Abstract
Rationale
Variability in the rate of nicotine metabolism, measured by the nicotine metabolite ratio (NMR), is associated with smoking behavior. However, data linking the NMR with nicotine dependence measured by the Fagerström Test for Nicotine Dependence (FTND) are mixed. Few past studies have examined alternative measures of nicotine dependence and how this relationship may vary by sex and race.
Objective
Using data from smokers undergoing eligibility evaluation for a smoking cessation clinical trial (n=833), this study examined variability in the relationship between NMR and nicotine dependence across sex and race and using three measures of nicotine dependence: FTND, time-to-first-cigarette (TTFC), and the Heaviness of Smoking Index (HSI).
Results
Controlling for sex and race, nicotine metabolism was associated with nicotine dependence only when using the HSI (p < .05). Male normal metabolizers of nicotine were more likely to have high nicotine dependence based on the FTND and HSI (p < .05), but NMR was not related to measures of nicotine dependence in women. For African Americans, the NMR was associated with nicotine dependence only for the TTFC (p < .05) but NMR was not associated with nicotine dependence among Caucasians. Post-hoc analyses indicated that the NMR was associated with cigarettes per day, overall and among men and Caucasians (p < .05).
Conclusions
While there was some variation in the relationship between nicotine metabolism and nicotine dependence across measures and sex and race, the results indicate that this relationship may be more attributable to the association between NMR and cigarettes per day.
Keywords: Nicotine metabolite ratio, nicotine dependence, race, sex, Fagerström, time-to-first cigarette, heaviness of smoking index, cigarettes per day
Introduction
Over the past several years, there has been growing interest in the potential influence of individual variability in the rate of nicotine metabolism, measured by the nicotine metabolite ratio (NMR), on a range of smoking phenotypes, including rate of smoking, smoking topography (e.g., number of puffs/cigarette), symptoms of craving and withdrawal, and the ability to quit smoking (Ray et al., 2009; West et al., 2011). Nicotine is metabolized into 3-hydroxycotinine (3-HC), which is then metabolized into cotinine exclusively by CYP2A6 (Nakajima et al., 1996; Dempsey et al., 2004). The NMR is the ratio between these two metabolites of nicotine (3-HC/COT). The NMR reflects CYP2A6 activity, including genetic and environmental influences, and is associated with CYP2A6 genotype in all ethnicities studied (Lea et al., 2006; Ho et al., 2009). NMR is a validated surrogate measure of total nicotine clearance, supported by the high correlation with nicotine clearance (Dempsey et al., 2004), due to the large role which CYP2A6 plays in the clearance of nicotine. The NMR is stable over time, and independent of the duration since last cigarette, due to the long half-life for cotinine and, at steady-state (i.e., in smokers), 3-HC is formation dependent (St Helens et al., 2012). Emerging from this literature is the growing possibility of using the NMR to personalize smoking cessation treatment selection and improve treatment response (Ray et al., 2009; Bough et al., 2012; Schnoll & Leone, 2011). However, a somewhat surprising result thus far concerns the lack of relationship found in past studies concerning the association between the NMR and measures of nicotine dependence. As described by West et al. (2011), more than a half-dozen studies have failed to show a significant relationship between the NMR and nicotine dependence measured by the Fagerström Test for Nicotine Dependence (FTND)3, or its derivatives. One potential interpretation of these results is that self-report measures of nicotine dependence and the NMR are unrelated, possibly representing divergent aspects of tobacco use and addiction.
Alternatively, these results may be due to the methodological approach taken in previous studies to evaluate this relationship. First, the vast majority of studies in this area have used the total FTND score to measure nicotine dependence. A recent study (Schnoll et al., 2013) found substantial variability in the prevalence of nicotine dependence (23%–64%) and in predictors of nicotine dependence depending on whether the FTND was used to define nicotine dependence vs. two component measures of the FTND: the Heaviness of Smoking Index (HSI; Kozlowski et al., 1994; 1981) and the time-to-first-cigarette (TTFC; Baker et al., 2007). Perhaps the relationship between nicotine dependence and the NMR varies depending upon the measure used to define nicotine dependence. Second, to the best of our knowledge, no study has examined whether sex or race moderate the relationship between the NMR and self-reported nicotine dependence. Several studies have reported that sex and race are associated with the NMR, with women vs. men and Caucasians vs. African Americans, having faster rates of nicotine metabolism (Benowitz et al., 2006; Schnoll et al., 2009; Kandel et al., 2007). Further, although the strength of the associations have been modest, being male and Caucasian increases the risk for higher nicotine dependence when each of these self-report measures of nicotine dependence is used (Schnoll et al., 2013). Consequently, it is plausible that assessing the relationship between NMR and nicotine dependence requires analyses that consider these factors in prediction models.
Using a large sample of smokers undergoing eligibility screening for a smoking cessation clinical trial, this study examined the relationship between the NMR and three measures of nicotine dependence – the FTND, HSI, and TTFC – and evaluated the association between the NMR and these measures of nicotine dependence across sex and race. A more detailed assessment of this relationship may help to better understand the nature of the association between nicotine metabolism and nicotine dependence and guide future studies in this area.
Methods
Data for this study were ascertained from intake/eligibility assessments conducted with smokers who were considering enrollment into an ongoing placebo-controlled, randomized clinical trial evaluating varenicline and transdermal nicotine for treating nicotine dependence, expected to be completed in 2014 (ClinicalTrials.gov Identifier: NCT01314001).
Procedures
Prospective participants responded to an advertisement for a free smoking cessation clinical trial. Potential subjects received a brief description of the trial and basic eligibility criteria were evaluated. Eligibility criteria for the trial, and for these analyses, were as follows: self-reported smoking of at least 10 cigarettes per day, a breath carbon monoxide value of >10ppm, age 18–65, interested in quitting smoking, absence of substance abuse or dependence, no current use of contraindicated medication including any smoking cessation medications, no medical contraindication (e.g., allergy to latex, uncontrolled hypertension), no history of a psychotic or bipolar disorder and no history of major depression in the past 6 months, no history of a suicide attempt, and a willingness to reside in the area for at least the next 12 months.
Those who were considered eligible during the phone screen were asked to attend an orientation session where they would learn more about the clinical trial and provide informed consent. If interested in proceeding, participants were scheduled to attend an in-person eligibility session that involved the collection of the blood sample (to determine NMR), the completion of smoking history and demographic measures, and the confirmation of study eligibility, including a urine drug and pregnancy (for women) test, a medical history and brief physical exam, and a psychiatric evaluation using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). All recruitment and screening procedures were standardized across the clinical trial sites and approved by the respective site Institutional Review Boards. The NMR, demographic, and smoking history data ascertained at this intake session and among participants who were considered eligible at this intake session were used for the present analyses.
Measures
A survey used in past clinical trials (Patterson et al., 2008) collected basic participant demographic (e.g., age, sex, race, education) and smoking history (e.g., number of cigarettes smoked per day, age started smoking) information. The FTND (Heatherton et al., 1981) was also administered to determine level of nicotine dependence. The FTND, a 6-item, self-report measure, is a widely-used scale for assessing level of nicotine dependence (Pérez-Rios et al., 2009). A total score out of 10 is compiled by summing the six items to reflect a higher level of nicotine dependence with higher total scale values. A cut-off of ≥ 5 on the FTND reflects high nicotine dependence (Schnoll et al., 2013). The TTFC and the HSI can be computed from individual items on the FTND (Baker et al., 2007; Garvey et al., 2000). For the TTFC, respondents indicate whether they smoke: within 5 minutes of waking, between 6 and 30 minutes of waking, between 31 and 60 minutes of waking, or greater than 60 minutes after waking, for which they are given a score of 0 (smoking more than 60 minutes after waking) to 3 (smoking within 5 minutes). A binary score can also be ascertained for the TTFC with a response of smoking the first cigarette of the day within 5 minutes of waking representing high nicotine dependence (Baker et al., 2007). For the HSI (computed by summing TTFC and the FTND item concerning cigarettes per day), respondents can receive a score from 0 to 6 and a binary score of ≥ 4 out of a possible 6 represents nicotine dependence (Garvey et al., 2000; Schnoll et al., 2013).
To identify the NMR, blood samples (10 ml) collected at the in-person eligibility visit were frozen until cotinine and 3′hydroxycotinine were assessed by liquid chromatography-tandem mass spectrometry (LC-MS) by the Tyndale laboratory (St. Helens et al., 2013). The limit of quantification was 1.0 ng/ml for both compounds. In addition to the continuous measure of the NMR, based on previous studies, participants were categorized in terms of NMR quartiles (i.e., lowest quartile equals slowest nicotine metabolism, vs. highest quartile equals fastest nicotine metabolism), and using a cut-off of >0.26 to differentiate normal (faster) metabolizers of nicotine from slow metabolizers of nicotine (Schnoll et al., 2009; Patterson et al., 2008).
Statistical Analysis
Sample characteristics were examined using descriptive statistics. Given the non-normal distribution of the NMR, it was log-transformed for analyses as usual for this and other metabolic ratios (Johnstone et al., 2006; Kandel et al., 2007; Schnoll et al., 2009). T-tests examined differences in NMR across sex and race. The distribution of NMR quartiles, and the distribution of slow vs. normal metabolizers using the 0.26 cut-off, across sex and race was analyzed using chi-square. T-tests were used to examine differences across sex and race in terms of FTND and HSI while ordinal logistic regression was used for TTFC. Chi-square was used to examine these relationships using cut-offs for the measures of high nicotine dependence (e.g., TTFC ≤ 5 minutes).
Following previous studies (e.g., Rubinstein et al., 2012), the relationship between the NMR and measures of nicotine dependence was assessed using linear regression. Models were examined using only the NMR as a predictor of the respective measure of nicotine dependence and then with sex and race included in models. Linear regression models were also examined for each measure of nicotine dependence including the main effects for NMR, sex, and race, and interaction terms. The raw scale for each measure of nicotine dependence was used for these analyses (e.g., 0–10 for FTND, 0–3 for TTFC, and 0–6 for HSI).
Lastly, using the categorical definition for NMR (i.e., > 0.26 for normal metabolizers) and categorical definitions of high nicotine dependence for each measure (i.e., TTFC ≤ 5 minutes, HSI ≥ 4, and FTND ≥ 5), chi-square analyses examined the relationship between nicotine metabolism and nicotine dependence. These analyses were performed for the entire sample and then separately for males vs. females and Caucasians vs. African Americans. Sex and race were not significantly related (p > .05), indicating that results concerning one variable were not confounded by the other. Finally, using these categorical definitions for NMR and high nicotine dependence for each measure, logistic regression examined the main effects of NMR, sex, and race, and interaction effects as predictors of each measure of nicotine dependence.
The present sample (N = 833) provided 80% power (α = .05) to detect an effect size (Cohen’s d) of 0.1 or a correlation of ≤ 0.1, indicating that the study was powered to detect relatively small effects.
Results
Sample Characteristics
Telephone screens were completed on 5,835 individuals; 3,087 (53%) of those phone screened were eligible and 2,879 (93%) of those eligible scheduled an orientation; 1,426 individuals attended orientation (50%) and 1,202 individuals (84%) attended the intake session; 897 individuals (75%) were considered eligible at the intake session and were assessed for NMR. The present analyses were conducted with 833 of these individuals who self-identified as Caucasian or African American (93%; since the numbers for the other racial/ethnic groups were extremely small).
Descriptive data on the sample are shown in Table 1. On average, participants were 46.0 years old (SD = 10.7 years). About 48% of the sample was female and about two-thirds of the sample was Caucasian. The average NMR was 0.39 (SD = .21) with 28% of the sample being classified as slow metabolizers and 72% of the sample being classified as normal metabolizers. On average, participants reported smoking 18.7 cigarettes per day (SD = 8.5), started smoking at about 16 years of age (SD = 4.3), and reported a mean FTND of 5.3 (SD = 2.0), a mean HSI of 3.4 (SD = 1.2), and a mean TTFC of 2.2 (SD = .84). Based on scale cutoffs, the rate of high nicotine dependence in the sample was: 68.8% for the FTND, 45.5% for the HSI, and 38% for the TTFC. All three measures of nicotine dependence were correlated (r = .75–.84) but were non-normally distributed in terms of skewness and kurtosis. However, tests of the proportional odds assumption were non-significant (p > .05), indicating that the NMR predicted uniformly across all categories and was a good predictor for ordered categories.
Table 1.
Characteristics of the Study Sample (N = 833)
| Variable | N (%), M (SD) | ||||
|---|---|---|---|---|---|
|
| |||||
| Men | Women | African American | Caucasian | Total | |
| Age | 45.7 (10.6) | 43.4 (10.8) | 46.7 (10.2) | 45.7 (11.0) | 46.0 (10.7) |
| Race | |||||
| Caucasian | 141 (32.3) | 152 (38.3) | 293 (35.2) | ||
| African American | 295 (67.7) | 245 (61.7) | 540 (64.8) | ||
| Marital Status | |||||
| Single | 137 (31.5) | 128 (32.2) | 139 (47.4) | 126 (23.4) | 265 (31.8) |
| Married/Living as married | 196 (45.1) | 150 (37.8) | 74 (25.3) | 272 (50.5) | 346 (41.6) |
| Divorced/Separated | 97 (22.3) | 97 (24.4) | 69 (23.6) | 125 (23.2) | 194 (23.2) |
| Widowed | 5 (1.2) | 22 (5.5) | 11 (3.8) | 16 (3.0) | 27 (3.3) |
| Education | |||||
| Some grade/high school | 32 (7.4) | 32 (8.1) | 39 (13.3) | 28 (5.2) | 64 (7.7) |
| High School Graduate/GED | 99 (22.8) | 85 (21.4) | 88 (30.0) | 96 (17.8) | 184 (22.0) |
| Some College/Technical School | 187 (43.0) | 163 (41.1) | 114 (38.9) | 236 (43.8) | 352 (42.2) |
| College Graduate | 117 (26.9) | 117 (29.5) | 52 (17.8) | 182 (33.8) | 234 (28.0) |
| Sex | |||||
| Female | 152 (51.9) | 245 (45.4) | 397 (47.7) | ||
| Male | 141 (48.1) | 295 (54.6) | 436 (52.3) | ||
| Cigarettes per Day | 19.6 (9.8) | 17.7 (6.6) | 16.6 (8.4) | 19.8 (8.3) | 18.7 (8.5) |
| Age Started Smoking | 16.3 (4.4) | 16.4 (4.1) | 16.9 (4.6) | 16.1 (4.0) | 16.4 (4.3) |
| NMR | 0.36 (.19) | 0.42 (.22) | 0.33 (.20) | 0.42 (.20) | 0.39 (0.21) |
| Rate of Nicotine Metabolism | |||||
| Slow (≤ 0.26) | 143 (32.8) | 94 (23.7) | 135 (46.1) | 102 (18.9) | 237 (28%) |
| Normal (> 0.26) | 293 (67.2) | 303 (76.3) | 158 (53.9) | 438 (81.1) | 596 (72%) |
| FTND | |||||
| < 5 | 145 (33.4) | 114 (28.7) | 82 (31.7) | 177 (32.9) | 259 (31.2) |
| ≥ 5 | 289 (66.6) | 283 (71.3) | 211 (72.0) | 361 (67.1) | 572 (68.8) |
| HSI | |||||
| < 4 | 243 (55.9) | 210 (52.9) | 165 (56.3) | 288 (53.4) | 453 (54.5) |
| ≥ 4 | 192 (44.1) | 187 (47.1) | 128 (43.7) | 251 (56.6) | 379 (45.5) |
| TTFC | |||||
| ≥ 6 minutes | 288 (66.2) | 228 (57.4) | 153 (52.2) | 363 (67.4) | 516 (62.0) |
| ≤ 5 minutes | 147 (33.8) | 169 (42.6) | 140 (47.8) | 176 (32.7) | 316 (38.0) |
| FTND CPD | |||||
| ≤ 10 | 31 (7.1) | 50 (12.6) | 51 (17.4) | 30 (5.6) | 81 (9.7) |
| 11–20 | 275 (63.2) | 255 (64.2) | 201 (68.6) | 329 (61.0) | 530 (63.7) |
| 21–30 | 106 (24.4) | 84 (21.2) | 33 (11.3) | 157 (29.1) | 190 (22.8) |
| >30 | 23 (5.3) | 8 (2.0) | 8 (2.7) | 23 (4.3) | 31 (3.7) |
Note. FTND = Fagerström Test for Nicotine Dependence; NMR = nicotine metabolite ratio; HSI = Heaviness of Smoking Index; TTFC = Time to first cigarette.
Nicotine Metabolism and Nicotine Dependence across Sex and Race
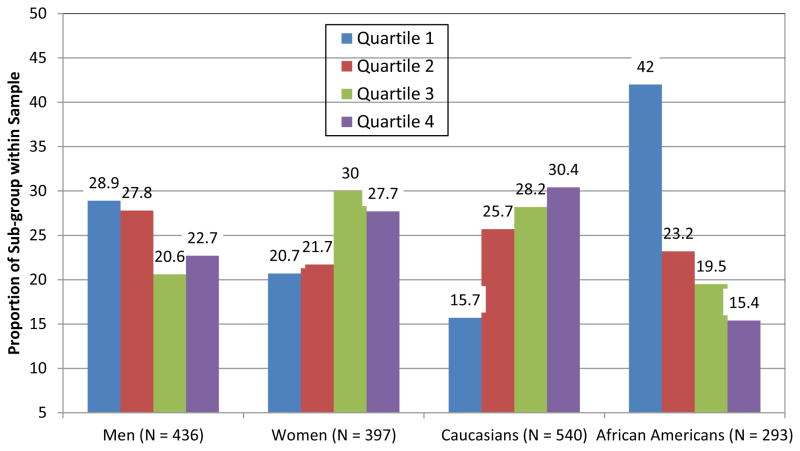
Both sex and race were associated with the NMR. Women had higher mean NMR than men (t[831] = −3.40, p < .001) and Caucasians had higher mean NMR than African Americans (t[831] = −7.53, p < .001; see Table 1). The distribution of quartiles across sex and race, showing that women (χ2[3] = 18.04, p < .001) and Caucasians (χ2[3] = 75.64, p < .001) were significantly more likely to be represented in the higher NMR quartiles, is shown in Figure 1. Based on the 0.26 cut-off, women were more likely to be normal metabolizers of nicotine than men (76% vs. 67%; χ2[1] = 8.49, p = .004) and Caucasians were more likely to be normal metabolizers of nicotine than African Americans (81% vs. 54%; χ2[1] = 69.0, p < .001).
Figure 1.
Distribution of NMR Quartiles within each Sex and Race Sub-Group
Note. The distribution of the quartiles within each sex and race sub-group showing women, vs. men, with a greater proportion of faster metabolizers and Caucasians, vs. African Americans, with a greater proportion of faster metabolizers.
The relationship between sex and nicotine dependence was not consistent. Sex was not associated with nicotine dependence measured by the FTND (t[823] = −1.08, p = .14) or the HSI (t[828] = 0.37, p =.65) but it was associated with TTFC (OR = .31; 95% CI: .05–.57, p = .02). As shown in Table 1, women were more likely to have high nicotine dependence defined by TTFC within 5 minutes of waking, vs. men (43% vs. 34%; χ2[1] = 7.05, p = .008). Likewise, race was not related to nicotine dependence measured by the FTND (t[636] = .70, p = .76) or the HSI (t[832] = −1.25, p =.11) but it was related to TTFC (OR = −.29; 95% CI: −.42–.15, p < .001). As seen in Table 1, African Americans were more likely to have high nicotine dependence defined as TTFC within 5 minutes of waking, vs. Caucasians (48% vs. 33%; χ2[1] = 18.78, p < .001).
The Association Between Nicotine Metabolism and Nicotine Dependence
Separate linear regression models assessing the relationship between the log NMR and each indicator of nicotine dependence indicated that nicotine metabolism was not associated with the FTND (β = .01; 95% CI: −.21–.23, p = .94) or TTFC (β =.03; 95% CI: −.06–.13, p = .49) but it was associated with the HSI (β =.16; 95% CI: .03–.30, p =.014)4. When sex and race were included in the models, NMR was still not associated with FTND (β = .01; 95% CI: −.22–.24, p = .92) or TTFC (β = .07; 95% CI: −.03–.17, p = .16) but the significant association with HSI remained (β = .16; 95% CI: .02–.27, p = .024). As shown in Table 2, normal metabolizers of nicotine were more likely to have high nicotine dependence measured by the HSI than slow nicotine metabolizers (48% vs. 40%; χ2[1] = 4.64, p = .03). The linear regression models, which included terms for main and interaction effects, indicated no significant interactions between NMR and sex or race for any measure of nicotine dependence (p’s > .05).
Table 2.
Rate of Nicotine Dependence Across Slow and Normal Metabolizers of Nicotine, Overall and by Sex and Race
| FTND | HSI | TTFC | FTND CPD | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| < 5 | ≥ 5 | χ2 | p | < 4 | ≥ 4 | χ2 | p | ≥ 6 mins | ≤ 5 mins | χ2 | p | ≤10 | 11–20 | 21–30 | > 30 | χ2 | p | |
| Overall | ||||||||||||||||||
| Slow | 34% | 66% | 1.53 | .22 | 60% | 40% | 4.64 | .03 | 63% | 37% | 0.10 | .75 | 15% | 63% | 20% | 2% | 13.3 | .004 |
| Normal | 30% | 70% | 52% | 48% | 62% | 38% | 8% | 64% | 24% | 5% | ||||||||
| Men | ||||||||||||||||||
| Slow | 41% | 59% | 5.24 | .02 | 66% | 34% | 8.42 | .004 | 69% | 31% | 0.87 | .35 | 12% | 66% | 20% | 2% | 12.6 | .006 |
| Normal | 30% | 70% | 51% | 49% | 65% | 35% | 5% | 62% | 26% | 7% | ||||||||
| Women | ||||||||||||||||||
| Slow | 24% | 76% | 1.09 | .30 | 52% | 48% | 0.03 | .86 | 53% | 47% | 0.91 | .34 | 19% | 60% | 20% | 1% | 5.2 | .16 |
| Normal | 30% | 70% | 53% | 47% | 59% | 41% | 11% | 66% | 21% | 2% | ||||||||
| Caucasian | ||||||||||||||||||
| Slow | 37% | 63% | 0.79 | .38 | 60% | 40% | 2.05 | .15 | 69% | 31% | 0.09 | .76 | 11% | 57% | 31% | 1% | 9.9 | .02 |
| Normal | 32% | 68% | 52% | 43% | 67% | 33% | 4% | 62% | 29% | 5% | ||||||||
| African American | ||||||||||||||||||
| Slow | 33% | 67% | 2.64 | .10 | 61% | 39% | 1.99 | .16 | 59% | 42% | 3.98 | .05 | 18% | 68% | 12% | 2% | 0.3 | .95 |
| Normal | 24% | 76% | 53% | 47% | 47% | 53% | 17% | 69% | 11% | 3% | ||||||||
Note. FTND = Fagerström Test for Nicotine Dependence; HSI = Heaviness of Smoking Index; TTFC = Time to first cigarette; CPD Cigarettes per day.
Table 2 shows the relationship between NMR and nicotine dependence using categorical cut-offs for the measures, overall and across race and sex. For men, there was a significant relationship between NMR and nicotine dependence for both the FTND and the HSI but not for TTFC. Male normal metabolizers of nicotine were more likely to show high nicotine dependence than male slow metabolizers of nicotine using the FTND (70% vs. 59%; χ2[1] = 5.24, p = .02) and the HSI (49% vs. 34%; χ2[1] = 8.42, p = .004). In contrast, for women, the NMR was not associated with nicotine dependence for any of the three measures. Lastly, for Caucasians, the NMR was not associated with nicotine dependence for any of the three measures. For African Americans, normal metabolizers of nicotine were more likely to exhibit high nicotine dependence than slow metabolizers of nicotine, but only when nicotine dependence was defined using the TTFC (53% vs. 42%; χ2[1] = 3.98, p = .05). Logistic regression models that included main and interaction effects for NMR, sex, and race were consistent with these results. In the model predicting high nicotine dependence based on the FTND, the main effect for sex (OR = 2.11, 95% CI: 1.18–3.77, p = .01) and the sex by NMR interaction effect (OR = 0.45, 95% CI: 0.23–0.89, p = .02) were significant. Likewise, in the model predicting high nicotine dependence based on the HSI, the main effect for sex (OR = 1.77, 95% CI: 1.04–3.03, p = .04) and the sex by NMR interaction effect (OR = 0.52, 95% CI: 0.28–0.97, p = .04) were significant.
Post-hoc Assessment Using the FTND Measure of Cigarettes per Day
Since the difference between the TTFC and HSI is the inclusion of the FTND item assessing cigarettes per day (FTND CPD; i.e., ≤ 10, 11–20, 21–30, ≥ 31), additional analyses were conducted. An ordinal logistic regression, predicting the FTND CPD item with log NMR was significant (OR = 0.25; 95% CI: .01–.50, p = .04), even when including sex and race in the model. There were no significant log NMR interactions with sex and race (p’s > .05), but main effects for sex (OR = −0.43; 95% CI: −.72 to −.15, p = .003) and race (OR = 1.01; 95% CI: .73–1.40, p < .001) were detected. Men and Caucasians reported smoking significantly more cigarettes per day (based on the FTND CPD item), than women and African Americans (see Table 1). When using the binary measure for nicotine metabolism, NMR was associated with the FTND CPD item (χ2[3] = 13.3, p = .004) and this relationship was evident for men and Caucasians (p’s < .02). However, there were no main or interaction effects found when these relationships were assessed in a multiple regression.
Discussion
The present study sought to examine whether the lack of relationship between nicotine metabolism, represented by the NMR, and nicotine dependence, measured by the FTND, reported in numerous previous studies could be attributable to a lack of consideration for the potential influence of sex and race and the common use of the FTND to define nicotine dependence. The results suggest that, in part, methodological and demographic issues may play a role in explaining past findings concerning the NMR and nicotine dependence. In particular, the results indicate that this relationship may be detected when nicotine dependence is defined by the HSI, overall, and that this relationship may be more robust in men. While West et al. (2011) suggested that the FTND reflects more of the behavioral aspects of tobacco use and addiction, whereas the NMR represents both behavioral and physiological aspects of tobacco use and addiction, the present results suggest an association when the HSI is used and categorical definitions for dependence and metabolism are utilized. However, post-hoc analyses for the FTND CPD item indicate that the NMR may also be considered an indicator of smoking rate, rather than as a broad measure of nicotine dependence.
The present study replicated past findings concerning the relationship between sex, race, and nicotine metabolism (e.g., Schnoll et al., 2009; Kandel et al., 2007). With regard to sex, the increased nicotine metabolism among women is likely attributable to sex hormones, with past studies showing that women using oral contraceptives show higher rates of nicotine metabolism than women who are not taking oral contraceptives (Benowitz et al., 2006).5 With regard to race, as with past studies (e.g., Kandel et al., 2007; St. Helen et al., 2012), Caucasians were more likely to be normal metabolizers of nicotine, compared to African Americans. These findings are consistent with previous studies showing that the frequency of CYP2A6 alleles, which influence variability in nicotine metabolism, vary across racial/ethnic groups (Binnington et al., 2012; Nakajima et al., 2006). Likewise, the present study showed that women and African Americans report higher levels of nicotine dependence, however this result was confined to the use of the TTFC measure of nicotine dependence. This result is consistent with studies concerning race (Hu et al., 2006; Schnoll et al., 2013) and sex (Goodwin et al., 2011; Schnoll et al., 2013) and further highlight the need to consider these characteristics in studies concerning nicotine dependence.
The assessment of the relationship between the NMR and measures of nicotine dependence, without consideration of race or sex, in the linear regression models, showed that these phenotypes were associated only when the HSI was used. Like many previous studies (e.g., Benowitz et al., 2003; Johnstone et al., 2006), the present study found no relationship between the NMR and nicotine dependence when measured by the FTND. Likewise, TTFC was not associated with the NMR. However, normal metabolizers of nicotine were more likely to have high nicotine dependence vs. slow metabolizers of nicotine when the HSI was used in linear and logistic regression models and in association analyses. This measure of nicotine dependence, which comprises smoking rate and TTFC, may be the measure that reflects the dimension of nicotine dependence that most highly converges with variability in the rate of nicotine metabolism. Interestingly, the HSI was previously found to be the optimal measure of nicotine dependence since it more accurately determined nicotine dependence than the TTFC (Schnoll et al., 2013). Relative to the FTND, the HSI may be more sensitive to an association with NMR since it excludes items that, given evolving public policy, have become less predictive of smoking behavior (e.g., smoking bans in public places). Likewise, the inclusion of smoking rate by the HSI may enhance the predictive value of this measure relative to TTFC. Yet, it is worth emphasizing that the post-hoc analyses concerning the CPD measure suggests that the NMR may simply be more reflective of smoking rate, rather than broader indices of nicotine dependence considered, including the HSI and the TTFC. Past studies have reported the significant association between NMR and smoking rate (Lerman et al., 2006; Schnoll et al., 2009) so it is also plausible that null findings concerning the NMR-nicotine dependence link are attributable to the lack of consideration of smoking rate within the measure of nicotine dependence.
Nevertheless, sex and race may need to be considered when assessing the relationship between the rate of nicotine metabolism and level of nicotine dependence (differences were noted even with the FTND CPD measure). In the association analysis and the regression models using categorical definitions for nicotine metabolism and nicotine dependence, significant sex by NMR interaction effects were found for both the FTND and the HSI. Among men, normal metabolizers of nicotine were more likely to have high nicotine dependence when using the FTND and the HSI (perhaps excluding cigarettes per day from the TTFC leads to a lack of association). In contrast, among women, regardless of the measure of nicotine dependence used, the NMR was not associated with nicotine dependence. These findings would suggest that, for men, nicotine metabolism and nicotine dependence represent a converging dimension of tobacco use and dependence but, for women, nicotine metabolism and nicotine dependence represent divergent aspects of tobacco use. Such sex differences speak to the issues of non-nicotine factors being more relevant to women than men in assessing nicotine dependence and are consistent with studies that have documented differences between men and women with regard to the self-reported reasons for smoking, the reinforcing qualities of smoking, responses to nicotine dependence treatments (including cessation, side effects, and adherence), responsiveness to smoking cues, and how genetic factors influence response to pharmacotherapies for nicotine dependence (Schnoll & Patterson, 2009; Perkins, 2001; 2009). For race, there was some indication that nicotine metabolism is associated with nicotine dependence (measured by the TTFC) for African Americans but not for Caucasians. However, the race x NMR terms within the regression models were not significant. On the one hand, these results suggest that race may influence the relationship between nicotine metabolism and nicotine dependence. But, on the other hand, in the present sample of relatively heavier smokers (≥ 10 cigarettes/day), these aspects of tobacco use and addiction may play similar roles for both racial groups. Additional research, especially with a more representative sample of African American smokers (since many are light smokers), is needed to more fully assess the role of race in this context.
Our findings should be considered in the context of limitations, including the use of self-report data for measures of nicotine dependence, the correlational analyses, the inclusion of treatment-seeking smokers, and the exclusion of other racial/ethnic groups. Further, given that the data were ascertained from a clinical trial with inclusion/exclusion criteria (e.g., currently smoking ≥ 10 cigarettes per day), especially exclusions related to the possible use of varenicline (e.g., about 18% of those screened were considered ineligible due to psychiatric co-morbidity), the generalizability of our findings may be limited. This may be particularly relevant concerning race since a relatively large proportion of African Americans are light smokers (i.e., report smoking < 10 cigarettes per day; Trinidad et al., 2009). Further, this study was restricted to examining three measures of nicotine dependence and, thus, it is unclear how using additional multi-factorial measures of nicotine dependence would influence the results and conclusions. Utilizing a sample from a clinical trial may also have yielded distributions for the measures of nicotine dependence that were non-normal, which can influence the results from the regression analyses. The present analyses should be repeated in a sample comprised of the general population of smokers so that measures used to define nicotine dependence will be more normally distributed (e.g., no cut-off for CPD). It is also worth mentioning that certain analyses used cut-offs based on convention or single studies and that these cut-offs may not represent the most ideal for differentiating nicotine dependence and nicotine metabolism. Lastly, a limitation of the approach taken is the possibility of type 1 error due to multiple testing. A Bonferonni correction would have reduced alpha to .03, in which case, certain models would not be statistically significant.
Nonetheless, the results of the present study suggest that a more complete understanding of the relationship between nicotine metabolism and nicotine dependence may require consideration of sex, race, and the measure used to define level of nicotine dependence. The HSI may be the most sensitive measure to detect this relationship, overall, and this relationship may be more robust in men. Yet, this finding does not necessarily indicate that the HSI is a superior measure of nicotine dependence, which is a complex construct that may be assessed using a broad range of criteria. Indeed, it is notable that smoking rate, as measured by the FTND CPD item, was likewise associated with NMR, and this finding suggests that the NMR may be associated primarily with this smoking phenotype rather than with broader measures of nicotine dependence. The overlap in variance between the NMR and the FTND CPD item, nevertheless, was relatively modest (~2%). Thus, while previous studies have shown the NMR to be predictive of cessation in men and women and in Caucasians and African Americans (e.g., Lerman et al., 2006; Ho et al., 2009; Schnoll et al., 2009), the ongoing prospective cessation trial from which the present data were ascertained will allow for further exploratory analyses of the predictive validity of the NMR for determining treatment response among specific subgroups of smokers. Thus, overall, the present findings help to broaden our understanding of the NMR and may help guide future research concerning nicotine metabolism and measures of nicotine dependence.
Acknowledgments
The authors would like to thank the participants and the following individuals who assisted in the implementation of this research project: Angela Pinto, Elissa Kranzler, Paul Sanborn, and Maria Novalen.
Footnotes
It is worth noting that Dr. Karl Fagerström recommends that the FTND be renamed the Fagerström Test for Cigarette Dependence; see Fagerström, 2012.
Since the distributions for the measures of nicotine dependence were non-normal, these analyses were also performed using ordinal regression and the results were identical.
In the present analysis, menopausal women had a significantly lower mean NMR vs. non-menopausal women. Menopausal status was not related to nicotine dependence and results from regression models were the same if menopausal status was considered.
Conflicts of Interest
Pfizer provided the study medication and placebo for this study free of charge but had no role in the current analyses or manuscript preparation. Drs. Schnoll, Dr. Tyndale, and Dr. George have served as consultants to pharmaceutical companies which make smoking cessation products.
References
- Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharm Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine & Tobacco Research. 2003;5:621–624. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- Binnington MJ, Zhu AZ, Renner CC, Lanier AP, Hatsukami DK, Benowitz NL, Tyndale RF. CYP2A6 and CYP2B6 genetic variation and its association with nicotine metabolism in South Western Alaska Native people. Pharmacogen Genomics. 2012;22:429–440. doi: 10.1097/FPC.0b013e3283527c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough KJ, Lerman C, Rose JE, et al. Biomarkers for smoking cessation. Clin Pharm Ther. 2013;93:526–538. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, Benowitz NL. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nic Tobacco Res. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Kinnunen T, Nordstrom BL, et al. Effects of nicotine gum dose by level of nicotine dependence. Nicotine Tob Res. 2000;2:53–63. doi: 10.1080/14622200050011303. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Pagura J, Spiwak R, Lemeshow AR, Sareen J. Predictors of persistent nicotine dependence among adults in the United States. Drug Alc Dep. 2011;118:127–33. doi: 10.1016/j.drugalcdep.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KA. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Ho M, Mwenifumbo J, Al Koudsi N, Okuyem K, Ahluwalia J, Benowitz N, Tyndale R. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85:635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Amer J Pub Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone E, Benowitz N, Cargill A, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharm Ther. 2006;80:319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Hu MC, Schaffran C, Udry JR, Benowitz NL. Urine nicotine metabolites and smoking behavior in a multiracial/multiethnic national sample of young adults. Amer J Epi. 2007;165:901–910. doi: 10.1093/aje/kwm010. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans T, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alc Dep. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Director J, Harford MA. Tobacco dependence and time to first cigarette of the day. Addict Beh. 1981;6:307–312. [Google Scholar]
- Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30:386–389. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharm Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Fukami T, Yamanaka H, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharm Ther. 80:282–297. doi: 10.1016/j.clpt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yamamoto T, Nunoya K, et al. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos. 1996;24:1212–1217. [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, et al. Toward personalized therapy for smoking cessation: A randomized placebo-controlled trial of bupropion. Clin Pharm Ther. 2008;84:320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- Pérez-Ríos M, Santiago-Pérez MI, Alonso B, Malvar A, Hervada X, de Leon J. Fagerstrom test for nicotine dependence vs. heavy smoking index in a general population survey. BMC Pub Health. 2009;30:493. doi: 10.1186/1471-2458-9-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Neb Symp Mot. 2009;55:143–169. doi: 10.1007/978-0-387-78748-0_9. [DOI] [PubMed] [Google Scholar]
- Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenetics. 2009;23:52–61. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Leone F. Biomarkers to optimize the treatment of nicotine dependence. Biomarkers Med. 2011;5:745–761. doi: 10.2217/bmm.11.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F. Sex heterogeneity in pharmacogenetic smoking cessation clinical trials. Drug Alc Dep. 2009;104S:S94–S99. doi: 10.1016/j.drugalcdep.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Tyndale R, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: A validation study. Pharm Biochem Beh. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Goren A, Annunziata K, Suaya J. Prevalence, predictors, and potential impact on health, productivity, and healthcare use of high nicotine dependence among adult smokers in the United States. Addict. 2013 doi: 10.1111/add.12285. [DOI] [PubMed] [Google Scholar]
- StHelen G, Dempsey D, Wilson M, Jacob P, 3rd, Benowitz NL. Racial differences in the relationship between tobacco dependence and nicotine and carcinogen exposure. Addict. 2013;108:607–617. doi: 10.1111/j.1360-0443.2012.04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Jacob P, 3rd, Benowitz NL. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2012;21:1105–1114. doi: 10.1158/1055-9965.EPI-12-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nic Tob Res. 2009;11:203–210. doi: 10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West O, Hajek P, McRobbie H. Systematic review of the relationship between the 3-hydroxycotinine/cotinine ratio and cigarette dependence. Psychopharmacology. 2011;21:313–322. doi: 10.1007/s00213-011-2341-1. [DOI] [PubMed] [Google Scholar]