Abstract
Few studies have investigated organochlorine pesticide residue content in freshwater plankton communities in Thailand. As a result, this study looks to examine the concentration of organochlorine pesticide residues in plankton collected from Khlong 7 (canal) at Rangsit agricultural area, central Thailand from June 2006 to February 2007. The results from this study show that plankton communities were composed of microphytoplankton, microzooplankton, and mesozooplankton. The average method recoveries varied from 84% to 103% with a relative standard deviation between 0.20% and 3.72%. The concentrations of organochlorine pesticide residues during a one-year-period were in the range of 0.10–3.65 ng/g wet wt and contained DDT and derivatives > Σ endosulfan > Σ HCH > Σ heptachlor > aldrin and dieldrin > endrin and endrin aldehyde > methoxychlor, respectively. Moreover, the residues of Σ HCH, DDT and derivatives, and methoxychlor were higher during wet season than dry season (t-test, p ≤ 0.05).
Keywords: Organochlorine, Pesticide, Residue, Plankton
Organochlorine pesticides (OCPs) have been used extensively worldwide since the early 1950s. However, due to their persistence in the environment, most of these pesticides are no longer allowed to be used in many countries, including Thailand. But due to the low price and ability to kill a broad spectrum of pests, farmers illegally use OCPs in agricultural fields resulting in pesticides entering local water bodies. These pesticides still persist in the environment (Anat and Paul 2000; Keithmaleesatti et al. 2007; Thirakhupt et al. 2006) and several cause imbalances in biota of the aquatic ecosystem (Favari et al. 2002). OCPs have often affected non-target organisms and the accumulation of phyto- and zoo-plankton at the base of the aquatic food web may increase resulting in significant concentrations in animals at higher trophic levels through the food web (Robinson et al. 1967; DeLorenzo et al. 2002).
In Thailand, there are few studies on organochlorine pesticide residues (OCPRs) content in freshwater plankton communities. This study dealt with 2 aims: (1) the study of plankton taxa in material collected by 80 μm plankton net and (2) the investigation of OCPRs concentration in Khlong 7, Rangsit agricultural area, Pathum Thani Province from June 2006 to February 2007.
Materials and Methods
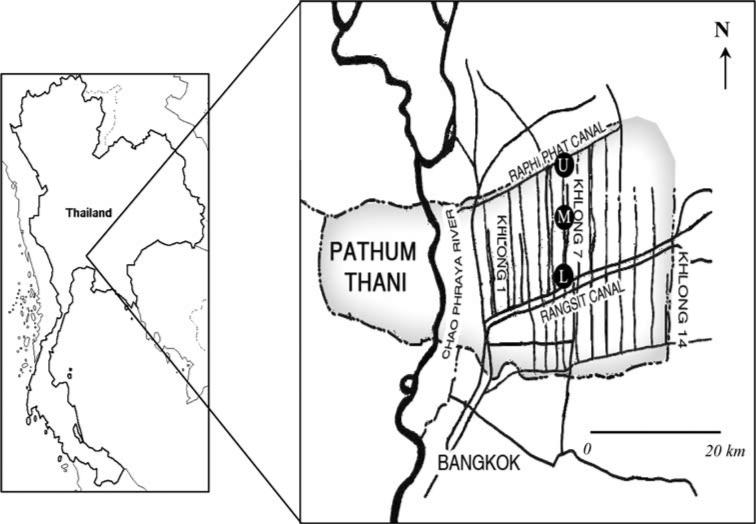
Rangsit agricultural area is located at the central part of Thailand in Pathum Thani Province. This agricultural area has a man-made irrigation-network-system consisting of 14 sub-canals (Khlong). These sub-canals are divided by Rangsit-Prayulasakdi canal into an upper and lower part. The study area is situated at Khlong 7, a 20-km sub-canal, on the upper part of the irrigation-network-system. Khlong 7 links Raphi Phat canal at the upstream side (14°12′38.00″N, 100°45′18.38″E) and Rangsit-Prayulasakdi canal at the downstream side (14°01′51.25″N, 100°45′21.25″E) (Fig. 1).
Fig. 1.
Map of Rangsit agricultural area, Pathum Thani Province, Thailand. The sampling stations are at Khlong 7; where (U) = upper stream, (M) = middle stream, and (L) = lower stream
Field samplings were conducted every 3 months from June 2006 to February 2007. Triplicate samples of plankton were collected from the upper stream (U), middle stream (M), and lower stream (L) of Khlong 7. The plankton samples were obtained with a No. 20 net with a mesh opening of 80 μm (APHA-AWWA-WPCF 1980). The plankton samples were taken using a No. 20 net with mesh opening of 80 μm (APHA-AWWA-WPCF 1980). After towing, a 50 mL sample of plankton was preserved in 2% neutral formalin in glass bottles at room temperature for species identification, and a liter of plankton was contained in polyethylene bottles and maintained below 4°C during transportation and storage until analysis.
The preserved planktons were identified to genus level under the conventional light microscope, using keys from Prescott (1978), Bold and Wynne (1985), Taylor (1987), Steidinger and Tangen (1997), Pennak (1989), Dodge and Lee (2000), Graham and Wilcox (2000), John et al. (2002), and Pechenik (2005).
Seventeen organochlorine pesticide standards for α-, γ-, β- and δ-HCH, heptachlor, heptachlor epoxide, aldrin, α-aendosulfan, β-endosulfan, endosulfan sulfate 4,4′-DDE, 4,4′-DDD, 4,4′-DDT, dieldrin, endrin, endrin aldehyde, and methoxychlor were obtained from Supelco (Bellefonte, PA, USA). A stock of the standard mixture containing 17 pesticides was prepared in 99% n-hexane at a concentration of 1000 ng/mL and stored at –4°C in a refrigerator. Working standard solutions were prepared at the concentration of 0.001–100 ng/mL and then diluted with 99% n-hexane.
Residue analysis solvents such as 95% and 99% n-hexane, dichloromethane, diethyl ether, and petroleum ether were pesticide grade solvents purchased from Lab-scan Asia Co. Ltd. All chemical reagents were purchased from Fluka Riedel-de Haën i.e. florisil (60–110 mesh) and sodium sulfate anhydrous (granular), which were heated overnight at 300°C. The 500 mg florisil SPE cartidges were purchased from Alltech Associates Inc.
All Pyrex® glassware was cleaned with laboratory detergent purchased from EMC-IMEX co., Ltd., then sequentially rinsed with distilled water and acetone. Finally, washed glassware was baked in an oven at 300°C overnight.
The method for plankton extraction was modified from DeLorenzo et al. (2002). The plankton mass was separated from an aliquot (30 mL) by centrifugation (2,500 rpm, or approximately 84g, for 30 min). The supernatant was decanted. The plankton pellet was then washed with deionized water and recentrifuged twice as before. Afterward, the plankton pellet was weighed using a 4-digit balance, dissolved in 2 mL methanol, and vortexed. An equal amount of hexane was then added and the contents were mixed. After phase separation, a 1-mL aliquot of hexane layer was transferred for clean up. A florisil SPE cartridge was applied for clean up using three fraction eluents: 10 mL of 6%, 15%, and 50% of diethyl ether in petroleum ether, respectively (Caleste and Irene 1997; Alvin and Lau 2004). The elution rate was 1 mL/min by gravity. The eluates were collected in a concentrator tube and the volume was reduced to 2 mL under a gentle stream of nitrogen for quantification with gas chromatography with micro electron capture detection (GC-μECD).
An Agilent 6890N GC equipped with micro Electron Capture Detector (μECD) was used for quantification. Compound separation was completed using DB-35MS fused silica capillary column (30 m length, 0.25 mm i.d., 0.25 μm film thickness) coated with 35% diphenyl polysiloxane (J&W Scientific). Sample quantification was performed using multiple external standards. A 1.0 μL sample was injected into the GC on splitless mode with 0.75 min vent delay. The injector and detector temperatures were maintained at 260 and 300°C, respectively. The oven temperature was initially maintained at 100°C for 2 min, and then programmed to increase at 12°C /min to 280°C and held for 10 min. Total run time was calculated to be 27.00 min. For optimum performance, the ultra-high-pure (UHP, 99.999%) helium was used as carrier gas with a flow rate at 2 mL/min linear velocity, and nitrogen (UHP) was set at 60 mL/min as make-up gas.
Organochlorine pesticides (OCPs) peaks and retention times were confirmed with DB-1701 fused silica capillary column (30 m length, 0.32 mm i.d., 0.25 μm film thickness) coated with 14% cyanopropylphenyl and 86% diphenyl polysiloxane (J&W Scientific). A calibration curve using the external mixed standard of 17 OCPs was performed for each compound to be quantified at concentrations of 1, 2, 5, 10, and 20 ng/mL. Calibration standards were run every 10 samples and all measurements were performed in the ranges of linearity found for each compound. The validation data of plankton showed essentially quantitative recovery [mean percent recovery in the range of 84–103% (n = 7)] and excellent precision (in the range of 0.20–3.72% RSD, relative standard deviation) for OCPRs in plankton. The method detection limits (MDLs) were in the range of 0.02–0.49 ng/g wet wt. The limit of detections (LODs) and the limit of quantitations (LOQs) were in the range of 0.001–0.05 ng/ mL and 0.002–0.20 ng/mL, respectively, for plankton samples (n = 51) taken throughout the sampling period. We considered the method to be reliable to quantify the concentration of OCPRs in plankton according to AOAC Peer Verified Methods Program (1993).
The statistical analysis was performed using SPSS software (Version 12.0). The mean comparison of OCPRs in plankton between wet season (June–November) and dry season (December to May) were determined by using independent sample t-test.
Results and Discussion
In this study, the plankton, captured in an 80 μm net, were identified by diversity of phyto- and zoo-plankton taxa in Khlong 7 Rangsit agricultural area, Pathum Thani Province, Thailand from June 2006 to May 2007. Under the conventional light microscope, the results showed that the plankton from three survey sites were mainly composed of micro-phytoplankton, microzooplankton, and mesozooplankton communities. Three genera of microphytoplanktons in the Phylum Cyanobacteria were found including Merismopedia sp., Anabaena spp., and Pseudanabaena sp. In the Phylum Euglenophyta, three genera of microphytoplanktons were found; Euglena sp., Phacus sp., and Strombomonas sp. The Phylum Dinophyta or dinoflagellates were dominated only by Gymnodinium sp. Four genera of microphytoplankton in the Phylum Chlorophyta were found such as Volvox sp. Ankistrodesmus sp., Tetraedron sp., and Pediastrum spp. Moreover, diatoms in the Phylum Bacillariophyta were occasionally found.
For microzooplankton in the Phylum Rotifera, Brachionus sp. and Euchlanis sp. were found, as well as Phylum Arthropoda, a number of Cladocera, and Daphnia sp. (mesozooplankton) and Cyclopoida, cyclopoid copepods (microzooplankton) communities were also abundant.
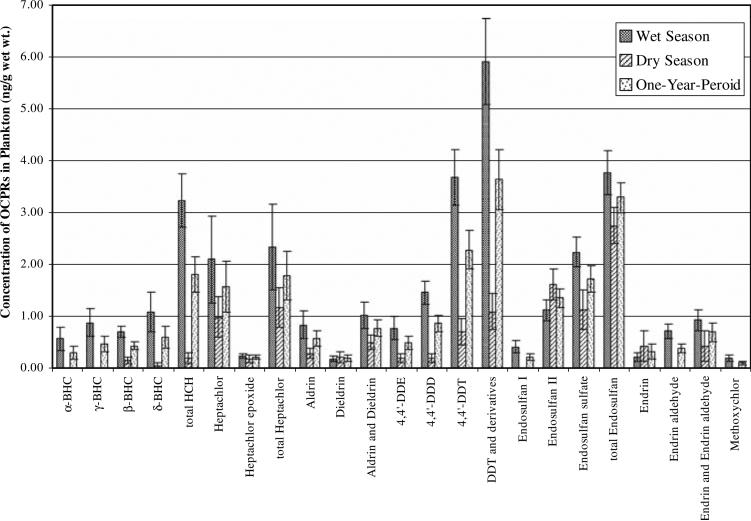
The mean values of OCPRs retained in plankton collected from three survey sites in Khlong 7 are shown in Table 1. Low standard error (S.E.) with respect to mean indicated that OCPRs composition did not differ considerably among sites. Table 1 and Fig. 2 show that the concentrations of OCPRs during a 1-year-period contained DDT and derivatives (3.65 ng/g wet wt) > Σ endosulfan (3.29 ng/g wet wt) > Σ HCH (1.80 ng/g wet wt) > Σ heptachlor (1.79 ng/g wet wt) > aldrin and dieldrin (0.77 ng/g wet wt) > Endrin and Endrin aldehyde (0.69 ng/g wet wt) > methoxychlor (0.10 ng/g wet wt), respectively.
Table 1.
The average concentration of OCPRs in plankton (phyto- and zoo-plankton) in the wet season (June–November), dry season (December–May), and one-year study period (June–May) at Khlong 7, Rangsit agricultural area, Pathum Thani Province, Thailand
| OCPs | Average concentration of OCPRs in plankton (mean ± SE) (ng/g wet wt) |
||
|---|---|---|---|
| Wet seasona (n = 27) | Dry season (n = 24) | One-year-period (n = 51) | |
| α-BHC | 0.57 ± 0.22 | <0.03 | 0.30 ± 0.12 |
| γ-BHC | 0.88 ± 0.26 | <0.05 | 0.47 ± 0.15 |
| β-BHC | 0.70 ± 0.11 | 0.14 ± 0.07 | 0.44 ± 0.08 |
| δ-BHC | 1.09 ± 0.39 | 0.05 ± 0.05 | 0.60 ± 0.22 |
| Σ HCH | 3.23 ± 0.50 | 0.20 ± 0.10 | 1.80 ± 0.34 |
| Heptachlor | 2.10 ± 0.84 | 0.99 ± 0.39 | 1.58 ± 0.48 |
| Heptachlor epoxide | 0.24 ± 0.04 | 0.18 ± 0.08 | 0.21 ± 0.04 |
| Σ Heptachlor | 2.34 ± 0.82 | 1.17 ± 0.38 | 1.79 ± 0.47 |
| Aldrin | 0.84 ± 0.26 | 0.28 ± 0.09 | 0.58 ± 0.15 |
| Dieldrin | 0.18 ± 0.06 | 0.22 ± 0.11 | 0.20 ± 0.06 |
| Aldrin and Dieldrin | 1.02 ± 0.26 | 0.50 ± 0.13 | 0.77 ± 0.15 |
| 4,4′-DDE | 0.77 ± 0.22 | 0.19 ± 0.09 | 0.50 ± 0.13 |
| 4,4′-DDD | 1.46 ± 0.23 | 0.20 ± 0.09 | 0.86 ± 0.16 |
| 4,4′-DDT | 3.69 ± 0.54 | 0.71 ± 0.26 | 2.28 ± 0.37 |
| DDT and derivatives | 5.92 ± 0.83 | 1.09 ± 0.35 | 3.65 ± 0.58 |
| Endosulfan I | 0.41 ± 0.12 | <0.003 | 0.22 ± 0.07 |
| Endosulfan II | 1.12 ± 0.20 | 1.62 ± 0.30 | 1.36 ± 0.18 |
| Endosulfan sulfate | 2.24 ± 0.29 | 1.13 ± 0.38 | 1.72 ± 0.25 |
| Σ Endosulfan | 3.77 ± 0.42 | 2.75 ± 0.35 | 3.29 ± 0.28 |
| Endrin | 0.21 ± 0.09 | 0.43 ± 0.30 | 0.31 ± 0.15 |
| Endrin aldehyde | 0.72 ± 0.14 | <0.01 | 0.38 ± 0.09 |
| Endrin and Endrin aldehyde | 0.93 ± 0.20 | 0.43 ± 0.30 | 0.69 ± 0.18 |
| Methoxychlor | 0.19 ± 0.06 | <0.01 | 0.10 ± 0.03 |
Statistical comparison between wet and dry season using independent samples t-test, the different letter in the same row indicates the significant difference at p ≤ 0.05
Fig. 2.
Comparison of average concentration of OCPRs in plankton in the wet season, the dry season, and the 1-year-period at Khlong 7, Rangsit agricultural area, Pathum Thani Province, Thailand
Five Phylum of phytoplankton, i.e. Cyanobacteria, Euglenophyta, Dinophyta, Chlorophyta, and Bacillariophyta and 2 orders in Phylum of Arthropoda, i.e. Cladocera and Cyclopoida, that were found in Khlong 7 Rangsit agricultural area were also similar to the Phylum found in Ignacio Ram'rez (IR) reservoir, Mexico (Favari et al. 2002). This may be because both areas were lentic aquatic ecosystems. However, there was no Phylum Rotifera found in Ignacio Ram'rez (IR) reservoir. Another study reported that major zooplankton communities identified from the paddy field in Pathum Thani Province were Rotifera, Cladocera, and Copepoda (Chittapun et al. 2007). The taxa related to this study may be due to the paddy fields in Rangsit agricultural area mainly using water from sub-canals (Khlongs) for growing rice causing the circulation of zooplanktons between paddy fields and sub- canals. Furthermore, Daphnia sp. is mainly recognized as a freshwater cladoceran, which is a very important component of zooplankton (Marti'nez-Jero'nimo and Marti'nez- Jero'nimo 2007; Chatmongkolkul and Chantangsi 2005) and cyclopoid copepods, which are commonly found swimming among macrophytes, (Sarvala 1998) such as water hyacinth, water morning glory, and neptunia, which are typically distributed in Khlong 7 (Siriwong et al. 2007).
The presence of OCPRs in plankton in this agricultural area, shown in Fig. 2, may have been a result of historical usage and some illegal usage at the present time. The statistical comparisons of OCPRs in plankton between wetand dry-seasons shows that the residues of Σ HCH, DDT and derivatives, and methoxychlor were higher in wet season than in dry season (independent samples t-test, p ≤ 0.05). This may be due to the heavy rain and runoff that can effectively transfer these compounds into the canals (Khlong 7) during the wet season. Although Σ HCH, DDT and derivatives, and methoxychlor were banned a few decades ago, residues are still present in soil and other terrestrial environments (Thirakhupt et al. 2006). During the course of this study, Σ endosulfan was still being illegally used. It was applied to every crop in the paddy fields, targeting the golden apple snail (Pomacea sp.). However, its residue was not significantly different between wet- and dry-seasons (independent samples t-test, p ≥ 0.05). Likewise, heptachlor and heptachlor epoxide, aldrin and dieldrin, and endrin and endrin aldehyde, which can be found in the soil around buildings and agricultural areas for the elimination of termites and to control pests (Thirakhupt et al. 2006), may be discharged into the canal. These pesticides could then accumulate in plankton resulting in residue during both the wet- and dry-seasons.
This study revealed that OCPRs are still persistent in plankton communities, which are the lowest trophic level of freshwater ecosystems of Khlong 7, Rangsit agricultural area. Although low concentrations were found, OCPRs could be transferred and magnified through the higher trophic levels. Therefore, the biomagnification of OCPRs through the food web should be considered in further studies.
Acknowledgements
This research was supported by the National Research Center of Excellence for Environmental and Hazardous Waste Management (NRC-EHWM), Chulalongkorn University. Funded in part by Thai Fogarty ITREOH Center, Chulalongkorn University, Bangkok, Thailand D43 TW007849-01 Fogarty International Center – National Institutes of Health – NIEHS. We would like to thank Dr. Ajcharaporn Piumsomboon for her advice on plankton identification and Mr. Saran Kiethmaleesatti for his field assistance.
Contributor Information
W. Siriwong, The College of Public Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand Thai Fogarty ITROEH Center at Chulalongkorn University, UMNDJ and Rutgers, Bangkok, Thailand.
K. Thirakhupt, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
D. Sitticharoenchai, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
M. Borjan, New Jersey Agricultural Experiment Station, Rutgers University, New Brunswick, NJ 08901, USA
M. Robson, Thai Fogarty ITROEH Center at Chulalongkorn University, UMNDJ and Rutgers, Bangkok, Thailand New Jersey Agricultural Experiment Station, Rutgers University, New Brunswick, NJ 08901, USA.
References
- Alvin CK, Lau S. Solid phase extraction cleanup for the determination of organochlorine pesticides in vegetable. Malays J Chem. 2004;6:39–47. [Google Scholar]
- Anat T, Paul FH. Pesticide use and occurance in Thailand. Malays J Chem. 2000;60:103–144. [Google Scholar]
- APHA-AWWA-WPCF . Standard methods for the examination of water and waste water. 15th edn. American Public Water Association; Washington, DC: 1980. [Google Scholar]
- Bold HC, Wynne MJ. Introduction to the algae. 2nd edn. Prentice-Hall; New Jersey: 1985. [Google Scholar]
- Caleste ML, Irene NDS. Extraction and clean-up methods for the determination of organochlorine pesticide residues in medical plants. J Chromatogr A. 1997;769:275–283. doi:10.1016/S0021-9673(97)00026-5. [Google Scholar]
- Chittapun S, Pholpunthin P, Sanoamuang L. Zooplankton diversity and composition during a crop cycle of three rice fields in Pathum Thani Province, Central Thailand.. The 3rd national conference on Algae and Plankton. Department of marine science; Bangkok, Thailand. March 21–23, 2007; Chulalongkorn University; 2007. p. 28. [Google Scholar]
- DeLorenzo ME, Taylor LA, Lund SA, Pennington PL, Strozier ED, Fulton MH. Toxicity and bioconcentration potential of the agricultural pesticide endosulfan in phytoplankton and zoo-plankton. B Environ Contam Toxicol. 2002;42:173–181. doi: 10.1007/s00244-001-0008-3. doi:10.1007/s00244-001-0008-3. [DOI] [PubMed] [Google Scholar]
- Dodge JD, Lee JJ. Phylum Dinofegellata Bütschli, 1985. In: Lee JJ, Leedale DF, Bradbury P, editors. An illustrated guide to the Protozoa. Kansas. Vol. 2. Allen Press; 2000. pp. 656–689. [Google Scholar]
- Favari L, Lo'pez E, Mart'nez-Tabche L, D'az-Pardow E. Effect of insecticides on plankton and fish of Ignacio Ramirez Reservoir (Mexico): a biochemical and biomagnification study. Ecotoxicol Environ Saf. 2002;51:177–186. doi: 10.1006/eesa.2002.2142. doi:10.1006/eesa.2002.2142. [DOI] [PubMed] [Google Scholar]
- Graham LE, Wilcox LW. Algae. Prentice-Hall; United State of America: 2000. [Google Scholar]
- John DM, Whitton BM, Brook AJ. The freshwater algae flora of the British Isles: an identification guide to freshwater and terrestrial algae. Cambridge University Press; United Kingdom: 2002. [Google Scholar]
- Keithmaleesatti S, Thirakhupt K, Pradatsudarasar A, Varanusupakul P, Kitana N, Robson M. Concentration of organochlorine in egg yolk and reproductive success of Egretta garzetta (Linnaeus, 1758) at Wat Tan-en non-hunting area, Phra Nakhorn Si Ayuthaya Province, Thailand. Ecotoxicol Environ Saf. 2007;68:79–83. doi: 10.1016/j.ecoenv.2006.08.004. doi:10.1016/j.ecoenv.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Marti'nez-Jero'nimo F, Marti'nez-Jero'nimo L. Chronic effect of NaCl salinity on a freshwater strain of Daphnia magna Straus (Crustacea: Cladocera): a demographic study. Ecotoxicol Environ Saf. 2007;67:411–416. doi: 10.1016/j.ecoenv.2006.08.009. doi:10.1016/j.ecoenv.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Pechenik JA. Biology of the invertebrates. 5th edn. McGraw-Hill; New York: 2005. [Google Scholar]
- Pennak RW. Fresh-water invertebrate of the United States: Protozoa to Mollusca. 3rd edn. Wiley; New York: 1989. [Google Scholar]
- Prescott GW. How to know the freshwater algae. 3rd edn. Wm. C Brown; Iowa: 1978. [Google Scholar]
- Robinson J, Richardson A, Crabtree AN, Coulson JC, Potts GR. Organochlorine residues in marine organisms. Nature. 1967;214:1307–1311. doi: 10.1038/2141307a0. doi:10.1038/2141307a0. [DOI] [PubMed] [Google Scholar]
- Sarvala J. Ecology and role of benthic copepods in northern lakes. J Marine Syst. 1998;15:75–86. doi:10.1016/S0924-7963(97)00040-7. [Google Scholar]
- Siriwong W, Thirakhupt K, Sitticharoenchai D, Robson M. Accumulation of organochlorine pesticide residues in aquatic plants. J Sci Res Chulalongkorn Univ. 2007;32:7–14. [Google Scholar]
- Steidinger KA, Tangen K. Dinoflagellates. In: Tomas CR, editor. Identifying marine phytoplankton. California. Academic Press; 1997. pp. 387–584. [Google Scholar]
- Taylor FJR. The biology of Dinoflagellates. Blackwell Scientific Publications; Great Britain: 1987. [Google Scholar]
- Thirakhupt K, Sitthicharoenchai D, Keithmaleesatti S, Siriwong W. Organochlorine pesticides and their usages in Thailand: a review. J Sci Res Chulalongkorn Univ. 2006;31:1–15. [Google Scholar]