Abstract
Rationale
Recent research has demonstrated that the drug, histamine, can function as a punisher of cocaine self-administration. However, little is known about how drug punishers affect the maximum reinforcing effectiveness of drugs as reinforcers.
Objective
The goal of the present study was to determine if histamine, when self-administered as a mixture with cocaine, could reduce cocaine’s maximum reinforcing effectiveness using two procedures designed for measuring reinforcing effectiveness.
Method
In the first experiment, rhesus monkeys were allowed to self-administer cocaine (0.1 mg/kg/inj) alone or as a mixture with histamine (0.012-0.05 mg/kg/inj) in a behavioral economic design. In the second experiment, monkeys were allowed to self-administer cocaine alone (0.006-0.56 mg/kg/inj) or as a mixture with histamine (0.025-0.1 mg/kg/inj) under a progressive-ratio schedule of reinforcement.
Results
In Experiment 1, histamine decreased the reinforcing effectiveness of cocaine in a dose-dependent manner as evidenced by increases in cocaine’s demand elasticity with increases in histamine dose. In Experiment 2, histamine decreased cocaine’s potency and effectiveness as a reinforcer in a dose-dependent manner as indicated by rightward and downward shifts, respectively, in the dose-response functions.
Conclusion
The reinforcing effectiveness of cocaine can be reduced by contingent self-administration of histamine. These results indicate that combining drug punishers with drug reinforcers reduces the maximum reinforcing effect of the drug reinforcer, which suggests a use for drug punishers as a deterrent to drug abuse (e.g., as mixtures with prescription medications with abuse potential).
Keywords: cocaine, histamine, monkey, punishment, reinforcement, progressive-ratio, behavioral economics, self-administration
Introduction
Punishment is operationally defined as a consequence of behavior that reduces the future probability of that behavior (Azrin and Holz 1966). Researchers have investigated the effects of punishment on drug self-administration using exteroceptive stimuli such as electric shock to reduce operant responding for drug injections (Deroche-Gamonet et al 2004; Grove and Schuster 1974; Johanson 1977). However, drugs, which produce interoceptive effects, can also function as punishers. For example, histamine has been shown to punish operant responding maintained by food and drugs in rats and monkeys, and does so in a dose-dependent manner (Goldberg 1980; Holtz et al 2013; Negus 2005; Podlesnik et al 2010; Woolverton 2003). Negus (2005), using monkeys in a food vs. cocaine choice procedure, reported that intravenous histamine injections punished both food and cocaine choice. More recently, Woolverton et al (2012) tested histamine as a punisher of cocaine self-administration with monkeys in a drug vs. drug choice procedure and found that the effectiveness of histamine as a punisher was dose-dependent and reduced by delay to its presentation. Taken together, these studies indicate that the effectiveness of histamine as a punisher depends on its dose and temporal contiguity to drug self-administration, results that are consistent with what has been reported with non-drug punishers (Azrin and Holz 1966).
Interestingly, while it is well established that punishment with histamine decreases food and drug self-administration, no study to our knowledge has quantitatively investigated the impact of punishment with histamine or any other drug on the maximum reinforcing effect (“reinforcing effectiveness,” henceforth) of a drug reinforcer. Measures of reinforcing effectiveness (e.g., progressive-ratio schedules of reinforcement) have a distinct advantage over fixed schedules of reinforcement in that that they can be used to rank order drugs in terms of how reinforcing they are (Hursh and Silberberg 2008; Richardson and Roberts 1996). Generally, a psychostimulant’s abuse potential is related to its effectiveness as a reinforcer (Freeman et al 2012; Wee et al 2005; Wilcox et al 2000; Woolverton 1995). If punishment with a drug can reduce the reinforcing effectiveness of another drug, it stands to reason that it may also reduce that drug reinforcer’s abuse potential. This approach could be particularly useful for reducing abuse of compounds with which drug punishers can be coupled (e.g., prescription medications).
The current study was designed to quantitatively determine the effects of punishment with histamine on the reinforcing effectiveness of cocaine. Specifically, monkeys were allowed to self-administer intravenous cocaine, either alone or as a mixture with a range of doses of histamine. Two procedures were used to index reinforcing effectiveness, behavioral economic (BE) demand curves and a progressive-ratio (PR) schedule of reinforcement. The BE approach relates the consumption of a reinforcer to its cost across a range of increasing prices, ultimately yielding a demand curve function. The recently introduced Exponential Model of Demand (Hursh and Silberberg 2008) is a descriptive model that permits scaling of reinforcer effectiveness as quantified through a rate constant defining the elasticity of demand across the entire range of prices (also see Banks et al 2011 for a recent relevant application). Alternatively, the PR approach uses a schedule that progressively increases the response requirement for a reinforcer within a session until the subject ceases to respond for the reinforcer (see Rowlett 2000 for a review). When multiple doses of a drug are tested, the asymptote in the dose-response function relating the drug’s dose to the subject’s breakpoints at each dose serves as the metric for reinforcing effectiveness. Both BE and PR approaches have distinct advantages that have been reviewed (Hursh and Roma 2013; Hursh and Silberberg 2008; Richardson and Roberts 1996; Rowlett 2000; Stafford et al 1998). The current study used both approaches with the rationale that if the effects of histamine on cocaine’s effectiveness as a reinforcer were similar across assays, then conclusions regarding the effects would be strengthened by convergent evidence. The hypothesis was that the addition of histamine would decrease cocaine’s effectiveness as a reinforcer in both tests in a manner that was related to histamine dose.
Methods
All animal-use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (1996).
Subjects
The subjects were five adult male rhesus monkeys (Macaca mulatta) weighing between 9.1 and 12.0 kg at the beginning of the study. Four monkeys (AV22, CJ82, M1388, and R99028) had a history of cocaine and D2 partial agonist self-administration (Freeman et al 2012). The fifth monkey (4199) had no history of drug self-administration prior to the current study. All monkeys were provided with sufficient food to maintain stable body weights (200-300 g/day, Teklad 25% Monkey Diet, Harlan/ Teklad, Madison, WI, USA) and had unlimited access to water via a lick-activated spout mounted in the cubicle wall. Fresh fruit was provided daily, and a vitamin supplement was given three times a week. Lighting was cycled to maintain 16 h of light and 8 h of dark, with lights on at 0600 hours.
Apparatus
Each monkey was fitted with a stainless-steel harness (E and H Engineering, Chicago, IL, USA) or a jacket (Lomir Biomedical, Malone, NY, USA) that was attached by a tether to the rear wall of the experimental cubicle (1.0 m3, Plaslabs, Lansing, MI, USA). The front door of the cubicle was constructed of transparent plastic, and the remaining walls were opaque. There were two response levers (PRL-001, BRS/LVE, Beltsville, MD, USA) mounted in steel boxes, 10 cm above the floor and 47.0 cm apart, on the interior of the door of each experimental cubicle. Four jeweled stimulus lights, two red and two white, were mounted above each lever on the faceplates of the lever boxes. Drug injections were delivered by a peristaltic infusion pump (Cole-Parmer, Chicago, IL, USA). Water was delivered by a separate peristaltic infusion pump through a sipper tube mounted 10 cm above the lever box associated with its delivery (see Procedure below for the purpose of including operant water delivery). A Macintosh computer with custom interface and software controlled all events in an experimental session and recorded data.
Surgery
Each monkey had a single-lumen catheter implanted according to the following protocol. The monkey was injected with a combination of atropine sulfate (0.04 mg/kg i.m.) and ketamine hydrochloride (10 mg/kg i.m.) followed 20–30 min later by inhaled isoflurane. When anesthesia was adequate, the catheter was surgically implanted into a major vein with the tip terminating near the right atrium of the heart. The distal end of the catheter was passed subcutaneously to the mid-scapular region, where it exited the monkey’s back. After surgery, the monkey was returned to the experimental cubicle. The catheter was threaded through the tether and connected to a single-lumen swivel (Lomir) mounted at the rear of the cubicle. An antibiotic (Kefzol; Eli Lilly & Company, Indianapolis, Indiana) was administered (22 mg/kg i.m.) twice daily for 7 days post-surgery to prevent infection. Over the course of the study, the catheter was filled with a solution of 40 units/ml heparin between sessions to prevent clotting at the catheter tip. If a catheter became nonfunctional during the experiment, it was removed, and the monkey was removed from the experiment for a 1-2 week period to allow the clearance of possible infections. After health was verified (i.e., no sign of systemic or local infection), a new catheter was implanted.
Procedure
Experiment 1: Behavioral Economic Demand Curves
Experimental sessions began at 12:00 P.M. each day, 7 days per week, and were signaled by illumination of the white lever lights. Each session lasted 4 h and began with two forced-choice (sampling) trials, one for each lever, followed by free-choice trials. During forced choice trials, only one of the two levers was active, and the active lever, signaled by the illumination of its pair of white lights, alternated in order to ensure equal exposure to the contingencies programmed for each lever. During free choice trials, both pairs of white lever lights were illuminated, and both levers were active. Following the completion of 10 responses on the active lever (i.e., FR10), the white lights were terminated and the red lights for that lever were illuminated for a 10-sec period that ran concurrently with the delivery of a reinforcer. Trials were separated by an 11-sec inter-trial interval (ITI) that ran concurrently with the delivery of the drug reinforcer. Responding on one lever resulted in a 10-sec drug injection, while responding on the other lever resulted in a 10-sec delivery of 2.0 ml of water through the sipper tube mounted in the door. The water alternative was incorporated into the design to serve as a control baseline for future studies that will investigate choice between cocaine or cocaine-histamine combinations and sweet liquid reinforcers (e.g., sucrose, saccharin). The right-left positions of the lever-response conditions (i.e., drug injection or water delivery) were counterbalanced across monkeys and remained fixed throughout the experiment within each monkey.
When drug intake stabilized at a response requirement, the FR was increased according to the following sequence: FR10, 32, 100, 320, 560, 1000, and 3200. Each response requirement was in effect until drug self-administration was stable, defined as 3 consecutive sessions in which the number of injections were within 20% of the 3-session mean with no upward or downward trends. Monkeys were tested under four drug conditions, in irregular order within and across monkeys. The four conditions were cocaine alone (0.1 mg/kg/inj) or that same dose of cocaine mixed with one of three concentrations of histamine that resulted in the following doses of histamine per injection: 0.012, 0.025, and 0.05 mg/kg/inj.
Experiment 2: Progressive Ratio
Four monkeys were used in Experiment 2 (4199, CJ82, M1388, and R99028). Experimental sessions for Experiment 2 were run daily beginning at the same time of day as Experiment 1. At the start of each session, the white lights were illuminated above both levers, and pressing the right lever resulted in the delivery of a 10-s drug injection. Responses on the left lever were counted but had no programmed consequences. During the injection, the white lights were extinguished, and the red lights were illuminated. Responding was trained and maintained with 0.1 mg/kg/inj cocaine under a PR schedule of reinforcement comparable to that described by Wilcox et al (2000). Test sessions were inserted into the daily sequence between two saline or two cocaine sessions. To prevent monkeys from learning this session sequence, a randomly determined saline or cocaine baseline session was inserted after every other test session. Thus, the final daily sequence of sessions was C, S, T, S, C, T, R, where “C”, “S”, “R”, and “T”, respectively represent sessions for cocaine baseline (0.1 mg/kg/inj), saline, randomly determined cocaine or saline, and tests.
All sessions consisted of 20 trials, with one injection available per trial. Because of response variability between monkeys, the set point of the initial response requirement was adjusted in each monkey to make the total number of injections during baseline drug sessions as comparable between monkeys as possible (range approximately 12–15 inj/session). The response requirement started at 12 responses per injection in monkey R99028, 50 responses in CJ82, 100 responses in M1388, and 200 responses in 4299. For all monkeys, the response requirement per injection doubled after every fourth trial. There was an ITI of 30 min duration after each injection during which lights were extinguished and levers were inactive. A subject had 30 min to complete a trial (limited hold [LH] 30 min). A trial ended with a drug injection or the expiration of the LH. If the response requirement was not completed for two consecutive trials (i.e., the LH expired) or the animal self-administered all 20 injections, the session ended.
For test sessions, the conditions made available were a range of cocaine doses (0.006 - 0.56 mg/kg/inj) alone or mixed with a range of histamine doses (0.025 – 0.1 mg/kg/inj). All test sessions were conducted in an irregular order within and across monkeys and were tested at least twice in each monkey, once with a saline session the day before and once with a cocaine session the day before. When the two test sessions of a condition showed high variability (each of the two determinations were different from the mean by ± three injections), the dose was re-tested twice, once after a saline and once after a cocaine baseline session and the data from the second determinations were used in the analysis. Unfortunately, monkey M1388 reached an endpoint of having no useable veins before all conditions could be completed. However, data collected in this subject up to that point are included in the current report.
Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD, USA), and histamine dihydrochloride was purchased commercially from Sigma-Aldrich (St. Louis, MO, USA). Final solutions were prepared using 0.9% saline, and mixtures of cocaine and histamine were prepared by adding both drugs to a single bag (i.e., a drug mixture). All doses are expressed as the salt forms of the drugs.
Data Analysis
For Experiment 1, data were analyzed using a behavioral economic demand curve approach which relates the consumption of a reinforcer (the number of injections per session) to its price (the response requirement for one drug injection) using a custom-designed GraphPad Prism 5.0 template freely available from the Institutes for Behavior Resources (www.ibrinc.org). Drug injections per session for each reinforcer condition were plotted as a function of response requirement and the data were fit with the Exponential Model of Demand introduced by Hursh and Silberberg (2008):
log Q = log Q0 + k (e −α · ( Q0 · C ) – 1),
where Q is the experimentally determined measure of consumption at any particular FR, Q0 is the predicted absolute consumption at price 0 and specifies the highest level of demand, k is the range of the exponential demand curve in log units shared across all individuals and conditions, α is the rate of decline in consumption as price is increased, and C is a measure of cost, expressed here as the FR value. The α statistic and derivatives thereof, which quantifies the elasticity of the demand curve, provides a measure of a reinforcer’s effectiveness (see Hursh and Silberberg 2008 and Hursh and Roma, 2013). In cases where the injections per session were 0, a value of 0.1 was entered in order to permit log transformation (for an example of this adjustment see Banks et al. 2011). Preliminary analyses revealed a value of 11 as the best-fit for the Exponential Model's k parameter, which was then held constant for formal analyses of all individual and aggregate demand curves.
For Experiment 1, there were two main dependent measures of interest. The first was baseline consumption, defined as the number of injections taken at the lowest response requirement (FR10). Because histamine can function as a punisher, the expectation was that histamine, when mixed with 0.1 mg/kg/inj cocaine, would reduce baseline consumption for cocaine relative to the cocaine alone condition. The second dependent measure was demand elasticity as expressed in the α term (and derivatives thereof) from the Exponential Model of Demand. These values were derived from analyses of aggregated mean demand curves of all monkeys' final three sessions per FR for cocaine alone and cocaine mixed with each dose of histamine. Hursh and colleagues (Hursh and Silberberg 2008; Hursh and Roma, 2013) have argued that reinforcing effectiveness or "essential value" of a reinforcer is inversely related to demand elasticity as quantified by α. Therefore, to make interpretation more intuitive, demand elasticity was graphically presented as its inverse (1/α) so that larger values reflected greater reinforcing effectiveness. Therefore, the expectation was that, if histamine reduced the reinforcing effectiveness of cocaine, then the essential value of cocaine alone would be significantly larger than at least one of the cocaine-histamine mixture conditions and at no point significantly smaller than any of the cocaine-histamine conditions. For the baseline consumption comparison, the effect of condition (Coc 0.1, Coc 0.1 + Hist 0.012, Coc 0.1 + Hist 0.025, Coc 0.1 + Hist 0.05) was analyzed via one-way Analysis of Variance (ANOVA) followed by post-hoc independent samples t tests. A Sum-of-Squares F test was first used to determine whether any of the conditions’ data differed from a single curve fit to all of the data, which was followed by post-hoc Sum-of-Squares F tests to compare demand elasticity (α) of each curve to each other. All post-hoc t tests and Sum-of-Squares F tests were subjected to Benjamini-Hochberg False Discovery Rate (FSD) stepwise Type I error correction (Benjamini and Hochberg, 1995). Statistical significance for all analyses was set at p < 0.05.
For Experiment 2, the mean number of injections per session was calculated individually from the two test sessions at a condition (i.e., cocaine alone or as a mixture with histamine). A dose of cocaine alone or as a combination with a dose of histamine was considered to be a reinforcer in a subject if the mean value for these two test sessions exceeded the mean value for that subject’s saline test sessions and the ranges did not overlap. For each monkey, the dose-effect data for cocaine alone and for the cocaine-histamine mixtures were fitted by non-linear regression (Sigmoidal Dose-Response; Variable Slope; Graphpad Prism 5.0), and the maximum injections and ED50 values for cocaine alone and for the cocaine-histamine mixtures were determined. Maximum injections are the measure of reinforcing effectiveness in a PR test (Rowlett 2000). Thus, a dose of histamine was considered to reduce cocaine’s reinforcing effectiveness in a subject if the maximum number of injections for that dose of histamine mixed with cocaine was lower than the maximum for cocaine alone, and the ranges did not overlap. ED50 values for conditions were generated from the regression analysis, and were used to provide a descriptive quantification of histamine’s effects on cocaine’s potency as a reinforcer. ED50 values were only calculated in cases where there were at least three data points for a condition (not including saline) with at least one data point above and one below the 50% point between saline and the highest point for that condition.
Results
Experiment 1
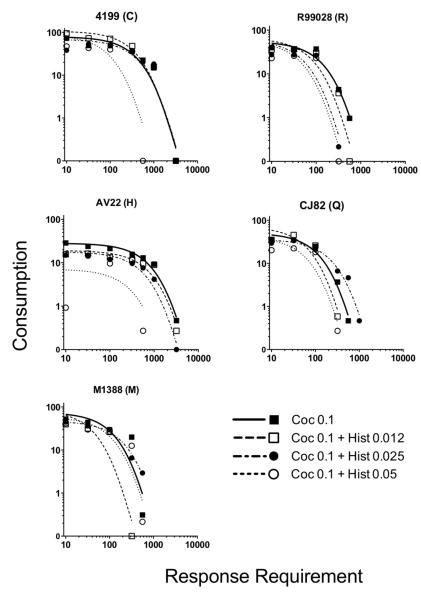
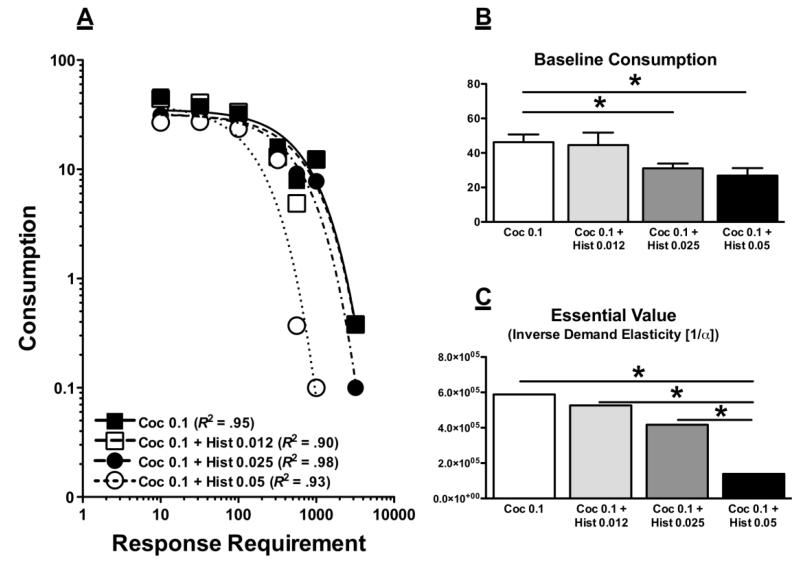
Figure 1 illustrates individual demand curves of cocaine consumption (mean injections per session) as a function of response requirement and histamine dose, and Table 1 lists individual subject baseline consumption, demand elasticity (α), and R2 values for all conditions. Cocaine functioned as a reinforcer in all subjects, and injections per session generally decreased with increases in response requirement for all conditions. Figure 2, Panel A illustrates the mean aggregated demand curves across monkeys for cocaine alone and cocaine mixed with various doses of histamine. The Exponential Model provided a good fit to the aggregated data (R2 range from .90-.98). A one-way ANOVA revealed a significant main effect of condition on baseline consumption (F(3,56) = 3.73, p = .016). As illustrated in Figure 2 Panel B, post-hoc tests revealed that 0.025 and 0.05 mg/kg/inj histamine significantly decreased consumption compared to cocaine alone (ts(28) > 2.80, ps < 0.05; all other ts(28) < 2.10, ps > 0.09). The essential values (1/α) of cocaine with each dose of histamine are presented in Figure 2, Panel C. Sum-of-Squares F Tests of the underlying α values confirmed that at least two conditions differed from each other (Fs(6,19) = 16.3, p < 0.0001), with post-hoc tests revealing that the essential value of cocaine combined with the high 0.05 mg/kg/inj histamine dose was significantly lower than that of cocaine alone or cocaine combined with 0.012 or 0.025 mg/kg/inj histamine (Fs(1,9) > 11.0, ps < 0.05).
Fig. 1.
Number of injections of 0.1 mg/kg/inj cocaine alone (solid line) and as a mixture with various doses of histamine (hatched lines) self-administered per session as a function of response requirement. Each panel represents an individual monkey, and each point represents the mean of three stable sessions.
Table 1.
Baseline consumption, demand elasticity (α), and R2 values of each condition for each monkey in Experiment 1. Coc = cocaine; Hist = histamine; BC = baseline consumption (i.e., mean injections received at FR10).
| Coc 0.1 | Coc 0.1 + | Coc 0.1 + | Coc 0.1 + | |
|---|---|---|---|---|
| Hist 0.025 | Hist 0.05 | Hist 0.1 | ||
|
| ||||
| Subject | BC / α / R2 | BC / α / R2 | BC / α / R2 | BC / α / R2 |
| 4199(C) | 74 / 1.2×10−6 / 0.98 | 96 / 9.7×10−7 / 0.99 | 38 / 1.3×10−6 / 0.96 | 47 / 5.5×10−6 / 0.73 |
| R99028 (R) | 40 / 5.3×10−6 /0.96 | 36 / 7.4×10−6 / 0.95 | 28 / 1.2×10−5 / 0.92 | 23 / 1.5×10−5 / 0.91 |
| AV22(H) | 29 / 1.9×10−6 / 0.99 | 16 / 2.4×10−6 / 0.98 | 15 / 4.1×10−6 / 0.99 | 2 / 1.4×10−5 / 0.31 |
| CJ82 (Q) | 36 / 7.0×10−6 / 0.99 | 35 / 8.6×10−6 / 0.96 | 30 / 5.0×10−6 / 0.98 | 20 / 1.5×10−5 / 0.94 |
| M1388(M) | 52 / 4.2×10−6 / 0.88 | 40 / 1.1×10−5 / 0..94 | 45 / 4.5×10−6 / 0.97 | 42 / 6.1×10−6 / 0.88 |
Fig. 2.
Panel A: Aggregated mean demand curves of all sessions from all monkeys for 0.1 mg/kg/inj of cocaine alone (solid line) and cocaine mixed with various doses of histamine (hatched lines). Panel B: Mean + SEM baseline consumption (i.e., at FR10) of all monkeys for cocaine alone and cocaine mixed with various doses of histamine. Panel C: "Essential Value" (inverse demand elasticity [1/α]) of the aggregated mean demand curves in Panel A for cocaine alone and cocaine mixed with various doses of histamine. * p ≤ 0.05.
Experiment 2
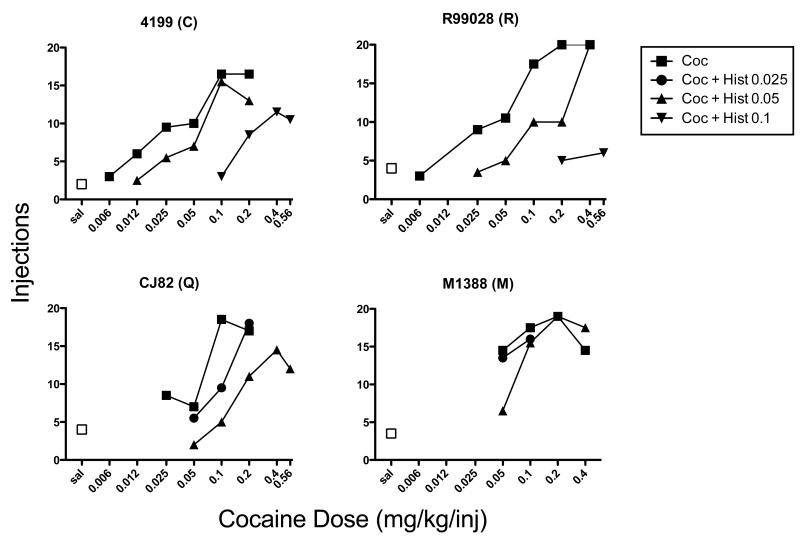
Figure 3 illustrates the number of injections each subject received of saline, cocaine, and cocaine mixed with various doses of histamine. Subjects took an average of 3.4 injections on the saline tests, with a range of 2 to 4 injections. Consistent with previous reports, the number of cocaine injections increased in a dose-dependent manner in each subject. In three of the four subjects (4199, R99028, and CJ82), adding histamine to cocaine shifted the cocaine dose-response function rightward and downward in a manner that was dependent on histamine dose. Unfortunately, subject M1388 ran out of useable veins before the conditions could be completed. Mean maximum injections per session and ED50 values for cocaine and cocaine mixed with histamine are presented in Table 2. Histamine decreased the maximum injections for cocaine at a dose of 0.1 mg/kg (histamine) in subjects 4199 and R99028 and at 0.05 mg/kg/inj in subject CJ82, indicating that it decreased the reinforcing effectiveness of cocaine. ED50 values for cocaine increased in an orderly fashion with increases in histamine dose, indicating that histamine decreased cocaine’s potency as a reinforcer.
Fig. 3.
Dose-response functions for self-administration of cocaine alone or cocaine mixed with various doses of histamine under a progressive ratio schedule of reinforcement. Each panel represents an individual monkey, and each data point is the mean value for two test sessions at each dose or dose combination of cocaine and histamine.
Table 2.
Maximum injections achieved and ED50 values for cocaine alone and cocaine mixed with various doses of histamine in each monkey in Experiment 2. The maximum injections value is the mean of the two test trials for the dose of cocaine alone or dose of cocaine mixed with histamine at which the highest number of injections were received by a monkey (i.e., the asymptote of the dose-response function). Coc = cocaine; Hist = histamine; Max = maximum injections achieved within the dose-response function.
| Coc Alone | Coc + | Coc + | Coc + | |
|---|---|---|---|---|
| Hist 0.025 | Hist 0.05 | Hist 0.1 | ||
|
| ||||
| Subject | Max / ED50 | Max / ED50 | Max / ED50 | Max / ED50 |
| 4199 (C) | 16.5 / 0.03 | -- / -- | 15.5 / 0.05 | 11.5* / 0.17 |
| R99028 (R) | 20 / 0.05 | -- / -- | 20 / 0.18 | 6* /-- |
| CJ82 (Q) | 18.5 / 0.06 | 18 / 0.11 | 14.5* / 0.17 | -- / -- |
| M1388(M) | 19 / -- | 16 / -- | 19 / -- | -- / -- |
-- indicates condition not run or insufficient data points for a calculation.
indicates that, within a subject, the mean maximum number of injections for a condition were less than those for cocaine alone with no overlap in range.
Discussion
The main finding of the present study was that histamine, a drug punisher, reduced cocaine’s effectiveness as a reinforcer. Confidence in this finding is supported by the fact that the result occurred using both BE and PR approaches, which use different analyses for measuring reinforcing effectiveness. The doses of histamine that were required to reduce the reinforcing effectiveness of cocaine in the current report (0.05-0.1 mg/kg/inj) were relatively high compared to those in previous studies that were effective in reducing choice for cocaine and food in rhesus monkeys (Negus 2005; Woolverton 2003). This may be due to differences in preparation. In the current study, responding was reinforced by cocaine on one lever only. However, Negus (2005) and Woolverton (2003) used choice procedures in which monkeys had the choice between punished and non-punished reinforcer options. Behavior may be more sensitive to punishment by histamine or any other stimulus when there is an alternative reinforcer available.
Another explanation for why relatively high doses of histamine were required to reduce cocaine’s effectiveness as a reinforcer is that histamine may function as a punisher of cocaine self-administration at doses that are lower than those required to reduce its maximum effectiveness as a reinforcer. This position is supported by the results of the PR tests in Experiment 2. Dose-response functions rendered from PR schedules of reinforcement can reveal changes in a drug’s potency as a reinforcer through lateral shifts in the dose-response function. Alternatively, these functions can also reveal changes in a drug’s reinforcing effectiveness through vertical shifts in the dose-response function’s asymptote. Thus, a treatment that decreases a drug’s potency as a reinforcer but not its effectiveness would be reflected in a rightward shift of the dose-response function with no vertical shift in the asymptote. In the current dataset, histamine decreased cocaine’s potency as a reinforcer in three monkeys (i.e., resulted in rightward shifts of the cocaine dose-response function) at doses that were not effective in reducing the maximum number of injections of cocaine (i.e., no change in the asymptotes; see CJ82, R99028, and 4199 in Figure 3). These results suggest that cocaine’s potency as a reinforcer is more sensitive to modulation by drug punishment than its effectiveness as a reinforcer. However, increasing the dose of histamine in these three monkeys did result in downward shifts in the dose-response functions for cocaine, indicating that drug punishers can decrease the reinforcing effectiveness of cocaine if the doses are sufficiently high. Unfortunately, the fourth monkey, M1388, ran out of useable veins before the end of the experiment, which prevented testing of a higher dose of histamine in this subject.
It should be noted that the rate-based measures used in the current report are limited in that they cannot distinguish between a stimulus’s punishing effect and potential non-specific rate-decreasing effects. Thus, an alternative interpretation to the current results is that histamine administration and accumulation over repeated injections constrained the subjects’ ability to respond at the rates necessary for acquiring cocaine injections at the higher response requirements. However, two characteristics of histamine make this interpretation unlikely. First, histamine has a short plasma elimination half-life (approximately 2 min in humans; Ind et al., 1982). The short inter-trial intervals in Experiment 1 (11 sec) make it impossible to rule out a role for histamine accumulation and non-specific effects on response rates. However, given that the inter-trial interval for Experiment 2 was 30 min, it is improbable that histamine accumulation had a direct effect on responding over trials, which is a strong indication that the decrease in cocaine injections observed with co-administration of histamine was related to its punishing effect. The second factor that argues against a non-specific effect of histamine on response rate is that systemically administered histamine does not enter the brain in appreciable amounts (Hershowit 1979), which eliminates the possibility of sedative or psychomotor effects that have been argued to constrain operant responding with centrally-acting drugs (see Lynch and Carroll, 2001).
In addition to providing tools for studying theoretical aspects of punishment, drug punishers have been used clinically to deter abuse of other drugs. For example, diphenoxylate, a narcotic that is used to treat diarrhea, is prescribed as a mixture with low doses of atropine, an anticholinergic drug with reported aversive effects, to deter intentional overdosing (Lomotil; Product Information). Similar punishment approaches have been used to deter alcohol (Antabuse) and buprenorphine (Suboxone) abuse (Barth et al 2010; Orman and Keating 2009). However, abuse of prescription opioids and stimulants is on the rise (Kaye and Darke 2012; Ling et al 2011), and most formulations of these prescription medications do not currently use punishment technologies to deter abuse. The findings in the current report provide evidence that drug punishers may be able to reduce the level of reinforcement achievable by prescription medications. Further characterization of drugs as punishers, including histamine, and identifying new classes of drugs that function as punishers may provide the groundwork for developing abuse-deterrent formulations for these compounds.
Acknowledgements
The current study was funded by National Institute on Drug Abuse grant R01 DA-019471 to W.L.W.
References
- Azrin NH, Holz WC. Punishment. In: Honig WK, editor. Operant behavior: areas of research and application. Prentice-Hall Inc.; Englewood Cliffs: 1966. pp. 380–447. [Google Scholar]
- Banks ML, Roma PG, Folk JE, Rice KC, Negus SS. Effects of the delta-opioid agonist SNC80 on the abuse liability of methadone in rhesus monkeys: A behavioral economic analysis. Psychopharm. 2011;216:431–439. doi: 10.1007/s00213-011-2235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth KS, Malcolm RJ. Disulfiram: an old therapeutic with new applications. CNS Neurol Disord Drug Targets. 2010;9:5–12. doi: 10.2174/187152710790966678. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Heal DJ, McCreary AC, Woolverton WL. Assessment of ropinirole as a reinforcer in rhesus monkeys. Drug Alcohol Depend. 2012;125:173–177. doi: 10.1016/j.drugalcdep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Histamine as a punisher in squirrel monkeys: effects of pentobarbital, chlordiazepoxide, and H1- and H2-receptor antagonists on behavior and cardiovascular responses. J Pharmacol Exp Ther. 1980;214:726–736. [PubMed] [Google Scholar]
- Grove RN, Schuster CR. Suppression of cocaine self-administration by extinction and punishment. Pharmacol Biochem Behav. 1974;2(199):2082–24403. doi: 10.1016/0091-3057(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Hershowitz BI. Histamine receptors. Annu Rev Pharmacol. 1979;19:203–244. doi: 10.1146/annurev.pa.19.040179.001223. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Anker JJ, Reiger PS, Claxton A, Carroll ME. Cocaine self-administration punished by i.v. histamine in rat models of high and low drug abuse vulnerability: Effects of saccharin preference, impulsivity, and sex. Physiol Behav. 2013 doi: 10.1016/j.physbeh.2013.08.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Behav. 2013;99:98–124. doi: 10.1002/jeab.7. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Ind PW, Brown MJ, Lhoste FJ, Macquin I, Dollery CT. Concentration effect relationships of infused histamine in normal volunteers. Agents Actions. 1982;12:12–16. doi: 10.1007/BF01965099. [DOI] [PubMed] [Google Scholar]
- Johanson CE. The effects of electric shock on responding maintained by cocaine in a choice procedure in the rhesus monkey. Psychopharm. 1977;53:277–282. doi: 10.1007/BF00492364. [DOI] [PubMed] [Google Scholar]
- Kaye S, Darke S. The diversion and misuse of pharmaceutical stimulants: what do we know and why should we care? Addiction. 2012;107:467–477. doi: 10.1111/j.1360-0443.2011.03720.x. [DOI] [PubMed] [Google Scholar]
- Li H, Gu S, Cai X, Speed WC, Pakstis AJ, Golub EI, Kidd JR, Kidd KK. Ethnic related selection for an ADH Class I variant with East Asia. PLoS One. 2008;3:e1881. doi: 10.1371/journal.pone.0001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: clinical issues and implications. Drug Alcohol Rev. 2011;30:300–305. doi: 10.1111/j.1465-3362.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of punishment on choice between cocaine and food. Psychopharm. 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- Orman JS, Keating GM. Buprenorphine/naloxone: a review of its use in the treatment of opioid dependence. Drugs. 2009;69:577–607. doi: 10.2165/00003495-200969050-00006. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Jimenez-Gomez C, Woods JH. A choice procedure to assess the aversive effects of drugs in rodents. J Exp Anal Behav. 2010;93:203–223. doi: 10.1901/jeab.2010.93-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Product Information: Lomotil, diphenoxylate-atropoine. GD Searle & Co; Chicago, IL: 1998. [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharm. 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharm. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Wee s, Carroll FI, Woolverton WL. A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants. Neuropsychopharm. 2005;31:351–362. doi: 10.1038/sj.npp.1300795. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: practical and theoretical concerns. Psychopharm. 2000;153:139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. A novel choice method for studying drugs as punishers. Pharmacol Biochem Behav. 2003;76:125–131. doi: 10.1016/s0091-3057(03)00219-3. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Freeman KB, Myerson J, Green L. Suppression of cocaine self-administration in monkeys: effects of delayed punishment. Psychopharm. 2012;220:509–517. doi: 10.1007/s00213-011-2501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL. Comparison of the reinforcing efficacy of cocaine and procaine in rhesus monkeys responding under a progressive-ratio schedule. Psychopharm. 1995;120:296–02. doi: 10.1007/BF02311177. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]