To the Editor
Chronic rhinosinusitis (CRS) is a common chronic disease with significant burden and impact on quality of life of affected individuals, characterized by inflammation of the nasal mucosa and paranasal sinuses. Patients can be subdivided into CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). Additionally, Aspirin Exacerbated Respiratory Disease (AERD) is a subgroup of CRSwNP characterized by the triad of CRSwNP, asthma, and hypersensitivity to aspirin or non-steroidal anti-inflammatory drugs (NSAIDs). Eosinophilia is evident in the nasal mucosa and submucosa of nasal polyps (NP) from patients in Europe and the US, but less so in Asian countries such as China or Japan (1). In this study we attempted, for the first time, to evaluate the role of another important inflammatory leukocyte, the basophil, in CRS. Basophils are granulocytes found mainly in the circulation and are known to have a role in allergic diseases, parasite expulsion and immunity against ectoparasites (2). Basophil numbers are elevated in the bronchial mucosa and submucosa of asthmatics, the nasal submucosa in allergic rhinitis, and in the skin of those with multiple inflammatory dermatologic conditions, including eczema and urticaria (2–5).
Although CRSwNP is a highly Th2-biased disease, making basophil involvement likely, there are no reports of basophils in CRS. In the current study we used 2D7, a mouse monoclonal antibody (mAB) against a basophil-specific intermediate form of major basic protein (6), to detect basophils in uncinate or polyp tissues obtained during surgery from CRS patients and control subjects as described previously (7). This antibody is shown to be a specific marker for basophils and does not recognized any other cell type (6). We employed the immunohistochemistry (IHC) protocol detailed in previous studies using 2D7 (3, 5). Mucosa and submucosa in the tissue biopsies were delineated, and the total number of positively stained cells in each field was counted in 50 random high power fields (hpf) with 100X magnification. Results are reported as the number of positive cells per square millimeter.
A tissue section was stained with Hematoxylin and Eosin (H&E). Eosinophils were identified based on their cellular features and distinct cytoplasmic eosinophilic granules. For all polyps samples, a serial section was stained with a tryptase mAB, a specific marker for mast cells as described previously (7). Cells were counted and data expressed as described above for basophils.
Data are presented as mean ± standard error of the mean (SEM). Comparisons between groups were assessed by ANOVA and Newman-Keuls multiple comparison tests. Correlations were measured using a Spearman Rank correlation test. All statistical analyses were performed using GraphPad Prism 5.0. A p value of less than 0.05 was considered statistically significant.
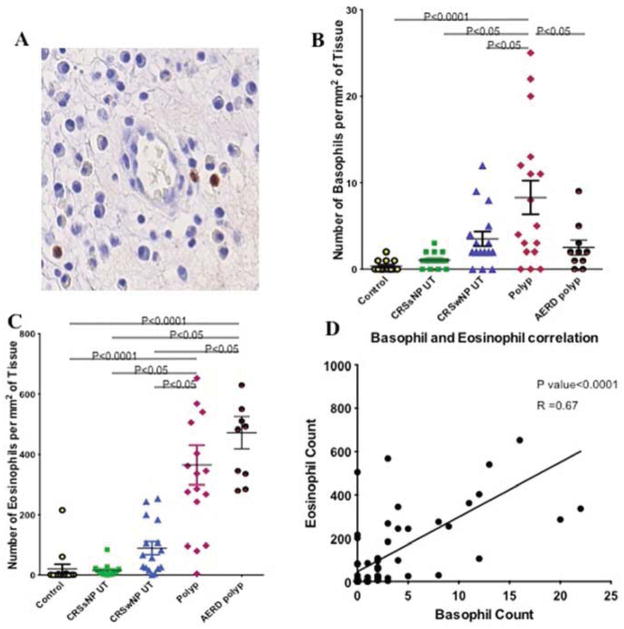
A total of 73 sections were labeled with 2D7, including those from 27 polyp patients (10 with AERD and 17 without AERD), and uncinate tissue (UT) from 16 CRSwNP patients, 15 CRSsNP patients and 15 control cases with no evidence of CRS. Cells were identified as basophils based on a brown cytoplasmic granular staining pattern (Figure 1A). Negative control slides were labeled with an isotype-matched non-immune antibody and showed no staining.
Figure 1.
2D7+ basophils in a polyp (arrow heads) (A). Comparison of basophil counts in polyp tissue from subjects with and without AERD, and in UT of controls, CRSwNP and CRSsNP patients (B). Comparison of eosinophil counts in polyp tissue of those with and without AERD and UT of controls, CRSwNP and CRSsNP patients (C). Correlation between basophils and eosinophils detected in all of the tissues evaluated (D).
As detailed in Table 1, there were no 2D7 basophils detected within the uncinate mucosa and submucosa of control subjects. A small number of these cells was detected in UT of CRSsNP patients and a greater number was detected in UT from CRSwNP patients, though the quantities detected were not significantly greater than in control UT.
Table 1.
Summary of cell count results in different tissues.
| Control | CRSsNP | CRSwNP | Polyp (non AERD) | Polyp (AERD) | |
|---|---|---|---|---|---|
| Basophils (cells per square millimeter) Mean (SEM) | 0.35(0.16) | 1.06(0.22) | 3.5(0.85) | 7.76(1.71)* | 2.50(0.86)† |
| Eosinophils (cells per square millimeter) Mean (SEM) | 20.5(15.57) | 14.73(5.49) | 89.31(21.49) | 365.1(65.46)** | 453(45)** |
| Mast cells (cells per square millimeter) Mean (SEM) | 25.21(3.4) | 29.60(0.35) |
p<0.05 for comparison by ANOVA and Newman-Keuls multiple comparison tests when compared to UT of CRSsNP and CRSwNP. And p<0.001 when compared to controls.
p<0.05 for comparison by ANOVA and Newman-Keuls multiple comparison tests when compared to UT of CRSwNP. And p<0.001 compared to UT of CRSsNP and controls
p<0.05 for comparison by ANOVA and Newman-Keuls multiple comparison tests when compared to non-AERD polyp.
The 2D7-positive cells per square millimeter of tissue were significantly higher in NP tissue of patients with CRSwNP compared to UT of control subjects or patients with CRSsNP and CRSwNP (Figure 1B). This count was also significantly higher when compared to the corresponding UT of the same CRSwNP patients; paired sets of polyp and UT tissues having been evaluated from 12 CRSwNP patients. Interestingly, the numbers of basophils detected in NP from AERD subjects were not higher than in normal or CRS UT, and were significantly lower than in NP from patients without AERD.
Eosinophil counts in UT of control patients with no evidence of sinusitis were significantly lower when compared to UT of patients with CRSwNP (Table 1). Polyp tissue had significantly higher numbers of eosinophils compared to UT of all groups, including controls, CRSsNP and CRSwNP. Polyps from subjects with AERD had significantly higher eosinophil counts compared to UT of all groups, with a trend toward higher levels than in non AERD polyps.
Basophil and eosinophil counts in all tissues (excluding AERD polyps) significantly correlated with one another (P<0.001, Spearman r=0.67), Figure 1D. But the Basophil and mast cell counts did not correlate.
Basophils and eosinophils utilize similar pathways for recruitment, and might be expected to be recruited to an inflammatory site in parallel (8). We therefore calculated the ratio of eosinophils to basophils by dividing the total number of eosinophils per hpf by the number of basophils per hpf in a fixed area in serially cut slides. The eosinophil to basophil ratio in normal UT had a mean value of 3.0; this value was 13.1 in CRSsNP UT and 49.0 in CRSwNP UT. Furthermore the magnitude of the ratio of eosinophils to basophils was highest in NP, with a mean of 89.2 in non-AERD polyps and 287.3 in AERD polyps. Values of this ratio were significantly higher in polyps when compared to UT of CRSwNP, CRSsNP, and control cases.
The blood basophil counts within 6 months of the time of surgery were available for 40 cases; 13 with analysis of polyp tissue and 27 with analysis of UT. There was no correlation between the blood basophil count and the number of basophils in either polyp or UT tissues. This appears to exclude systemic basophilia as a likely cause of the observed increase of basophils in the polyp tissue. We also correlated the basophil numbers in polyps with total serum IgE (available in 13 cases), presence of anosmia or hyposmia as a marker for disease severity and positive skin test. None of these comparisons were significant (both including and excluding AERD polyps). Potentially other patient-related factors, such as treatment, might have influenced the number of basophils. We compared the number of basophils in UT and polyp of patients who were on oral steroids at the time of surgery compared to those who were not (both including and excluding AERD cases); the results were not statistically significant.
In this letter we report that there is a trend for increased numbers of basophils in UT from patients with CRS and that nasal polyp tissue in non AERD patients contains clearly elevated numbers of basophils compared with UT from both patients and controls; polyps from patients with AERD had a trend toward elevated basophils, but significantly fewer basophils than non AERD polyps. As expected based upon reports in the literature (9), eosinophils were also elevated in UT from CRS patients, and were especially prominent in nasal polyp tissue from subjects both with and without AERD. The lower number of detected basophils in AERD polyps raises the question as to whether these cells were never recruited to these polyp samples or could not be detected by 2D7. Basophils recruited to AERD nasal polyp tissue may be activated rapidly in vivo, may degranulate more quickly and more completely after homing to the polyp and thus lose their granule proteins, including the protein detected by 2D7, as described previously in skin biopsies obtained during the late phase response to allergen challenge (10).
The number of tissue basophils correlated with the number of tissue eosinophils, suggesting that these cells are associated in the inflammation found in CRS. Despite this significant correlation, there was a relative enrichment of eosinophils compared to basophils in polyp tissue, as the eosinophil/basophil ratio was close to 100 in polyps, a value significantly higher than UT from CRS patients. This ratio in nasal polyp tissue is also markedly higher than ratios calculated from counts reported in the literature using other respiratory tissues; the ratio was about 10 in the bronchial mucosa in asthma in one study (11) and about 20 in nasal submucosa in allergic rhinitis at the peak of season in another (5). This significant enrichment of eosinophils in nasal polyps compared to basophils might reflect selective recruitment of eosinophils to the polyp or sustained survival of eosinophils compared to basophils in polyp tissue and when compared to other respiratory tissues undergoing an allergic response. It is also possible that our measurements of basophils are underestimates due to degranulation as mentioned above or for another reason. For example, it is possible that recruited basophils might exit the polyp into the nasal cavity preferentially compared to eosinophils. There are multiple studies demonstrating basophils in respiratory secretions in asthma during asthmatic attacks or after allergen challenge and allergic rhinitis in the peak of allergy season (5, 12). In one study, basophil numbers in the sputum increased by almost 200-fold from baseline values during the allergy season compared to eosinophils that increased by approximately 30-fold (4). This could be indicative of a higher tendency of basophils to migrate into the airway lumen.
The presence of basophils in nasal polyp tissue raises the questions about their modes of activation and potential role in perpetuating or enhancing nasal polyp inflammation. The level of IL-3 mRNA, a major cytokine for development and survival of basophils is reportedly not elevated in CRS nasal and polyp tissue (13), a conclusion confirmed in a microarray analysis done by our group (14). However, as supported by the presence of basophils in IL-3-deficient mice (15), these cells can be expanded by other cytokines, such as thymic stromal lymphopoietin (16) and IL-33 (17), the former of which is significantly expressed in nasal polyp epithelial cells extracted from CRS patients (18). Basophils can be activated by cross-linking of allergen-specific IgE as well as via bacterial peptidoglycans through TLR-2 (2), or by formyl-methionyl-leucyl-phenylalanine (19) or serine proteases through other receptors (2). Thus, bacterial infection or colonization of the sinus mucosa, commonly observed in CRS, can theoretically activate the basophils we have detected in NP tissue.
Once recruited and activated, basophils can potentially exert inflammatory effects through several mediators. Basophils are a remarkable source of vasoactive histamine and leukotrienes that can cause tissue edema, and hence contribute to nasal congestion. Improvement of nasal congestion in response to leukotriene antagonists suggests an important role for these mediators, derived from basophils and other cells, in the symptomatology of CRS patients.
Basophils can also participate in recruiting other leukocytes to sites of allergic inflammation. Basophils secrete large quantities of IL-4 (20) and IL-13 (2), cytokines that are known to promote goblet cell hyperplasia and mucus production in asthma. Hyperplasia of goblet cells, submucosal glands and excessive mucus secretion are all features that are commonly seen in CRS patients. IL-4 can also increase the expression of integrin receptors like vascular cell adhesion molecule-1 (VCAM-1) that can bind VLA-4 expressing leukocytes including lymphocytes, monocytes and eosinophils (21), and is shown to be correlated with the presence of eosinophils in CRS (22). It should thus be considered that basophils may be involved in recruitment of other inflammatory cells to the polyp and promoting inflammation in CRS.
In conclusion, we found a significantly higher number of basophils in nasal polyp tissue compared to UT of controls and CRS patients in CRSwNP patients without AERD. In an environment rich in potential activators, and considering their ability to produce multiple inflammatory mediators, basophils may thus make an important contribution to the pathogenesis and symptomatology of CRS.
Acknowledgments
Funding: NIH R37 HL068546, R01 HL078860, R01 AI072570, R21 HL113913
We acknowledge NIH grants; R37HL068546, R01 HL078860, R21 HL113913 and the Ernest S. Bazley Trust for funding.
List of Abbreviations
- CRS
Chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- IHC
Immunohistochemistry
- NP
Nasal polyp
- UT
Uncinate tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. The Journal of allergy and clinical immunology. 2008;122(5):961–8. doi: 10.1016/j.jaci.2008.07.008. Epub 2008/09/23. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder JT. Basophils: emerging roles in the pathogenesis of allergic disease. Immunological reviews. 2011;242(1):144–60. doi: 10.1111/j.1600-065X.2011.01023.x. Epub 2011/06/21. [DOI] [PubMed] [Google Scholar]
- 3.Nouri-Aria KT, Irani AM, Jacobson MR, O’Brien F, Varga EM, Till SJ, et al. Basophil recruitment and IL-4 production during human allergen-induced late asthma. J Allergy Clin Immunol. 2001;108(2):205–11. doi: 10.1067/mai.2001.117175. Epub 2001/08/10. [DOI] [PubMed] [Google Scholar]
- 4.Gauvreau GM, Lee JM, Watson RM, Irani AM, Schwartz LB, O’Byrne PM. Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am J Respir Crit Care Med. 2000;161(5):1473–8. doi: 10.1164/ajrccm.161.5.9908090. Epub 2000/05/12. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DR, Irani AM, Walker SM, Jacobson MR, Mackay IS, Schwartz LB, et al. Grass pollen immunotherapy inhibits seasonal increases in basophils and eosinophils in the nasal epithelium. Clin Exp Allergy. 2001;31(11):1705–13. doi: 10.1046/j.1365-2222.2001.01231.x. Epub 2001/11/07. [DOI] [PubMed] [Google Scholar]
- 6.Plager DA, Weiss EA, Kephart GM, Mocharla RM, Matsumoto R, Checkel JL, et al. Identification of basophils by a mAb directed against pro-major basic protein 1. The Journal of allergy and clinical immunology. 2006;117(3):626–34. doi: 10.1016/j.jaci.2005.10.023. Epub 2006/03/09. [DOI] [PubMed] [Google Scholar]
- 7.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130(2):410–20. e5. doi: 10.1016/j.jaci.2012.02.046. Epub 2012/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochner BS, Schleimer RP. Mast cells, basophils, and eosinophils: distinct but overlapping pathways for recruitment. Immunol Rev. 2001;179:5–15. doi: 10.1034/j.1600-065x.2001.790101.x. Epub 2001/04/09. [DOI] [PubMed] [Google Scholar]
- 9.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope. 2011;121(10):2262–7. doi: 10.1002/lary.21969. Epub 2011/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irani AM, Huang C, Xia HZ, Kepley C, Nafie A, Fouda ED, et al. Immunohistochemical detection of human basophils in late-phase skin reactions. The Journal of allergy and clinical immunology. 1998;101(3):354–62. doi: 10.1016/S0091-6749(98)70248-9. Epub 1998/04/03. [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane AJ, Kon OM, Smith SJ, Zeibecoglou K, Khan LN, Barata LT, et al. Basophils, eosinophils, and mast cells in atopic and nonatopic asthma and in late-phase allergic reactions in the lung and skin. J Allergy Clin Immunol. 2000;105(1 Pt 1):99–107. doi: 10.1016/s0091-6749(00)90184-2. Epub 2000/01/12. [DOI] [PubMed] [Google Scholar]
- 12.Hastie R, Heroy JH, 3rd, Levy DA. Basophil leukocytes and mast cells in human nasal secretions and scrapings studied by light microscopy. Lab Invest. 1979;40(5):554–61. Epub 1979/05/01. [PubMed] [Google Scholar]
- 13.Rudack C, Stoll W, Bachert C. Cytokines in nasal polyposis, acute and chronic sinusitis. Am J Rhinol. 1998;12(6):383–8. doi: 10.2500/105065898780708008. Epub 1999/01/12. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri S, Lin DC, Rosati M, Carter RG, Norton JE, Suh L, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012;67(7):920–8. doi: 10.1111/j.1398-9995.2012.02848.x. Epub 2012/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392(6671):90–3. doi: 10.1038/32190. Epub 1998/03/24. [DOI] [PubMed] [Google Scholar]
- 16.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10(7):697–705. doi: 10.1038/ni.1740. Epub 2009/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181(9):5981–9. doi: 10.4049/jimmunol.181.9.5981. Epub 2008/10/23. [DOI] [PubMed] [Google Scholar]
- 18.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.04.005. Epub 2013/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siraganian RP, Hook WA. Mechanism of histamine release by formyl methionine-containing peptides. J Immunol. 1977;119(6):2078–83. Epub 1977/12/01. [PubMed] [Google Scholar]
- 20.MacGlashan D, Jr, White JM, Huang SK, Ono SJ, Schroeder JT, Lichtenstein LM. Secretion of IL-4 from human basophils. The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152(6):3006–16. Epub 1994/03/15. [PubMed] [Google Scholar]
- 21.Ying S, Meng Q, Barata LT, Robinson DS, Durham SR, Kay AB. Associations between IL-13 and IL-4 (mRNA and protein), vascular cell adhesion molecule-1 expression, and the infiltration of eosinophils, macrophages, and T cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J Immunol. 1997;158(10):5050–7. Epub 1997/05/15. [PubMed] [Google Scholar]
- 22.Jahnsen FL, Haraldsen G, Aanesen JP, Haye R, Brandtzaeg P. Eosinophil infiltration is related to increased expression of vascular cell adhesion molecule-1 in nasal polyps. Am J Respir Cell Mol Biol. 1995;12(6):624–32. doi: 10.1165/ajrcmb.12.6.7539273. Epub 1995/06/01. [DOI] [PubMed] [Google Scholar]