Abstract
Background
Mast cells play a critical role in inflammatory skin diseases through releasing pro-inflammatory mediators; however, few therapies directly target these cells. In 1878, the use of topical Thymol, a now recognized potent agonist for Transient Receptor Potential (TRP) channels, was first described to treat eczema and psoriasis.
Objective
We sought to determine the mechanisms through which thymol may alter skin inflammation.
Methods
We examined the effect of topical thymol on IgE-dependent responses using a mast cell–dependent passive cutaneous anaphylaxis (PCA) model as well as in vitro cultured mast cells.
Results
Thymol dose-dependently inhibited PCA when administered topically 24 hours prior to antigen challenge but provoked an ear swelling response directly on application. This direct effect was associated with local mast cell degranulation and was absent in histamine-deficient mice. However, unlike with PCA responses, there was no late phase swelling. In vitro, thymol directly trigged calcium flux in mast cells via TRP-channel activation, along with degranulation and cytokine transcription. However, no cytokine protein was produced. Instead, thymol induced a significant increase in apoptotic cell death that was seen both in vitro and in vivo.
Conclusions
We propose that the efficacy of thymol in reducing IgE-dependent responses is through promotion of activation-induced apoptotic cell death of mast cells and that this likely explains the clinical benefits observed in early clinical reports.
Keywords: mast cell, thymol, calcium, passive cutaneous anaphylaxis
Introduction
Mast cells have an important role in the pathogenesis of allergic disease.1, 2 As tissue resident cells, they are strategically situated at host-environment interfaces such as the skin, airway, and gastrointestinal tract ready to respond to immunogenic stimuli.3 In a variety of acute and chronic conditions, mast cell accumulation, activation, and release of pro-inflammatory mediators are critical to initiate and propagate inflammation.4 Several approaches to treating allergic disease have targeted pathways that involve mast cells and their mediators, including desensitization through immunotherapy, leukotriene and histamine receptor inhibitors, tyrosine kinase inhibitors, and anti-IgE antibodies.5-7 Mast cells are long-lived cells and survive repeated activation;8 altering their proliferation and survival with a view to reducing their numbers has recently been proposed as a potential approach for therapeutic intervention for allergic diseases.8, 9
Clinically, these cells are well recognized for their participation in the pathogenesis of inflammatory skin diseases, such as atopic dermatitis (AD). Indeed, AD is characterized by a dramatic increase in the number of IgE+ mast cells present in the skin.10, 11 In atopic diseases, their activation occurs in part by cross-linking the high-affinity IgE receptor, FcεRI, leading to histamine-containing granule release and de novo production of arachidonic acid metabolites, cytokines, and chemokines that alter vascular permeability and promote skin inflammation.12-17 In addition to IgE receptors and other activating receptors, mast cells have recently been shown to also express several transient receptor potential (TRP) channels that function to sense environmental changes, including temperature, pressure, and other sensations. Since the skin is the one of the primary barriers interacting with environmental stressors, regulating TRP channel signals may be able to modulate mast cell-mediated skin inflammation.
In the British Journal of Medicine in 1878, Henry Radcliffe Crocker reported that topical thymol, now a known TRP-channel agonist, could be used as a remedy for patients with eczema with improvement noted in advanced lesions unresponsive to conventional therapy.18 Crocker applied topical thymol either as an ointment dissolved in vaseline or a lotion dissolved in a mixture of ethanol and glycerin and referred to as “stimulant therapy,” since tingling occurred upon initial application and this was followed by rapid improvement of the skin lesions.18 Thymol is a monocyclic phenolic compound found in thyme (Thymus vulgaris), part of the Lamiacea family of plants.19 It is widely used at low concentrations in antiseptic mouthwashes20, in part for its antibiotic,21, 22 antifungal,23 and antioxidant 24, 25 properties. In the last few years, studies revealed that thymol is a ligand for the TRPV3 and TRPA1 channels,26, 27 members of this diverse family of temperature-sensitive cation channels.28 Despite this very early clinical report, the use of thymol in treating skin inflammation has not been further pursued, perhaps because the underlying mechanisms are unknown. Studies investigating the effect of thymol in vitro have shown that low thymol concentrations of can promote calcium mobilization29-31 and protect cells from DNA damage,32, 33 radiation-induced cytotoxicity,25 and oxidative stress.34 Conversely, at higher concentrations, it inhibits cell proliferation and can induce apoptosis in human and murine cancer cell lines.29, 35-38 Thinking about the effects of thymol in the context of TRP-expressing mast cell functions, we sought to examine the effects of thymol on allergen-triggered skin inflammation.
Our findings demonstrate thymol treatment leads to sustained calcium flux in mast cells and a significant reduction in their survival. Uncontrolled calcium signaling is a hallmark mechanism that diminishes cell survival by promotion of activation-induced cell death (AICD)-associated apoptosis.39 While calcium flux is also a hallmark of IgE stimulation via FcεRI, mast cells are resistant to AICD due to the concomitant production of nitric oxide production.40 Conversely, thapsigargin, a calcium pump inhibitor which robustly mobilizes calcium, has been shown to drive AICD in mast cells.41 Here we show that thymol promotes calcium signaling in mast cells via TRP activation and that thymol-activated mast cells undergo apoptosis likely through AICD. Functionally, this induced death is sufficient to prevent anaphylactic responses upon antigen exposure in IgE primed animals. Taken together, our findings suggest that promoting mast cell death could be a novel approach to limiting atopic disease. Furthermore, our study provides the first mechanistic insights into the previously observed clinical benefits of topical thymol.
Methods
Reagents
Thymol, ruthenium red, HC-030031, 2-APB, ionomycin, anti-DNP-IgE, DNP-HSA, and probenecid were purchased from Sigma-Aldrich (St. Louis, MO). Annexin V, Sytox, and Fluo-4-AM were purchased from Invitrogen (Carlsbad, CA). Anti-CD117 and anti-CD16/32 were purchased from BD Pharmingen (San Diego, CA), and anti-FcεRI from eBioscience (San Diego, CA).
Animals
C57/BL6 and BALB/c mice (4-8 weeks old) were obtained from Taconic Farms (Hudson, NY). HDC-/- mice, deficient in histamine, were previously described.42 All animal studies were performed under guidelines for care and welfare by IACUC under protocols approved by the Northwestern University Animal Care and Use Committee.
Ear Swelling
For thymol-induced ear swelling, 10 μL of thymol or DMSO was administered to both sides of the ear and for passive cutaneous anaphylaxis, anti-DNP-IgE (100 ng) was intradermally injected into a mouse ear followed 24 hours later by topical thymol (20 μL per ear) followed 24 hours later by intravenous injection of DNP-HSA (100 μg). Ear swelling was measured with thickness gauge calipers.
Histology
Mice were euthanized 12 and 24 hours after thymol-induced ear swelling. Ear tissue was fixed in formalin and embedded in paraffin. Tissue sections were stained with pinacyanol erythrosinate (PE) as previously described.43 Mast cell degranulation was determined by counting cells with dense granules and compact shape versus those with dispersed granules outside the cell. 20 high-powered fields were assessed per sample in a blinded fashion.
MC cultures
MC/9 cells were obtained from American Type Culture Collection and bone marrow-derived mast cells (BMMC) were obtained from C57/BL6, as previously described.44
β-Hexosaminidase Assay
MC/9 cells were incubated with thymol or 48/80 (50 μg/mL) for 40 minutes at 37°C. The supernatants and cell lysates were collected. Degranulation was assessed by measuring the release of β-Hexosaminidase as previously described.45
Real-time RT-PCR
Total RNA and cDNA were prepared as previously described.46 Gene expression was determined using specific Taqman probes (Applied Biosystems, Foster City, CA). β-actin was used as a housekeeping gene for analysis of changes in cycle threshold. Fold induction for treated samples was determined based on vehicle treated samples.
Cytokine Measurement
MC/9 cells were incubated with thymol or ionomycin (1 μg/mL) for 18 hours. The cells were centrifuged and supernatant was collected and analyzed by standard ELISA.
Annexin-V/Sytox
BMMCs were incubated with thymol for 6 hours and stained with Annexin V according to the manufacturer's protocol (Invitrogen). The cells were co-stained with Sytox and analyzed by flow cytometry.
Intraperitoneal Thymol
C57/BL6 mice were injected i.p. with thymol (100 μg in 200 μL PBS + 0.5% EtOH). At the indicated time points, mice were euthanized and the peritoneal cavity was lavaged with cold PBS (6 mL) and collected. 107 cells from this were blocked with anti-CD16/32, stained with anti-c-kit (CD117)/anti-FcεRI, then stained with annexin-V/Sytox, and analyzed by flow cytometry.
Caspase-3 Activation
BMMCs were incubated with thymol for 6 or 24 hours and stained for activated capsase-3 according to the manufacturer's instructions (BD Pharmingen), then analyzed by flow cytometry.
DNA fragmentation/clumping
BMMCs were incubated with thymol for 24 hours. For DNA clumping, cells were labeled with Hoechst 33342 and assessed by fluorescence microscopy. For DNA fragmentation, cells were processed and analyzed as previously described.47
Calcium Flux
BMMCs were loaded with 1.5 μM Fluo-4 for 30 minutes at 37°C in loading buffer (Ca2+/Mg2+-free HBSS + 2 mM Probenecid + 0.1% BSA). The cells were washed and incubated at 37°C for 25 minutes in loading buffer +1.8 mM anhydrous CaCl2. The cells were allowed to equilibrate to room temperature and analyzed by flow cytometry for 25 seconds to establish a baseline before thymol was added and analyzed for 60 seconds. Data was analyzed using the Flowjo kinetics platform to visualize a change in FITC over time. Inhibitors for were incubated with MCs for 1 hour at 37°C. For dose curve and inhibitor studies, flux data was divided into pre- and post-stimulation subsets and median values were subtracted from each other to determine Δ median FITC. Relative calcium flux was determined by comparison to maximal flux for wildtype BMMCs.
Statistics
Data provided as mean ± SEM. Statistical significance was determined using 2-tailed student t test, ANOVA (Dunnet's test), or non-linear regression, as appropriate. All analysis was done using GraphPad Prism (La Jolla, CA)
Results
Thymol suppresses mast cell-mediated passive cutaneous anaphylaxis
In order to first test whether thymol could modulate mast cell-mediated skin inflammation in vivo, we investigated the effect of thymol on responses during passive cutaneous anaphylaxis (PCA), an animal model of skin inflammation mediated by antigen-specific IgE, that we and others have demonstrated is highly mast cell dependent.44, 48 PCA was induced by intradermal sensitization of anti-DNP-IgE in the ear followed by systemic intravenous DNP-HSA challenge to cross-link the anti-DNP IgE bound on the surface of mast cells, where a biphasic inflammatory response occurs with initial ear swelling 1 hour after challenge from histamine release followed by cytokine-mediated secondary response approximately 24 hours later.49 The previously published protocol was modified to allow for topical thymol treatment 24 hours prior to antigen-specific DNP-HSA challenge at 48 hours. Topical thymol dose-dependently suppressed PCA [Fig 1a] with significant differences at both the early (1 hour) and the late (24 hours) phase of the PCA response [Fig 1b], suggesting that topical thymol treatment indeed could modulate mast cell function in such a way as to diminish mast cell responses upon triggering by antigen-specific IgE.
Figure 1. Thymol inhibits passive cutaneous anaphylaxis.
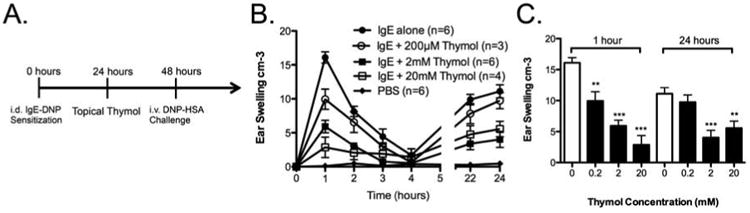
A, Schematic of PCA with thymol treatment. B, Ear thickness measurements after DNP-HSA challenge. C, Comparison of PCA-induced ear thickness at 1 and 24 hours. n=3-6 mice per group from 2 independent experiments, **=p < 0.01, ***=p<0.005 by 2-way ANOVA.
Thymol induced histamine-dependent ear swelling associated with mast cell degranulation
To next test the direct effect of thymol on cutaneous mast cells in the absence of other stimuli, such as antigen-specific IgE in the previous experiment, we measured the ear swelling response after topical thymol administration. When administered alone to the mouse ear, topical thymol can directly activate mast cells, since a dose-dependent immediate ear swelling occurred in both C57BL/6 and BALB/c mice resolved by 24 hours and remained unchanged for 48 hours [Fig 2a]; these results suggested that thymol can directly activate mast cells. It is not clear why there were strain to strain differences in the threshold concentration of thymol needed to elicit ear swelling, with BALB/c mice requiring a ten-fold greater concentration. We next assessed the ratio of intact mast cells to degranulated mast cells from pinacyanol-erythrosinate–stained paraffin-embedded ear tissue 24 hours after topical thymol and observed a significant increase in the percent of degranulated MCs at higher concentrations of thymol [Fig 2b-c], suggesting that thymol activates mast cells in vivo to degranulate. Since immediate ear swelling is dependent on histamine release from degranulated mast cells, we further examined the requirement for histamine in thymol-induced ear swelling by examining histidine decarboxylase knockout (HDC-/-) mice, which lack the ability to convert histidine to histamine.42 Thymol had minimal effect on ear swelling in HDC-/- mice as compared to wildtype mice, similar to vehicle alone [Fig 2d], suggesting that histamine was necessary for thymol-induced ear swelling. Similar results were found for mast cell deficient mice (data not shown). No discernable abnormality was observed either grossly or histologically 24 or 48 hours after topical thymol, suggesting a targeted effect on cutaneous mast cells. Interestingly, despite inducing acute cutaneous mast cell activation within an hour of administration, no sustained late phase activation was observed after thymol stimulation alone, unlike seen with PCA, suggesting that mast cell activation may have been halted prematurely.
Figure 2. Topical thymol directly promotes local mast cell activation.
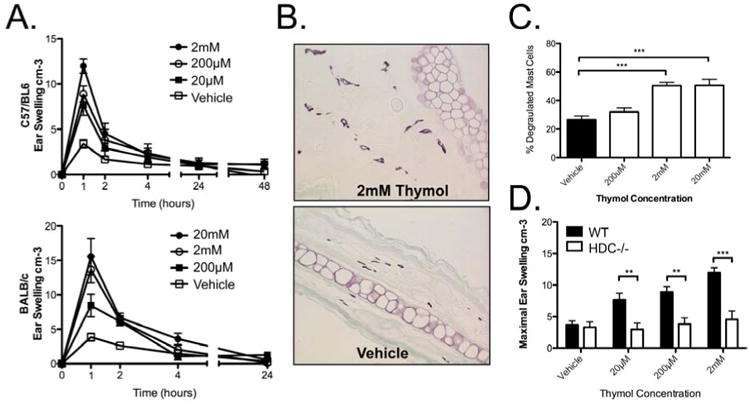
A, Topical thymol-induced ear swelling in C57/BL6 and BALB/c mice. B, Representative ear histology. C, Quantification for degranulated mast cells by PE stain in BALB/c mice. D, Ear thickness 1 hour after topical thymol in C57/BL6 and HDC-/- mice. n=6-21 mice per group from 3 (A, D) and 2 (C) independent experiments., **=p < 0.01, ***=p<0.005 by 1-way ANOVA.
Thymol induced calcium flux in BMMCs
Mast cell degranulation and histamine production is Ca2+ dependent and thymol has previously been shown to mobilize calcium stores in glioblastoma,29 osteoblastoma,31 and pituitary GH3 cells.30 To test whether thymol directly affects mast cell Ca2+ flux, BMMCs were load with fluo-4-AM and analyzed by flow cytometry before and after thymol stimulation. Calcium flux was determined on Flowjo software using the kinetics platform to assess change in FITC over time. Indeed, thymol induced a dose-dependent calcium flux in mast cells [Fig 3a/b], consistent with the previous experiments suggesting that thymol activates mast cells.
Figure 3. Thymol induced calcium flux in mast cells.
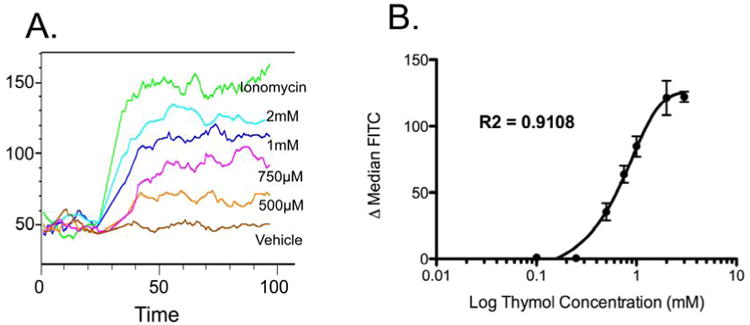
A, Representative kinetics plot. B, Graphical representation of difference between median pre- and post-stimulation FITC signal. n = 4-6 samples per group from 4 independent experiments.
Thymol induced calcium flux is partially mediated by triggering TRPA1
Our data show that thymol regulates calcium flux in mast cells. Since thymol is known to transduce signaling through TRP channels and several TRP channels are expressed on mast cells, we focused on testing whether thymol-induced calcium flux was activated by the thymol-activated TRPA1 channel,26, 27 which has previously been shown to be expressed on mast cells.50 First, we assessed thymol-induced calcium flux after pre-incubation with Ruthenium Red, a non-specific TRP channel inhibitor. Ruthenium red dose-dependently decreased thymol-induced calcium flux [Fig 4a], suggesting that a TRP channel may be partially responsible for the interaction of thymol with mast cells. We then tested a previously described selective antagonist for TRPA1, HC-030031,51 along with 2-APB, a pharmacologic antagonist of IP3R-mediated Ca2+ release.52 After pre-incubation with MCs, HC-030031 and 2-APB dose-dependently decreased thymol-induced calcium flux [Fig 4b-c]. Importantly, ionomycin-induced calcium flux was maintained after inhibitor treatment, suggesting a targeted effect (data not shown). Therefore, thymol acts via TRPA1 and IP3R to intracellularly mobilize calcium within the mast cell.
Figure 4. Thymol induced calcium flux through TRPA1 and not TRPV3.
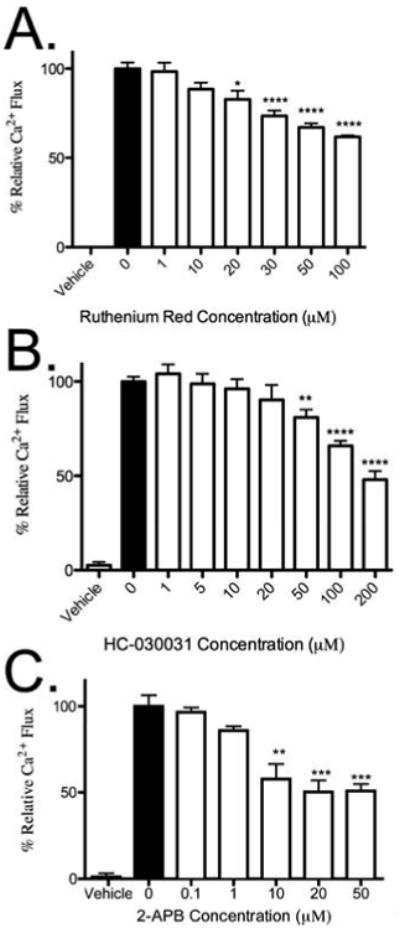
A, Relative calcium flux from 800 μM thymol in BMMCs after 1-hour incubation with Ruthenium Red. B, Relative calcium flux from 800 μM thymol in BMMCs after 1-hour incubation with HC-030031. C, Relative calcium flux from 800 μM thymol in BMMCs after 1-hour incubation with 2-APB. n = 3 samples per group from 3 independent experiments. **=p < 0.01, ***=p<0.005 by 1-way ANOVA.
Thymol induced mast cell degranulation and gene transcription in vitro but prevented protein production
To better understand the downstream effect of direct thymol activation on mast cell-degranulation and cytokine production, we examined in vitro degranulation as well as cytokine mRNA expression and protein production after treating MC/9 cells, a murine fetal liver-derived IL-3 dependent mast cell line. As shown in Fig 5a, ≥2 mM thymol induced comparable degranulation to the control secretagogue 48/80, as assessed by β-Hexosaminidase release after 40-minute incubation with thymol. We also observed a dose-dependent increase in IL-6 and IL-13 gene transcription [Fig 5b] after 3 hour incubation. While we expected that we would also observe increased IL-6 and IL-13 protein production, as is typical with upregulated mRNA transcripts, we surprisingly observed no increases in IL-6 and IL-13 protein in the supernatants of thymol-treated cells, unlike upon treatment with the Ca2+ ionophore, ionomycin [Fig 5c]. Although thymol was able to activate MCs in vitro, characterized by degranulation and gene transcription, the lack of cytokine production in the supernatant suggested that thymol may also function to inhibit the cytokine protein production/release pathway from mast cells, or perhaps affect mast cell numbers at this later time point by altering their survival.
Figure 5. Thymol activated mast cells in vitro but prevented secretion of protein.
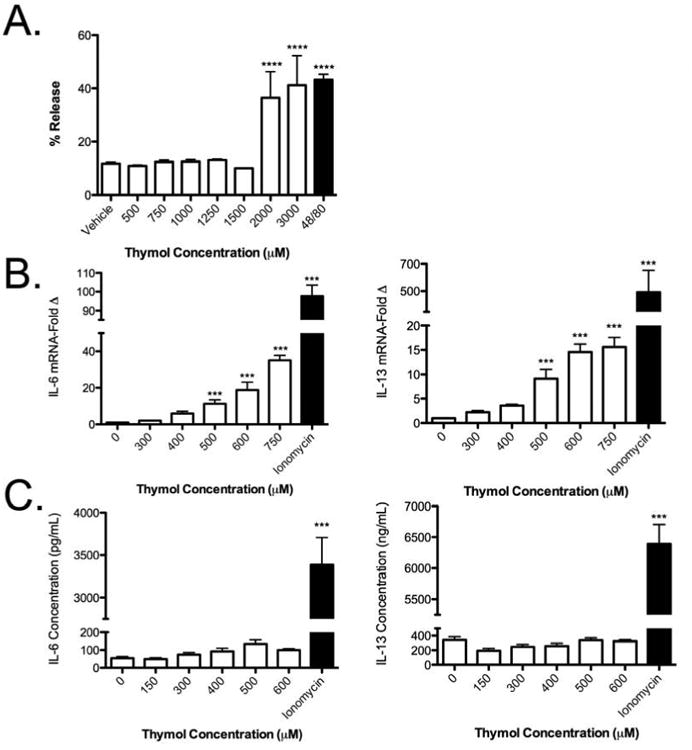
A, Percent release of β-hexosaminidase in MC/9 cells after 40-minute stimulation with thymol. B, Gene expression after 3-hour stimulation with thymol or ionomycin (1 μg/mL) expressed as fold induction over vehicle. C, Cytokine quantification of supernantants after 18-hour stimulation with thymol or ionomycin (1 μg/mL). n = 3 samples per group from 3 independent experiments. ***=p<0.005, ****=p<0.001 by 1-way ANOVA.
Thymol decreased BMMC viability via apoptosis
To distinguish between the possibilities that thymol inhibited cytokine production/release or affected mast cell survival after initial activation, we next examined the effect of thymol on mast cell viability. Here, we utilized BMMCs as opposed to MC/9 cells because of their low turnover and high relative baseline viability. Thymol was incubated with BMMCs and analyzed by flow cytometry for viability by Sytox labeling. At 24 hours [Fig 6a], thymol induced a dose-dependent decrease in mast cell viability.
Figure 6. Thymol decreased mast cell viability in vivo and in vitro by apoptosis.
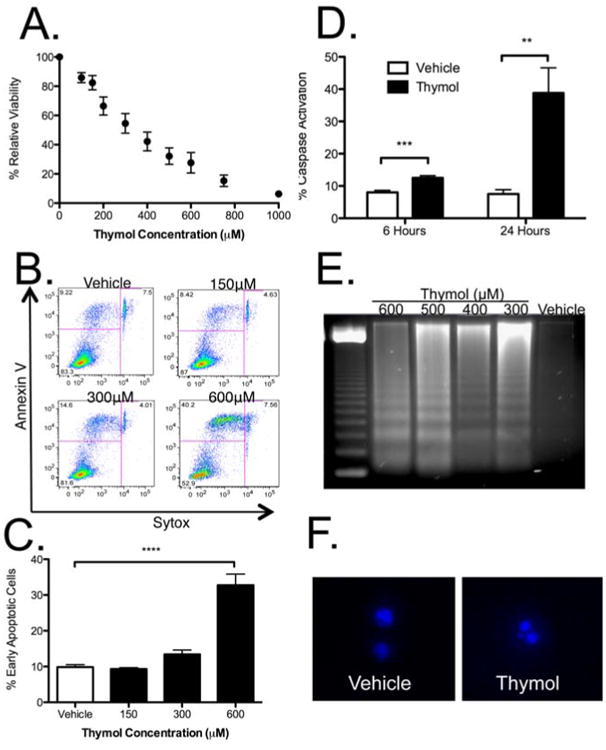
A, Viability of BMMCs after 24 hours of thymol treatment. B, Flow cytometry for early (Annexin V+/Sytox-) and late (Annexin V+/Sytox+) apoptotic BMMC after thymol incubation for 6 hours. C, Quantification of early apoptotic cells after 6 hours of thymol treatment. D, Intracellular staining for active capsase-3 after 6- and 24-hour thymol incubation. E, DNA fragmentation after 24 hours of thymol treatment. F, Hoechst 33342+ staining after 24 hours of thymol incubation. n = 3 samples per group in A, C and D, from 3-4 independent experiments. ***=p<0.005, ****=p<0.001 by 1-way ANOVA.
To assess whether thymol induced mast cell apoptosis, we examined the effect of thymol on cell membrane scrambling, which occurs early in apoptosis, by measuring phosphatidylserine exposure on the cell surface. As shown in representative flow plots [Fig 6b] and graphically [Fig 6c], 6-hour incubation with thymol led to a dose-dependent increase in early apoptotic cells, (annexin-V-positive/Sytox-negative). In addition, thymol induced a significant and time-dependent increase in intracellular caspase-3 activation, another readout of apoptosis induction [Fig 6d]. Two late hallmarks of apoptosis–DNA fragmentation [Fig 6e] and clumping [Fig 6f]– were also both seen after 24 hours of thymol. Therefore, thymol decreased mast cell viability in vitro by inducing apoptosis.
Thymol decreased intraperitoneal mast cell viability via apoptosis
Considering that we observed a therapeutic suppression of specific-antigen–dependent mast cell responses by thymol in our PCA model even though thymol itself activated mast cells, we postulated whether thymol-induced mast cell apoptosis by AICD might underlie the suppression of the PCA reaction we observed. We initially examined the density of cutaneous mast cells 12 hours after topical thymol (20mM) in BALB/c mice from pinacyanol-erythrosinate–stained paraffin-embedded ear tissue. We observed a significant decreased in mast cell density, which suggested that mast cells were depleted by thymol [Fig 7a]. To further examine if thymol induced mast cell apoptosis in vivo, we chose to focus on intraperitoneal mast cells, given that this is a rich source of mast cells and because of limitations in isolating and analyzing functional cutaneous mast cells. For this experiment, 100 μg thymol was administered i.p. to C57BL/6 mice. The mice were euthanized at various time points and the peritoneum was lavaged with cold PBS. The recovered fluid was analyzed for mast cell content based on the percent of viable double positive c-kit/FcεRI cells. Thymol induced a time-dependent statistical decrease in mast cells over a 12-hour period [Fig 7b]. By 1 hour, a significant increase in annexin-V-positive/Sytox-negative mast cells was detectable, suggesting apoptosis had occurred [Fig 7c]. Therefore, thymol reduced mast cell viability in vivo through AICD-induced apoptosis, providing an explanation for how thymol can suppress mast cell-dependent inflammation in vivo.
Figure 7. Thymol decreased mast cell viability in vivo by apoptosis.
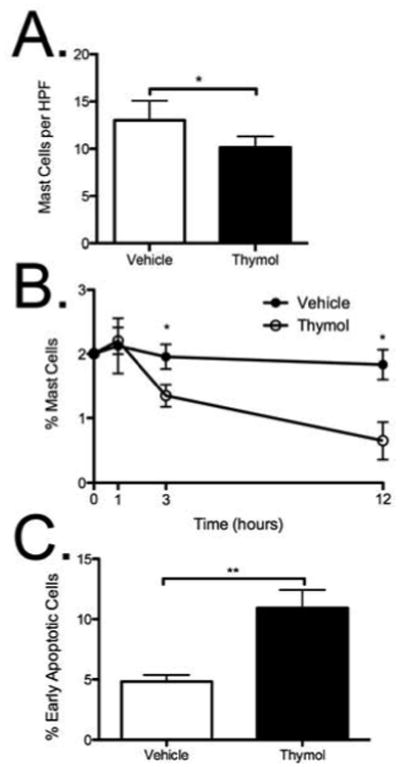
A, Quantification of mast cell density by PE stain in BALB/c mice 12 hours after topical thymol. B, Percent mast cells (c-kit+/FcεRI+) detectable after i.p. injection of thymol (100 μg/animal) in peritoneal lavage fluid. C, Frequency of early apoptotic (Annexin V+/Sytox-) mast cells from peritoneal lavage fluid at 1 hour. n = 3-7 mice per group, *=p<0.05, **=p<0.01 by 1-way ANOVA.
Discussion
In this study, we sought to elucidate a possible mechanism for the beneficial effects of thymol on mast cell-associated skin inflammation, such as in eczema.18 Severe AD is often refractory to standard topical therapy53 and for these patients, cyclosporine, tacrolimus, systemic steroids, azathioprine, and omalizumab are treatment considerations.53, 54 One limitation of these medications is their broad immunomodulatory effect with additional side effects and risks, particularly with systemic administration, driving a need for new therapies. The effector phase of many allergic diseases is characterized by antigen-driven mast cell activation causing degranulation with histamine release and de novo production of pro-inflammatory cytokines. We therefore utilized PCA to better understand the efficacy of thymol, as it is a useful model to evaluate localized antigen-driven mast cell-mediated allergic reactions, a recognized component in eczema pathogenesis.14-17 Our data demonstrates a novel anti-allergic property of thymol to induce mast cell apoptosis, limiting their subsequent ability to elicit IgE-mediated responses.
Interestingly, thymol clearly promotes an initial mast cell activation, characterized by a robust increase in intracellular calcium, which was inhibited by non-selective TRP channel antagonist, Ruthenium Red, a selective TRPA1 antagonist, HC-030031, and the IP3R antagonist, 2-APB, defining this as a likely pathway through which thymol elicits effects on mast cells. While mast cells are known to express TRPA1, the functional effects of TRPA1 ligands on mast cells have not previously been shown. It remains possible that additional mechanisms are important since we were unable to fully ablate the entire calcium flux response with any of these well-characterized inhibitors. Indeed, we were unable to reverse the decreased cell viability using Ruthenium Red, HC-030031, or 2-APB (data not shown), although this experimental approach is confounded by the intrinsic inhibitory effects of these agents on cell viability generally, since Ca2+ signaling is necessary for cell homeostasis. Additionally, it is possible that ablation of PCA by thymol could occur by depletion of granule contents prior to antigen challenge, however our data demonstrates that thymol induces cell death in mast cells that is preceded by sustained calcium flux via TRPA1.
Several studies have assessed different mechanisms for targeted induction of apoptosis in mast cells.8 Much of the mechanistic research has focused on the intrinsic pathway and the role of the Bcl-2 family,4 as well as death receptors, CD95R55 and TRAIL-R,56 and inhibitory receptor CD300a57 in the extrinsic pathway. Recent human and animal model studies support the concept that apoptosis carries important therapeutic considerations.58-60 AICD acts a homeostatic mechanism for immune cleanup, classically associated with the removal of activated T cells after clonal expansion in an immune response.61 Mast cells possess a long life span in part due to their resistance to AICD and apoptotic death via several counter-regulatory mechanisms, including nitric oxide-mediated Cav1.2 L-type Ca2+ channel activation, 62 and SHP-1 directed upregulation of ERK1/2 and Bcl-xL.63 However, mast cells have been shown to exhibit AICD in response to thapsigargin, a stimulator of store-operated Ca2+ channel (SOC) entry and selective inhibitor of the sarco/endoplasmic Ca2+-ATPase (SERCA) pump, necessary for replenishing endoplasmic reticulum (ER) stores when sustained cytosolic calcium is required.41 It remains to be determined whether thymol targets similar calcium stores to induce AICD but in pituitary GH3 cells, thymol was shown to induce a rise in intracellular Ca2+ by triggering both external Ca2+ influx via SOC entry and internal Ca2+ release from the ER.30 Our data suggests that mast cell stimulation with thymol leads to mobilization of Ca2+ that activates degranulation and begins gene transcription; however continued exposure to thymol leads to sustained elevation of cytosolic calcium and thus AICD-associated apoptosis.
Although the role of mast cells in eczema is not entirely clear, our mechanistic findings would seem to explain the two key observations made by Crocker—the “stimulatory” nature of thymol is likely explained by the initial activation of mast cells and the release of preformed granules into surrounding tissue while the benefits of prolonged exposure may be explained by loss of tissue-resident mast cells that confer IgE-mediated allergen sensitivity due to apoptosis. Crocker also made reference to the benefit of thymol therapy in patients who were refractory to other therapies of that time. Importantly, many AD patients today remain difficult to treat; even with use of topical glucocorticoids or immunosuppression.64 Anti-IgE therapy has recently been shown to be therapeutically beneficial in such patients,65 suggesting that targeting the IgE/mast cell pathway may be clinically advantageous.
In conclusion, our study demonstrates that thymol promotes an activation-induced apoptotic death of mast cells, heralded by a robust mobilization of calcium, which is likely mediated by TRPA1. The consequence of this response is a depletion of mast cells, leading to a therapeutic suppression of subsequent IgE-dependent responses to antigen. Based on these mechanistic findings, topical thymol may be a potential therapeutic for the ablation of mast cell-associated skin inflammation, including eczema.
Key Messages.
Topical application of thymol reduces IgE-mediated responses to antigen.
Mechanistically, mast cells are sent into activation-induced death due to sustained calcium activation.
Acknowledgments
We acknowledge Miller Scientific Communications for editorial assistance. This work was supported by NIH/NIAID R01 AI072570 (PJB).
Abbreviations
- PCA
Passive Cutaneous Anaphylaxis
- AD
Atopic Dermatitis
- AICD
Activation-induced Cell Death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunological reviews. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nature reviews Immunology. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 3.Tsai M, Grimbaldeston M, Galli SJ. Mast cells and immunoregulation/immunomodulation. Advances in experimental medicine and biology. 2011;716:186–211. doi: 10.1007/978-1-4419-9533-9_11. [DOI] [PubMed] [Google Scholar]
- 4.Ekoff M, Nilsson G. Mast cell apoptosis and survival. Advances in experimental medicine and biology. 2011;716:47–60. doi: 10.1007/978-1-4419-9533-9_4. [DOI] [PubMed] [Google Scholar]
- 5.Kay AB. Allergy and allergic diseases. Second of two parts. The New England journal of medicine. 2001;344:109–13. doi: 10.1056/NEJM200101113440206. [DOI] [PubMed] [Google Scholar]
- 6.Kay AB. Allergy and allergic diseases. First of two parts. The New England journal of medicine. 2001;344:30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 7.Luskova P, Draber P. Modulation of the Fcepsilon receptor I signaling by tyrosine kinase inhibitors: search for therapeutic targets of inflammatory and allergy diseases. Current pharmaceutical design. 2004;10:1727–37. doi: 10.2174/1381612043384538. [DOI] [PubMed] [Google Scholar]
- 8.Karra L, Berent-Maoz B, Ben-Zimra M, Levi-Schaffer F. Are we ready to downregulate mast cells? Current opinion in immunology. 2009;21:708–14. doi: 10.1016/j.coi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Kovarova M, Rivera J. A molecular understanding of mast cell activation and the promise of anti-allergic therapeutics. Current medicinal chemistry. 2004;11:2083–91. doi: 10.2174/0929867043364801. [DOI] [PubMed] [Google Scholar]
- 10.Alenius H, Laouini D, Woodward A, Mizoguchi E, Bhan AK, Castigli E, et al. Mast cells regulate IFN-gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. The Journal of allergy and clinical immunology. 2002;109:106–13. doi: 10.1067/mai.2002.120553. [DOI] [PubMed] [Google Scholar]
- 11.Tanei R, Hasegawa Y, Sawabe M. Abundant immunoglobulin E-positive cells in skin lesions support an allergic etiology of atopic dermatitis in the elderly. Journal of the European Academy of Dermatology and Venereology : JEADV. 2012 doi: 10.1111/j.1468-3083.2012.04612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navi D, Saegusa J, Liu FT. Mast cells and immunological skin diseases. Clinical reviews in allergy & immunology. 2007;33:144–55. doi: 10.1007/s12016-007-0029-4. [DOI] [PubMed] [Google Scholar]
- 13.Liu FT, Goodarzi H, Chen HY. IgE, Mast Cells, and Eosinophils in Atopic Dermatitis. Clinical reviews in allergy & immunology. 2011 doi: 10.1007/s12016-011-8252-4. [DOI] [PubMed] [Google Scholar]
- 14.Soter NA. Morphology of atopic eczema. Allergy. 1989;44(Suppl 9):16–9. doi: 10.1111/j.1398-9995.1989.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 15.Irani AM, Sampson HA, Schwartz LB. Mast cells in atopic dermatitis. Allergy. 1989;44(Suppl 9):31–4. [PubMed] [Google Scholar]
- 16.Gombert M, Dieu-Nosjean MC, Winterberg F, Bunemann E, Kubitza RC, Da Cunha L, et al. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. Journal of immunology. 2005;174:5082–91. doi: 10.4049/jimmunol.174.8.5082. [DOI] [PubMed] [Google Scholar]
- 17.Horsmanheimo L, Harvima IT, Jarvikallio A, Harvima RJ, Naukkarinen A, Horsmanheimo M. Mast cells are one major source of interleukin-4 in atopic dermatitis. The British journal of dermatology. 1994;131:348–53. doi: 10.1111/j.1365-2133.1994.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 18.Crocker HR. Thymol as a Remedy in Skin-Diseases. Br Med J. 1878;1:225–6. doi: 10.1136/bmj.1.894.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baser KH. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Current pharmaceutical design. 2008;14:3106–19. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- 20.Basch E, Ulbricht C, Hammerness P, Bevins A, Sollars D. Thyme (Thymus vulgaris L.), thymol. Journal of herbal pharmacotherapy. 2004;4:49–67. [PubMed] [Google Scholar]
- 21.Burt S. Essential oils: their antibacterial properties and potential applications in foods--a review. International journal of food microbiology. 2004;94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro S, Guggenheim B. The action of thymol on oral bacteria. Oral microbiology and immunology. 1995;10:241–6. doi: 10.1111/j.1399-302x.1995.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 23.Guo N, Liu J, Wu X, Bi X, Meng R, Wang X, et al. Antifungal activity of thymol against clinical isolates of fluconazole-sensitive and -resistant Candida albicans. Journal of medical microbiology. 2009;58:1074–9. doi: 10.1099/jmm.0.008052-0. [DOI] [PubMed] [Google Scholar]
- 24.Kim DO, Lee CY. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Critical reviews in food science and nutrition. 2004;44:253–73. doi: 10.1080/10408690490464960. [DOI] [PubMed] [Google Scholar]
- 25.Archana PR, Nageshwar Rao B, Ballal M, Satish Rao BS. Thymol, a naturally occurring monocyclic dietary phenolic compound protects Chinese hamster lung fibroblasts from radiation-induced cytotoxicity. Mutation research. 2009;680:70–7. doi: 10.1016/j.mrgentox.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nature neuroscience. 2006;9:628–35. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 27.Lee SP, Buber MT, Yang Q, Cerne R, Cortes RY, Sprous DG, et al. Thymol and related alkyl phenols activate the hTRPA1 channel. British journal of pharmacology. 2008;153:1739–49. doi: 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nature reviews Drug discovery. 2011;10:601–20. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 29.Hsu SS, Lin KL, Chou CT, Chiang AJ, Liang WZ, Chang HT, et al. Effect of thymol on Ca(2+) homeostasis and viability in human glioblastoma cells. European journal of pharmacology. 2011 doi: 10.1016/j.ejphar.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Shen AY, Huang MH, Wang TS, Wu HM, Kang YF, Chen CL. Thymol-evoked Ca+ mobilization and ion currents in pituitary GH3 cells. Natural product communications. 2009;4:749–52. [PubMed] [Google Scholar]
- 31.Chang HT, Hsu SS, Chou CT, Cheng JS, Wang JL, Lin KL, et al. Effect of thymol on Ca2+ homeostasis and viability in MG63 human osteosarcoma cells. Pharmacology. 2011;88:201–12. doi: 10.1159/000331864. [DOI] [PubMed] [Google Scholar]
- 32.Slamenova D, Horvathova E, Sramkova M, Marsalkova L. DNA-protective effects of two components of essential plant oils carvacrol and thymol on mammalian cells cultured in vitro. Neoplasma. 2007;54:108–12. [PubMed] [Google Scholar]
- 33.Horvathova E, Turcaniova V, Slamenova D. Comparative study of DNA-damaging and DNA-protective effects of selected components of essential plant oils in human leukemic cells K562. Neoplasma. 2007;54:478–83. [PubMed] [Google Scholar]
- 34.Mahmud H, Mauro D, Foller M, Lang F. Inhibitory effect of thymol on suicidal erythrocyte death. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2009;24:407–14. doi: 10.1159/000257433. [DOI] [PubMed] [Google Scholar]
- 35.Xuan NT, Shumilina E, Schmid E, Bhavsar SK, Rexhepaj R, Gotz F, et al. Role of acidic sphingomyelinase in thymol-mediated dendritic cell death. Molecular nutrition & food research. 2010;54:1833–41. doi: 10.1002/mnfr.200900577. [DOI] [PubMed] [Google Scholar]
- 36.Deb DD, Parimala G, Saravana Devi S, Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chemico-biological interactions. 2011;193:97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Stammati A, Bonsi P, Zucco F, Moezelaar R, Alakomi HL, von Wright A. Toxicity of selected plant volatiles in microbial and mammalian short-term assays. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1999;37:813–23. doi: 10.1016/s0278-6915(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 38.Satooka H, Kubo I. Effects of thymol on B16-F10 melanoma cells. J Agric Food Chem. 2012;60:2746–52. doi: 10.1021/jf204525b. [DOI] [PubMed] [Google Scholar]
- 39.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405–34. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 40.Inoue T, Suzuki Y, Yoshimaru T, Ra C. Nitric oxide protects mast cells from activation-induced cell death: the role of the phosphatidylinositol-3 kinase-Akt-endothelial nitric oxide synthase pathway. Journal of leukocyte biology. 2008;83:1218–29. doi: 10.1189/jlb.1007667. [DOI] [PubMed] [Google Scholar]
- 41.Soboloff J, Berger SA. Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. The Journal of biological chemistry. 2002;277:13812–20. doi: 10.1074/jbc.M112129200. [DOI] [PubMed] [Google Scholar]
- 42.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS letters. 2001;502:53–6. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 43.Bensley SH. Pinacyanol erthrosinate as a stain for mast cells. Stain technology. 1952;27:269–73. doi: 10.3109/10520295209105090. [DOI] [PubMed] [Google Scholar]
- 44.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. The Journal of biological chemistry. 2000;275:4429–34. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 46.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. The Journal of allergy and clinical immunology. 2009;123:231–8. e4. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong J, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Analytical biochemistry. 1994;218:314–9. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- 48.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. The Journal of experimental medicine. 2007;204:2797–802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malaviya R, Uckun FM. Role of STAT6 in IgE receptor/FcepsilonRI-mediated late phase allergic responses of mast cells. Journal of immunology. 2002;168:421–6. doi: 10.4049/jimmunol.168.1.421. [DOI] [PubMed] [Google Scholar]
- 50.Prasad P, Yanagihara AA, Small-Howard AL, Turner H, Stokes AJ. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol. 2008;181:5024–34. doi: 10.4049/jimmunol.181.7.5024. [DOI] [PubMed] [Google Scholar]
- 51.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, et al. TRPA1 contributes to cold hypersensitivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15165–74. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma HT, Beaven MA. Regulators of Ca(2+) signaling in mast cells: potential targets for treatment of mast cell-related diseases? Advances in experimental medicine and biology. 2011;716:62–90. doi: 10.1007/978-1-4419-9533-9_5. [DOI] [PubMed] [Google Scholar]
- 53.Andres C, Belloni B, Mempel M, Ring J. Omalizumab for patients with severe and therapy-refractory atopic eczema? Current allergy and asthma reports. 2008;8:179–80. doi: 10.1007/s11882-008-0029-3. [DOI] [PubMed] [Google Scholar]
- 54.Darsow U, Lubbe J, Taieb A, Seidenari S, Wollenberg A, Calza AM, et al. Position paper on diagnosis and treatment of atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2005;19:286–95. doi: 10.1111/j.1468-3083.2005.01249.x. [DOI] [PubMed] [Google Scholar]
- 55.Hartmann K, Wagelie-Steffen AL, von Stebut E, Metcalfe DD. Fas (CD95, APO-1) antigen expression and function in murine mast cells. Journal of immunology. 1997;159:4006–14. [PubMed] [Google Scholar]
- 56.Berent-Maoz B, Piliponsky AM, Daigle I, Simon HU, Levi-Schaffer F. Human mast cells undergo TRAIL-induced apoptosis. Journal of immunology. 2006;176:2272–8. doi: 10.4049/jimmunol.176.4.2272. [DOI] [PubMed] [Google Scholar]
- 57.Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. Journal of immunology. 2005;175:7989–95. doi: 10.4049/jimmunol.175.12.7989. [DOI] [PubMed] [Google Scholar]
- 58.Ma Z, Tovar JP, Kwong KY, Paek D. Pimecrolimus induces apoptosis of mast cells in a murine model of cutaneous mastocytosis. International archives of allergy and immunology. 2010;153:413–8. doi: 10.1159/000316353. [DOI] [PubMed] [Google Scholar]
- 59.Karlberg M, Ekoff M, Huang DC, Mustonen P, Harvima IT, Nilsson G. The BH3-mimetic ABT-737 induces mast cell apoptosis in vitro and in vivo: potential for therapeutics. Journal of immunology. 2010;185:2555–62. doi: 10.4049/jimmunol.0903656. [DOI] [PubMed] [Google Scholar]
- 60.Juurikivi A, Sandler C, Lindstedt KA, Kovanen PT, Juutilainen T, Leskinen MJ, et al. Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis. Annals of the rheumatic diseases. 2005;64:1126–31. doi: 10.1136/ard.2004.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brenner D, Krammer PH, Arnold R. Concepts of activated T cell death. Critical reviews in oncology/hematology. 2008;66:52–64. doi: 10.1016/j.critrevonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki Y, Inoue T, Ra C. Endothelial nitric oxide synthase is essential for nitric oxide generation, L-type Ca2+ channel activation and survival in RBL-2H3 mast cells. Biochimica et biophysica acta. 2010;1803:372–85. doi: 10.1016/j.bbamcr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Inoue T, Suzuki Y, Mizuno K, Nakata K, Yoshimaru T, Ra C. SHP-1 exhibits a pro-apoptotic function in antigen-stimulated mast cells: positive regulation of mitochondrial death pathways and negative regulation of survival signaling pathways. Molecular immunology. 2009;47:222–32. doi: 10.1016/j.molimm.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 64.Paller AS, Simpson EL, Eichenfield LF, Ellis CN, Mancini AJ. Treatment strategies for atopic dermatitis: optimizing the available therapeutic options. Semin Cutan Med Surg. 2012;31:S10–7. doi: 10.1016/j.sder.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Vigo PG, Girgis KR, Pfuetze BL, Critchlow ME, Fisher J, Hussain I. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J Am Acad Dermatol. 2006;55:168–70. doi: 10.1016/j.jaad.2005.12.045. [DOI] [PubMed] [Google Scholar]


