Abstract
17β-estradiol (E2) has been implicated to play a critical role in neuroprotection, synaptic plasticity, and cognitive function. Classically, the role of gonadal-derived E2 in these events is well established, but the role of brain-derived E2 is less clear. To address this issue, we investigated the expression, localization, and modulation of aromatase and local E2 levels in the hippocampus following global cerebral ischemia (GCI) in adult ovariectomized rats. Immunohistochemistry (IHC) revealed that the hippocampal regions CA1, CA3 and dentate gyrus (DG) exhibited high levels of immunoreactive aromatase staining, with aromatase being co-localized primarily in neurons in non-ischemic animals. Following GCI, aromatase became highly expressed in GFAP-positive astrocytes in the hippocampal CA1 region at 2–3 days post GCI reperfusion. An ELISA for E2 and IHC for E2 confirmed the GCI-induced elevation of local E2 in the CA1 region and that the increase in local E2 occurred in astrocytes. Furthermore, central administration of aromatase antisense (AS) oligonucleotides, but not missense (MS) oligonucleotides, blocked the increase in aromatase and local E2 in astrocytes after GCI, and resulted in a significant increase in GCI-induced hippocampal CA1 region neuronal cell death and neuroinflammation. As a whole, these results suggest that brain-derived E2 exerts important neuroprotective and anti-inflammatory actions in the hippocampal CA1 region following GCI.
Introduction
17β-Estradiol (E2, estrogen) is a steroid hormone that has been implicated to be neuroprotective against a variety of neurodegenerative disorders, including stroke, Alzheimer’s disease (AD) and Parkinson’s disease, although controversy exists [1–4]. With respect to stroke, studies in rats, mice and gerbils found a sex difference in brain injury following cerebral ischemia, with young adult female animals having smaller infarct volume as compared to young adult males [1, 5, 6]. Similarly, a number of studies have documented sex differences in stroke risk and outcome in humans, with women generally protected against stroke, at least until menopause [7, 8]. Many groups, including our own, have shown that exogenous administration of E2 dramatically reduces infarct volume in cortex and hippocampus following focal or global cerebral ischemia (GCI) in ovariectomized female mice, rats and gerbils, and in male rats and gerbils [1, 9–13].
It has been generally assumed that the neuroprotective effects of E2 are primarily due to ovarian-derived E2. However, work by a number of laboratories has shown that certain areas of the brain exhibit high expression of the E2 generating enzyme, aromatase, which has raised the possibility that brain-derived E2 may have important roles in the CNS. For instance, work within the last decade in rodents, birds, monkeys, and humans has shown that forebrain structures, in particular the hippocampus CA1–CA3 regions, exhibits high expression of aromatase as indicated by in situ hybridization, RT-PCR and immunohistochemical analysis, and can produce significant levels of E2 levels that are equivalent to or even higher than that observed in the circulation [14–22]. It should be noted that the cerebral cortex has also been reported to express aromatase [16, 23, 24], and thus brain-derived E2 may also regulate cortical functions. In support of this possibility, global aromatase knockout mice have been reported to have greater cortical damage following focal cerebral ischemia than wild type ovariectomized mice, suggesting that brain-derived E2 may have neuroprotective actions in the cerebral cortex [25].
With respect to the hippocampus, treatment of cultured mouse hippocampal neurons with an aromatase inhibitor has been reported to result in a significant decrease in axon outgrowth and dendritic spines in the CA1 region [19, 21, 26–28], as well as a significant decrease of long-term potentiation (LTP) amplitude, dendritic spines and synapses in hippocampal slices in vitro [29, 30]. These results suggest that local E2 in the hippocampus may modulate synaptic function. Interestingly, studies in songbirds have also shown that inhibiting aromatase by intracerebral administration of aromatase inhibitors results in increased damage and apoptosis in the brain after a penetrating injury [31, 32]. Aromatase inhibition has also been reported to result in increased hippocampal damage in male rats following excitotoxic injury [33].
It is well known that the hippocampal CA1 region is highly vulnerable to GCI, which can occur after cardiac arrest, asphyxiation, and hypotensive shock [34, 35], and can lead to significant neuronal damage, cognitive defect and mortality. It is currently unknown whether brain-derived E2 in the hippocampal CA1 region has a neuroprotective role against GCI, and whether it can modulate neuroinflammation that occurs after GCI. To address these deficits in our knowledge, the goals of the current study were: 1) to access whether aromatase and local E2 levels change in the hippocampus following GCI, 2) to determine the cell types containing aromatase and local E2 expression in ischemic and non-ischemic animals, and 3) to assess whether antisense oligonucleotide knockdown of aromatase and local E2 levels in the hippocampus affects GCI-induced neurodegeneration and inflammation in ovariectomized rats.
Materials and Methods
Animal Model of Global Cerebral Ischemia
All procedures were approved by the Georgia Regents University Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health guidelines for animal research. Adult Sprague Dawley female SD rats were obtained from Harlan Inc. and studied at 3 months of age. All the rats were bilaterally ovariectomized one week before the induction of GCI. GCI was performed by four-vessel occlusion (4-VO) as described previously [36–38]. In brief, both common carotid arteries (CCAs) of the rat were separated under anesthesia and a silastic ligature was placed loosely around each artery before the incision was closed. Immediately after this procedure, both vertebral arteries at the level of the alar foramina were permanently electrocauterized with bipolar cauterization. After 24 h recovery, both CCAs were exposed under light anesthesia with isoflurane and occluded with aneurysm clips to induce 8 min transient forebrain ischemia. Successful forebrain ischemia was ensured by monitoring the pupils for dilation and being unresponsive to light, and loss of righting reflex of each animal during cerebral ischemia. The clips were then removed, and blood flow through the carotid arteries was inspected and confirmed before the wound was sutured. Normal rectal temperature was maintained with a heat lamp and thermal blanket during surgical procedures. Animals in the sham group underwent identical procedures except that the CCAs were exposed, but there were no occlusion.
Intracerebroventricular (ICV) Antisense Administration
Alzet osmotic mini-pumps (model 1007D, 7 day release; Durect Corporation, Cupertino, CA) were filled with 20 nmol of HPLC-purified aromatase antisense oligodeoxynucleotide (AS-ODN, 5′-ATCAGCAAGTCCTCGAGCAT-3′, synthesized by Integrated DNA Technologies, Inc.) or scrambled missense (MS, 5′-CCGCGAAAATCGCTTTAGCA-3′) in sterile 0.9% saline. The last 3 bases on both the 5′ and 3′ were end-phosphorothioated to limit ODN degradation. For the cannula and osmotic pump implantation, the animals were fixed in a stereotaxic frame under anesthesia and the skull was exposed following a midline incision. The mini-pumps connected to the Alzet infusion cannula (Brain Infusion Kit 2; Durect Corporation) were implanted subcutaneously under the upper back skin three days before ischemia. The cannula was implanted into the lateral cerebral ventricle based on the following stereotaxic coordinates: anterior/posterior −0.8 mm, medial/lateral −1.5 mm, dorsal/ventral −3.5 mm.
Hippocampal Sample Preparation
As described previously [37], animals were sacrificed under anesthesia at the specific time points to process brain tissue and homogenates. Whole brains were removed quickly and the hippocampal CA1 tissues were microdissected from both sides of the hippocampal fissure and immediately frozen in dry ice. Tissues were homogenized using a glass homogenizer in ice-cold homogenization medium consisting of 50 mM HEPES, pH 7.4, 150 mM NaCl, 12 mM β-glycerophosphate, 3 mM dithiotheitol (DTT), 2 mM sodium orthovanadate (Na3VO4), 1 mM EGTA, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% Triton X-100, and Halt Protease/Phosphatase Inhibitor Cocktail (Pierce, Thermo Scientific). The homogenates were centrifuged at 15,000×g for 30 min at 4°C and supernatants were collected. Protein concentrations were determined by the Modified Lowry Protein Assay (Pierce, Rockford, ILL), and the samples were aliquoted and stored at −80°C until use.
Western blotting
For Western blot analysis, protein samples were boiled with Laemmli loading buffer for 5 min. Equal amounts of protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 4–20% Tris-glycine gel, and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore). The membranes were blocked in Odyssey Blocking Buffer for 1 h and incubated with primary antibodies at 4°C overnight. The antibodies against aromatase (sc-14245) and β-actin (sc-130656) were from Santa Cruz Biotechnology, Inc. using a 1:200 dilution. The membrane was then washed with PBS containing 0.1% Tween 20 to remove unbound antibody, followed by incubation with Alexa Fluor 680 goat anti-rabbit IgG or donkey anti-goat IgG for 1 h at room temperature. Bound proteins were visualized using the Odyssey Imaging System (LI-COR Bioscience, Lincoln, NB) and semi-quantitative analyses of the signals were performed with the Image J analysis software (Version 1.30v; NIH, USA). A mean ± SE was calculated from the data for graphical presentation and statistical comparison.
Quantification of 17β-estradiol Levels
17β-estradiol levels in hippocampal CA1 tissue were measured using a sensitive ELISA method as described previously by others [39]. The 17β-Estradiol ELISA (ADI-900-174, Enzo Life Sciences, Farmingdale, NY) used in our studies has a detection range of 15.6–1000 pg/ml, and a sensitivity of 14.0 pg/ml. The cross reactivity of the ELISA for other steroids is very low (testosterone = 0.01%, progesterone = 0.001%, estradiol-17alpha = 0.032%, ethinylestradiol-7alpha = 0.043%). Briefly, 100 μl of hippocampal sample was added to the bottom of the appropriate wells followed by the addition of 50 μl 17β-estradiol conjugated to alkaline phosphatase and 50 μl sheep polyclonal antibody to 17β-estradiol. The plate was then incubated at room temperature with shaking (~500 rpm) for 2 h. After 3 washes with 400 μl of wash buffer, 200 μl of the pNpp substrate solution was added into each well and incubated for 1 h at room temperature without shaking. Stop solution (50 μl) was added afterwards and the optical density was read at 405 nm. 17β-estradiol levels in the protein samples were determined based on an established standard and expressed as fmol/mg of sample protein.
Immunofluorescence Staining
At various time points after ischemic reperfusion, the animals were deeply anesthetized with isoflurane and perfused transcardially with 0.9% ice-cold saline followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). The brains were post-fixed, cryoprotected with 30% sucrose in 0.1 M PB (pH 7.4) at 4°C until they sank. The tissues were then embedded in optimal cutting temperature (OCT) compound and 20 μm-thick frozen coronal sections were cut through the coronal plane of the dorsal hippocampus (~2.5–4.5 mm posterior from bregma). Sections containing intact hippocampus were collected and utilized for double/triple immunofluorescence staining as described previously [40]. Briefly, coronal brain sections were blocked with 10% normal donkey serum for 1 h at room temperature in PBS containing 0.1% Triton X-100, followed by incubation with appropriate primary antibodies overnight. The following primary antibodies were used in different combinations: rabbit anti-NeuN (1:500, NBP1-92716, Novus Biologicals); mouse anti-GFAP (1:500, G3893, Sigma), rabbit anti-17β-estradiol (1:2, BioGenex); goat anti-aromatase (1:50, sc-14245) and mouse anti-Iba1 (1:50, sc-32725) from Santa Cruz Biotechnology. After primary antibody incubation, sections were washed 4 × 10 min at room temperature, followed by incubation with proper Alexa Fluor 594/647/488 donkey anti-goat/rabbit/mouse secondary antibody (1:500; Invitrogen Corporation, Carlsbad, CA) for 1 h at room temperature. Sections were then washed with PBS and briefly with water, and mounted with water-based mounting medium containing anti-fading agents (Biomeda, Fischer Scientific, Pittsburgh, PA). All the Confocal images were captured on an LSM510 Meta confocal laser microscope (Carl Zeiss, Germany) using either a 5X or 40X oil immersion Neofluor objective (NA, 1.3) with the image size set at 1024 X 1024 pixels. The captured images were viewed and analyzed using LSM510 Meta imaging software.
Histological Analysis
Histological examination was performed on free-floating coronal brain sections by NeuN staining, as described in detail previously by our laboratory [40]. Briefly, seven days after cerebral ischemia or sham ischemia, animals were deeply anesthetized and brain sections were prepared as mentioned above and stained with an antibody against NeuN (1:500, Millipore Bioscience). Images were captured on an LSM510 Meta confocal laser microscope (Carl Zeiss, Thornwood, NY). NeuN positive pyramidal cells with intact and round nuclei were counted as surviving cells. For quantitative assessment, the number of surviving neurons per 250 μm length of medial CA1 pyramidal cell layer was counted bilaterally in 3–5 representative sections (200 μm apart) per animal. The cell counts were then averaged to provide a single value for each animal. A Mean ± SE was calculated from the data in each group and statistical analysis performed as described below.
Statistical Analysis
All values were described as mean ± SE. Statistical analysis was performed using one-way analysis of variance (ANOVA) with SigmaStat 3.0 software (SPSS, Inc., Chicago, IL), followed by Student-Newman-Keuls post-hoc tests to determine group differences. When groups were compared to the sham group, Dunnett’s test was adopted for post-hoc analyses after ANOVA. Probability values of P < 0.05 were accepted to be statistically significant.
Results
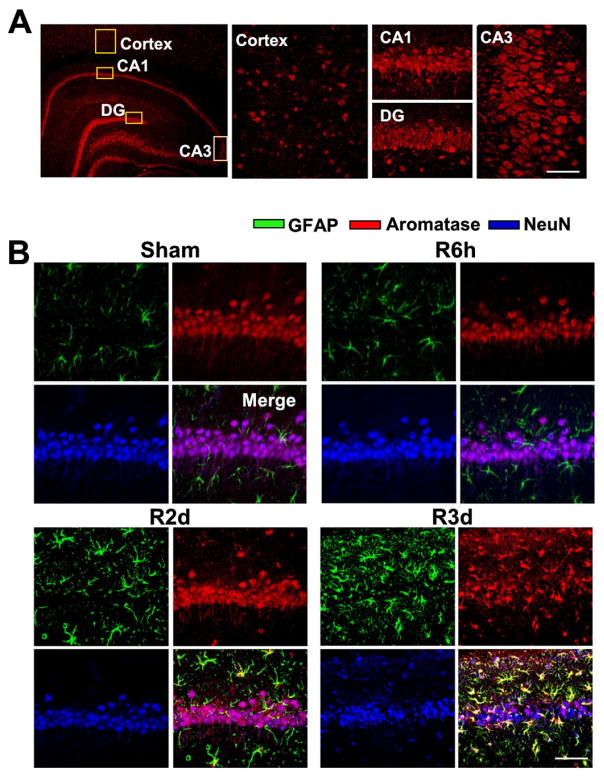
We first examined aromatase protein expression in the hippocampus and cortex of adult ovariectomized rats using immunohistochemistry. As shown in Figure 1A, the hippocampal regions CA1, CA3 and dentate gyrus (DG) showed high levels of immunoreactive aromatase staining, while the cortex displayed only moderate staining. We next examined the cell type of expression of aromatase in the hippocampal CA1 region and its temporal pattern following GCI. As shown in Figure 1B, triple immunohistochemical co-localization results for aromatase, a neuron (NeuN)-marker, and an astrocyte (GFAP)-marker revealed that non-ischemic sham animals display high aromatase immunostaining in neurons in the hippocampal CA1 region, with little co-localization observed in astrocytes. Similarly, at 6h reperfusion after GCI, aromatase was still primarily expressed in neurons in the CA1 region. However, at 2d reperfusion after GCI, in addition to neuronal co-localization of aromatase in the CA1 region, there was an increase of aromatase protein expression in GFAP-positive reactive astrocytes in the CA1 region. At 3d reperfusion after GCI, there is a very robust increase in GFAP-positive reactive astrocytes in the CA1 region that highly express aromatase protein.
Fig. 1. Aromatase protein expression in ovariectomized rat brain.
(A) Immunofluorescence staining for aromatase (red) in the hippocampus and cerebral cortex of ovariectomized adult rat brain. (B) Confocal analysis for GFAP (green), aromatase (red) and NeuN (blue), and merged images in the hippocampal CA1 region following sham ischemia and global cerebral ischemia (GCI) reperfusion (R) for 6 hours, 2 days and 3 days. Note that aromatase protein expression was elevated in astrocytes at 2–3 days after GCI reperfusion, as compared to sham animals. Representative confocal images are shown from 4–5 rats per group (Magnification: ×40, Scale bar = 50 μm).
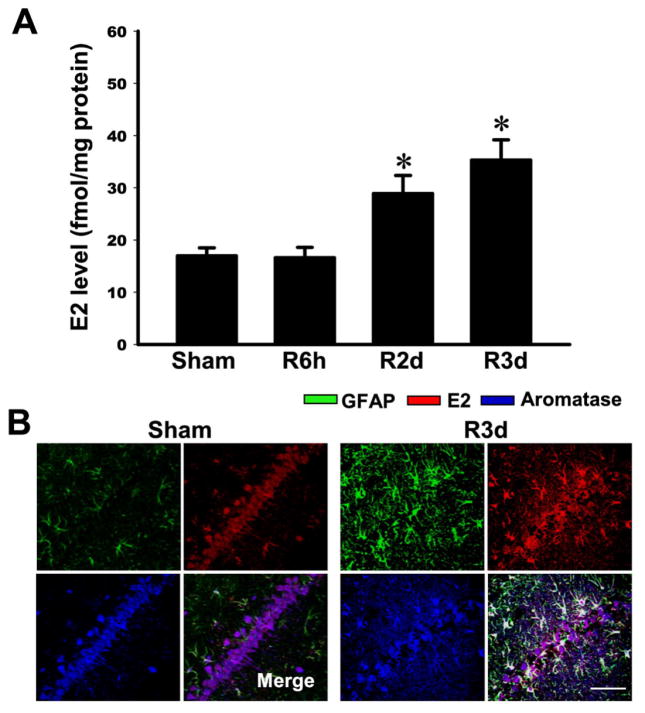
We next determined whether local E2 levels increase in the hippocampal CA1 region following GCI and whether the E2 increase occurs in aromatase-positive cells. Two approaches were used to measure E2. The first approach involved use of a highly sensitive ELISA to measure E2 from CA1 tissue homogenates (sham, reperfusion GCI 6h, 2d and 3d), while the second approach utilized triple immunohistochemistry with an E2 antibody, GFAP antibody, and aromatase antibody to determine co-localization of the three signals in CA1 region using CA1 sections (3d reperfusion GCI). As shown in Figure 2A, measurement of E2 levels in the CA1 region by a specific ELISA revealed that E2 levels at 6h reperfusion after GCI were similar to sham controls. In contrast, E2 levels showed a significant elevation at 2d and 3d reperfusion after GCI in the CA1 region as compared to the sham controls. Triple immunohistochemistry for E2, aromatase and GFAP in CA1 sections from sham animals revealed significant co-localization of E2 and aromatase, which was mainly observed in non-GFAP-positive cells (Figure 2B). This agrees with results of Fig. 1B that showed sham animals have aromatase primarily co-localized in neurons and not in astrocytes. Examination of CA1 sections at 3d reperfusion after GCI using triple immunohistochemistry revealed that all three signals (E2, aromatase and GFAP) are highly expressed and co-localized, which demonstrates that aromatase-positive reactive astrocytes in the hippocampal CA1 region show a robust increased generation of E2 at 3d reperfusion after GCI (Figure 2B). This finding suggests that the enhanced E2 levels observed in the CA1 region by ELISA at 3d reperfusion after GCI is due predominantly to the increased E2 production by aromatase-positive reactive astrocytes in the CA1 region.
Fig. 2. Elevation of E2 levels in the hippocampal CA1 region at 2–3 days post global cerebral ischemia (GCI) reperfusion in ovariectomized rats.
(A) E2 levels were measured by a highly sensitive and specific E2 ELISA using protein samples from hippocampal CA1 region following different time of reperfusion. Data are expressed as mean±SE from 4–5 rats per group. *P<0.05 vs. sham. (B) Representative confocal analyses indicate that E2 colocalizes with aromatase protein in astrocytes at 3 days after GCI reperfusion (n= 4–5 per group, Magnification: ×40, Scale bar = 50 μm).
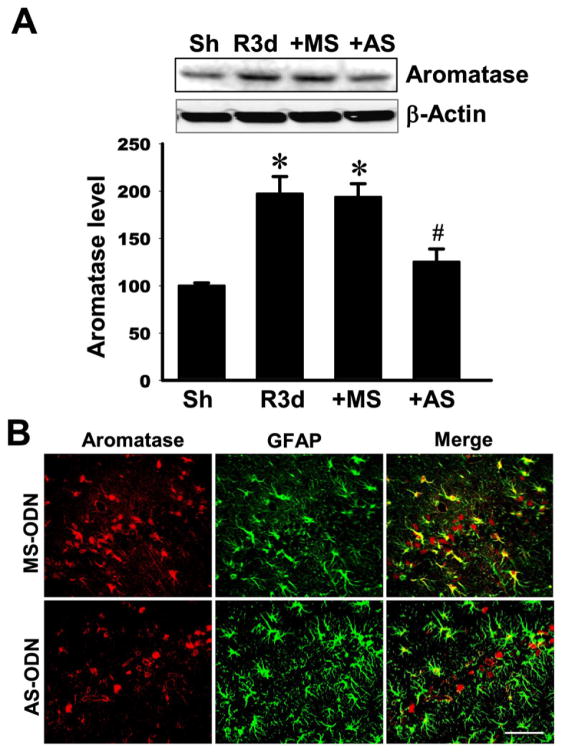
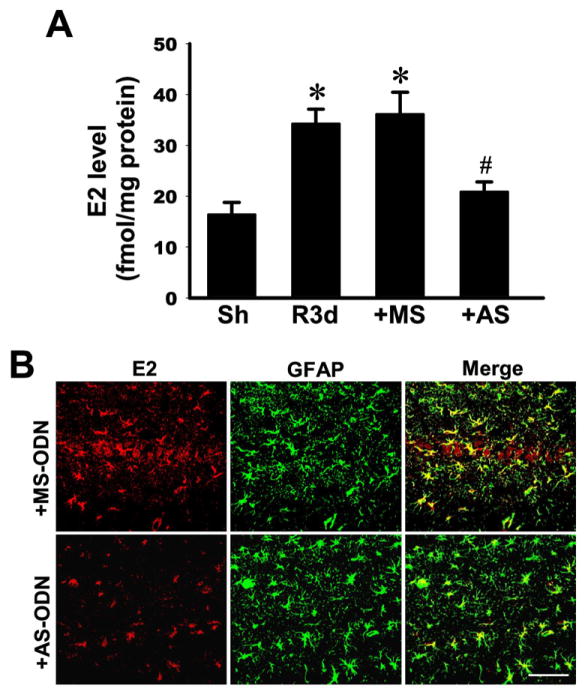
We next examined whether the elevation of aromatase and local E2 in the hippocampal CA1 region after GCI has a neuroprotective or anti-inflammatory role. To answer this question, we utilized an antisense oligonucleotide knockdown approach to knockdown aromatase protein levels in the hippocampal CA1 region. Toward this end, missense scrambled (MS) or antisense (AS) oligonucleotides to aromatase were administered intracebroventrically (icv) into the lateral ventricle continuously via a mini-osmotic pump (implanted subcutaneously in upper mid-back region). MS or AS oligonucleotides were administered continuously icv beginning 3 days before GCI and continuing until the time of sacrifice. As shown in Figure 3A, aromatase immunoreactive protein levels in the hippocampal CA1 region at 3d reperfusion (R3d) were significantly increased as compared to the sham control group (Sh). MS oligonucleotides administration had no significant effect upon aromatase immunoreactive protein levels in the CA1 region. In contrast, AS oligonucleotides to aromatase significantly attenuated aromatase immunoreactive protein levels in the hippocampal CA1 region, as compared to MS control and the R3d groups. Double immunohistochemistry confirmed that AS administration markedly suppressed aromatase immunoreactive protein in the hippocampal CA region at 3d reperfusion after GCI, with much of the previously observed increased in aromatase expression in GFAP-positive reactive astrocytes after GCI markedly attenuated (Figure 3B). The effect of aromatase antisense knockdown upon local E2 levels in the hippocampal CA1 region was next examined using a specific ELISA and immunohistochemistry approaches. As shown in Figure 4A, MS oligonucleotide treatment had no effect upon the reperfusion 3d increase of E2 in the hippocampal CA1 region. In contrast, AS treatment markedly attenuated local E2 levels in the CA1 region, as compared to the R3d and MS control groups. In further confirmation of this finding, double immunohistochemistry for E2 and GFAP also showed strongly reduced E2 staining in GFAP-positive astrocytes at reperfusion 3d in the AS-treated group, as compared to the MS control group (Figure 4B).
Fig. 3. Effectiveness of antisense oligodeoxynucleotides (AS-ODN) knockdown of aromatase protein level in the hippocampal CA1 region at 3 days after global cerebral ischemia (GCI) reperfusion.
(A) Protein samples from sham, GCI reperfusion 3 days, GCI reperfusion 3 days plus aromatase AS-ODN or mismatch scramble (MS)-ODN were analyzed by Western blotting with antibodies specific for aromatase and β-Actin. *P<0.05 vs. sham. #P<0.05 vs. MS group. N=4–5. (B) Representative confocal images showing profound attenuation of aromatase protein expression in astrocytes at 3 days after GCI reperfusion (Magnification: ×40, Scale bar = 50 μm).
Fig. 4. Effectiveness of aromatase antisense oligodeoxynucleotide (AS-ODN) knockdown in reducing brain-derived E2 levels in the hippocampal CA1 region at 3 days post global cerebral ischemia (GCI) reperfusion.
(A) E2 levels in the indicated groups were measured by a specific E2 ELISA using protein samples from hippocampal CA1 region at 3 days post GCI reperfusion. Data were expressed as mean ± SE from 4–5 rats per group. *P<0.05 vs. sham, #P<0.05 vs. MS group. N=3–5. (B) Representative confocal images showing the reduction of brain-derived E2 levels in CA1 region astrocytes at 3 days after GCI reperfusion (Magnification: ×40, Scale bar = 50 μm).
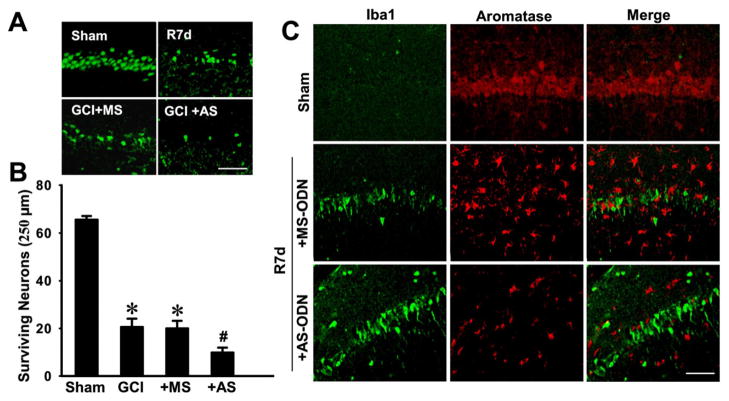
We next examined whether AS knockdown of aromatase and corresponding reduction of local E2 levels in the hippocampal CA1 affected neuronal cell survival and/or microglial activation after GCI. As shown in Figure 5A&B, GCI induced significant neuronal cell death at 7-days after GCI as indicated by a decrease in NeuN-positive surviving neurons in the hippocampal CA1 region, as compared to sham control. MS oligonucleotide administration had no effect upon the number of surviving neurons as compared to the GCI control. In contrast, the AS oligonucleotide group showed a greater loss of surviving neurons as compared to the MS and GCI control groups. Furthermore, double immunostaining of hippocampal CA1 sections at 7 d after GCI for aromatase and iba1, a marker of activated microglia, revealed that aromatase is not colocalized in activated microglia (Fig 5C). The results also revealed that aromatase AS knockdown animals had increased iba1 staining in the CA1 region as compared to the MS control, suggesting that, in addition to a neuroprotective effect, brain-derived E2 may also exert an anti-inflammatory effect following ischemic injury.
Fig. 5. Antisense oligodeoxynucleotide (AS-ODN) knockdown of aromatase and brain-derived E2 levels in the hippocampal CA1 region of ovariectomized rats leads to enhanced neuronal damage and microglia activation following global cerebral ischemia (GCI).
(A) Typical staining with NeuN of hippocampal sections from sham, GCI reperfusion 7 days (R7d), or GCI reperfusion 7 days aromatase AS-ODN or MS-ODN groups. (B) Quantitative analyses of the number of surviving neurons per 250μm length of medial CA1. NeuN-positive CA1 pyramidal cells showing intact and round nuclei were counted as surviving cells. Data are means±SE. *P<0.05 vs. sham, #P<0.05 vs. MS group. N = 5 per group. (C) Typical confocal microscopy images showing the double staining of iba1 and aromatase in hippocampal CA1 region from sham and GCI post reperfusion 7 days rats pretreated with aromatase AS-ODN or MS-ODN. Note that aromatase knockdown in ovariectomized rats leads to enhanced microglia activation, and that aromatase was not expressed in activated microglial cells. Scale bar, 50 μm. Magnification, 40×.
Discussion
It has been known for some time that gonadal-derived and exogenous E2 can protect the vulnerable hippocampal CA1 region from GCI-induced neuronal damage and cell death [1, 40–42]. The results of the current study add to our understanding by revealing for the first time an important neuroprotective and anti-inflammatory role of local brain-derived E2 in the rat hippocampus CA1 region following GCI. Clinically, GCI can occur due to cardiac arrest, asphyxiation, and hypotensive shock. Of these, cardiac arrest is the most prevalent and remains a leading cause of neurological damage, cognitive defects and mortality. Post-mortem studies have shown that the human hippocampus, like rodents, displays significant expression of aromatase, and thus a role for local-derived E2 in neuroprotection and anti-inflammatory actions in the hippocampus may also extend to humans [16, 43].
The current study used an antisense oligonucleotide knockdown approach to decrease aromatase expression and local E2 levels in the hippocampal CA1 region. Previous work by our group and others have validated that fluorescent-tagged oligonucleotides injected into the lateral ventricle reach and are predominantly localized in the hippocampal CA1 region [40]. In the current study, we confirmed the effectiveness of the AS knockdown of aromatase (and local E2) via several approaches that included 1) Western blot for aromatase, 2) immunohistochemistry for aromatase and E2, and 3) a highly specific and sensitive ELISA for E2. Using these approaches, we confirmed that chronic aromatase AS treatment (but not MS oligonucleotide treatment) resulted in a significant attenuation of aromatase expression and local E2 levels in the hippocampal CA1 region. We should mention that preliminary studies using a different approach, icv administration of an aromatase inhibitor, letrozole, confirmed a neuroprotective and anti-inflammatory role of brain-derived E2 in the hippocampus (unpublished observations). In addition to the neuroprotective and anti-inflammatory role for local E2 revealed in our study, work by other investigators has provided evidence that brain-derived E2 can also regulate synaptic plasticity [19, 21, 26–28], LTP [29, 30], and protect the hippocampus from excitotoxic damage [33].
An important question is which cell type in the hippocampus mediates the neuroprotective and anti-inflammatory effects of local-derived E2 observed in our study? To address this issue, we used double or triple immunohistochemistry for aromatase or E2 and cell-specific markers for astrocytes (GFAP), neurons (NeuN), and activated microglial (iba1) so as to identify the cell type of E2 production in the non-ischemic and ischemic states. Our studies revealed that aromatase and E2 are co-localized in both neurons and activated astrocytes, but do not appear to be expressed/produced in activated microglia in the CA1 region. Under basal, non-ischemic conditions (e.g. in sham animals), aromatase and E2 were co-localized predominantly in neurons in the hippocampal CA1 region, with little co-localization/expression found in astrocytes. In contrast, at 3d after ischemic reperfusion, the situation is reversed, with aromatase and E2 expression predominantly observed in reactive astrocytes, with less co-localization/expression observed in neurons.
Currently, we cannot determine conclusively whether the neuroprotective and anti-inflammatory effects of local E2 observed in our study are due to neuronal- or astrocyte-derived E2, or both. However, we did observe an ischemia-induced increase of hippocampal CA1 region E2 levels by ELISA and immunostaining at 2d and 3d after GCI reperfusion. Based on co-localization immunohistochemistry studies, the increased E2 levels appeared to be due exclusively to increased aromatase expression and E2 generation in reactive astrocytes. Administration of aromatase antisense oligonucleotides blocked the astrocyte-derived increase of E2 after GCI, an effect that was correlated with enhanced neurodegeneration and neuroinflammation following GCI. This finding suggests that astrocytes are the major source of the increased local E2 after GCI, and that astrocyte-derived E2 may mediate beneficial neuroprotective and anti-inflammatory actions in the hippocampal CA1 region following GCI. Nevertheless, a role for neuronal-derived E2 cannot be entirely ruled out. Previous in vitro studies using highly purified hippocampal neurons have shown that neuron-generated E2 can also be strongly neuroprotective [44]. Furthermore, our aromatase antisense knockdown approach, in addition to attenuating the astrocyte-generated E2 after GCI, likely would also reduce basal neuron-localized aromatase/E2, since it was initiated 3 days prior to GCI. Thus, while we believe that astrocyte-derived aromatase and E2 may in large part mediate the neuroprotective and anti-inflammatory effects we observed in the hippocampal CA1 region after GCI, we cannot completely exclude a role for neuronal-derived E2. Further studies will be needed in the future to fully address this issue.
Finally, previous work has suggested that crosstalk may exist between gonadal/exogenous E2 and brain-derived E2. For instance, a recent in vitro study found that the ability of exogenous E2 to enhance synaptic plasticity and exert neuroprotection in hippocampal H19-7 cells was lost if local E2 production by the hippocampal cells was inhibited by aromatase inhibitor treatment [45]. This finding raises the possibility that the beneficial neural effects of exogenous E2 may require priming, mediation and/or coordination by local brain-derived E2. Further work is needed to address this issue.
In conclusion, the current study provides evidence of an important role for brain-derived E2 in exerting neuroprotection and anti-inflammatory actions in the hippocampal CA1 region after GCI. Astrocyte-derived E2 increased significantly after GCI and may mediate the observed neuroprotective anti-inflammatory effects, although a role for neuron-derived E2 cannot entirely be ruled out. Taken as a whole, these findings add to a growing body of literature suggesting that brain-derived E2 has important roles and functions in the brain in a variety of species.
Highlights.
Aromatase is highly expressed in neurons in the hippocampus in non-ischemic rats.
Aromatase and local 17beta-estradiol increase in hippocampal astrocytes after global cerebral ischemia.
In vivo knockdown of aromatase leads to greater global ischemic damage and increased microglial activation in the hippocampus
Brain-derived 17beta-estradiol exerts neuroprotection and anti-inflammatory effects in the hippocampus following global cerebral ischemia
Acknowledgments
This research was supported by Research Grant (NS050730) from the National Institutes of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao J, Brinton RD. Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer’s disease. Adv Pharmacol. 2012;64:327–71. doi: 10.1016/B978-0-12-394816-8.00010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpkins JW, Singh M, Brock C, Etgen AM. Neuroprotection and estrogen receptors. Neuroendocrinology. 2012;96:119–30. doi: 10.1159/000338409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourque M, Dluzen DE, Di Paolo T. Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson’s disease. Front Neuroendocrinol. 2012;33:169–78. doi: 10.1016/j.yfrne.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–65. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 6.Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, et al. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- 7.Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. ILAR J. 2004;45:147–59. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- 8.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–9. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 9.Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28:8430–41. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–30. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 11.Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, et al. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–8. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Shughrue PJ, Merchenthaler I. Estrogen prevents the loss of CA1 hippocampal neurons in gerbils after ischemic injury. Neuroscience. 2003;116:851–61. doi: 10.1016/s0306-4522(02)00790-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784:321–4. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]
- 14.Veiga S, Azcoitia I, Garcia-Segura LM. Extragonadal synthesis of estradiol is protective against kainic acid excitotoxic damage to the hippocampus. Neuroreport. 2005;16:1599–603. doi: 10.1097/01.wnr.0000179081.39659.7d. [DOI] [PubMed] [Google Scholar]
- 15.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–47. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Higaki S, Takumi K, Itoh M, Watanabe G, Taya K, Shimizu K, et al. Response of ERbeta and aromatase expression in the monkey hippocampal formation to ovariectomy and menopause. Neurosci Res. 2012;72:148–54. doi: 10.1016/j.neures.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, et al. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–27. doi: 10.1016/j.brainres.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fester L, Prange-Kiel J, Jarry H, Rune GM. Estrogen synthesis in the hippocampus. Cell Tissue Res. 2011;345:285–94. doi: 10.1007/s00441-011-1221-7. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Segura LM. Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol. 2008;20:705–12. doi: 10.1111/j.1365-2826.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 21.Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, et al. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochimica et biophysica acta. 2010;1800:1030–44. doi: 10.1016/j.bbagen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Brain Res Mol Brain Res. 1994;24:227–37. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 23.Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem Mol Biol. 1999;70:237–41. doi: 10.1016/s0960-0760(99)00114-4. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes P. Estrogen receptor ss activity modulates synaptic signaling and structure. J Neurosci. 2010;30:13454–60. doi: 10.1523/JNEUROSCI.3264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–5. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–21. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–42. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 28.von Schassen C, Fester L, Prange-Kiel J, Lohse C, Huber C, Bottner M, et al. Oestrogen synthesis in the hippocampus: role in axon outgrowth. J Neuroendocrinol. 2006;18:847–56. doi: 10.1111/j.1365-2826.2006.01484.x. [DOI] [PubMed] [Google Scholar]
- 29.Grassi S, Tozzi A, Costa C, Tantucci M, Colcelli E, Scarduzio M, et al. Neural 17beta-estradiol facilitates long-term potentiation in the hippocampal CA1 region. Neuroscience. 2011;192:67–73. doi: 10.1016/j.neuroscience.2011.06.078. [DOI] [PubMed] [Google Scholar]
- 30.Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, et al. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–26. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16:676–83. doi: 10.1111/j.1365-2826.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 32.Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56:97–105. doi: 10.1002/glia.20594. [DOI] [PubMed] [Google Scholar]
- 33.Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47:318–29. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- 34.Neumann JT, Cohan CH, Dave KR, Wright CB, Perez-Pinzon MA. Global cerebral ischemia: synaptic and cognitive dysfunction. Curr Drug Targets. 2013;14:20–35. doi: 10.2174/138945013804806514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–72. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 37.Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28:8430–41. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang QG, Wang RM, Scott E, Han D, Dong Y, Tu JY, et al. Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause. Brain. 2013;136:1432–45. doi: 10.1093/brain/awt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S, Asakawa T, Ding S, Liao L, Zhang L, Shen J, et al. Chaihu-shugan-san administration ameliorates perimenopausal anxiety and depression in rats. PLoS One. 2013;8:e72428. doi: 10.1371/journal.pone.0072428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–36. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–24. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merchenthaler I, Dellovade TL, Shughrue PJ. Neuroprotection by estrogen in animal models of global and focal ischemia. Annals of the New York Academy of Sciences. 2003;1007:89–100. doi: 10.1196/annals.1286.009. [DOI] [PubMed] [Google Scholar]
- 43.Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. doi: 10.1016/j.brainres.2009.09.111. [DOI] [PubMed] [Google Scholar]
- 44.Cui J, Wang Y, Dong Q, Wu S, Xiao X, Hu J, et al. Morphine protects against intracellular amyloid toxicity by inducing estradiol release and upregulation of Hsp70. J Neurosci. 2011;31:16227–40. doi: 10.1523/JNEUROSCI.3915-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chamniansawat S, Chongthammakun S. A priming role of local estrogen on exogenous estrogen-mediated synaptic plasticity and neuroprotection. Exp Mol Med. 2012;44:403–11. doi: 10.3858/emm.2012.44.6.046. [DOI] [PMC free article] [PubMed] [Google Scholar]