Abstract
PURPOSE
To investigate changes of whole eye axial biometry during accommodation using ultra-long scan depth optical coherence tomography (UL-OCT).
DESIGN
Prospective, observational case series.
METHODS
Twenty-one adult subjects were enrolled. Using UL-OCT, the left eye of each subject was imaged with relaxed (0 D) and accommodative stimuli (+6 D). Full eye biometry included central corneal thickness (CCT), anterior chamber depth (ACD), lens thickness, vitreous length and axial length (AL).
RESULTS
During accommodation (+6 D), the axial biometry of the whole eye changed significantly. Compared to the rest state, ACD at the accommodative state decreased significantly from 3.128 ± 0.305 mm to 2.961 ± 0.298 mm (paired t-test, P < 0.001). The lens thickness increased significantly from 3.723 ± 0.237 mm to 3.963 ± 0.234 mm (P < 0.001). The vitreous length decreased significantly from 17.129 ± 0.864 mm to 17.057± 0.848 mm (P < 0.001). AL was 24.519 ± 0.917 mm at the rest state and increased to 24.545±0.915 mm with +6 D accommodation stimulus. The elongated AL of 26.1 ± 13.4 μm between the rest and accommodative states was significant (P < 0.001).
CONCLUSIONS
During accommodation, whole eye axial biometry changed, including a decrease in ACD and vitreous length, and an increase in lens thickness and AL. UL-OCT provides an alternative method that is suitable for full eye biometry during accommodation.
Keywords: axial biometry, whole eye, optical coherence tomography, accommodation
INTRODUCTION
Human accommodation is a dynamic process that compensates the retinal defocus for near working vision. During accommodation, the accommodation apparatus makes various responses for sharp focusing. With the classical theory, the axial biometry of the whole eye changes, including a decrease in anterior chamber depth (ACD) and vitreous length, and an increase in lens thickness.1 However, changes in the axial length (AL) during accommodation remains controversial,2–6 possibly due to the uncertainty of the crystalline refractive index for calculating AL or the technical limitations of performing full eye biometry during accommodation.
Whether the refractive index changes during accommodation remains unsettled. Research evidence and mathematic modeling have demonstrated that the refractive index may change during accommodation.7–9 For instance, Gullstrand’s intracapsular mechanism of accommodation assumed that the average refractive index of crystalline lens increased by 0.0015 per diopter during accommodation.7, 8 Le Grand et al. considered the average refractive index of the crystalline lens to increase 0.001 per diopter during accommodation.9 Dubbelman et al. demonstrated that the average refractive index of the crystalline lens increased 0.0013 per diopter during accommodation.7 On the contrary, other studies showed that the gradient refractive index remained constant with accommodation.10–13
Measurement techniques for full eye biometry during accommodation need to be based on some assumptions to yield results. With A-scan ultrasonography, no change in AL was observed during accommodation2–4 whereas other studies reported an increase.5, 6 Using partial coherence tomography, including the IOLMaster (Carl Zeiss Meditec, Dublin, CA) and Lenstar (Haag-Streit AG, Koeniz, Switzerland), AL has been found to increase during accommodation.10, 14, 15 With advances in whole eye imaging using ultra-long scan depth optical coherence tomography (UL-OCT),16–21 each compartmental dimension of the whole eye can be measured. This may provide an alternative way for revisiting axial elongation during accommodation. The repeatability of our UL-OCT method for measuring the whole biometry has been validated, and the AL results agreed well with the IOLMaster results. The goal of this study was to investigate changes in axial biometry of the whole eye during accommodation using UL-OCT.
METHODS
Twenty-one healthy adult subjects (10 males and 11 females) were recruited into this study. The mean age was 27.4 ± 4.5 years (ranging from 19 to 35 years). The mean refractive error was −0.9 ± 1.14 diopters (ranged from 0 to −3.00 diopters). There were 10 myopic subjects with a mean refractive error of 1.95 ± 0.88 diopters and 11 emmetropic subjects. The exclusion criteria included subjects older than 35 years; those with any systemic disease or anterior or posterior segment pathology of the eye, including a history of laser treatment; and those with trauma or who had received eye surgery. Approval was obtained from the institutional review board for human research at the University of Miami. All subjects participated in this prospective study signed consent forms and were treated in accordance with the tenets of the Declaration of Helsinki.
The use of UL-OCT has been reported previously and the details of the system have been documented elsewhere.20, 21 Briefly, the system included a superluminescent diode light source (SLD, InPhenix, IPSDD0808, Livermore, CA, USA). The center wavelength is 840 nm with a bandwidth of 50 nm. The spectrometer consisted of a line array complementary metal–oxide–semiconductor (CMOS) camera (Basler Sprint spL4096-140k; Basler AG, Germany), a collimating lens (f = 50 mm, OZ Optics, Ottawa, Canada), a 1,800 lines/mm transmission grating and an image enlargement lens (Schneider Optics, f = 240 mm, Hauppauge, NY). A switchable reference arm with four mirrors was used to sequentially acquire four images.21 The scan depth of each single reference arm was 12.57 mm. Overlaying the first and second reference arms for the anterior segment, the four switchable reference arms obtained an equivalent scan depth of 37.71 mm in air without an image gap.20, 21 By imaging a mirror at different depths we obtained the point spread function (PSF). The full width at half-maximum intensity of the PSF was determined as the resolution of the OCT system in air. The measured axial resolution was 7.7 μm in the air. The resolution for ocular biometry was 5.6 μm, assuming a refractive error of 1.38.
A Badal system mounted into the sample arm provided the vision targets and accommodative stimulus during imaging as detailed elsewhere.22 The vision target of a white Snellen letter “E” was displayed by a light-emitting diode (LED) screen on a black background. The measured eye fixed on the vision target, and the other eye was sheltered. The OCT measuring beam was congruent to the fixation target, which ensured biometry was conducted on the fixation/visual axis. The spherical refractive error of each subject was corrected by the Badal target system before the subject was imaged. The left eye of each subject was imaged at a baseline status of 0D and an accommodative status of +6 D stimulus. The measurements were repeated twice for both states during a single study visit. All measurements were performed by the same researcher (YS).
An automated custom software was used to reconstruct full eye images, and this software has been validated against a manual method as previously reported.21 Refractive indices of the cornea, aqueous humor, crystalline lens and vitreous were 1.387,23 1.342,24 1.40825 and 1.34124 at 840 nm wavelength, respectively. Structural boundary locations were detected along the central axis up to the retinal pigment epithelium (RPE). Optical path lengths of the central corneal thickness (CCT), ACD (distance between corneal endothelium and anterior lens surface), LT and VL were obtained from a longitudinal reflectivity profile processed from central 190 A-scans after unusable A-scans due to the specular reflex on the apex (Figure 1). The geometrical lengths of every compartment were calculated based on the cited refractive indices. The locations of the anterior and posterior poles of the crystalline lens were measured as the distances from the anterior corneal surface to each of these poles.
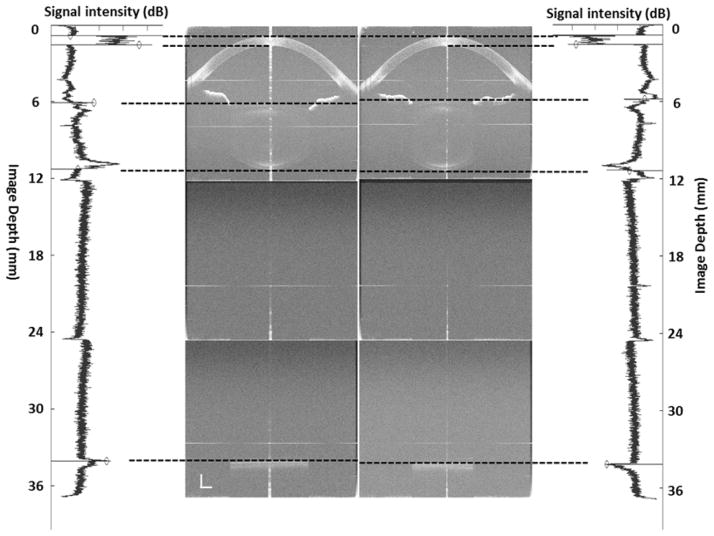
Figure 1.
The left side shows the optical length of whole eye imaging with ultra-long scan depth optical coherence tomography from a 25-year-old subject with myopia (−1.0 diopter) and the corresponding longitudinal reflectivity profiles produced by custom-developed software at the rest state. The right side shows the optical length of whole eye imaging with ultra-long scan depth optical coherence tomography from the same subject and the corresponding longitudinal reflectivity profiles produced by custom-developed software during accommodation (+6D). During accommodation, the axial biometry of the whole eye changes, including a decrease in ACD and VL, and an increase in LT and AL (paired t-test, p < 0.001). At the wavelength of 840 nm, the refractive indices of the cornea, aqueous humor, crystalline lens and vitreous were 1.387, 1.342, 1.408 and 1.341, respectively. Bars = 1 mm.
ACD: anterior chamber depth; LT: lens thickness; VL: vitreous length; AL: axial length.
The method for whole eye biometry using our ultra-long scan depth OCT has been validated.21 In the published study, a model eye (OEMI-7, Ocular Instruments Inc., Bellevue, WA) was imaged with an ultra-long scan depth-OCT to validate our method for OCT full eye biometry. The measurement obtained with the ultra-long scan depth-OCT was highly correlated with the geometry of the model eye in all measured parameters. Repeated measurements were performed in a group of 37 subjects. The coefficients of repeatability (CoRs) of CCT, ACD, lens thickness, vitreous length and AL were 0.021 mm, 0.067 mm, 0.071 mm, 0.084 mm and 0.075 mm, respectively. The CoR% ranged from 0.3% to 3.9% and the ICC ranged from 0.946 to 0.999. Measurements obtained from the UL-OCT in that study were compared with measurements obtained using the IOLMaster and a strong relation was documented.21
A statistical software package (SPSS for Windows 17.0, SPSS Inc., Chicago, IL) was used for descriptive statistics and data analysis. All data are presented as the mean ± standard deviation. Paired t-tests were used to access changes in each parameter between the baseline and accommodative conditions. P < 0.05 was considered as significantly different between measurements.
RESULTS
The geometric lengths of all elements of the whole eye biometry at rest and accommodative states were obtained (Table 1, Figure 2). Compared to the rest state, ACD at the accommodative state decreased significantly from 3.128 ± 0.305 mm (median 3.149) to 2.961 ± 0.298 mm (median 2.987) (P < 0.001). Lens thickness increased significantly from 3.723 ± 0.237 mm (median 3.661) to 3.989 ± 0.234 mm (median 3.964). Vitreous length decreased significantly from 17.129 ± 0.864 mm (median 16.946) to 17.057± 0.848 mm (median 16.794) (P < 0.001). AL was 24.519 ± 0.917 mm (median 24.335) at the rest state and increased to 24.545 ± 0.915 mm (median 24.353) in the +6 D accommodative stimulus. The elongated AL of 26.1 ± 13.4 μm between rest and accommodative states was significant (P < 0.001). During accommodation, the anterior crystalline lens pole moved forward by 0.167 mm, which was approximately 1.7 times the backward movement of the posterior crystalline lens pole. There were significant correlations between the increase in lens thickness and the total decrease in ACD (r = −0.72, P < 0.05) and VL (r = −0.64, P < 0.05). However, there was no correlation between the increase in lens thickness and an increase in AL (r = −0.15, P > 0.05). No significant change was found in CCT during accommodation (P > 0.05). Between myopic and emmetropic subjects, no significant differences were found for changes in ACD, crystalline lens thickness, vitreous length, or AL (P > 0.05).
Table 1.
The axial biometry (mean ± SD, mm) of the whole eye at the baseline (0D) and the accommodative condition (+6D) measured with ultra-long scan depth optical coherence tomography in 21 left eyes of 21 subjects.
| 0D
|
+6D
|
P | Δ | |||||
|---|---|---|---|---|---|---|---|---|
| OPL | mean | median | OPL | mean | median | |||
| CCT | 0.747 ± 0.035 | 0.539 ± 0.025 | 0.538 | 0.747 ± 0.034 | 0.538 ± 0.024 | 0.538 | 0.416 | 0.000 ± 0.003 |
| ACD | 4.198 ± 0.409 | 3.128 ± 0.305 | 3.149 | 3.973 ± 0.400 | 2.961 ± 0.298 | 2.987 | <0.001 | −0.167 ± 0.078 |
| LT | 5.242 ± 0.334 | 3.723 ± 0.237 | 3.661 | 5.616 ± 0.330 | 3.989 ± 0.234 | 3.964 | <0.001 | 0.265 ± 0.104 |
| VL | 22.970 ± 1.159 | 17.129 ± 0.864 | 16.946 | 22.874 ± 1.138 | 17.057 ± 0.848 | 16.794 | <0.001 | −0.072 ± 0.076 |
| AL | 33.158 ± 1.221 | 24.519 ± 0.917 | 24.335 | 33.210 ± 1.2019 | 24.545 ± 0.915 | 24.353 | <0.001 | 0.026 ± 0.013 |
OPL: optical path length; CCT: central corneal thickness; ACD: anterior chamber depth; LT: lens thickness; VL: vitreous length; AL: axial length. P: paired t-test. Δ: the change between the baseline (0D) and the accommodative condition (+6D).
Figure 2.
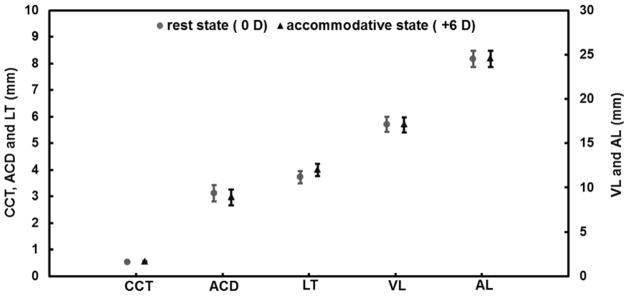
Inter-session variations of each element of the axial biometry at rest and the accommodative states measured with ultra-long scan depth optical coherence tomography in the 21 left eyes of the 21 subjects. Compared to the rest state, ACD and VL decreased significantly (paired t-test, P < 0.001, respectively) and LT and AL increased significantly (paired t-test, P < 0.001). Bars = standard deviation.
CCT: central corneal thickness; ACD: anterior chamber depth; LT: lens thickness; VL: vitreous length; AL: axial length. Bars = standard deviation.
DISCUSSION
During accommodation, the axial biometry of the whole eye undergoes changes such as a decrease in ACD and vitreous length, and an increase in lens thickness. The crystalline lens position shifts during accommodation, while the crystalline lens undergoes a change in shape. Our results showed that the ACD decreased by approximately 28 μm/D; LT increased by approximately 40 μm/D; vitreous length decreased by approximately 12 μm/D; and AL increased by 4 μm/D; all of which were in agreement with previous studies.3, 7, 10, 15 The decrease in ACD with accommodation was 63% of the increase in lens thickness, and the decrease in vitreous length with accommodation was 27% of the increase in lens thickness. The increase in lens thickness (0.265 ± 0.104 μm) was compensated for by a decrease in ACD (−0.167 ± 0.078 μm) and VL (−0.072 ± 0.076 μm), indicating that AL (0.026 ± 0.013 μm) increased. The decrease in ACD and vitreous length indicated that the anterior surface of the crystalline lens moved forward, while the posterior surface of the crystalline lens moved backward. The decrease in the vitreous length was due to the backward movement of the posterior lens pole, which was larger than that of the AL. The amount that the crystalline lens moved forward was approximately 1.7 times greater than the amount that the crystalline lens moved backward, which was smaller than the result of three times that reported by Drexler et al. using partial coherence interferometry.26 The result of Dubbelman et al. was five times as much forward movement of the crystalline lens as backward.7 Kasthurirangan et al. reported that the anterior segment depth (ACD + lens thickness) did not change using MRI.27 A possible reason for this finding was that the resolution limit of the MRI technique was only 0.156 mm and the expected changes were of 0.1 mm or less.
Changes in the axial biometry of the whole eye during accommodation could be explained by the Helmholtz’s theory.1 During accommodation, the ciliary muscle contracts, the ciliary ring shrinks and the zonules are relaxed, which relieves tension on the crystalline lens capsule. Due to the elasticity of the crystalline lens capsule, the crystalline lens is more spherical in shape, which increases in lens thickness, and the anterior surface of the crystalline lens moves forward while the posterior surface of the crystalline lens moves backward. The increase in AL is within the range of previously published data (Table 2). However, the increase in AL cannot be explained by the Helmholtz’s theory. To explain this phenomenon, some researchers hypothesize that the contraction of the ciliary muscle generates an inward pull force towards the choroid and sclera adjacent to the ciliary body.14, 15 To maintain a constant ocular volume, the posterior portion of the globe displaces rearward, which results in an increase in axial length. Due to reduced ocular rigidity in the myopic eye, greater elongation was observed.15 Inversely, Schmid et al. found that there was no difference in ocular rigidity between emmetropic and myopic children.28 In addition, during accommodation, a decrease in intraocular pressure (IOP) was observed in both emmetropic and myopic eyes and there was a significant positive association between changes in AL and changes in IOP.29–31 Although choroidal thickness was not measured in this study, it may need to be considered in future studies of the elongation of the eye during accommodation. Wallman et al. first described changes in eye length by choroidal thickness changes in chicks.32 New evidence found that subfoveal choroidal changes may contribute to an increase in the axial length during accommodation.33 Our research on elongation of the axial length during accommodation together with the evidence of choroidal thinning during near work33, 34 may add insightful information to better understand the development of myopia.
Table 2.
Axial length measurements of eyes in the present study and previous studies
| Author | Device | AL(0D) | Stimulus | ΔAL | ΔAL/D |
|---|---|---|---|---|---|
| Current study | UL-OCT | 24.52 ± 0.92 | 6.0D | 0.026 | 0.0044 |
| Read et al.10 | LENSTAR | 23.70 ± 0.75 | 6.0D | 0.024 | 0.0040 |
| Drexler et al.14 | Partial coherence interferometry | 23.84 ± 0.74 | 5.1D | 0.013 | 0.0025 |
| Mallen et al.15 | IOLMaster | 23.25 ± 0.66 | 2.0D | 0.014 | 0.0070 |
| 23.25 ± 0.66 | 4.0D | 0.026 | 0.0060 | ||
| 23.25 ± 0.66 | 6.0D | 0.037 | 0.0061 |
AL: axial length (mm). ΔAL: the change of AL between the baseline and the accommodative condition in reference paper. ΔAL/D: the AL change per diopter.
During accommodation, changes in ACD, vitreous length and lens thickness are well documented. However, changes in axial length are still under debate, and no consensus has been reached. One hypothesis is that ciliary muscle contraction causes AL elongation during accommodation.35, 36 Another possible mechanism is that the refractive index of the crystalline lens may increase during accommodation, which might explain measurement artifacts of axial lengths.7–9 However, in previous studies, de Castro et al. found that the lens gradient refractive index remained constant during accommodation,11, 12 suggesting that the average refractive index may not change during accommodation. This theory was applied to calculate AL changes in the present study and others.10, 13–15 Atchison et al. reported two accommodated model eyes with two different lenses (shell lens and gradient index lens). Assuming there is no change in axial length with accommodation and the refractive index of the lens is stable, Atchison et al. calculated that the error in the axial length was 18 μm by the shell lens and 26 μm by the gradient index lens for an accommodation of 10.9D.13 Read et al. used a similar method to correct AL results obtained with Lenstar. Corrected results of 5.2 μm and 7.4 μm were obtained for +3D and +6D accommodation states, respectively.10 In the present study, measurements were taken during the rest state and accommodative state. Assuming there was no change in the refractive index of the crystalline lens during accommodation, a 26 μm increase in AL was noted. Instead of the single light beam measurement by the IOLMaster and Lenstar, UL-OCT provides a B-scan image of axial compartments of the whole eye. The optical path lengths of CCT, ACD, lens thickness, vitreous length and AL can be obtained, and the geometrical dimensions can be directly calculated. The elongation values obtained using UL-OCT matches well with previous findings using other methods. OCT can also provide other information such as the pupil response to accommodative stimulation and crystalline lens curvature; however, we did not measure these parameters in the present study.22 With the development of UL-OCT,16 three dimensional scanning and real-time imaging of the whole eye can further improve the usefulness of UL-OCT for studying accommodation.
There are limitations in the present study. First, we only studied changes in emmetropes and low myopes subjects. In high myopes, the value of the changes may not be the same. The amount of crystalline lens moving forward and backward and the values of increased lens thickness were significantly smaller for near vision tasks compared to far vision tasks.34 Second, choroidal thickness was found to be decreased during accommodation, which may account for certain increases in AL.37 In the present study, this change was not taken into account due to the inability to measure the choroidal thickness with the current settings. Third, our sample size may be relatively small; although our results generally agreed with previous reports. Fourth, we asked the subject to fixate on the target during imaging, which ensured that the measuring beam was aimed at the visual axis, presumably on the fovea. The image of the retina did not show the details of the retina due to the scanning probe, which used a telecentric optical design; therefore, no confirmation of the retinal position was made. Because we used a retinal pigment epithelium boundary, the measurement may not be affected heavily if the position was not actually on the fovea. With a resolution of 5.6 μm, the axial elongation of 26 μm was unlikely due to measurement error. Fifth, we used a published mean value of the indices of refraction to estimate the full eye biometry, which may have induced measurement errors. Lastly, the accommodative lag should be considered in future research.
In conclusion, during accommodation, whole eye axial biometry changed, including a decrease in ACD and vitreous length, and an increase in lens thickness and AL. UL-OCT provides an alternative method that may be suitable for full eye biometry during accommodation.
Acknowledgments
Funding / Support
This study was supported by research grants in part from the NIH EY021012, EY021336 and EY020607S, NIH Center Grant P30 EY014801, Research to Prevent Blindness (RPB). A visiting scholar award for Dr. Jianguang Zhong was provided by the Hangzhou First People’s Hospital, Hangzhou, China. The funding organizations had no role in the design or conduct of this study.
Biographies
 Jianguang Zhong, MD, is currently a chief physician of ophthalmology in Hangzhou First People’s Hospital, Hangzhou, Zhejiang, China. He graduated from Zhejiang University in 1994 majoring in clinical medicine. Following graduation, Dr. Zhong completed a residency in ophthalmology at Hangzhou First People’s Hospital. In 2000, he completed a master’s degree in ophthalmology. From September 2012, he spent one year at Bascom Palmer Eye Institute, doing research regarding the clinical application of optical coherence tomography. Dr. Zhong’s current research interests include full eye biometry measurement and slit-lamp functional imaging.
Jianguang Zhong, MD, is currently a chief physician of ophthalmology in Hangzhou First People’s Hospital, Hangzhou, Zhejiang, China. He graduated from Zhejiang University in 1994 majoring in clinical medicine. Following graduation, Dr. Zhong completed a residency in ophthalmology at Hangzhou First People’s Hospital. In 2000, he completed a master’s degree in ophthalmology. From September 2012, he spent one year at Bascom Palmer Eye Institute, doing research regarding the clinical application of optical coherence tomography. Dr. Zhong’s current research interests include full eye biometry measurement and slit-lamp functional imaging.
 Jianhua Wang, MD, PhD, is currently a Tenured Associate Professor at the Bascom Palmer Eye Institute, University of Miami. He is also scientific co-director of experimental imaging laboratory. Dr. Wang graduated from Zhejiang Medical University, Hangzhou, China in 1988. In 2003, he obtained his PhD in Vision Science from University of Waterloo, Waterloo, Canada. Dr. Wang’s current research interests include clinical applications of ophthalmic structural and functional imaging.
Jianhua Wang, MD, PhD, is currently a Tenured Associate Professor at the Bascom Palmer Eye Institute, University of Miami. He is also scientific co-director of experimental imaging laboratory. Dr. Wang graduated from Zhejiang Medical University, Hangzhou, China in 1988. In 2003, he obtained his PhD in Vision Science from University of Waterloo, Waterloo, Canada. Dr. Wang’s current research interests include clinical applications of ophthalmic structural and functional imaging.
Footnotes
Financial Disclosures
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST. The authors report the following financial disclosure: The University of Miami and Dr. Wang hold a provisional patent (WO 2013/081902) used in the study.
Contributions of the authors
Design of the study (JZ, JW, YS, AT, HJ, CL, HZ); data collection (JZ, YS, AT, JW), analysis and interpretation of the data (JZ, JW, YS, AT, HJ, CL, HZ, ZX), preparation, review and approval of the manuscript (JZ, JW, YS, AT, HJ, CL, HZ, ZX).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.von Helmholtz H. Uber die akkommodation des auges. Arch Ophthalmol. 1855;1:1–84. [Google Scholar]
- 2.Van der Heijde GL, Weber J. Accommodation used to determine ultrasound velocity in the human lens. Optom Vis Sci. 1989;66(12):830–833. doi: 10.1097/00006324-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Garner LF, Yap MK. Changes in ocular dimensions and refraction with accommodation. Ophthalmic Physiol Opt. 1997;17(1):12–17. [PubMed] [Google Scholar]
- 4.Beauchamp R, Mitchell B. Ultrasound measures of vitreous chamber depth during ocular accommodation. Am J Optom Physiol Opt. 1985;62(8):523–532. doi: 10.1097/00006324-198508000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande S. Ocular biometric study during accommodation. International Journal of Medical and Clinical Research. 2012;3(4):150–153. [Google Scholar]
- 6.Shum PJ, Ko LS, Ng CL, Lin SL. A biometric study of ocular changes during accommodation. Am J Ophthalmol. 1993;115(1):76–81. doi: 10.1016/s0002-9394(14)73528-7. [DOI] [PubMed] [Google Scholar]
- 7.Dubbelman M, Van der Heijde GL, Weeber HA, Vrensen GF. Changes in the internal structure of the human crystalline lens with age and accommodation. Vision Res. 2003;43(22):2363–2375. doi: 10.1016/s0042-6989(03)00428-0. [DOI] [PubMed] [Google Scholar]
- 8.Gullstrand A. How I found the mechanism of intracapsular accommodation. Nobel lecture. 1911 Dec 11; Available at External link http://www.nobelprize.org/nobel_prizes/medicine/laureates/1911/gullstrand-lecture.pdf.
- 9.Le Grand Y. Physiological optics. Berlin ; New York: Springer-Verlag; 1980. pp. 86–87. [Google Scholar]
- 10.Read SA, Collins MJ, Woodman EC, Cheong SH. Axial length changes during accommodation in myopes and emmetropes. Optom Vis Sci. 2010;87(9):656–662. doi: 10.1097/OPX.0b013e3181e87dd3. [DOI] [PubMed] [Google Scholar]
- 11.de Castro A, Ortiz S, Gambra E, Siedlecki D, Marcos S. Three-dimensional reconstruction of the crystalline lens gradient index distribution from OCT imaging. Opt Express. 2010;18(21):21905–21917. doi: 10.1364/OE.18.021905. [DOI] [PubMed] [Google Scholar]
- 12.de Castro A, Birkenfeld J, Maceo B, et al. Influence of shape and gradient refractive index in the accommodative changes of spherical aberration in nonhuman primate crystalline lenses. Invest Ophthalmol Vis Sci. 2013;54(9):6197–6207. doi: 10.1167/iovs.13-11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atchison DA, Smith G. Possible errors in determining axial length changes during accommodation with the IOLMaster. Optom Vis Sci. 2004;81(4):283–286. doi: 10.1097/00006324-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Drexler W, Findl O, Schmetterer L, Hitzenberger CK, Fercher AF. Eye elongation during accommodation in humans: differences between emmetropes and myopes. Invest Ophthalmol Vis Sci. 1998;39(11):2140–2147. [PubMed] [Google Scholar]
- 15.Mallen EA, Kashyap P, Hampson KM. Transient axial length change during the accommodation response in young adults. Invest Ophthalmol Vis Sci. 2006;47(3):1251–1254. doi: 10.1167/iovs.05-1086. [DOI] [PubMed] [Google Scholar]
- 16.Grulkowski I, Liu JJ, Potsaid B, et al. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed Opt Express. 2012;3(11):2733–2751. doi: 10.1364/BOE.3.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De FC, Ruggeri M, Manns F, Ho A, Parel JM. In vivo measurement of the average refractive index of the human crystalline lens using optical coherence tomography. Opt Lett. 2013;38(2):85–87. doi: 10.1364/OL.38.000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggeri M, Uhlhorn SR, De FC, Ho A, Manns F, Parel JM. Imaging and full-length biometry of the eye during accommodation using spectral domain OCT with an optical switch. Biomed Opt Express. 2012;3(7):1506–1520. doi: 10.1364/BOE.3.001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai C, Zhou C, Fan S, et al. Optical coherence tomography for whole eye segment imaging. Opt Express. 2012;20(6):6109–6115. doi: 10.1364/OE.20.006109. [DOI] [PubMed] [Google Scholar]
- 20.Tao A, Shao Y, Zhong J, Jiang H, Shen M, Wang J. Versatile optical coherence tomography for imaging the human eye. Biomed Opt Express. 2013;4:1031–1044. doi: 10.1364/BOE.4.001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong J, Shao Y, Tao A, et al. Axial biometry of the entire eye using ultra-long scan depth optical coherence tomography. American Journal of Ophthalmology. 2013 doi: 10.1016/j.ajo.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao Y, Tao A, Jiang H, et al. Simultaneous real-time imaging of the ocular anterior segment including the ciliary muscle during accommodation. Biomed Opt Express. 2013;4(3):466–480. doi: 10.1364/BOE.4.000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephen RU, Fabrice M, Hassan T, Pascal R, Jean-Marie P. Corneal group refractive index measurement using low-coherence interferometry. SPIE. 1998;3246:14–21. (1998) [Google Scholar]
- 24.Atchison DA, Smith G. Chromatic dispersions of the ocular media of human eyes. J Opt Soc Am A Opt Image Sci Vis. 2005;22(1):29–37. doi: 10.1364/josaa.22.000029. [DOI] [PubMed] [Google Scholar]
- 25.Uhlhorn SR, Borja D, Manns F, Parel JM. Refractive index measurement of the isolated crystalline lens using optical coherence tomography. Vision Res. 2008;48(27):2732–2738. doi: 10.1016/j.visres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drexler W, Baumgartner A, Findl O, Hitzenberger CK, Fercher AF. Biometric investigation of changes in the anterior eye segment during accommodation. Vision Res. 1997;37(19):2789–2800. doi: 10.1016/s0042-6989(97)00066-7. [DOI] [PubMed] [Google Scholar]
- 27.Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J Vis. 2011;11(3) doi: 10.1167/11.3.19. [DOI] [PubMed] [Google Scholar]
- 28.Schmid KL, Li RW, Edwards MH, Lew JK. The expandability of the eye in childhood myopia. Curr Eye Res. 2003;26(2):65–71. doi: 10.1076/ceyr.26.2.65.14513. [DOI] [PubMed] [Google Scholar]
- 29.Read SA, Collins MJ, Becker H, et al. Changes in intraocular pressure and ocular pulse amplitude with accommodation. Br J Ophthalmol. 2010;94(3):332–335. doi: 10.1136/bjo.2009.166355. [DOI] [PubMed] [Google Scholar]
- 30.Read SA, Collins MJ, Annis-Brown T, et al. The short-term influence of elevated intraocular pressure on axial length. Ophthalmic Physiol Opt. 2011;31(4):398–403. doi: 10.1111/j.1475-1313.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 31.Read SA, Collins MJ. The short-term influence of exercise on axial length and intraocular pressure. Eye (Lond) 2011;25(6):767–774. doi: 10.1038/eye.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 33.Woodman EC, Read SA, Collins MJ. Axial length and choroidal thickness changes accompanying prolonged accommodation in myopes and emmetropes. Vision Res. 2012;72:34–41. doi: 10.1016/j.visres.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Woodman EC, Read SA, Collins MJ, et al. Axial elongation following prolonged near work in myopes and emmetropes. Br J Ophthalmol. 2011;95(5):652–656. doi: 10.1136/bjo.2010.180323. [DOI] [PubMed] [Google Scholar]
- 35.Jeon S, Lee WK, Lee K, Moon NJ. Diminished ciliary muscle movement on accommodation in myopia. Exp Eye Res. 2012;105:9–14. doi: 10.1016/j.exer.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard AL, Davies LN. In vivo analysis of ciliary muscle morphologic changes with accommodation and axial ametropia. Invest Ophthalmol Vis Sci. 2010;51(12):6882–6889. doi: 10.1167/iovs.10-5787. [DOI] [PubMed] [Google Scholar]
- 37.Woodman EC, Read SA, Collins MJ. Axial length and choroidal thickness changes accompanying prolonged accommodation in myopes and emmetropes. Vision Res. 2012;72:34–41. doi: 10.1016/j.visres.2012.09.009. [DOI] [PubMed] [Google Scholar]