Abstract
Purpose
Chemotherapy-induced peripheral neuropathy (CIPN) occurs in as high as 70% of patients receiving certain types of chemotherapy agents. The FDA has yet to approve a therapy for CIPN. The aim of this multicenter, phase III, randomized, double-blind, placebo-controlled trial was to investigate the efficacy of 2% ketamine plus 4% amitriptyline cream (KA cream) for reducing chemotherapy-induced peripheral neuropathy (CIPN).
Methods
Cancer survivors who completed chemotherapy at least one month prior and had CIPN (≥4 out of 10) were enrolled (N=462). CIPN was assessed using average scores from a 7-day daily diary that asks patients to rate the average “pain, numbness, or tingling in [their] hands and feet over the past 24 hours” on an 11-point numeric rating scale at baseline and 6-weeks post intervention. ANCOVA was used to measure differences in 6-week CIPN with effects including baseline CIPN, KA treatment arm, and previous taxane therapy (Y/N).
Results
The KA treatment showed no effect on 6-week CIPN scores (adjusted mean difference = −0.17, p = 0.363).
Conclusions
This study suggests that two percent ketamine plus 4% amitriptyline cream does not decrease CIPN symptoms in cancer survivors.
Keywords: chemotherapy-induced peripheral neuropathy, ketamine, amitriptyline
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of chemotherapeutics such as taxanes, vinca alkaloids, platinum agents, and epothilones[1,2]. The prevalence of CIPN may be as high as 70% and depends on type, dose, and duration of therapy[1]. Symptoms include impaired sensory and motor function and pain in the hands and feet. A recent study reported that one third of patients with CIPN rated neuropathic symptoms as their most troublesome cancer-related side effect, demonstrating a significant impact on quality of life[3]. Although many interventions have shown promise for preventing and treating CIPN in small, early-phase studies, no treatment for CIPN is currently FDA approved.
Duloxetine was effective for treatment of existing painful CIPN in in a recently published phase III clinical trial[4]. Although, this is encouraging, systemic anti-depressants are often associated with toxicities including fatigue, dry mouth, dizziness, and constipation. A topical treatment option would be beneficial for patients who do not tolerate systemic treatments well. Further, local, topical administration of effective agents would reduce systemic adsorption and side effects while maximizing local dosage and treatment effects[5], allowing for the safe combination of multiple drugs that could not be given in combination systemically.
Multiple pathophysiological mechanisms underlie neuropathy[6]. Combining drugs that target different aspects of errant neurotransmission may increase efficacy. Three separate randomized controlled clinical trials support the strategy of combining anti-epileptic drugs with either opioids or tricyclic antidepressants to alleviate painful diabetic neuropathy[7–9]. Further, studies have demonstrated the efficacy of topical analgesics in treating painful neuropathy. For example, separate and combined topical administration of doxepin and capsaicin[10] or ketamine and amitriptyline were shown to decrease neuropathic pain in patients with various diagnoses, including post-herpetic and diabetic neuralgia[11,12].
This phase III study was designed to evaluate the efficacy and safety of a 2% ketamine plus 4% amitriptyline cream (KA cream) for reducing CIPN symptoms in patients who have completed chemotherapy. This drug combination was chosen because ketamine and amitriptyline target multiple, separate neuropathy-inducing mechanisms[13–16]. In fact, together they potentially target four pathways of nociception, including NMDA and Na+ channels, serotonin/norepinephrine, and opioid receptors. Furthermore, this drug combination has been shown to have analgesic effects in patients with diabetic neuropathy[12,11].
Methods
Study Design and Participants
This study was a multicenter phase III, double-blind, randomized, placebo-controlled clinical trial. The University of Rochester Research Subjects Review Board as well as local community clinical oncology sites approved the protocol and informed consent documents. Informed consent was obtained from all patients prior to enrolment in the study.
The University of Rochester Cancer Center Community Clinical Oncology Program (URCC CCOP) recruited 462 participants at 23 geographically unique sites throughout the United States. Eligible patients had chemotherapy-induced neuropathy symptoms, including pain, numbness, or tingling. All participants were recruited to the study at least one month after they completed chemotherapy to prevent inclusion of participants whose CIPN would likely regress on its own. Prior to enrollment in the study, patients completed a daily diary that asked them to rate the “pain, numbness, or tingling in [their] hands and feet over the past 24 hours” on an 11-point numeric rating scale (NRS) ranging from 0 [not at all] – 10 [as bad as you can imagine]. Participants were instructed to answer this question in relation to any of the three symptoms in either their hands or feet, whichever area was affected. Patients with average seven-day pain, numbness, and tingling ratings from the diary of ≥ 4 were enrolled in the study. A cut-off of ≥ 4 on numeric rating scales for chronic pain is standard inclusion criteria in many chronic pain clinical trials[17]. Because pain is a major component of the primary neuropathy outcome, this cut-off was chosen here. Eligible patients were at least 18 years of age and were required to speak and understand English. Karnofsky performance status eligibility was > 60. Subjects were excluded based on the following criteria: any known hypersensitivity to a component of the cream; clinical evidence of pre-existing peripheral neuropathy resulting from another reason; use of other topical treatments or neurological procedures (e.g., blocks); glaucoma or urinary retention; clinically significant depression; pregnancy; treatment with monoamine oxidase inhibitors, barbiturates, anticholinergic agents, sympathomimetic drugs, or inhibitors of the CP450 2D6 system; open skin lesions in the region the cream was to be applied; creatinine >2 mg/dL within 30 days prior to the screening visit; or any co-morbid condition that the investigator thought could interfere with efficacy or safety. Patients were allowed to take pain medications as long as the dosage was stable for at least two weeks prior to initiating the study.
Randomization and Blinding
Patients were randomized using a computer-generated random number sequence with a block size of four. Randomization was stratified based on study site and two treatment regimen groups: those who had received taxanes (taxane) and those who had not received taxanes (non-taxane). Treatment assignments were blinded to the study investigators and patients. The KA and placebo creams were supplied in identical tubes. The creams looked identical and had similar consistencies and odors.
Procedures and Assessments
Subjects were instructed to apply up to, but not exceeding, four grams of KA cream two times per day to each area with pain, numbness, and/or tingling. A measuring device was provided to assist in dispensing the proper amount of the cream.
Patients completed the seven-day daily pain, numbness, and tingling diary starting one week prior to entry into the study and at three and six weeks after study enrollment. The daily scores were averaged to calculate the pain, numbness, and tingling score for each data point. This average score at six weeks was the primary outcome. Secondary measures included a pain item (worst pain over the past 24hr on a 0–10 NRS [0 = no pain, 10 = worst pain you can imagine]) as part of a symptom inventory that was adapted from the MD Anderson Symptom Inventory (MDASI)[18] at baseline and weeks three and six.
Adverse events (AEs) were assessed over the phone at weeks 2 and 5 and in person at week 7 by asking the participants the open ended question “How are you feeling.” AEs were reported regardless of whether they were deemed to be related to the treatment by the investigator. AEs were graded using the most current version of the National Cancer Institute-Current Toxicity Criteria.
Statistical analysis
All analyses were performed on an intent-to-treat basis. Differences in baseline characteristics between treatment groups were tested using t-tests for continuous variables and likelihood ratio tests for nominal data. The primary analysis outlined in the protocol was an analysis of covariance (ANCOVA) to assess changes in pain, numbness, and tingling from baseline to week six, with week-six pain, numbness, and tingling score as the response, baseline pain, numbness, and tingling score as the covariate, treatment group, taxane group (i.e. subject on taxanes or not), and treatment-group*taxane-group interaction as factors. If the treatment-group*taxane-group interaction was insignificant at the 0.05 significance level, the protocol specified that the model be refit without the interaction. If the interaction was significant, then two ANCOVAs would be performed separately for the taxane and non-taxane groups, with a Bonferroni-adjusted 0.025 significance level. According to the protocol, missing week-six data were to be handled by the last value carried forward (LVCF) method, but we chose instead to use multiple imputation (MI) under the missing at random (MAR) assumption because LVCF is known to produce biases[19]. SAS PROC MI was used to generate 100 imputations separately for each combination of treatment group and taxane group using Markov Chain Monte-Carlo (MCMC) with the EM Posterior Mode for starting values, a Jeffrey’s prior distribution, and 200 burn-in iterations. SAS PROC MIANALYZE was used to combine the estimates across the imputations. Pain from the symptom inventory was assessed as a secondary outcome using a similar analysis as the primary outcome.
The sample size was set to power the study so that a treatment effect could be detected for each chemotherapy group (taxane and non-taxane) if a significant interaction between treatment and chemotherapy groups occurred. Since two treatment comparisons were proposed, the Bonferroni adjustment for multiple comparisons was used. In the absence of information in the literature, we conservatively chose a zero correlation for the power calculation. A sample size of 80 patients in the KA cream and placebo groups (within each of the two taxane groups) provides approximately 80% power with a two-sided alpha of 0.025 to detect mean differences of 1.15 points on the pain, numbness, and tingling numerical rating scale (SD = 2.35[20]). Thus, enrollment goals were set at a minimum of 160 patients per chemotherapy group. SAS Version 9.2 and R Version 2.14 were used for the analyses as appropriate.
Results
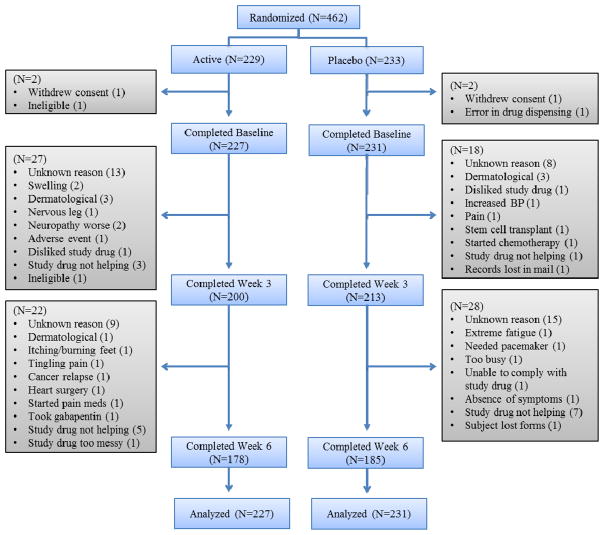
Four hundred sixty-two subjects were enrolled in the study, with 229 randomized to the ketamine-amitriptyline (KA) cream group and 233 to the placebo group. Baseline characteristics of the study sample are shown in Table 1. There were no statistically significant differences in baseline characteristics between treatment groups, including whether the patients had received a taxane or non-taxane chemotherapy. Two subjects were excluded from the KA cream group (one withdrew consent and one ineligible) and 2 subjects were excluded from the placebo group (one withdrew consent and one error in drug dispensing), leaving 227 and 231 subjects’ data analyzed on an intent to treat basis in the treatment and placebo groups, respectively (Figure 1). Four subjects in the active group and three subjects in the placebo group withdrew from the study for skin-related reasons, including itching and rash. Other reasons for withdrawal included increased blood pressure (placebo group), and subjects starting chemotherapy or undergoing a stem cell transplant.
Table 1.
Baseline Characteristics
| All (%) | Active | Placebo | ||
|---|---|---|---|---|
| Race | White | 405 (88%) | 199 | 206 |
| African American | 38 (8%) | 20 | 18 | |
| Other | 18 (4%) | 10 | 8 | |
| Gender | Female | 327 (71%) | 156 | 171 |
| Marital Status | Married | 325 (71%) | 165 | 160 |
| Divorced | 45 (10%) | 20 | 25 | |
| Separated | 5 (1%) | 2 | 3 | |
| Single | 44 (9%) | 23 | 21 | |
| Widowed | 42 (9%) | 19 | 23 | |
| Ethnicity | Hispanic or latino | 13 (3%) | 7 | 6 |
| Not hispanic or latino | 445 (96%) | 221 | 224 | |
| Unknown | 3 (1%) | 1 | 2 | |
| Education | Completed graduate trainng | 47 (10%) | 28 | 19 |
| Standard college or university (4 Years) | 108 (23%) | 53 | 55 | |
| Partial college training (< 4 years) | 156 (34%) | 76 | 80 | |
| High school graduate | 128 (28%) | 63 | 65 | |
| Not high school educated | 22 (5%) | 9 | 13 | |
| Previous Treatment | Previous surgery | 393 (85%) | 198 | 195 |
| Previous chemotherapy | 460 (100%) | 229 | 231 | |
| Previous radiation therapy | 223 (48%) | 114 | 109 | |
| Site | Hematologic | 42 (9%) | 24 | 18 |
| Head & neck | 12 (3%) | 7 | 5 | |
| Lung | 35 (8%) | 12 | 23 | |
| GI | 124 (27%) | 55 | 69 | |
| Genitourinary tract | 24 (5%) | 15 | 9 | |
| Gynecologic | 31 (7%) | 15 | 16 | |
| Breast | 184 (40%) | 96 | 88 | |
| Skin | 2 (0.4%) | 1 | 1 | |
| Melanoma | 1 (0.2%) | 0 | 1 | |
| Soft tissue sarcoma | 3 (0.7%) | 2 | 1 | |
| Bone & cartilaginous | 2 (0.4%) | 2 | 0 | |
| Cardiac | 1 (0.2%) | 0 | 1 | |
| Other | 9 (1%) | 5 | 4 | |
| Regimen | Taxanes | 246 (53%) | 126 | 120 |
Figure 1.
Consort Diagram.
Mean baseline pain, numbness, and tingling scores were 6.55 (95% confidence interval = 6.35, 6.76) in the KA group and 6.47 (95% confidence interval = 6.28, 6.66) in the placebo group. Mean week-six pain, numbness, and tingling scores were 4.93 (95% confidence interval = 4.59, 5.27) in the KA group and 5.19 (95% confidence interval = 4.89, 5.49) in the placebo group (Table 2). In the first ANCOVA outlined by the protocol to investigate the changes in pain, numbness, and tingling, the treatment-group*taxane-group interaction was not statistically significant (p = 0.132), so the interaction term was removed and the ANCOVA was reanalyzed, and it was therefore unnecessary to perform analyses by taxane group. The parameter estimates from the final ANCOVA are presented in Table 3. The KA treatment did not significantly reduce the pain, numbness, and tingling scores (active-placebo mean = −0.174, 95% CI = −0.548, 0.201, p = 0.363). Similar non-significant results were found at 3 weeks (data not shown). Those in the taxane group, however, did show a larger reduction of pain, numbness, and tingling than those in the non-taxane group, and this difference was statistically significant (taxane – non-taxane mean = −0.398, 95% CI = −0.782, −0.015, p = 0.042).
Table 2.
Descriptive statistics for pain, numbness, and tingling scores
| KA cream | Placebo | |||
|---|---|---|---|---|
| Mean [95% CI] | N obs | Mean [95% CI] | N obs | |
| Baseline | 6.55 [6.35, 6.76] | 227 | 6.47 [6.28, 6.66] | 231 |
| Week 6 | 4.93 [4.59, 5.27] | 178 | 5.19 [4.89, 5.49] | 184 |
N obs represents the number of patients with evaluable data at each time point. Missing data were imputed using multiple imputation method.
Table 3.
Summary of ANCOVA parameters predicting week six pain, numbness, and tingling scores.
| Parameter | Estimate | Std Error | 95% Confidence Limits | P-Value |
|---|---|---|---|---|
| Baseline pain, numbness, tingling | 0.804 | 0.062 | 0.682, 0.926 | <.0001 |
| Treatment Arm: Active - Control | −0.174 | 0.190 | −0.548, 0.201 | 0.363 |
| Taxane group: Taxanes - nonTaxanes | −0.398 | 0.195 | −0.782, −0.015 | 0.042 |
Mean baseline pain scores were 5.77 (95% confidence interval = 5.41, 6.15) in the KA group and 5.51 (95% confidence interval = 5.14, 5.88) in the placebo group. Mean week-six pain scores were 4.64 (95% confidence interval = 4.23, 5.05) in the KA group and 4.68 (95% confidence interval = 4.28, 5.08) in the placebo group (Table 4). The same ANCOVA was performed for pain as in the primary analysis. In this case the Arm*taxane interaction was not significant and therefore removed from the model. Similarly to pain, numbness, and tingling, the KA treatment did not significantly reduce pain when assessed alone (active-placebo mean = −0.208, 95% CI = −0.694, 0.278, p = 0.400) (Table 5).
Table 4.
Descriptive statistics for pain
| KA cream | Placebo | |||
|---|---|---|---|---|
| Mean [95% CI] | N obs | Mean [95% CI] | N obs | |
| Baseline | 5.78 [5.41, 6.16] | 223 | 5.51[5.14, 5.88] | 227 |
| Week 6 | 4.64 [4.23, 5.05] | 179 | 4.68 [4.28, 5.08] | 190 |
N obs represents the number of patients with evaluable data at each time point. Missing data were imputed using multiple imputation method.
Table 5.
Summary of ANCOVA parameters predicting week six pain.
| Parameter | Estimate | Std Error | 95% Confidence Limits | P-Value |
|---|---|---|---|---|
| Baseline pain | 0.505 | 0.044 | 0.419, 0.590 | <.0001 |
| Treatment Arm: Active – Control | −0.208 | 0.247 | −0.694, 0.278 | 0.400 |
The primary analysis was repeated adjusting for baseline characteristics including age, sex, Karnofsky performance score, whether a patient had previous surgery, and time since completion of chemotherapy. Adjusting for these characteristics could decrease the variability in the estimate and detect a difference that was not detected by the primary outcome. This analysis; however, was consistent with the primary analysis and did not show a difference between arms in 6-week pain numbness and tingling scores. Further, when this analysis was repeated as a complete case analysis, the KA cream still had no effect on pain, numbness, and tingling.
Adverse events (AEs) were assessed in the intent to treat population (n=458). Two hundred ninety-five AEs were reported during the study; 147 occurred in the KA group and 158 occurred in the placebo group. Eight serious AEs were reported, with four in each arm. Twenty-one AEs were severe; ten occurred in the KA group and 11 in the placebo group. Five of the severe AEs were classified as musculoskeletal, two as swelling, and one as fatigue. See Table 6 for the number of events by treatment arm in the most common AE classes, including musculoskeletal, gastrointestinal, skin, and neurological. The percent of subjects reporting AEs of all classes was similar between arms, although the study was not powered to detect differences in AEs.
Table 6.
Incidence of AEs in classes with more than 10 events.
| AE class | KA cream N (%)(total n=227) | Placebo cream N (%)(total n=231) | p-value |
|---|---|---|---|
| Musculoskeletal | 30 (13%) | 33 (14%) | 0.80 |
| Gastrointestinal | 16 (7%) | 27 (12%) | 0.13 |
| Skin | 23 (10%) | 15 (6%) | 0.26 |
| Neurological | 18 (8%) | 13 (6%) | 0.48 |
| Pulmonary | 7 (3%) | 16 (7%) | 0.09 |
| Fatigue | 8 (4%) | 11 (5%) | 0.65 |
| Cardiac | 9 (4%) | 8 (3%) | 1.0 |
| Endocrine | 9 (4%) | 6 (3%) | 0.61 |
| Insomnia | 5 (2%) | 7 (3%) | 0.77 |
| Allergic | 7 (3%) | 5 (2%) | 0.77 |
Discussion
This double-blind, placebo-controlled clinical trial demonstrated that 2% ketamine plus 4% amitriptyline (KA) cream did not alleviate chemotherapy–induced pain, numbness, and tingling in cancer survivors. Further, secondary analyses assessing pain alone also showed no benefit for KA cream. Although many interventions show promise of efficacy for painful CIPN in smaller trials, this topical treatment joins many others that have proven ineffective in large confirmatory trials. Calcium and magnesium infusions were shown to decrease the incidence of oxaliplatin-induced neuropathy in a retrospective cohort study[21] and a smaller randomized placebo-controlled study[22], but these results have yet to be confirmed in a phase III study. Multiple clinical trials suggest that glutathione may decrease the severity of CIPN[23–25], but again, these results have not been confirmed in a large trial. Small studies have identified other possible treatments for CIPN including the selective serotonin norepinephrine reuptake inhibitor, venlafaxine[26,27], the anti-epileptic drug, pregabalin[28], α-lipoic acid[29,30], and acetyl L-carnitine[31]. However, the efficacy of these agents for reducing CIPN has not been proven in large phase III studies, and some of these agents have been proven ineffective in large confirmatory trials. In fact, Hershman et al. recently reported a phase III placebo-controlled study that demonstrated that acetyl L-carnitine when given in conjunction with taxane therapy for 24 weeks actually increased the severity of neuropathy [32]. Many other agents, including gabapentin[33], nortriptyline[34], amitriptyline[35,36], and lamotrigine[37] that reduce painful neuropathy in diabetic or post-herpetic neuropathy, have shown little effect on painful CIPN. Duloxetine is the only treatment that has been proven effective for painful CIPN in a phase III randomized, placebo-controlled study[4]. Further investigation of interventions to reduce CIPN symptoms, including pain, is clearly needed.
Because different types of chemotherapy cause neuropathy through different molecular mechanisms[38,39], neuropathy caused by different chemotherapy agents may respond differently to different medications. Based on the demographic of patients treated at the CCOP sites, we knew that patients who had received taxanes would represent the largest subset of patients recruited to the trial. Thus, we investigated the effects of the KA cream, taking into account whether the patients had received taxanes or not. Regardless of treatment group, the group of patients who had received taxanes did report a larger reduction in pain, numbness, and tingling after six weeks on the trial than those who had received other chemotherapy agents. This could be due to differences in the taxane and non-taxane groups. For example, the taxane group consisted of more women than the non-taxane group (89.9% and 49.8% female, respectively), although this difference was not statistically significant. Alternatively, neuropathy from taxane treatment could be more likely to resolve on its own than neuropathy resulting from other chemotherapy agents, producing the larger decrease in the taxane group during the duration of this trial. This is unlikely, however, considering that the mean time elapsed between the completion of chemotherapy and the beginning of the study was similar for the taxane and non-taxane groups (mean difference = 15.6 days, p = 0.79). Finally, this result could be a chance finding considering the p-value is close to 0.05 (0.042) and this is a secondary analysis of the trial.
The treatment-group*taxane-group interaction was not significant in the original ANCOVA described by the protocol, suggesting that the cause for the difference in decreased pain, numbness, and tingling between the taxane and non-taxane groups is not related to the KA cream. Thus, the KA cream did not benefit the subset of patients who received taxane chemotherapy. The proportion of subjects who correctly guessed their treatment assignment was not statistically different between placebo and treatment groups (p =0.20), suggesting that the blind was maintained in the study.
Information other than whether patients received a taxane or non-taxane chemotherapy treatment was not collected during this study. This lack of data limits our ability to generate hypothesis about how neuropathy induced by other chemotherapy regimens may respond to KA cream.
Since the initiation of this trial, Barton et al. published a study investigating a topical treatment for CIPN consisting of amitriptyline, ketamine, and a third ingredient, baclofen[40]. In this study, the topical treatment had only a “trending” effect on sensory neuropathy measured by the EORTC subscale (p=0.053). Thus, both trials suggest that topical formulations containing amitriptyline plus ketamine are likely not clinically beneficial, at least at the concentrations or with the cream bases used in these trials. The trending result obtained in Barton et al.’s study suggests that adding baclofen to the mixture may be promising. Further, the ketamine and amitriptyline concentrations used in that trial were lower (1.5% ketamine and 3% amitriptyline) than in this one. Our trial used 2% ketamine plus 4% amitriptyline and few serious or severe adverse events occurred. Further, similar numbers of AEs occurred in the KA and placebo groups suggesting that 2% ketamine plus 4% amitriptyline cream is likely safe and well-tolerated, although the study was not powered to detect differences in AE rates. Thus, higher concentrations of ketamine plus amitriptyline in conjunction with baclofen may be well tolerated and beneficial to these patients and worth investigation.
The duloxetine trial[4], which is the only successful phase III confirmatory trial for CIPN to date, focused on painful CIPN and used the Brief Pain Inventory-Short Form as the primary outcome. Our trial and the ketamine-amitriptyline-baclofen trial[40] used tools that assess pain, numbness, and tingling simultaneously for the primary outcome. Considering ketamine and amitriptyline are both analgesics, it is possible that the effects of the ketamine plus amitriptyline containing creams may have been diluted when all three neuropathy symptoms were assessed together. As a secondary analysis, we evaluated pain separately using a 0 to 10 NRS symptom inventory question that was evaluated once at each assessment. Future studies evaluating the effects of analgesic compounds on CIPN should assess neuropathic pain separately using comprehensive tools like the McGill pain inventory. This strategy could identify an important subset of patients who benefit from these topical analgesics, even if the drugs appear to lack efficacy in a larger population of patients with varying CIPN symptoms.
As the evidence stands now, topical formulations containing amitriptyline plus ketamine are not recommended for reducing CIPN. Future research should focus on identifying novel topical agents that are efficacious for treating CIPN and evaluating painful CIPN separately in addition to rating overall CIPN symptoms.
Acknowledgments
Supported by: The National Cancer Institute (U10CA37420)
We thank the patients, clinicians and researchers of the University of Rochester Community Clinical Oncology Program who contributed to this study.
Footnotes
Clinical Trial Registration number: NCT00471445
Disclosure:
The authors have no conflicts of interest to discuss. This work was funded by the National Cancer Institute (U10CA37420). Epicept provided the drug and placebo creams.
References
- 1.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12 (9):619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 2.Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol. 2008;66 (3):218–228. doi: 10.1016/j.critrevonc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Kautio AL, Haanpaa M, Kautiainen H, Kalso E, Saarto T. Burden of chemotherapy-induced neuropathy-a cross-sectional study. Support Care Cancer. 2010;19(12) doi: 10.1007/s00520-010-1043-2. [DOI] [PubMed] [Google Scholar]
- 4.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama. 2013;309 (13):1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorge LL, Feres CC, Teles VE. Topical preparations for pain relief: efficacy and patient adherence. J Pain Res. 2011;4:11–24. doi: 10.2147/JPR.S9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stillman M. Clinical approach to patients with neuropathic pain. Cleve Clin J Med. 2006;73 (8):726–728. 729–730, 733–726. doi: 10.3949/ccjm.73.8.726. [DOI] [PubMed] [Google Scholar]
- 7.Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374 (9697):1252–1261. doi: 10.1016/S0140-6736(09)61081-3. [DOI] [PubMed] [Google Scholar]
- 8.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352 (13):1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 9.Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain. 2008;12 (6):804–813. doi: 10.1016/j.ejpain.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 10.McCleane G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: a randomized, double-blind, placebo-controlled study. Br J Clin Pharmacol. 2000;49 (6):574–579. doi: 10.1046/j.1365-2125.2000.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical amitriptyline and ketamine in neuropathic pain syndromes: an open-label study. J Pain. 2005;6 (10):644–649. doi: 10.1016/j.jpain.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Lynch ME, Clark AJ, Sawynok J. A pilot study examining topical amitriptyline, ketamine, and a combination of both in the treatment of neuropathic pain. Clin J Pain. 2003;19 (5):323–328. doi: 10.1097/00002508-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Smith DJ, Pekoe GM, Martin LL, Coalgate B. The interaction of ketamine with the opiate receptor. Life Sci. 1980;26 (10):789–795. doi: 10.1016/0024-3205(80)90285-4. [DOI] [PubMed] [Google Scholar]
- 14.Davies SN, Alford ST, Coan EJ, Lester RA, Collingridge GL. Ketamine blocks an NMDA receptor-mediated component of synaptic transmission in rat hippocampus in a voltage-dependent manner. Neurosci Lett. 1988;92 (2):213–217. doi: 10.1016/0304-3940(88)90063-8. [DOI] [PubMed] [Google Scholar]
- 15.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340 (2–3):249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 16.Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C., 3rd Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. 1998;284 (1):208–214. [PubMed] [Google Scholar]
- 17.Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, Jensen MP, Katz NP, Raja SN, Rappaport BA, Rowbotham MC, Backonja MM, Baron R, Bellamy N, Bhagwagar Z, Costello A, Cowan P, Fang WC, Hertz S, Jay GW, Junor R, Kerns RD, Kerwin R, Kopecky EA, Lissin D, Malamut R, Markman JD, McDermott MP, Munera C, Porter L, Rauschkolb C, Rice AS, Sampaio C, Skljarevski V, Sommerville K, Stacey BR, Steigerwald I, Tobias J, Trentacosti AM, Wasan AD, Wells GA, Williams J, Witter J, Ziegler D. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012;153 (6):1148–1158. doi: 10.1016/j.pain.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89 (7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Molenberghs GKM. Missing data in clinical studies. John Wiley and Sons; West Sussex: 2007. [Google Scholar]
- 20.Dworkin RH, Corbin AE, Young JP, Jr, Sharma U, LaMoreaux L, Bockbrader H, Garofalo EA, Poole RM. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60 (8):1274–1283. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- 21.Gamelin L, Boisdron-Celle M, Delva R, Guerin-Meyer V, Ifrah N, Morel A, Gamelin E. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. 2004;10 (12 Pt 1):4055–4061. doi: 10.1158/1078-0432.CCR-03-0666. [DOI] [PubMed] [Google Scholar]
- 22.Grothey A, Nikcevich DA, Sloan JA, Kugler JW, Silberstein PT, Dentchev T, Wender DB, Novotny PJ, Chitaley U, Alberts SR, Loprinzi CL. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol. 2011;29 (4):421–427. doi: 10.1200/JCO.2010.31.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombo N, Bini S, Miceli D, Bogliun G, Marzorati L, Cavaletti G, Parmigiani F, Venturino P, Tedeschi M, Frattola L, Buratti C, Mangioni C. Weekly cisplatin +/− glutathione in relapsed ovarian carcinoma. Int J Gynecol Cancer. 1995;5 (2):81–86. doi: 10.1046/j.1525-1438.1995.05020081.x. [DOI] [PubMed] [Google Scholar]
- 24.Smyth JF, Bowman A, Perren T, Wilkinson P, Prescott RJ, Quinn KJ, Tedeschi M. Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: results of a double-blind, randomised trial. Ann Oncol. 1997;8 (6):569–573. doi: 10.1023/a:1008211226339. [DOI] [PubMed] [Google Scholar]
- 25.Milla P, Airoldi M, Weber G, Drescher A, Jaehde U, Cattel L. Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs. 2009;20 (5):396–402. doi: 10.1097/CAD.0b013e32832a2dc1. [DOI] [PubMed] [Google Scholar]
- 26.Durand JP, Alexandre J, Guillevin L, Goldwasser F. Clinical activity of venlafaxine and topiramate against oxaliplatin-induced disabling permanent neuropathy. Anticancer Drugs. 2005;16 (5):587–591. doi: 10.1097/00001813-200506000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Durand JP, Deplanque G, Montheil V, Gornet JM, Scotte F, Mir O, Cessot A, Coriat R, Raymond E, Mitry E, Herait P, Yataghene Y, Goldwasser F. Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2011;23(1):200–205. doi: 10.1093/annonc/mdr045. [DOI] [PubMed] [Google Scholar]
- 28.Saif MW, Syrigos K, Kaley K, Isufi I. Role of pregabalin in treatment of oxaliplatin-induced sensory neuropathy. Anticancer Res. 2010;30 (7):2927–2933. [PubMed] [Google Scholar]
- 29.Gedlicka C, Kornek GV, Schmid K, Scheithauer W. Amelioration of docetaxel/cisplatin induced polyneuropathy by alpha-lipoic acid. Ann Oncol. 2003;14 (2):339–340. doi: 10.1093/annonc/mdg051. [DOI] [PubMed] [Google Scholar]
- 30.Gedlicka C, Scheithauer W, Schull B, Kornek GV. Effective treatment of oxaliplatin-induced cumulative polyneuropathy with alpha-lipoic acid. J Clin Oncol. 2002;20 (15):3359–3361. doi: 10.1200/JCO.2002.99.502. [DOI] [PubMed] [Google Scholar]
- 31.Maestri A, De Pasquale Ceratti A, Cundari S, Zanna C, Cortesi E, Crino L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori. 2005;91 (2):135–138. doi: 10.1177/030089160509100206. [DOI] [PubMed] [Google Scholar]
- 32.Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, Hansen L, Lew DL, Greenlee H, Fehrenbacher L, Wade JL, Wong SF, Hortobagyi GN, Meyskens FL, Albain KS. Randomized placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol. 2013;31(20):2627–33. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110 (9):2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 34.Hammack JE, Michalak JC, Loprinzi CL, Sloan JA, Novotny PJ, Soori GS, Tirona MT, Rowland KM, Jr, Stella PJ, Johnson JA. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98 (1–2):195–203. doi: 10.1016/s0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 35.Kautio AL, Haanpaa M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35 (1):31–39. doi: 10.1016/j.jpainsymman.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 36.Kautio AL, Haanpaa M, Leminen A, Kalso E, Kautiainen H, Saarto T. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res. 2009;29 (7):2601–2606. [PubMed] [Google Scholar]
- 37.Rao RD, Flynn PJ, Sloan JA, Wong GY, Novotny P, Johnson DB, Gross HM, Renno SI, Nashawaty M, Loprinzi CL. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer. 2008;112 (12):2802–2808. doi: 10.1002/cncr.23482. [DOI] [PubMed] [Google Scholar]
- 38.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249 (1):9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 39.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13 (1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 40.Barton DL, Wos EJ, Qin R, Mattar BI, Green NB, Lanier KS, Bearden JD, 3rd, Kugler JW, Hoff KL, Reddy PS, Rowland KM, Jr, Riepl M, Christensen B, Loprinzi CL. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer. 2011;19 (6):833–841. doi: 10.1007/s00520-010-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]