Abstract
The HIV-1 Tat protein is secreted by infected cells. Extracellular Tat can affect bystander uninfected T cells and induce numerous biological responses such as apoptosis and cytokine secretion. Tat is likely involved in several immune disorders during AIDS. Nevertheless, it is not known whether Tat triggers cell responses directly upon binding to signaling receptors at the plasma membrane or after delivery to the cytosol. The pathway that enables Tat to reach the cytosol is also unclear. Here we visualized Tat within T-cell–coated pits and endosomes. Moreover, inhibitors of clathrin/AP-2–mediated uptake such as chlorpromazine, activated RhoA, or dominant-negative mutants of Eps15, intersectin, dynamin, or rab5 impaired Tat delivery to the cytosol by preventing its endocytosis. Molecules neutralizing low endosomal pH or Hsp90 inhibitors abolished Tat entry at a later stage by blocking its endosomal translocation, as directly shown using a cell-free translocation assay. Finally, endosomal pH neutralization prevented Tat from inducing T-cell responses such as NF-κB activation, apoptosis, and interleukin secretion, indicating that cytosolic delivery is required for Tat signaling. Hence, Tat enters T cells essentially like diphtheria toxin, using clathrin-mediated endocytosis before low-pH–induced and Hsp90-assisted endosomal translocation. Cell responses are then induced from the cytosol.
INTRODUCTION
Tat is a strong trans-activator that enables productive transcription from the HIV-1 long terminal repeat (LTR) and is required for HIV-1 replication (Rubartelli et al., 1998; Watson and Edwards, 1999; Noonan and Albini, 2000). Albeit devoid of signal sequence, it is released by infected cells and nanomolar Tat concentrations were measured in the sera of HIV-1–infected patients (Xiao et al., 2000). Exogenous Tat can affect monocytes, endothelial cells and neurons, but one of its main targets is the T cell (Rubartelli et al., 1998; Watson and Edwards, 1999; Noonan and Albini, 2000). Indeed, Tat induces IL-2 and IL-8 hypersecretion by T cells (Ott et al., 1997, 1998) and can also trigger their apoptosis (Li et al., 1995; Chen et al., 2002). Circulating Tat is thus thought to be involved in AIDS development (Rubartelli et al., 1998; Watson and Edwards, 1999). Consistently, evaluations of Tat-containing vaccines have yielded encouraging results (Voss et al., 2003).
Tat has the capacity to enter the cytosol from the outside medium, like several bacterial toxins such as diphtheria and cholera toxin catalytic subunits (Falnes and Sandvig, 2000). This property was demonstrated in pioneer studies by showing that extracellular Tat could trans-activate reporter genes placed under the control of HIV-1 LTR (Frankel and Pabo, 1988; Mann and Frankel, 1991). This finding was later confirmed using several Tat fusion proteins and different readouts for monitoring Tat cytosolic delivery (Fawell et al., 1994). Nevertheless, contrary to bacterial toxins, the overall pathway enabling extracellular Tat to access the cytosol remains elusive, although endocytosis seems to be required (Bonifaci et al., 1995; Frankel et al., 1988). Caveolae have been shown to mediate the internalization of Tat fusion proteins by HeLa cells (Fittipaldi et al., 2003) but Tat endocytosis by T cells cannot take place from caveolae because T cells do not express caveolin and are devoid of caveolae (Fra et al., 1994; Lamaze et al., 2001).
We focused on Tat uptake by T cells. Tat can bind to several receptors at the plasma membrane, such as CD26 (Gutheil et al., 1994), CXCR4 (Xiao et al., 2000), heparan sulfate proteoglycans (Tyagi et al., 2001), and the low-density lipoprotein receptor–related protein (LRP) (Liu et al., 2000). Most of these receptors are endocytosed by T cells. For instance, the LRP is internalized via the clathrin-dependent pathway (Orlandi and Fishman, 1998). The behavior of CXCR4 appears more complex because it can be found within both coated pits (Signoret et al., 1997) and detergent-resistant plasma membrane domains (rafts; Nguyen and Taub, 2002). A fraction of cell-surface CD26 is also found within rafts (Salgado et al., 2003). Because a raft-mediated, clathrin-independent endocytosis pathway exists in T lymphocytes (Lamaze et al., 2001), the identified receptors could drive Tat endocytosis through either a clathrin-dependent or a raft-mediated pathway, and the initial Tat uptake pathway in T cells remains to be identified.
Regarding downstream Tat intracellular routing, no information is currently available for any cell type on Tat translocation site(s) that could enable cytosolic delivery or on the mechanism underlying this trans-membrane transport. Cellular proteins involved in this process, if any, remain to be identified.
The last unclear issue related to Tat intracellular transport is whether Tat triggers cell responses such as IL-2 secretion by T cells through mere binding to signaling receptors at the plasma membrane (Secchiero et al., 2000) or after its delivery to the cytosol (Ott et al., 1997).
In this study, we delineated the pathway that enables extracellular Tat to get into T cells and identified low endosomal pH and cytosolic Hsp90 as key regulators of the translocation process. We also showed that Tat has to reach the cytosol to elicit cell responses.
MATERIALS AND METHODS
Plasmids and Transfections
HIV-1 LTR (from –485 to +78) was inserted into pGL3 (Promega, Madison, WI) upstream from Firefly luciferase gene. The control vector pRL-TK (Promega) contains a thymidine kinase promoter upstream from Renilla luciferase gene. Plasmids pEGFP-C1 or -C2 (CLONTECH, Palo Alto, CA) containing Eps15DIIIΔ2 or Eps15Δ95/295 (Benmerah et al., 1999), intersectin SH3A domain (Simpson et al., 1999), or RhoA (WT, G14V, or T19N; Ory et al., 2000) have been described as well as pCMV5-DynaminII (WT or K44A; Lamaze et al., 2001), and pcDNA3.1 containing Tat (Ott et al., 1997) or rab5a (WT, Q79L or S34N; Mukhopadhyay et al., 1997). Jurkat human T cells (clone E6-1) were cultivated in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS, Invitrogen). Cells (9 × 106) were electroporated with 7 μg of pGL3-LTR, 0.3 μg of pRL-TK, and an effector plasmid as indicated. The total DNA for each transfection was normalized to 56 μg with pCI vector (Promega). To examine trans-activation by extracellular Tat, cells were treated with 200 nM Tat and 100 μM chloroquine (CQ) at 18 h posttransfection (Mann and Frankel, 1991). Luciferase activities were measured 24 h after adding Tat and CQ. Firefly activity in the presence of Tat {Firefly(Tat)} was corrected for activity in its absence {Firefly(Ctrol)}, normalizing the data using Renilla activity (Wei et al., 1998). Hence, trans-activation = Firefly(Tat)/Renilla(Tat)–Firefly(Ctrol)/Renilla(Ctrol). Renilla activity was essentially insensitive to the different cell treatments performed in this study. Transfection efficiencies were determined using pEGFP-C2 and fluorescence-activated cell sorting (FACS) analysis. They ranged from 40 to 60%. Expression levels were monitored for dynamin and rab5 transfectants using immunoblotting. Plasmid doses of 14, 28, and 56 μg yielded overexpression of ∼5-, 12- and 30-fold above endogenous protein levels, respectively. Assays for trans-activation by intracellular Tat in the presence of drug or cotransfected plasmid were performed using pcDNA3.1-Tat (7 μg), which was cotransfected with luciferase plasmids.
Proteins and Antibodies
Tat was expressed in Escherichia coli BL21 (λDE3) transfected with Pet11d-Tat (HXB3, BH-10 isolate, 86 residues; Bonifaci et al., 1995). After purification from E. coli cytosol on heparin-agarose (Chang et al., 1997), it was reduced and denaturated before refolding by reverse-phase HPLC, and lyophilization (Ott et al., 1998). Tat was resuspended in citrate-buffered saline (150 mM NaCl, 50 mM sodium citrate, pH 7.0) before use. This preparation was endotoxin-free and gave results identical to those obtained using chemically synthesized Tat (Tecnogen, Piana di Monte Verna, Italy). Buffers were degased before adding Tat to minimize its oxidation. Transferrin (Tf) was conjugated to Cy5 using a labeling kit (Amersham Biosciences, Piscataway, NJ). Low-density lipoproteins (LDL) were purified from human blood (EFS, Montpellier, France) and made fluorescent using 3,3′-dioctadecyloxacarbocyanine as described (Pitas et al., 1981). Ricin (Sigma, St. Louis, MO) was complexed to colloidal gold as indicated (Beaumelle et al., 1990). Horseradish peroxidase (HRP), LDL, Tat, and Tf were radiolabeled with 125I-Na (ICN Radiochemicals, Irvine, CA) using Iodogen (Pierce, Rockford, IL; Alami et al., 1998). 125I-Tat (specific activity ∼1 μCi/μg) was stored at –80°C in citrate-buffered saline supplemented with BSA. Purified rabbit anti-rab5a, goat anti-rab7, antidynamin and anti-IκBα antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-CD95 from R&D Systems (Minneapolis, MN). The IgG1 monoclonal antibody (mAb) 6C4 against lysobisphosphatidic acid (LBPA) has been described (Kobayashi et al., 1998). Anti–Lamp-1, -2 and -3 mAbs (IgG1) were from the Iowa Developmental Studies Hybridoma Bank. Anti-GM130 (IgG1), anti-Hsp90, and anti-BIP mAbs were from Transduction Laboratories (Lexington, KY). Anti-Tat mAb (IgG2) was from Advanced Biotechnologies (Columbia, MD) and sheep anti-human TGN46 antibodies from Serotec (Raleigh, NC). Chicken anti-human mannose 6-phosphate receptor (M6PR) antibodies were a gift of Henri Rochefort (Montpellier, France). Except when otherwise indicated, secondary and tertiary antibodies for immunofluorescence were from Nordic Immunological Labs (Tilburg, The Netherlands). Other antibodies were from Immunotech (Beckman-Coulter, Fullerton, CA).
Cytosol Preparation
Cytosol was purified from exponentially growing HeLa S3 cells that were first washed twice in PBS and then once in 5 mM KCl, 2 mM MgCl2, 10 mM Pipes, pH 7.2, containing protease inhibitors (Complete; Roche Applied Science, Indianapolis, IN). They were finally resuspended in one volume of the latter buffer. Lysis was performed using a nitrogen cavitation apparatus (20 min, 50 psi) and then a glass homogenizer. Isotonicity was restored by adding 0.1 volume of 1.1 M KCl, 10 mM Pipes, pH 7.2, before centrifugation at 800 × g for 5 min. The supernatant was further centrifuged at 20,000 × g for 30 min and finally at 100,000 × g for 1 h. The resulting cytosol was aliquoted and stored at –80°C.
Internalization and Cell-free Translocation Assays
Continuous uptake assays were performed by incubating cells at 37°C with 50 nM 125I-Tat or 10 nM 125I-Tf in labeling medium (RPMI containing 5% FCS for Tat or 0.05% BSA for Tf) for the indicated times. Cells were then washed at 4°C and resuspended in Pronase (0.3% in PBS, supplemented with 10 μg/ml heparin in the case of Tat in order to favor its dissociation from the membrane; Tyagi et al., 2001). After 40 min on ice, cells were spun through dibutylphthalate (Alami et al., 1998). Plasma membrane-bound 125I-Tf and 125I-Tat were removed with more than 95 and 85% efficiency by the Pronase or Pronase/heparin treatment, respectively. Cell associated-counts insensitive to these treatments were taken to represent internalized 125I-ligand. The presence of excess (1 μM) unlabeled ligand during labeling decreased cell-associated radioactivity by >90%.
To study the effect of drugs on initial uptake, cells were pretreated with the drug for 30 min at 37°C in labeling medium. The 125I-ligand was then added for 1.5 h (125I-Tat) or 5 min (125I-Tf), before removing plasma membrane-associated proteins by Pronase scraping as described above.
For prebound ligand uptake assays, cells were labeled at 4°C for 30 min with 50 nM 125I-Tat or 10 nM 125I-Tf in labeling medium, washed, and then incubated at 37°C in labeling medium for the indicated times to allow endocytosis. Cells were then cooled to 4°C before Pronase scraping.
Endosomes were purified for the translocation assay essentially as indicated earlier (Beaumelle et al., 1992). Briefly, Jurkat cells were incubated at 37°C for 6 h with 125I-Tat or 45 min with control tracers (125I-LDL, 125I -Tf, or 125I -HRP). LDL were added (150 μg/ml) for the last 45 min of labeling. Cells were then treated with ricin-gold on ice and lysed. Crude membranes from the postnuclear supernatant were separated on a sucrose gradient. This fractionation procedure enables isolation of endosomes lightened by cointernalization of LDL and devoid of contamination by plasma membrane vesicles burdened by ricin-gold (Beaumelle et al., 1992). Endosomes were collected, concentrated by ultracentrifugation, and finally resuspended in 110 mM KCl, 20 mM MgCl2, 20 mM Pipes, pH 7.1. Cytosol was added (at 1 mg protein/ml) together with an ATP regeneration system (5 mM ATP, 5 mM MgCl2, 5 mM creatine phosphate, 60 U/ml creatine phosphokinase). Translocation was assayed for 0–120 min at 37°C before cooling and ultracentrifugation on a sucrose cushion. Endosome and supernatant radioactivities were assayed and translocation activity was calculated using the increase (%) in the supernatant/endosome radioactivity ratio (Beaumelle et al., 1992).
Immunofluorescence and Electron Microscopy
Cells were incubated for 6 h at 37°C with 50 nM Tat in RPMI/5% FCS. For fluorescence microscopy, 100 nM Tf-Cy5 was added for the last 40 min. Cells were washed, fixed, permeabilized, and then labeled with antibodies (Roumier et al., 2001). Anti-Tat mAb was revealed using goat antimurine IgG2, and then Alexa546-labeled donkey anti-goat IgG antibodies (Molecular Probes, Eugene, OR). Anti-Lamps antibodies, anti-GM130, and anti-LBPA were detected using a rabbit anti-mouse IgG1 and then fluorescein-labeled swine anti-rabbit antibodies. The anti-M6PR and anti-TGN46 antibodies were revealed using fluorescein-conjugated rabbit anti-chicken and anti-sheep antibodies, respectively. In experiments involving goat anti-rab7 antibodies, they were detected using fluorescein-labeled donkey anti-goat antibodies, whereas anti-Tat mAb were revealed using rabbit anti-mouse and then rhodamine-labeled swine anti-rabbit antibodies. Cells were finally mounted and examined under a Leica TCS 4D confocal microscope (Morlon-Guyot et al., 2003). The acousto-optical tunable filter of the instrument was used together with the detectors to equilibrate fluorescence levels and avoid passage from one channel to the other. No red labeling was observed in the absence of Tat. Endocytosis efficiencies were assessed using images from >10 cells and the ImageQuant software (Amersham Biosciences). Colocalizations were quantified (n >10 cells) using the Metamorph software package (Universal Imaging, Downingtown, PA). Threshold was set manually.
For electron microscopy, after incubation with Tat, cells were cooled to 4°C for successive labeling with anti-Tat mAb, then rabbit anti-mouse antibodies (Dako), and finally protein-A conjugated to 5-nm diameter colloidal gold (Hans Geuze, The Netherlands), before processing for conventional epon sectioning (Beaumelle et al., 1990).
Assays for Apoptosis and IκBα
To monitor Tat-induced T-cell apoptosis, Jurkat cells (0.33 × 106/ml) were cultivated for 24 h in the presence of 7 nM HIV-1 Tat, 10 nM bafilomycin A1 (Baf), 10 μM CQ, or l00 ng/ml anti-CD95. They were then labeled with annexinV-fluorescein as recommended by the manufacturer (Roche Applied Science), before FACS analysis. For IκBα quantitation, cells were kept overnight in RPMI supplemented with 1% FCS to increase the IκBα endogenous level and then transferred to RPMI/10% FCS for treatment with 7 nM Tat, 0.1 nM TNF-α, 100 nM Baf, or 50 μM CQ, as indicated. Cells were then washed and lysed (Manna and Aggarwal, 2000), and soluble proteins were precipitated before SDS/PAGE and immunoblotting.
Immunoassays for Cytokine Release
Peripheral blood mononuclear cells were obtained from human blood (EFS) by Ficoll-Paque+ gradient centrifugation and resuspended in RPMI containing 10% pooled human AB+ serum. Peripheral blood lymphocytes were purified using adherence-mediated depletion of monocytes. T cells were further enriched through immunomagnetic negative selection with Dynabeads M450 (Dynal, Lake Success, NY) coated with mAbs against CD14, CD19, CD20, and CD32. T cells were >90% pure according to FACS analysis using a fluorescent anti-CD3 antibody and were seeded on plates precoated with 3 μg/ml anti-CD3 and 1 μg/ml anti-CD28 mAbs (Ott et al., 1998). Tat (250 nM) was added and supernatants were harvested after 24 h. Cytokine concentrations were determined by ELISA using Immunotech kits (Beckman-Coulter). Experiments were performed using blood from six different adult donors.
RESULTS
Preliminary Characterization of Tat Uptake by T cells
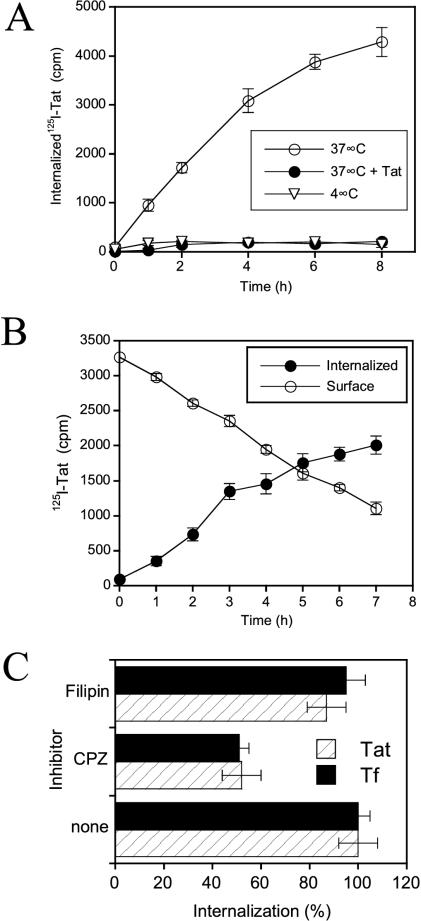
We first examined the uptake of 125I-Tat by Jurkat T cells. Surprisingly slow internalization kinetics were observed at 37°C over an 8-h continuous labeling period (Figure 1A). Internalization was blocked at 4°C, indicating that endocytosis might be required. Intracellular accumulation was also prevented by excess unlabeled Tat, showing that the labeling procedure did not affect Tat binding properties. When 125I-Tat was prebound at 4°C, intracellular accumulation at 37°C was also found to proceed very slowly, i.e., over several hours (Figure 1B).
Figure 1.
Characteristics of Tat internalization by Jurkat T lymphocytes. (A) Continuous uptake protocol. Cells were incubated at the indicated temperature with 125I-Tat for different times and internalization was quantified as Pronase/heparin-resistant cell radioactivity. Where indicated, 1 μM unlabeled Tat was present during cell labeling. (B) Prebinding technique. 125I-Tat was prebound at 4°C, excess ligand was washed out, and cells were incubated at 37°C. Radioactivity at the cell surface and in the intracellular fraction was then determined using Pronase scraping. (C) Effect of inhibitors. Cells were pretreated for 30 min at 37°C with 20 μM chlorpromazine (CPZ) or 1 μg/ml filipin, before adding the 125I-ligand. Cells were further incubated for 1.5 h (Tat) or 5 min (Tf) before scraping surface-bound ligand. “100% internalization” refers to uptake in the absence of drug.
In a first attempt to identify the Tat internalization pathway, we used drugs that have already been used on T cells to discriminate between clathrin/AP-2–dependent and raft-mediated endocytosis. Quantitatively, most clathrin-mediated uptake relies on the AP-2 adaptor complex (Traub, 2003). Chlorpromazine disrupts the assembly of AP-2 and clathrin, thereby inhibiting uptake by the clathrin/AP-2 pathway without perturbing internalization via lipid rafts, while filipin is a sterol-binding agent that disrupts rafts and prevents internalization by this pathway (Subtil et al., 1994; Orlandi and Fishman, 1998). Tf was used as a tracer for clathrin/AP-2-dependent endocytosis (Traub, 2003) in these uptake inhibition experiments.
Chlorpromazine decreased the efficiency of 125I-Tf endocytosis by 50% (Figure 1C), without affecting cell viability. Filipin did not significantly impair Tf uptake. These results are in line with previous reports examining Tf endocytosis by T cells (Subtil et al., 1994; Orlandi and Fishman, 1998). Just like Tf, Tat internalization was reduced to 50% by chlorpromazine, whereas filipin had no effect (Figure 1C). These pharmacological data therefore suggested that T cells endocytose Tat via a clathrin/AP-2–dependent pathway.
Tat Is Endocytosed via a Clathrin Pathway
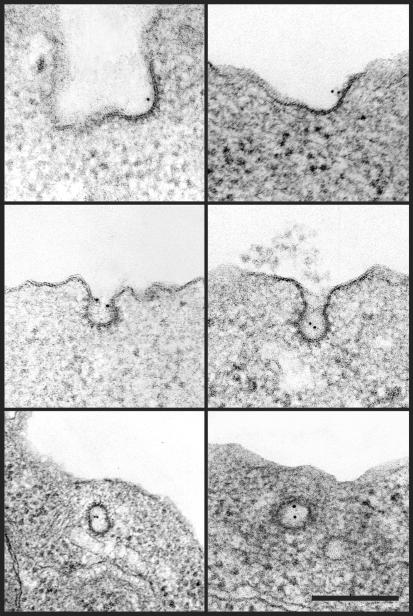
We then used immunogold labeling and electron microscopy to assess Tat localization at the lymphocyte surface. Remarkably, upon 37°C cell labeling, Tat was associated with coated pits at the plasma membrane (Figure 2). Quantitative analysis showed that of 180 plasma membrane–bound gold particles counted, 34% were in coated pits. Because in T cells, as in most cell types, these structures represent ∼2% of the plasma membrane surface (Foti et al., 1997), the efficiency of Tat concentration within coated pits further indicated that Tat could enter T cells using a clathrin-dependent pathway.
Figure 2.
Tat localized to coated pits on the T-cell plasma membrane. Jurkat cells were incubated for 6 h at 37°C with 50 nM Tat, cooled to 4°C, and labeled with an anti-Tat mAb revealed using 5-nm-diameter gold before fixation and preparation for electronmicroscopic examination. No cell labeling was obtained in the absence of Tat. Bar, 200 nm.
To examine this issue more directly, we used dominant-negative inhibitors of clathrin-dependent or raft-mediated endocytosis pathways. These effectors are thought to be more specific than drugs and have been validated in a number of studies (Johannes and Lamaze, 2002). Eps15 is a constituent of plasma membrane clathrin-coated pits that is constitutively associated with AP-2, and overexpression of dominant-negative mutants of Eps15 selectively inhibits clathrin/AP-2–dependent endocytosis (Benmerah et al., 1999), whereas internalization mediated by lipid rafts remains unaffected (Lamaze et al., 2001). Intersectin is involved in the formation of constricted coated pits, and overexpression of the intersectin SH3A domain impairs coated pit–mediated uptake (Simpson et al., 1999). The K44A point mutation within the dynamin GTPase site blocks endocytosis by preventing scission of vesicles, whether coated or not, from the plasma membrane (Lamaze et al., 2001). Mutants of RhoA GTPase are also powerful tools to discriminate between the clathrin/AP-2–dependent or –independent endocytosis pathway. Overexpression of RhoA WT does not affect endocytosis from either coated pits or lipid rafts. When a permanently activated mutant is used, the clathrin/AP-2 pathway is inhibited, whereas the raft pathway remains unaffected. Conversely, internalization from rafts, but not clathrin/AP-2–dependent endocytosis is blocked upon overexpression of the inactivated, GDP-bound, RhoA T19N mutant in T cells (Lamaze et al., 2001).
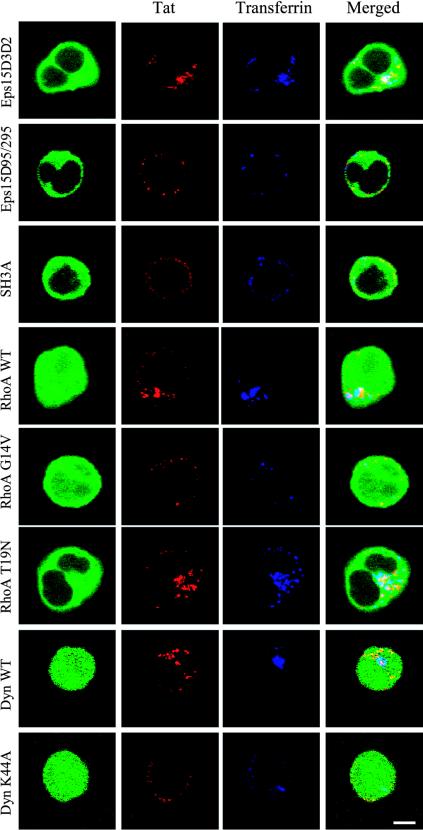
To evaluate whether Tat endocytosis proceeds via a clathrin pathway, we individually and transiently overexpressed these constructs in Jurkat cells before assessing Tat internalization by immunofluorescence. Cells transfected with the control version of Eps15 (Eps15D3Δ2) or dynamin (WT) internalized both Tat and Tf (Figure 3). As detailed below, endocytosed Tat partly colocalized with Tf. Dominant-negative constructions impairing coated vesicle formation, i.e., Eps15Δ95/295, the intersectin SH3A domain and dynamin K44A all prevented, with approximately the same efficiency (by 60–80%), Tf and Tat uptake. RhoA WT, or its inactivated form RhoA T19N, did not affect Tat or Tf internalization, which was inhibited by 60–70% upon expression of an activated version, i.e., RhoA G14V (Figure 3). The finding that RhoA T19N did not impair Tat endocytosis suggested that Tat is not significantly internalized via the raft-pathway. Taken together these results indicated that T cells endocytosed Tat through an Eps15-, intersectin- and dynamin-dependent route, most presumably by the well-characterized clathrin/AP-2–mediated endocytosis pathway.
Figure 3.
Tat is endocytosed via coated pits. Cells were transiently transfected with a vector (56 μg) allowing overexpression of an EGFP-tagged protein (Eps15D3Δ2, Eps15Δ95/295, intersectin SH3A domain, RhoA WT, G14V, or T19N), or with an EGFP vector (6 μg) and another coding for dynamin II-WT or dynamin II-K44A (50 μg). After 24 h, 50 nM Tat was added for 6 h, and 100 nM Tf-Cy5 for the last 40 min to label early endosomes. Cells were then washed, fixed, processed for immunofluorescence detection of Tat, and examined under a confocal microscope. Representative median optical sections were recorded. Bar, 5 μm.
Tat Uptake by Coated Pits Is Required for Subsequent Transport to the Cytosol
Albeit electronmicroscopic examination and data from internalization assays using molecular effectors provide compelling evidence that T cells use coated pits to endocytose Tat, this finding does not necessarily mean that Tat has to be taken up by a clathrin-dependent pathway before cytosolic delivery. In other words, endocytosis might be a dead end for Tat if it could reach the cytosol directly through the plasma membrane. Such direct transport might indeed take place in the case of Tat protein transduction domain (PTD; Green et al., 2003). To explore this possibility, we examined the ability of the clathrin pathway effectors described above to affect extracellular Tat access to the cytosol, as monitored using a conventional trans-activation assay. This assay is based on the use of two transfected luciferases. The Firefly gene is placed under the control of HIV-1 LTR that will be trans-activated by Tat, whereas the Renilla gene is driven by a Tat-insensitive promoter.
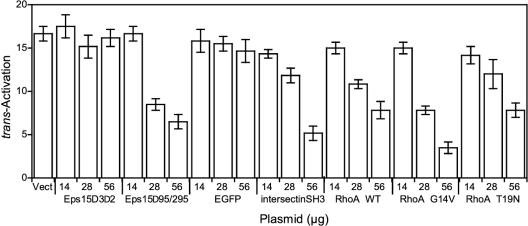
We first performed control experiments to monitor the effects of effector overexpression on trans-activation by cytosolically expressed Tat. Among the constructions, dominant-negative dynamin (K44A) only significantly affected trans-activation by cytosolic Tat, inhibiting this process by ∼63%. The basis of this inhibition is still not clear, but dynamin is already known to have multiple functions within the cell (McNiven et al., 2000). This effect prevented further use of this mutant in the trans-activation assay.
trans-activation by extracellular Tat was not significantly affected by overexpression of the control constructions Eps15-D3Δ2 or EGFP (Figure 4). Conversely, increasing doses of dominant-negative versions, Eps15-Δ95/295 and the intersectin-SH3A domain, resulted in a dose-dependent decrease in Tat trans-activation, reaching 60–70% inhibition. Furthermore, chlorpromazine inhibited by 85% trans-activation by extracellular Tat (unpublished data). Hence, trans-activation by exogenous Tat is severely impaired when Eps15-, intersectin- or AP-2–dependent endocytosis is blocked. Surprisingly, overexpression of RhoA WT inhibited trans-activation by extracellular Tat (Figure 4). This effect, which plateaued at 50%, was detected in this assay but not when monitoring endocytosis microscopically (Figure 3). This was likely due to the fact that cells were allowed to internalize Tat for 24 h in the trans-activation assay, allowing cumulative effects, whereas the incubation time was only 6 h for the immunofluorescence experiments. The inactivated mutant of RhoA (RhoAT19N), behaved just like the wild-type in the trans-activation assay and therefore did not show any specific effect (Figure 4). This indicated that rafts are not involved in Tat delivery to the cytosol. Strong inhibition of trans-activation (by 75%) was observed when using the activated version of RhoA (RhoA G14V), which prevents clathrin/AP-2–dependent uptake.
Figure 4.
Internalization by clathrin-dependent endocytosis is required for Tat to reach the cytosol. Tat cytosolic delivery was monitored using the trans-activation assay. Cells were transfected with luciferase plasmids and the indicated amount of vector coding for EGFP alone (Vect) or an EGFP-tagged protein, as indicated. After 18 h, 100 μM CQ and 200 nM Tat were added. Luciferase activities were assayed 24 h later, and trans-activation was monitored as the increase in the Firefly/Renilla luciferase activity ratio.
Collectively, the data obtained using the trans-activation assay and molecular effectors of clathrin-dependent endocytosis confirmed the morphological evidence that coated pits are the doorstep for subsequent Tat delivery to the cytosol in T cells.
Rab5 Regulates Tat Delivery to the Cytosol
To confirm the need for Tat endocytosis to reach the cytosol, we assessed the involvement of the small GTPase rab5 in this transport. Rab5 was indeed identified as a key component regulating endocytosis, including ligand uptake from the plasma membrane to early endosomes, and homotypic fusion between early endosomes (Gruenberg, 2001). Mutants in the GTPase site of rab5 are known to affect endocytosis efficiency. Indeed, dominant-negative GDP-binding mutants decrease the uptake efficiency of recycling ligands, i.e., Tf (Stenmark et al., 1994) as well as LDL that are destined to lysosomes (Vitelli et al., 1997). The constitutively active GTPase-deficient mutant rab5Q79L, initially reported to increase the Tf internalization rate (Stenmark et al., 1994), did not show any effect on this process in a more recent study (Ceresa et al., 2001).
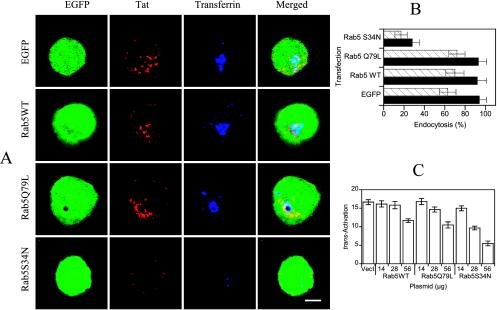
We morphologically examined the effects of overexpressing rab5 mutants on Tat endocytosis. We found that rab5 WT or the activated version did not significantly alter Tat or Tf internalization, whereas conversely, their endocytosis was strongly inhibited (by ∼70%) in cells overexpressing rab5S34N, a dominant-negative mutant of rab5 (Figure 5, A and B). These results indicated that rab5 controls Tat endocytosis.
Figure 5.
Rab5 modulates Tat internalization by T cells. (A) Dependence on rab5 of Tat and Tf endocytosis. Cells were cotransfected with a rab5 expression vector (50 μg) and an EGFP plasmid (6 μg), as indicated. After 24 h, Tat was added for 6 h, and Tf-Cy5 for the last 40 min. Cells were then processed for immunofluorescence detection of Tat. Median optical sections were recorded. A characteristic enlarged endosome induced by rab5Q79L (Ceresa et al., 2001; Stenmark et al., 1994) is clearly noticeable. Bar, 5 μm. (B) Quantification. Images as shown in A were quantified using ImageQuant. The percentage of endosome-associated fluorescence over total cell fluorescence was calculated for n >10 transfected cells. The results are expressed as mean ± SEM for Tf (closed bars) and Tat (hatched bars). (C) Rab5 controls Tat delivery to the cytosol. Cells were transfected with the luciferase plasmids and a vector, either empty (Vect), or coding for the indicated mutant of rab5. Cells were then treated with Tat and CQ before assaying luciferase activities to measure the trans-activation efficiency.
The next question was whether rab5 regulates exogenous Tat delivery to the cytosol. This issue was assessed using the trans-activation assay to monitor cytosolic delivery. Here again, overexpressing rab5 WT or its activated mutant (rab5Q79L) produced essentially the same effect, inhibiting trans-activation by extracellular Tat by 38% for the highest dose. A stronger 70% inhibition of trans-activation was observed for the same dose of dominant-negative rab5 (Figure 5C). Because these effectors did not affect trans-activation by intracellular Tat (unpublished data), we concluded that rab5 regulates Tat delivery to the cytosol in T cells. Altogether, trans-activation data obtained using rab5 mutants and effectors regulating clathrin-mediated uptake showed that extracellular Tat transits through the endocytic pathway on its way to the cytosol.
Tat Transport along the Endocytic Pathway
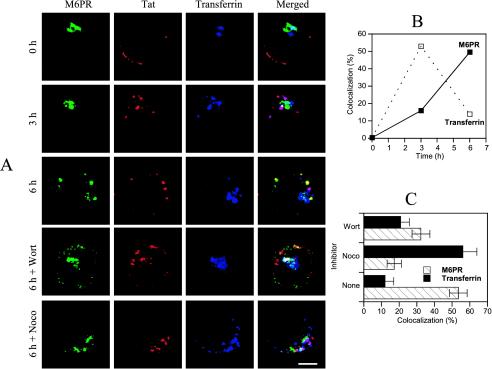
We therefore examined Tat transport along the endocytic network in T cells, initially using Tf and M6PR as early and late endosome markers, respectively (Gruenberg, 2001). M6PR can also be found to some extent in early endosomes (Hirst et al., 1998). It was therefore not surprising to observe some labeling overlap between the internalized Tf and M6PR in T cells (Figure 6A). After initial binding at the plasma membrane, Tat concentrated within early endosomes after 3 h. At that time, it began being detected in late endosomes (Tf–, M6PR+). Tat accumulated within these structures after 5–6 h of labeling (Figure 6, A and B). This slow progression along the endocytic pathway is consistent with the internalization kinetics observed using radiolabeled Tat (Figure 1, A and B). Tat delivery to late endosomes could be strongly impaired using the microtubule-perturbating agent nocodazole, which blocks ligand transfer from early to late endosomes in most cell types (Mallard et al., 1998). Wortmannin, which inhibits the phosphatidylinositol-3-kinase implicated in this transport (Gruenberg, 2001), was less efficient than nocodazole in preventing Tat delivery to late endosomes (Figure 6, A and C).
Figure 6.
Time course of Tat delivery to early and late endosomes. (A) Jurkat cells were incubated with Tat for the indicated times. Tf-Cy5 was added for the last 40 min. Cells were then washed, fixed, and permeabilized for detection of Tat and M6PR by immunofluorescence. When indicated, 100 nM wortmannin (Wort) or 10 μM nocodazole (Noco) were present during cell labeling. Bar, 5 μm. (B) Time-dependent colocalization of Tat with Tf or M6PR. The percentage of Tat pixels positive for Tf or M6PR is shown. (C) Effect of nocodazole or wortmannin on Tat colocalization with Tf or M6PR.
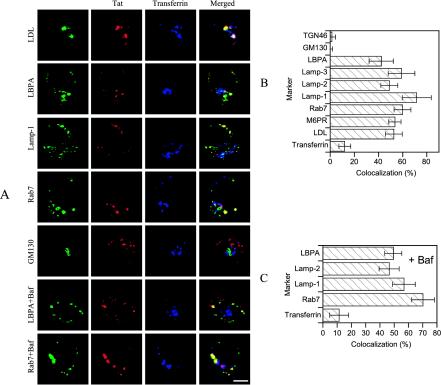
We then used additional markers to identify the endocytic elements in which Tat became concentrated after 6 h of uptake by T cells (Figure 7, A and B). Tat was internalized in the same structures as fluorescent LDL that follow the degradation pathway to late endosomes and lysosomes (Vitelli et al., 1997). Furthermore, Tat colocalized with established late endosome/lysosome markers, such as the lysosomal glycoproteins Lamp-1, -2, and -3, the lipid LBPA, and rab7, a bona fide late endosome marker (Gruenberg, 2001). Tat-containing structures were negative for GM130 or TGN46 (Figure 7, A and B), indicating that Tat is not transported to the Golgi apparatus. Hence, Tat specifically accumulated within late endosomes (i.e., rab7+ and M6PR+ structures) during uptake.
Figure 7.
Internalized Tat concentrated within late endosomes. (A) Cells were labeled for 6 h with Tat and for the last 40 min with Tf-Cy5. They were then processed for Tat, LBPA, Lamp-1, rab7, or GM130 detection. When indicated, (Baf or LDL), 100 nM Baf was present during cell labeling or 20 μg/ml fluorescent LDL were added for the last hour. Bar, 5 μm. (B) Colocalization of internalized Tat with different markers (images for M6PR are presented in Figure 6A, and those for Lamp-2, Lamp-3, and TGN46 are not shown). (C) Colocalization in the presence of Baf (images for Lamp-1 and Lamp-2 are not shown).
Tat Transits through Late Endosomes on Its Way to the Cytosol
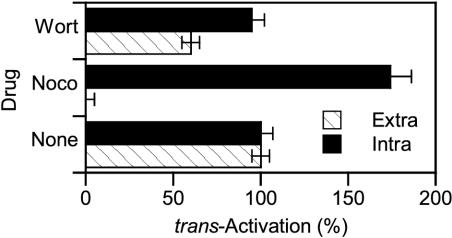
Again, the fact that Tat was detected up to late endosomes does not imply that it has to go that deep in the endocytic pathway to translocate to the cytosol. To evaluate this requirement, we monitored the effects of nocodazole and wortmannin, which impaired Tat delivery to late endosomes (Figure 6, A and C), on trans-activation by extracellular Tat.
During control experiments using cytosolic Tat expression, wortmannin had no effect, whereas nocodazole enhanced Tat trans-activation by ∼70% (Figure 8). This was likely due to the interaction of Tat with microtubules (Chen et al., 2002). Hence, nocodazole, by releasing a fraction of normally microtubule-bound Tat molecules, likely allowed them to reach the nucleus. When extracellular Tat was used, both drugs inhibited trans-activation, although nocodazole was clearly more efficient. These data are in agreement with the respective abilities of these drugs to prevent Tat transfer to late endosomes (Figure 6C) and suggest that this transport is required for subsequent Tat delivery to the cytosol.
Figure 8.
Inhibition of Tat transport to late endosomes impairs its delivery to the cytosol. Cells were transfected with the luciferase plasmids and, when indicated, (Intra) a Tat-coding vector (28 μg). After 18 h, cells were treated for 24 h with 100 μM CQ in the presence of 10 μM nocodazole (Noco) or 100 nM wortmannin (Wort) and, when indicated (Extra), 200 nM Tat. The trans-activation value obtained in the absence of drug was set at 100%. Specific effects on trans-activation by extracellular Tat take place during the endocytosis/translocation steps.
Neutralization of Low Endosomal pH Inhibits Tat Delivery to the Cytosol
We then wondered whether the acidic endosomal pH was involved to any extent in Tat delivery to the cytosol. Indeed, it is well documented that several bacterial toxins, such as diphtheria toxin (DT), exploit this low lumenal pH to trigger conformational changes leading to membrane insertion and translocation of the catalytic subunit to the cytosol (Beaumelle et al., 1992; Ratts et al., 2003).
This issue was difficult to assess because, except in one report (Jiang et al., 1996), trans-activation by extracellular Tat, which is the most conventional and easy test to monitor cytosolic delivery, requires the presence of CQ (Figure 9A and several articles reviewed in Noonan and Albini, 2000). This requirement for CQ was thought to result from the ability of this weak base to neutralize acidic compartments, thereby protecting Tat from lysosomal degradation (Mann and Frankel, 1991; Noonan and Albini, 2000). Nevertheless, a number of other molecules can neutralize endo-lysosomal pH. Baf is considered to be the most specific, because it directly inhibits the vacuolar H+-ATPase responsible for acidification (Clague et al., 1994). Monensin and nigericin, which are protonophores, methylamine and ammonium chloride which are weak bases, are also known to neutralize the endosome lumen (Alami et al., 1998).
Figure 9.
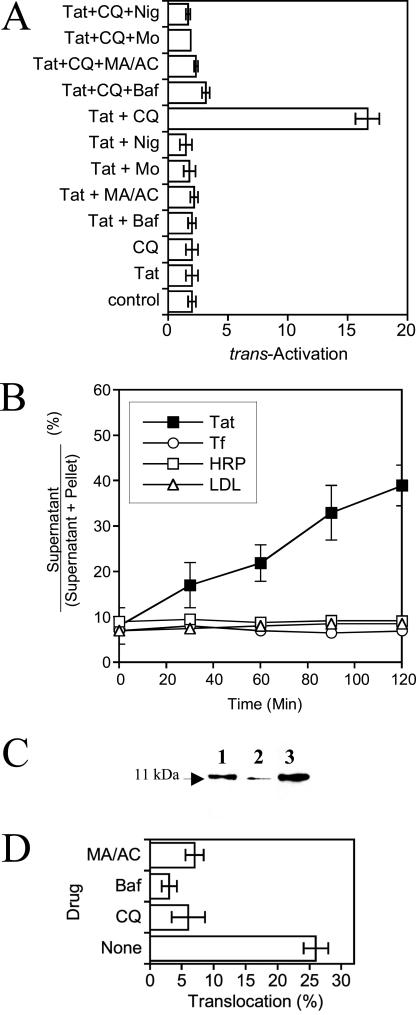
Endosome neutralization inhibits Tat translocation. (A) CQ is required for trans-activation by extracellular Tat. Jurkat cells were transfected with plasmids encoding luciferases. After 18 h, cells were treated with Tat, Baf (100 nM), nigericin (Nig, 5 μM), monensin (Mo, 50 μM), methylamine/ammonium chloride (MA/AC, 20 mM each), or CQ (100 μM), as indicated. Luciferase activities were assayed 24 h later. (B) Cell-free translocation of 125I-Tat from Jurkat endosomes. Cells were labeled with the indicated 125I tracer before endosome isolation. Endosomes prepared from cells labeled with 125I- HRP, 125I-Tf, or 125I-LDL, were used as negative controls. Translocation was assayed for the indicated times in the presence of cytosol and an ATP-regenerating system. After ultracentrifugation on a sucrose cushion, supernatant and pellet were counted. (C) Visualization of translocated material. SDS/PAGE analysis of endosomal (lane 1) and translocated material from 125I-Tat–labeled endosomes after 0 h (lane 2) or 2 h (lane 3) of translocation. Gels were revealed using storage phosphor screens. (D) Cell-free Tat translocation is inhibited by endosome neutralization. Translocation was assayed for 90 min in the absence or presence of 500 nM Baf, 50 μM CQ, or MA/AC (20 mM each), as indicated. The endosome suspension was then ultracentrifuged. Translocation is expressed as the increase in the medium/endosome radioactivity ratio (%).
Although all these molecules effectively neutralized endosomes from Jurkat cells (unpublished data), when the trans-activation assay was performed in the presence of one of these drugs, it was surprising to find that CQ was the only one enabling significant trans-activation by extracellular Tat (Figure 9A). In contrast to exogenous Tat, cytosolically expressed Tat did not require CQ for efficient trans-activation in T cells (unpublished data), as shown earlier for HeLa cells (Frankel and Pabo, 1988). Hence, the CQ effect that allows trans-activation by extracellular Tat takes place in the endocytic pathway. We did not observe significant Tat degradation during uptake by T cells (unpublished data) and could therefore not test whether CQ increased its intracellular stability as shown earlier in HeLa cells (Frankel and Pabo, 1988).
Unexpectedly, when the trans-activation assay was performed in the presence of any other endosome neutralizing drug, in addition to 100 μM CQ, trans-activation by extracellular Tat was entirely abolished (Figure 9A). None of these drugs significantly affected trans-activation by intracellular Tat (unpublished data), indicating that they also acted during Tat endocytosis and/or translocation. Although these pharmacological data indicated that low endosomal pH was required for exogenous Tat to reach the cytosol, CQ had, compared with the other molecules, a specific effect that is only observed in the trans-activation assay (see below).
How could endosome neutralizing drugs block Tat passage into the cytosol? One possibility is that they prevented Tat cytosolic access by inhibiting its delivery to late endocytic structures which, according to pharmacological data (Figure 8), appeared to be a favorite translocation site. Drugs such as Baf, which neutralize the endosome lumen, have indeed been shown to impair early to late endosome transport (Clague et al., 1994).
We therefore examined, using confocal microscopy, whether Tat routing within Jurkat cells was affected by the presence of Baf. We found that Tat delivery to late endocytic elements, as identified using Lamp-1, Lamp-2, LBPA, or rab7, was as efficient in treated cells compared with control cells (Figure 7, A and C). Tat was not significantly retained within early endosomes (Tf+) in Baf-treated cells. Similar images were obtained using CQ (not depicted). Hence, drugs neutralizing endosomes did not block Tat delivery to late endosomes. This finding is in agreement with previous studies (van Weert et al., 1995) and indicated that these drugs prevented Tat delivery to the cytosol by affecting a late stage of the entry process, most likely the translocation step.
Endocytosed Tat Requires Low-Endosomal pH to Translocate
To directly assay Tat translocation, we used a purified endosome preparation that contains both early and late endocytic vesicles (Beaumelle and Hopkins, 1989) and a cell-free translocation assay. We previously used this assay to study DT (Beaumelle et al., 1992) and Pseudomonas exotoxin (Alami et al., 1998) translocation across the endosome membrane. Cells were labeled with 125I-Tat or control tracers, 125I-Tf, 125I- HRP, or 125I-LDL before endosome isolation. At onset of the translocation assay, <10% of radiolabeled tracers were found in the supernatant after endosome centrifugation (Figure 9B). On incubation at 37°C, no release of any control tracer could be detected, whereas some 125I-Tat was translocated from endosomes to the medium. Tat translocation proceeded linearly with time, and ∼30% of endosomal 125I-Tat was translocated in 2 h (Figure 9B). Tat was not processed during uptake or translocation (Figure 9C). From the endocytosis efficiency and translocation activity, we calculated that, when cell labeling equilibrium is reached (i.e., after more than 6 h), 3–5% of cell-associated Tat molecules reach the cytosol in 1 h.
Tat translocation was strongly inhibited (by 70–90%) in the presence of Baf, CQ, or ammonium chloride/methylamine (Figure 9D). These results show that Tat crosses the endosome membrane in response to low-pH exposure.
Tat Translocation Requires Cytosolic Hsp90
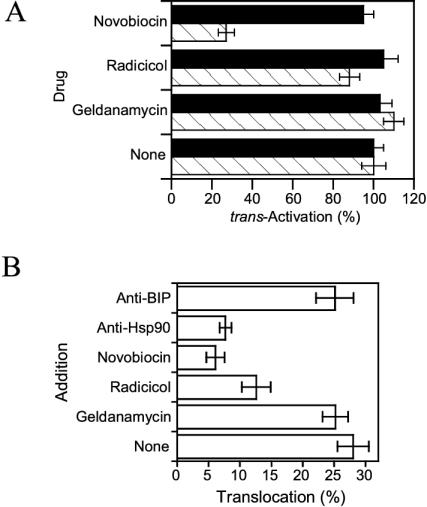
The process used by Tat to cross the endosome membrane upon low pH exposure is similar to that used by DT A-chain, which is the prototype of toxins entering cells from endosomes (Beaumelle et al., 1992; Ratts et al., 2003). Because DT A-chain translocation was recently found to require cytosolic Hsp90 (Ratts et al., 2003), we examined whether this chaperone was also involved in Tat trans-membrane transport. To this end, we first used well-characterized inhibitors of this chaperone. Novobiocin inhibits Hsp90 through binding to both its C- and N-terminal ATP-binding sites, whereas geldanamycin and radicicol only block the latter (Picard, 2002). None of these drugs significantly affected trans-activation by intracellular Tat. Novobiocin inhibited by ∼70% trans-activation by extracellular Tat, whereas geldanamycin or radicicol had no significant effect (Figure 10A). These results suggested that Hsp90 chaperone activity is required for Tat translocation.
Figure 10.
Tat translocation requires cytosolic Hsp90. (A) Effect of Hsp90 inhibitors on trans-activation by intracellular or extracellular Tat. Cells were transfected with the luciferase plasmids and (closed bars; intracellular) a Tat-coding vector (28 μg). After 18 h, cells were treated for 24 h with 100 μM CQ in the presence of 10 nM geldanamycin, 10 nM radicicol, 100 μM novobiocin, or 200 nM Tat (hatched bars; extracellular). The trans-activation value obtained in the absence of drug was set at 100%. (B) Effect of Hsp 90 inhibitors on cell-free Tat translocation. Cells were labeled with 125I-Tat before endosome isolation. Translocation was assayed for 90 min in the presence of 10 μM geldanamycin, 10 μM radicicol, 600 μM novobiocin, or 1 μg/ml of mAb, as indicated.
We used the cell-free translocation assay to more specifically assess the role of Hsp90 in Tat trans-membrane transport. Higher drug concentrations could be used in this assay, so the effects were more pronounced. Novobiocin was again the most active inhibitor, inhibiting Tat translocation by 80%. Radicicol inhibited translocation by 55%, and geldanamycin once more had no effect. Moreover, translocation was inhibited by 70% using an anti-Hsp90 mAb (directed against the C-terminal domain), although not affected by a control, anti-BIP mAb (Figure 10B). These data confirmed that Hsp90 chaperone activity is required for Tat transport through the endosome membrane.
Tat Triggers Cell Responses after Delivery to the Cytosol
It was not clear whether Tat triggered lymphocyte responses upon plasma membrane-binding (Secchiero et al., 2000) or after delivery to the cytosol (Ott et al., 1997). Drugs neutralizing endosomal pH enabled us to explore this issue because they blocked the latter pathway by preventing Tat exit from endosomes (Figure 9D), without affecting Tat binding, according to confocal microscopy examination (unpublished data).
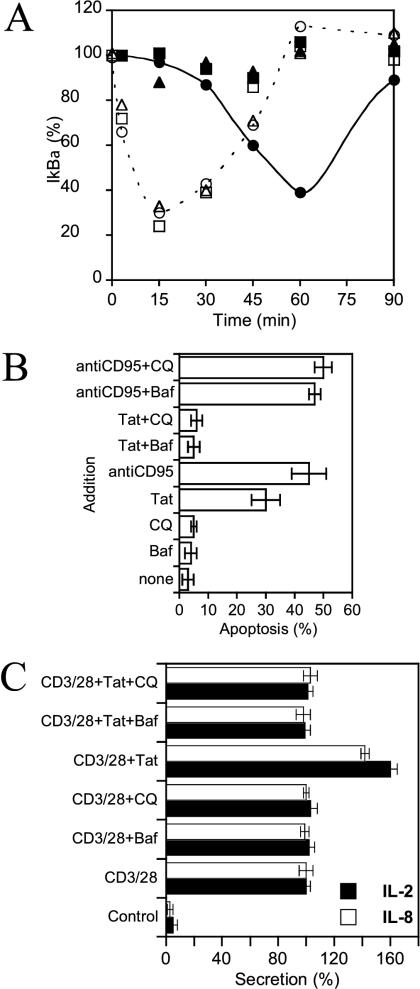
We therefore assessed the effects of CQ and Baf on three key established biological effects of exogenous Tat on T cells. A large number of Tat immune and proinflammatory responses are mediated by activation of the transcription factor NF-κB resulting from IκBα degradation. Such degradation can also be triggered by TNF-α, a cytokine that signals directly from the plasma membrane (Latz et al., 2002) and that we used as control inducer. When applied to Jurkat cells, TNF-α quickly induced massive IκBα degradation, which peaked after 15 min before resynthesis (Figure 11A; Manna and Aggarwal, 2000). Tat clearly needed more time to trigger IκBα degradation, which peaked after 1 h. Moreover, in Baf- or CQ-treated cells, IκBα degradation induced by TNF-α was unaffected, whereas that induced by Tat was entirely inhibited (Figure 11A). The kinetic difference between the effects of Tat and TNF-α as well as the ability of endosome neutralizing drugs to prevent IκBα degradation when induced by Tat, but not by TNF-α, strongly suggested that endocytosis and translocation from acidified endosomes are required for Tat to elicit this cell response.
Figure 11.
Tat delivery to the cytosol is required for its signaling activity. (A) Kinetics of IκBα degradation. Jurkat cells were treated with Tat (closed symbols) or TNF-α (open symbols), in the absence of drug (circles), or in the presence of Baf (triangles), or CQ (squares) for the indicated times before lysis and SDS/PAGE. Quantitative analysis of IκBα was then performed using duplicate immunoblots. IκBα levels are expressed as percentages of the amount present in untreated cells. Most error bars fall within the symbol size. Baf or CQ alone did not affect the IκBα level (unpublished data). (B) Apoptosis of Jurkat T cells. Cells were cultivated for 24 h in the presence of Tat, Baf, CQ, or an anti-CD95 antibody, as indicated. Apoptosis was then assayed using annexinV-fluorescein binding and FACS analysis. (C) Interleukin secretion. Primary T cells were purified by immunomagnetic negative selection and stimulated with anti-CD3/CD28 mAbs in the presence or absence of Tat. Supernatants were harvested after 24 h and assayed for IL-2 and IL-8. Where indicated, 10 nM Baf or 0.6 μM CQ were added 20 min before Tat. “100% secretion” refers to levels obtained using anti-CD3/CD28 stimulation. These amounts were 2 and 0.8 ng/ml on average for IL-2 and IL-8, respectively.
This requirement was confirmed when we examined the ability of Tat to cause Jurkat cell apoptosis (Li et al., 1995; Chen et al., 2002). An anti-CD95 antibody, which stimulates the Fas apoptosis pathway upon binding to CD95 at the plasma membrane (Aragane et al., 1998), was the control inducer. Apoptosis induction by Tat was abrogated by CQ or Baf, whereas apoptosis triggered from the plasma membrane by anti-CD95 remained insensitive to these drugs (Figure 11B).
Several perturbations of the immune system during AIDS likely result from the ability of Tat to alter the cytokine balance by inducing the secretion of key cytokines by peripheral blood mononuclear cells, and T cells in particular (Rubartelli et al., 1998). We used primary T cells to explore whether retaining Tat within endosomes prevented it from inducing cytokine secretion. Similar data were obtained on monocytes (not depicted). A conventional way to obtain optimal stimulation of T cells is to cross-link CD3 and CD28. This results in IL-2 and IL-8 secretion (Figure 11C). Tat addition to T cells after CD3 and CD28 costimulation enhanced secretion of both interleukins by 40–60%. These values are consistent with previous findings (Ott et al., 1997, 1998). Endosomal neutralization by Baf or CQ prevented Tat-induced interleukin release without interfering with secretion when triggered from the plasma membrane by CD3 and CD28 cross-linking (Figure 11C). These results with primary T cells validate our former data obtained on the Jurkat cell line.
Altogether, the data obtained in experiments to monitor IκBα degradation, apoptosis induction and interleukin secretion show that Tat has to reach the cytosol before triggering cell responses. These findings, obtained using different readouts for monitoring Tat delivery to the cytosol, also corroborated the results of the trans-activation and translocation assays and confirmed that Tat relies on low endosomal pH to translocate to the cytosol.
DISCUSSION
We showed here that exogenous Tat enters T cells using clathrin-mediated endocytosis, depending on Eps15, intersectin, and dynamin before a translocation step which is induced by low endosomal pH, assisted by Hsp90 and enables delivery to the cytosol, from which Tat will elicit cell responses.
Throughout this study, we never obtained evidence of direct Tat transport through the plasma membrane to reach the cytosol. This possibility can be dismissed in the light of results obtained with inhibitors such as chlorpromazine, nocodazole, and endosome-neutralizing drugs. These agents, which block Tat at different stages of its intracellular transport toward the cytosol, all specifically blocked trans-activation by exogenous Tat, but not by intracellular Tat. The finding that Tat does not directly cross the plasma membrane is in agreement with recent data obtained for the PTD, indicating that this peptide is endocytosed and that the diffuse cytosolic staining observed previously upon PTD addition to cells was a fixation artifact (Green et al., 2003). Endocytosis also seems to be required for the PTD to reach the cytosol (Richard et al., 2003).
Molecular inhibitors of clathrin-dependent endocytosis enabled us to confirm morphological evidence that Tat enters T cells via coated pits. It was surprising to observe a promiscuous ligand such as Tat taken up solely by a clathrin-, Eps15-, intersectin- and dynamin-dependent pathway. Data from internalization inhibition experiments performed using mAbs indicated that, to enter T cells, Tat used several receptors identified in other cell types, including CD26, CXCR4, and the LRP (unpublished data). Among these receptors, LRP (Orlandi and Fishman, 1998) and CXCR4 (Signoret et al., 1997) are endocytosed via coated pits and might therefore drive Tat through this pathway.
Clathrin-dependent uptake usually enables fast internalization. Maximum Tf accumulation in Jurkat cells was reached in 10–15 min for instance (not depicted), whereas several hours were required for Tat (Figure 1). Moreover, Tat-EGFP internalization by HeLa cells also proceeded >10 h, while this fusion protein enters HeLa cells using caveolae (Fittipaldi et al., 2003), structures that where recently shown to enable reasonably fast uptake, i.e., in <1 h (Peters et al., 2003). Tat endocytosis via caveolae or coated pits likely involves different receptors. Hence Tat, but neither its cell binding moiety nor its internalization pathway, appears to be directly responsible for this slow intracellular accumulation, which probably reflects the fact that Tat is able to interact with several proteins within its vicinity, hampering progression along the endocytic pathway.
Lymphocytes are devoid of caveolae (Fra et al., 1994; Lamaze et al., 2001). The finding that Tat uses coated pits to enter T cells (this study), although preferring caveolae for endocytosis by HeLa cells (Fittipaldi et al., 2003), is reminiscent of the cholera toxin internalization phenotype, because this toxin is endocytosed by the clathrin pathway in cells expressing low levels of caveolin-1, whereas it is internalized via a clathrin-independent route in cells with high caveolin-1 (Singh et al., 2003).
Intracellular routing of exogenous Tat has not yet been visualized. It remains to be assessed within epithelial cells. The pathway followed by Tat in these cells can hardly be anticipated because caveolae can target cargos to different subcellular compartments such as the Golgi, the ER, or late endosomes (Nabi and Le, 2003; Peters et al., 2003). We chose to study Tat uptake by lymphocytes because they are one of its main targets during AIDS (Rubartelli et al., 1998). We did not observe Tat transport to the Golgi or the ER, suggesting that Tat does not take a retrograde route from early endosomes as is the case for cholera and shiga toxins (Falnes and Sandvig, 2000). Instead, we found that Tat accumulated within late endosomes upon internalization by T cells (Figures 6 and 7). Pharmacological data indicated that these structures are the favorite Tat translocation site within T cells. It might be concluded that receptors such as CXCR4, which follow the degradation pathway (Signoret et al., 1997), provide more adequate Tat intracellular routing than recycling receptors such as the LRP, which from the sorting endosome is directed to the recycling compartment in T cells (Alami et al., 1998). Nevertheless we found, using artificial membranes, that Tat could start inserting itself into membranes when pH drops to 5.5, whereas sorting endosomes often show in Jurkat cells lumenal pH values below 5 (unpublished data). Hence, once delivered to sorting endosomes, and irrespective of the receptor used to drive it there, Tat likely inserts into the membrane, allowing it to follow the default pathway to late endosomes (Gruenberg, 2001).
Several bacterial toxins such as the DT A-chain, Clostridium botulinum C2 toxin enzyme component and cytotoxic necrotizing factor 1 (CNF1) rely on low endosomal pH to enter the cytosol. Cytosolic delivery of the former toxins is insensitive to nocodazole (Lemichez et al., 1997; Barth et al., 2000), indicating that they translocate from early endosomes, whereas nocodazole blocks CNF1 access to the cytosol in most cell types, suggesting that this toxin has to reach a late endocytic compartment for translocation (Contamin et al., 2000). Nocodazole also prevented Tat delivery to the cytosol (Figure 8). The reasons why CNF1 and Tat have to reach late endosomes for translocation is not yet clear. When Tat was prebound to cells at 4°C before exposing them to low pH in order to mimick the endosomal lumen (Contamin et al., 2000), we found that some Tat molecules could be translocated to the cytosol (unpublished data). Nevertheless, such artificially induced Tat translocation through the plasma membrane proceeded very slowly compared with natural endosomal translocation. Hence, after acid-driven membrane insertion, late stages of the Tat translocation process may rely on specific cellular proteins or lipids only found within late endosomes.
Tat (this study) joins DT A-chain (Ratts et al., 2003) and C2 toxin enzyme component (Haug et al., 2003) on the growing list of endosome-translocating toxins relying on low endosomal pH and cytosolic Hsp90 to reach the cytosol. Translocation of these toxins was nevertheless differentially affected by Hsp90 inhibitors. This might indicate that molecular complexes enabling trans-membrane transport are different in each case. Indeed, Hsp90 is known to interplay with a diversity of cochaperones and regulators (Picard, 2002). It is not yet clear whether Hsp90 directly interacts with incoming toxins.
Albeit the chaperone activity of Hsp90 is required to complete translocation, low-pH triggered membrane insertion is a strict prerequisite to initiate and possibly energize Tat trans-membrane transport. This result from trans-activation and translocation assays (Figure 9) was corroborated by the ability of endosome neutralizing drugs to prevent exogenous-Tat signaling, as monitored using three different readouts (Figure 11). Soluble Tat thus triggers cell responses after delivery to the cytosol and not just upon plasma membrane binding, as observed when Tat was coated on plastic (Secchiero et al., 2000). Our finding is consistent with the fact that Tat, whether cytosolically expressed or extracellular, can trans-activate the IL-2 gene (Ott et al., 1997). Hence, Tat primary targets initiating cell responses are likely cytosolic and/or nuclear. Tat affects a large number of cell activities and transduction pathways, providing compelling evidence that this protein behaves as a viral toxin (Rubartelli et al., 1998; Chen et al., 2002). We further completed this picture by showing here that Tat uses a bacterial toxin strategy to enter cells.
Acknowledgments
We are grateful to Drs. Alejandro Barbieri, Moncef Benkirane, Alexandre Benmerah, Clare Futter, Cécile Gauthier-Rouvière, Jean Gruenberg, Christophe Lamaze, Susan Michelson, Peter McPherson, Emanuele Papini, Henri Rochefort, Anna Rubartelli and Sandy Schmid and for their kind gift of reagents. We thank Fabrice Raynaud, Fabrice Mérezègue, and Brigitte N′Guyen for technical assistance. This work was supported by grants from the CNRS (Cell Biology Program) and by the Agence Nationale de Recherches sur le SIDA.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–12–0921. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–12–0921.
Abbreviations used: Baf, bafilomycin; CQ, chloroquine; CNF1, cytotoxic necrotizing factor 1; DT, diphtheria toxin; FACS, fluorescence-activated cell sorting; FCS, fetal calf serum; HRP, horseradish peroxidase; LDL, low-density lipoproteins; LBPA, lysobisphosphatidic acid; LRP, LDL receptor-related protein; LTR, long terminal repeat; mAb, monoclonal antibody; M6PR, mannose 6-phosphate receptor; PTD, protein transduction domain; Tf, transferrin.
References
- Alami, M., Taupiac, M.P., Reggio, H., Bienvenue, A., and Beaumelle, B. (1998). Involvement of ATP-dependent Pseudomonas exotoxin translocation from a late recycling compartment in lymphocyte intoxication procedure. Mol. Biol. Cell 9, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragane, Y., Kulms, D., Metze, D., Wilkes, G., Poppelmann, B., Luger, T.A., and Schwarz, T. (1998). Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J. Cell Biol. 140, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth, H., Blocker, D., Behlke, J., Bergsma-Schutter, W., Brisson, A., Benz, R., and Aktories, K. (2000). Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J. Biol. Chem. 275, 18704–18711. [DOI] [PubMed] [Google Scholar]
- Beaumelle, B., Bensammar, L., and Bienvenue, A. (1992). Selective translocation of the A chain of diphtheria toxin across the membrane of purified endosomes. J. Biol. Chem. 267, 11525–11531. [PubMed] [Google Scholar]
- Beaumelle, B., Gibson, A., and Hopkins, C.R. (1990). Isolation and preliminary characterization of the major membrane boundaries of the endocytic pathway in lymphocytes. J. Cell Biol. 111, 1811–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumelle, B., and Hopkins, C.R. (1989). High-yield isolation of functionally competent endosomes from mouse lymphocytes. Biochem J. 264, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah, A., Bayrou, M., Cerf-Bensussan, N., and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303–1311. [DOI] [PubMed] [Google Scholar]
- Bonifaci, N., Sitia, R., and Rubartelli, A. (1995). Nuclear translocation of an exogenous fusion protein containing HIV Tat requires unfolding. AIDS 9, 995–1000. [DOI] [PubMed] [Google Scholar]
- Ceresa, B.P., Lotscher, M., and Schmid, S.L. (2001). Receptor and membrane recycling can occur with unaltered efficiency despite dramatic Rab5(q79l)-induced changes in endosome geometry. J. Biol. Chem. 276, 9649–9654. [DOI] [PubMed] [Google Scholar]
- Chang, H.C., Samaniego, F., Nair, B.C., Buonaguro, L., and Ensoli, B. (1997). HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11, 1421–1431. [DOI] [PubMed] [Google Scholar]
- Chen, D., Wang, M., Zhou, S., and Zhou, Q. (2002). HIV-1 Tat targets microtubules to induce apoptosis, a process promoted by the pro-apoptotic Bcl-2 relative Bim. EMBO J. 21, 6801–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague, M.J., Urbe, S., Aniento, F., and Gruenberg, J. (1994). Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 269, 21–24. [PubMed] [Google Scholar]
- Contamin, S., Galmiche, A., Doye, A., Flatau, G., Benmerah, A., and Boquet, P. (2000). The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol. Biol. Cell 11, 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falnes, P.O., and Sandvig, K. (2000). Penetration of protein toxins into cells. Curr. Opin. Cell Biol. 12, 407–413. [DOI] [PubMed] [Google Scholar]
- Fawell, S., Seery, J., Daikh, Y., Moore, C., Chen, L.L., Pepinsky, B., and Barsoum, J. (1994). Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 91, 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi, A., Ferrari, A., Zoppe, M., Arcangeli, C., Pellegrini, V., Beltram, F., and Giacca, M. (2003). Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 tat fusion proteins. J. Biol. Chem. 36, 34141–34149. [DOI] [PubMed] [Google Scholar]
- Foti, M., Mangasarian, A., Piguet, V., Lew, D.P., Krause, K.H., Trono, D., and Carpentier, J.L. (1997). Nef-mediated clathrin-coated pit formation. J. Cell Biol. 139, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra, A.M., Williamson, E., Simons, K., and Parton, R.G. (1994). Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 269, 30745–30748. [PubMed] [Google Scholar]
- Frankel, A.D., Bredt, D.S., and Pabo, C.O. (1988). Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science 240, 70–73. [DOI] [PubMed] [Google Scholar]
- Frankel, A.D., and Pabo, C.O. (1988). Cellular uptake of the Tat protein from human immunodeficiency virus. Cell 55, 1189–1193. [DOI] [PubMed] [Google Scholar]
- Green, I., Christison, R., Voyce, C.J., Bundell, K.R., and Lindsay, M.A. (2003). Protein transduction domains: are they delivering? Trends Pharmacol. Sci. 24, 213–215. [DOI] [PubMed] [Google Scholar]
- Gruenberg, J. (2001). The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell. Biol. 2, 721–730. [DOI] [PubMed] [Google Scholar]
- Gutheil, W.G., Subramanyam, M., Flentke, G.R., Sanford, D.G., Munoz, E., Huber, B.T., and Bachovchin, W.W. (1994). Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat's immunosuppressive activity. Proc. Natl. Acad. Sci. USA 91, 6594–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug, G., Leemhuis, J., Tiemann, D., Meyer, D.K., Aktories, K., and Barth, H. (2003). The host cell chaperone Hsp90 is essential for translocation of the binary Clostridium botulinum C2 toxin into the cytosol. J. Biol. Chem. 278, 32266–32274. [DOI] [PubMed] [Google Scholar]
- Hirst, J., Futter, C.E., and Hopkins, C.R. (1998). The kinetics of mannose 6-phosphate receptor trafficking in the endocytic pathway in HEp-2 cells: the receptor enters and rapidly leaves multivesicular endosomes without accumulating in a prelysosomal compartment. Mol. Biol. Cell 9, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, M.C., Lin, J.K., and Chen, S.S.L. (1996). Inhibition of HIV-1 Tat-mediated transactivation by quinacrine and chloroquine. Biochem. Biophys. Res. Commun. 226, 1–7. [DOI] [PubMed] [Google Scholar]
- Johannes, L., and Lamaze, C. (2002). Clathrin-dependent or not: is it still the question? Traffic 3, 443–451. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., Stang, E., Fang, K.S., de Moerloose, P., Parton, R.G., and Gruenberg, J. (1998). A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 392, 193–197. [DOI] [PubMed] [Google Scholar]
- Lamaze, C., Dujeancourt, A., Baba, T., Lo, C.G., Benmerah, A., and Dautry-Varsat, A. (2001). Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 7, 661–71. [DOI] [PubMed] [Google Scholar]
- Latz, E., Visintin, A., Lien, E., Fitzgerald, K.A., Monks, B.G., Kurt-Jones, E.A., Golenbock, D.T., and Espevik, T. (2002). Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277, 47834–47843. [DOI] [PubMed] [Google Scholar]
- Lemichez, E., Bomsel, M., Devilliers, G., vanderSpek, J., Murphy, J.R., Lukianov, E.V., Olsnes, S., and Boquet, P. (1997). Membrane translocation of diphtheria toxin fragment A exploits early to late endosome trafficking machinery. Mol. Microbiol.. 23, 445–457. [DOI] [PubMed] [Google Scholar]
- Li, C., Friedman, D., Wang, C., Metelev, V., and Pardee, A. (1995). Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268, 429–439. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Jones, M., Hingtgen, C.M., Bu, G., Laribee, N., Tanzi, R.E., Moir, R.D., Nath, A., and He, J.J. (2000). Uptake of HIV-1 Tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat. Med. 6, 1380–1387. [DOI] [PubMed] [Google Scholar]
- Mallard, F., Antony, C., Tenza, D., Salamero, J., Goud, B., and Johannes, L. (1998). Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 143, 973–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, D.A., and Frankel, A.D. (1991). Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 10, 1733–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna, S.K., and Aggarwal, B.B. (2000). Differential requirement for p56lck in HIV-tat versus TNF-induced cellular responses: effects on NF-kappa B, activator protein-1, c-Jun N-terminal kinase, and apoptosis. J. Immunol. 164, 5156–5166. [DOI] [PubMed] [Google Scholar]
- McNiven, M.A., Cao, H., Pitts, K.R., and Yoon, Y. (2000). The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci. 25, 115–120. [DOI] [PubMed] [Google Scholar]
- Morlon-Guyot, J., Helmy, M., Lombard-Frasca, S., Pignol, D., Pieroni, G., and Beaumelle, B. (2003). Identification of the ricin lipase site and implication in cytotoxicity. J. Biol. Chem. 278, 17006–17011. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, A., Barbieri, A.M., Funato, K., Roberts, R., and Stahl, P.D. (1997). Sequential actions of Rab5 and Rab7 regulate endocytosis in the Xenopus oocyte. J. Cell Biol. 136, 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi, I.R., and Le, P.U. (2003). Caveolae/raft-dependent endocytosis. J. Cell Biol. 161, 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, D.H., and Taub, D. (2002). CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168, 4121–4126. [DOI] [PubMed] [Google Scholar]
- Noonan, D., and Albini, A. (2000). From the outside in: extracellular activities of HIV Tat. Adv. Pharmacol. 48, 229–250. [DOI] [PubMed] [Google Scholar]
- Orlandi, P.A., and Fishman, P.H. (1998). Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory, S., Munari-Silem, Y., Fort, P., and Jurdic, P. (2000). Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J. Cell Sci. 113, 1177–1188. [DOI] [PubMed] [Google Scholar]
- Ott, M., Emiliani, S., Van Lint, C., Herbein, G., Lovett, J., Chirmule, N., McCloskey, T., Pahwa, S., and Verdin, E. (1997). Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science 275, 1481–1485. [DOI] [PubMed] [Google Scholar]
- Ott, M., Lovett, J.L., Mueller, L., and Verdin, E. (1998). Superinduction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-kappaB factors. J. Immunol. 160, 2872–2880. [PubMed] [Google Scholar]
- Peters, P.J. et al. (2003). Trafficking of prion proteins through a caveolae-mediated endosomal pathway. J. Cell Biol. 162, 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard, D. (2002). Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol. Life Sci. 59, 1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas, R.E., Innerarity, T.L., Weinstein, J.N., and Mahley, R.W. (1981). Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis 1, 177–185. [DOI] [PubMed] [Google Scholar]
- Ratts, R., Zeng, H., Berg, E.A., Blue, C., McComb, M.E., Costello, C.E., vander-Spek, J.C., and Murphy, J.R. (2003). The cytosolic entry of diphtheria toxin catalytic domain requires a host cell cytosolic translocation factor complex. J. Cell Biol. 160, 1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, J.P., Melikov, K., Vives, E., Ramos, C., Verbeure, B., Gait, M.J., Chernomordik, L.V., and Lebleu, B. (2003). Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 278, 585–590. [DOI] [PubMed] [Google Scholar]
- Roumier, A., Olivo-Marin, J.C., Arpin, M., Michel, F., Martin, M., Mangeat, P., Acuto, O., Dautry-Varsat, A., and Alcover, A. (2001). The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity 15, 715–728. [DOI] [PubMed] [Google Scholar]
- Rubartelli, A., Poggi, A., Sitia, R., and Zocchi, M.R. (1998). HIV-I Tat: a polypeptide for all seasons. Immunol. Today 19, 543–545. [DOI] [PubMed] [Google Scholar]
- Salgado, F.J., Lojo, J., Alonso-Lebrero, J.L., Lluis, C., Franco, R., Cordero, O.J., and Nogueira, M. (2003). A role for interleukin-12 in the regulation of T cell plasma membrane compartmentation. J. Biol. Chem. 278, 24849–24857. [DOI] [PubMed] [Google Scholar]
- Secchiero, P., Zella, D., Curreli, S., Mirandola, P., Capitani, S., Gallo, R.C., and Zauli, G. (2000). Pivotal role of cyclic nucleoside phosphodiesterase 4 in Tat-mediated CD4+ T cell hyperactivation and HIV type 1 replication. Proc. Natl. Acad. Sci. USA 97, 14620–14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret, N. et al. (1997). Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J. Cell Biol. 139, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, F., Hussain, N.K., Qualmann, B., Kelly, R.B., Kay, B.K., McPherson, P.S., and Schmid, S.L. (1999). SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat. Cell Biol. 1, 119–124. [DOI] [PubMed] [Google Scholar]
- Singh, R.D., Puri, V., Valiyaveettil, J.T., Marks, D.L., Bittman, R., and Pagano, R.E. (2003). Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 14, 3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., Valencia, A., Martinez, O., Ullrich, O., Goud, B., and Zerial, M. (1994). Distinct structural elements of Rab5 define its functional specificity. EMBO J. 13, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil, A., Hemar, A., and Dautry-varsat, A. (1994). rapid endocytosis of interleukin 2 receptor when clathrin-coated pit endocytosis is inhibited. J. Cell Sci. 107, 3461–3468. [DOI] [PubMed] [Google Scholar]
- Traub, L.M. (2003). Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 163, 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi, M., Rusnati, M., Presta, M., and Giacca, M. (2001). Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276, 3254–3261. [DOI] [PubMed] [Google Scholar]
- van Weert, A.W.M., Dunn, K.W., Geuze, H.J., Maxfield, F.R., and Stoorvogel, W. (1995). Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J. Cell Biol. 130, 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli, R., Santillo, M., Lattero, D., Chiariello, M., Bifulco, M., Bruni, C.B., and Bucci, C. (1997). Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272, 4391–4397. [DOI] [PubMed] [Google Scholar]
- Voss, G., Manson, K., Montefiori, D., Watkins, D.I., Heeney, J., Wyand, M., Cohen, J., and Bruck, C. (2003). Prevention of disease induced by a partially heterologous AIDS virus in rhesus monkeys by using an adjuvanted multicomponent protein vaccine. J Virol. 77, 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, K., and Edwards, R.J. (1999). HIV-1-trans-activating (Tat) protein: both a target and a tool in therapeutic approaches. Biochem. Pharmacol. 58, 1521–1528. [DOI] [PubMed] [Google Scholar]
- Wei, P., Garber, M.E., Fang, S.M., Fischer, W.H., and Jones, K.A. (1998). A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92, 451–462. [DOI] [PubMed] [Google Scholar]
- Xiao, H., Neuveut, C., Tiffany, H.L., Benkirane, M., Rich, E.A., Murphy, P.M., and Jeang, K.T. (2000). Selective CXCR4 antagonism by tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc. Natl. Acad. Sci. USA 97, 11466–11471. [DOI] [PMC free article] [PubMed] [Google Scholar]