharis caught from Mae Pa ranges from 0.044 to 1.089 mg/kg. Meanwhile in F. limnocharis caught from Mae Tao, the range is from 0.266 to 4.626 mg/kg. Site-related comparison shows that testicular cadmium concentration in Mae Tao frogs are between 2.2 and 6.1 times higher than Mae Pa frogs. For frogs from both sites, the testicular cadmium concentrations are the highest during the late dry season.
The results for whole organismal cadmium are shown in Fig. 1d. Whole organismal cadmium concentration in F. limnocharis caught from Mae Pa ranges from 0.024 to 0.045 mg/kg. On the other hand, in F. limnocharis caught from Mae Tao, the range is from 0.180 to 0.549 mg/kg. To compare, whole organismal cadmium concentration in Mae Tao frogs are between 7.5 and 14.5 times higher than Mae Pa frogs. Seasonally, Mae Pa frogs caught during the early dry season showed the highest whole organismal cadmium concentration. For Mae Tao, the highest whole organismal cadmium concentration is shown in frogs caught during the late dry season.
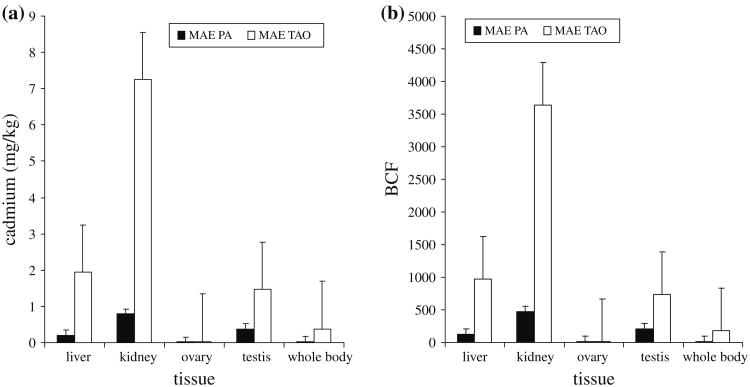
Overall comparisons also show that in both sites, renal cadmium concentrations are higher than hepatic cadmium. Renal cadmium concentration in Mae Pa frogs are between 2.9 and 6.8 times higher than hepatic cadmium concentration. In Mae Tao frogs, the renal cadmium concentrations are between 2.7 and 9.5 times higher than hepatic cadmium concentration. Tissue-by-tissue comparison showed that in both sites, renal cadmium concentration is the highest while ovarian cadmium concentration is the lowest (Fig. 2a). The result is in line with bioconcentration factor analysis where for both sites, kidney showed the highest cadmium bioconcentration factor (Fig. 2b). The average cadmium bioconcentration factor in kidney is 467.75 for Mae Pa frogs and 3,672.32 for Mae Tao frogs.
Fig. 2. Comparison of a average cadmium level and b cadmium bioconcentration factor in F. limnocharis caught from Mae Sot, Tak.
Loumbourdis and Vogiatzis (2002) reported that liver is one of the main target organs of cadmium accumulation in Rana ridibunda. In another study, Loumbourdis and Wray (1998) found that Rana ridibunda liver has higher cadmium concentration than carcass. Pérez-Coll et al. (1997) found out that 26% of the cadmium uptake is deposited into the liver. Foran et al. (2002) stated that cadmium can accumulate and be retained in the liver. However, our sets of results showed a different trend. While accumulation of cadmium in the liver is quite high, it is clear that renal cadmium accumulation is even more apparent. This may be because the liver is the primary accumulation site while the kidney is the final accumulation site. Loumbourdis et al. (2007) reported that cadmium may gain entry into hepatocytes via endocytosis mediated by Fe binding protein such as ferritin. In the liver, cadmium will bind with metallothionein or stay as free cadmium in the hepatocytes. Free cadmium from liver is then released into the gastrointestinal lumen by the secretion of bile contents in the bile duct. Some of the cadmium is removed by feces while most will enter the blood stream through the enterohepatic circulation. Cadmium will then be transported to the various target organs, especially kidney. Therefore, throughout the life span of the frog, cadmium will be continually accumulated in the liver, and then transported to the kidney. So far, there has been no account on whether cadmium in the kidney is excreted or not. Hence, it is assumed that all cadmium that accumulates in the kidney will be retained there. High cadmium accumulation in the kidney is also reported by various other studies. The highest renal cadmium accumulations were found in Gobio gobio (Bervoets and Blust 2003), Salmo trutta (Olsvik et al. 2000), Rana ridibunda (Loumbourdis et al. 2007), Pleurodeles waltl (Flament et al. 2003) and Gasterosteus aculeatus (Bervoets et al. 2001).
While kidney showed higher cadmium accumulation than liver, we also found that both are actually suitable indicators for biomonitoring of cadmium accumulation. This is because both hepatic and renal cadmium levels are significantly higher in F. limnocharis caught from the contaminated site as compared to those from the reference site.
Among reproductive tissues, ovarian cadmium showed very little accumulation. To add, the differences in ovarian cadmium accumulation between reference and contaminated sites were also not significant. This shows that the ovary probably is not a suitable organ to be used in biomonitoring of cadmium accumulation. However, this study found out that high cadmium accumulation is found in the testis. This is shown by the high testicular cadmium and high testicular cadmium bioconcentration factor in F. limnocharis from the impacted site as compared to those from the reference site. This is expected because cadmium is a known toxicant directly targets testis (Lee 1983).
In this study, all the frogs used for the analysis of whole organismal cadmium weighed less than two grams. In these frogs, the use of organs, especially kidney and testis was rather difficult. Therefore, for these small frogs, whole body cadmium analyses were performed. We have included the use of small frogs in this research because we anticipate that in the future, not all field sampling activities will be able to have a yield of large frogs. In this case, instead of determining cadmium accumulation in organs and tissues, whole organismal cadmium accumulation may be a better choice of analysis. In our study, we found out that there were significant differences in whole organismal cadmium level and whole organismal cadmium bioaccumulation factor between F. limnocharis caught from contaminated site with those from reference site. Frogs from contaminated site had higher whole organismal cadmium level and whole organismal cadmium bioaccumulation factor. Therefore, in cases when organ cadmium accumulation determination in large frogs is not available, the use of whole organismal cadmium in small frogs is also considered as suitable indicator for biomonitoring of cadmium accumulation.
When we compared cadmium accumulation according to season, we found that the highest cadmium accumulation occurred either during the late dry season or during the early rainy season. This is because these two seasons are the active season when reproductive tissues are developing and when reproduction actually takes place. Zug et al. (2001) stated that rainfall is one of the major determinants of timing of reproduction. AmphibiaWeb (2008) confirmed this by stating that the breeding of the F. limnocharis is triggered by rain and it is usually the first species to come to the calling sites. In order for the rice frog to be the first species to come to the calling sites during the early rainy season, their ovaries and testes will have to start developing and maturing during the late dry season. Reproductive tissues development and maturation requires energy, hence the frogs would have to increase food and water intake during the late dry season for these purposes. During the early rainy season, the actual breeding occurs, and again these efforts require a lot of energy. Zug et al. (2001) stated that egg development would constitute a large portion of their overall energy budget. Therefore, it is during these two seasons, more food and water were consumed. And with increased food and water consumption, there was also a chance of increased uptake of cadmium along with it. This would explain the high cadmium concentration in the liver, kidney and testes of the frogs during the late dry and early rainy seasons.
In conclusion, this research found that frogs from the contaminated site had higher hepatic, renal, testicular and whole organismal cadmium when compared to frogs from the reference site. The results also showed that the kidney is the greatest cadmium-accumulating organ. We also found that frogs caught during the late dry and early rainy seasons tend to have higher tissue and organismal cadmium than those caught during late rainy and early dry seasons. Therefore, when using F. limnocharis in biomonitoring cadmium accumulation, types of tissues used and seasonal sampling period should be taken into consideration.
Acknowledgments
This research is part of a graduate research project titled “Using the Rice Frog (Fejervarya limnocharis) as Sentinel Species for Cadmium Contamination in Tak Province, Thailand”. Financial support of this work was obtained from the National Center of Excellence for Environmental and Hazardous Waste Management (NCE-EHWM), the 90th Anniversary of Chulalongkorn University Fund and a new staff development grant (Ratchadaphiseksomphot Endowment Fund), and the MUA-TRF research grant (MRG4980120) to NK. An educational grant from the Malaysian Ministry of Higher Education to MSO is fully acknowledged. Additional support from NIH Fogarty ITREOH, D43 TW007849.
Contributor Information
Mohd Sham Othman, International Postgraduate Programs in Environmental Management, Graduate School, Chulalongkorn University, Bangkok, Thailand; National Center of Excellence for Environmental and Hazardous Waste Management (NCE-EHWM), Chulalongkorn University, Bangkok, Thailand; Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand; Environmental Health Program, Faculty of Allied Health, Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia.
Wichase Khonsue, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand.
Jirarach Kitana, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand.
Kumthorn Thirakhupt, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand.
Mark Gregory Robson, Email: robson@aesop.rutgers.edu, National Center of Excellence for Environmental and Hazardous Waste Management (NCE-EHWM), Chulalongkorn University, Bangkok, Thailand; School of Environmental and Biological Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ, USA; Thai Fogarty International Training and Research in Environmental and Occupational Health Center, Chulalongkorn University, Bangkok, Thailand.
Noppadon Kitana, Email: nkitana@hotmail.com, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand.
References
- AmphibiaWeb. [Accessed 6 Jan 2008];Fejervarya limnocharis, alpine cricket frog. 2008 Available via DIALOG http://amphibiaweb.org.
- Bervoets L, Blust R. Metal concentrations in water, sediment and gudgeon (Gobio gobio) from a pollution gradient: relationship with fish condition factor. Environ Pollut. 2003;126:9–19. doi: 10.1016/S0269-7491(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Bervoets L, Blust R, Verheyen R. Accumulation of metals in tissues of three-spined stickleback (Gasterousteus aculeatus) from natural fresh waters. Ecotoxicol Environ Saf. 2001;48:117–127. doi: 10.1006/eesa.2000.2010. [DOI] [PubMed] [Google Scholar]
- Burger J, Campbell KR, Murray S, Campbell TS, Gaines KF, Jeitner C, Shukla T, Burke S, Gochfeld M. Metal levels in blood, muscle and liver of water snakes (Nerodia spp) from New Jersey, Tennessee and South Carolina. Sci Total Environ. 2007;373:56–563. doi: 10.1016/j.scitotenv.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Flament S, Kuntz S, Chesnel A, Grillier-Vuissoz I, Tankozic C, Penrad-Mobayed M, Auque G, Shirali P, Schroeder H, Chardard D. Effect of cadmium on gonadogenesis and metamorphosis in Pleurodeles waltl (urodele amphibian) Aquat Toxicol. 2003;64:143–153. doi: 10.1016/S0166-445X(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Foran CM, Peterson BN, Benson WH. Influence of parental and developmental cadmium exposure on endocrine and reproductive function in Japanese medaka (Oryzias latipes) Comp Biochem Physiol C. 2002;133:345–354. doi: 10.1016/s1532-0456(02)00128-x. [DOI] [PubMed] [Google Scholar]
- Francis PC, Birge WJ, Black JA. Effects of cadmium-enriched sediment on fish and amphibian embryo-larval stages. Ecotoxicol Environ Saf. 1984;8:378–387. doi: 10.1016/0147-6513(84)90006-X. [DOI] [PubMed] [Google Scholar]
- IUCN(International Union for Conservation of Nature), Conservation International and NatureServe. [Accessed 6 Jan 2008];Global amphibian assessment. 2006 Available via www.globalamphibians.org.
- Lee IP. Effects of environmental metals on male reproduction. In: Clarkson TW, Nordberg GF, Sager PR, editors. Reproductive and developmental toxicity of metals. Plenum Press; New York: 1983. pp. 253–278. [Google Scholar]
- Loumbourdis NS, Vogiatzis AK. Impact of cadmium on liver pigmentary system of the frog Rana ridibunda. Ecotoxicol Environ Saf. 2002;53:52–58. doi: 10.1006/eesa.2002.2153. [DOI] [PubMed] [Google Scholar]
- Loumbourdis NS, Wray D. Heavy metal concentration in the frog Rana ridibunda from a small river of Macedonia, Northern Greece. Environ Int. 1998;24:427–431. doi: 10.1016/S0160-4120(98)00021-X. [DOI] [Google Scholar]
- Loumbourdis NS, Kostaropoulos I, Theodoropoulou B, Kalmanti D. Heavy metal accumulation and metallothionein concentration in the frog Rana ridibunda after exposure to chromium or a mixture of chromium and cadmium. Environ Pollut. 2007;145:787–792. doi: 10.1016/j.envpol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Olsvik PA, Gundersen P, Andersen RA, Zachariassen KE. Metal accumulation and metallothionein in two populations of brown trout, Salmo trutta, exposed to different natural water environments during a run-off episode. Aquat Toxicol. 2000;50:301–316. doi: 10.1016/S0166-445X(00)00094-1. [DOI] [PubMed] [Google Scholar]
- Pérez-Coll CS, Herkovits J, Fridman O, Daniel P, D'Eramo JL. Metallothionein and cadmium uptake by the liver in Bufo arenarum. Environ Pollut. 1997;97:311–315. doi: 10.1016/S0269-7491(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Rie MT. Assessment of the effects of groundwater pollution on a sentinel species, Chrysemys picta, on Cape Cod, MA: tissue contaminant levels and hepatic and reproductive bioindicators. Department of Biology, Boston University; Boston: 2000. [Google Scholar]
- Simmons RW, Pongsakul P, Saiyasitpanich D, Klinphoklap S. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: implications for public health. Environ Geochem Health. 2005;27:501–511. doi: 10.1007/s10653-005-7857-z. [DOI] [PubMed] [Google Scholar]
- Zug GR, Vitt LJ, Caldwell JP. Herpetology an introductory biology of amphibians and reptiles. 2nd. Academic Press; San Diego: 2001. [Google Scholar]