Abstract
Malaria kills close to a million people every year, mostly children under the age of five. In the drive towards the development of an effective vaccine and new chemotherapeutic targets for malaria, field-based studies on human malaria infection and laboratory-based studies using animal models of malaria offer complementary opportunities to further our understanding of the mechanisms behind malaria infection and pathology. We outline here the parallels between the Plasmodium chabaudi mouse model of malaria and human malaria. We will highlight the contribution of P. chabaudi to our understanding of malaria in particular, how the immune response in malaria infection is initiated and regulated, its role in pathology, and how immunological memory is maintained. We will also discuss areas where new tools have opened up potential areas of exploration using this invaluable model system.
The rodent malarias: from the gallery forest to the lab
In the years following the discovery by Vincke in 1948 that malaria parasites infect African rodents, these parasites (Box 1) have been used extensively in laboratory research on malaria. Their use has informed almost every subdiscipline of malariology, including parasite genetics, genomics, immunology, evolutionary biology, and ecological studies. It is not our intent to cover all of these areas, but instead to discuss some of the most fruitful areas to date and high-light some of the crucial parallels uncovered between human malaria and experimental malaria. Although various Plasmodium species infecting rodents differ in their life-cycle characteristics (Table 1), there is significant conservation of both genetic and phenotypic traits between the human malaria parasites and their rodent counterparts [1], which makes them attractive as models for human malaria.
Box 1. P chabaudi subspecies and clones.
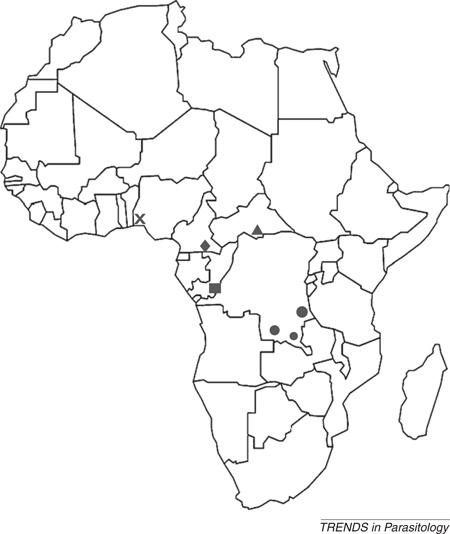
Plasmodium chabaudi is one of four species of African rodent malaria parasites discovered in Sub-Saharan Central Africa during the late 1940s to mid 1960s, the others being Plasmodium berghei Vincke & Lips, 1948; Plasmodium vinckei Rhodain, 1952; and Plasmodium yoelii, Landau & Killick-Kendrick, 1966. The locations where these species were discovered are shown in Figure I. The natural mammalian hosts for all these species are the African thicket rats Grammomys surdaster (P. berghei) or Thamnomys rutilans (all other species). The natural insect vector is known only for P. berghei and P. vinckei vinckei, sporozoites of which were found in the salivary glands of Anopheles dureni mosquitoes. There are three subspecies of P. chabaudi, each isolated in a different location; P. c. chabaudi from the Central African Republic, P. c. adami from Congo Brazzaville, and an as-yet uncharacterized subspecies, P. c. ssp from Cameroon. This article considers P. c. chabaudi, unless otherwise stated.
There is abundant genetic polymorphism within all the rodent malaria species, but to date it is the genetically distinct cloned lines of P. chabaudi that have been characterized most extensively. Originally defined by isoenzyme analysis, the cloned lines of P. chabaudi have formed the basis for numerous elegant investigations into the genetics of malaria parasites. Such studies were initiated in Edinburgh by Geoffrey Beale’s laboratory. P. chabaudi was chosen as the most suitable rodent parasite for genetic studies of the basis of anti-malarial drug resistance by Walliker et al. because wild type P. chabaudi (unlike P. yoelii) is inherently sensitive to chloroquine [107] and can also be easily transmitted through mosquitoes in the laboratory, an advantage the rodent malaria parasites hold to this day over the human parasite species.
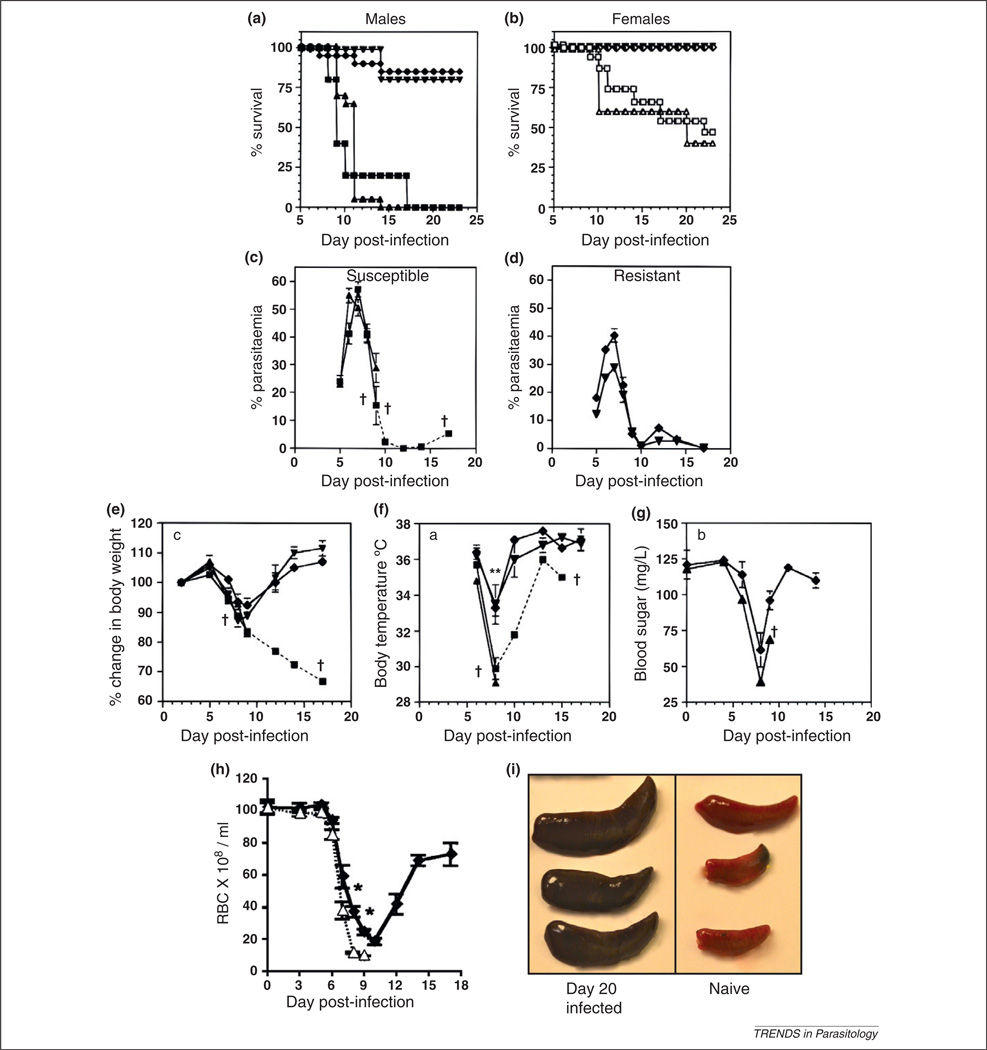
The P. chabaudi model of malaria mirrors many of the pathological and immunological aspects of human malaria infections, in general making it an excellent tool to further our knowledge of human malaria infections and to identify new targets for therapeutic interventions. However, there are some limitations to this model, including the organ of sequestration in mice (the liver rather than the brain), the level of circulating parasitemia required to observe pathogenic symptoms of malaria in mice (higher than for human malaria infections), some differences in pathogenic symptoms such as the development of hypothermia rather than fever, and the discrepancy of parasitemia profiles between mice (Figure 1) and the more chronic parasitemia profile in the Thamnomys natural host [108].
Figure I. Distribution of rodent malaria parasites in Africa. Cross, Plasmodium yoelii nigeriensis, Plasmodium vinckei brucechwatti; triangle, Plasmodium yoelii yoelii, Plasmodium chabaudi chabaudi, Plasmodium vinckei petteri; diamond, Plasmodium yoelii subsp. Plasmodium chabaudi subsp. Plasmodium vinckei subsp.; square, Plasmodium yoelii killicki, Plasmodium chabaudi adami, Plasmodium vinckei lentum; circle, Plasmodium berghei, Plasmodium vinckei vinckei.
Table 1.
Life-cycle characteristics of rodent malaria parasites
| Parasite | Area of isolationa | Sporogony temperature (°C) (optimum) |
Sporozoite length (mean; µM) |
Synchrony in blood |
Erythrocyte preference |
Liver stage (h) |
|---|---|---|---|---|---|---|
| P. berghei | Katanga (●) | 19–21 | 11.0 | Asynchronous | Reticulocytes | 50 |
| P. y. yoelii | Central African Republic (▲) | 24 | 14.0 | Asynchronous | Reticulocytesb | 43–50 |
| P. y. killicki | Brazzaville (■) | 22–24 | 14.0 | Asynchronous | Reticulocytes | 46–50 |
| P. y. nigeriensis | Nigeria (x) | 24 | 16.7 | Asynchronous | Reticulocytes | 47–50 |
| P. v. vinckei | Katanga (●) | 20–21 | 15.0 | Synchronous | Normocytes | 53–61 |
| P. v. lentum | Brazzaville (■) | 24–25 | 21.0 | Synchronous | Normocytes | <72 |
| P. v. brucechwatti | Nigeria (x) | 24–26 | 14.7 | Synchronous | Normocytes | 61–65 |
| P. v. petteri | Central African Republic (▲) | 24–26 | 16.2 | Synchronous | Normocytes | 53–61 |
| P. c. chabaudi | Central African Republic (▲) | 26 | 11.7 | Synchronous | Normocytes | 52–53 |
| P. c. adami | Brazzaville (■) | 24–26 | 11.7 | Synchronous | Normocytes | ?c |
|
P. y. subsp. P. c. subsp. P. v. subsp. |
Cameroon (◆) | ? | ? | ? | ? | ? |
Symbols represent location on the map in Box 1, Figure I.
P. y. yoelii strains 17XL and YM can invade normocytes.
? = undetermined.
The ability to manipulate every aspect of host and parasite genetics and environment in mouse models makes them invaluable in malariology. Here we discuss the contribution of the rodent malaria parasite Plasmodium chabaudi, described by Landau in 1965 [2], to our understanding of human malaria. One of the most useful attributes of P. chabaudi is the variety of well-characterized cloned lines (Box 1) [3]. These lines are all synchronous (unlike P. berghei ANKA or P. yoelii [4]) and exhibit a spectrum of disease severity [5], different levels of sequestration [6] and transmissibility to mosquitoes [7]. The most commonly used line is P. chabaudi chabaudi (AS), which is generally non-lethal, similar to malaria caused by both P. vivax and P. falciparum in humans (less than 0.5% lethality), but sequesters (adheres) on vascular endothelium, induces a chronic infection [8], and can be relatively easily transmitted through Anopheles stephensi mosquitoes.
P. chabaudi displays striking hematological similarities to P. falciparum
Different Plasmodium spp invade erythrocytes at particular stages in red blood cell (RBC) development; the availability of the preferred RBC type determines much of the dynamics of infection. P. falciparum invades RBCs of all ages, whereas P. vivax displays a preference for immature RBCs (reticulocytes). The preferences for the rodent parasites are detailed in Table 1, but it is notable that, as with P. falciparum, P. chabaudi invades both normocytes and reticulocytes [9]; however, unlike the other rodent Plasmodium spp, P. chabaudi-infected red blood cells (iRBC) adhere to host endothelial cells at the schizont stage (sequestration) [6,10] and to uninfected RBCs (rosetting) [11]. These adhesion phenotypes are associated with cerebral malaria, however, P. chabaudi does not sequester in the brain, but in the liver; nevertheless, in IL-10 deficient mice it induces cerebral edema and hemorrhage, suggesting that inflammation alone leads to these symptoms [12]. Although sequestration of mature schizonts has been observed in other rodent malaria parasite species in some strains of mice [13], the phenomenon is much more apparent in P. chabaudi infection in which mature schizonts are almost never observed in the peripheral blood circulation [12], as with P. falciparum. Endothelial sequestration of iRBCs takes place via binding to host endothelial receptors such as CD36 for both P. falciparum [14] and P. chabaudi iRBCs [15], and to intercellular and vascular cellular adhesion molecules (ICAM and VCAM).
P. vivax, P. knowlesi and all of the rodent malaria parasites express a large family of variant surface proteins named the Plasmodium interspersed repeat family (PIRS) that may be structurally homologous to the RIFIN and STEVOR proteins in P. falciparum [16]. One hypothesis for their function is that they are ligands for the binding of iRBCs to endothelial cells and to uninfected RBCs [16]. P. chabaudi has the smallest set of pir genes, and contains approximately 135 cir sequences [16], making this species attractive for studies in this area (Box 2).
Box 2. Important fields open to investigation in Plasmodium chabaudi.
Anemia: the mechanisms of the four processes contributing to severe anemia (dyserythropoiesis), erythropoietic suppression, phagocytosis of infected and uninfected RBCs) remain unclear.
Antigenic variation and immune evasion: although rodent parasites do not contain any homolog to PfEMP1, they do have a large family of variant proteins, encoded in similar chromosomal locations near the telomeres, termed the cir genes in P. chabaudi, which contain significantly fewer (~130) of these than P. yoelii (~800), making them easier to study for their role in immune evasion.
Basic biology: understanding the entry and exit of the parasite and of the function of various Plasmodium proteins has been opened up by development of transgenic P. chabaudi, and linkage group selection (LGS) for genetic studies.
Ligands of sequestration: sequestration is mediated by PIR proteins (analogous to STEVORs and RIFINs of P. falciparum), and other candidates, in conjunction with their host endothelial ligands (CD36, ICAM, VCAM) as well as inflammatory mediators in various syndromes attributed to sequestration. The role of sequestration in cerebral pathology can also be studied in P. chabaudi because inflammation may be enough to induce cerebral edema in this model, while sequestration is only seen in other tissues.
Pre-erythrocytic stages: mechanisms of entry into liver, adhesion and immune escape, CD8+ T cell responses and memory (although a very elegant series of publications has elucidated some mechanisms in the P. yoelii model).
Virulence: using genetic techniques, the host and parasite genes involved in determining the relative virulence of a parasite strain/host combination can be determined much more clearly than in human studies.
Pregnancy and malaria: mice have been shown to have increased chances of dying and losing their embryos in case of P. chabaudi infection. This creates the opportunity to study the mechanisms of parasite tropism for the placenta and the serious consequences for tens of thousands of mothers.
P. chabaudi can be used to dissect mechanisms of pathology in malaria infection
Mice infected with P. chabaudi display an array of symptoms which start late in the peak of infection and resolve as the parasite is cleared (Figure 1, Table 2) [17]. The pathogenesis of malaria infection in humans is multi-factorial, but may be considered to centre around three interlinked phenomena: (i) immune response-induced severe malarial anemia (SMA) [18], (ii) sequestration of iRBCs to activated endothelial cells [14], and (iii) metabolic acidosis, one of the strongest predictors of death from human malaria [19]; all these symptoms can be linked to the systemic inflammation induced by the parasite.
Figure 1.
Pathological features of P. chabaudi AS infection. The survival of mice aged 6–10 weeks infected intraperitoneally with 105 P. chabaudi AS is dependent on mouse genetic background and differs between males (a) and females (b). Results are presented as the percentages of 5–15 mice per group surviving on a particular day; % circulating parasitemia (monitored by Giemsa-stained thin blood smears) peaks between days 7–9 and is higher in susceptible male mice [A/J and DBA/2 (c)] than in resistant male mice [B10.A and B10.D2 (d)]. Results are presented as the arithmetic means of 5–10 mice per group ± SEM. One surviving A/J male mouse which died at day 17 postinfection is represented with a broken line. During infection mice lose weight (e), become hypothermic (f) and hypoglycemic (g); all these parameters return to normal levels upon clearance of the peak of parasitemia. Data presented are the mean values ± SEM for five mice per group. Mice also become anemic from P. chabaudi infection (h). Resistant C57BL/6 (◆) and susceptible A/J (Δ) male mice were infected with 106 P. chabaudi AS intraperitoneally and the number of circulating RBCs monitored for 18 d postinfection. Data represent means ± SEM for seven mice per group. The development of splenomegaly is shown in (i) (T. LeFevre, unpublished). Three naïve spleens are shown on the right, and three spleens from male C57BL/6 mice 20 d postinfection with 105 P. chabaudi are shown on the left; the darker color in the latter indicates the accumulation of hemozoin, the insoluble parasite by-product. (a–g) modified from Cross and Langhorne [17] with permission. A/J males (■), B10.A males (◆), DBA/2 males (▲), B10.D2 males (▼), A/J females (□), DBA/2 females (△), B10.A females (◇) and B10.D2 females (▽). *, significantly different (P < 0.05); †, mortality. (h) reproduced from Chang et al. [24], with permission.
Table 2.
Comparison of the pathogenesis of P. chabaudi and human infectious Plasmodium
| Pathogenesis | P. falciparum | P. vivax | P. chabaudi |
|---|---|---|---|
| Fever | Constant, or irregular peaks of fever initially, often followed by a tertian pattern (36–48 h cyclical) of fever | Occurs every 48 h, the length of the synchronous erythrocytic cycle | Infected mice gradually develop hypothermia |
| Anemia and Thrombocytopenia | Development of SMA with dyserythropoeisis, erythrophagocytosis and suppression of hematopoiesis. Patients also become thrombocytopenic |
Development of SMA with dyserythropoiesis, erythrophagocytosis and suppression of hematopoiesis. Patients also become thrombocytopenic |
Development of lethal anemia with dyserythropoiesis and suppression of hematopoiesis Induces thrombocytopenia which recovers after the peak of parasitemia |
| Cerebral malaria | iRBCs sequester onto the endothelial lining of the brain correlates with cerebral edema, hemorrhage, inflammation, and blockage of blood flow | Not generally associated with cerebral malaria but some reports in the literature | Not generally associated with cerebral malaria Cerebral symptoms (edema, hemorrhage) have been observed in C57BL/6 IL10−/−mice |
| Splenomegaly | Splenomegaly present | Splenomegaly present | Observable splenomegaly from day 5 postinfection peaking at day 14. Does not directly correlate with parasitemia or sequestration |
| Hypoglycemia | Infection induces hypoglycemia | Infection induces hypoglycemia | Infection induces hypoglycemia |
| Respiratory distress and metabolic acidosis | Acute respiratory distress syndrome (ARDS) may be lethal. Induction of metabolic acidosis contributing to ARDS |
Induces lung injury and respiratory distress. Less acute than in falciparum malaria. Induction of metabolic acidosis leading to respiratory distress |
Genetically susceptible mice (BALB/c RAG−/− or C57BL/6 IL-10−/−) or those infected with virulent strains of P. chabaudi can develop signs of respiratory distress (Tracey J. Lamb, unpublished). Other mouse models may show a more analogous syndrome. Induction of hyperlactemia (P. chabaudi adami). The level of acidosis is not known |
| Placental malaria | Cytoadherence of iRBCs in placenta, low birth weight. Increased risk of perinatal mortality | Rarely cytoadherence of iRBCs in placenta. Associated with low birth weight | Cytoadherence of iRBCs on the placenta, inflammation and fetal loss |
In contrast to the human malarias where cyclical schizogony leads to fever, mice infected with P. chabaudi develop hypothermia (Table 2). Other syndromes of malaria studied thus far are analogous to those in human malaria infection. Anemia occurs in malaria-infected individuals due to a combination of death of infected erythrocytes, suppression of erythropoiesis, clearance of uninfected RBCs by phagocytosis, and dyserythropoiesis (abnormal development and early release of RBC progeni- tor cells) [18]. These same processes have been shown to take place in malarial anemia in mice infected with P. chabaudi [20,21] making this a good model in which to examine possible mechanisms behind disrupted hematopoiesis in human malarial anemia.
The utility of the P. chabaudi model to studies of human health was elegantly shown by McDevitt and colleagues [22] who demonstrated the pivotal role of macrophage migration inhibitory factor (MIF) in promoting anemia via the suppression of erythroid progenitor development. Suppression of erythropoiesis in the P. chabaudi system [23] can, in part, be controlled by the kidney hormone erythropoietin which induces reticulocytosis [24]. Clearly, much more can be gleaned from this model regarding the mechanisms of SMA (Box 2).
Host genetics influences the severity of malaria in both humans and mice
In endemic areas, malaria represents a spectrum of disease as a result of the complex interplay between environmental factors (such as transmission intensity) and host and parasite genetics. At least one large genetic study is currently ongoing in humans, but the influence of host genetics on malaria severity can be seen in studies using the P. chabaudi mouse model of malaria. Infection with P. chabaudi (clone AS) leads to a peak parasitemia of 20–50% of iRBCs between days seven and ten postinfection, followed by recrudescence (Figure 1), dropping to a sub-patent level after one month (detected by blood transfer) and for two subsequent months [8]. The percentage of iRBCs in circulation depends on many factors, including the nutritional status, sex and strain of the mice (Figure 1a).
The use of quantitative trait locus (QTL) analysis of crosses between susceptible and resistant mouse strains (A/J, lethal and C57BL/6, resistant; Figure 1a,b) to determine systematically the host genes responsible for susceptibility led to the identification of genetic markers linked resistance and susceptibility phenotypes. These regions are located on chromosomal regions named chabaudi resistance (Char) loci [25,26]. Ten Char loci have been defined to date, and these regions include genes encoding proinflammatory cytokines and signaling pathways as well as immune cell adhesion molecules.
The best example where this approach has been validated in human studies was the identification of CHAR3 which is associated with control of post-peak parasitemia. This region contains TNF and LTA genes and the MHC complex [27]. Furthermore CHAR4 encodes NF-κBp105, a key signaling molecule leading to TNF production [28]. The role of the proinflammatory cytokine tumor necrosis factor (TNF)-α in mediating the severity of malaria infection is supported by the association of the TNF 376A promoter polymorphism which leads to enhanced binding of OCT-1 and upregulation of TNF-α [29]. MHC haplotypes are also clearly linked to susceptibility, and human MHC alleles HLA-Bw53 and DRB1*1302-DQB1*0501 are associated with resistance to severe malaria in humans [30]. Furthermore, the Char8 locus is syntenic to human chromosome region 5q31–33 [31], and both of these regions predispose their respective host to higher parasitemias. The striking similarities played by host genetics in the severity of infections in both humans and mice underlines the importance of host genetic factors in variations in malaria pathogenesis.
Unraveling parasite genetics using the P. chabaudi mouse model
Parasite genetics are also a major determinant in the pathogenesis of malaria infection. P. falciparum as a species consists of many different strains, some of which have been shown to differ in characteristics such as rosetting, which shows a positive correlation with the severity of malaria [32]. Although P. chabaudi clone AS is normally the first choice of clone for laboratory researchers working with the P. chabaudi mouse model of malaria (it is the best-characterized P. chabaudi clone), more- or less-virulent clones can be used to tease apart the genetic and immunological mechanisms behind the virulence of malaria infection. Other studies of parasite genetics using mutated clones with drug-resistant phenotypes have identified underlying drug-resistance genotypes [33], and P. chabaudi has proved to be an ideal model for this because orthologous genes have been shown to control the same drug-resistance phenotypes in both P. chabaudi and P. falciparum [33,34], highlighting the conservation of genetic function between the species [35].
The technique of linkage group selection (LGS) was developed to improve the previous gene discovery strategies (namely QTL mapping of phenotype-associated genomic locations). LGS has subsequently also been used to identify genes encoding differences in the growth rates of strains of Plasmodium parasites [36] and can be applied to discovering novel vaccine candidate antigens, as confirmed by data reaffirming the importance of the merozoite surface protein 1 locus (msp1) in protective immunity [37].
Immune mechanisms
Due to similarities with the human immune response to Plasmodium infections summarized in Table 3, the immunology of P. chabaudi blood-stage infections has been well-studied. Study of the initiation and regulation of the immune response, and immunological memory to P. chabaudi, have contributed useful hypotheses for further study in human malaria that are highlighted here as examples of what can be achieved.
Table 3.
Comparison of P. chabaudi and P. falciparum immune responses
| Cell type | P. falciparum | P. chabaudi |
|---|---|---|
| Dendritic cells |
P. falciparum: unclear; suppressive at times, but activate at low doses P. vivax: sporozoites activate DCs to kill hepatic stages Produce cytokines IL-12, IL10 |
Activation of CD11c+CD8+DCs (Th1), and later post-peak of infection CD11c+CD8-DCs (IL-4, IL-10) Antigen is processed within a 3–4 h time frame Produce IL-12, IL6, IL-10 and TNF |
| Macrophages/monocytes | Monocytes phagocytose iRBCs. Macrophages sense parasite products such as GPI anchors and parasite DNA trapped in hemozoin, and produce inflammatory cytokines TNF, IL-6 and IL-12p40 |
Produce IL-12 – associated with resistance. Monocytes contribute to parasite clearance |
| CD4+ T cells | Mix of Th1 cells producing IFN-γ with lower levels of Th2 and Th17 cells Th2 cells producing IL-4 Balance of IL-10 and TNF crucial Tregs correlated with susceptibility to infection |
Th1 cells produce IFN-γ; Tregs, and IL-10+ T cells regulate pathogenesis; TNF and IFN-γ are crucial for clearance but cause pathology |
| B cells | Cytophilic antibody (IgG1) correlates with decreased parasitemia Antibody to parasite variants correlates to exposure, protection Disorganization of splenic architecture |
Produce cytophilic antibody (IgG2a in mice) and IgG1 Large short-lived component to the antibody response and temporary disorganization in splenic architecture |
| Memory cells | Specific Th1 cells shown to correlate with protection Memory B cells accumulate with repeated infections |
Effector memory Th1 cells develop, not exhausted Functional and long-lived B cells generated |
The initiation of the immune response to malaria
Dendritic cells (DCs) are professional antigen-presenting cells that initiate the activation of naïve T cells; however, our understanding of the response of DCs to Plasmodium iRBCs is incomplete, with some publications suggesting that maturation and antigen-presentation capabilities of DCs are impaired when exposed to P. falciparum iRBCs [38]. As evidence, it has been shown that DCs in the circulation of acute P. falciparum patients in Kenya have decreased levels of MHC on their surface [39], suggesting that their ability to present malaria antigen to T cells is impaired. However, the conclusion that DCs are refractory to activation by intact iRBCs has been challenged by studies of P. chabaudi experimental malaria which have demonstrated the production of proinflammatory cytokines such as IL-12 and TNF-α [40] in response to intact iRBCs in vitro.
Although it is clear that uptake, processing and presentation of P. chabaudi antigens takes place well enough to provoke a large parasite-specific adaptive immune response [40–43], it appears that the window for activation of Th1 cells by antigen-presenting cells closes after several days of infection as CD8+ DCs undergo apoptosis and are replaced by CD8− DCs [42]. The various mechanisms of inhibition described for human DCs exposed to P. falciparum iRBCs [38], and in rodent malaria models [44], occur after the immune response has been initiated, providing much-needed regulation of the substantial and potentially pathogenic T cell response. Indeed, human DCs exposed to low levels of P. falciparum iRBCs become activated and produce IL-12 (Table 3), supporting this hypothesis [45]. In addition to DCs, inflammatory monocytes can contribute to parasite clearance (Table 3) but, unlike classical DCs [42], they do not contribute to T cell activation during infection [46]. Belayev and colleagues discovered a myelolymphoid progenitor cell that is expanded in response to IFN-γ, and this may be the precursor of this parasitocidal monocyte [21].
The receptors mediating recognition of malaria parasites have been investigated, but no definitive receptor has been found. Antigen-presenting cells can be activated by free merozoites, iRBCs [47] and/or parasite products released during schizogony [48]. Recognition of parasite products is known to include Toll-like receptor 2 (TLR2) (GPI anchors) [49] and TLR9 (parasite DNA in combination with insoluble hemozoin crystals [50] or nucleosomal proteins [51]). The in vivo significance of TLR-mediated recognition in human malaria is supported by reports of TLR polymorphism associations with resistance and susceptibility to human malaria [52,53] and expression of TLRs on antigen-presenting cells in the circulation, which increase on the surface during P. falciparum infection [54]. Experimental malaria in mice deficient for various TLRs or MyD88, an adaptor protein downstream of TLRs, IL-18R and IL-1R (C. Voisine and J. Langhorne, unpublished) [40,52,55,56], has demonstrated some redundancy in TLR usage. Only when MyD88 is knocked out are substantial systemic reductions in the inflammatory immune response, including IL-12, observed [57]. However, the consequences of this are still unclear because MyD88−/− mice still display disrupted splenic architecture [58] and similar parasitemias to wild type mice [57].
The regulation of the immune response in Plasmodium infection
Observations of T and B cell responses in P. chabaudi-infected mice to date are consistent with immune responses measured in the peripheral blood of humans [59]. The concentration of malaria-specific antibodies in sera increases as individual infections progress, and IgG has been shown to clear both P. falciparum and P. chabaudi infection [60]. CD4+ T cells have been shown to rescue immunodeficient animals from lethal blood-stage P. chabaudi infection [61], and T cells may also modulate susceptibility to reinfection in humans [62].
Malaria infection is associated with the development of a Th1 response typified by the production of IFN-γ from natural killer (NK) cells [63] and T cells [64] (γδ and αβ T cells), and this holds true for P. chabaudi infection (Table 3) [65,66]. The Th1 response in P. chabaudi infection dampens after the peak of parasitemia when specific antibody is generated [65]. The mechanisms behind this observation have been investigated with the aim of understanding how to reduce inflammation-related pathology in human malaria infection.
A favorable ratio of TNF-α/IL-10 correlates with better outcomes for several severe malaria syndromes in humans with P. falciparum [67], suggesting that regulatory cytokines are a key component in reducing pathology. The P. chabaudi model has provided evidence of the crucial role of the regulatory cytokines TGF-β and IL-10 in modulating the magnitude of damaging immunopathology in malaria [68]. Regulatory T cells can promote parasite establishment in both P. falciparum [69] and P. chabaudi infection [70], but the mechanisms of action are not known. Other T cell subsets can produce IL-10 in P. falciparum infection [64], and IL-10+ effector T cells play a role in P. yoelii infection [71], suggesting that they arise from the Th1 response. The main source(s) of protective regulatory cytokines in malaria infection remains to be revealed.
Studies of memory to P. chabaudi explain important phenomena in P. falciparum infection
Immune memory exists in malaria infection, as evidenced by the increase in resistance to malaria pathogenesis with age. However, complete (and strain-specific) clinical immunity is partially dependent on constant exposure to Plasmodium parasites. Reinfection with the same clone of P. falciparum in humans [72,73] or P. chabaudi in mice [74,75] results in similar reductions in circulating parasitemia. Furthermore, intermediate levels of protection to non-homologous secondary infections have been observed in both human and mouse malaria [75,76]. However, children are susceptible to repeated infections, even though B and T cell memory responses tested seem to develop normally [62,77], suggesting that exposure to a genetically diverse population of parasites plays a significant role in life-long susceptibility to parasitemia [78,79]. Therefore, polymorphic antigens may account for recrudescence, chronic infection [16] and susceptibility to repeated infections [80]. In the P. chabaudi mouse model, strain-specific immunity is mediated to a large degree by polymorphism in the msp1 gene [81,82]. This gene is also polymorphic amongst P. falciparum strains, possibly as a result of host immune selection pressure [83]. Fortunately, there are also domains of merozoite surface protein 1 (MSP1) that are functionally conserved and have been successfully used in vaccine trials and basic research.
Although immunity to different strains of malaria accrues with age, resistance to disease may be short-lived, although this remains to be well-documented in humans [84]. Epidemiological studies of malaria-specific antibodies suggest that they may not be long-lived following clearance of infection [85]. For example, high levels of MSP1-specific IgG in a patient normally suggests recent infection, not cumulative infection [86]. Recent data showing a correlation between the accumulation of atypical memory B cells and exposure suggest that these B cells may be less responsive, impairing memory responses [87]; these cells have not yet been identified in mice.
Nevertheless, the conclusion that defective B cells cause short-lived protection seems to be too simplistic. In mice, there are two phases of Plasmodium-specific antibody production and decay: a peak in the month after the infection, corresponding to a peak of short-lived extrafollicular antibody-producing cells in the spleen, and a subsequent stable phase [8,88]. MSP1-specific plasma cells accumulate dramatically in the acute phase primarily in the bone marrow, and the low levels that remain after the response suggest that there are long-lived antibody producing cells [89,90]. The acute-phase and long-lived plasma cells can be distinguished by their location and surface markers, generating the testable hypotheses that longlived malaria-specific plasma cells do survive at some level in humans also, although it is unclear whether the low levels remaining after the peak are detectable with current methods, or are sufficient to contribute to protection, and whether they may be depleted by subsequent infections [89,91].
Data from Thailand and Madagascar have identified memory B cells in individuals occasionally exposed to malaria and showed that they can be long-lived [77,92] and that serum antibody levels do increase with age [93]. Although there are caveats to these studies, such as the sensitivity of testing small volumes of blood for low frequencies of memory B cells [89], and lack of access to the bone marrow in people, the finding that memory B cells can accumulate over years of exposure is in direct contrast to work in the P. yoelii model that suggests that B and T cells specific to malaria infection are deleted on reinfection [91,94]. P. chabaudi MSP1-specific memory B and T cells are also maintained over the long term [90,95]. Given that the peak of T cell proliferation is at day 6 and the peak of B and T cell apoptosis is at day 12 postinfection [96,97], the data suggest that deletion observed at day 7 may be due to normal contraction of the immune response and may provide the opportunity for the generation of acquired immunity and memory. Development of a B cell receptor transgenic mouse may further elucidate this matter.
The number of memory T cells with the ability to make IFN-γ has been shown to correlate with protection against P. falciparum malaria [62]. P. chabaudi infection and P. falciparum both generate MSP1-specific memory T cells with a high proportion of effector memory as opposed to a central memory phenotype [95,98]. Effector memory T cells may require antigen to maintain their protective function. Given that P. vivax has a dormant liver stage, a constant source of CS antigen, it is interesting that the CS-specific T cell responses of P. vivax outlive those of P. falciparum [99]. It has been proposed that the absolute number of specific memory T cells required for protection against the liver stages of malaria is relatively high [100], potentially leading to a high threshold of protection, easily lost in the face of multiple infections. Given that both the quantity and function of T cells combine to confer protection, measurement of reactivation of lymphocyte populations in vivo by reinfection may represent a complementary approach for measuring responsive lymphocytes [101,102]. Further technical advances, such as human MHC-tetramers for affected populations, may be required to advance our knowledge in this area. The mechanisms behind the decay of immunity are currently being elucidated in P. chabaudi, but given that there is a decay of T cell memory over time [103], and that memory T cells with chronic exposure protect best [61], the loss of immunity may be due to a loss of malaria-specific T cell function in the absence of continual stimulation, and ongoing variation of the parasite.
Concluding remarks
Owing to the extensive similarities between P. chabaudi-infected mice and human infection with Plasmodium spp, as well as the spectrum of models available from different combinations of mouse and parasite variants, P. chabaudi offers an unrivalled system for laboratory investigations of malaria. Recent advances in genetic manipulation of P. chabaudi, specifically the ability to perform transfection [104], offer multiple opportunities, as suggested in Box 2, including for the study of pre-erythrocytic immunology [105,106]. The many parallels between data on P. chabaudi experimental malaria and human malaria suggest that the use of this species in laboratory experiments will continue to yield further important contributions to our understanding of the immunobiology of malaria for years to come.
Acknowledgments
The authors would like to thank Julie Gutman for insightful discussion and Alberto Moreno and Richard Carter for critical reading of the manuscript. The authors apologize to those authors whose work could not be cited due to space constraints.
References
- 1.Walliker D, et al. Genetic recombination in malaria parasites. Nature. 1971;232:561–562. doi: 10.1038/232561a0. [DOI] [PubMed] [Google Scholar]
- 2.Landau I. [Description of Plasmodium chabaudi N. Sp. Parasite of African Rodents] C.R. Hebd. Seances Acad. Sci. 1965;260:3758–3761. [PubMed] [Google Scholar]
- 3.Carter R. Studies on enzyme variation in the murine malaria parasites Plasmodium berghei, P. yoelii, P. vinckei and P. chabaudi by starch gel electrophoresis. Parasitology. 1978;76:241–267. doi: 10.1017/s0031182000048137. [DOI] [PubMed] [Google Scholar]
- 4.Bagnaresi P, et al. Unlike the synchronous Plasmodium falciparum and P. chabaudi infection, the P. berghei and P. yoelii asynchronous infections are not affected by melatonin. Int. J. Gen. Med. 2009;2:47–55. doi: 10.2147/ijgm.s3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell AS, et al. Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution. 2006;60:1358–1371. [PubMed] [Google Scholar]
- 6.Gilks CF, et al. Relationships between sequestration, antigenic variation and chronic parasitism in Plasmodium chabaudi chabaudi – a rodent malaria model. Parasite Immunol. 1990;12:45–64. doi: 10.1111/j.1365-3024.1990.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 7.Gadsby N, et al. A study on pathogenicity and mosquito transmission success in the rodent malaria parasite Plasmodium chabaudi adami . Int. J. Parasitol. 2009;39:347–354. doi: 10.1016/j.ijpara.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Achtman AH, et al. Malaria-specific antibody responses and parasite persistence after infection of mice with Plasmodium chabaudi chabaudi . Parasite Immunol. 2007;29:435–444. doi: 10.1111/j.1365-3024.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 9.Carter R, Walliker D. New observations on the malaria parasites of rodents of the Central African Republic – Plasmodium vinckei petteri subsp. nov. and Plasmodium chabaudi Landau, 1965. Ann. Trop. Med. Parasitol. 1975;69:187–196. doi: 10.1080/00034983.1975.11687000. [DOI] [PubMed] [Google Scholar]
- 10.Cox J, et al. Plasmodium chabaudi: a rodent malaria model for in vivo and in vitro cytoadherence of malaria parasites in the absence of knobs. Parasite Immunol. 1987;9:543–561. doi: 10.1111/j.1365-3024.1987.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 11.Mackinnon MJ, et al. Plasmodium chabaudi: rosetting in a rodent malaria model. Exp. Parasitol. 2002;101:121–128. doi: 10.1016/s0014-4894(02)00103-0. [DOI] [PubMed] [Google Scholar]
- 12.Sanni LA, et al. Cerebral edema and cerebral hemorrhages in interleukin-10-deficient mice infected with Plasmodium chabaudi . Infect. Immun. 2004;72:3054–3058. doi: 10.1128/IAI.72.5.3054-3058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coquelin F, et al. Final stage of maturation of the erythrocytic schizonts of rodent Plasmodium in the lungs. C.R. Acad. Sci. III. 1999;322:55–62. doi: 10.1016/s0764-4469(99)80017-1. [DOI] [PubMed] [Google Scholar]
- 14.Heddini A, et al. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect. Immun. 2001;69:5849–5856. doi: 10.1128/IAI.69.9.5849-5856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mota MM, et al. Plasmodium chabaudi-infected erythrocytes adhere to CD36 and bind to microvascular endothelial cells in an organ-specific way. Infect. Immun. 2000;68:4135–4144. doi: 10.1128/iai.68.7.4135-4144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham D, et al. The pir multigene family of Plasmodium: antigenic variation and beyond. Mol. Biochem. Parasitol. 2010;170:65–73. doi: 10.1016/j.molbiopara.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Cross CE, Langhorne J. Plasmodium chabaudi chabaudi (AS): inflammatory cytokines and pathology in an erythrocytic-stage infection in mice. Exp. Parasitol. 1998;90:220–229. doi: 10.1006/expr.1998.4335. [DOI] [PubMed] [Google Scholar]
- 18.Lamikanra AA, et al. Malarial anemia: of mice and men. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- 19.Mackintosh CL, et al. Clinical features and pathogenesis of severe malaria. Trends Parasitol. 2004;20:597–603. doi: 10.1016/j.pt.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Mohan K, Stevenson MM. Dyserythropoiesis and severe anaemia associated with malaria correlate with deficient interleukin-12 production. Br. J. Haematol. 1998;103:942–949. doi: 10.1046/j.1365-2141.1998.01126.x. [DOI] [PubMed] [Google Scholar]
- 21.Belyaev NN, et al. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat. Immunol. 2010;11:477–485. doi: 10.1038/ni.1869. [DOI] [PubMed] [Google Scholar]
- 22.McDevitt MA, et al. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J. Exp. Med. 2006;203:1185–1196. doi: 10.1084/jem.20052398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang KH, et al. Inappropriately low reticulocytosis in severe malarial anemia correlates with suppression in the development of late erythroid precursors. Blood. 2004;103:3727–3735. doi: 10.1182/blood-2003-08-2887. [DOI] [PubMed] [Google Scholar]
- 24.Chang KH, et al. Modulation of the course and outcome of blood-stage malaria by erythropoietin-induced reticulocytosis. J. Infect. Dis. 2004;189:735–743. doi: 10.1086/381458. [DOI] [PubMed] [Google Scholar]
- 25.Min-Oo G, et al. Mapping of Char10 a novel malaria susceptibility locus on mouse chromosome 9. Genes Immun. 2010;11:113–123. doi: 10.1038/gene.2009.78. [DOI] [PubMed] [Google Scholar]
- 26.Fortin A, et al. Complex genetic control of susceptibility to malaria in mice. Genes Immun. 2002;3:177–186. doi: 10.1038/sj.gene.6363841. [DOI] [PubMed] [Google Scholar]
- 27.Burt RA, et al. Temporal expression of an H2-linked locus in host response to mouse malaria. Immunogenetics. 1999;50:278–285. doi: 10.1007/s002510050603. [DOI] [PubMed] [Google Scholar]
- 28.Fortin A, et al. Identification of a new malaria susceptibility locus ( Char4 ) in recombinant congenic strains of mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10793–10798. doi: 10.1073/pnas.191288998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight JC, et al. A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nat. Genet. 1999;22:145–150. doi: 10.1038/9649. [DOI] [PubMed] [Google Scholar]
- 30.Hill AV, et al. Common West African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 31.Rihet P, et al. Malaria in humans: Plasmodium falciparum blood infection levels are linked to chromosome 5q31-q33. Am. J. Hum. Genet. 1998;63:498–505. doi: 10.1086/301967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heddini A, et al. Binding of Plasmodium falciparum-infected erythrocytes to soluble platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): frequent recognition by clinical isolates. Am. J. Trop. Med. Hyg. 2001;65:47–51. doi: 10.4269/ajtmh.2001.65.47. [DOI] [PubMed] [Google Scholar]
- 33.Borges S, et al. Genome-wide scan reveals amplification of mdr1 as a common denominator of resistance to mefloquine, lumefantrine and artemisinin in P. chabaudi malaria parasites. Antimicrob. Agents Chemother. 2011;55:4858–4865. doi: 10.1128/AAC.01748-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Q, Saul A. The dihydrofolate reductase domain of rodent malarias: point mutations and pyrimethamine resistance. Mol. Biochem. Parasitol. 1994;65:361–363. doi: 10.1016/0166-6851(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 35.Janse CJ, et al. A genotype and phenotype database of genetically modified malaria-parasites. Trends Parasitol. 2011;27:31–39. doi: 10.1016/j.pt.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Pattaradilokrat S, et al. Gene encoding erythrocyte binding ligand linked to blood stage multiplication rate phenotype in Plasmodium yoelii yoelii . Proc. Natl. Acad. Sci. U.S.A. 2009;106:7161–7166. doi: 10.1073/pnas.0811430106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrin LH, et al. Plasmodium falciparum: characterization of defined antigens by monoclonal antibodies. Clin. Exp. Immunol. 1980;41:91–96. [PMC free article] [PubMed] [Google Scholar]
- 38.Urban BC, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 39.Urban BC, et al. Peripheral blood dendritic cells in children with acute Plasmodium falciparum malaria. Blood. 2001;98:2859–2861. doi: 10.1182/blood.v98.9.2859. [DOI] [PubMed] [Google Scholar]
- 40.Seixas E, et al. The interaction between DC and Plasmodium berghei/chabaudi-infected erythrocytes in mice involves direct cellto- cell contact, internalization and TLR. Eur. J. Immunol. 2009;39:1850–1863. doi: 10.1002/eji.200838403. [DOI] [PubMed] [Google Scholar]
- 41.Voisine C, et al. Classical CD11c+ dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. Int. J. Parasitol. 2010;40:711–719. doi: 10.1016/j.ijpara.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Sponaas AM, et al. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J. Exp. Med. 2006;203:1427–1433. doi: 10.1084/jem.20052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ing R, et al. Interaction of mouse dendritic cells and malariainfected erythrocytes: uptake, maturation, and antigen presentation. J. Immunol. 2006;176:441–450. doi: 10.4049/jimmunol.176.1.441. [DOI] [PubMed] [Google Scholar]
- 44.Lundie RJ. Antigen presentation in immunity to murine malaria. Curr. Opin. Immunol. 2011;23:119–123. doi: 10.1016/j.coi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Elliott SR, et al. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect. Immun. 2007;75:3621–3632. doi: 10.1128/IAI.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sponaas AM, et al. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood. 2009;114:5522–5531. doi: 10.1182/blood-2009-04-217489. [DOI] [PubMed] [Google Scholar]
- 47.Langhorne J, et al. The response of CD4+ T cells to Plasmodium chabaudi chabaudi. Immunol. Rev. 1989;112:71–94. doi: 10.1111/j.1600-065x.1989.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 48.Korbel DS, et al. Killer Ig-like receptor (KIR) genotype predicts the capacity of human KIR-positive CD56dim NK cells to respond to pathogen-associated signals. J. Immunol. 2009;182:6426–6434. doi: 10.4049/jimmunol.0804224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horowitz A, et al. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J. Immunol. 2010;185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 50.Korbel DS, et al. Natural killer cells and innate immunity to protozoan pathogens. Int. J. Parasitol. 2004;34:1517–1528. doi: 10.1016/j.ijpara.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Gowda NM, et al. The nucleosome (histone–DNA complex) is the TLR9-specific immunostimulatory component of Plasmodium falciparum that activates DCs. PLoS ONE. 2011;6:e20398. doi: 10.1371/journal.pone.0020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franklin BS, et al. Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5789–5794. doi: 10.1073/pnas.0809742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langhorne J, et al. Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection. Immunol. Rev. 2004;201:35–47. doi: 10.1111/j.0105-2896.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 54.Ropert C, et al. Role of TLRs/MyD88 in host resistance and pathogenesis during protozoan infection: lessons from malaria. Semin. Immunopathol. 2008;30:41–51. doi: 10.1007/s00281-007-0103-2. [DOI] [PubMed] [Google Scholar]
- 55.Cramer JP, et al. MyD88/IL-18-dependent pathways rather than TLRs control early parasitaemia in non-lethal Plasmodium yoelii infection. Microbes Infect. 2008;10:1259–1265. doi: 10.1016/j.micinf.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 56.Kordes M, et al. Caspase-1 activation of interleukin-1beta (IL-1b) and IL-18 is dispensable for induction of experimental cerebral malaria. Infect. Immun. 2011;79:3633–3641. doi: 10.1128/IAI.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franklin BS, et al. MyD88-dependent activation of dendritic cells and CD4(+) T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes Infect. 2007;9:881–890. doi: 10.1016/j.micinf.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Cadman ET, et al. Alterations of splenic architecture in malaria are induced independently of Toll-like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infect. Immun. 2008;76:3924–3931. doi: 10.1128/IAI.00372-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langhorne J, et al. Immunity to malaria: more questions than answers. Nat. Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 60.Meding SJ, Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi . Eur. J. Immunol. 1991;21:1433–1438. doi: 10.1002/eji.1830210616. [DOI] [PubMed] [Google Scholar]
- 61.Stephens R, et al. Malaria-specific transgenic CD4(+) T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood. 2005;106:1676–1684. doi: 10.1182/blood-2004-10-4047. [DOI] [PubMed] [Google Scholar]
- 62.Todryk SM, et al. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE. 2008;3:e2027. doi: 10.1371/journal.pone.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 2002;169:2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 64.Walther M, et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5:e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langhorne J, et al. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi : distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int. Immunol. 1989;1:416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- 66.Ing R, Stevenson MM. Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice. Infect. Immun. 2009;77:770–782. doi: 10.1128/IAI.00994-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.May J, et al. Plasma interleukin-10:tumor necrosis factor (TNF)-alpha ratio is associated with TNF promoter variants and predicts malarial complications. J. Infect. Dis. 2000;182:1570–1573. doi: 10.1086/315857. [DOI] [PubMed] [Google Scholar]
- 68.Li C, et al. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10-deficient mice are ameliorated by anti-tumor necrosis factor alpha and exacerbated by anti-transforming growth factor beta antibodies. Infect. Immun. 2003;71:4850–4856. doi: 10.1128/IAI.71.9.4850-4856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walther M, et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Berretta F, et al. IL-2 contributes to maintaining a balance between CD4+Foxp3+ regulatory T Cells and effector CD4+ T cells required for immune control of blood-stage malaria infection. J. Immunol. 2011;186:4862–4871. doi: 10.4049/jimmunol.1003777. [DOI] [PubMed] [Google Scholar]
- 71.Couper KN, et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Contamin H, et al. Different genetic characteristics of Plasmodium falciparum isolates collected during successive clinical malaria episodes in Senegalese children. Am. J. Trop. Med. Hyg. 1996;54:632–643. doi: 10.4269/ajtmh.1996.54.632. [DOI] [PubMed] [Google Scholar]
- 73.Newbold CI, et al. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 1992;75:281–292. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 74.Cheesman S, et al. Mixed strain infections and strain-specific protective immunity in the rodent malaria parasite Plasmodium chabaudi chabaudi in mice. Infect. Immun. 2006;74:2996–3001. doi: 10.1128/IAI.74.5.2996-3001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jarra W, Brown KN. Protective immunity to malaria: studies with cloned lines of Plasmodium chabaudi and P. berghei in CBA/Ca miceIThe effectiveness and inter- and intra-species specificity of immunity induced by infection. Parasite Immunol. 1985;7:595–606. doi: 10.1111/j.1365-3024.1985.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 76.Collins WE, Jeffery GM. A retrospective examination of sporozoite-induced and trophozoite-induced infections with Plasmodium ovale: development of parasitologic and clinical immunity during primary infection. Am. J. Trop. Med. Hyg. 2002;66:492–502. doi: 10.4269/ajtmh.2002.66.492. [DOI] [PubMed] [Google Scholar]
- 77.Wipasa J, et al. Long-lived antibody and B cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax . PLoS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bull PC, et al. Plasmodium falciparum antigenic variation: relationships between in vivo selection, acquired antibody response, and disease severity. J. Infect. Dis. 2005;192:1119–1126. doi: 10.1086/432761. [DOI] [PubMed] [Google Scholar]
- 79.McKenzie FE, et al. Strain theory of malaria: the first 50 years. Adv. Parasitol. 2008;66:1–46. doi: 10.1016/S0065-308X(08)00201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kinyanjui SM, et al. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J. Infect. Dis. 2004;190:1527–1533. doi: 10.1086/424675. [DOI] [PubMed] [Google Scholar]
- 81.Cheesman S, et al. A single parasite gene determines strainspecific protective immunity against malaria: the role of the merozoite surface protein I. Int. J. Parasitol. 2010;40:951–961. doi: 10.1016/j.ijpara.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Pattaradilokrat S, et al. Linkage group selection: towards identifying genes controlling strain specific protective immunity in malaria. PLoS ONE. 2007;2:e857. doi: 10.1371/journal.pone.0000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hughes AL. Positive selection and interallelic recombination at the merozoite surface antigen-1 (MSA-1) locus of Plasmodium falciparum . Mol. Biol. Evol. 1992;9:381–393. doi: 10.1093/oxfordjournals.molbev.a040730. [DOI] [PubMed] [Google Scholar]
- 84.Struik SS, Riley EM. Does malaria suffer from lack of memory? Immunol. Rev. 2004;201:268–290. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 85.Achtman AH, et al. Longevity of the immune response and memory to blood-stage malaria infection. Curr. Top. Microbiol. Immunol. 2005;297:71–102. doi: 10.1007/3-540-29967-x_3. [DOI] [PubMed] [Google Scholar]
- 86.Drakeley CJ, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weiss GE, et al. A positive correlation between atypical memory B cells and Plasmodium falciparum transmission intensity in cross-sectional studies in Peru and Mali. PLoS ONE. 2011;6:e15983. doi: 10.1371/journal.pone.0015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Achtman AH, et al. Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J. Immunol. 2003;171:317–324. doi: 10.4049/jimmunol.171.1.317. [DOI] [PubMed] [Google Scholar]
- 89.Nduati EW, et al. Distinct kinetics of memory B-cell and plasma-cell responses in peripheral blood following a blood-stage Plasmodium chabaudi infection in mice. PLoS ONE. 2010;5:e15007. doi: 10.1371/journal.pone.0015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ndungu FM, et al. Functional memory B cells and long-lived plasma cells are generated after a single Plasmodium chabaudi infection in mice. PLoS Pathog. 2009;5:e1000690. doi: 10.1371/journal.ppat.1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wykes MN, et al. Plasmodium yoelii can ablate vaccineinduced long-term protection in mice. J. Immunol. 2005;175:2510–2516. doi: 10.4049/jimmunol.175.4.2510. [DOI] [PubMed] [Google Scholar]
- 92.Migot F, et al. Anti-malaria antibody-producing B cell frequencies in adults after a Plasmodium falciparum outbreak in Madagascar. Clin. Exp. Immunol. 1995;102:529–534. doi: 10.1111/j.1365-2249.1995.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiss GE, et al. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 2010;6:e1000912. doi: 10.1371/journal.ppat.1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirunpetcharat C, Good MF. Deletion of Plasmodium berghei-specific CD4+ T cells adoptively transferred into recipient mice after challenge with homologous parasite. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1715–1720. doi: 10.1073/pnas.95.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stephens R, Langhorne J. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog. 2010;6:e1001208. doi: 10.1371/journal.ppat.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Helmby H, et al. Cellular changes and apoptosis in the spleens and peripheral blood of mice infected with blood-stage Plasmodium chabaudi chabaudi AS. Infect. Immun. 2000;68:1485–1490. doi: 10.1128/iai.68.3.1485-1490.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanchez-Torres L, et al. Mouse splenic CD4+ and CD8+ T cells undergo extensive apoptosis during a Plasmodium chabaudi chabaudi AS infection. Parasite Immunol. 2001;23:617–626. doi: 10.1046/j.1365-3024.2001.00422.x. [DOI] [PubMed] [Google Scholar]
- 98.Chelimo K, et al. Age-Related differences in naturally acquired T cell memory to Plasmodium falciparum merozoite surface protein 1. PLoS ONE. 2011;6:e24852. doi: 10.1371/journal.pone.0024852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zevering Y, et al. Life-spans of human T-cell responses to determinants from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium vivax . Proc. Natl. Acad. Sci. U.S.A. 1994;91:6118–6122. doi: 10.1073/pnas.91.13.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmidt NW, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kinyanjui SM, et al. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short halflife. Malaria J. 2007;6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stephens R, et al. Germinal centre and marginal zone B cells expand quickly in a second Plasmodium chabaudi malaria infection producing mature plasma cells. Parasite Immunol. 2009;31:20–31. doi: 10.1111/j.1365-3024.2008.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Freitas do Rosario AP, et al. Gradual decline in malariaspecific memory T cell responses leads to failure to maintain longterm protective immunity to Plasmodium chabaudi AS despite persistence of B cell memory and circulating antibody. J. Immunol. 2008;181:8344–8355. doi: 10.4049/jimmunol.181.12.8344. [DOI] [PubMed] [Google Scholar]
- 104.Spence PJ, et al. Transformation of the rodent malaria parasite Plasmodium chabaudi . Nat. Protoc. 2011;6:553–561. doi: 10.1038/nprot.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fonseca L, et al. Cytokine responses of CD4+ T cells during a Plasmodium chabaudi chabaudi (ER) blood-stage infection in mice initiated by the natural route of infection. Malaria J. 2007;6:77. doi: 10.1186/1475-2875-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Culleton RL, et al. Strain-specific immunity induced by immunization with pre-erythrocytic stages of Plasmodium chabaudi . Parasite Immunol. 2011;33:73–78. doi: 10.1111/j.1365-3024.2010.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carter R. Effect of PABA on chloroquine resistance in Plasmodium berghei yoelii . Nature. 1972;238:98–99. doi: 10.1038/238098b0. [DOI] [PubMed] [Google Scholar]
- 108.Landau I, Chabaud A. Plasmodium species infecting Thamnomys rutilans: a zoological study. Adv. Parasitol. 1994;33:49–90. doi: 10.1016/s0065-308x(08)60411-x. [DOI] [PubMed] [Google Scholar]