Abstract
Since basic scientific studies in the 1990's revealed dramatic gender differences in neurological damage from cerebral ischemia, significant evidence has accumulated for a neuroprotective role of ovarian-derived 17β-estradiol (E2). Intriguingly, observational studies have further suggested that early and prolonged loss of ovarian E2 (premature menopause) leads to a doubled lifetime risk for dementia and a five-fold increased risk of mortality from neurological disorders, but some controversy remains. Here, we briefly summarize and analyze clinical cohort studies assessing the detrimental neurological outcomes of premature menopause. Furthermore, we discuss current basic science studies elucidating the molecular mechanisms underlying the enhanced risk of neurological disease in prematurely menopausal women and the “window of opportunity” for estrogen benefit. Finally, we highlight four critical issues in the field that require collaboration between basic scientists and clinicians for successful resolution, with the ultimate goal of maintaining optimal neurological health in prematurely menopausal women.
Keywords: Estradiol, Estrogen, Dementia, Menopause, Neurological Disease
1. Introduction - Premature Menopause May Enhance the Risk of Neurological Disease
Menopause is a phase of female reproductive senescence characterized by depletion of ovarian follicles and cessation of menstruation, beginning at a median age of 51 in developed countries (Armstrong et al, 2004; Kato et al, 1998; Rocca et al, 2009). However, a significant number of women enter menopause early (< 45) or prematurely (< 40) due to hysterectomy ± oophorectomy, premature ovarian insufficiency, or iatrogenic damage from chemotherapeutics, radiation, or surgery [for review, see (Jin et al, 2012; Shuster et al, 2010)]. Work by several groups has provided evidence that early menopause may negatively impact neurological health [See Table 1 for Summary]. For instance, the Mayo Clinic Cohort Study of Oophorectomy and Aging revealed that surgical induction of early menopause enhanced the risk of ischemic stroke, doubled the lifetime risk of dementia, and increased the risk of mortality from neurological disorders five-fold (Rivera et al, 2009; Rocca et al, 2007; Rocca et al, 2006; Rocca et al, 2010; Rocca et al, 2011; Rocca et al, 2012b; Rocca et al, 2009; Shuster et al, 2008; Shuster et al, 2010). Intriguingly, these detrimental effects increased as the age of menopausal onset decreased. Likewise, the Nurses Health Study found that premenopausal oophorectomy was associated with significantly higher risks for cognitive impairment, dementia, depression, anxiety, stroke, and mortality (Parker, 2010; Parker, 2013; Parker et al, 2009). In addition, a recent publication, which combined data from two longitudinal studies (Religious Orders Study and Rush Memory and Aging Project), also found that earlier age at surgical menopause was associated with faster decline in global cognition (episodic and semantic memory) and an increased presence of Alzheimer's disease neuropathology (β-amyloid plaques) (Bove et al, 2013). Finally, a Danish cohort study of over 2 million women echoed the findings of American cohort studies and extended the results to include hysterectomy, suggesting that premenopausal hysterectomy and premenopausal hysterectomy with either unilateral or bilateral oophorectomy were all associated with a enhanced risk of developing early-onset dementia (Phung et al, 2010).
Table 1. Surgical Menopause Cohort Studies and Neurological Outcomes.
Average age at surgery and length of follow-up are shown in years. Note that cohort studies finding an enhanced risk of neurological disease and mortality in surgically menopausal women had significantly longer length of follow-up.
| Study | Cohort Size | Type of Surgical Menopause | Average Age at Surgery (or Study Initiation) | Length of Follow-Up | Findings |
|---|---|---|---|---|---|
| Mayo Clinic Cohort of Oophorectomy and Aging | 2,365 | Bilateral Oophorectomy | 44 | 25 | ↑ Risk of Dementia, Stroke, Parkinsonism, and Mortality from Neurological Disorders |
| Nurses Health Study | 29,380 | Bilateral Oophorectomy | 43–47 | 24 | ↑ Risk of Dementia, Stroke, Depression, Anxiety, and Mortality |
| Religious Orders Study and Rush Memory and Aging Project | 1884 | Not Available | 42.7 | 18 | ↑ Cognitive Decline and Neural β-Amyloid Plaques |
| Danish Nationwide Cohort Study | 2,313,838 | Unilateral or Bilateral | |||
| Oophorectomy ± Hysterectomy | 40 | Until Death or Diagnosis of Dementia | ↑ Risk of Early-Onset Dementia in ALL Types of Surgical Menopause Occurring Before 50 Years of Age | ||
| California Teachers Study (CTS) | 42,004 | Bilateral Oophorectomy | <45 and ≥45 | 11.3 | No ↑ Risk of Cancer Mortality, Cardiovascular Mortality, or All-Cause Mortality for Either Group |
| Women's Health Initiative Observational Study (WHIOS) | 25,448 | Bilateral Oophorectomy | 49 | 7.6 | No ↑ Risk of Cardiovascular Disease, Stroke, Hip Fracture, or All-Cause Mortality |
On the other hand, some studies failed to find any deleterious effects of premature menopause (Table 1). For instance, the California Teachers Study (CTS) found that bilateral oophorectomy did not increase the risk of mortality from cancer, cardiovascular disease, or all-causes (Duan et al, 2012). In addition, the Women's Health Initiative Observational Study (WHIOS) failed to find negative consequences of premenopausal oophorectomy on cardiovascular disease, stroke, hip fracture or total mortality (Jacoby et al, 2011). However, both studies had several caveats that may explain the lack of any negative effects. First, women with bilateral oophorectomy in the WHIOS study were an average age of 49 at the time of surgery and, therefore, cannot be characterized as either early or prematurely menopausal. In addition, these women were compared to hysterectomized women instead of women who had all of their reproductive organs intact. Since hysterectomy is thought to interfere with ovarian function (Nahas et al, 2003; Siddle et al, 1987; Xiangying et al, 2006), and since the aforementioned Danish cohort study found that hysterectomy alone was associated with a 38% increased risk of dementia (Phung et al, 2010; Rocca & Ulrich, 2012), using hysterectomized women as referents could potentially confound the results by making significant differences between groups smaller and harder to detect. Finally, both the CTS and the WHIOS suffered from a potentially insufficient length of follow-up (mean of 11.3 years and 7.6 years, respectively), which is significantly shorter than the 20- to 30-year follow-up periods utilized in the other observational studies described above (Rocca & Ulrich, 2012). It is thus possible that longer follow periods may be necessary to observe negative neurological consequences in the CTS and WHIOS studies.
2. Estrogen Replacement Reverses Many, But Not All of the Detrimental Effects of Premature Menopause
The hallmark of premature menopause is an early and prolonged loss of circulating ovarian hormones. In particular, early and prolonged loss of E2 has been proposed to potentially underlie many of the negative neurological outcomes associated with premature menopause. This premise is based on the fact that a vast breadth of clinical and basic science studies have suggested that E2 is neuroprotective [for review see (Brann et al, 2007; Scott et al, 2012)]. In support of an underlying role for E2 loss in the detrimental neurological effects of premature menopause, several studies have shown that early estrogen therapy (ET) prevents many of the neurological sequelae associated with premature menopause. For instance, the enhanced risk of dementia was not observed in oophorectomized women who received ET from the time of oophorectomy until the median age of natural menopausal onset (Rocca et al, 2007). Likewise, the increased risk of stroke, cardiovascular disease and all-cause mortality observed in premenopausally oophorectomized women was not observed in those who initiated early ET (Parker, 2013; Parker et al, 2013; Rocca et al, 2012a). Unfortunately, the increased risk of depression, anxiety, and parkinsonism was not normalized in bilaterally oophorectomized women subjected to timely ET, but the reason for this discrepancy remains unknown (Rocca & Ulrich, 2012; Shuster et al, 2010). As such, extended deprivation of other ovarian hormones, such as progesterone and androgens, may also play a role in the enhanced risk of neurological disease in prematurely menopausal women [for review, see (Acosta et al, 2013; Morrison et al, 2006; Singh & Su, 2013a; Singh & Su, 2013b)]. However, in comparison to E2, the evidence implicating these alternative hormones is less abundant, and more work is needed.
3. Unlocking the Underlying Mechanisms for Enhanced Neurological Risks – Clues from Basic Science Research
3.1 Brain Hypersensitivity to Pathologic Stressors
Despite significant evidence of the neurological implications of prolonged loss of ovarian function, an important question remains unanswered: how exactly does premature menopause enhance the risk for dementia, neurological disease, and all-cause mortality? With respect to the increased risks of dementia and neurological disease, recent studies have provided some tantalizing clues that may help unravel this mystery [See Figure 1 for Summary]. Work in an animal model of premature menopause (long-term ovariectomy) revealed that the hippocampus, an area critical in learning and memory, becomes hypersensitive to ischemic injury following surgical menopause (Zhang et al, 2013). A similar hypersensitivity to stroke damage has also been observed in the cerebral cortex (Ding et al, 2013). Intriguingly, surgically menopausal rats also demonstrated a profound hippocampal induction of Alzheimer's disease (AD)-related proteins, increased amyloidogenesis, and worse cognitive outcome after ischemic stress (Zhang et al, 2013). Further work showed that the brain's hypersensitivity also extended to an AD-relevant insult, as the hippocampus of surgically menopausal rats was profoundly hypersensitive to the neurotoxic effects of amyloid-beta 1-42 (Ab1-42), the most amyloidogenic form of the Ab peptide. As such, enhanced sensitivity of the brain to various stressors after premature and prolonged loss of ovarian-derived E2 is a plausible reason for the increased risk of neurological diseases and mortality from these disorders.
Figure 1. Molecular Mechanisms Underlying Enhanced Risk of Neurological Disease Following Premature Menopause.
See text for further details. CNS, Central Nervous System; Dkk1, Dickkopf-1, E2, 17β-Estradiol, Wnt, Wingless.
3.2. Potential Underlying Molecular Mechanisms
With respect to the molecular mechanisms underlying these phenomena, additional studies in CA1 hippocampal neurons of surgically menopausal rats revealed basal up-regulation of the neurodegenerative protein Dkk1, basal antagonism of pro-survival Wnt/β-catenin signaling, and basal acetylation/stabilization of the stress sensor p53, which sensitizes cells to stress (Raz et al, 2011; Scott et al, 2013). Further work revealed that the hippocampal hypersensitivity, AD-related protein induction, and enhanced amyloidogenesis might also depend on activation of the NADPH oxidase/superoxide/C-Jun N-terminal kinase pathway, a stress-activated intracellular signaling cascade that further enhances oxidative stress and promotes apoptosis in neurons, through both transcriptional and post-translational mechanisms (Zhang et al, 2013). Brinton and colleagues have also provided compelling evidence that E2 has an additional critical role in sustaining the brain's bioenergetic capacity by preserving glucose metabolism and mitochondrial function [for review, see (Rettberg et al, 2013)]. Their research showed that ovariectomy in a pre-clinical model of AD was paralleled by a shift to neuronal utilization of ketone bodies as an energy source rather than the more common, and much more effective, energy source, glucose (Hamilton et al, 2011; Yao et al, 2010; Yao et al, 2012). This shift led to a concomitant decline in brain glucose transport and metabolism, and interestingly, occurred simultaneously with compromised oxidative phosphorylation, signs of mitochondrial oxidative stress and an accumulation of Aβoligomers in the mitochondria (Yao & Brinton, 2012). Importantly, all of these effects were reversed either in part or in full by ET given immediately following ovariectomy (Ding et al, 2013; Yao et al, 2012). Conversely, they suggest, if E2 is not replaced and the ketogenic utilization is allowed to continue, eventually fatty acids in myelinated axons may be oxidized and degraded to provide the brain with more alternative fuel, further compromising brain function through the destruction of white matter (Yao & Brinton, 2012).
3.3 Timing of ET and Clinical Implications
Finally, from a therapeutic viewpoint, timing of ET also appears important. Work by our group using the surgical menopause animal model, revealed that low dose ET at the end of the long-term ovariectomy period could not prevent the hippocampal hypersensitivity and enhanced post-ischemic amyloidogenesis observed following surgical menopause. However, if ET was initiated early, at the time of ovariectomy, and maintained throughout the entire ovariectomy period, it completely prevented these events, indicating that, at least in rats, early ET following surgical menopause is critical for preventing the neurological sequelae associated with prolonged loss of ovarian function. Furthermore, our laboratory recently uncovered a possible mechanism to explain this timing effect, as estrogen receptor alpha (ERα), the key mediator of E2's neuroprotective effects, was selectively degraded in hippocampal neurons of surgically menopausal and aged, reproductively senescent female rats that failed to receive timely low dose ET (Zhang et al, 2011). A similar loss of ERα has now been demonstrated in the cerebral cortex of surgically menopausal rats as well (Ding et al, 2013). Collectively, these studies suggest that the timing of ET following menopausal onset may be critical to its success, with early initiation required to reap maximum neurological benefit and avert adverse events. These findings are consistent with the previously proposed “critical period” and “healthy cell bias” hypotheses, which state that there is a window of opportunity for E2 to yield neurological benefit following menopause, and once this window closes, exogenous E2 may become detrimental to the brain (Brinton, 2005; Brinton, 2008; Maki, 2006; Sherwin, 2007). Thus, as a whole, the above basic science findings provide insights into potential basic mechanisms that may contribute to the increased risk of dementia, neurological disease, and mortality observed in women following premature menopause, and they provide increased evidence that early ET following menopausal onset is crucial in order to prevent the negative neurological effects associated with prolonged loss of ovarian function (Figure 1).
4. Conclusion - Bridging the Gap
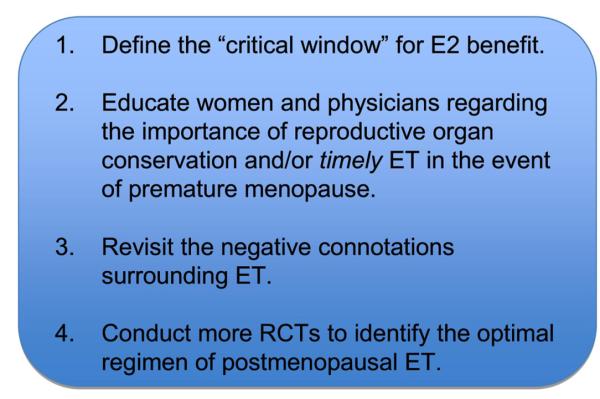
In closing, while prolonged loss of ovarian-derived E2 appears to incur a significantly increased risk for dementia and mortality from neurological disorders, more work is needed to bridge the gap between the bench and the bedside (Figure 2). First, while timely postmenopausal ET may neutralize many detrimental neurological effects, the “window of opportunity” for E2 benefit has yet to be firmly defined. Along these lines, a crucial question is: what is the timeframe for the “window of opportunity” for E2 benefit after menopausal onset in women? Months? Years? Secondly, both women and physicians should be educated regarding the importance of reproductive organ conservation, and/or timely ET to offset the increased neurological risks in the event of hysterectomy/oophorectomy, premature ovarian insufficiency, or iatrogenic premature menopause. It is encouraging to note that the current Global Consensus Statement on Menopausal Therapy, which was endorsed by the North American Menopause Society, International Menopause Society, European Menopause and Andropause Society, The Endocrine Society, and the American Society for Reproductive Medicine, states, “In women with premature ovarian insufficiency, systemic hormone therapy is recommended at least until the average age of the natural menopause” (de Villiers et al, 2013). Third, the negative connotation surrounding ET needs to be revisited. Understandably, the negative results of the Women's Health Initiative (WHI) have made physicians hesitant to prescribe—and postmenopausal women hesitant to take—hormone-based therapy, owing to the perceived increased risk of adverse events, such as breast cancer and stroke. However, the negative connotation of ET that derived from these highly sensationalized results has led to unfortunate negative outcomes. Using an intricate algorithm, a recent study suggested that approximately 40,292 to 48,835 excess premature deaths of hysterectomized women can be attributed to avoidance of timely ET following publication of the negative WHI results 10 years ago (Sarrel et al, 2013). Considering the volume of basic scientific research touting the benefits of timely postmenopausal ET and the 10-year follow-up of the WHI estrogen alone trial, which showed no increased risk of breast cancer, cardiovascular disease, or stroke, and a decreased risk of mortality for the youngest group of postmenopausal women (aged 50–59) (LaCroix et al, 2011), perhaps opinions regarding ET should be re-examined.
Figure 2. Bridging the Gap.
These four crucial areas need to be addressed by both scientists and clinicians in order to optimize postmenopausal ET and better protect the neurological health of prematurely menopausal women. E2, 17β-Estradiol; ET, Estrogen Therapy; RCT, Randomized Controlled Clinical Trials.
Fourth, while costly and labor-intensive, more randomized, clinical trials of postmenopausal E2-based therapy should be conducted in order to solidify the optimal ET regimen. Thanks to clinical studies following the WHI, such as E3N and ESTHER, we now know that E2 is a superior to CEEs, with respect to neurological benefit, and that transdermal administration of E2 appears safer than the oral route, with respect to risk of venous thromboembolism (Canonico et al, 2010; Canonico et al, 2007). Ongoing studies, such as the Kronos Early Estrogen Prevention Study (KEEPS) and Early versus Late Intervention Trial with Estradiol (ELITE), will hopefully yield more insight regarding the effectiveness of ET with regard to optimal type of estrogen administered and time of initiation following menopausal onset (Harman et al, 2005; Henderson & Brinton, 2010; Menon & Vongpatanasin, 2006; Miller et al, 2009). Finally, pharmacological development is underway for neuroSERMs (selective estrogen receptor modifiers), which could theoretically provide all the neurological benefits of E2 without exerting carcinogenic or feminizing side effects through other estrogen-responsive tissues (Brinton, 2004; Simpkins et al, 2009). As such, successful resolution of these critical issues through collaboration between basic scientists and clinicians and implementation of safe, effective, E2-based therapies hold much promise for the neurological health of postmenopausal women.
Highlights
Premature loss of ovarian estradiol enhances the risk of neurological disease.
Premature menopause may cause brain hypersensitivity to pathologic stressors.
Timely estrogen therapy may avert these negative neurological sequelae.
Basic scientists and clinicians should collaborate to address these crucial issues.
Acknowledgements
The authors' work described in this review was supported by Research Grant NS050730 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA, to D.W.B., Scientist Development Grant 10SDG256009 from the American Heart Association to Q.Z., and Pre-Doctoral Fellowship 12PRE11530009 from the American Heart Association to E.L.S.
Abbreviations
- Aβ
Beta-Amyloid
- AD
Alzheimer's disease
- CEEs
Conjugated Equine Estrogens
- CTS
California Teachers Study
- Dkk1
Dickkopf-1
- E2 or Estrogen
17β-Estradiol
- ERα
Estrogen Receptor Alpha
- ET
Estrogen Therapy
- MPA
Medroxyprogesterone Acetate
- MRI
Magnetic Resonance Imaging
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- SERMs
Selective Estrogen Receptor Modifiers
- WHI
Women's Health Initiative
- WHIMS
Women's Health Initiative Memory Study
- WHIOS
Women's Health Initiative Observational Study
- WHISCA
Women's Health Initiative Study of Cognitive Aging
- Wnt
Wingless
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. An update on the cognitive impact of clinically-used hormone therapies in the female rat: Models, mazes, and mechanisms. Brain research. 2013;1514:18–39. doi: 10.1016/j.brainres.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2013 doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Requirements of a brain selective estrogen: advances and remaining challenges for developing a NeuroSERM. Journal of Alzheimer's disease : JAD. 2004;6:S27–35. doi: 10.3233/jad-2004-6s607. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Annals of the New York Academy of Sciences. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends in neurosciences. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico M, Fournier A, Carcaillon L, Olie V, Plu-Bureau G, Oger E, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F, Scarabin PY. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:340–345. doi: 10.1161/ATVBAHA.109.196022. [DOI] [PubMed] [Google Scholar]
- Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PY, Estrogen, Thromboembolism Risk Study G Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- de Villiers TJ, Gass ML, Haines CJ, Hall JE, Lobo RA, Pierroz DD, Rees M. Global Consensus Statement on menopausal hormone therapy. Maturitas. 2013;74:391–392. doi: 10.1016/j.maturitas.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Ding F, Yao J, Zhao L, Mao Z, Chen S, Brinton RD. Ovariectomy induces a shift in fuel availability and metabolism in the hippocampus of the female transgenic model of familial Alzheimer's. PloS one. 2013;8:e59825. doi: 10.1371/journal.pone.0059825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Xu X, Koebnick C, Lacey JV, Jr., Sullivan-Halley J, Templeman C, Marshall SF, Neuhausen SL, Ursin G, Bernstein L, Henderson KD. Bilateral oophorectomy is not associated with increased mortality: the California Teachers Study. Fertility and sterility. 2012;97:111–117. doi: 10.1016/j.fertnstert.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RT, Rettberg JR, Mao Z, To J, Zhao L, Appt SE, Register TC, Kaplan JR, Brinton RD. Hippocampal responsiveness to 17beta-estradiol and equol after long-term ovariectomy: implication for a therapeutic window of opportunity. Brain research. 2011;1379:11–22. doi: 10.1016/j.brainres.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric : the journal of the International Menopause Society. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Progress in brain research. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby VL, Grady D, Wactawski-Wende J, Manson JE, Allison MA, Kuppermann M, Sarto GE, Robbins J, Phillips L, Martin LW, O'Sullivan MJ, Jackson R, Rodabough RJ, Stefanick ML. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women's Health Initiative Observational Study. Archives of internal medicine. 2011;171:760–768. doi: 10.1001/archinternmed.2011.121. [DOI] [PubMed] [Google Scholar]
- Jin M, Yu Y, Huang H. An update on primary ovarian insufficiency. Science China Life sciences. 2012;55:677–686. doi: 10.1007/s11427-012-4355-2. [DOI] [PubMed] [Google Scholar]
- Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. Journal of clinical epidemiology. 1998;51:1271–1276. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J, Investigators WHI Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Menon DV, Vongpatanasin W. Effects of transdermal estrogen replacement therapy on cardiovascular risk factors. Treatments in endocrinology. 2006;5:37–51. doi: 10.2165/00024677-200605010-00005. [DOI] [PubMed] [Google Scholar]
- Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, Lobo RA, Manson JE, Merriam GR, Naftolin F, Santoro N, Taylor HS, Harman SM. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) Journal of cardiovascular translational research. 2009;2:228–239. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas E, Pontes A, Traiman P, NahasNeto J, Dalben I, De Luca L. Inhibin B and ovarian function after total abdominal hysterectomy in women of reproductive age. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2003;17:125–131. [PubMed] [Google Scholar]
- Parker WH. Bilateral oophorectomy versus ovarian conservation: effects on long-term women's health. Journal of minimally invasive gynecology. 2010;17:161–166. doi: 10.1016/j.jmig.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Parker WH. Ovarian conservation versus bilateral oophorectomy at the time of hysterectomy for benign disease. Menopause. 2013 doi: 10.1097/GME.0b013e31829be0a0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstetrics and gynecology. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, Berek JS, Manson JE. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study. Obstetrics and gynecology. 2013;121:709–716. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dementia and geriatric cognitive disorders. 2010;30:43–50. doi: 10.1159/000314681. [DOI] [PubMed] [Google Scholar]
- Raz L, Zhang QG, Han D, Dong Y, De Sevilla L, Brann DW. Acetylation of the pro-apoptotic factor, p53 in the hippocampus following cerebral ischemia and modulation by estrogen. PloS one. 2011;6:e27039. doi: 10.1371/journal.pone.0027039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettberg JR, Yao J, Brinton RD. Estrogen: A master regulator of bioenergetic systems in the brain and body. Frontiers in neuroendocrinology. 2013 doi: 10.1016/j.yfrne.2013.08.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera CM, Grossardt BR, Rhodes DJ, Rocca WA. Increased mortality for neurological and mental diseases following early bilateral oophorectomy. Neuroepidemiology. 2009;33:32–40. doi: 10.1159/000211951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. The lancet oncology. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD., Jr. Premature menopause or early menopause and risk of ischemic stroke. Menopause. 2012a;19:272–277. doi: 10.1097/gme.0b013e31822a9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neuro-degenerative diseases. 2010;7:163–166. doi: 10.1159/000289229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain research. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neuro-degenerative diseases. 2012b;10:175–178. doi: 10.1159/000334764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ., 3rd Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Women's health. 2009;5:39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Ulrich LG. Oophorectomy for whom and at what age? Primum non nocere. Maturitas. 2012;71:1–2. doi: 10.1016/j.maturitas.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59 years. American journal of public health. 2013;103:1583–1588. doi: 10.2105/AJPH.2013.301295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Frontiers in neuroendocrinology. 2012;33:85–104. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EL, Zhang QG, Han D, Desai BN, Brann DW. Long-term estrogen deprivation leads to elevation of Dickkopf-1 and dysregulation of Wnt/beta-Catenin signaling in hippocampal CA1 neurons. Steroids. 2013;78:624–632. doi: 10.1016/j.steroids.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? Journal of neuroendocrinology. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause international. 2008;14:111–116. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle N, Sarrel P, Whitehead M. The effect of hysterectomy on the age at ovarian failure: identification of a subgroup of women with premature loss of ovarian function and literature review. Fertility and sterility. 1987;47:94–100. doi: 10.1016/s0015-0282(16)49942-5. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Perez E, Wang X, Yang S, Wen Y, Singh M. The potential for estrogens in preventing Alzheimer's disease and vascular dementia. Therapeutic advances in neurological disorders. 2009;2:31–49. doi: 10.1177/1756285608100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Su C. Progesterone and neuroprotection. Hormones and behavior. 2013a;63:284–290. doi: 10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Su C. Progesterone-induced neuroprotection: Factors that may predict therapeutic efficacy. Brain research. 2013b;1514:98–106. doi: 10.1016/j.brainres.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiangying H, Lili H, Yifu S. The effect of hysterectomy on ovarian blood supply and endocrine function. Climacteric : the journal of the International Menopause Society. 2006;9:283–289. doi: 10.1080/13697130600865774. [DOI] [PubMed] [Google Scholar]
- Yao J, Brinton RD. Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer's disease. Advances in pharmacology. 2012;64:327–371. doi: 10.1016/B978-0-12-394816-8.00010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochimica et biophysica acta. 2010;1800:1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial beta-amyloid. Neurobiology of aging. 2012;33:1507–1521. doi: 10.1016/j.neurobiolaging.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E617–624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Wang RM, Scott E, Han D, Dong Y, Tu JY, Yang F, Reddy Sareddy G, Vadlamudi RK, Brann DW. Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause. Brain : a journal of neurology. 2013;136:1432–1445. doi: 10.1093/brain/awt046. [DOI] [PMC free article] [PubMed] [Google Scholar]