Abstract
Objective
The purpose of this study was to evaluate the effects of three different surface conditioning methods on the shear bond strength (SBS) of metal brackets bonded directly to gold alloy with chemically cured resin.
Methods
Two hundred ten type III gold alloy specimens were randomly divided into six groups according to the combination of three different surface conditioning methods (aluminum oxide sandblasting only, application of a metal primer after aluminum oxide sandblasting, silica coating and silanation) and thermocycling (with thermocycling, without thermocycling). After performing surface conditioning of specimens in accordance with each experimental condition, metal brackets were bonded to all specimens using a chemically cured resin. The SBS was measured at the moment of bracket debonding, and the resin remnants on the specimen surface were evaluated using the adhesive remnant index.
Results
Application of metal primer after aluminum oxide sandblasting yielded a higher bond strength than that with aluminum oxide sandblasting alone (p < 0.001), and silica coating and silanation yielded a higher bond strength than that with metal primer after aluminum oxide sandblasting (p < 0.001). There was no significant change in SBS after thermocycling in all groups.
Conclusions
With silica coating and silanation, clinically satisfactory bond strength can be attained when metal brackets are directly bonded to gold alloys using a chemically cured resin.
Keywords: Bonding, Biomaterial science, Silicoating, Chemically cured resin
INTRODUCTION
Orthodontic treatment is being performed more frequently in middle-aged patients, and middle-aged patients typically exhibit more dental restorations and a poorer periodontal status than adolescents and young adults. Orthodontic bands are typically used for restored teeth in which direct bonding of the bracket is difficult. However, dental plaque may accumulate around the band margins1 as a result of the difficulty of shaping the bands to tooth surfaces. Although surface conditioning techniques and adhesion materials have been developed for direct bracket bonding to the metal surface, the bond strength of the adhesive to gold alloy is less satisfactory than that of the adhesive to an enamel surface prepared with the classical acid etching method.
Adhesion is defined as "the maintained status of two materials at the interface by mechanical and chemical bonding forces." Acid etching of enamel is a good example of a method to enhance mechanical bond strength. Bonding agents with desirable flowability infiltrate into fine irregularities formed at the enamel surface by acid etching, and efficient and strong adhesion is achieved with the micromechanical lock effect.2 However, acid etching cannot be used with a metal surface. Therefore, use of green stone3 and diamond burs for macromechanical retention, and aluminum oxide sandblasting4 for micromechanical retention, are widely applied in clinical situations. In addition to the methods used for enhanced mechanical retention, use of a metal primer for chemical bonding between the metal surface and adhesion material has been studied.5,6 The bond strength with these methods may not equal that with enamel etching, and there is controversy over the sustainability of the enhanced bond strength obtained by the application of metal primer.6 However, more and more orthodontists are using direct bonding to metal restorations because of improved oral hygiene, as well as convenience to the practitioner, compared with the conventional method using bands. Recently, chemicophysical bonding using a silica coating and silanation (silicoating),7,8 which can enhance mechanical and chemical bonds, has been introduced in the field of orthodontics. Chemically cured resin is more fully polymerized than light-cured resin, particularly in the central area of the bracket, when a metal bracket is bonded to gold alloy.9 This suggests that the chemically cured resin may be superior in this circumstance. Metal primer is popular among orthodontists because it can be applied easily after routine sandblasting using aluminum oxide. To date, there has been no study comparing the bond strength attained by different surface conditioning methods including sandblasting, application of metal primer, and silicoating, together with the use of chemically cured resin, for orthodontic bonding.
The purpose of this study was to compare the shear bond strength (SBS) of three surface conditioning methods including aluminum oxide sandblasting only, aluminum oxide sandblasting and application of metal primer, and silicoating when a metal bracket was bonded to a gold alloy surface using chemically cured resin. The change in the SBS after thermocycling was also investigated to examine the influence of the stress caused by repetitive temperature changes, which is the primary cause of bond strength weakening in clinical practice. The adhesive remnant index (ARI)10 was also evaluated.
MATERIALS AND METHODS
Fabrication of experimental specimens
Plate-shaped specimens of type III dental gold alloys used for casting (Goldenian C-48; Shinhung, Seoul, Korea) were placed at the bottom of a cuboidal silicon mold (2.4 × 2.4 × 1.9 cm). As the type III dental gold alloy is primarily used for onlay, crown, and crown and bridge restorations in small areas, and assuming that the adherend was a posterior tooth restoration, the type III gold alloy was considered appropriate for specimens in this investigation. The gold alloy used in the present study contained 48% gold, 38.2% silver, 4.0% palladium, and 9.8% microelements. Orthodontic acrylic resin (Dentsply, York, PA, USA) was poured into the silicon mold. After the resin blocks were completely cured, the mold was removed and the specimen was located on the upper surface of the block.
The gold alloy surface was sequentially polished with nos. 120, 1,000, 2,000, and 3,000 sandpaper (single-disc polisher; Gang Hae Industry, Gwangju, Korea) under a spray of water, and then finely polished with a rouge for dental alloys (Hi-rouge®; High Dental Korea, Seoul, Korea) and a cotton wheel (Dae-Myung Dental, Seoul, Korea) to achieve a flat surface on which to bond the bracket to the specimen surface. A 5 × 5 mm adhesion area was delimited by applying scotch tape to the final polished gold alloy surface. The total number of gold alloy specimens used in this experiment was 210. The specimens were randomly divided into three groups of 70 samples each, and each group was treated with one of the three surface conditioning methods-aluminum oxide sandblasting (AS), aluminum oxide sandblasting plus metal primer (MP), and silicoating (SC). Half of the specimens of each group underwent thermocycling-aluminum oxide sandblasting with thermocycling (AST), aluminum oxide sandblasting plus metal primer with thermocycling (MPT), and silicoating with thermocycling (SCT). Finally, the gold alloy specimens were classified into six groups consisting of 35 samples per group (Table 1).
Table 1.
Experimental groups classified by surface conditioning method and thermocycling
AS, Aluminum oxide sandblasting; AST, AS with thermocycling; MP, AS plus metal primer; MPT, MP with thermocycling; SC, silicoating; SCT, SC with thermocycling.
Bracket bonding
The surfaces of the specimens in the AS, AST, MP, and MPT groups were roughened with 50 µm aluminum oxide (blasting medium; Dentaurum GmbH & Co. KG, Ispringen, Germany) by using an intraoral sandblaster (Microetcher; Danville Materials Inc., San Ramon, CA, USA) at a distance of 1 cm and pressure of 3.6 kgf/cm2 for 10 s. Afterward, the scotch tape was removed from the surfaces of the specimens, and the surfaces were rinsed and dried thoroughly with an air-water spray for 10 s each. A uniform coat of metal primer (Reliance Orthodontic Products, Itasca, IL, USA) was painted onto the gold alloy specimens in the MP and MPT groups with a microbrush. The specimens were then allowed to dry for 30 s according to the manufacturer's instructions.
In the SC and SCT groups, the specimens were sandblasted with 30 µm silicon dioxide (CoJet Sand; 3M ESPE, St. Paul, MN, USA) by using an intraoral sandblaster (Microetcher) at a distance of 1 cm and pressure of 3.6 kgf/cm2 for 10 s. Consequently, the gold alloy surfaces of the specimens became roughened and silica-coated. Afterward, the scotch tape was removed from the specimens, and dry air was lightly applied for 5 s. Silane (ESPE Sil; 3M ESPE) agent was applied with a microbrush and evaporated for 5 min according to the manufacturer's instructions.
Standard metal brackets for the upper central incisor (standard edgewise bracket; TOMY Inc., Tokyo, Japan) with a base area of approximately 14.22 mm2 were bonded to all specimens. The bracket bases were not specifically conditioned prior to bracket bonding. The bonding adhesive used in this study was a chemically cured resin (Unite™; 3M Unitek, Monrovia, CA, USA). The bracket base and gold alloy surfaces were coated with resin primer, and resin adhesive paste was applied to the bracket bases, according to the manufacturer's instructions. The brackets were subsequently positioned and bonded to the gold alloy surfaces with constant pressure, and the excess resin extruded at the bracket-alloy interfaces was carefully removed with a dental explorer. Specimens were kept at room temperature for 4 min, allowing chemical curing to take place. After this process, they were stored in a water bath (Jeio Tech., Daejeon, Korea) at 36.5℃ with 100% relative humidity for 24 h to achieve complete curing. Each step in the procedure such as the fabrication of gold alloy blocks, surface conditioning, and bracket bonding was carried out by the same researcher for consistency and reproducibility of the experiment.
Thermocycling
The specimens in the AST, MPT, and SCT groups underwent thermocycling based on the work of Bishara et al.11 after storage in the water bath for 24 h. For thermocycling, 5℃ and 55℃ distilled water baths (thermocycler; Gang Hae Industry) were used. The samples were immersed in each bath for 20 s, which was considered one cycle of thermocycling, for 1,000 thermocycles.
Measurement of SBS
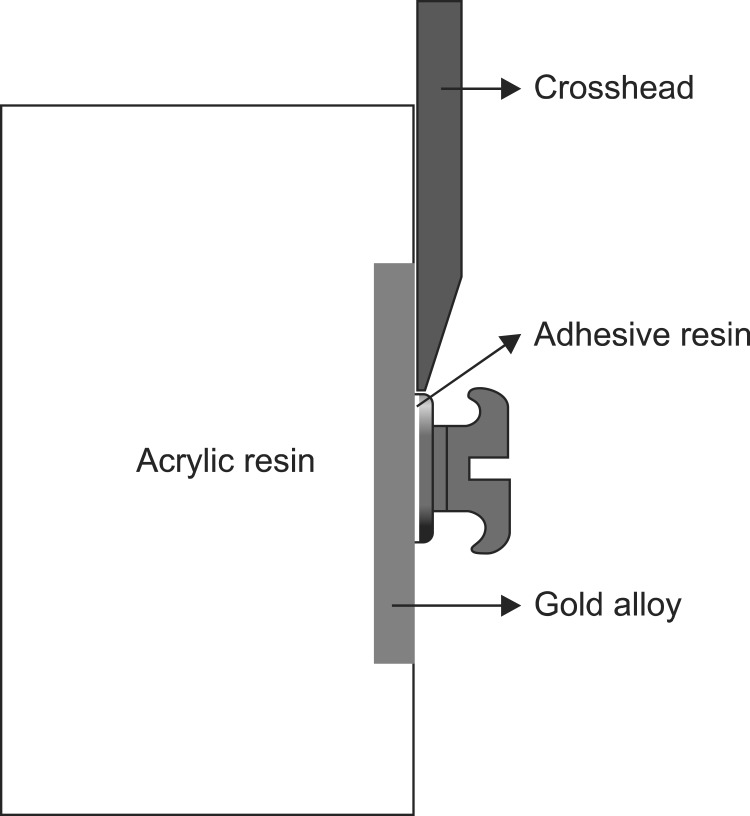
A universal testing machine with a 500 N load cell (Lloyd Instruments®; Ametek, Berwyn, PA, USA) was used to measure the SBS of each specimen. The acrylic resin blocks were fixed in the lower jig vice. The anterior/posterior and right/left positions of the acrylic resin blocks were adjusted so that the adhesive face between the bracket and the specimen was perpendicular to the crosshead (Figure 1). The crosshead speed for the SBS measurement was 1 mm/min, and the maximum load at the moment of bracket debonding was measured. The SBS (MPa) of each specimen was measured by dividing this numerical value (N) by the base area (mm2) of the bracket.
Figure 1.
Schematic diagram of specimen used in experiment, and crosshead position for shear bond strength measurement.
ARI measurement
The adhesion surface of each gold alloy specimen was photographed with a digital camera (Nikon D90; 105 mm micro lens, 1/125, F25, ISO 200, -0.3 eV; Nikon, Tokyo, Japan) after measuring the SBS. Using an image processing software (Photoshop CS5 Extended 12.0 [Adobe systems Inc., San Jose, CA, USA]), each image was cropped, leaving only the 5 × 5 mm adhesion area, and a grid comprising horizontal and vertical lines at a width of 1 mm was superimposed on the image. The ARI of the edited image observed on a monitor was used to evaluate the resin remnants. The criteria for determining the ARI10 are shown in Table 2.
Table 2.
Criteria for determination of the ARI10
ARI, Adhesive remnant index.
Statistical analysis
The Shapiro-Wilk test was performed to confirm that all six groups followed a normal distribution. Two-way analysis of variance (ANOVA) was used to assess the effect of the surface conditioning method and thermocycling process on the SBS. The Kruskal-Wallis H test was used to investigate the statistical significance of the ARI among the different surface conditioning methods, and the Mann-Whitney U test was performed with Bonferroni correction for multiple comparisons. The Mann-Whitney U test was used to evaluate the effect of thermocycling on the ARI, and the correlation between the SBS and ARI in each group was evaluated using Spearman correlation analysis. All statistical analyses were performed using SPSS program ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The mean value and standard deviation of the SBS for each group, and the statistical significance of the SBS value among the groups, are shown in Table 3. The mean SBS values of the AS, MP, and SC groups were 14.0, 18.7, and 21.9 MPa, respectively. SBS was significantly higher in the MP than AS group (p < 0.001), and in the SC than MP group (p < 0.001) (Table 3).
Table 3.
The mean value and standard deviation of shear bond strength in each group with the statistical significance between groups
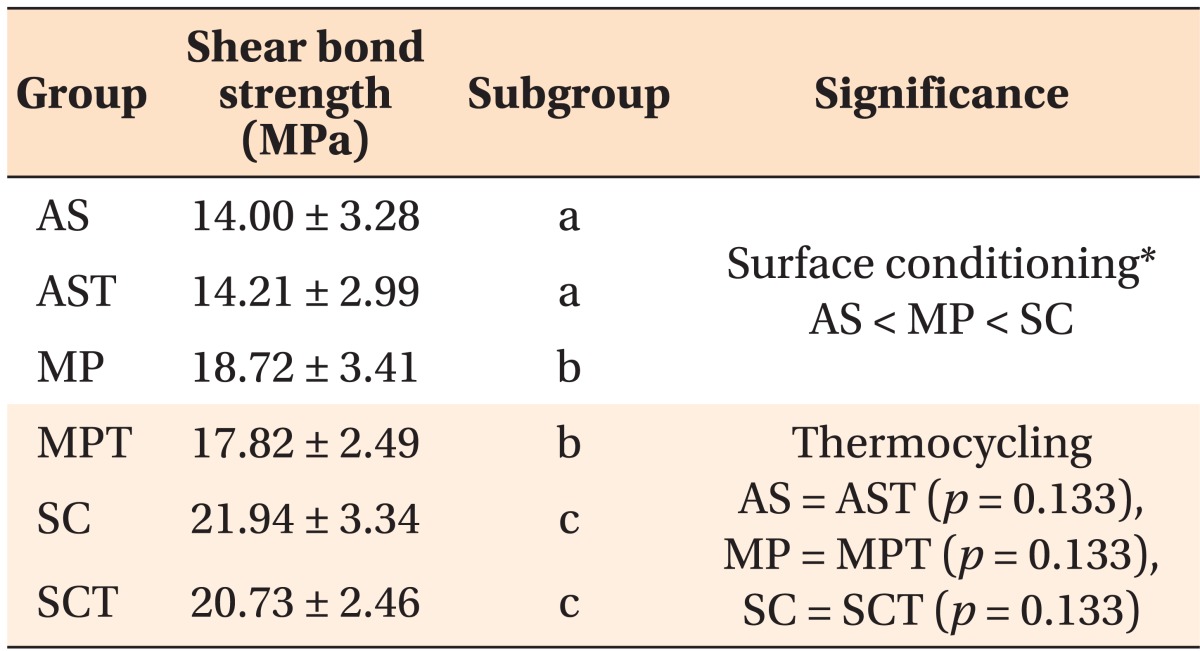
The shear bond strength value is presented as mean ± standard deviation.
Measurements with the same subgroup letter indicate no statistically significant difference using multiple comparisons with Tukey's test at a significance level of p > 0.05.
*Significant result after two-way analysis of variance.
AS, Aluminum oxide sandblasting; AST, AS with thermocycling; MP, AS plus metal primer; MPT, MP with thermocycling; SC, silicoating; SCT, SC with thermocycling.
Although the mean SBS values of the MPT and SCT groups decreased after thermocycling, no significant difference in SBS was observed after thermocycling (p = 0.133) (Table 3). Further, there was no interaction between the surface conditioning method and thermocycling process (p = 0.340).
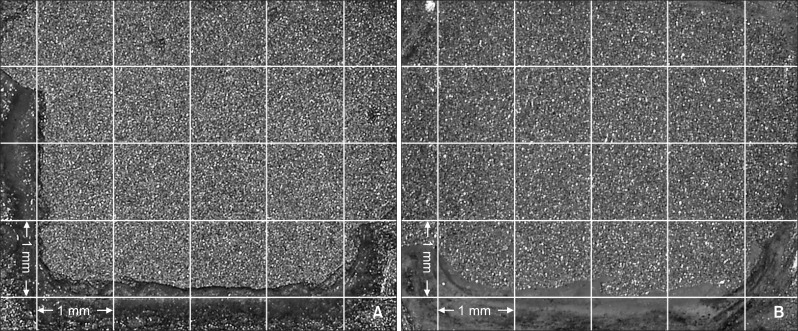
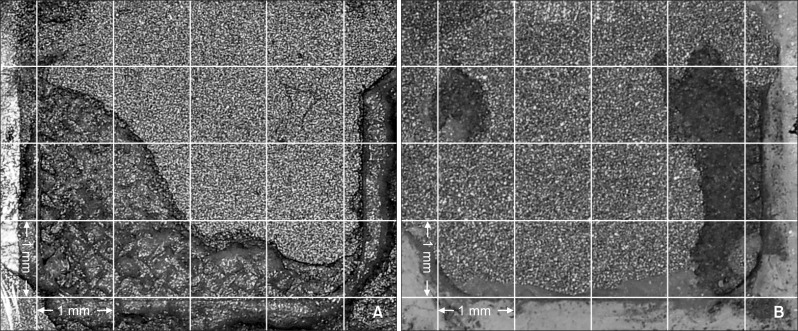
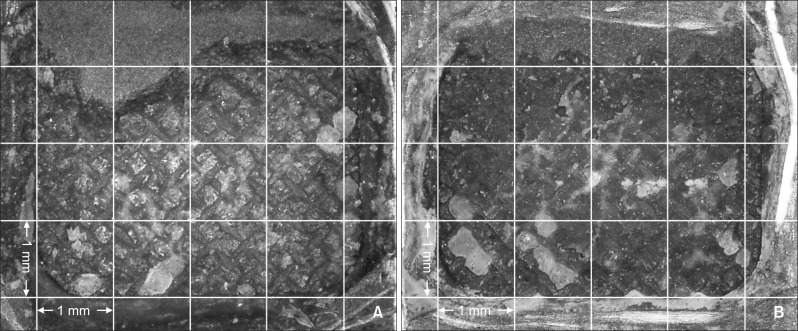
The distribution of the ARI scores in each group is shown in Table 4. The most frequent ARI score observed in each group was as follows: AS and AST, 0; MP and MPT, 1; and SC and SCT, 2 (Table 4, Figures 2, 3, and 4).
Table 4.
ARI of each experimental group with the statistical significance between groups
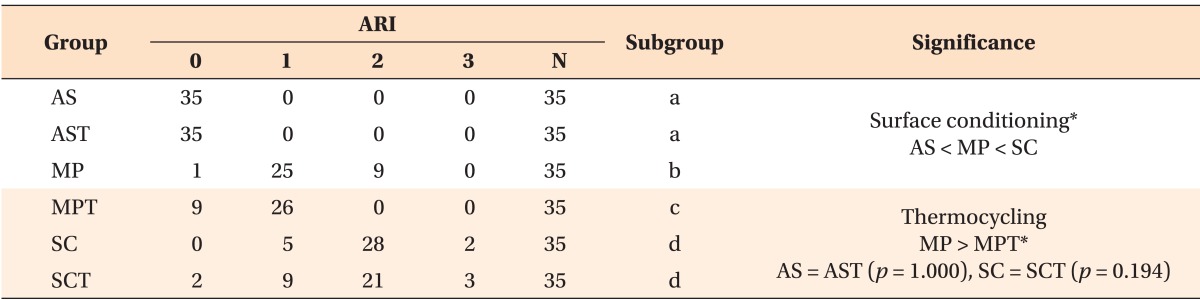
Measurements with the same subgroup letter indicate no statistically significant difference using Mann-Whitney U test at a significance level of p > 0.05.
*Significant result after Mann-Whitney U test.
ARI, Adhesive remnant index; AS, aluminum oxide sandblasting; AST, AS with thermocycling; MP, AS plus metal primer; MPT, MP with thermocycling; SC, silicoating; SCT, SC with thermocycling.
Figure 2.
Adhesion surface of gold alloy in the aluminum oxide sandblasting (AS) group. A, AS group; B, AS with thermocycling (AST) group. An adhesive remnant index of 0 was most frequently observed in AS and AST groups.
Figure 3.
Adhesion surface of gold alloy in the aluminum oxide sandblasting plus metal primer (MP) group. A, MP group; B, MP with thermocycling (MPT) group. An adhesive remnant index of 1 was most frequently observed in MP and MPT groups.
Figure 4.
Adhesion surface of gold alloy in the silicoating (SC) group. A, SC group; B, SC with thermocycling (SCT) group. An adhesive remnant index of 2 was most frequently observed in SC and SCT groups.
The results of the Kruskal-Wallis H test and the Mann-Whitney U test with Bonferroni correction (p < 0.0167) revealed significant differences in the ARI among the three surface conditioning methods with and without application of the thermocycling process. The Mann-Whitney U test revealed no statistical differences in the ARI between the AS and AST groups (p = 1.0) and SC and SCT groups (p = 0.194), although the ARI was significantly greater in the MP than MPT group (Table 4).
The SC and SCT groups exhibited higher SBS values than the MP and MPT groups, respectively, and the MP and MPT groups showed higher SBS values than the AS and AST groups, respectively (Table 3). Similar results were revealed for the ARI (Table 4). Accordingly, the correlation between the SBS and ARI was investigated, and a moderately positive correlation was observed between the SBS and ARI (Spearman correlation coefficient = 0.637, p < 0.05).
DISCUSSION
This study investigated bond strength of gold alloy treated with three different surface conditioning methods with or without thermocycling and directly bonded to metal brackets with chemically cured resin. The specimens treated with silica coating and silination yielded the highest SBS values, and the SBS values of each of the three surfaces were not significantly reduced by application of a thermocycling procedure.
Several studies7,12,13 have reported that sandblasting of metal with aluminum oxide effectively enhances micromechanical retention between the metal and adhesive resin. Although the present study did not compare bond strengths before and after aluminum oxide sandblasting of the gold alloy, there was no significant change in SBS values of gold alloy treated with aluminum oxide sandblasting before and after thermocycling; this result was similar to that of previous study.13
The effect on bond strength of chemical conditioning methods using metal primers has also been evaluated.6,8,14 Various commercialized metal primers are available; with the metal primer containing 4-META (methacryloxyethyl trimellitic anhydride), the acid anhydride group combines with the hydroxyl groups and oxygen atoms in the metal oxide layer, and the methacrylate group polymerizes with the composite resin.8,12 By this mechanism, the metal primer containing 4-META increases the bond strength between the metal and the composite resin.8,12
In the present study, sufficient bond strength was maintained after thermocycling when the metal primer was used after sandblasting. This result corresponds with that of the investigation by Matsumura et al.15 in which the samples were thermocycled after V-primer or Metaltite application. However, Lee et al.6 found that metal primers including V-Primer, Metaltite, and Alloy Primer significantly increased the bond strength of gold alloy, but that bond strength of the specimens treated with all three types of primer decreased after thermocycling, and concluded that metal primer had no advantage over aluminum oxide sandblasting in bond strength. This result is contrary to the result of the present study, and the reason appears to be the use of different resin types in each study. Yoshida and Atsuta16 reported that the effectiveness of the primers for noble metals may be influenced by the initiator system of the resin bonded to the metal. According to their study, when V-Primer was applied to gold alloy, the tri-n-butylborane resin and benzoyl peroxide-amine resin exhibited higher bond strengths than the camphoroquinone-amine resin to gold alloy, with or without thermocycling.16 Lee et al.6 used a light-cured resin containing camphoroquinone as the photoinitiator. There is no previous study evaluating whether the bond strength of gold alloy specimens treated with the metal primer containing 4-META is maintained after thermocycling. Therefore, no data is available for direct comparison with the present study results.
If abrasive particles with a silicated surface are blasted onto a metal surface at high energies, kinetic energy is converted into thermal energy, and the high temperature formed by the impact of the abrasive particles results in silica being incorporated and embedded into the metal to a depth of 15 µm.17 Atsü et al.18 reported that the silica particles were incorporated into a metal surface by blasting pressure when the metal surface was blasted with 30 µm diameter aluminum oxide chemically modified with silica, thereby forming a fine roughened surface. Watanabe et al.19 verified that a silica layer was formed on the surface of dental gold alloys by using electron microprobe analysis element mapping.
When the silane is applied to the silica layer formed on the metal surface, silane and silica combine strongly by a dehydration reaction, separating the hydroxyl group.8 Silanes have dual reactivity. Non-hydrolyzable functional groups can combine with the resin composite monomers containing C=C double bonds, and the hydrolyzable alkoxy group can bond with hydroxyl group-rich inorganic substrates such as silica.18,20
Silane for conditioning purposes is supplied as a liquid dissolved in a volatile solvent such as alcohol; thus, application is straightforward. However, sufficient time is required for evaporation of the solvent, and for the chemical reaction between silica and silane to take place, to achieve adequate bond strength. Therefore, an appropriate time interval is necessary for a successful silicoating procedure.21 Manufacturers recommend 5 min of silanation before applying the adhesive for extraoral use, and 1 min for intraoral use. In this study, a time interval of 5 min after silane application was selected to avoid the effect of insufficient time for the evaporation of the solvent and chemical reaction. In clinical applications, 1 min after silane application may be adequate. Because silicoating is achieved by incorporation of silica particles into the metal surface, this method is less affected by the gold alloy composition and oxide layer formation, compared with metal primer application after aluminum oxide sandblasting.8,22 In the present study, the silicoating group exhibited an SBS value of 20.73 ± 2.46 (mean ± standard deviation) MPa after thermocycling, which represents a higher bond strength than that of the metal primer application after aluminum oxide sandblasting. This bond strength is comparable to that of a metal bracket bonded to an enamel surface.23 Further, this SBS value is greater than the SBS value of 13.63 ± 2.55 MPa reported in the study of Jung et al.8 in which light-cured resin was used with silicoating.
Typically, the weaker the bond between the adherend and the adhesive, the fewer resin remnants will remain on the adherend surface21,24; this trend was also observed in the present study. More resin remnants were observed on the gold alloy specimens in the SC than MP group, and in the MP than AS group. This trend was maintained after thermocycling. According to a study by Jung et al.8, the ARI was higher with silicoating than aluminum oxide sandblasting; however, there was no statistical significance. Jung et al.8 regarded the cause of the result as the use of light-cured resin adhesive in their study, and reported that the insignificant difference in ARI might have been produced by uncured resin remaining nonuniformly on the specimen surface.
Although bond strength was increased by treatment with a metal primer after aluminum oxide sandblasting, greater bond strength was attained with silicoating. Based on this result, the success rate of direct bonding to gold alloy restorations or prostheses may be increased by application of silicoating to the surfaces of gold alloys.
Silicoating may increase bond strength with both light-cured resin8 and chemically cured resin. However, bond strength and ARI may be affected by resin type. This should be studied in the future. Further, the brackets were bonded to flat surfaces of the gold alloy specimens in the present study. In practice, the surface of gold alloy restorations is not flat, so gap formation between the gold alloy surface and the bracket is inevitable and bond strengths are expected to be less than those obtained in this in vitro study. Clinical studies are required regarding bond failure rate with irregular surfaces.
CONCLUSION
When a chemically cured resin is used to bond metal brackets to gold alloys treated with surface conditioning methods including aluminum oxide sand-blasting alone, metal primer application after aluminum oxide sandblasting, and silicoating: 1. the highest SBS can be attained with the silicoating technique; 2. metal primer application after sandblasting increases SBS compared with sandblasting alone; 3. SBS is maintained after thermocycling for all surface conditioning methods.
Footnotes
This study was supported by a research fund from Chosun University, 2011.
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Boyd RL, Baumrind S. Periodontal considerations in the use of bonds or bands on molars in adolescents and adults. Angle Orthod. 1992;62:117–126. doi: 10.1043/0003-3219(1992)062<0117:PCITUO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215–235. [PubMed] [Google Scholar]
- 3.Wood DP, Jordan RE, Way DC, Galil KA. Bonding to porcelain and gold. Am J Orthod. 1986;89:194–205. doi: 10.1016/0002-9416(86)90032-1. [DOI] [PubMed] [Google Scholar]
- 4.Zachrisson BU, Buyukyilmaz T. Recent advances in bonding to gold, amalgam and porcelain. J Clin Orthod. 1993;27:661–675. [Google Scholar]
- 5.Antoniadou M, Kern M, Strub JR. Effect of a new metal primer on the bond strength between a resin cement and two high-noble alloys. J Prosthet Dent. 2000;84:554–560. doi: 10.1067/mpr.2000.109986. [DOI] [PubMed] [Google Scholar]
- 6.Lee YK, Cha JY, Yu HS, Hwang CJ. Effect of metal primer and thermocycling on shear bonding strength between the orthodontic bracket and gold alloy. Korean J Orthod. 2009;39:320–329. [Google Scholar]
- 7.Nergiz I, Schmage P, Herrmann W, Ozcan M. Effect of alloy type and surface conditioning on roughness and bond strength of metal brackets. Am J Orthod Dentofacial Orthop. 2004;125:42–50. doi: 10.1016/s0889-5406(03)00507-9. [DOI] [PubMed] [Google Scholar]
- 8.Jung MH, Chung SH, Shon WJ. Shear bond strength between gold alloy and orthodontic metal bracket using light emitting diode curing light. Korean J Orthod. 2010;40:27–33. [Google Scholar]
- 9.Shon WJ, Kim TW, Chung SH, Jung MH. The effects of primer precuring on the shear bond strength between gold alloy surfaces and metal brackets. Eur J Orthod. 2012;34:72–76. doi: 10.1093/ejo/cjq163. [DOI] [PubMed] [Google Scholar]
- 10.Artun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod. 1984;85:333–340. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 11.Bishara SE, Ajlouni R, Laffoon JF. Effect of thermocycling on the shear bond strength of a cyanoacrylate orthodontic adhesive. Am J Orthod Dentofacial Orthop. 2003;123:21–24. doi: 10.1067/mod.2003.1. [DOI] [PubMed] [Google Scholar]
- 12.Büyükyilmaz T, Zachrisson BU. Improved orthodontic bonding to silver amalgam. Part 2. Lathe-cut, admixed, and spherical amalgams with different intermediate resins. Angle Orthod. 1998;68:337–344. doi: 10.1043/0003-3219(1998)068<0337:IOBTSA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Büyükyilmaz T, Zachrisson YO, Zachrisson BU. Improving orthodontic bonding to gold alloy. Am J Orthod Dentofacial Orthop. 1995;108:510–518. doi: 10.1016/s0889-5406(95)70051-x. [DOI] [PubMed] [Google Scholar]
- 14.Bulbul M, Kesim B. The effect of primers on shear bond strength of acrylic resins to different types of metals. J Prosthet Dent. 2010;103:303–308. doi: 10.1016/S0022-3913(10)60063-7. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura H, Shimoe S, Nagano K, Atsuta M. Effect of noble metal conditioners on bonding between prosthetic composite material and silver-palladium-copper-gold alloy. J Prosthet Dent. 1999;81:710–714. doi: 10.1016/s0022-3913(99)70111-3. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, Atsuta M. Effect of MMA-PMMA resin polymerization initiators on the bond strengths of adhesive primers for noble metal. Dent Mater. 1999;15:332–336. doi: 10.1016/s0109-5641(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 17.Sun R, Suansuwan N, Kilpatrick N, Swain M. Characterisation of tribochemically assisted bonding of composite resin to porcelain and metal. J Dent. 2000;28:441–445. doi: 10.1016/s0300-5712(00)00006-3. [DOI] [PubMed] [Google Scholar]
- 18.Atsü SS, Gelgör IE, Sahin V. Effects of silica coating and silane surface conditioning on the bond strength of metal and ceramic brackets to enamel. Angle Orthod. 2006;76:857–862. doi: 10.1043/0003-3219(2006)076[0857:EOSCAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Ino S, Okada S, Katsumata Y, Hamano N, Hojo S, et al. Influence of simplified silica coating method on the bonding strength of resin cement to dental alloy. Dent Mater J. 2008;27:16–20. doi: 10.4012/dmj.27.16. [DOI] [PubMed] [Google Scholar]
- 20.Matinlinna JP, Lassila LV, Ozcan M, Yli-Urpo A, Vallittu PK. An introduction to silanes and their clinical applications in dentistry. Int J Prosthodont. 2004;17:155–164. [PubMed] [Google Scholar]
- 21.Jung MH, Shon WJ, Park YS, Chung SH. Effects of silanation time on shear bond strength between a gold alloy surface and metal bracket. Korean J Orthod. 2013;43:127–133. doi: 10.4041/kjod.2013.43.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider W, Powers JM, Pierpont HP. Bond strength of composites to etched and silica-coated porcelain fusing alloys. Dent Mater. 1992;8:211–215. doi: 10.1016/0109-5641(92)90086-r. [DOI] [PubMed] [Google Scholar]
- 23.Cozza P, Martucci L, De Toffol L, Penco SI. Shear bond strength of metal brackets on enamel. Angle Orthod. 2006;76:851–856. doi: 10.1043/0003-3219(2006)076[0851:SBSOMB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Patusco VC, Montenegro G, Lenza MA, Alves de Carvalho A. Bond strength of metallic brackets after dental bleaching. Angle Orthod. 2009;79:122–126. doi: 10.2319/072507-345.1. [DOI] [PubMed] [Google Scholar]