Abstract
AIM
Children born very preterm are at risk for impaired motor performance ranging from cerebral palsy (CP) to milder abnormalities, such as developmental coordination disorder. White matter abnormalities (WMA) at term have been associated with CP in very preterm children; however, little is known about the impact of WMA on the range of motor impairments. The aim of this study was to assess whether WMA were predictive of all levels of motor impairments in very preterm children.
METHOD
Two hundred and twenty-seven very preterm infants (<30wks’ gestational age or birthweight <1250g) had brain magnetic resonance imaging at term-equivalent age to assess for WMA, which were categorized as nil, mild, or moderate to severe. At 5 years of age children were classified as having a moderate to severe motor impairment if they were below the 5th centile or mild to severe motor impairment if their score placed them no higher than the 15th centile on the Movement Assessment Battery for Children (MABC). WMA (nil vs mild and nil vs moderate–severe) were explored as predictors of motor impairment using logistic regression. Analyses were repeated adjusting for the effects of other perinatal variables and excluding children with CP.
RESULTS
Of the 193 very preterm children (97 males, 96 females) assessed with the MABC, 53 (27%) were classified as having a moderate to severe motor impairment and 96 (50%) a mild to severe motor impairment. WMA were predictive of motor impairment in very preterm children, with mild versus no WMA increasing the odds of moderate to severe motor impairment by over fivefold (odds ratio [OR] 5.6; 95% confidence interval [CI] 1.9–16.1; p=0.002) and mild to severe impairment by twofold (OR 2.2; 95% CI 1.1–4.2; p=0.02). Compared with no WMA, moderate to severe WMA increased the odds for moderate to severe impairment 19-fold (OR 19.4; 95% CI 5.6–66.7; p<0.001) and for mild to severe motor impairment ninefold (OR 9.4; 95% CI 3.2–28.1; p<0.001). Results remained similar after controlling for several potential confounders and after excluding 14 children who had CP at age 2 years.
INTERPRETATION
WMA predict motor impairment at 5 years, with rates of impairment increasing with more severe WMA. Very preterm children with any WMA at term require follow-up throughout childhood.
Children born preterm are at an increased risk of a range of motor impairments ranging from mild motor problems, such as coordination difficulties, to cerebral palsy (CP).1,2 The risk of developing CP increases with decreasing gestational age with rates ranging from 14.6% for children born at 22 to 27 weeks’ gestation, to 6.2% for children born at 28 to 31 weeks’ gestation, to 0.1% in children born at term.1 Very preterm children also suffer from an increased risk for non-CP motor problems, such as developmental coordination disorder.2 The DSM-IV refers to developmental coordination disorder as impaired motor coordination that (1) is substantially below expectation given the child’s age and intellectual ability, (2) significantly interferes with daily activities and academic achievement, and (3) is not associated with CP or other general medical condition.3 Given the difficulty in operationalizing the DSM-IV diagnostic criteria associated with this disorder, for this paper the term ‘non-CP motor impairment’ is preferred. In preterm cohorts, the rate of non-CP motor impairment has been reported to be as high as 70%,4–6 although a recent systematic review estimates that 41% (95% CI 32.1–48.9) of children born very preterm have mild to moderate motor impairments.2 Identifying preterm infants who are at high risk of both CP and non-CP motor impairments is important for enrolling children in surveillance programmes, early implementation of targeted intervention, and counselling families, to try to reduce the burden of this condition as the child develops.
Magnetic resonance imaging (MRI) is a powerful tool for understanding brain injury in preterm infants and identifying the neural correlates of neurosensory and neurobehavioural impairments.7–15 Using MRI in the neonatal period, our group and others have shown that most children born very preterm display diffuse white matter abnormalities (WMA).8,10,11,13 These abnormalities include white matter signal abnormality, loss of white matter, ventricular dilatation, and thinning of the corpus callosum, and are predictive of early motor delay and CP.10,15 We have previously reported a relationship between early motor delay at 2 years (Woodward et al.10) and WMA but we are unaware of other studies that relate WMA to later motor impairment. Prediction of early delay does not necessarily translate to later impairment. This study directly addresses this issue, which is important given motor impairment is one of the most common consequences of being born preterm.2 The aim of this study was to assess whether neonatal WMA were predictive of more general motor impairment in very preterm children at 5 years of age. Of particular importance, we hypothesized that increasing severity of WMA at term-equivalent age would predict a range of motor impairment at age 5 years, including and excluding children diagnosed with CP.
METHOD
Participants
Two-hundred and twenty-seven preterm infants (gestational age <30wk or birthweight <1250g), of 348 who were eligible, were recruited as part of the Victorian Infant Brain Study longitudinal follow-up study of preterm children born between 2001 and 2003. Children with significant genetic or congenital abnormalities likely to affect brain function or development were excluded. Infants were born at, or transferred shortly after birth to, the Royal Women’s Hospital, Victoria, Australia. Infants were enrolled before term-equivalent age after parental consent was obtained. This study was approved by the Royal Women’s Hospital and Royal Children’s Hospital ethics committees. At 2 years of age, children had a comprehensive neurodevelopmental assessment, including evaluation by a paediatrician for the presence of CP.
Perinatal data collection
Extensive perinatal data including gestational age, birthweight z-score (to capture growth restriction in utero), sex, and bronchopulmonary dysplasia (defined as oxygen requirement at 36wks’ postmenstrual age) were collected following consent to participate.
At 24 months of age, parents were asked to complete a questionnaire to elicit sociodemographic information. Social risk was assessed using an index comprising six aspects of social status including family structure, education of primary caregiver, occupation of primary income earner, employment status of primary income earner, language spoken at home, and maternal age at birth.16,17 Children were divided into those at a higher social risk (index ≥2) and those at a lower social risk (index <2). At 24 months of age, all children had a standardized paediatric neurological evaluation, which included an assessment of muscle tone, power and reflexes. CP and its severity were diagnosed with the use of standard criteria: location or body part impaired (e.g. diplegia or hemiplegia), degree of impairment of muscle tone and reflexes, and the effects on ambulation.18
MRI
Brain MRI was performed at term-equivalent age (approx. 38–42wks’ gestation). The MRI was performed without sedation or anaesthesia using previously published techniques and sequences.19 To summarize, infants were fed, swaddled, and placed in a Vac Fix beanbag (S&S Par Scientific, Odense, Denmark) designed to keep the infant still and supported in the 1.5T General Electric Signa LX Echospeed System MR scanner (Milwaukee, WI, USA). An established qualitative scoring system was used to classify WMA as normal, mild, or moderate to severe based upon a grading system of five scales, including (1) the nature and extent of white matter signal abnormality, (2) periventricular white matter volume loss, (3) the presence of any cystic abnormalities, (4) ventricular dilatation, and (5) thinning of the corpus callosum.8,10 All scans were scored by two raters who were blind to clinical status, with 94% interrater agreement.9
Outcome measures
At 5 years’ corrected age, motor functioning was assessed using the Movement Assessment Battery for Children (MABC),20 which is a standardized measure of gross and fine motor development. This test is frequently used in the assessment of motor impairment,2 and has high reliability and validity. We used the total score from the MABC, which is expressed as a centile, with higher centiles indicating better motor performance. Children were classified as having a moderate to severe motor impairment if their score placed them in the 5th centile or below on the MABC, or mild to severe motor impairment if their score was not more than the 15th centile. Children who were too impaired to complete the assessment were assigned an MABC score less than the 5th centile. All assessors were unaware of perinatal details of the participants, including MRI findings. Age was corrected for gestational age to avoid a bias towards lower scores of very preterm children.21
Analysis
Data were analysed using Stata 11.0 (Stata Corp., College Station, Texas, USA). Means and standard deviations as well as medians and interquartile ranges for the MABC are presented according to WMA grade. The linearity of the relationship between the proportion impaired and WMA was assessed using a χ2 test for a linear trend (χ2linear trend). Severity levels of WMA (mild vs nil and moderate to severe vs nil) were explored by logistic regression as predictors of motor impairments at 5 years.22 The odds ratio (OR) is an approximation to the relative risk.22 Results from regression models are presented as OR and 95% confidence intervals (CI) from an unadjusted analysis as well as adjusted for perinatal factors known to be related to neurodevelopment, including sex, gestational age, birthweight z-score, bronchopulmonary dysplasia, and social risk category (higher or lower) to assess the additional effect of WMA over and above these other factors.2,9,10 Analyses were repeated with children with a diagnosis of CP at 2 years excluded, to estimate the relationship between WMA and non-CP motor impairment. All analyses were fitted using generalized estimating equations with an exchangeable correlation structure and robust (sandwich) estimators of standard error to allow for the clustering of twins.23 Positive and negative predictive values for any WMA and moderate to severe WMA in predicting motor impairment were also assessed.
RESULTS
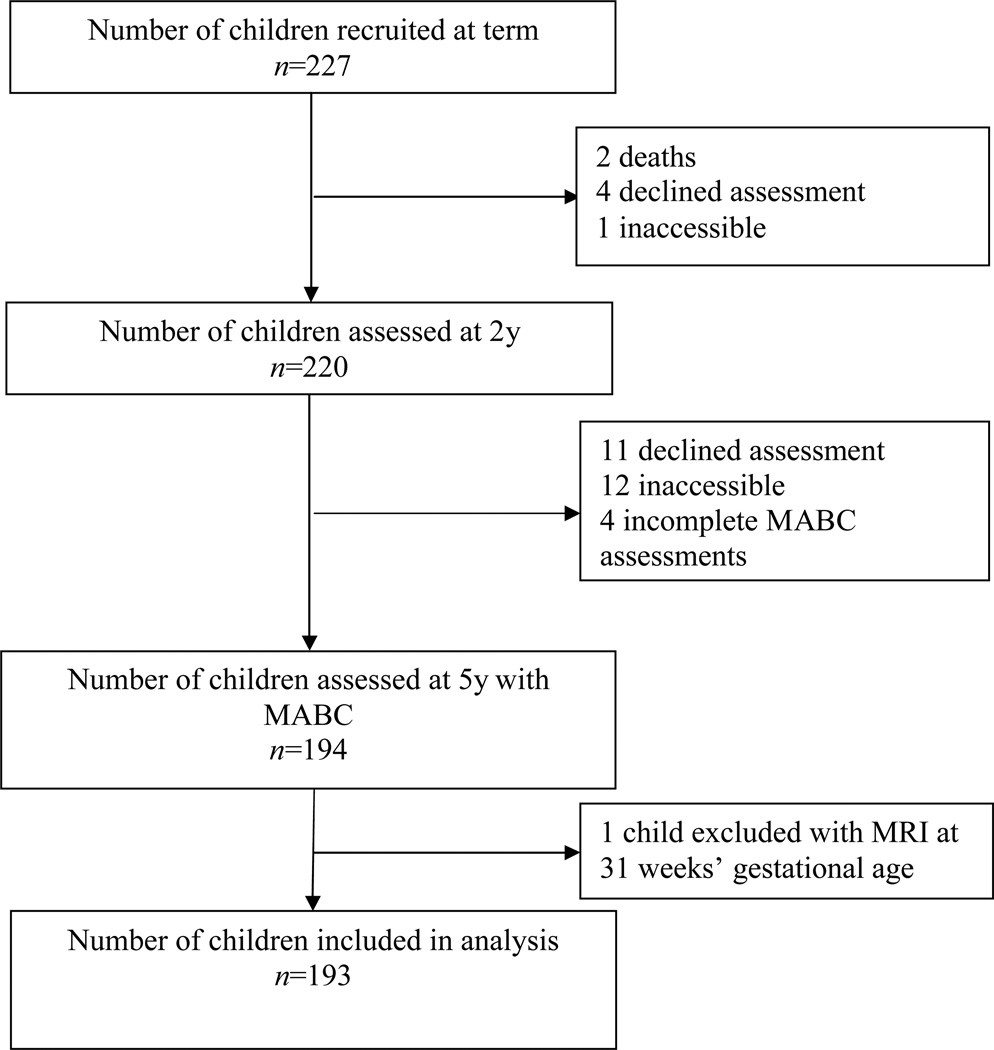
Of the initial 227 infants recruited for this study, 193 were included in this analysis (Fig. 1). The mean gestational age for the sample was 27.5 weeks and mean birthweight was 962g; other demographic features are shown in Table I. Most demographic variables were similar between the 193 children who were included compared with 34 non-included, except for the latter group who had more necrotizing entercolitis (25%) and cystic periventricular leukomalacia (15%). MRI-defined WMA in the newborn period was mild in 53% (n=103) and moderate to severe in 16% (n=30). Fifteen children had CP, of whom six had quadriplegia, six had diplegia, one had hemiplegia, and one had monoplegia. Five children were classified as having severe CP, three as moderate, and seven as mild.
Figure 1.
Recruitment and follow-up from term to 5 years. MABC, Movement Assessment Battery for Children; MRI, magnetic resonance imaging.
Table I.
Neonatal and demographic characteristics of participants
| Characteristic | All participants (n=193) |
Excluding infants with cerebral palsy at 2y (n=178) |
|---|---|---|
| Gestational age, wk | ||
| Mean (SD) | 27.5 (1.9) | 27.5 (1.9) |
| Range | 22–32 | 22–32 |
| Birthweight, g | ||
| Mean (SD) | 962 (220) | 965 (221) |
| Range | 414–1425 | 414–1425 |
| Birthweight z-score | ||
| Mean (SD) | −0.55 (0.93) | −0.54 (0.93) |
| Range | −3.54 to 1.15 | −3.54 to 1.15 |
| n (%) | n (%) | |
| Males/Females | 97/96 | 89/89 |
| Twins/triplets | 77 (39) | 73 (41) |
| High social risk (score≥2)a | 115 (60) | 105 (60) |
| Number missing | 2 | 2 |
| Bronchopulmonary dysplasiaa | 62 (32) | 57 (32) |
| Number missing | 1 | 1 |
| Postnatal corticosteroidsa | 17 (9) | 15 (8) |
| Number missing | 1 | 1 |
| Necrotizing enterocolitis (including suspected) | 16 (8) | 13 (7) |
| Sepsisa | 54 (33) | 47 (31) |
| Number missing | 27 | 24 |
| Grade 3/4 intraventricular haemorrhage | 7 (4) | 6 (3) |
| Cystic periventricular leukomalacia | 7 (4) | 5 (3) |
| White matter abnormalities on MRI | ||
| Nil | 61 (32) | 61 (34) |
| Mild | 102 (53) | 94 (53) |
| Moderate–severe | 30 (16) | 23 (13) |
Percentages of those with data available. MRI, magnetic resonance imaging.
The mean and median centile ranks on the MABC are shown in Table II. The mean and median ranks varied by group, with the highest scores in children with no WMA and the lowest scores in those with moderate to severe WMA. Of the 193 children, 53 (27%) were classified as having a moderate to severe motor impairment and 96 (50%) as having a mild to severe motor impairment (Table II), much higher than the expected rates of 5 and 15% based on the centile cut-offs. The proportion of infants with both moderate to severe and mild to severe motor impairment increased with increasing severity of WMA for the group overall (χ2linear trend=27.9, p<0.001, and χ2linear trend=20.4, p<0.001 respectively), and when children with CP were excluded (χ2linear trend=18.2, p<0.001, and χ2linear trend=14.7, p<0.001 respectively). Of note, in the no WMA group there were only four children (7%) who had a moderate to severe motor impairment and a further 19 (31%) who had a mild motor impairment.
Table II.
Movement Assessment Battery for Children (MABC) scores for infants with nil, mild, and moderate to severe white matter abnormalities (WMA)
| Motor impairment | Nil | WMA mild |
WMA moderate– severe |
Total sample |
|---|---|---|---|---|
| All participants | (n=61) | (n=102) | (n=30) | (n=193) |
| MABC centile | ||||
| Mean (SD) | 30.0 (23.4) | 22.3 (23.7) | 6.2 (6.2) | 22.2 (23.1) |
| Median (IQR) | 21 (12–46) | 15 (4–32) | 4 (1–10) | 16 (5–32) |
| Range | 1–93 | 1–93 | 1–19 | 1–93 |
| Moderate–severe impairment, n (%) | 4 (7) | 31 (30) | 18 (60) | 53 (27) |
| Mild–severe impairment, n (%) | 19 (31) | 52 (51) | 25 (83) | 96 (50) |
| Excluding infants with CP at 2y | (n=61) | (n=94) | (n=23) | (n=178) |
| MABC centile | ||||
| Mean (SD) | 30.0 (23.4) | 23.4 (23.9) | 7.7 (6.4) | 23.6 (23.2) |
| Median (IQR) | 21 (12–46) | 17 (5–32) | 6 (1–14) | 16 (6–32) |
| Range | 1–93 | 1–93 | 1–19 | 1–93 |
| Moderate–severe impairment, n (%) | 4 (7) | 25 (27) | 11 (48) | 40 (22) |
| Mild–severe impairment, n (%) | 19 (31) | 46 (49) | 18 (78) | 83 (47) |
Moderate–severe impairment is defined as MABC total impairment score ≤5th centile; mild–severe impairment is defined as MABC total impairment score ≤15th centile. IQR, interquartile range; CP cerebral palsy.
WMA were predictive of motor impairment in very preterm children (Table III), with mild WMA increasing the odds of moderate to severe motor impairment more than fivefold (unadjusted OR 5.6; 95% CI 1.9–16.3; p=0.002) and mild to severe impairment twofold (OR 2.2; 95% CI 1.1–4.2, p=0.02) compared with infants with no WMA. Moderate to severe WMA increased the odds of moderate to severe motor impairment more than 19-fold (OR 19.4; 95% CI 5.6–66.7; p<0.001) and for mild to severe motor impairment ninefold (OR 9.4; 95% CI 3.2–28.1; p<0.001) compared with infants with no WMA. Results remained similar after controlling for sex, social risk, bronchopulmonary dysplasia, gestational age, and birthweight z-scores, and after excluding children with CP at 2 years of age.
Table III.
Severity of white matter abnormalities as predictors of motor impairment at 5y
| Motor impairment | White matter abnormalitya |
Unadjusted odds ratiob (95% CI) |
p value | Adjusted odds ratioc (95% CI) |
p value |
|---|---|---|---|---|---|
| All participants (n=193) | |||||
| Moderate–severe impairment | Mild | 5.6 (1.9–16.3) | 0.002 | 6.6 (2.0–21.9) | 0.002 |
| Moderate–severe | 19.4 (5.6–66.7) | <0.001 | 38.4 (8.8–167.0) | <0.001 | |
| Mild–severe impairment | Mild | 2.2 (1.1–4.2) | 0.02 | 2.0 (1.0–4.1) | 0.06 |
| Moderate–severe | 9.4 (3.2–28.1) | <0.001 | 10.2 (3.2–32.7) | <0.001 | |
| Excluding infants with cerebral palsy at 2y (n=178) | |||||
| Moderate–severe impairment | Mild | 4.5 (1.5–12.8) | 0.006 | 4.9 (1.5–16.5) | 0.01 |
| Moderate–severe | 10.7 (3.0–38.5) | <0.001 | 20.1 (4.4–92.6) | <0.001 | |
| Mild–severe impairment | Mild | 2.0 (1.0–3.9) | 0.04 | 1.8 (0.9–3.6) | 0.12 |
| Moderate–severe | 6.2 (2.0–18.6) | 0.001 | 6.3 (1.9–20.8) | 0.002 | |
Grade of white matter abnormality compared with no white matter abnormality;
Results adjusted for clustering of multiples only;
Results adjusted for sex, gestational age at birth, birthweight z-score, high social risk, and bronchopulmonary dysplasia (n=190 and 175 in the analysis including and excluding children with cerebral palsy at 2y respectively); Moderate–severe impairment is defined as Movement Assessment Battery for Children total impairment score ≤5th centile; mild–severe impairment is defined as Movement Assessment Battery for Children total impairment score ≤15th centile.
CI, confidence interval.
The positive predictive value for WMA predicting motor impairment was high for moderate to severe motor impairment and mild to severe motor impairment; however, the negative predictive value was low (Table IV). On the other hand, the negative predictive value for moderate to severe WMA was high and the positive predictive value low for any motor impairment.
Table IV.
Positive predictive value (PPV) and negative predictive value (NPV) of WMA predicting motor impairment
| Motor impairment | Any WMA | Moderate–severe WMA | ||
|---|---|---|---|---|
| PPV | NPV | PPV | NPV | |
| All participants (n=193) | ||||
| Moderate–severe impairment | 92.5 (31.8–97.9) | 40.7 (32.5–49.3) | 34.0 (21.5–48.3) | 91.4 (85.5–95.5) |
| Mild–severe impairment | 80.2 (70.8–87.6) | 43.3 (33.3–53.8) | 26.0 (17.6–36.0) | 94.9 (88.4–98.3) |
| Excluding infants with cerebral palsy at 2y (n=178) | ||||
| Moderate–severe impairment | 90.0 (76.3–97.2) | 43.3 (33.0–50.0) | 27.5 (14.6–43.9) | 91.3 (85.3–95.4) |
| Mild–severe impairment | 77.1 (66.6–85.6) | 44.2 (34.0–54.8) | 21.7 (13.4–32.1) | 94.7 (88.1–98.3) |
Values are mean (95% confidence intervals). WMA, white matter abnormalities.
DISCUSSION
This study provides further evidence of the high rate of motor impairment in very preterm children, both including and excluding CP. In particular, our rates of 27% for moderate to severe motor impairment and 50% for mild to severe impairment, excluding children with CP, are consistent with recent findings.2 These rates are approximately three to four times higher than those observed in the general population, reflecting the magnitude of motor coordination difficulties in addition to CP in the preterm population. The implications of motor impairment including CP are considerable, potentially affecting fitness and health, sport participation, social skills, emotional well-being, and self-esteem, as well as cognitive functioning and academic achievement.24–26 Given the significance of motor coordination problems in the preterm population, it is critical that we determine the neuromechanisms underpinning this pattern of deficits and that we become better at identifying children at elevated risk for motor coordination problems to enable referral to early intervention programmes that are tailored to address fine and gross motor development. Although evidence so far suggests that early intervention is not effective in improving motor outcomes in preterm children as a whole,24,25,27,28 more specific motor remediation programmes may be beneficial as has been demonstrated in other populations such as CP29 and adult stroke.30
We have previously reported that MRI-defined WMA are independently associated with neurobehavioral impairments at 2 years’ corrected age, including motor delay and CP,13 suggesting that qualitative ratings of brain abnormalities in the neonatal period may be predictive of later motor impairments. Although cystic periventricular leukomalacia diagnosed on cranial ultrasound has high specificity in predicting motor delay and CP, it is now a rare complication of being born preterm,31 it does not explain the high rates of motor delay in very preterm children, and has low sensitivity in relation to motor delay.10 We have reported that using qualitative scoring of MRI-defined WMA in the neonatal period is the strongest predictor of motor delay at 2 years of age; it is more strongly associated with motor delay than traditional predictors of outcome including gestational age, birthweight, bronchopulmonary dysplasia, and postnatal corticosteroids.10 However, limited data have been evaluated on the relation of WMA on MRI to later motor outcomes. Examining the issue in this study, we found that the rate of motor impairment at age 5 years increased with increasing severity of WMA, both including and excluding those with an earlier diagnosis of CP. The rate of moderate to severe motor impairment in very preterm children without MRI-defined WMA was only 7%, close to the expected rate of 5% on the MABC, in contrast to 60% of children with moderate to severe WMA. As expected, the rates of motor impairment were higher across all WMA groups when the cut-off was lowered to include children with mild motor impairment, but the relationship between WMA and motor impairments remained. The strong association between neonatal WMA and motor impairment is unlikely to be indirectly due to other perinatal risk factors as OR and significance levels remained stable after adjusting for a range of risk factors. Of note, 19 (31%) children without WMA had mild to severe motor impairment. Multiple complex mechanisms are likely to contribute to motor impairment in this population and further analyses to investigate this issue are beyond the scope of this paper.
Consistent with the notion that development is multi-determined and is not dependent on a single factor, our findings indicate that motor development in very preterm children is not solely related to neonatal WMA. For instance, very preterm children without WMA had a rate of mild motor impairments that was over double that expected in the general population (31% vs 15%), suggesting that there are other factors associated with preterm birth that lead to an increased risk of at least mild motor impairments. Furthermore, not all children with moderate to severe WMA went on to develop motor coordination difficulties. Genetic, environmental, and neural reorganization factors are all likely to play a significant role in motor development.
The qualitative rating system used in this study to classify neonatal WMA covers a broad range of elements including cystic and signal abnormality, white matter volume loss, ventricular dilatation, myelination of the posterior limb of the internal capsule, and integrity of the corpus callosum.8,10 As such, it is difficult to speculate confidently on the neural mechanisms that are related to motor impairment in this population. White matter lesions and signal abnormalities are often located in close proximity to corticospinal tracts, and it is reasonable to expect that these alterations have a greater influence on motor function. Ventricular dilatation, white matter loss, and delayed myelination refer to more diffuse alterations that are likely to compromise neural connectivity for a range of functional systems, including the motor system. Longitudinal neuroimaging and the use of more advanced imaging techniques such as diffusion tensor imaging are needed to further our understanding of how the preterm brain develops under stress and reorganizes after injury.
The strengths of this study are that it included a large cohort of very preterm children who had an MRI at term and who were prospectively followed from birth to 5 years. It also had high rates of follow-up (85% of eligible children were seen at 5y), and assessors were blinded to perinatal and MRI data for all evaluations. A limitation of the study was that CP diagnosis was determined at 2 years of age; however, we have previously reported that the level of agreement for CP classification between 2 and 8 years of age is high in extremely preterm infants.32 A further limitation was that the sample size was fixed by the recruitment of 227 infants for the original study, which had 2-year outcomes as a major endpoint. We may have been underpowered to find some clinically important associations.
Findings from this study have important implications for both clinicians and researchers. When counselling parents about outcomes for their children born preterm, it is important that the clinician considers not only the more severe developmental problems such as CP but also the risk of other motor impairments; MRI at term helps to identify those at greater risk of motor problems. Given the high rate of both CP and non-CP motor impairments in infants born preterm and their potential impact on other areas of function, further research is needed to understand the pathways that lead to all levels of motor problems. Our study findings would support the role of cerebral white matter injury in this pathway.
What this paper adds
WMA (assessed using MRI) in the neonatal period are predictive of motor impairments at 5 years of age in preterm children.
Rates of motor impairment increase with more severe WMA; however, 31% of children with no abnormality had a mild to severe motor impairment.
ACKNOWLEDGEMENTS
We acknowledge the contributions of the Victorian Infant Brain Studies team, especially Merilyn Bear. This study was funded by the National Health and Medical Research Council (project grant 237117; senior research fellowship [PJA] 628371), The Royal Women’s Hospital Research Foundation, The Brockhoff Foundation, The Murdoch Childrens Research Institute, and the Cerebral Palsy Alliance Fellowship (to AJS).
ABBREVIATIONS
- MABC
Movement Assessment Battery for Children
- WMA
White matter abnormalities
REFERENCES
- 1.Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol. 2008;50:334–340. doi: 10.1111/j.1469-8749.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- 2.Williams JW, Lee KJ, Anderson PJ. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52:232–237. doi: 10.1111/j.1469-8749.2009.03544.x. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn, Text Revision, (DSM-IV-TR) Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 4.Jongmans M, Mercuri E, de Vries L, Dubowitz L, Henderson SE. Minor neurological signs and perceptual-motor difficulties in prematurely born children. Arch Dis Child Fetal Neonatal Ed. 1997;76:F9–F14. doi: 10.1136/fn.76.1.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salt A, D’Amore A, Ahluwalia J, et al. Outcome at 2 years for very low birthweight infants in a geographical population: risk factors, cost, and impact of congenital anomalies. Early Hum Dev. 2006;82:125–133. doi: 10.1016/j.earlhumdev.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Schmidhauser J, Caflisch J, Rousson V, Bucher HU, Largo RH, Latal B. Impaired motor performance and movement quality in very-low-birthweight children at 6 years of age. Dev Med Child Neurol. 2006;48:718–722. doi: 10.1017/S001216220600154X. [DOI] [PubMed] [Google Scholar]
- 7.Beauchamp MH, Thompson DK, Howard K, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- 8.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 9.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 10.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 11.Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 12.Kurdahi Badr L, Bookheimer S, Purdy I, Deeb M. Predictors of neurodevelopmental outcome for preterm infants with brain injury: MRI, medical and environmental factors. Early Hum Dev. 2009;85:279–284. doi: 10.1016/j.earlhumdev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boardman JP, Craven C, Valappil S, et al. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. NeuroImage. 2010;52:409–414. doi: 10.1016/j.neuroimage.2010.04.261. [DOI] [PubMed] [Google Scholar]
- 14.Glass HC, Fujimoto S, Ceppi-Cozzio C, et al. White-matter injury is associated with impaired gaze in premature infants. Pediatr Neurol. 2008;38:10–15. doi: 10.1016/j.pediatrneurol.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Roberts G, Howard K, Spittle AJ, Brown NC, Anderson PJ, Doyle LW. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health. 2008;44:276–280. doi: 10.1111/j.1440-1754.2007.01251.x. [DOI] [PubMed] [Google Scholar]
- 17.Treyvaud K, Anderson VA, Howard K, et al. Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics. 2009;123:555–561. doi: 10.1542/peds.2008-0477. [DOI] [PubMed] [Google Scholar]
- 18.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 19.Cheong JLY, Hunt RW, Anderson PJ, et al. Head growth in preterm infants: Correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics. 2008;121:e1534–e1540. doi: 10.1542/peds.2007-2671. [DOI] [PubMed] [Google Scholar]
- 20.Henderson SE, Sugden DA. Movement Assessment Battery for Children (Movement ABC) London: Harcourt Assessment; 1992. [Google Scholar]
- 21.Rickards AL, Kitchen WH, Doyle LW, Kelly EA. Correction of developmental and intelligence test scores for premature birth. Aust Paediatr J. 1989;25:127–129. doi: 10.1111/j.1440-1754.1989.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 22.Kirkwood B, Sterne JAC. Essential Medical Statistics. Massachusetts: Blackwell Science; 2003. [Google Scholar]
- 23.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 24.Dewey D, Kaplan BJ, Crawford SG, Wilson BN. Developmental coordination disorder: associated problems in attention, learning, and psychosocial adjustment. Hum Mov Sci. 2002;21:905–918. doi: 10.1016/s0167-9457(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 25.Miyahara M, Piek J. Self-esteem of children and adolescents with physical disabilities: quantitative evidence from meta-analysis. J Dev Phys Disabil. 2006;18:219–234. [Google Scholar]
- 26.Skinner RA, Piek JP. Psychosocial implications of poor motor coordination in children and adolescents. Hum Mov Sci. 2001;20:73–94. doi: 10.1016/s0167-9457(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 27.Orton J, Spittle A, Doyle L, Anderson P, Boyd R. Do early intervention programmes improve cognitive and motor outcomes for preterm infants after discharge? A systematic review. Dev Med Child Neurol. 2009;51:851–859. doi: 10.1111/j.1469-8749.2009.03414.x. [DOI] [PubMed] [Google Scholar]
- 28.Spittle AJ, Orton J, Doyle LW, Boyd R. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev. 2007;2:CD005495. doi: 10.1002/14651858.CD005495.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Taub E, Ramsey SL, DeLuca S, Echols K. Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics. 2004;113:305–312. doi: 10.1542/peds.113.2.305. [DOI] [PubMed] [Google Scholar]
- 30.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamrick SE, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004;145:593–599. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Roberts G, Anderson PJ, Doyle LW the Victorian Infant Collaborative Study Group. The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very preterm children born in 1997. Arch Dis Child. 2010;95:786–790. doi: 10.1136/adc.2009.160283. [DOI] [PubMed] [Google Scholar]