Abstract
Estrogens play an important role in the regulation of normal physiology, aging and many disease states. Although the nuclear estrogen receptors have classically been described to function as ligand-activated transcription factors mediating genomic effects in hormonally regulated tissues, more recent studies reveal that estrogens also mediate rapid signaling events traditionally associated with G protein-coupled receptors. The G protein-coupled estrogen receptor GPER (formerly GPR30) has now become recognized as a major mediator of estrogen’s rapid cellular effects throughout the body. With the discovery of selective synthetic ligands for GPER, both agonists and antagonists, as well as the use of GPER knockout mice, significant advances have been made in our understanding of GPER function at the cellular, tissue and organismal levels. In many instances, the protective/beneficial effects of estrogen are mimicked by selective GPER agonism and are absent or reduced in GPER knockout mice, suggesting an essential or at least parallel role for GPER in the actions of estrogen. In this review, we will discuss recent advances and our current understanding of the role of GPER and certain drugs such as SERMs and SERDs in physiology and disease. We will also highlight novel opportunities for clinical development towards GPER-targeted therapeutics, for molecular imaging, as well as for theranostic approaches and personalized medicine.
Keywords: 17 -estradiol; Cardioprotection; Cardiovascular; Endothelium; endoplasmic reticulum, Endothelin, eNOS; Endothelial Nitric Oxide Synthase, epidermal growth factor receptor, EGFR, extracellular signal-regulated kinase, ERK; Endothelium; endothelial cells; ER; ER; ER; ERE; Estradiol; Estrogen; Estrogen Receptor; Genomic Signaling; GPCR; GPER; GPR30; MAPK; Non-Genomic Signaling; Personalized Medicine; Phosphatidylinositol-3-OH Kinase; PI3K; Plasma Membrane; Raloxifene; SERM; SERD; Tamoxifen; Theranostics; Vascular Smooth Muscle Cell
1. Introduction
Historically, cellular responses to estrogens and estrogenic compounds, as well as other steroids, have been described in terms of the “classical” nuclear receptors and in the case of estrogen, the estrogen receptors α(ERα) (King and Greene, 1984; Green et al., 1986; Greene et al., 1986) and β (ERβ) (Kuiper et al., 1996). These receptors mediate cellular effects on gene expression known as “genomic” signaling, through the formation of receptor homo- or heterodimers, and binding to estrogen response elements (ERE) in the promoter and regulatory regions of target genes (Edwards, 2005). Nuclear ERs (even in the absence of ligand binding or direct binding to DNA) also interact with other classes of transcription factors and DNA modifying proteins through complex protein-protein interactions, thus regulating gene expression and cell function (Schultz-Norton et al., 2011). Such mechanisms are the topic of many of the articles in this special issue.
In addition to genomic cellular effects, estrogen, as well as other steroids, mediates a variety of “rapid” cellular responses to physiological concentrations of estrogens, responses that occur on a time frame of seconds to minutes, inconsistent with de novo transcription and protein synthesis (Falkenstein et al., 2000). In fact, some of the earliest cellular effects of estrogen were rapid effects on cAMP synthesis (Szego and Davis, 1967) and calcium mobilization (Pietras and Szego, 1975). These rapid estrogen-mediated effects are transmitted via enzymatic pathways and ion channels through the activation of what are generically denoted as membrane-associated ERs (mER), and are referred to as “non-genomic” or “extra-nuclear” pathways (Fu and Simoncini, 2008; Levin, 2009). It should however be noted that any absolute distinction between genomic and non-genomic effects is rather arbitrary as many intracellular signaling pathways result in the modulation of gene expression (Ho et al., 2009). As a result, the combination of these multiple cellular actions allows for the fine-tuning of estrogen-mediated regulation of gene expression (Bjornstrom and Sjoberg, 2005). In addition, ERs also undergo extensive post-translational modifications including phosphorylation, acetylation, sumoylation and palmitoylation that modulate their function (Anbalagan et al., 2012). Thus, the ultimate cellular response to estrogen stimulation results from a complex interplay of transcriptional and non-transcriptional events.
In addition to the classical nuclear estrogen receptors, a now extensive body of literature over the last ~10 years has identified and characterized the functions of a 7-transmembrane spanning G protein-coupled receptor, GPER (previously named GPR30), predominantly in the rapid actions of estrogen (Filardo et al., 2000; Prossnitz et al., 2008a; Prossnitz et al., 2008b; Prossnitz and Barton, 2011; Filardo and Thomas, 2012), although effects on gene expression have also been described (Prossnitz and Maggiolini, 2009; Vivacqua et al., 2012). GPER was identified by a number of laboratories between 1996-1998 as an orphan receptor with no known ligand, and thus named GPR30, belonging to the family of 7-transmembrane spanning G protein-coupled receptors. The receptor cDNA was identified from multiple sources including B lymphocytes (Owman et al., 1996; Kvingedal and Smeland, 1997), ER-positive breast cancer cells (Carmeci et al., 1997), human endothelial cells exposed to fluid shear stress (Takada et al., 1997) as well as database mining (O’Dowd et al., 1998) and degenerate oligonucleotide screening of genomic DNA (Feng and Gregor, 1997). However, in 2000, pioneering studies by Filardo and colleagues demonstrated that the expression of GPER was required for the rapid estrogen-mediated activation of ERK1/2 (Filardo et al., 2000) and subsequently in 2002 cAMP generation (Filardo et al., 2002). In 2005, estrogen binding to GPER was demonstrated by multiple groups (Revankar et al., 2005; Thomas et al., 2005) and in 2006, the first GPER-selective agonist was described (Bologa et al., 2006). This and the subsequent identification of GPER-selective antagonists (Dennis et al., 2009; Dennis et al., 2011) led to an increasing number of studies addressing the potential cellular and physiological functions of GPER. To date, functions for GPER have been described in almost every physiological system, including reproductive, endocrine, urinary, nervous, immune, musculoskeletal and cardiovascular (Prossnitz and Barton, 2011). Thus, combined with the actions of estrogen through the classical ERs, GPER serves to add to the complexity of mechanisms involved in the physiological responses to estrogen.
Endogenous estrogens are protective for multiple diseases prior to menopause (Rettberg et al., 2013), not the least of which are cardiovascular disease and atherosclerosis, based in part on the beneficial effects of estrogen on blood pressure and cholesterol profiles (Meyer et al., 2011b). In addition to beneficial metabolic effects (e.g. cholesterol regulation (Faulds et al., 2012)), estrogens exert multiple direct beneficial effects on the heart and arterial wall, including vasodilation, inhibition of smooth muscle cell proliferation, inhibition of inflammation, antioxidant effects, and endothelial/cardiac cell survival following injury (Meyer et al., 2006; Meyer and Barton, 2009; Meyer et al., 2009; Knowlton and Lee, 2012). Although nuclear ERs contribute to several of these effects, presumably by regulating ERE-containing genes, the actions of non-nuclear ERα have also been demonstrated (Chambliss et al., 2010; Wu et al., 2011; Banerjee et al., 2013). However, more recent studies have demonstrated that GPER also activates multiple signaling pathways in cardiovascular and immune cells that either acutely regulate cellular function, or possibly modulate gene expression through ERE-independent mechanisms (Lindsey and Chappell, 2011; Prossnitz and Barton, 2011; Filardo and Thomas, 2012; Han et al., 2013). In this review, we will discuss recent advances in our understanding of how GPER contributes to the beneficial effects of estrogen in the cardiovascular and immune systems with an outlook to possible clinical applications.
2. GPER Ligands and Signaling Mechanisms
2.1 GPER Ligands
Following the seminal report of rapid estrogen signaling requiring the expression of GPER in 2000 (Filardo et al., 2000), many studies have demonstrated specific estrogen binding to cells (or membranes from cells) expressing GPER. In 2005, Thomas et al. reported selective binding of tritiated estradiol to membrane preparations of cells endogenously expressing or transfected to express GPER, with an affinity of ~3 nM (Thomas et al., 2005). In that same year, Revankar et al. utilized a fluorescent estrogen derivative to demonstrate specific estrogen binding to various cancer cell lines and GPER-transfected COS cells with an affinity of ~6 nM, which was directly proportional to the level of receptor expression (Revankar et al., 2005). It should be noted that since GPER, like most GPCRs, has not yet been purified to homogeneity, such studies provide a correlation between ligand binding and presence of GPER, although imaging studies with fluorescent estrogen derivatives demonstrate strong colocalization of receptor and fluorescent estrogen, suggesting GPER likely represents the site of estrogen binding. Since these original studies, many other groups have similarly reported estrogen binding to GPER-expressing cells or membrane preparations (Wang et al., 2008b; Liu et al., 2009; Lappano et al., 2010). Importantly, within the steroid family, estrogen binding to GPER exhibits high selectivity (>1000×) over testosterone, cortisol, and progesterone (Thomas et al., 2005), and although studies have suggested a requirement for GPER expression in certain rapid actions of aldosterone (Ding et al., 2009; Gros et al., 2011), a recent study was unable to detect aldosterone binding in membranes when estrogen binding was readily demonstrable (Cheng et al., 2013). Furthermore, although estrone and 17α-estradiol show poor binding activity towards GPER (Thomas et al., 2005), estriol at μM concentrations has been reported to act as an antagonist (Lappano et al., 2010), and 2-methoxy-estradiol has recently been reported to bind to GPER (Koganti et al., 2013).
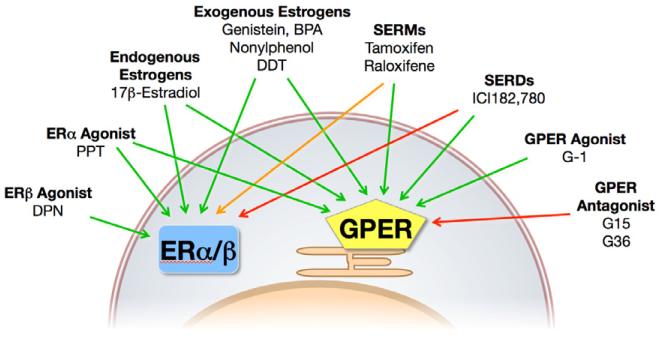
With the wealth of synthetic and natural estrogen substances in existence, it is perhaps not surprising that a large number have been shown to interact with GPER. Of the therapeutic anti-estrogens, ICI182,780 (a selective estrogen receptor downregulator, SERD) was first demonstrated to interact with GPER, but surprisingly, as opposed to its antagonistic action towards ERα/β, ICI182,780 acted as an agonist towards GPER (Filardo et al., 2000), with subsequent binding studies revealing an affinity of ~30 nM (Fig. 1) (Thomas and Dong, 2006). Similarly, 4-hydroxytamoxifen (the active metabolite of tamoxifen, a selective estrogen receptor modulator, SERM) also acts as a GPER agonist (Revankar et al., 2005; Vivacqua et al., 2006b), and recently raloxifene has also been demonstrated to activate GPER in cells deficient for ERα (Petrie et al., 2013), consistent with the actions of a series of benzothiophene SERMs in neuroprotection (Abdelhamid et al., 2011). Many synthetic compounds from the pesticide and plastics industries known to have estrogenic effects have also been demonstrated to bind and/or activate GPER, including atrazine (Albanito et al., 2008), bisphenol A (Dong et al., 2011; Chevalier et al., 2012; Pupo et al., 2012; Sheng et al., 2013), daidzein (Kajta et al., 2013), zearalonone, nonphenol, kepone, p,p’-DDT, o,p’-DDE and 2,2′,5′,-PCB-4-OH (Thomas and Dong, 2006). Finally, a number of phytoestrogens display agonist activity towards GPER, including genistein (Maggiolini et al., 2004; Thomas and Dong, 2006; Vivacqua et al., 2006a), quercetin (Maggiolini et al., 2004), equol (Rowlands et al., 2011), resveratrol (Dong et al., 2013), oleuropein, and hydroxytyrosol (Chimento et al., 2013).
Figure 1.
Ligands of GPER and ERs. Agonists (green arrows), partial agonists (SERMS, orange arrow) and antagonists (red arrows) of GPER and the classical estrogen receptors ER and ER. SERMS and SERDs that f. SERMS and SERDs that function as ER antagonists in many tissues act as agonists of GPER. GPER-selective ligands (G-1, G15 and G36) are highly selective for GPER over ERs. Modified from (Meyer et al., 2011b), with permission of Elsevier Publishers.
New synthetic and screening approaches have identified a number of compounds that display selectivity among the various estrogen receptors (ERα, ERβ, and GPER). The first GPER-selective ligand identified was G-1, which displays an affinity for GPER of ~10 nM with no significant binding or function at either ERα or ERβ at concentrations as high as 10 μM (Bologa et al., 2006; Dennis et al., 2011). Subsequent studies identified a related compound lacking the ethanone moiety, G15, which functioned as a highly selective GPER antagonist (Dennis et al., 2009). As a result of low levels of activity against ERα or ERβ at concentrations of ~10 μM, a derivative (G36) was synthesized that restored the steric bulk of G-1, maintaining antagonism while restoring the ER counterselectivity to levels similar to those of G-1 (Dennis et al., 2011). Together these three compounds have now been used in over 150 publications to study the effects of GPER in systems from cells to tissues and organs to live animals and have served as the basis of GPER-selective radioimaging agents (Nayak et al., 2010; Ramesh et al., 2010; Burai et al., 2012). STX, a diphenylacrylamide analog of tamoxifen, had been demonstrated to exert rapid neurological effects through a Gq protein-coupled mechanism unrelated to classical ERs (Qiu et al., 2003), suggesting GPER might be involved. However, although the effects of STX persist in a GPER knockout mouse (as well as in ERα/ERβ double knockout mice) (Qiu et al., 2008), STX has been demonstrated to mimic estrogen-mediated effects on the SF-1 transcription factor by GPER (Lin et al., 2009). MIBE (ethyl 3-[5-(2-ethoxycarbonyl-1-methylvinyloxy)-1-methyl-1H-indol-3-yl] but-2-enoate) has been described as an antagonist of both ERα and GPER, although the affinity for both receptors is greater than 10 μM (Lappano et al., 2012b). Two additional compounds, GPER-L1 and GPER-L2 have been reported to function as GPER-selective agonists, with binding affinities of ~100 nM (Lappano et al., 2012a). Interestingly, the widely used ERα-selective (410-fold over ERβ) agonist PPT has recently been shown also to be capable of acting as an agonist of GPER (at concentrations of 10-100 nM), while the ERβ-selective agonist DPN displayed no corresponding activity towards GPER (up to concentrations of 10 μM) (Petrie et al., 2013). Thus although PPT remains selective for ERα over both ERβ and GPER, care needed in the interpretation of results employing receptor-selective ligands, particularly with respect to the concentrations and doses of these compounds used (Washburn et al., 2013). Nevertheless, the increasing spectrum of GPER/ER-modulating compounds suggests that many new opportunities exist in their experimental use and perhaps clinical development.
Although the amino acid sequence of GPER bears homology to the chemokine receptor subfamily of GPCRs (Owman et al., 1996; Feng and Gregor, 1997), early studies found no activation in response to a panel of chemokines (Owman et al., 1996). However, there is evidence to suggest that a chemokine (CCL-18) modulates GPER activity, possibly through direct binding to GPER (Feng and Gregor, 1997). Thus, CCL-18 could act as an endogenous inhibitor of GPER, similar to the actions of IL-1ra, which attenuates the activation of IL-1R by IL-1β (Dripps et al., 1991).
2.2 GPER-mediated Signaling Mechanisms
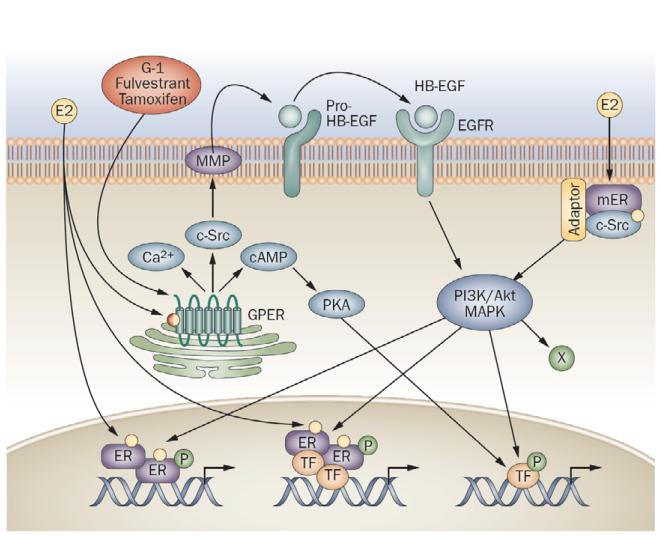
In the original report of estrogen-mediated rapid signaling by GPER, Filardo et al. (Filardo et al., 2000) described a mechanism of ERK1/2 activation by GPER employing transactivation of the EGFR (Fig. 2) (Daub et al., 1996). Consistent with early events of G protein-coupled receptor-mediated signaling, GPER acted via a Gβγ-dependent, pertussis toxin-sensitive pathway, implying the involvement of Gαi/o proteins. However, inhibition of EGFR kinase activity or downregulating HB-EGF with CRM-197 or scavenging free HB-EGF with antibodies also prevented estrogen-mediated signaling, suggesting the involvement of a transactivation of the EGFR by metalloproteinase-mediated release of HB-EGF, a mechanism subsequently confirmed by many studies (Revankar et al., 2005; Peyton and Thomas, 2011; Li et al., 2013b). Furthermore, a role for fibronectin-recruited α5β1 integrin has been implicated in the transactivation of the EGFR (Quinn et al., 2009) as has a role for sphingosine kinase (Sukocheva et al., 2006). In addition to these pathways, GPER has also been demonstrated to mediate Gαs protein activation (Thomas et al., 2005) leading to adenylyl cyclase activation and cAMP accumulation (Filardo et al., 2002), which further leads to PKA activation (Zucchetti et al., 2013) and transcriptional activation of CREB (Kanda and Watanabe, 2004) as well as vascular effects (Lindsey et al., 2013b) among other sequellae. Additional downstream pathways reported to be activated by GPER include PI3K (Revankar et al., 2005; Petrie et al., 2013), PKC (Goswami et al., 2011), calcium mobilization (Revankar et al., 2005; Tica et al., 2011), and other ion channels (Fraser et al., 2010; Goswami et al., 2011). Interestingly, many studies have observed that a vast majority of GPER is expressed in intracellular membranes (Revankar et al., 2005; Otto et al., 2008; Filardo and Thomas, 2012), with little expressed at the cell surface in most cells and cell lines likely due to both inefficient export mechanisms, which involve receptor activity-modifying protein 3 (RAMP3) (Bouschet et al., 2012; Lenhart et al., 2013), as well as constitutive GPER internalization (Cheng et al., 2011a; Cheng et al., 2011b). Furthermore, differentially permeable estrogen derivatives reveal that membrane permeability of the ligand is required for rapid signaling by GPER, suggesting the intracellular pool is functional (Revankar et al., 2007). A pathological role for RAMP3 (Receptor Activity-modifying Protein 3) in the trafficking of GPER has been demonstrated through the G-1-mediated attenuation of cardiac hypertrophy and perivascular fibrosis in a mouse model prone to heart disease (Lenhart et al., 2013). Finally, analyses of serum-stimulated human endothelial cells identified PALS1-associated tight junction protein and FUN14 domain containing 2 as GPER-interacting partners, with functional or trafficking roles yet to be described (Zazzu, 2011).
Figure 2.
Signaling pathways initiated by GPER and ERs. Endogenous estrogens, including 17 -estradiol (E2), represent nonselective activators of the three known ERs, ER, ER and GPER. Estrogen activates nuclear ERs, inducing dimerization of the receptors and binding of receptor dimers to target gene promoters. Alternatively, activated ERs modulate the function of other classes of transcription factors (TFs) through protein-protein interactions. Subpopulations of ERs at the plasma membrane following activation by estrogen interact with adaptor proteins (adaptor) and signaling molecules such as c-src, which mediates rapid signaling via PI3K/Akt and MAPK pathways. GPER, which is predominantly localized intracellularly, can be activated by estrogen, or selective GPER agonists (such as G-1), but also by selective estrogen receptor downregulators (such as fulvestrant), or selective estrogen receptor modulators (such as tamoxifen and raloxifene). GPER activation stimulates cAMP production, calcium mobilization and c-src, which activates MMPs. These MMPs cleave pro-HB-EGF, releasing free HB-EGF that transactivates EGFR, which in turn activates MAPK and PI3K/Akt pathways that can induce additional rapid (nongenomic) effects (X), or genomic effects regulating gene transcription. Estrogen-mediated transcriptional regulation may involve phosphorylation (P) of ER or other TFs that may directly interact with ER, or bind independently of ER within the promoters of target genes. Abbreviations: E2, 17 - estradiol; EGFR, epidermal growth factor receptor; ER, estrogen receptor; GPER, G-protein-coupled ER; MMP, matrix metalloproteinase; pro-HB-EGF, pro-heparin-binding epidermal growth factor; TF, transcription factor. Figure reproduced from (Prossnitz and Barton, 2011).
Beyond rapid signaling effects, GPER also regulates gene expression, as demonstrated by some of the earliest studies on genomic signaling by GPER (Kanda and Watanabe, 2003a; Kanda and Watanabe, 2003b; Kanda and Watanabe, 2004; Maggiolini et al., 2004; Ylikomi et al., 2004). These and subsequent studies revealed the upregulation of the immediate early gene c-fos (and other genes) by estrogen (O’Brien et al., 2006; Vivacqua et al., 2006b; Albanito et al., 2007; Prossnitz and Maggiolini, 2009), as had been demonstrated almost 20 years earlier in the rat uterus (Weisz and Bresciani, 1988) and rat aortic vascular smooth muscle (Orimo et al., 1993). In addition, estrogen and the GPER-selective agonist have been demonstrated to upregulate the expression of cell cycle regulators such as cyclins A, D1 and E (Albanito et al., 2007), connective tissue growth factor (Pandey et al., 2009), Early growth response-1 (Vivacqua et al., 2012), and VEGF (De Francesco et al., 2013), in many cases through EGFR- and ERK-dependent mechanisms. The latter mechanism is also involved in the cleavage of cyclin E, associated with tumor progression and resistance to anti-estrogens, and induced by tamoxifen and G-1 in GPER-expressing cells (Li et al., 2013b). Interestingly, recent studies by Maggiolini and colleagues have suggested that GPER localization to the nucleus via an importin-dependent mechanism is involved in its transcriptional activity (Pupo et al., 2013). In addition, GPER in a complex with EGFR has been localized to the cyclin D1 promoter (Madeo and Maggiolini, 2010). How two integral membrane proteins are localized to DNA within the nucleus is an intriguing question and in need of further investigation.
3. Interactions of GPER with Other Receptors
3.1 Lipid Signaling via Nuclear Steroid Hormone Receptors: Interactions of GPER
Estrogen Receptor (ER)
Co-expression of ERα and ERβ with GPER suggests the possibility of interactions between these receptors and their signaling pathways (Prossnitz and Barton, 2011). Functional cross-talk has been reported, where GPER expression is required along with ERα for estrogen-mediated activity in cancer cells (Albanito et al., 2007) or for inhibiting ERα-mediated functions in uterine epithelial cells (Gao et al., 2011). Functional cross-talk between ERα and GPER is also evident from functional vascular studies in porcine coronaries, where acute NO-dependent vasodilation is observed only with ERα-selective agonists such as PPT, but is completely abrogated when ERα, ERβ, and GPER are activated simultaneously by estrogen (Traupe et al., 2007). The recent discovery that responses to PPT, which is often employed as an ERα-selective agonist (relative to ERβ), also activated GPER complicates our understanding of PPT-mediated responses (Petrie et al., 2013), as they may, at least in part, involve GPER, depending on the receptors expressed and concentrations used. A redundancy for the receptors mediating effects of estrogen is also suggested by work by Mauvais-Jarvis and colleagues (Tiano et al., 2011), who reported that the protective effects of estrogen on pancreatic islets are comparable and independent of selective activation of ERα, ERβ, or GPER (Tiano et al., 2011). Thus, cross-talk between GPER and estrogen receptors (and possibly other steroid receptors) appears to be likely and should be considered when assessing possible pathogenic and therapeutic questions related to activation or inhibition of estrogen receptors (Barton, 2012). Receptor cross-talk and functional redundancies are likely to be important for understanding the actions of drugs targeting either one or several estrogen receptors. ERα-36, a membrane-localized splice variant of the human ER α identified in breast cancer cells as a dominant negative effector of full length ERα (Wang et al., 2005), has also been proposed as an effector of GPER-dependent signaling (Wang et al., 2005; Wang et al., 2006). Its function remains controversial and in vivo data in particular are scarce. Lindsey and coworkers reported renoprotective effects of G-1 but did not detect ERα-36 (whereas ERα-46 and ERα-66 were abundantly expressed) (Lindsey et al., 2011). Thus, in vivo evidence for a direct role of ERα-36 in mediating the effects of GPER is still lacking. Whether ERα-36 has indirect effects such as cross-talk involving GPER remains to be determined. Finally, the orphan nuclear receptor estrogen receptor-related receptor α (ERRα) has been implicated in GPER-mediated signaling as GPER activation causes transcriptional activation of ERRα protein synthesis and regulates ERRα-mediated downstream effects and cell proliferation (Li et al., 2010).
Glucocorticoid Receptor (GR)
Glucocorticoids are important regulators of energy and bone metabolism (Tisdale, 2002; Meyer et al., 2011a) and a role for GPER in maintaining metabolism has been suggested as GPER activation reduces food intake (Washburn et al., 2013) and stimulates insulin secretion (Sharma and Prossnitz, 2011), whereas GPER-deficient mice are obese and insulin-resistant (Haas et al., 2009; Ford et al., 2011; Meyer et al., 2011a; Sharma et al., 2013). GPER has also been demonstrated to negatively regulate the transcriptional activity of glucocorticoid receptor (Ylikomi et al., 2004) and may thereby also indirectly affect energy metabolism, expenditure, and overall energy homeostasis and body weight through GR activity.
Mineralocorticoid Receptor (MR)
Aldosterone, a potent vasoconstrictor and mitogen synthesized by the adrenal gland and by vascular smooth muscle cells (Matsuzawa et al., 2013), has been proposed to act through GPER for certain functions (Gros et al., 2011; Gros et al., 2013). Although aldosterone does not bind to GPER (Cheng et al., 2013), thus appearing to exclude the possibility of GPER being a true aldosterone receptor, it is possible that, similar to what has been reported for estrogen receptors, a functional cross-talk between MR and GPER exists. This likely also underlies the recently reported vagal tone-activating effect of central aldosterone infusion (Brailoiu et al., 2013). On the other hand, since both deletion of vascular MR (McCurley et al., 2012; Schiffrin, 2013; Pruthi et al., 2014) and pharmacological activation of GPER (Haas et al., 2009) lower blood pressure, whereas aldosterone in most studies has the contrary effect by causing vasoconstriction and thereby increasing blood pressure, these effects would argue against a role for aldosterone as a main co-factor mediating GPER-dependent effects. Finally, since aldosterone is produced via AT1 receptor-dependent mechanisms (Volpe et al., 1997), the inhibitory effect of GPER activation on AT1 receptor expression (Koganti et al., 2013) may represent a novel mechanism for how aldosterone and GPER interact.
Vitamin D Receptor (VDR)
As a secosteroid, vitamin D (cholecalciferol) maintains bone function and growth through activation of the vitamin D receptor (VDR) (Norman and Powell, 2014). GPER expression can be detected in human bone (Heino et al., 2008) and GPER-dependent regulation of bone growth has been reported (Heino et al., 2008; Ford et al., 2011; Ren and Wu, 2012). An inflammation-dependent interaction between vitamin D receptor signaling and GPER is strongly suggested by the estrogen-dependent inhibitory effects of vitamin D on autoimmune encephalitis, an animal model of multiple sclerosis, where the effect of vitamin D is completely abrogated in female mice lacking GPER (Subramanian et al., 2012). GPER/VDR interactions are also likely to be involved in the beneficial effects of vitamin D on bone structure in postmenopausal women, which have been shown to be estrogen-dependent (Durusu Tanriover et al., 2010).
3.2 Other Receptors Interacting with GPER
In the ovary, GPER function is dependent on the transmembrane GPCR mediating the effects of luteinizing hormone, a heterodimeric glycoprotein, which shares the same α-subunit as FSH, TSH, TSH, and hCG (Dufau, 1998). Luteinizing hormone was recently shown to regulate GPER expression at the mRNA and protein level in human granulosa cells (Pavlik et al., 2011). Interactions and cross-talk between GPER and growth factor receptors, particularly EGFR and IGFR, have been recently summarized by Maggiolini and colleagues (Bartella et al., 2012; Lappano et al., 2013). In addition, functions of GPER may be affected, both in health and disease, by transcription factor-induced reprogramming of its expression pattern, which has been recently described for ERα in breast cancer (Ross-Innes et al., 2012).
4. New Aspects of GPER Function in the Cardiovascular System
The vascular physiology of GPER, including the initial studies demonstrating a role in the regulation of vascular tone and protection from myocardial reperfusion injury, has been recently reviewed in a number of articles (Fig. 3) (Meyer et al., 2011b; Chakrabarti et al., 2013; Han et al., 2013; Holm and Nilsson, 2013). GPER was originally cloned as an orphan GPCR after induction by fluid shear stress in human vascular endothelial cells, as well as from different cancer cell lines (Prossnitz and Barton, 2011). Shear stress is a strong inducer of expression of the enzyme responsible for production of the vasodilator nitric oxide (NO) (Fleming, 2010), suggesting that GPER function might be linked to the L-arginine/NO pathway (Meyer et al., 2011b). Indeed, numerous laboratories have demonstrated NO- and endothelium-dependent vasodilation via activation of GPER, effects that are abrogated by endothelial denudation or treatment with NO synthase inhibitors such as L-NAME (Haynes et al., 2002; Moriarty et al., 2006; Meyer et al., 2011b; Barton et al., 2013; Lindsey et al., 2013b). Several recent studies have provided further insights into how GPER functions in vascular endothelial cells. In both cerebrovascular as well as acute kidney injury, estrogen-mediated protection is in part mediated via GPER in both males and females (Hutchens et al., 2012; Murata et al., 2013). In mesenteric resistance arteries, which dilate partly via endothelium-dependent hyperpolarization, vasodilator responses via GPER involve endothelium-derived NO (Lindsey et al., 2013b). Finally, GPER has been identified as an inhibitory regulator of pro-inflammatory proteins in endothelial cells (Chakrabarti and Davidge, 2012) and consistent with this, chronic G-1 treatment improves endothelium-dependent vasomotion in disease conditions associated with vascular inflammation, such as diabetes mellitus (Li et al., 2012).
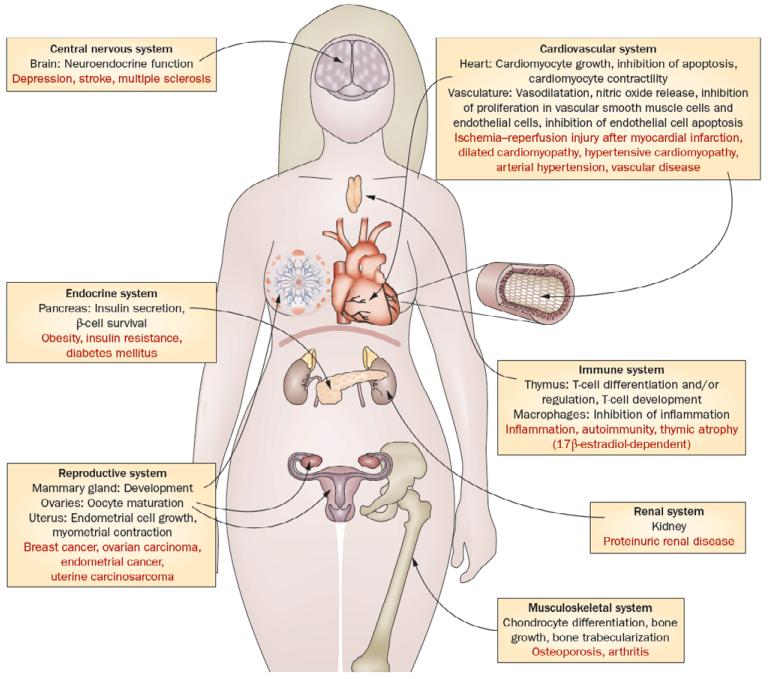
Figure 3.
Current experimental and clinical evidence implicates a physiological role (shown in black) for GPER in neuroendocrine and cerebral functions, immunity and immune cell function, metabolic and endocrine regulation, vascular, myocardial, and kidney function, as well as development and reproductive functions. In addition, data obtained in experimental models of disease and/or tissue from patients with disease suggest roles for GPER in pathological conditions (shown in red), such as diabetes mellitus, arterial hypertension, proteinuric renal disease, osteoporosis, arthritis, immune diseases, such as multiple sclerosis, and cancer. Targeting GPER activity with highly selective ligands in humans may represent a novel approach for the treatment of these conditions, for molecular imaging, as well as for theranostic approaches and personalized medicine. Figure reproduced from (Prossnitz and Barton, 2011).
Rapid, non-genomic effects of estrogen, an agonist for ERα, ERβ, and GPER, have been demonstrated in human coronary and internal mammary arteries of male and female patients (Mugge et al., 1993; Haas et al., 2007), suggesting a possible role for GPER in regulating vascular and organ function of males. Indeed, vascular smooth muscle internal mammary arteries of male patients express GPER (Haas et al., 2007) and GPER-dependent effects in arteries and organs of animals of both sexes have been demonstrated (Prossnitz and Barton, 2011). Pharmacological stimulation of GPER utilizing G-1 has no effect in intact arteries or renal cells derived from GPER null mice (Haas et al., 2009; Hofmeister et al., 2012), confirming the specificity of this ligand-receptor interaction. Pharmacological inhibition of GPER utilizing antagonists such as G15 results in vasoconstriction (Meyer et al., 2012a); (Lindsey et al., 2009; Yu et al., 2011), suggesting that endogenous GPER has vasodilatory actions. However, the lack of hypertension in GPER knockout mice suggests (yet unidentified) counter-regulatory mechanisms facilitating vasodilation, possibly involving central (Brailoiu et al., 2012), cardiac, or renal effects or alternatively modulating responses in the autonomous nervous system.
Using mice deficient for GPER, several recent studies performed in male mice have revealed that endogenous GPER functions as an endogenous inhibitor of the activity of endothelium-dependent contracting factor (EDCF) (Meyer et al., 2012a), as well as the newly discovered adipose-derived contracting factor (ADCF) formed in perivascular fat (Meyer et al., 2013), with both the EDCF and the ADCF vasoconstrictor pathways being entirely dependent on cyclooxygenase activity. As cyclooxygenase-derived vasoconstrictor prostanoids have been shown to accelerate progression of experimental atherosclerosis (Belton and Fitzgerald, 2003), endogenous GPER might represent a novel mechanism protecting from atherosclerosis. Atheroprotective mechanisms via endogenous GPER might also involve interference with adipogenesis, inflammation, and dyslipidemia, three of the key factors strongly implicated in atherogenesis (Barton, 2013), since male GPER-deficient mice exhibit increased adipogenesis, inflammatory activation, and increased circulating lipid levels (Sharma et al., 2013). GPER deficiency results not only in visceral obesity but also in perivascular adipose formation (Meyer et al., 2013), potentially involving a direct inhibitory effect of GPER on mitotic clonal expansion of adipocytes (Zhu et al., 2013). Unlike activation of ERα, which stimulates endothelial cell proliferation, angiogenesis, and wound healing (Haynes et al., 2002; Kim and Bender, 2009), selective agonists for GPER inhibit endothelial cell proliferation, effects that have also been demonstrated in studies using ICI 182,780 and genistein, agonists of GPER (Filardo et al., 2000; Petrie et al., 2013), to interfere with endothelial cell proliferation (Holm et al., 2011).
GPER is highly expressed in vascular smooth muscle cells (Haas et al., 2009), a key cell required for the development of atherogenesis (Barton et al., 2007). In human arteries, as well as cultured human vascular smooth muscle cells, expression of GPER can be detected (Haas et al., 2007; Haas et al., 2009) and its activation results in cell growth inhibition (Haas et al., 2009), with comparable effects demonstrated in porcine coronary vascular smooth muscle cells (Li et al., 2013a). In rat vascular smooth muscle cells, GPER is expressed at a comparable level in both males and females, and at a much higher level than ERβ (Ma et al., 2010). Activation of vascular smooth muscle cell GPER also results in rapid ERK phosphorylation (Haas et al., 2009; Gros et al., 2011), an effect enhanced by adenoviral overexpression of GPER (Gros et al., 2011). GPER expression also interferes with the contractile response to agonists for other GPCRs, including endothelin receptors (Meyer et al., 2010) and in intact arteries, endogenous GPER suppresses endothelin-mediated changes in intracellular calcium and endothelin-mediated vasoconstriction, as the response is enhanced in GPER knockout mice (Meyer et al., 2012b). The role of GPER in the regulation of intracellular calcium has now been confirmed in cultured vascular smooth muscle cells, demonstrating an involvement of voltage-sensitive calcium changes (Holm et al., 2013). Moreover, a role for estrogen-dependent GPER-mediated calcium mobilization has been demonstrated in micro-dissected renal tubules and cortical collecting ducts, effects that were absent in GPER knockout mice (Hofmeister et al., 2012). These findings corroborate a novel and previously unknown role for GPER in non-genomic effects of estrogens on intracellular calcium.
Recent reports have extended our knowledge about GPER functions in vascular smooth muscle and regulation of arterial tone. In mesenteric resistance arteries, which partly dilate via endothelium-dependent hyperpolarization, Lindsey and colleagues have recently identified vascular smooth muscle adenylyl cyclase/cAMP signaling as contributing to relaxation (Lindsey et al., 2013b). A role for GPER/G-1-mediated EGFR transactivation, as observed in many cancer cells (Filardo et al., 2000), in vascular relaxation is supported by a recent study showing that two different EGFR antagonists abolished the relaxant effects of G-1 (Jang et al., 2013).
Several factors appear to affect vascular GPER function. Aging in normotensive and hypertensive rats results in a downregulation of GPER in both males and females, and is associated with a reduced relaxation to G-1 or E2 (Lindsey et al., 2013a). As has been shown for other agonists, estrogen receptor-selective agonists display a marked anatomic heterogeneity in arteries of female rodents, with some vessels responding negligibly while others show rapid relaxation in response to activation of ERs or GPER (Reslan et al., 2013). A similar heterogeneity has recently been reported to be present in human uterine and placental arteries (Corcoran et al., 2013). The implications of such heterogeneity for vascular protection and susceptibility to disease development have been recently discussed (Barton et al., 2013). Activation of GPER is also protective to the heart, as it improves diastolic function and left ventricular remodeling in hypertensive rats (Wang et al., 2012) and ameliorates cardiac function in a model of isoproterenol-induced heart failure (Kang et al., 2012). Preliminary reports have also demonstrated that GPER activity, but not that of ERα or ERβ, is critical in the protective effects of estrogen on myocardial reperfusion injury (Bopassa et al., 2011; Bopassa et al., 2012a; Bopassa et al., 2012b) with the identification of multiple differentially up- and down-regulated mitochondrial proteins in GPER KO mice (Bopassa et al., 2013). Finally, responses to treatment with G-1 may show a sexual dimorphism, as recently reported by Broughton et al. in a stroke model (Broughton et al., 2014) and by Caron’s group using a genetic model of cardiac hypertrophy and fibrosis (Lenhart et al., 2013).
5. GPER in Immunity and Inflammation
Estrogen and, as more recently demonstrated, drugs commonly used as “anti-estrogens”, exert multiple effects upon the development and function of the immune system (Ray and Ficek, 2012; Bonds and Midoro-Horiuti, 2013; Sakiani et al., 2013), as exemplified by the developmental effects of estrogen in estrogen-promoted atrophy of the thymus during pregnancy (Pernis, 2007). Expression of GPER in multiple immune cells, including B and T cells, monocytes/macrophages and neutrophils, suggested that some estrogenic effects in the immune system could be mediated by GPER (Wang et al., 2008a; Blasko et al., 2009; Rettew et al., 2010; Cabas et al., 2013). A role for GPER in estrogen-mediated thymic atrophy was first suggested employing ERα, ERβ, and GPER knockout mice (Wang et al., 2008a). Whereas ERα was shown to mediate exclusively the early developmental blockage of thymocyte development, GPER was required for thymocyte apoptosis that preferentially occurs in T cell receptor double-positive thymocytes. Furthermore, G-1 induced thymic atrophy and thymocyte apoptosis, but not a developmental blockage. Although supported by an increased apoptosis rate for naïve T cells in GPER knockout mice (Isensee et al., 2009) distinct from those used in the original study (Wang et al., 2008a), others, however, have not observed similar effects with G-1 (Otto et al., 2008) or in ovariectomized GPER knockout mice (Martensson et al., 2009) distinct from the other two GPER knockout mice employed, suggesting further clarification is necessary.
Estrogens have also more recently received increased attention as potential anti-inflammatory/anti-apoptotic therapeutic agents for stroke, reperfusion injury associated with myocardial infarction, and autoimmune diseases, particularly multiple sclerosis (Niino et al., 2009). Employing a widely used murine model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), estrogen-mediated protection was significantly decreased in GPER knockout mice (Wang et al., 2009), whereas in studies that employed G-1 treatment with the EAE model, protection against clinical and hi stological manifestations of EAE was similar to that of estrogen (Blasko et al., 2009; Wang et al., 2009). Importantly, the effects of G-1 were completely absent in GPER knockout mice, demonstrating a requirement for GPER, and as the protective effects of estrogen were partially lost, this suggests that estrogen acts through independent yet overlapping mechanisms via both ERα and GPER, that can be completely mimicked through G-1 activation of GPER in the absence of ERα-mediated effects (Wang et al., 2009). Whereas G-1 was demonstrated in one study to enhance the suppressive activity of CD4+Foxp3+ T regulatory cells through upregulation of programmed death 1 (Wang et al., 2009), in another study G-1 was observed to inhibit inflammatory cytokine production of macrophages (Blasko et al., 2009). These data are consistent with a more recent study that has also demonstrated the estrogen-induced inhibition of LPS-induced TNFα production in bone marrow-derived macrophages is mediated by GPER through calcium signaling but not ERα activation (Liu et al., 2013). In addition, the therapeutic effect of ethinyl estradiol in established disease was demonstrated to require expression of GPER but not ERα, and was associated with the production of the anti-inflammatory cytokine IL-10 (Yates et al., 2010). Demonstrating this effect explicitly, G-1 was shown to elicit de novo IL-10 production in pro-inflammatory Th17-polarized cells in vitro as well as in vivo following G-1 administration (Brunsing and Prossnitz, 2011). Furthermore, using ex vivo cultures of purified CD4+ T cells, G-1 elicited Foxp3 expression (a marker of natural and induced regulatory T cells) under Th17-polarizing conditions, which exist in most autoimmune diseases (Brunsing et al., 2013). Lastly, GPER has recently been shown to modulate vertebrate (fish) granulocyte (neutrophil) functions, including reducing the respiratory burst and pro- and anti-inflammatory gene expression through a cAMP/protein kinase A/CREB-mediated pathway (Cabas et al., 2013). Together, these reports suggest that endogenous GPER conveys immunoprotective effects, which, like the direct generally anti-inflammatory effects of G-1 on the immune system, are multifaceted and complex.
As many cardiovascular diseases involve st rong inflammatory and immune components (e.g. atherosclerosis (Moore et al., 2013) and reperfusion injury (Frangogiannis, 2006)), the functions of GPER could have additional important roles, beyond the direct effects on the endothelium and smooth muscle discussed above, in the development and progression of these conditions. Estrogen has long been recognized for its anti-atherogenic effects, reflecting prevention of atherosclerosis through inhibition of inflammation and endothelial/vascular dysfunction (Resanovic et al., 2013), and in the early stages of atherosclerosis, where estrogen-dependent protective effects are independent of ERα (Villablanca et al., 2009). One recently reported additional direct immunomodulatory effect of GPER in the endothelium is the attenuation of TNFα-induced upregulation of pro-inflammatory adhesion proteins such as ICAM-1 and VCAM-1 (Chakrabarti and Davidge, 2012), likely to result in decreased leukocyte adherence, important in atherogenesis and other vascular diseases (Golias et al., 2007). GPER deficiency has also been shown to result in insulin resistance, dyslipidemia, obesity, and increased circulating pro-inflammatory cytokines (Haas et al., 2009; Sharma et al., 2013), all likely to contribute to vascular dysfunction and disease (Meyer and Barton, 2009; Meyer et al., 2011b), suggesting that GPER contributes in important ways to a healthy metabolism and inflammatory state. Immune effects via GPER have also been shown to be involved in the reduction of infarct size and improvement of peripheral immunosuppression following stroke with estrogen deficiency in female mice (Zhang et al., 2010). Similar effects following myocardial infarction are possible and thus, in addition to the direct cardioprotective effects of G-1 observed in myocardial ischemia-reperfusion models involving activation of PI3K/Akt and ERK1/2 (Deschamps and Murphy, 2009; Bopassa et al., 2010; Patel et al., 2010; Weil et al., 2010), GPER may play additional important roles in the immune system that might have profound effects on the course of diseases with a strong underlying inflammatory component, such as atheroclerotic vascular disease (Meyer and Barton, 2009).
6. Outlook and Potential Clinical Applications
It has been almost 20 years since the first report identifying and cloning GPR30 (Owman et al., 1996), yet only over the past few years, with the publication rate of scientific reports on GPER now exceeding 10 per month on average, has our understanding of GPER’s cellular functions in physiology and disease truly begun to advance. GPR30 was officially designated G protein-coupled estrogen receptor (GPER) by the International Union of Basic and Clinical Pharmacology in 2007 based on the preponderance of results demonstrating estrogen action through GPER (Alexander et al., 2011). However, despite academic laboratories providing molecular and pharmacological tools (Huryn et al., 2013) as well as GPER knockout mice (Wang et al., 2008a; Martensson et al., 2009), deciphering the unique roles of GPER as an estrogen receptor in health and disease remains challenging due to the complexity of physiological responses to estrogen and the multiple receptors and interacting partners involved. These issues have been further complicated at times by seemingly controversial research findings, leading to premature conclusions (Tiano et al., 2011; Barton, 2012), such as statements that GPER is “a receptor for aldosterone, but not estrogen” (Funder, 2011). The field of non-genomic (“non-classical”) effects mediated by ERα and its many splice variants with varying cellular localization, as well as the multitude of ligands with widely varying effects depending on cellular context, has experienced similar difficulties (Heldring et al., 2007; Kim et al., 2011). Further complications arise from sex differences in GPER and sex steroid receptor expression and function (Barton, 2012; Miller, 2014), only now beginning to be investigated (Lenhart et al., 2013; Broughton et al., 2014).
In the future, the measurement of GPER expression levels, its cellular and tissue distribution, or the use GPER agonists or antagonists may provide new approaches in diagnostic, prognostic, or theranostic evaluation (Smith et al., 2007; Smith et al., 2009), drug therapy, or molecular imaging in disease (Fowler, 2014). With regard to clinical effects mediated by GPER, it is now clear that previous views must be reassessed regarding SERMs, such as raloxifene, and SERDs, such as ICI182,780 (Fulvestrant or Faslodex®). Instead of acting solely as ER-modulating agents (Barton, 2012), these drugs, known and clinically used as cancer therapeutics, also have the potential to act as agonists for GPER in vivo. The fact that GPER activation causes vasodilation is consistent with the hypotensive side effects observed in some patients receiving Faslodex® (Vergote and Abram, 2006). The understanding of fulvestrant action has however been further complicated by the recent finding that this compound may also act as an ERα agonist when the activation function-2 (AF-2) of ERα is mutated (Borjesson et al., 2011; Moverare-Skrtic et al., 2014). Finally, the question as to whether polymorphisms of the GPER gene, which maps to chromosome 7p22, a locus also associated with resistant arterial hypertension in genetic linkage studies in humans (Lafferty et al., 2000; Haas et al., 2009), may be associated with changes in cardiovascular disease risk, is beginning to be addressed in arterial hypertension (Feldman et al., 2013) as is the relationship of GPER polymorphisms to human seminoma risk (Chevalier et al., 2014). Finally, information related to GPER (e.g. responses to certain treatments, expression in tumors (van’t Veer and Bernards, 2008; Venkatakrishnan et al., 2013)) may also be used for theranostic purposes that could provide optimization of current treatments and allow personalized medicine for many diseases (Shastry, 2006), including cancer and cardiovascular diseases (Ozdemir et al., 2006; Offit, 2011).
Acknowledgments
Funding
Original work by the authors is supported by NIH R01grants CA127731 and CA163890 (E.R.P.) and Swiss National Science Foundation grants 108 258 and 122 504 (M.B.).
Table of Abbreviations
- eNOS
Endothelial nitric oxide synthase
- ERK1/2
Extracellular signal-related kinases-1/2
- ERα
Estrogen receptor alpha
- ERβ
Estrogen receptor beta
- GPER
G protein-coupled estrogen receptor 1
- GPR30
G protein-coupled receptor 30
- MAPK
Mitogen-activated protein kinase
- mER
Membrane-associated estrogen receptor (α or β)
- NO
Nitric oxide
- PI3K
Phosphatidylinositol-3-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
ERP is an inventor on patents for GPER-targeted ligands and imaging agents owned by the University of New Mexico.
References
- Abdelhamid R, Luo J, Vandevrede L, Kundu I, Michalsen B, Litosh VA, Schiefer IT, Gherezghiher T, Yao P, Qin Z, Thatcher GR. Benzothiophene selective estrogen receptor modulators provide neuroprotection by a novel GPR30-dependent mechanism. ACS Chem. Neurosci. 2011;2:256–268. doi: 10.1021/cn100106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanito L, Lappano R, Madeo A, Chimento A, Prossnitz ER, Cappello AR, Dolce V, Abonante S, Pezzi V, Maggiolini M. G-protein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ. Health Perspect. 2008;116:1648–1655. doi: 10.1289/ehp.11297. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br. J. Pharmacol. 2011;164(Suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbalagan M, Huderson B, Murphy L, Rowan BG. Post-translational modifications of nuclear receptors and human disease. Nucl Recept Signal. 2012;10:e001. doi: 10.1621/nrs.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Chambliss KL, Mineo C, Shaul PW. Recent insights into non-nuclear actions of estrogen receptor alpha. Steroids. 2013 doi: 10.1016/j.steroids.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Bartella V, De Marco P, Malaguarnera R, Belfiore A, Maggiolini M. New advances on the functional cross-talk between insulin-like growth factor-I and estrogen signaling in cancer. Cell. Signal. 2012;24:1515–1521. doi: 10.1016/j.cellsig.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Barton M. Position paper: The membrane estrogen receptor GPER--Clues and questions. Steroids. 2012;77:935–942. doi: 10.1016/j.steroids.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Barton M. Prevention and endothelial therapy of coronary artery disease. Curr. Opin. Pharmacol. 2013;13:226–241. doi: 10.1016/j.coph.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Barton M, Meyer MR, Prossnitz ER. Alike but not the same: anatomic heterogeneity of estrogen receptor-mediated vasodilation. J. Cardiovasc. Pharmacol. 2013;62:22–25. doi: 10.1097/FJC.0b013e31829709d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M, Minotti R, Haas E. Inflammation and atherosclerosis. Circ. Res. 2007;101:750–751. doi: 10.1161/CIRCRESAHA.107.162487. [DOI] [PubMed] [Google Scholar]
- Belton O, Fitzgerald DJ. Cyclooxygenase isoforms and atherosclerosis. Expert Rev. Mol. Med. 2003;5:1–18. doi: 10.1017/S1462399403005842. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J. Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr. Opin. Allergy Clin. Immunol. 2013;13:92–99. doi: 10.1097/ACI.0b013e32835a6dd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H16–23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopassa JC, Leeb-Lundberg LF, Olde B, Toro L, Stefani E. Loss of rapid estrogen-Induced cardioprotection in GPER−/− mice after ischemia and reperfusion. Biophys. J. 2011;100:294a–295a. [Google Scholar]
- Bopassa JC, Lu R, Singh H, Olde B, Krust A, Leeb-Lundberg LF, Toro L, Stefani E. G protein-coupled Estrogen Receptor 1, but not Estrogen Receptors alpha and beta, mediates rapid estrogen-induced cardioprotection during ischemia/reperfusion Iinjury in male mice. Biophys. J. 2012a;102:141a. [Google Scholar]
- Bopassa JC, Lu R, Singh H, Olde B, Leeb-Lundberg LF, Toro L, Stefani E. Identification of mitochondrial proteins regulated during activation of GPER1-leading to cardioprotection. Biophys. J. 2013;104:657a. [Google Scholar]
- Bopassa JC, Lu R, Singh H, Zilb erstein NF, Olde B, Krust A, Leeb-Lundberg LF, Toro L, Stefani E. Rapid activation of G protein-coupled Estrogen Receptor 1 protects the heart against ischemia-reperfusion injury by inhibiting the mPTP opening via PKC/AKT/ERK/GSK-3b pathways. Circ. Res. 2012b;111:A213. [Google Scholar]
- Borjesson AE, Windahl SH, Lagerquist MK, Engdahl C, Frenkel B, Moverare-Skrtic S, Sjogren K, Kindblom JM, Stubelius A, Islander U, Antal MC, Krust A, Chambon P, Ohlsson C. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6288–6293. doi: 10.1073/pnas.1100454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouschet T, Martin S, Henley JM. Regulation of calcium sensing receptor trafficking by RAMPs. Adv. Exp. Med. Biol. 2012;744:39–48. doi: 10.1007/978-1-4614-2364-5_4. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Arterburn JB, Oprea TI, Chitravanshi VC, Brailoiu E. Bradycardic effects mediated by activation of G protein-coupled estrogen receptor (GPER) in rat nucleus ambiguus. Exp. Physiol. 2012;98:679–691. doi: 10.1113/expphysiol.2012.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu GC, Benamar K, Arterburn JB, Gao E, Rabinowitz JE, Koch WJ, Brailoiu E. Aldosterone increases cardiac vagal tone via GPER activation. J. Physiol. 2013 doi: 10.1113/jphysiol.2013.257204. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Brait VH, Ah Kim H, Lee S, Chu HX, Gardiner-Mann CV, Guida E, Evans MA, Miller AA, Arumugam TV, Drummond GR, Sobey CG. Sex-Dependent Effects of G Protein-Coupled Estrogen Receptor Activity on Outcome After Ischemic Stroke. Stroke. 2014 doi: 10.1161/STROKEAHA.113.001499. epub. [DOI] [PubMed] [Google Scholar]
- Brunsing RL, Owens KS, Prossnitz ER. The G protein-coupled estrogen receptor (GPER) agonist G-1 expands the regulatory T-cell population under TH17-polarizing conditions. J. Immunother. 2013;36:190–196. doi: 10.1097/CJI.0b013e31828d8e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsing RL, Prossnitz ER. Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology. 2011;134:93–106. doi: 10.1111/j.1365-2567.2011.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burai R, Ramesh C, Nayak T, Dennis MK, Bryant B, Prossnitz ER, Arterburn JB. Synthesis and characterization of tricarbonyl-Re/Tc(I) chelate probes targeting the G protein-coupled estrogen receptor GPER/GPR30. PLoS One. 2012;7:e46861. doi: 10.1371/journal.pone.0046861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabas I, Rodenas MC, Abellan E, Meseguer J, Mulero V, Garcia-Ayala A. Estrogen signaling through the G protein-coupled estrogen receptor regulates granulocyte activation in fish. J. Immunol. 2013;191:4628–4639. doi: 10.4049/jimmunol.1301613. [DOI] [PubMed] [Google Scholar]
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Davidge ST. G-protein coupled receptor 30 (GPR30): a novel regulator of endothelial inflammation. PLoS One. 2012;7:e52357. doi: 10.1371/journal.pone.0052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Morton JS, Davidge ST. Mechanisms of Estrogen Effects on the Endothelium: An Overview. Can. J. Cardiol. 2013 doi: 10.1016/j.cjca.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J. Clin. Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SB, Dong J, Pang Y, Larocca J, Hixon M, Thomas P, Filardo EJ. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol. Cell. Endocrinol. 2013;382:950–959. doi: 10.1016/j.mce.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Graeber CT, Quinn JA, Filardo EJ. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids. 2011a;76:892–896. doi: 10.1016/j.steroids.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Quinn JA, Graeber CT, Filardo EJ. Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-Golgi-proteasome pathway. J. Biol. Chem. 2011b;286:22441–22455. doi: 10.1074/jbc.M111.224071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Bouskine A, Fenichel P. Bisphenol A promotes testicular seminoma cell proliferation through GPER/GPR30. Int. J. Cancer. 2012;130:241–242. doi: 10.1002/ijc.25972. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Paul-Bellon R, Camparo P, Michiels JF, Chevallier D, Fenichel P. Genetic Variants of GPER/GPR30, a Novel Estrogen-Related G Protein Receptor, Are Associated with Human Seminoma. Int J Mol Sci. 2014;15:1574–1589. doi: 10.3390/ijms15011574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento A, Casaburi I, Rosano C, Avena P, De Luca A, Campana C, Martire E, Santolla MF, Maggiolini M, Pezzi V, Sirianni R. Oleuropein and hydroxytyrosol activate GPER/GPR30-dependent pathways leading to apoptosis of ER-negative SKBR3 breast cancer cells. Mol Nutr Food Res. 2013 doi: 10.1002/mnfr.201300323. epub. [DOI] [PubMed] [Google Scholar]
- Corcoran JJ, Nicholson C, Sweeney M, Charnock JC, Robson SC, Westwood M, Taggart MJ. Human uterine and placental arteries exhibit tissue-specific acute responses to 17beta-oestradiol and oestrogen-receptor specific agonists. Mol. Hum. Reprod. 2013 doi: 10.1093/molehr/gat095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini M. HIF-1alpha/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs) Breast Cancer Res. 2013;15:R64. doi: 10.1186/bcr3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J. Steroid Biochem. Mol. Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1806–1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Gros R, Limbird LE, Chorazyczewski J, Feldman RD. Estradiol-mediated ERK phosphorylation and apoptosis in vascular smooth muscle cells requires GPR30. Am. J. Physiol. Cell Physiol. 2009;297:C1178–1187. doi: 10.1152/ajpcell.00185.2009. [DOI] [PubMed] [Google Scholar]
- Dong S, Terasaka S, Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ. Pollut. 2011;159:212–218. doi: 10.1016/j.envpol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Dong WH, Chen JC, He YL, Xu JJ, Mei YA. Resveratrol inhibits K(v)2.2 currents through the estrogen receptor GPR30-mediated PKC pathway. Am. J. Physiol. Cell Physiol. 2013;305:C547–557. doi: 10.1152/ajpcell.00146.2013. [DOI] [PubMed] [Google Scholar]
- Dripps DJ, Brandhuber BJ, Thompson RC, Eisenberg SP. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J. Biol. Chem. 1991;266:10331–10336. [PubMed] [Google Scholar]
- Dufau ML. The luteinizing hormone receptor. Annu. Rev. Physiol. 1998;60:461–496. doi: 10.1146/annurev.physiol.60.1.461. [DOI] [PubMed] [Google Scholar]
- Durusu Tanriover M, Bora Tatar G, Uluturk TD, Dayangac Erden D, Tanriover A, Kilicarslan A, Oz SG, Erdem Yurter H, Sozen T, Sain Guven G. Evaluation of the effects of vitamin D receptor and estrogen receptor 1 gene polymorphisms on bone mineral density in postmenopausal women. Clin. Rheumatol. 2010;29:1285–1293. doi: 10.1007/s10067-010-1548-6. [DOI] [PubMed] [Google Scholar]
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu. Rev. Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol. Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J. Endocrinol. 2012;212:3–12. doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- Feldman RD, Gros R, Hegele RA, Ding Q, Hussain Y, Ban MR, McIntyre AD. A common hypofunctional genetic variant of GPER: Increased blood pressure in female carriers and increased expression in female hypertensive patients. Hypertension. 2013;62:A352. [Google Scholar]
- Feng Y, Gregor P. Cloning of a novel member of the G protein-coupled receptor family related to peptide receptors. Biochem. Biophys. Res. Commun. 1997;231:651–654. doi: 10.1006/bbrc.1997.6161. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frac kelton AR, Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh JT, Clegg D, Zerwekh J, Oz OK. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J. Bone Miner. Res. 2011;26:298–307. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AM. A Molecular Approach to Breast Imaging. J. Nucl. Med. 2014 doi: 10.2967/jnumed.113.126102. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr. Med. Chem. 2006;13:1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Ozerlat-Gunduz I, Onkal R, Diss JK, Latchman DS, Djamgoz MB. Estrogen and non-genomic upregulation of voltage-gated Na(+) channel activity in MDA-MB-231 human breast cancer cells: role in adhesion. J. Cell. Physiol. 2010;224:527–539. doi: 10.1002/jcp.22154. [DOI] [PubMed] [Google Scholar]
- Fu XD, Simoncini T. Extra-nuclear signaling of estrogen receptors. IUBMB Life. 2008;60:502–510. doi: 10.1002/iub.80. [DOI] [PubMed] [Google Scholar]
- Funder JW. Medicine. The genetics of primary aldosteronism. Science. 2011;331:685–686. doi: 10.1126/science.1202887. [DOI] [PubMed] [Google Scholar]
- Gao F, Ma X, Ostmann AB, Das SK. GPR30 Activation Opposes Estrogen-Dependent Uterine Growth via Inhibition of Stromal ERK1/2 and Estrogen Receptor Alpha (ER{alpha}) Phosphorylation Signals. Endocrinology. 2011 doi: 10.1210/en.2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golias C, Tsoutsi E, Matziridis A, Makridis P, Batistatou A, Charalabopoulos K. Review. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo. 2007;21:757–769. [PubMed] [Google Scholar]
- Goswami C, Kuhn J, Dina OA, Fernandez-Ballester G, Levine JD, Ferrer-Montiel A, Hucho T. Estrogen destabilizes microtubules through an ion-conductivity-independent TRPV1 pathway. J. Neurochem. 2011;117:995–1008. doi: 10.1111/j.1471-4159.2011.07270.x. [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Greene G, Krust A, Goffin C, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human oestrogen receptor cDNA. J. Steroid Biochem. 1986;24:77–83. doi: 10.1016/0022-4731(86)90035-x. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am. J. Physiol. Cell Physiol. 2013;304:C532–540. doi: 10.1152/ajpcell.00203.2012. [DOI] [PubMed] [Google Scholar]
- Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension. 2011;57:442–451. doi: 10.1161/HYPERTENSIONAHA.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ. Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- Han G, Li F, Yu X, White RE. GPER: a novel target for non-genomic estrogen action in the cardiovascular system. Pharmacol. Res. 2013;71:53–60. doi: 10.1016/j.phrs.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Li L, Russell KS, Bender JR. Rapid vascular cell responses to estrogen and membrane receptors. Vascul. Pharmacol. 2002;38:99–108. doi: 10.1016/s0306-3623(02)00133-7. [DOI] [PubMed] [Google Scholar]
- Heino TJ, Chagin AS, Savendahl L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J. Endocrinol. 2008;197:R1–6. doi: 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol. Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Ho MK, Su Y, Yeung WW, Wong YH. Regulation of transcription factors by heterotrimeric G proteins. Curr. Mol. Pharmacol. 2009;2:19–31. doi: 10.2174/1874467210902010019. [DOI] [PubMed] [Google Scholar]
- Hofmeister MV, Damkier HH, Christensen BM, Olde B, Fredrik Leeb-Lundberg LM, Fenton RA, Praetorius HA, Praetorius J. 17beta-Estradiol induces nongenomic effects in renal intercalated cells through G protein-coupled estrogen receptor 1. Am. J. Physiol. Renal Physiol. 2012;302:F358–368. doi: 10.1152/ajprenal.00343.2011. [DOI] [PubMed] [Google Scholar]
- Holm A, Baldetorp B, Olde B, Leeb-Lundberg LM, Nilsson BO. The GPER1 Agonist G-1 Attenuates Endothelial Cell Proliferation by Inhibiting DNA Synthesis and Accumulating Cells in the S and G2 Phases of the Cell Cycle. J. Vasc. Res. 2011;48:327–335. doi: 10.1159/000322578. [DOI] [PubMed] [Google Scholar]
- Holm A, Hellstrand P, Olde B, Svensson D, Leeb-Lundberg LM, Nilsson BO. The G protein-coupled estrogen receptor 1 (GPER1/GPR30) agonist G-1 regulates vascular smooth muscle cell Ca(2)(+) handling. J. Vasc. Res. 2013;50:421–429. doi: 10.1159/000354252. [DOI] [PubMed] [Google Scholar]
- Holm A, Nilsson BO. Identification and characterization of new mechanisms in vascular oestrogen signalling. Basic Clin. Pharmacol. Toxicol. 2013;113:287–293. doi: 10.1111/bcpt.12118. [DOI] [PubMed] [Google Scholar]
- Huryn DM, Resnick LO, Wipf P. Contributions of Academic Laboratories to the Discovery and Development of Chemical Biology Tools. J. Med. Chem. 2013;56:7161–7176. doi: 10.1021/jm400132d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens MP, Fujiyoshi T, Komers R, Herson PS, n S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am. J. Physiol. Renal Physiol. 2012;303:F377–385. doi: 10.1152/ajprenal.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- Jang EJ, Seok YM, Arterburn JB, Olatunji LA, Kim IK. GPER-1 agonist G1 induces vasorelaxation through activation of epidermal growth factor receptor-dependent signalling pathway. J. Pharm. Pharmacol. 2013;65:1488–1499. doi: 10.1111/jphp.12113. [DOI] [PubMed] [Google Scholar]
- Kajta M, Rzemieniec J, Litwa E, Lason W, Lenartowicz M, Krzeptowski W, Wojtowicz AK. The key involvement of estrogen receptor beta and G-protein-coupled receptor 30 in the neuroprotective action of daidzein. Neuroscience. 2013;238:345–360. doi: 10.1016/j.neuroscience.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17Beta-estradiol enhances the production of nerve growth factor in THP-1-derived macrophages or peripheral blood monocyte-derived macrophages. J. Invest. Dermatol. 2003a;121:771–780. doi: 10.1046/j.1523-1747.2003.12487.x. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17beta-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J. Invest. Dermatol. 2003b;121:1500–1509. doi: 10.1111/j.1523-1747.2003.12617.x. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17beta-estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. J. Invest. Dermatol. 2004;123:319–328. doi: 10.1111/j.0022-202X.2004.12645.x. [DOI] [PubMed] [Google Scholar]
- Kang S, Liu Y, Sun D, Zhou C, Liu A, Xu C, Hao Y, Li D, Yan C, Sun H. Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS One. 2012;7:e48185. doi: 10.1371/journal.pone.0048185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Mol. Cell. Endocrinol. 2009;308:3–8. doi: 10.1016/j.mce.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Toomre D, Bender JR. Splice isoform estrogen receptors as integral transmembrane proteins. Mol. Biol. Cell. 2011;22:4415–4423. doi: 10.1091/mbc.E11-05-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012;135:54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganti S, Snyder R, Gumaste U, Karamyan VT, Thekkumkara T. 2-Methoxyestradiol binding of GPR30 down-regulates angiotensin AT receptor. Eur. J. Pharmacol. 2013;723C:131–140. doi: 10.1016/j.ejphar.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvingedal AM, Smeland EB. A novel put ative G-protein-coupled receptor expressed in lung, heart and lymphoid tissue. FEBS Lett. 1997;407:59–62. doi: 10.1016/s0014-5793(97)00278-0. [DOI] [PubMed] [Google Scholar]
- Lafferty AR, Torpy DJ, Stowasser M, Taymans SE, Lin JP, Huggard P, Gordon RD, Stratakis CA. A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22) J. Med. Genet. 2000;37:831–835. doi: 10.1136/jmg.37.11.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappano R, De Marco P, De Francesco EM, Chimento A, Pezzi V, Maggiolini M. Cross-talk between GPER and growth factor signaling. J. Steroid Biochem. Mol. Biol. 2013;137:50–56. doi: 10.1016/j.jsbmb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Lappano R, Rosano C, De Marco P, De Francesco EM, Pezzi V, Maggiolini M. Estriol acts as a GPR30 antagonist in estrogen receptor-negative breast cancer cells. Mol. Cell. Endocrinol. 2010;320:162–170. doi: 10.1016/j.mce.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Lappano R, Rosano C, Santolla MF, Pupo M, De Francesco EM, De Marco P, Ponassi M, Spallarossa A, Ranise A, Maggiolini M. Two novel GPER agonists induce gene expression changes and growth effects in cancer cells. Curr. Cancer Drug Targets. 2012a;12:531–542. doi: 10.2174/156800912800673284. [DOI] [PubMed] [Google Scholar]
- Lappano R, Santolla MF, Pupo M, Sinicropi MS, Caruso A, Rosano C, Maggiolini M. MIBE acts as antagonist ligand of both estrogen receptor alpha and GPER in breast cancer cells. Breast Cancer Res. 2012b;14:R12. doi: 10.1186/bcr3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart PM, Broselid S, Barrick CJ, Leeb-Lundberg LM, Caron KM. G-protein-coupled receptor 30 interacts with receptor activity-modifying protein 3 and confers sex-dependent cardioprotection. J. Mol. Endocrinol. 2013;51:191–202. doi: 10.1530/JME-13-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol. Metab. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yu X, Szynkarski CK, Meng C, Zhou B, Barhoumi R, White RE, Heaps CL, Stallone JN, Han G. Activation of GPER Induces Differentiation and Inhibition of Coronary Artery Smooth Muscle Cell Proliferation. PLoS One. 2013a;8:e64771. doi: 10.1371/journal.pone.0064771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Birnbaumer L, Teng CT. Regulation of ERRalpha gene expression by estrogen receptor agonists and antagonists in SKBR3 breast cancer cells: differential molecular mechanisms mediated by g protein-coupled receptor GPR30/GPER-1. Mol. Endocrinol. 2010;24:969–980. doi: 10.1210/me.2009-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen Y, Zhu ZX, Liu XH, Yang L, Wan L, Lei TW, Wang XD. 4-Hydroxytamoxifen-stimulated processing of cyclin E is mediated via G protein-coupled receptor 30 (GPR30) and accompanied by enhanced migration in MCF-7 breast cancer cells. Toxicology. 2013b;309:61–65. doi: 10.1016/j.tox.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Li ZL, Liu JC, Liu SB, Li XQ, Yi DH, Zhao MG. Improvement of vascular function by acute and chronic treatment with the GPR30 agonist G1 in experimental diabetes mellitus. PLoS One. 2012;7:e38787. doi: 10.1371/journal.pone.0038787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin BC, Suzawa M, Blind RD, Tobias SC, Bulun SE, Scanlan TS, Ingraham HA. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res. 2009;69:5415–5423. doi: 10.1158/0008-5472.CAN-08-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Chappell MC. Evidence that the G protein-coupled membrane receptor GPR30 contributes to the cardiovascular actions of estrogen. Gend. Med. 2011;8:343–354. doi: 10.1016/j.genm.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, da Silva AS, Silva MS, Chappell MC. Reduced vasorelaxation to estradiol and G-1 in aged female and adult male rats is associated with GPR30 downregulation. Am. J. Physiol. Endocrinol. Metab. 2013a;305:E113–118. doi: 10.1152/ajpendo.00649.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids. 2013b doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension. 2011;58:665–671. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhao Y, Xie K, Sun X, Gao Y, Wang Z. Estrogen-induced nongenomic calcium signaling inhibits lipopolysaccharide-stimulated Tumor Necrosis Factor alpha production in macrophages. PLoS One. 2013;8:e83072. doi: 10.1371/journal.pone.0083072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu P, Sham KW, Yuen JM, Xie C, Zhang Y, Liu Y, Li S, Huang X, Cheng CH, Lin H. Identification of a membrane estrogen receptor in zebrafish with homology to mammalian GPER and its high expression in early germ cells of the testis. Biol. Reprod. 2009;80:1253–1261. doi: 10.1095/biolreprod.108.070250. [DOI] [PubMed] [Google Scholar]
- Ma Y, Qiao X, Falone AE, Reslan OM, Sheppard SJ, Khalil RA. Gender-specific reduction in contraction is associated with increased estrogen receptor expression in single vascular smooth muscle cells of female rat. Cell. Physiol. Biochem. 2010;26:457–470. doi: 10.1159/000320569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo A, Maggiolini M. Nuclear alternate estrogen receptor GPR30 mediates 17beta-estradiol-induced gene expression and migration in breast cancer-associated fibroblasts. Cancer Res. 2010;70:6036–6046. doi: 10.1158/0008-5472.CAN-10-0408. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J. Biol. Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y, Suematsu S, Saito J, Omura M, Nishikawa T. Vascular Aldosterone Production at the Pre-Diabetic Stage of Young Otsuka Long-Evans Tokushima Fatty (OLETF) Rats, Compared with Long-Evans Tokushima Otsuka (LETO) Rats. Molecules. 2013;18:15636–15647. doi: 10.3390/molecules181215636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat. Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Amann K, Field AS, Hu C, Hathaway HJ, Kanagy NL, Walker MK, Barton M, Prossnitz ER. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension. 2012a;59:507–512. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI182,780. Pharmacology. 2010;86:58–64. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Barton M. ERa, ERb, and gpER: Novel aspects of estrogen receptor signaling in atherosclerosis. Cardiovasc. Res. 2009;83:605–610. doi: 10.1093/cvr/cvp187. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011a doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Field AS, Kanagy NL, Barton M, Prossnitz ER. GPER regulates endothelin-dependent vascular tone and intracellular calcium. Life Sci. 2012b;91:623–627. doi: 10.1016/j.lfs.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Fredette NC, Barton M, Prossnitz ER. Regulation of vascular smooth muscle tone by adipose-derived contracting factor. PLoS One. 2013;8:e79245. doi: 10.1371/journal.pone.0079245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol. Cell. Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul. Pharmacol. 2011b;55:17–25. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM. Why are sex and gender important to basic physiology, translational and individualized medicine? Am. J. Physiol. Heart Circ. Physiol. 2014 doi: 10.1152/ajpheart.00994.2013. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Borjesson AE, Farman HH, Sjogren K, Windahl SH, Lagerquist MK, Andersson A, Stubelius A, Carlsten H, Gustafsson JA, Ohlsson C. The estrogen receptor antagonist ICI 182,780 can act both as an agonist and an inverse agonist when estrogen receptor alpha AF-2 is modified. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1180–1185. doi: 10.1073/pnas.1322910111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc. Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- Murata T, Dietrich HH, Xiang C, Dacey RG., Jr. G Protein-Coupled Estrogen Receptor Agonist Improves Cerebral Microvascular Function After Hypoxia/Reoxygenation Injury in Male and Female Rats. Stroke. 2013 doi: 10.1161/STROKEAHA.112.678177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak TK, Dennis MK, Ramesh C, Burai R, Atcher RW, Sklar LA, Norenberg JP, Hathaway HJ, Arterburn JB, Prossnitz ER. Influence of charge on cell permeability and tumor imaging of GPR30-targeted 111In-labeled nonsteroidal imaging agents. ACS Chem. Biol. 2010;5:681–690. doi: 10.1021/cb1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niino M, Hirotani M, Fukazawa T, Kikuchi S, Sasaki H. Estrogens as potential therapeutic agents in multiple sclerosis. Cent. Nerv. Syst. Agents Med. Chem. 2009;9:87–94. doi: 10.2174/187152409788452054. [DOI] [PubMed] [Google Scholar]
- Norman PE, Powell JT. Vitamin d and cardiovascular disease. Circ. Res. 2014;114:379–393. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J. Biol. Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- O’Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, Kolakowski LF, Jr, George SR. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47:310–313. doi: 10.1006/geno.1998.5095. [DOI] [PubMed] [Google Scholar]
- Offit K. Personalized medicine: new genomics, old lessons. Hum. Genet. 2011;130:3–14. doi: 10.1007/s00439-011-1028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Inoue S, Ikegami A, Hosoi T, Akishita M, Ouchi Y, Muramatsu M, Orimo H. Vascular smooth muscle cells as target for estrogen. Biochem. Biophys. Res. Commun. 1993;195:730–736. doi: 10.1006/bbrc.1993.2106. [DOI] [PubMed] [Google Scholar]
- Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. GPR30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- Owman C, Blay P, Nilsson C, Lolait SJ. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochem. Biophys. Res. Commun. 1996;228:285–292. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- Ozdemir V, Williams-Jones B, Glatt SJ, Tsuang MT, Lohr JB, Reist C. Shifting emphasis from pharmacogenomics to theragnostics. Nat. Biotechnol. 2006;24:942–946. doi: 10.1038/nbt0806-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey DP, Lappano R, Albanito L, Madeo A, Maggiolini M, Picard D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO J. 2009;28:523–532. doi: 10.1038/emboj.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VH, Chen J, Ramanjaneya M, Karteris E, Zachariades E, Thomas P, Been M, Randeva HS. G-protein coupled estrogen receptor 1 expression in rat and human heart: Protective role during ischaemic stress. Int. J. Mol. Med. 2010;26:193–199. doi: 10.3892/ijmm_00000452. [DOI] [PubMed] [Google Scholar]
- Pavlik R, Wypior G, Hecht S, Papadopoulos P, Kupka M, Thaler C, Wiest I, Pestka A, Friese K, Jeschke U. Induction of G protein-coupled estrogen receptor (GPER) and nuclear steroid hormone receptors by gonadotropins in human granulosa cells. Histochem. Cell Biol. 2011;136:289–299. doi: 10.1007/s00418-011-0846-7. [DOI] [PubMed] [Google Scholar]
- Pernis AB. Estrogen and CD4+ T cells. Curr. Opin. Rheumatol. 2007;19:414–420. doi: 10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, Smith HO, Hathaway HJ, Prossnitz ER. G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet. Gynecol. Int. 2013;2013:472720. doi: 10.1155/2013/472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton C, Thomas P. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio) Biol. Reprod. 2011;85:42–50. doi: 10.1095/biolreprod.110.088765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Endometrial cell calcium and oestrogen action. Nature. 1975;253:357–359. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu. Rev. Physiol. 2008a;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol. Cell. Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol. Sci. 2008b;29:116–123. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler. Thromb. Vasc. Biol. 2014;34:355–364. doi: 10.1161/ATVBAHA.113.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupo M, Pisano A, Lappano R, Santolla MF, De Francesco EM, Abonante S, Rosano C, Maggiolini M. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 2012;120:1177–1182. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupo M, Vivacqua A, Perrotta I, Pisano A, Aquila S, Abonante S, Gasperi-Campani A, Pezzi V, Maggiolini M. The nuclear localization signal is required for nuclear GPER translocation and function in breast Cancer-Associated Fibroblasts (CAFs) Mol. Cell. Endocrinol. 2013;376:23–32. doi: 10.1016/j.mce.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JA, Graeber CT, Frackelton AR, Jr., Kim M, Schwarzbauer JE, Filardo EJ. Coordinate regulation of estrogen-mediated fibronectin matrix assembly and epidermal growth factor receptor transactivation by the G-protein-coupled receptor, GPR30. Mol. Endocrinol. 2009 doi: 10.1210/me.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh C, Nayak TK, Burai R, Dennis MK, Hathaway HJ, Sklar LA, Prossnitz ER, Arterburn JB. Synthesis and characterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30. J. Med. Chem. 2010;53:1004–1014. doi: 10.1021/jm9011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Ficek M. Immunomodulatory effects of anti-estrogenic drugs. Acta. Pharm. 2012;62:141–155. doi: 10.2478/v10007-012-0012-3. [DOI] [PubMed] [Google Scholar]
- Ren J, Wu JH. 17beta-estradiol rapidly activates calcium release from intracellular stores via the GPR30 pathway and MAPK phosphorylation in osteocyte-like MLO-Y4 cells. Calcif. Tissue Int. 2012;90:411–419. doi: 10.1007/s00223-012-9581-x. [DOI] [PubMed] [Google Scholar]
- Resanovic I, Rizzo M, Zafirovic S, Bjelogrlic P, Perovic M, Savic K, Patti AM, Isenovic RE. Anti-atherogenic effects of 17beta-estradiol. Horm. Metab. Res. 2013;45:701–708. doi: 10.1055/s-0033-1343478. [DOI] [PubMed] [Google Scholar]
- Reslan OM, Yin Z, do Nascimento GR, Khalil RA. Subtype-specific Estrogen Receptor-mediated Vasodilator Activity in the Cephalic, Thoracic, and Abdominal Vasculature of Female Rat. J. Cardiovasc. Pharmacol. 2013;62:26–40. doi: 10.1097/FJC.0b013e31828bc88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettberg JR, Yao J, Brinton RD. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol. 2013 doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettew JA, McCall S.H.t., Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol. Cell. Endocrinol. 2010;328:87–92. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, Sklar LA, Arterburn JB, Prossnitz ER. Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem. Biol. 2007;2:536–544. doi: 10.1021/cb700072n. [DOI] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, Green AR, Ali S, Chin SF, Palmieri C, Caldas C, Carroll JS. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481:389–393. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands DJ, Chapple S, Siow RC, Mann GE. Equol-Stimulated Mitochondrial Reactive Oxygen Species Activate Endothelial Nitric Oxide Synthase and Redox Signaling in Endothelial Cells: Roles for F-Actin and GPR30. Hypertension. 2011;57:833–840. doi: 10.1161/HYPERTENSIONAHA.110.162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat. Rev. Endocrinol. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Vascular mineralocorticoi d receptors regulate blood pressure effects on myogenic tone and role in aging. Circ. Res. 2013;112:415–417. doi: 10.1161/CIRCRESAHA.113.300883. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, Ziegler YS, Nardulli AM. ERalpha-associated protein networks. Trends Endocrinol. Metab. 2011;22:124–129. doi: 10.1016/j.tem.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154:4136–4145. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology. 2011;152:3030–3039. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry BS. Pharmacogenetics and the concept of individualized medicine. Pharmacogenomics J. 2006;6:16–21. doi: 10.1038/sj.tpj.6500338. [DOI] [PubMed] [Google Scholar]
- Sheng ZG, Huang W, Liu YX, Zhu BZ. Bisphenol A at a low concentration boosts mouse spermatogonial cell proliferation by inducing the G protein-coupled receptor 30 expression. Toxicol. Appl. Pharmacol. 2013;267:88–94. doi: 10.1016/j.taap.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Smith HO, Arias-Pulido H, Kuo DY, Howard T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE, Prossnitz ER. GPR30 predicts poor survival for ovarian cancer. Gynecol. Oncol. 2009;114:465–471. doi: 10.1016/j.ygyno.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am. J. Obstet. Gynecol. 2007;196:386.e381–311. doi: 10.1016/j.ajog.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Miller LM, Grafe MR, Vandenbark AA, Offner H. Contribution of GPR30 for 1,25 dihydroxyvitamin D(3) protection in EAE. Metab. Brain Dis. 2012;27:29–35. doi: 10.1007/s11011-011-9266-6. [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J. Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc. Natl. Acad. Sci. U. S. A. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem. Biophys. Res. Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, Mauvais-Jarvis F. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J. Clin. Invest. 2011;121:3331–3342. doi: 10.1172/JCI44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tica AA, Dun EC, Tica OS, Gao X, Arterburn JB, Brailoiu GC, Oprea TI, Brailoiu E. G protein-coupled estrogen receptor 1-mediated effects in the rat myometrium. Am. J. Physiol. Cell Physiol. 2011;301:C1262–1269. doi: 10.1152/ajpcell.00501.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale MJ. Biochemical mechanisms of cellular catabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:401–405. doi: 10.1097/00075197-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension. 2007;49:1364–1370. doi: 10.1161/HYPERTENSIONAHA.106.081554. [DOI] [PubMed] [Google Scholar]
- van’t Veer LJ, Bernards R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature. 2008;452:564–570. doi: 10.1038/nature06915. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- Vergote I, Abram P. Fulvestrant, a new treatment option for advanced breast cancer: tolerability versus existing agents. Ann. Oncol. 2006;17:200–204. doi: 10.1093/annonc/mdj047. [DOI] [PubMed] [Google Scholar]
- Villablanca AC, Tenwolde A, Lee M, Huck M, Mumenthaler S, Rutledge JC. 17beta-estradiol prevents early-stage atherosclerosis in estrogen receptor-alpha deficient female mice. J. Cardiovasc. Transl. Res. 2009;2:289–299. doi: 10.1007/s12265-009-9103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Ando S, Maggiolini M. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the G protein-coupled receptor GPR30. Mol. Pharmacol. 2006a;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17b-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol. Endocrinol. 2006b;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Romeo E, De Marco P, De Francesco EM, Abonante S, Maggiolini M. GPER mediates the Egr-1 expression induced by 17beta-estradiol and 4-hydroxitamoxifen in breast and endometrial cancer cells. Breast Cancer Res. Treat. 2012;133:1025–1035. doi: 10.1007/s10549-011-1901-8. [DOI] [PubMed] [Google Scholar]
- Volpe M, Gigante B, Enea I, Porcellini A, Russo R, Lee MA, Magri P, Condorelli G, Savoia C, Lindpaintner K, Rubattu S. Role of tissue renin in the regulation of aldosterone biosynthesis in the adrenal cortex of nephrectomized rats. Circ. Res. 1997;81:857–864. doi: 10.1161/01.res.81.5.857. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbark AA, Offner H. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J. Immunol. 2009;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol. Endocrinol. 2008a;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, Roy SK. G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology. 2008b;149:4452–4461. doi: 10.1210/en.2008-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc. Res. 2012;94:96–104. doi: 10.1093/cvr/cvs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem. Biophys. Res. Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn N, Borgquist A, Wang K, Jeffery GS, Kelly MJ, Wagner EJ. Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Neuroendocrinology. 2013;97:160–175. doi: 10.1159/000338669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery. 2010;148:436–443. doi: 10.1016/j.surg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Weisz A, Bresciani F. Estrogen induces expression of c-fos and c-myc protooncogenes in rat uterus. Mol. Endocrinol. 1988;2:816–824. doi: 10.1210/mend-2-9-816. [DOI] [PubMed] [Google Scholar]
- Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear Estrogen Receptor Signaling in Endothelium. J. Biol. Chem. 2011;286:14737–14743. doi: 10.1074/jbc.R110.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikomi T, Vienonen A, Ahola TM. G protein-coupled receptor 30 down-regulates cofactor expression and interferes with the transcriptional activity of glucocorticoid. Eur. J. Biochem. 2004;271:4159–4168. doi: 10.1111/j.1432-1033.2004.04353.x. [DOI] [PubMed] [Google Scholar]
- Yu X, Ma H, Barman SA, Liu AT, Sellers M, Stallone JN, Prossnitz ER, White RE, Han G. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. Am. J. Physiol. Endocrinol. Metab. 2011;301:E882–888. doi: 10.1152/ajpendo.00037.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazzu V. Dissertation Germany. Humboldt University Berlin; 2011. The human G protein-coupled receptor GPR30: interaction partners and expression analysis in endothelial cells; pp. 1–123. [Google Scholar]
- Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J. Immunol. 2010;184:4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Yuen JM, Sham KW, Cheng CH. GPER mediates the inhibitory actions of estrogen on adipogenesis in 3T3-L1 cells through perturbation of mitotic clonal expansion. Gen. Comp. Endocrinol. 2013;193:19–26. doi: 10.1016/j.ygcen.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Zucchetti AE, Barosso IR, Boaglio AC, Basiglio CL, Misczuk G, Larocca MC, Ruiz ML, Davio CA, Roma MG, Crocenzi FA, Pozzi EJ. G protein-coupled receptor30-adenylyl cyclase-protein kinase A pathway is involved in estradiol 17ss-d-glucuronide-induced cholestasis. Hepatology. 2013 doi: 10.1002/hep.26752. [DOI] [PubMed] [Google Scholar]