Abstract
Sirtuins are nicotinamide adenine dinucleotide (NAD+)-dependent deacylases that have traditionally been linked with calorie restriction and aging in mammals. These proteins also play an important role in maintaining neuronal health during aging. During neuronal development, the SIR2 ortholog SIRT1 is structurally important, promoting axonal elongation, neurite outgrowth and dendritic branching. This sirtuin also plays a role in memory formation by modulating synaptic plasticity. Hypothalamic functions that affect feeding behavior, endocrine function and circadian rhythmicity are all regulated by SIRT1. Finally, SIRT1 plays protective roles in several neurodegenerative diseases including Alzheimer’s, Parkinson’s and motor neuron diseases, which may relate to its functions in metabolism, stress resistance and genomic stability. Drugs that activate SIRT1 may offer a promising approach to treat these disorders.
Keywords: sirtuin, histone deacetylase, aging, cognitive function, neurodevelopment, neurodegeneration, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, motor neuron disease, multiple sclerosis
Introduction
The mammalian sirtuins are deacylase proteins that have been shown to regulate diverse cellular processes including aging, inflammation and stress resistance (Brunet et al., 2004; Imai et al., 2000; Kaeberlein et al., 1999; Kim et al., 1999; North and Verdin, 2004). These enzymes were originally identified as genetic silencing factors in yeast (Klar et al., 1979; Rine et al., 1979) and can act on histones and other substrates in the presence of nicotinamide adenine dinucleotide (NAD+) (Imai et al., 2000; Massudi et al., 2012; Yoshino et al., 2011).
The sirtuins are categorized as class III histone deacetylases (HDACs) and the posttranslational modification of histone substrates can modulate chromatin condensation and gene transcription. Deacetylation of lysine residues is often associated with transcriptional repression, however many sirtuin substrates are non-histone proteins and several sirtuins do not have deacetylase activity (Dokmanovic et al., 2007; Gregoretti et al., 2004).
There are seven sirtuins in mammals with varied subcellular localization and enzymatic activity (Frye, 1999, 2000). SIRT1, SIRT2 and SIRT3 exhibit NAD-dependent deacetylase activity (Imai et al., 2000; Jeong et al., 2012; North et al., 2003; Onyango et al., 2002; Tanny et al., 1999). SIRT4 is an ADP-ribosyltransferase (Haigis et al., 2006), whereas SIRT5 has NAD-dependent demalonylase, desuccincylase and deacetylase activities (Du et al., 2011; Nakagawa et al., 2009; Peng et al., 2011). SIRT6 was initially shown to have deacetylase and ADP-ribosyltransferase activities (Liszt et al., 2005; Michishita et al., 2008; Mostoslavsky et al., 2006) and more recently has been shown to catalyze the hydrolysis of fatty acyl lysine residues, which may affect protein secretion (Park et al., 2012). SIRT7 is an NAD-dependent deacetylase (Barber et al., 2012; Vakhrusheva et al., 2008).
The sirtuins reside in different subcellular compartments, influencing their in vivo substrates and cellular functions. SIRT1 was initially described as a nuclear protein (Mouchiroud et al., 2013) that may also shuttle to the cytoplasm during neuronal differentiation and neurite outgrowth (Hisahara et al., 2008; Sugino et al., 2010; Tanno et al., 2007), tumor progression (Byles et al., 2010; Ramsey et al., 2008) and apoptosis (Jin et al., 2007). SIRT2 is a cytoplasmic protein that can deacetylate tubulin (North et al., 2003) but has also been described in the nucleus during cell cycle progression (Canto et al., 2012), cancer (Braidy et al., 2013), and bacterial infection (Eskandarian et al., 2013). SIRT3, SIRT4 and SIRT5 are mitochondrial sirtuins, however they each have distinct enzymatic activities within this organelle (Du et al., 2011; Haigis et al., 2006; Nakagawa et al., 2009). SIRT6 associates with chromatin in the nucleus (Liszt et al., 2005; Mostoslavsky et al., 2006) and is enriched in the nucleolus during the G1 phase of the cell cycle (Ardestani and Liang, 2012), and SIRT7 is a nucleolar protein (Ford et al., 2006).
Although there has been some controversy regarding the functions of sirtuins in calorie restriction and aging, many recent papers have reaffirmed the important roles of these proteins in both processes (reviewed in (Guarente, 2013). For example, SIRT3 was shown to be essential for calorie restriction to protect the neurons in the cochlea against oxidative damage and thus forestall hearing loss in mice (Someya et al., 2010).
Sirtuins have been recently shown to have many important functions during development, influencing brain structure through axon elongation (Li et al., 2013), neurite outgrowth (Sugino et al., 2010), and dendritic branching (Ferrante et al., 1997) as well as cellular fate of neuronal precursor cells (Prozorovski et al., 2008; Rafalski et al., 2013). These proteins also play a major role in the hypothalamus, affecting circadian rhythmicity (Asher et al., 2008; Bellet et al., 2013; Bellet et al., 2011; Chang and Guarente, 2013; Nakahata et al., 2008; Nakahata et al., 2009), endocrine function (Cohen et al., 2009) and feeding behaviors (Ramadori et al., 2011; Sasaki et al., 2010; Satoh et al., 2010). In the adult brain, SIRT1 can also modulate synaptic plasticity and memory formation (Gao et al., 2010; Michan et al., 2010). In addition to its importance during normal brain aging, SIRT1 has also been shown to ameliorate a number of neurodegenerative disorders including Alzheimer’s (Donmez et al., 2010; Min et al., 2010; Qin et al., 2006), Parkinson’s (Donmez et al., 2012; Mudo et al., 2012), Huntington’s disease (Jeong et al., 2012; Jiang et al., 2012), motor neuron diseases (Han et al., 2012; Kim et al., 2007; Montie et al., 2011) and multiple sclerosis (Fonseca-Kelly et al., 2012; Shindler et al., 2010; Shindler et al., 2007) in animal models of these diseases. Current therapies for these neurologic disorders are not curative and there is great interest in targeting sirtuin pathways pharmacologically.
Small molecules like resveratrol and synthetic SIRT1 activators were shown to activate the enzyme directly via an allosteric site adjacent to the catalytic domain (Hubbard et al., 2013). Although we discuss resveratrol throughout this review, it is important to remember the caveat that small molecules may also hit non-SIRT1 neuronal targets to affect biological outcomes in vivo. Resveratrol inhibits cAMP-degrading phosphodiesterases and activates the CamKKβ-AMPK pathway by increasing cAMP and activating Epac1. In addition to increasing intracellular calcium levels this pathway also increases NAD+ and SIRT1 activity (Park et al., 2012). SIRT2 inhibitors have also been shown to be protective in animal models of Parkinson’s and Huntington’s diseases (Chopra et al., 2012; Outeiro et al., 2007). In this review, we will present an overview of SIRT1 function in the brain during neurodevelopment, normal aging and neurodegenerative disease.
Sirtuin 1 and normal brain aging
Neuronal structure
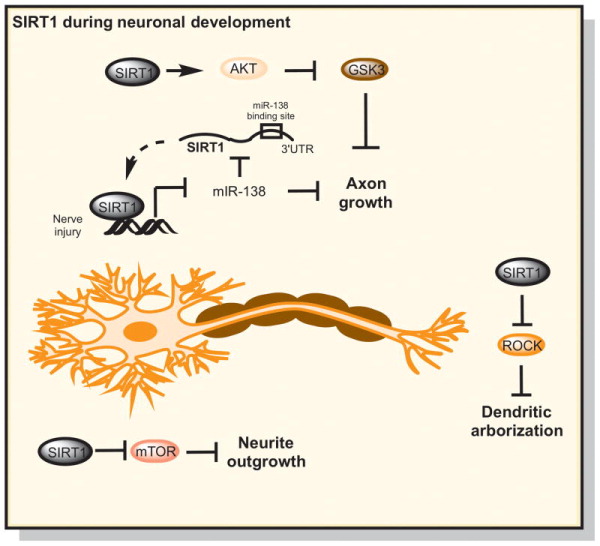
The early development of neurons begins with neurite process elongation followed by axon differentiation, dendritic arborization and synapse formation (Ferrante et al., 1997). Using cell culture models and in utero electroporation, SIRT1 has been shown to promote neurite outgrowth and the subcellular localization of this deacetylase is critical for its function (Figure 1). In PC12 cells, cytoplasmic SIRT1 stimulates NGF-dependent neurite outgrowth, however this effect is abolished when nuclear localized constructs or catalytically inactive mutants are analyzed (Sugino et al., 2010). A second study has confirmed and expanded on these observations reporting that SIRT1 briefly localizes to the nucleus during differentiation from neuronal precursor cells (NPCs) and SIRT1 inhibition decreases neurite length in culture and in mouse brain following in utero electroporation (Hisahara et al., 2008). A third study linked neurite outgrowth and neuronal viability with the downregulation of mammalian target of rapamycin (mTOR) (Guo et al., 2011).
Figure 1. The influence of sirtuin 1 on neuronal architecture. The role of SIRT1 on structural features of neuronal cells are depicted.
SIRT1 can promote axon development by deacetylating Akt and inhibiting Gsk3 affecting microtubule dynamics during axon elongation (Li et al., 2013). SIRT1 also interacts with microRNA-138 via a negative feedback loop and this microRNA suppresses axon growth (Liu et al., 2013). SIRT1 also affects dendritic arborization (Michan et al., 2010) and dendritic spine morphology by inhibition of ROCK kinase activity (Ferrante et al., 1997). Cytoplasmic SIRT1 can stimulate NGF-dependent neurite outgrowth (Hisahara et al., 2008; Sugino et al., 2010), which is due to downregulation of mTOR and inhibition of its downstream effectors (Guo et al., 2011).
SIRT1 has also been shown to promote axon development in embryonic hippocampal neurons. SIRT1 activates Akt, the upstream inhibitory kinase of glycogen synthase kinase 3 (GSK3), and promotes axonogenesis in cultured hippocampal neurons (Li et al., 2013). In embryonic cortical neurons, SIRT1 is the target of microRNA-138, a small noncoding RNA that molecule suppresses axon growth (Liu et al., 2013). In response to peripheral nerve injury, SIRT1 is also able to suppress miR-138 creating a negative feedback loop.
Dendritic arborization is another important aspect of neuronal development because the degree of branching affects the number of synaptic inputs that each neuron can integrate. SIRT1 KO mice have been shown to have a reduced dendritic arbor but similar distribution of dendritic spines relative to wildtype mice (Michan et al., 2010). A second study corroborated these findings in neuronal culture and also found that the architecture of neuronal spines differed with SIRT1 overexpression and that SIRT1 catalytic activity was important for the maintainance of dendritic arborization by inhibition of ROCK kinase activity (Ferrante et al., 1997). These studies support a role for SIRT1 in the regulation and maintenance of dendritic growth.
Learning and memory
The development of synapses and modulation of their strength is important for memory formation and sirtuins play an important role in this process. SIRT1 knockout animals have morphologic alterations in neuronal structure, exhibiting dendrites with decreased complexity and shorter branches by Golgi staining (Michan et al., 2010). Studies using electron microscopy to examine synaptic morphology show that inhibiting SIRT1 decreases synaptic inputs to hippocampal neurons (Dietrich et al., 2010). Functionally, SIRT1 knockout mice have impaired hippocampal-dependent memory that is associated with decreased long-term potentiation in the CA1 region of the hippocampus. The expression of hippocampal genes was examined by microarray analysis and genes for synaptic function, membrane fusion, myelination, and metabolic function were altered in knockout mice (Michan et al., 2010).
Brain-specific SIRT1 knockout mice have decreased synaptic plasticity (Figure 2). SIRT1 interacts with a repressor complex containing the transcription factor YY that regulates mIR-134 and this brain specific microRNA regulates cAMP response binding protein (CREB) expression and brain-derived neurotrophic factor (BDNF) (Gao et al., 2010). The relationship between SIRT1 and these targets is shown in figure 2. A subsequent study examining whether these alterations were reproducible using a pharmacologic approach (with attending caveats for non-SIRT1 effects) rather than genetic deletion confirmed that levels of miR-124 and miR-134 decreased after treatment with resveratrol (Zhao et al., 2013). Both CREB and BDNF increased while memory formation and long-term potentiation (LTP) induction improved in treated animals (Zhao et al., 2013).
Figure 2. The regulation of learning and memory by sirtuin 1.
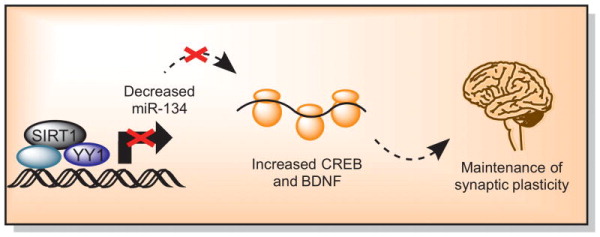
In the hippocampus, SIRT1 affects synaptic plasticity via a repressor complex containing the transcription factor YY, which regulates microRNA-134 (mIR-134). This brain specific microRNA regulates cAMP response binding protein (CREB) expression and brain-derived neurotrophic factor (BDNF) (Gao et al., 2010). These proteins are important for synapse formation and long-term potentiation. SIRT1 knockout mice have impaired hippocampal-dependent memory that is associated with decreased long-term potentiation in the CA1 region of the hippocampus (Gao et al., 2010).
Hypothalamic function
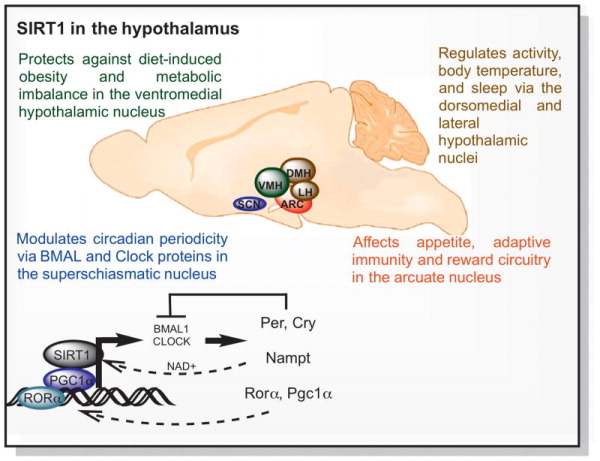
The hypothalamus regulates physiology and behavior by sensing metabolic changes and coordinating neuroendocrine responses that modulate appetite, temperature, circadian control and hormonal release to maintain homeostasis (Coppari, 2012). SIRT1 is expressed in several hypothalamic regions that regulate the response to food availability and circadian behaviors including the arcuate (ARC), ventromedial (VMH), dorsomedial (DMH), lateral hypothalamic (LH) and paraventricular nucleus (PVN) as well as the suprachiasmatic nucleus (SCN) (Ramadori et al., 2008; Satoh et al., 2010). The role of SIRT1 in these brain regions is illustrated in figure 3.
Figure 3. Sirtuin1 and hypothalamic function.
The hypothalamus regulates physiology and behavior by coordinating neuroendocrine responses that modulate appetite, temperature, circadian control and hormonal release to maintain homeostasis (Coppari, 2012). In the ventromedial hypothalamic nucleus, SIRT1 increases energy expenditure and protects against diet-induced obesity under fed high-fat diets (Ramadori et al., 2011; Ramadori et al., 2010). SIRT1 also regulates activity and body temperature in the dorsomedial and lateral hypothalamus (Satoh et al., 2010). In arcuate nucleus, SIRT1 modulates appetite, adaptive immunity and reward circuitry (Dietrich et al., 2010; Matarese et al., 2013). In the superschiasmatic nucleus of the hypothalamus, SIRT1 levels decrease with aging, affecting the activity pattern and circadian period. Overexpressing brain SIRT1 activates the transcription of BMAL and CLOCK proteins enabling animals to be protected from aging related changes to the central circadian clock (Chang and Guarente, 2013).
The VMH nucleus regulates the balance between glucose and insulin, and steroidogenic factor 1 (SF1)-expressing neurons within this nucleus are particularly important for coordinating the autonomic response to high fat diet and obesity. SF1 neurons express SIRT1 (Ramadori et al., 2011) and send excitatory projections to pro-opiomelanocortin (POMC) cells in the arcuate nucleus of the hypothalamus that release α-melanocyte-stimulating hormone and cocaine-and-amphetamine-regulated transcript peptides to promote satiety (Coppari, 2012; Dietrich et al., 2010). The arcuate nucleus also contains a second population of neurons that inhibit POMC neurons, producing neuropeptide Y (NPY), agouti-related peptide (AgRP) and GABA to promote feeding behaviors (Dietrich et al., 2010).
SIRT1 plays an important role in regulating neuronal function in several hypothalamic regions (Figure 3). Mice that lack SIRT1 in SF1 or POMC neurons have decreased energy expenditure and are more sensitive to diet-induced obesity when fed high-fat diets (Ramadori et al., 2011; Ramadori et al., 2010). Knocking out SIRT1 in hypothalamic AgRP neurons decreases food intake (Dietrich et al., 2010), an effect that is recapitulated when pharmacologic or siRNA is used to inhibit SIRT1 nonspecifically in the hypothalamus, causing decreased weight and food intake (Cakir et al., 2009). The mechanism for how SIRT1 regulates the feeding response to caloric restriction is attributed to the deacetylation of FOXO and may also affect mTOR/S6K signaling (Cakir et al., 2009). SIRT1 also modulates the response to NPY, a neurotransmitter that increases food intake, by activating FOXO in response to phosphorylation by minibrain/Dyrk1a kinase (Hong et al., 2012).
SIRT1 is also a critical mediator of the response to ghrelin, a peptide hormone that stimulates food intake. Ghrelin increases SIRT1 activity causing p53 deacetylation and AMPK activation, affecting fatty acid metabolism and feeding behavior (Velasquez et al., 2011). Under caloric restriction, SIRT1 increases levels of the orexin type 2 receptor (Ox2r) in DMH and LH neurons increasing responsiveness to ghrelin (Satoh et al., 2010). The extended life span of SIRT1 brain-specific transgenic mice has also been attributed to upregulation of Ox2r due to deacetylation of the transcription factor Nk2 homeobox 1 (Satoh et al., 2013).
Other functions attributed to hypothalamic AgRP neurons including regulation of adaptive immunity, exploratory drive and response to cocaine are also altered in mice when SIRT1 is ablated in these cells (Dietrich et al., 2012; Matarese et al., 2013). Immunologic changes are caused by disinhibition of POMC neurons that project to the thoracic spinal cord, increasing sympathetic tone. Deletion of SIRT1 in AgRP neurons creates a proinflammatory environment, with enhanced effector T-cell activity and decreased regulatory T-cell function (Matarese et al., 2013). AgRP neurons also innervate the ventral tegmental area (VTA) of the midbrain, and SIRT1 deletion results in higher levels of dopamine in the forebrain and facilitates long-term potentiation in a spike-timing dependent protocol (Dietrich et al., 2012).
In addition to its role in metabolic regulation of feeding behavior and energy expenditure SIRT1 has also been linked with circadian control. It was originally shown to regulate circadian rhythm in peripheral tissues, and BMAL1 and PER2 were identified as SIRT1 targets in the liver (Asher et al., 2008; Nakahata et al., 2008). In the superschiasmatic nucleus of the hypothalamus, SIRT1 levels decrease with aging, and functional circadian decline is suppressed by over-expressing SIRT1 in the brain (Chang and Guarente, 2013). SIRT1 affects BMAL1 transcription by acting cooperatively with PGC1α at RORα-binding sites. BMAL1 is important for maintaining functional connections between neurons, synaptic terminals, regulating cerebral redox homeostasis, linking circadian clock proteins with neurodegeneration (Musiek et al., 2013). BMAL1 and CLOCK proteins form heterodimers that activate the transcription of cryptochrome, period and NAMPT, an enzyme that synthesizes NAD+ (Asher et al., 2008; Nakahata et al., 2008; Nakahata et al., 2009). Circadian regulation of NAD+ was recently found to be a major regulator of metabolism and mitochondrial oxidation and metabolism across the daily cycles of fasting and feeding (Peek et al., 2013).
SIRT1 in neurodegenerative disorders
Alzheimer’s disease
Over 35 million people have dementia worldwide and Alzheimer’s disease (AD) is the most common cause of cognitive impairment (Gomes et al., 2013). Familial AD is an autosomal dominant neurologic disorder that affects individuals in their 50s and 60s and is associated with mutations in the genes for amyloid precursor protein (APP), presenilin 1 (PS1) or presenilin 2 (PS2). APP is cleaved by β- and γ-secretase complexes leading to the formation of amyloid-β (aβ) peptides that coalesce to form extracellular plaques. Tau protein also becomes abnormally aggregated during AD pathogenesis and forms neurofibrillary tangles within cells.
One of the initial observations raising the possibility that sirtuins might ameliorate AD pathology was the finding that resveratrol can reduce aβ-induced toxicity in cell lines (Conte et al., 2003; Jang and Surh, 2003; Sun et al., 2001) and hippocampal neurons (Han et al., 2004). Parallel studies in vivo showed that resveratrol could also reduce plaque formation in transgenic mouse models of AD (Karuppagounder et al., 2009; Vingtdeux et al., 2010). In order to link sirtuins more definitively with decreased of amyloid toxicity, lentiviral infection with SIRT1 was performed and decreased aβ-neurotoxicity was observed due to decreased microglial expression of NF-κB (Chen et al., 2005). A second group identified an alternate mechanism using cell culture and murine models that neuronal SIRT1 expression promoted a non-amyloidogenic APP processing pathway by decreasing levels of ROCK1 kinase (Qin et al., 2006). In vivo evidence supporting the idea that SIRT1 overexpression can decrease plaque burden and improve behavioral phenotypes was performed in the APP/PS1 mouse model. The retinoic acid receptor β was identified as a SIRT1 substrate that up-regulates alpha-secretase activity by increasing ADAM10 gene. This results in greater processing of APP along a non-amyloidogenic pathway (Donmez et al., 2010).
SIRT1 has also been shown to ameliorate tau pathology in the p25 (Kim et al., 2007) and P301L mouse models (Min et al., 2010; Min et al., 2012) affecting cognitive impairment and mortality. Tau protein is acetylated at multiple residues early in the disease process and deacetylation by SIRT1 may allow ubiquitin ligases to target tau for degradation, promoting clearance rather than pathologic intracellular aggregation of this protein (Cohen et al., 2011; Min et al., 2010). These mechanisms are schematically represented in figure 4.
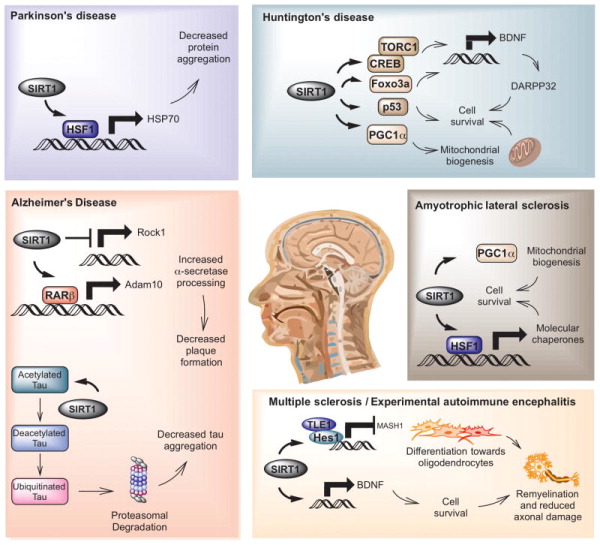
Figure 4. Major targets and mechanisms of SIRT1 in mouse models of neurodegenerative disease.
Alzheimer’s disease (highlighted in red) In Alzheimer’s disease, SIRT1 has been shown to promote non-amyloidogenic APP processing pathway by decreasing levels of ROCK1 kinase (Qin et al., 2006). SIRT1 may also target the retinoic acid receptor β, which activates ADAM10 to facilitate processing of APP along a non-amyloidogenic pathway (Donmez et al., 2010). SIRT1 has also been shown to directly deacetylate tau in several tauopathy models enabling ubiquitin ligases to promote clearance of this protein (Cohen et al., 2011; Min et al., 2010). Parkinson’s disease (highlighted in blue) SIRT1 has been shown to deacetylate HSF, which induces transcription of molecular chaperones that promote protein folding (Donmez et al., 2012; Raynes et al., 2012; Westerheide et al., 2009). In addition to its effects on the heat shock response, SIRT1 may also function to regulate autophagy and mitophagy, which may affect α-synuclein toxicity in the context of PD (Sampaio-Marques et al., 2012; Wu et al., 2011). There is also evidence that PGC1α may be a relevant target in mouse models of PD (Mudo et al., 2012). Huntington’s disease (highlighted in green) Several different molecular mechanisms account for the protective effect of SIRT1 overexpression against mutant huntingtin toxicity (Jeong et al., 2012; Jiang et al., 2012). Mutant huntingtin protein was found to inhibit the enzymatic activity of SIRT1 during HD pathogenesis. One proposed mechanism is that SIRT1 deacetylates TORC1, facilitating BDNF transcription through CREB (Jeong et al., 2012). An alternate explanation for the protective effect of SIRT1 in HD mice was that this protein might maintain TrkB signaling and DARPP32 levels as HD progresses. Foxo3a deacetylation was another SIRT1 target implicated in promoting cell survival in the HD models (Jiang et al., 2012). Amyotrophic lateral sclerosis (highlighted in beige) The proposed mechanism for SIRT1’s activity in Amyotrophic lateral sclerosis parallels one of the pathways observed in PD. SIRT1 has been shown to deacetylate HSF1, which increases transcription of molecular chaperones including HSP70 and HSP25 that help to maintain intracellular protein homeostasis, reducing toxicity to motor neurons (Han et al., 2012; Raynes et al., 2012; van Ham et al., 2008; Westerheide et al., 2009). It has also been shown to affect mitochondrial biogenesis in cell culture models of ALS and this may be due to deacetylation of PGC1α (Wang et al.) Multiple sclerosis (highlighted in orange) In experimental autoimmune encephalitis (EAE), the mouse model for multiple sclerosis (MS), the mechanism of protection in whole animal models has not been completely elucidated. In adult neuronal precursor cells (NPCs), SIRT1 binds Hes1 under oxidative conditions, inhibiting Mash1 transcription and driving NPCs toward astroglial differentiation (Prozorovski et al., 2008) and increasing the oligodendrocyte population may help remyelinate lesions, reduce axonal damage or decrease astrogliosis. An alternate approach has identified increased BDNF and NAMPT as possible targets to explain neuroprotection due to SIRT1 overexpression in EAE (Nimmagadda et al., 2013).
While these studies support the idea that SIRT1 may influence both Aβ and neurofibrillary tau pathology, more work is needed to investigate whether SIRT1 activators can be used to treat patients with AD. The majority of patients with AD have the late onset disorder that is not associated with the same mutations in APP, PS1 and PS2 that are found in familial AD and are used to study the disease in mouse and cell-based models. The clinicaltrials.gov registry indicates that there are several ongoing or recently completed clinical trials testing various formulations of resveratrol in AD patients. More specific SIRT1 activators, when they are available, will be invaluable in assessing whether SIRT1 activating compounds are efficacious in humans.
Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurologic disease affecting over 600,000 patients in the United States (Kowal et al., 2013). Patients develop tremors, rigidity and postural instability due to the loss of dopaminergic neurons in the substantia nigra of the midbrain. Inclusions of α-synuclein and ubiquitin are a pathologic hallmark of the disease (Blesa et al., 2012).
A substantial body of literature supports the hypothesis that SIRT1 is protective in cell culture and animal models of PD (Albani et al., 2009; Blanchet et al., 2008; Donmez et al., 2012; Raynes et al., 2012; van Ham et al., 2008; Wu et al., 2011). Several mechanisms for this effect have been proposed as SIRT1 deacetylates heat shock factor 1 (HSF1), which increases transcription of molecular chaperones such as heat shock protein 70 (Donmez et al., 2012; Raynes et al., 2012; Westerheide et al., 2009). Another relevant mechanistic pathway may be that SIRT1 can deacetylate PGC1α as overexpressing PGC1α or resveratrol protect dopaminergic neurons against MPTP-induced cell degeneration (Mudo et al., 2012). SIRT1 may also function to regulate autophagy and mitophagy, which may affect α-synuclein toxicity in the context of PD (Sampaio-Marques et al., 2012; Wu et al., 2011). Several SIRT1 targets that are relevant to its effects in PD are illustrated in figure 4.
Huntington’s disease
Huntington’s disease (HD) is a rare movement disorder that causes chorea, loss of motor coordination, cognitive dysfunction and dementia. It is an autosomal dominant disease caused by the expansion of a CAG repeat that codes for a polyglutamine stretch in the huntingtin protein that affects its aggregation propensity (La Spada, 2012)
SIRT1 has been evaluated as a potential pharmacologic target for HD in multiple animal and cell culture systems with mixed results depending on the organism, HD model and endpoints measured for evaluation. In Drosophila models, SIRT1 inhibition using either genetic or pharmacologic strategies can suppress pathogenesis of HD (Pallos et al., 2008). In C. elegans models, the converse is true and SIRT1 overexpression or resveratrol treatment is protective (Parker et al., 2005; Parker et al., 2012). In mammalian systems, studies using pharmacologic agents have not shown conclusive results with several studies indicating SIRT1 may be protective (Kumar et al., 2006) and others showing a modest improvement in motor deficits or peripheral tissues without a clear improvement in survival (Hathorn et al., 2011; Ho et al., 2010).
Genetic models of SIRT1 overexpression have yielded greater clarity with two studies showing that sirtuins can protect against mutant huntingtin toxicity in three different mouse models of HD (Jeong et al., 2012; Jiang et al., 2012). One proposed mechanism is that SIRT1 deacetylates the CREB coactivator TORC1, facilitating its nuclear localization and BDNF transcriptional activation via CREB (Jeong et al., 2012). Mutant huntingtin protein was found to inhibit the enzymatic activity of SIRT1 during HD pathogenesis. An alternate explanation for the protective effect of SIRT1 in HD mice was that this protein might maintain TrkB signaling and DARPP32 levels as HD progresses. Foxo3a deacetylation was another SIRT1 target implicated in promoting cell survival in the HD models (Jiang et al., 2012). The major mechanisms for neuroprotection identified in mouse models of HD are illustrated in figure 4. Thus several different molecular mechanisms may explain the improvements in HD symptoms in these genetic models, and future experiments will clarify which targets are critical components of the neuroprotective phenotype and whether these findings can be translated into new therapies for patients with HD.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive disease that affects motor neurons, compromising muscle strength and coordination. Over the past few decades a number of mutations in a functionally diverse set of genes have been identified including SOD1 (Rosen et al., 1993), TDP43 (Arai et al., 2006; Neumann et al., 2006), FUS (Kwiatkowski et al., 2009), VCP (Johnson et al., 2010), UBQLN2 (Deng et al.) and C9ORF72 (DeJesus-Hernandez et al.; Renton et al.). There are many proposed etiologies for ALS including RNA metabolism, mitochondrial dysfunction, oxidative damage, protein misfolding, glutamate-mediated excitotoxicity and axonal transport defects (Cozzolino et al., 2012).
Several studies using resveratrol and lentiviral injection have indicated that SIRT1 may be protective in tissue culture (Wang et al.; Yanez et al.) and mouse models of ALS (Han et al., 2012; Kim et al., 2007). Method of delivery is an important consideration as two studies have examined the effects of resveratrol in whole animal SOD mouse models. Intraperitoneal injection of the compound yielded significant improvements in morbidity and mortality (Han et al., 2012) whereas dietary treatment with resveratrol was not found to improve disease progression (Markert et al.).
The proposed mechanism for SIRT1’s activity in motor neuron disease parallels its role in mouse models of PD. SIRT1 has been shown to deacetylate HSF1, which increases transcription of molecular chaperones including HSP70 and HSP25 that help to maintain intracellular protein homeostasis, reducing toxicity to motor neurons (Han et al., 2012; Raynes et al., 2012; van Ham et al., 2008; Westerheide et al., 2009). In cell culture models of ALS, SIRT1 activation may increase mitochondrial biogenesis through PGC1α (Wang et al.). These mechanisms are illustrated in figure 4. SIRT1 is altered both in the SOD mutant mouse lines (Kim et al., 2007; Lee et al.) as well as in patient samples (Korner et al., 2013) and therefore may be promising both as a disease marker and a therapeutic target for ALS.
Spinal and bulbar muscular atrophy
Spinal and bulbar muscular atrophy (SBMA) is a rare neurologic disorder that causes muscle weakness and endocrine problems in males due to its effects on motor and sensory neurons. Also known as Kennedy’s disease, this disorder is caused by a CAG trinucleotide expansion in the amino-terminal region of the androgen receptor (Brinkmann; La Spada et al., 1991; Merry). Testosterone reduction by surgical or chemical methods improves the disease phenotype in animal models (Katsuno et al., 2003; Katsuno et al., 2002), but has not been shown to improve symptoms in patients with SBMA (Fernandez-Rhodes et al.; Katsuno et al.).
Recent work has shown that the androgen receptor (AR) can be repressed through deacetylation by SIRT1 (Fu et al., 2006). SIRT1 can also deacetylate the polyglutamine-expanded androgen receptor in SBMA (Montie et al., 2011). If the androgen receptor is a SIRT1 substrate in patients, then this finding has great therapeutic potential because SIRT1 activation may have fewer side effects relative to androgen withdrawal and other therapies that have been explored for SBMA treatment.
Multiple sclerosis
Multiple sclerosis (MS) is a chronic, demyelinating disorder characterized by intermittent episodes of focal inflammation and neurologic dysfunction. The disease often leads to gradual decline and permanent neuronal damage. One of the most established models for MS is experimental autoimmune encephalitis (EAE), an induced disorder where T cells react against myelin antigens and form demyelinating lesions in the CNS of mice (Olitsky and Yager, 1949).
Several studies show resveratrol may be protective in mouse models of MS. In a relapsing-remitting EAE model, resveratrol was able to reduce neuronal damage but did not affect inflammation (Shindler et al., 2010; Shindler et al., 2007). In chronic EAE models, resveratrol treatment has also delayed symptom onset, decreased neuronal loss and paralysis (Fonseca-Kelly et al., 2012; Singh et al., 2007). In these studies the mechanism of action for resveratrol was not established and could be related to the activity of SIRT1 or other proteins including AMPK or PGC1α.
A more recent study used genetic overexpression of SIRT1 in a chronic EAE model and found that clinical symptoms were improved with less axonal injury and fewer apoptotic cells observed in the presence of SIRT1 overexpression. This protective phenotype was associated with increased brain-derived neurotrophic factor and NAD levels (Nimmagadda et al., 2013). Other studies examining the effect of SIRT1 in adult neuronal precursor pools (NPCs) have not shown as clear a picture regarding the effect of SIRT1 on EAE. In a study examining the influence of redox state on neural precursor cell (NPC) differentiation, SIRT1 levels increased in glial fibrillary acidic protein (GFAP) positive cells near EAE inflammatory lesions. This group found that SIRT1 increases in NPCs and binds Hes1 under oxidative conditions, inhibiting Mash1 transcription and driving NPCs toward astroglial differentiation (Prozorovski et al., 2008). This pathway may comprise part of the endogenous response to demyelinating disorders such as MS and is depicted in figure 4. A subsequent study showed that deleting SIRT1 in NPC and neural progenitors prior to induction delayed onset of paralysis in a chronic EAE model (Rafalski et al., 2013). Taken together, these results suggest that increasing the oligodendrocyte population may help remyelinate lesions, reduce axonal damage or decrease astrogliosis (Rafalski et al., 2013), however the specificity of these genetic models for NPCs may be less clinically relevant as this small population of cells may be difficult to target pharmacologically.
Prion disease
Prion diseases are a group of fatal neurodegenerative conditions resulting from accumulation of misfolded prion protein that causes degeneration and apoptosis of neurons. These diseases are transmitted when infectious, conformationally altered prion (PrPsc) converts normal cellular prion (PrP) into a pathologic form that has high β-sheet content (Prusiner, 1998). The disease can be modeled in vitro by inoculating cells with synthetic peptides from the disordered N-terminal domain of the prion protein, which causes aggregation of endogenous cellular prion. Disease manifestations are modeled in vivo by direct intracranial inoculation of infectious material or by using transgenic mouse lines that overexpress mutant prion protein. In mouse strains with genetically deleted SIRT1, the progagation of prion protein was delayed (Chen et al., 2008). Other models do not show the same effect, with genetic and pharmacologic studies in both nematode and in vitro models of prion toxicity showing protection against neurodegeneration in the presence of increased SIRT1 activity (Bizat et al., 2010; Jeong et al., 2013; Seo et al., 2012). Mechanisitically SIRT1-mediated deacetylation of p53 and p65 may regulate apoptotic signaling proteins affecting cell survival (Seo et al., 2012). It has also been shown that SIRT1 may protect against prion disease by inducing autophagy, which decreases mitochondrial impairment in neurons (Jeong et al., 2013). Further work will be needed to resolve the differences in outcomes between these studies and to clarify whether modulating sirtuin activity is likely to affect the severity or outcome of prion infection in patients.
SIRT1 in psychiatric disorders
A number of genetic association studies have evaluated the relationship between SIRT1 haplotypes and psychiatric disorders including schizophrenia, bipolar disorder, anxiety disorder, major depressive disorder and drug induced psychosis and several SIRT1 single nucleotide polymorphisms (SNPs) were overrepresented in patients with depression and anxiety disorders (Kishi et al., 2011a; Kishi et al., 2011b; Kishi et al., 2010; Libert et al., 2011). Another approach investigating a link between sirtuins and mood disorders found that levels of SIRT1, SIRT2 and SIRT6 transcripts decreased in peripheral white blood cells during depressive and remissive states of patients with major depression and bipolar disorder (Abe et al., 2011).
In animal models, genetic modulation of SIRT1 levels correlates with anxiety and exploratory drive and is mechanistically linked with serotonin levels in the brain. SIRT1 deacetylates NHLH2, a transcription factor that regulates monoamine oxidase A (MAO-A). This enzyme converts serotonin to 5-hydroxyindole-acetic acid (5-HIAA). Thus, mice lacking brain SIRT1 have low levels of MAO-A and high levels of its substrate, serotonin, whereas mice over-expressing brain SIRT1 are serotonin deficient. Moreover, behavioral differences in the mice can be normalized by treatment with anxiolytic or antidepressant medications used in patients such as MAO inhibitors and selective serotonin reuptake inhibitors (Libert et al., 2011).
SIRT1 may also play an important role in addiction and substance abuse. Cocaine or morphine administration increases SIRT1 expression in the nucleus accumbens, a brain region that regulates motivation and reward (Ferguson et al., 2013; Renthal et al., 2009). Chronic drug administration increases binding of activator protein 1 (AP1) sites in the sirtuin promoter with ΔFosB, a transcription factor that modulates addiction associated behaviors and induces expression of SIRT1 and SIRT2. Importantly viral-mediated over-expression of SIRT1 in this region enhanced the reward response, while knockdown decreased it (Ferguson et al., 2013). Resveratrol was also found to have acute effects on cocaine-mediated neurotransmission by enhancing dopamine neurotransmission (Shuto et al., 2013). In contrast, in the AgRP neurons of the hypothalamus, knockdown of SIRT1 enhanced responses to cocaine (Dietrich et al., 2012). In summary, SIRT1 plays important roles in behavioral processes by affecting neurotransmitter processing in multiple brain circuits.
Conclusions and future directions
Sirtuins play a critical role in the maintenance of neural systems and behavior during normal aging. SIRT1 affects neuronal morphology during development and also is important in maintaining metabolic and circadian homeostasis during aging. Due to its hypothalamic effects and peripheral targets, modulation of SIRT1 activity may be an effective treatment strategy for diabetes and obesity. It is also a promising therapeutic target for a number of neurodegenerative disorders where it affects proteostasis and facilitates aggregate clearance.
Modulation of mammalian sirtuins like SIRT1 may thus be a valuable strategy to slow the trajectory of aging related degenerative changes in the central nervous system and neurologic disorders. However much still needs to be learned about the safety and efficacy profiles of sirtuin activators. One exciting new finding in the field of aging research is that the levels of NAD+, the cosubstrate for SIRT1-7, decreases with aging in mice (Mouchiroud et al., 2013; Ramsey et al., 2008), rats (Braidy et al., 2013), worms (Mouchiroud et al., 2013), and even humans (Massudi et al., 2012). This reduction in NAD+ is expected to reduce the activities of SIRT1-7, and may thus compromise protection against neurological decline and neurodegenerative diseases. Moreover, supplementation of the diet with of NAD+ precursors, such as nicotinamide mononucleotide or nicotinamide riboside, can restore NAD+ levels and reverse untoward metabolic phenotypes in aging mice (Canto et al., 2012; Gomes et al., 2013; Yoshino et al., 2011). The decline in NAD+ may be due to chronic activation of poly-ADP-ribose polymerase by DNA damage, since PARP inhibition can also restore aging-depleted stores of NAD+ (Bai et al., 2011; Gomes et al., 2013; Mouchiroud et al., 2013).
It remains to be seen if this kind of NAD+ depletion occurs in neurodegenerative diseases. But this is a real possibility, since these diseases feature a high degree of chronic DNA damage (Adamec et al., 1999; Ferrante et al., 1997; Robison and Bradley, 1984). One exciting possibility is that sirtuin activators in combination with NAD+ supplementation will provide a one-two punch to prevent or even treat neurodegenerative diseases. This possibility would provide a novel broad-based approach, which is complementary to developing disease-targeted strategies.
Acknowledgments
We would like to acknowledge the Harvard Neurodiscovery Center Pilot Projects program (AH), NIH and the Glenn Foundation for Medical Research for support (LG). LG consults for GSK, Chronos, Elysiumhealth and Segterra. We would also like to thank Dr. Masaki Igarashi for helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Uchida S, Otsuki K, Hobara T, Yamagata H, Higuchi F, Shibata T, Watanabe Y. Altered sirtuin deacetylase gene expression in patients with a mood disorder. J Psychiatr Res. 2011;45:1106–1112. doi: 10.1016/j.jpsychires.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Adamec E, Vonsattel JP, Nixon RA. DNA strand breaks in Alzheimer’s disease. Brain Res. 1999;849:67–77. doi: 10.1016/s0006-8993(99)02004-1. [DOI] [PubMed] [Google Scholar]
- Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Ardestani PM, Liang F. Sub-cellular localization, expression and functions of Sirt6 during the cell cycle in HeLa cells. Nucleus. 2012;3:442–451. doi: 10.4161/nucl.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen K, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487:114–118. doi: 10.1038/nature11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Nakahata Y, Boudjelal M, Watts E, Mossakowska DE, Edwards KA, Cervantes M, Astarita G, Loh C, Ellis JL, et al. Pharmacological modulation of circadian rhythms by synthetic activators of the deacetylase SIRT1. Proc Natl Acad Sci U S A. 2013;110:3333–3338. doi: 10.1073/pnas.1214266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Orozco-Solis R, Sahar S, Eckel-Mahan K, Sassone-Corsi P. The time of metabolism: NAD+, SIRT1, and the circadian clock. Cold Spring Harb Symp Quant Biol. 2011;76:31–38. doi: 10.1101/sqb.2011.76.010520. [DOI] [PubMed] [Google Scholar]
- Bizat N, Peyrin JM, Haik S, Cochois V, Beaudry P, Laplanche JL, Neri C. Neuron dysfunction is induced by prion protein with an insertional mutation via a Fyn kinase and reversed by sirtuin activation in Caenorhabditis elegans. J Neurosci. 2010;30:5394–5403. doi: 10.1523/JNEUROSCI.5831-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet J, Longpre F, Bureau G, Morissette M, DiPaolo T, Bronchti G, Martinoli MG. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1243–1250. doi: 10.1016/j.pnpbp.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol. 2012;2012:845618. doi: 10.1155/2012/845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Poljak A, Grant R, Jayasena T, Mansour H, Chan-Ling T, Guillemin GJ, Smythe G, Sachdev P. Mapping NAD metabolism in the brain of ageing Wistar rats: potential targets for influencing brain senescence. Biogerontology. 2013 doi: 10.1007/s10522-013-9489-5. [DOI] [PubMed] [Google Scholar]
- Brinkmann AO. Molecular mechanisms of androgen action--a historical perspective. Methods Mol Biol. 776:3–24. doi: 10.1007/978-1-61779-243-4_1. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Byles V, Chmilewski LK, Wang J, Zhu L, Forman LW, Faller DV, Dai Y. Aberrant cytoplasm localization and protein stability of SIRT1 is regulated by PI3K/IGF-1R signaling in human cancer cells. Int J Biol Sci. 2010;6:599–612. doi: 10.7150/ijbs.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One. 2009;4:e8322. doi: 10.1371/journal.pone.0008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Guarente L. SIRT1 Mediates Central Circadian Control in the SCN by a Mechanism that Decays with Aging. Cell. 2013;153:1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Hutter G, Bruno J, Govindarajan A, Easlon E, Lin SJ, Aguzzi A, Lindquist S, Guarente L. The role of calorie restriction and SIRT1 in prion-mediated neurodegeneration. Exp Gerontol. 2008;43:1086–1093. doi: 10.1016/j.exger.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, Cipicchio PM, Lauver MA, Choi SH, Silverman RB, et al. The Sirtuin 2 Inhibitor AK-7 Is Neuroprotective in Huntington’s Disease Mouse Models. Cell Rep. 2012;2:1492–1497. doi: 10.1016/j.celrep.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee VM. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte A, Pellegrini S, Tagliazucchi D. Synergistic protection of PC12 cells from beta-amyloid toxicity by resveratrol and catechin. Brain Res Bull. 2003;62:29–38. doi: 10.1016/j.brainresbull.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Coppari R. Metabolic actions of hypothalamic SIRT1. Trends Endocrinol Metab. 2012;23:179–185. doi: 10.1016/j.tem.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino M, Pesaresi MG, Gerbino V, Grosskreutz J, Carri MT. Amyotrophic lateral sclerosis: new insights into underlying molecular mechanisms and opportunities for therapeutic intervention. Antioxid Redox Signal. 2012;17:1277–1330. doi: 10.1089/ars.2011.4328. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, Xu AW, Souza DO, Gao Q, Diano S, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 2010;30:11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araujo I, Liu ZW, Horvath TL. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat Neurosci. 2012;15:1108–1110. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L. SIRT1 Protects against alpha-Synuclein Aggregation by Activating Molecular Chaperones. J Neurosci. 2012;32:124–132. doi: 10.1523/JNEUROSCI.3442-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandarian HA, Impens F, Nahori MA, Soubigou G, Coppee JY, Cossart P, Hamon MA. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science. 2013;341:1238858. doi: 10.1126/science.1238858. [DOI] [PubMed] [Google Scholar]
- Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, Renthal W, Neve R, Liu X, Shao N, et al. Essential Role of SIRT1 Signaling in the Nucleus Accumbens in Cocaine and Morphine Action. J Neurosci. 2013;33:16088–16098. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Rhodes LE, Kokkinis AD, White MJ, Watts CA, Auh S, Jeffries NO, Shrader JA, Lehky TJ, Li L, Ryder JE, et al. Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomised placebo-controlled trial. Lancet Neurol. 10:140–147. doi: 10.1016/S1474-4422(10)70321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Jr, Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- Fonseca-Kelly Z, Nassrallah M, Uribe J, Khan RS, Dine K, Dutt M, Shindler KS. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front Neurol. 2012;3:84. doi: 10.3389/fneur.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20:1075–1080. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Fu M, Liu M, Sauve AA, Jiao X, Zhang X, Wu X, Powell MJ, Yang T, Gu W, Avantaggiati ML, et al. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al. Declining NAD(+) Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013;27:2072–2085. doi: 10.1101/gad.227439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011;89:1723–1736. doi: 10.1002/jnr.22725. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Han S, Choi JR, Soon Shin K, Kang SJ. Resveratrol upregulated heat shock proteins and extended the survival of G93A-SOD1 mice. Brain Res. 2012;1483:112–117. doi: 10.1016/j.brainres.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathorn T, Snyder-Keller A, Messer A. Nicotinamide improves motor deficits and upregulates PGC-1alpha and BDNF gene expression in a mouse model of Huntington’s disease. Neurobiol Dis. 2011;41:43–50. doi: 10.1016/j.nbd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A. 2008;105:15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp Neurol. 2010;225:74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Hong SH, Lee KS, Kwak SJ, Kim AK, Bai H, Jung MS, Kwon OY, Song WJ, Tatar M, Yu K. Minibrain/Dyrk1a regulates food intake through the Sir2-FOXO-sNPF/NPY pathway in Drosophila and mammals. PLoS Genet. 2012;8:e1002857. doi: 10.1371/journal.pgen.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, ESY, Lamming DW, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR, 3rd, Bordone L, Guarente L, Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2012;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JK, Moon MH, Lee YJ, Seol JW, Park SY. Autophagy induced by the class III histone deacetylase Sirt1 prevents prion peptide neurotoxicity. Neurobiol Aging. 2013;34:146–156. doi: 10.1016/j.neurobiolaging.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q, Hou Z, Cai H, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2012 doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Doyu M, Minamiyama M, Sang C, Kobayashi Y, Inukai A, Sobue G. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med. 2003;9:768–773. doi: 10.1038/nm878. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, Sang C, Kobayashi Y, Doyu M, Sobue G. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35:843–854. doi: 10.1016/s0896-6273(02)00834-6. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Banno H, Suzuki K, Takeuchi Y, Kawashima M, Yabe I, Sasaki H, Aoki M, Morita M, Nakano I, et al. Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 9:875–884. doi: 10.1016/S1474-4422(10)70182-4. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. Embo J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Benguria A, Lai CY, Jazwinski SM. Modulation of life-span by histone deacetylase genes in Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:3125–3136. doi: 10.1091/mbc.10.10.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Fukuo Y, Kitajima T, Okochi T, Yamanouchi Y, Kinoshita Y, Kawashima K, Inada T, Kunugi H, Kato T, et al. SIRT1 gene, schizophrenia and bipolar disorder in the Japanese population: an association study. Genes Brain Behav. 2011a;10:257–263. doi: 10.1111/j.1601-183X.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- Kishi T, Fukuo Y, Okochi T, Kitajima T, Ujike H, Inada T, Yamada M, Uchimura N, Sora I, Iyo M, et al. No significant association between SIRT1 gene and methamphetamine-induced psychosis in the Japanese population. Hum Psychopharmacol. 2011b;26:445–450. doi: 10.1002/hup.1223. [DOI] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Kitajima T, Okochi T, Okumura T, Tsunoka T, Yamanouchi Y, Kinoshita Y, Kawashima K, Fukuo Y, et al. SIRT1 gene is associated with major depressive disorder in the Japanese population. J Affect Disord. 2010;126:167–173. doi: 10.1016/j.jad.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Klar AJ, Fogel S, Macleod K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics. 1979;93:37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner S, Boselt S, Thau N, Rath KJ, Dengler R, Petri S. Differential sirtuin expression patterns in amyotrophic lateral sclerosis (ALS) postmortem tissue: neuroprotective or neurotoxic properties of sirtuins in ALS? Neurodegener Dis. 2013;11:141–152. doi: 10.1159/000338048. [DOI] [PubMed] [Google Scholar]
- Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28:311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- Kumar P, Padi SS, Naidu PS, Kumar A. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol. 2006;17:485–492. doi: 10.1097/00008877-200609000-00014. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- La Spada AR. Finding a sirtuin truth in Huntington’s disease. Nat Med. 2012;18:24–26. doi: 10.1038/nm.2624. [DOI] [PubMed] [Google Scholar]
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Lee JC, Shin JH, Park BW, Kim GS, Kim JC, Kang KS, Cha CI. Region-specific changes in the immunoreactivity of SIRT1 expression in the central nervous system of SOD1(G93A) transgenic mice as an in vivo model of amyotrophic lateral sclerosis. Brain Res. 1433:20–28. doi: 10.1016/j.brainres.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Li XH, Chen C, Tu Y, Sun HT, Zhao ML, Cheng SX, Qu Y, Zhang S. Sirt1 promotes axonogenesis by deacetylation of Akt and inactivation of GSK3. Mol Neurobiol. 2013;48:490–499. doi: 10.1007/s12035-013-8437-3. [DOI] [PubMed] [Google Scholar]
- Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S, Preisig M, Otowa T, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- Liu CM, Wang RY, Saijilafu, Jiao ZX, Zhang BY, Zhou FQ. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev. 2013;27:1473–1483. doi: 10.1101/gad.209619.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert CD, Kim E, Gifondorwa DJ, Childers MK, Milligan CE. A single-dose resveratrol treatment in a mouse model of amyotrophic lateral sclerosis. J Med Food. 13:1081–1085. doi: 10.1089/jmf.2009.0243. [DOI] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Procaccini C, Menale C, Kim JG, Kim JD, Diano S, Diano N, De Rosa V, Dietrich MO, Horvath TL. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell responses. Proc Natl Acad Sci U S A. 2013;110:6193–6198. doi: 10.1073/pnas.1210644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry DE. Attacking the flank: targeting new pathways in SBMA. Nat Med. 18:1461–1463. doi: 10.1038/nm.2967. [DOI] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Sohn P, Devidze N, Zhou Y, Tracy T, Gan L. Sirt1 deficiency in the brain increases tau acetylation and exacerbates cognitive deficits in a mouse model of tauopathy. New Orleans, LA. 2012; Neuroscience Meeting Planner.Society for Neuroscience; 2012. [Google Scholar]
- Montie HL, Pestell RG, Merry DE. SIRT1 Modulates Aggregation and Toxicity through Deacetylation of the Androgen Receptor in Cell Models of SBMA. J Neurosci. 2011;31:17425–17436. doi: 10.1523/JNEUROSCI.3958-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudo G, Makela J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Malkia A, Bonomo A, Kairisalo M, et al. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nimmagadda VK, Bever CT, Vattikunta NR, Talat S, Ahmad V, Nagalla NK, Trisler D, Judge SI, Royal W, 3rd, Chandrasekaran K, et al. Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets. J Immunol. 2013;190:4595–4607. doi: 10.4049/jimmunol.1202584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5:224. doi: 10.1186/gb-2004-5-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olitsky PK, Yager RH. Experimental disseminated encephalomyelitis in white mice. J Exp Med. 1949;90:213–224. doi: 10.1084/jem.90.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, Marsh JL. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum Mol Genet. 2008;17:3767–3775. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Parker JA, Vazquez-Manrique RP, Tourette C, Farina F, Offner N, Mukhopadhyay A, Orfila AM, Darbois A, Menet S, Tissenbaum HA, Neri C. Integration of beta-catenin, sirtuin, and FOXO signaling protects from mutant huntingtin toxicity. J Neurosci. 2012;32:12630–12640. doi: 10.1523/JNEUROSCI.0277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. Circadian Clock NAD+ Cycle Drives Mitochondrial Oxidative Metabolism in Mice. Science. 2013 doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Rafalski VA, Ho PP, Brett JO, Ucar D, Dugas JC, Pollina EA, Chow LM, Ibrahim A, Baker SJ, Barres BA, et al. Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nat Cell Biol. 2013;15:614–624. doi: 10.1038/ncb2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, Vianna CR, Sinclair DA, Elias CF, Coppari R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes R, Leckey BD, Jr, Nguyen K, Westerheide SD. Heat shock and caloric restriction have a synergistic effect on the heat shock response in a sir2.1-dependent manner in Caenorhabditis elegans. J Biol Chem. 2012;287:29045–29053. doi: 10.1074/jbc.M112.353714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J, Strathern JN, Hicks JB, Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979;93:877–901. doi: 10.1093/genetics/93.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison SH, Bradley WG. DNA damage and chronic neuronal degenerations. J Neurol Sci. 1984;64:11–20. doi: 10.1016/0022-510x(84)90051-0. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Sampaio-Marques B, Felgueiras C, Silva A, Rodrigues M, Tenreiro S, Franssens V, Reichert AS, Outeiro TF, Winderickx J, Ludovico P. SNCA (alpha-synuclein)-induced toxicity in yeast cells is dependent on sirtuin 2 (Sir2)-mediated mitophagy. Autophagy. 2012;8:1494–1509. doi: 10.4161/auto.21275. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kim HJ, Kobayashi M, Kitamura YI, Yokota-Hashimoto H, Shiuchi T, Minokoshi Y, Kitamura T. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology. 2010;151:2556–2566. doi: 10.1210/en.2009-1319. [DOI] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JS, Moon MH, Jeong JK, Seol JW, Lee YJ, Park BH, Park SY. SIRT1, a histone deacetylase, regulates prion protein-induced neuronal cell death. Neurobiol Aging. 2012;33:1110–1120. doi: 10.1016/j.neurobiolaging.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol. 2010;30:328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Rex TS, Elliott P, Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:3602–3609. doi: 10.1167/iovs.07-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto T, Kuroiwa M, Koga Y, Kawahara Y, Sotogaku N, Toyomasu K, Nishi A. Acute effects of resveratrol to enhance cocaine-induced dopamine neurotransmission in the striatum. Neurosci Lett. 2013;542:107–112. doi: 10.1016/j.neulet.2013.02.050. [DOI] [PubMed] [Google Scholar]
- Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino T, Maruyama M, Tanno M, Kuno A, Houkin K, Horio Y. Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth factor-induced neurite outgrowth in PC12 cells. FEBS Lett. 2010;584:2821–2826. doi: 10.1016/j.febslet.2010.04.063. [DOI] [PubMed] [Google Scholar]
- Sun AY, Draczynska-Lusiak B, Sun GY. Oxidized lipoproteins, beta amyloid peptides and Alzheimer’s disease. Neurotox Res. 2001;3:167–178. doi: 10.1007/BF03033189. [DOI] [PubMed] [Google Scholar]
- Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99:735–745. doi: 10.1016/s0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez DA, Martinez G, Romero A, Vazquez MJ, Boit KD, Dopeso-Reyes IG, Lopez M, Vidal A, Nogueiras R, Dieguez C. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes. 2011;60:1177–1185. doi: 10.2337/db10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Tang L, Zhang N, Fan D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci Lett. 503:250–255. doi: 10.1016/j.neulet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez M, Galan L, Matias-Guiu J, Vela A, Guerrero A, Garcia AG. CSF from amyotrophic lateral sclerosis patients produces glutamate independent death of rat motor brain cortical neurons: protection by resveratrol but not riluzole. Brain Res. 1423:77–86. doi: 10.1016/j.brainres.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM, Gao J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435:597–602. doi: 10.1016/j.bbrc.2013.05.025. [DOI] [PubMed] [Google Scholar]