Abstract
Agonist-stimulated internalization followed by recycling to the cell membrane play an important role in fine-tuning the activity of chemokine receptors. Because the recycling of chemokine receptors is critical for the reestablishment of the cellular responsiveness to ligand, it is crucial to understand the mechanisms underlying the receptor recycling and resensitization. In the present study, we have demonstrated that the chemokine receptor CXCR2 associated with myosin Vb and Rab11-family interacting protein 2 (FIP2) in a ligand-dependent manner. Truncation of the C-terminal domain of the receptor did not affect the association, suggesting that the interactions occur upstream of the C terminus of CXCR2. After ligand stimulation, the internalized CXCR2 colocalized with myosin Vb and Rab11-FIP2 in Rab11a-positive vesicles. The colocalization lasted for ∼2 h, and little colocalization was observed after 4 h of ligand stimulation. CXCR2 also colocalized with myosin Vb tail or Rab11-FIP2 (129–512), the N-terminal–truncated mutants of myosin Vb and Rab11-FIP2, respectively, but in a highly condensed manner. Expression of the enhanced green fluorescent protein-tagged myosin Vb tail significantly retarded the recycling and resensitization of CXCR2. CXCR2 recycling was also reduced by the expression Rab11-FIP2 (129–512). Moreover, expression of the myosin Vb tail reduced CXCR2- and CXCR4-mediated chemotaxis. These data indicate that Rab11-FIP2 and myosin Vb regulate CXCR2 recycling and receptor-mediated chemotaxis and that passage of internalized CXCR2 through Rab11a-positive recycling system is critical for physiological response to a chemokine.
INTRODUCTION
Chemokine receptors belong to the large family of seven-transmembrane G protein-coupled receptors (GPCRs) that function in immune and inflammatory response by regulating the activation and migration of leukocytes, immune cell development, and angiogenesis (Nagasawa et al., 1996; Luster 1998; Belperio et al., 2000; Murphy et al., 2000; Zlotnik and Yoshie, 2000). Some of them (e.g., CCR5 and CXCR4) participate in HIV infection of CD4+ T lymphocytes as coreceptors (Berger et al., 1999). Ligand binding to the chemokine receptors triggers various signaling cascades, including activation of G proteins, phosphotidylinositol 3-kinase, Janus kinase/signal transducers and activators of transcription proteins, the Rho-p160 ROCK axis, and the MAPK pathway (Wu et al., 1993; Ganju et al., 1998; Mellado et al., 1998; Vicente-Manzanares et al., 1999; Vicente-Manzanares et al., 2002). Chemokine activation of these intracellular signals is often accompanied by chemokine receptor internalization and trafficking back to the cell membrane. The intracellular trafficking of chemokine receptors controls their activities, and the balance between the chemokine receptor recycling and degradation dictates the leukocyte responsiveness to chemokines (Sabroe et al., 1997; Asagoe et al., 1998; Khandaker et al., 1998; Mack et al., 1998).
Ligand stimulated chemokine receptor internalization can be accomplished through the formation of clathrin-coated pits and/or through formation of lipid rafts (Signoret et al., 1997; Yang et al., 1999; Mueller et al., 2002; Venkatesan et al., 2003). The coated pits internalize as coated vesicles and the later fuse to early endosomes after uncoating (Wu et al., 2001). In the early endosomes, the receptors undergo a dephosphorylation by specific protein phosphatases such as protein phosphatase 2A (Fan et al., 2001a). The dephosphorylated receptors are either delivered to recycling endosomes or to late endosomes/lysosomes for degradation, depending upon the presence of the extracellular ligands (Fan et al., 2003). The recycling and degradation rate may vary among different chemokine receptors (Chuntharapai and Kim, 1995; Zaslaver et al., 2001). Although mechanisms underlying ligand-induced receptor endocytosis have been extensively studied, the mechanisms for the recycling of chemokine receptors are still poorly understood. Given the central role of chemokine receptor recycling in a continuous functional response to chemokines, it is critical to identify the processes that control chemokine receptor recycling.
We have previously demonstrated that the internalized chemokine receptor CXCR2 colocalized with transferrin (Fan et al., 2003), suggesting that some, if not all, of the recycling systems for transferrin are used by CXCR2. The recycling of CXCR2 is regulated by Rab11a, a member of the Rab GTPase family that also plays a role in the recycling of transferrin receptor and certain other GPCRs such as the M4 muscarinic receptor (Wang et al., 2000; Volpicelli et al., 2002). CXCR2 internalized into Rab11a-positive recycling endosomes, and expression of a dominant negative mutant of Rab11a (Rab11a-S25N) inhibited the recycling of CXCR2 (Fan et al., 2003). The molecular mechanisms by which Rab11a performs its regulatory role are still poorly understood. Previous studies have identified a number of Rab11a interacting proteins, including myosin Vb (Lapierre et al., 2001), Rab11-binding protein/Rabphilin 11 (Mammoto et al., 1999; Zeng et al., 1999), and a group of Rab11-family interacting proteins (Rab11-FIPs) such as pp75/Rip11, Rab11-FIP1, Rab11-FIP2, Rab11-FIP3, Rab11-FIP4, and Rab-coupling protein (Prekeris et al., 2000; Hales et al., 2001; Lindsay et al., 2002; Wallace et al., 2002). Among these Rab11 interacting proteins, myosin Vb and Rab11-FIP2 are of particular interest because both proteins associate with each other and Rab11a (Lapierre et al., 2001; Hales et al., 2002). Expression of green fluorescent protein (GFP)-myosin Vb tail, which lacks the motor domain, retarded the transferrin recycling and caused concentration of transferrin receptors in pericentrosomal vesicles (Lapierre et al., 2001). Also, expression of the myosin Vb tail induced a concentration of M4 muscarinic acetylcholine receptors in perinuclear endosomes and impaired receptor recycling (Volpicelli et al., 2002). Rab11-FIP2 also plays a role in the recycling of transferrin receptor and IgA receptor (Hales et al., 2002). Expression of a truncation mutant of Rab11-FIP2, lacking its amino terminal C2-domain [Rab11-FIP2 (129–512)], retarded plasma membrane recycling (Hales et al., 2002). Because the mechanisms that control transferrin receptor recycling do not generalize to all GPCRs (Cao et al., 1999), and because different GPCR subtypes exhibit distinct intracellular trafficking pathways (Trejo and Coughlin, 1999; Anborgh et al., 2000; Krudewig et al., 2000; Tsao and von Zastrow, 2000; Innamorati et al., 2001), it is crucial to characterize the recycling mechanisms for the chemokine receptors. In this article, we demonstrate that myosin Vb and Rab11-FIP2 coimmunoprecipitated with the chemokine receptor CXCR2 in a ligand-dependent manner. The internalized CXCR2 colocalized with myosin Vb and Rab11-FIP2 in the Rab11a-positive recycling system. However, expression of either the myosin Vb tail or Rab11-FIP2 (129–512) resulted in condensation of CXCR2 in Rab11a-positive vesicles and retardation of the receptor recycling. We provide evidence that the recycling of CXCR2 is required for the receptor resensitization and the receptor-mediated chemotaxis.
MATERIALS AND METHODS
Plasmids
The plasmids of CXCR2, 331T, IL323,324/AA, enhanced green fluorescent protein (EGFP)-Rab11a, full-length EGFP-myosin Vb, EGFP-myosin Vb tail, full-length EGFP-Rab11-FIP2, EGFP-Rab11-FIP2 (129–512), and DsRed2-myosin Vb tail were constructed as described previously (Mueller et al., 1995; Fan et al., 2001b; Lapierre et al., 2001; Hales et al., 2002).
Cell Culture and Transfection
Human embryonic kidney (HEK)293 cells and rat basophilic leukemia (RBL)-2H3 cells were both grown in DMEM, containing 10% fetal bovine serum and a 1:100 dilution of penicillin/streptomycin, at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were transfected with plasmid containing CXCR2 by using LipofectAMINE Plus reagent (Invitrogen, Carlsbad, CA). Stably transfected cells were selected with 560 μg/ml Geneticin (G418) and evaluated for receptor expression by using 125I-CXCL1 (#NEX-321; PerkinElmer Life Sciences, Boston, MA) binding assay. The stable cell lines were transfected with plasmids for EGFP-myosin Vb, EGFP-Rab11-FIP2, or DsRed2-myosin Vb tail by using LipofectAMINE Plus reagent (Invitrogen) in serum-free medium for 3 h and recovered in serum-containing medium for 24 h at which time GFP fluorescence was present in >80% of HEK293 cells and >20% of RBL-2H3 cells.
Confocal Visualization of Agonist-induced Internalization of CXCR2
Confocal microscopy was performed on an LSM-410 laser scanning microscope (Carl Zeiss, Thornwood, NY) by using a 63× 1.3 numerical aperture oil immersion lens (Carl Zeiss). HEK293 cells stably expressing CXCR2 were transfected with plasmid for EGFP-tagged myosin Vb or EGFP-Rab11-FIP2. HEK293 cells stably expressing CXCR2 were cotransfected with EGFP-Rab11a and DsRed2-myosin Vb tail. HEK293 cells stably expressing CXCR2 were transfected with plasmid for EGFP-tagged myosin Va tail. RBL-2H3 cells were cotransfected with plasmids for CXCR2 and DsRed2-myosin Vb tail. Cells were treated with carrier buffer or CXCL1 for various time intervals and fixed with methanol. Cells were washed with phosphate-buffered saline (PBS) and incubated with an antibody mixture containing a mouse monoclonal CXCR2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and a rabbit Rab11a antibody (Zymed Laboratories, South San Francisco, CA) for 30 min. Cells were washed and incubated with a antibody mixture containing a Cy3-conjugated anti-mouse antibody (Molecular Probes, Eugene, OR) and a Cy5-conjugated anti-rabbit antibody (Molecular Probes) for 30 min. Confocal microscopy was performed using dual excitation (488 nm for EGFP, 568 nm for Cy3, and 647 nm for Cy5) and emission (515–540 nm for EGFP, 590–610 nm for Cy3, and 660–685 for Cy5) filter sets. Quantification for the colocalization of myosin Vb or Rab11-FIP2 with CXCR2 was performed in 15 fields at each time point by using the MetaMorph Imaging System (Universal Imaging, Downingtown, PA). In the quantification, only the cells expressing both CXCR2 and either myosin Vb or Rab11-FIP2 were counted. We calculated the percentage of EGFP-myosin Vb or EGFP-Rab11-FIP2 colocalized with CXCR2.
Fluorescence-activated Cell Sorting (FACS) Analysis
HEK293 cells stably expressing CXCR2 were transfected with plasmids for the wild-type or mutant myosin Vb and Rab11-FIP2 as indicated. Cells were incubated in HEPES (20 mM)-buffered DMEM at 37°C for 30 min in the presence or absence of CXCL1 (200 ng/ml). Cells were washed in ice-cold medium followed by continued incubation in ligand-free medium at 37°C for 60 min. Cells were incubated with a monoclonal phycoerythrin-conjugated CXCR2 antibody (BD PharMingen, San Diego, CA) at 4°C for 60 min. Cells were washed and fixed in 2% formaldehyde in phosphate-buffered saline and analyzed in FACScan equipped with CellQuest software (BD Biosciences, San Jose, CA).
Coimmunoprecipitation and Western Blot
For the coimmunoprecipitation of CXCR2 with EGFP-myosin Vb or EGFP-Rab11-FIP2, HEK293 cells stably expressing CXCR2 were transfected with plasmids for EGFP-tagged myosin Vb or EGFP-Rab11-FIP2. Cells were treated with CXCL1 (200 ng/ml) for various time intervals, washed three times with ice-cold PBS, and lysed in 1 ml of immunoprecipitation buffer containing PBS (pH.7.0), 0.1% sodium deoxylcholate, 0.01% SDS, and 1% NP-40. The cell debris was removed by centrifugation (15,000 × g, 15 min). The supernatant was precleared by incubation with 40 μl of protein A/G agarose (Pierce Chemical, Rockford, IL) for 1 h at 4°C to reduce nonspecific binding. After removing the protein A/G agarose by centrifugation (15,000 × g, 1 min), the cleared supernatant was collected and 10 μl of mouse monoclonal anti-CXCR2 antibody (Santa Cruz Biotechnology) was added for overnight precipitation at 4°C. Protein A/G (40 μl) was then added and incubation was continued at 4°C for 2 h. The protein A/G–antibody–antigen complex was then collected by washing three times with ice-cold immunoprecipitation buffer. The final pellet was resuspended in 40 μl of SDS sample buffer containing 5% β-mercaptoethanol and heated to 50°C for 10 min. Forty microliters of this preparation was separated by 10% SDS-PAGE, and the proteins on the gel were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The coprecipitated EGFP-tagged myosin Vb or Rab11-FIP2 was detected by Western blotting by using a rabbit specific anti-GFP antibody (Santa Cruz Biotechnology). For the coimmunoprecipitation of CXCR2 with the endogenous Rab11-FIP2, HEK293 cells stably expressing CXCR2 were treated with or without CXCL1 (200 ng/ml) for 60 min and then lysed in the immunoprecipitation buffer. CXCR2 was immunoprecipitated as described above, and coprecipitated Rab11-FIP2 was detected by Western blotting.
Chemotaxis Assay
A 96-well chemotaxis chamber (Neuroprobe, Gaithersburg, MD) was used for chemotaxis assays, and the lower compartment of the chamber was loaded with 400-μl aliquots of 1 mg/ml ovalbumin/DMEM (chemotaxis buffer) or CXCL1 diluted in the chemotaxis buffer (1–250 ng/ml). Polycarbonate membranes (10-μm pore size) were coated on both sides with 20 μg/μl human collagen type IV, incubated for 2 h at 37°C, and then stored at 4°C overnight. To prepare HEK293 cells for chemotaxis assay, cells were removed from the culture dish by trypsinization, washed with Hanks' solution, and incubated in 10% fetal bovine serum/DMEM for 2 h at 37°C to allow time for restoration of receptors. Cells (5 × 105 in 100 μl) were loaded into the top of each well of a 96-well chemotaxis chamber. The bottom of each well contained 600 μl of prewarmed chemotaxis buffer with different concentrations of ligand. The plate was then incubated for 240 min at 37°C in a 5% CO2 atmosphere, and cells migrating to the lower chamber were counted after being stained with a Diff-Quik kit. Cell chemotaxis was quantified by counting the number of migrating cells present in 10 microscope fields (20× objective).
Intracellular Calcium Mobilization Assay
HEK293 cells stably expressing CXCR2 were transfected with plasmids for vector, full-length myosin Vb, or myosin Vb tail. Cells were released by shaking, collected by centrifugation at 300 × g for 5 min and washed with incubation buffer (Hanks' buffer containing 5 mM HEPES). Cells were resuspended at 2 × 106 cells/ml and incubated with 2.5 μM Fluo-3 (Molecular Probes) for 30 min at 37°C. After incubation, the cells were washed once with the incubation buffer containing 2 mM CaCl2. The cells were finally adjusted to 2 × 106 cells/ml. Cells were stimulated with CXCL1 (200 ng/ml) and intracellular Ca2+ mobilization experiments were performed as described previously. For the receptor desensitization assay, cells were incubated with CXCL1 (200 ng/ml) for 30 min while loading with Fluo-3. The ligand was removed by repeated wash of the cells, and CXCL1 (200 ng/ml)-induced intracellular Ca2+ mobilization was measured. For the receptor resensitization assay, cells were incubated with CXCL1 (200 ng/ml) for 30 min, followed by repeated wash with ligand-free incubation buffer, and the recovery of the cells was allowed in the reaction buffer for 60 min, before being loaded with Fluo-3. CXCL1-induced Ca2+ mobilization was measured as described previously (Fan et al., 2001b). To compare the receptor resensitization between the control cells, cells expressing full-length myosin Vb, and cells expressing myosin Vb tail, the time taken to recover 80% of the mobilized Ca2+ (t0.80) (Fan et al., 2001b) and the potency of the Ca2+ mobilization (peak Ca2+ concentration – basal Ca2+ concentration) were calculated.
RESULTS
Colocalization of CXCR2 with Myosin Vb in Rab11a-positive Vesicles
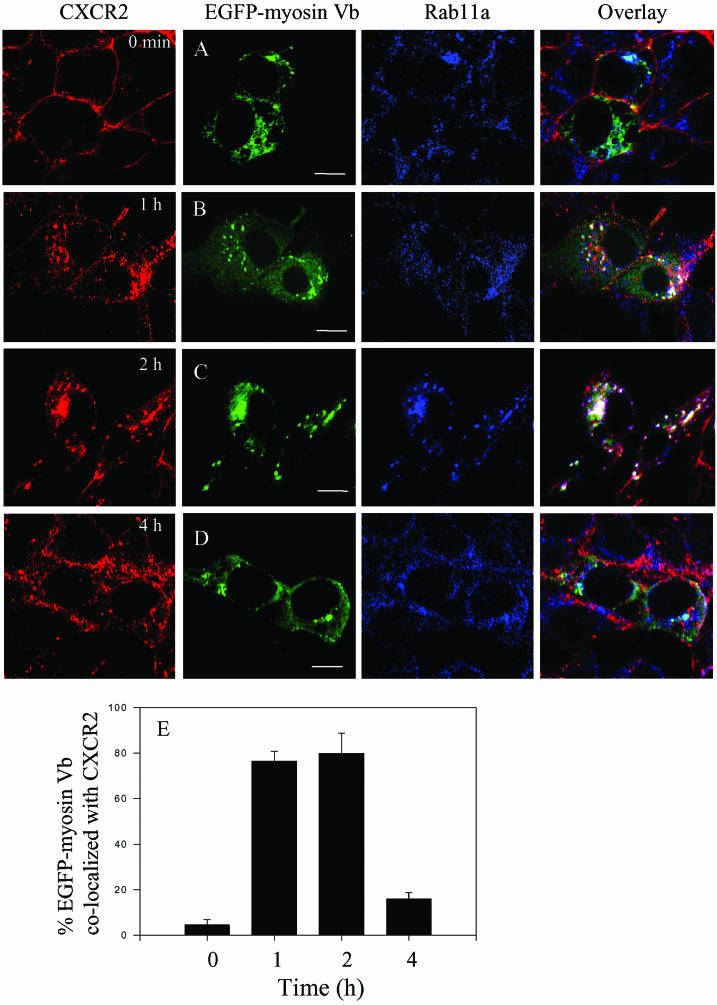
We have demonstrated that CXCR2 was internalized into Rab11a-positive vesicles in response to ligand stimulation (Fan et al., 2003). Encouraged by the recent findings that the membrane recycling system was regulated by myosin Vb (Lapierre et al., 2001), a Rab11a interacting protein, we sought to examine whether CXCR2 colocalized with myosin Vb in Rab11a-positive vesicles after ligand stimulation. HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for EGFP-tagged myosin Vb. Cells were treated with CXCL1 (200 ng/ml) for different lengths of time (0–240 min), and the subcellular localization of CXCR2, EGFP-myosin Vb, and Rab11a was visualized by confocal microscopy. As shown in Figure 1, before exposure of the cells to ligand, CXCR2 was expressed predominantly on the cell surface, whereas myosin Vb and Rab11a were in the internal vesicles (Figure 1A). In response to CXCL1 stimulation for 60 min, CXCR2 was mostly internalized and partially colocalized with both Rab11a and myosin Vb (Figure 1B). The colocalization of CXCR2 with myosin Vb in Rab11a-positive vesicles lasted for ∼2 h (Figure 1C). However, continued ligand treatment resulted in the reduction of the colocalization of CXCR2 with myosin Vb and Rab11a. After 4-h exposure to ligand, little colocalization between CXCR2 and myosin Vb was observed (Figure 1D). Using the MetaMorph imaging system, we quantified the percentage of the EGFP-myosin Vb colocalized with CXCR2. Approximately 80% of the EGFP-myosin Vb colocalized with the internalized CXCR2 (Figure 1E) during the peak colocalization (1–2 h after ligand stimulation). The percentage of EGFP-myosin Vb colocalized with CXCR2 was remarkably reduced to ∼15% after prolonged ligand treatment (4 h) (Figure 1E). This is consistent with our previous data showing the reduction of CXCR2 colocalization with Rab11a after 4 h of ligand treatment (Fan et al., 2003). We have hypothesized that the recycling of CXCR2 is blocked in the continued presence of ligand. The receptors are transported to late endosomes, because an increased colocalization of the receptor with Rab7, a late endosome marker, was observed during this period of time (4 h) (Fan et al., 2003).
Figure 1.
Agonist-dependent colocalization of CXCR2 with myosin Vb in Rab11a-positive endosomes. HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-myosin Vb. Cells were incubated in the absence (A) or presence of CXCL1 (200 ng/ml) at 37°C for 1 h (B), 2 h (C), and 4 h (D), and then fixed with methanol. Cells were incubated with an antibody mixture containing a mouse monoclonal anti-CXCR2 antibody (1:50 diluted) and a rabbit anti-Rab11a (1:50 diluted) antibody at room temperature for 30 min, followed by incubation with a mixture of secondary antibodies containing a Cy3-conjugated anti-mouse antibody and a Cy5-conjugated anti-rabbit antibody at room temperature for 30 min. Representative laser-scanning confocal micrographs from three independent experiments demonstrating the distribution of CXCR2 (red), EGFP-myosin Vb (green), and Rab11a (blue) are shown. The colocalization between CXCR2, EGFP-myosin Vb, and Rab11a was shown in white color. Images were processed using Photoshop software (Adobe Systems, San Jose, CA). Bars, 10 μm. Quantification of the percentage of the EGFP-myosin Vb colocalized with CXCR2 (E) from 15 fields was performed using the MetaMorph Imaging system (Universal Imaging).
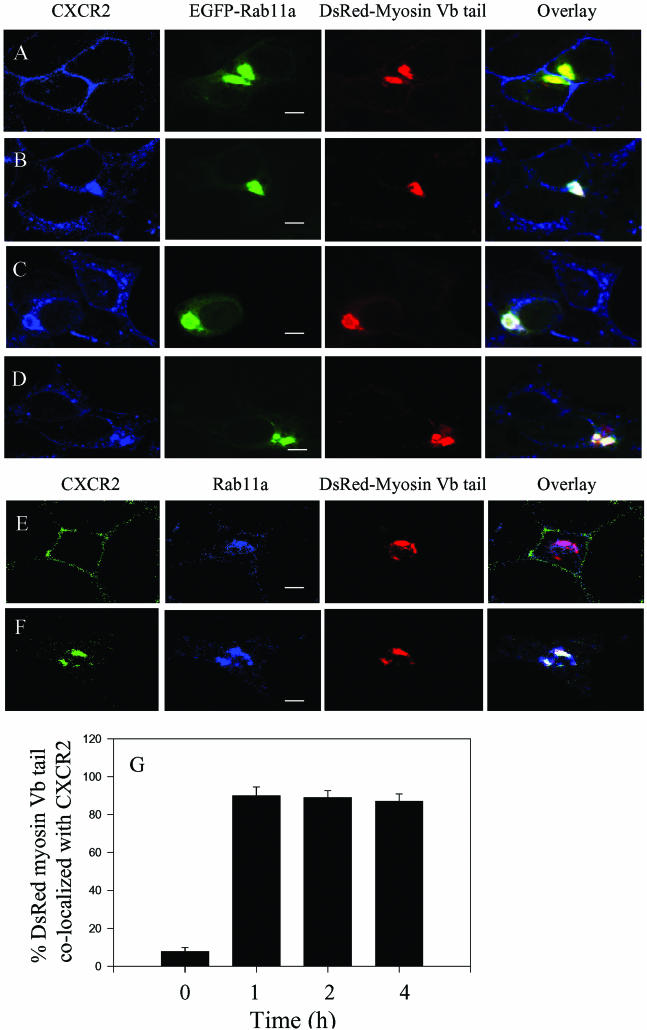
Previous studies have shown that expression of myosin Vb tail induced condensation of Rab11a in the perinuclear recycling endosomal compartments (Lapierre et al., 2001). To test whether expression of the myosin Vb tail results in concentration of CXCR2 in Rab11a-positive vesicles, HEK293 cells stably expressing CXCR2 were cotransfected with EGFP-Rab11a and DsRed2-myosin Vb tail. The subcellular localization of CXCR2, myosin Vb tail and Rab11a was visualized by confocal microscopy after ligand stimulation. As shown in Figure 2A, expression of the myosin Vb tail resulted in condensation of Rab11a, consistent with the previous reports in other cell types (Lapierre et al., 2001; Volpicelli et al., 2002). In response to CXCL1 treatment, CXCR2 was internalized and codistributed with the EGFP-Rab11a and the DsRed2-myosin Vb tail in a highly condensed manner (Figure 2, B–D). However, unlike the colocalization of CXCR2 with the full-length myosin Vb, which is reduced after prolonged ligand treatment (4 h), CXCR2 exhibited constant colocalization with myosin Vb tail and Rab11a even after 4 h of ligand treatment (Figure 2D). We quantified the percentage of the DsRed-myosin Vb tail colocalized with CXCR2. Approximately 90% of DsRed-myosin Vb tail were colocalized with CXCR2 after ligand treatment for 1, 2, and 4 h (Figure 2G). To determine whether a similar phenomenon can be observed in other cell types, we examined the colocalization of CXCR2 with DsRed2-myosin Vb tail and the endogenous Rab11a in RBL-2H3 cells, a cell line that is widely used for the signaling and trafficking of chemokine receptors. As shown in Figure 2, E and F, expression of the DsRed2-myosin Vb tail in RBL-2H3 cells also resulted in colocalization of CXCR2 with Rab11a and the myosin Vb in a highly condensed manner. In contrast to the results with the myosin Vb tail, GFP-myosin Va tail expressed in HEK293 cells showed no effect on the distribution of CXCR2 (our unpublished data), suggesting the specific involvement of myosin Vb in CXCR2 trafficking.
Figure 2.
Colocalization of CXCR2 with the myosin Vb tail in Rab11a-positive vesicles. HEK293 cells (A–D) or RBL-2H3 cells (E and F) stably expressing CXCR2 were transiently transfected with plasmids of DsRed2-myosin Vb tail and EGFP-Rab11a (A–D) or with plasmid of DsRed2-myosin Vb tail (E and F). Cells were treated with CXCL1 (200 ng/ml) at 37°C for various time intervals as indicated and then fixed with methanol. Cells were incubated with a rabbit CXCR2 antibody (1:50 diluted) (A–D) or an antibody mixture containing a mouse monoclonal anti-CXCR2 antibody (1:50 diluted) and a rabbit anti-Rab11a antibody (1:50 diluted) (E and F) at room temperature for 30 min. After washing, the cells were incubated with a Cy5 conjugated anti-rabbit antibody (A–D) or a mixture of secondary antibodies containing a fluorescein isothiocyanate-conjugated anti-mouse antibody and a Cy5-conjugated anti-rabbit antibody at room temperature for 30 min. Representative laser-scanning confocal micrographs from three independent experiments demonstrating the distribution of CXCR2 (blue), DsRed2-myosin Vb tail (red), and EGFP-Rab11a (green) in HEK293 cells, or the distribution of CXCR2 (green), DsRed-myosin Vb tail (red), and endogenous Rab11a (blue) in RBL-2H3 cells are shown. The colocalization between CXCR2, DsRed-myosin Vb tail, and Rab11a is shown in white. Images were processed using Photoshop software (Adobe Systems). Bars, 10 μm. Quantification of the percentage of the DsRed-myosin Vb tail colocalized with CXCR2 from 15 fields in HEK293 cells was performed using the MetaMorph Imaging system (G).
Colocalization of CXCR2 with Rab11-FIP2 in Rab11a-positive Vesicles
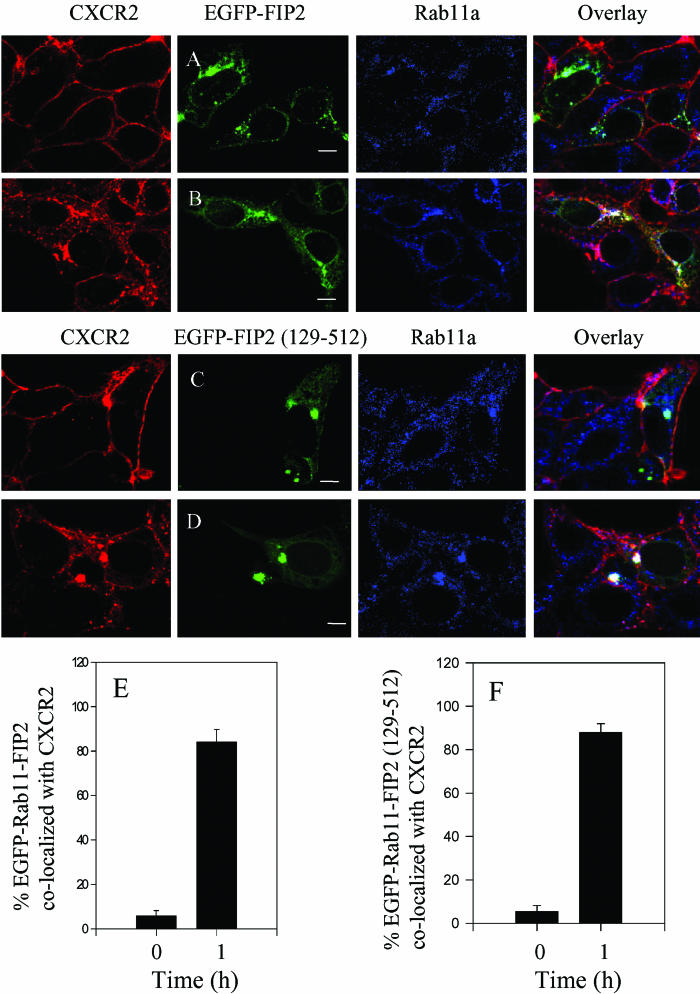
Rab11-FIP2 interacts with Rab11a and myosin Vb and plays a role in plasma membrane recycling (Hales et al., 2001, 2002). To determine whether the internalized CXCR2 colocalizes with Rab11-FIP2 in the recycling system, we transfected HEK293 cells stably expressing CXCR2 with a construct of EGFP-Rab11-FIP2, treated the cells with CXCL1, and visualized the subcellular localization of CXCR2, Rab11-FIP2, and Rab11a by confocal microscopy. As shown in Figure 3A, before ligand stimulation, CXCR2 was expressed predominantly on the cell surface, whereas Rab11-FIP2 was in the internal vesicles. In response to CXCL1 stimulation for 60 min, CXCR2 was mostly internalized and colocalized with both Rab11-FIP2 and Rab11a (Figure 3B). We observed that after prolonged ligand treatment (4 h), the colocalization of CXCR2 with Rab11-FIP2 and Rab11a was remarkably reduced (our unpublished data), consistent with the results for the colocalization of CXCR2 with myosin Vb (Figure 1). The percentage of EGFP-Rab11-FIP2 colocalized with CXCR2 was quantified as described above. Approximately 80% of EGFP-Rab11-FIP2 colocalized with CXCR2 after ligand treatment for 60 min, whereas only ∼5% of EGFP-Rab11-FIP2 colocalized with CXCR2 before ligand stimulation (Figure 3E). We also examined the colocalization of CXCR2 with EGFP-Rab11-FIP2 (129–512), which lacks its amino-terminal C2 domain and functions as a dominant negative acting truncation that causes accumulation of Rab11a and disrupts IgA trafficking in Madin-Darby canine kidney cells and transferrin trafficking in HeLa cells (Hales et al., 2002). As shown in Figure 3C, before ligand stimulation, CXCR2 was expressed on the cell surface, whereas the EGFP-Rab11-FIP2 (129–512) was concentrated and colocalized with Rab11a in the perinuclear compartments. After ligand stimulation for 60 min, the internalized CXCR2 colocalized with Rab11-FIP2 (129–512) and Rab11a in a highly condensed manner (Figure 3D). Approximately 80% of EGFP-Rab11-FIP2 (129–512) colocalized with CXCR2 after ligand treatment for 60 min. In contrast, only ∼5% of EGFP-Rab11-FIP2 (129–512) colocalized with the receptor before ligand stimulation (Figure 3E). These data suggest the involvement of Rab11-FIP2 in CXCR2 trafficking.
Figure 3.
Agonist-dependent colocalization of CXCR2 with the wild type and mutant Rab11-FIP2 in Rab11a-positive vesicles. HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-tagged Rab11-FIP2 (A and B) or Rab11-FIP2 (129–512) (C and D). Cells were treated with (B and D) or without (A and C) CXCL1 (200 ng/ml) at 37°C for 60 min and then fixed with methanol. Cells were incubated with an antibody mixture containing a mouse monoclonal anti-CXCR2 antibody (1:50 diluted) and a rabbit anti-Rab11a (1:50 diluted) antibody at room temperature for 30 min, followed by incubation with a mixture of secondary antibodies containing a Cy3-conjugated anti-mouse antibody and a Cy5-conjugated anti-rabbit antibody at room temperature for 30 min. Representative laser-scanning confocal micrographs from three independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab11-FIP2 (green), or EGFP-Rab11-FIP2 (129–512) (green), and Rab11a (blue) are shown. The colocalization between CXCR2 and EGFP-Rab11-FIP2, or EGFP-Rab11-FIP2 (129–512) and Rab11a is shown in white. Images were processed using Photoshop software (Adobe Systems). Bars, 10 μm. Quantification of the percentage of the EGFP-Rab11-FIP2 (E) or EGFP-Rab11-FIP2 (129–512) (F) colocalized with CXCR2 from 15 fields in HEK293 cells was performed using the MetaMorph Imaging system.
Association of CXCR2 with Myosin Vb and Rab11-FIP2
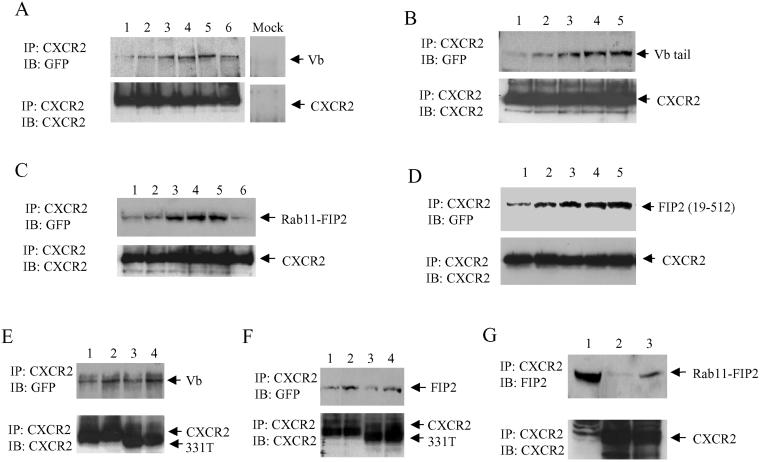
Because of the colocalization of CXCR2 with myosin Vb and Rab11-FIP2 in Rab11a-positive vesicles, we sought to determine whether myosin Vb and Rab11-FIP2 associate with CXCR2. To address this question, we immunoprecipitated CXCR2 from the cell lysate of HEK293 cells stably expressing CXCR2 and transiently expressing either EGFP-myosin Vb or EGFP-Rab11-FIP2. Coprecipitated EGFP-myosin Vb and EGFP-Rab11-FIP2 were determined by Western blotting. As shown in Figure 4, A and C, CXCR2 associated with myosin Vb or Rab11-FIP2 in response to ligand treatment in a time-dependent manner, which peaked with 60 min of ligand stimulation, and the association decreased after 2 h of incubation. By comparing the density of the immunoblots for myosin Vb and Rab11-FIP2 coprecipitated with CXCR2 from 1 ml of cell lysate and the immunoblots of myosin Vb and Rab11-FIP2 in 40 μl of cell lysate, we estimated that ∼4% myosin Vb or Rab11-FIP2 were coprecipitated with CXCR2 (our unpublished data). We also determined whether myosin Vb tail and Rab11-FIP2 coimmunoprecipitate with CXCR2. CXCR2 was immunoprecipitated from the cell lysate of HEK293 cells stably expressing CXCR2 and transiently expressing either EGFP-myosin Vb tail or EGFP-Rab11-FIP2 (129–512). Coprecipitated EGFP-myosin Vb tail and EGFP-Rab11-FIP2 (129–512) were determined by Western blotting. As shown in Figure 4, B and D, CXCR2 associated with myosin Vb tail or Rab11-FIP2 (129–512) in the same time course as the receptor association with the full-length myosin Vb or Rab11-FIP2. To determine whether CXCR2 associates with the endogenous Rab11-FIP2, we treated HEK293 cells stably expressing CXCR2 with or without CXCL1 for 60 min, immunoprecipitated CXCR2 from the cell lysate, subjected the precipitate to SDS-PAGE under reducing conditions, and detected the coprecipitated Rab11-FIP2 by Western blotting by using a specific Rab11-FIP2 antibody. Consistent with the above-mentioned results, CXCR2 associated with the endogenous Rab11-FIP2 in an agonist-dependent manner (Figure 4G). However, we did not observe a ligand-dependent association of CXCR2 with pp75/Rip11 (our unpublished data). These data indicate a ligand-dependent interaction between CXCR2 and Rab11-FIP2/myosin Vb complex.
Figure 4.
Coimmunoprecipitation of myosin Vb and Rab11-FIP2 with CXCR2. (A) HEK293 cells stably expressing CXCR2 were transfected with the plasmid for EGFP-myosin Vb. Cells were exposed to CXCL1 (200 ng/ml) for 0 min (lane 1), 15 min (lane 2), 30 min (lane 3), 60 min (lane 4), 120 min (lane 5), or 240 min (lane 6). CXCR2 was immunoprecipitated from the cell lysate by using a mouse monoclonal CXCR2 antibody. A preimmune mouse serum was used as a negative control (mock). Proteins were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. Coprecipitated EGFP-myosin Vb was detected using a rabbit polyclonal anti-GFP antibody (top). The membrane was stripped and reblotted with a rabbit CXCR2 antibody to confirm equal loading (bottom). (B) HEK293 cells stably expressing CXCR2 were transfected with the plasmid for EGFP-myosin Vb tail. Cells were exposed to CXCL1 (200 ng/ml) for 0 min (lane 1), 15 min (lane 2), 30 min (lane 3), 60 min (lane 4), and 120 min (lane 5). CXCR2 was immunoprecipitated from the cell lysate and the coprecipitated EGFP-myosin Vb tail was detected by Western blotting as described above. (C) HEK293 cells stably expressing CXCR2 were transfected with the plasmid for EGFP-Rab11-FIP2. Cells were exposed to CXCL1 (200 ng/ml) for 0 min (lane 1), 15 min (lane 2), 30 min (lane 3), 60 min (lane 4), 120 min (lane 5), or 240 min (lane 6). CXCR2 was immunoprecipitated from the cell lysate as described above. Coprecipitated EGFP-Rab11-FIP2 was detected using a rabbit polyclonal anti-GFP antibody (top). The membrane was stripped and reblotted with a mouse monoclonal anti-CXCR2 antibody to confirm equal loading (bottom). (D) HEK293 cells stably expressing CXCR2 were transfected with the plasmid for EGFP-Rab11-Fip2 (129–512). Cells were exposed to CXCL1 (200 ng/ml) for 0 min (lane 1), 15 min (lane 2), 30 min (lane 3), 60 min (lane 4), and 120 min (lane 5). CXCR2 was immunoprecipitated from the cell lysate and the coprecipitated EGFP-Rab11-FIP2 (129–512) was detected by Western blotting as described above. (E) HEK293 cells stably expressing wild-type CXCR2 (lanes 1 and 2) or 331T (lanes 3 and 4) were transfected with the plasmid for EGFP-myosin Vb. Cells were incubated with (lanes 2 and 4) or without (lanes 1 and 3) CXCL1 (200 ng/ml) for 60 min. CXCR2 was immunoprecipitated from the cell lysate and the coprecipitated EGFP-myosin Vb was detected by Western blotting as described above. (F) HEK293 cells stably expressing wild-type CXCR2 (lanes 1 and 2) or 331T (lanes 3 and 4) were transfected with plasmids for EGFP-Rab11-FIP2. Cells were treated with (lanes 2 and 4) or without (lanes 1 and 3) CXCL1 (200 ng/ml) for 60 min, and CXCR2 was immunoprecipitated from the cell lysate. Coprecipitated EGFP-Rab11-FIP2 was detected by Western blotting as described above. (G) HEK293 cells stably expressing CXCR2 were treated with (lanes 3) or without (lanes 2) CXCL1 (200 ng/ml) for 60 min. Lysates were made, and CXCR2 immunoprecipitation was performed as described above. Lysate was loaded in one lane of the gel as an immunoblot control (lane 1). The membranes were immunoblotted with an anti-Rab11-FIP2 antibody. The membrane was stripped and reblotted with a mouse monoclonal anti-CXCR2 antibody to confirm equal loading (bottom). The results represent one of three independent experiments. IP, immunoprecipitation; IB, immunoblotting.
CXCR2 undergoes phosphorylation in response to ligand stimulation (Mueller et al., 1995). We have demonstrated previously that a C-terminal truncated mutant of CXCR2–331T, which eliminates all of the phosphorylation sites, exhibited normal internalization in HEK293 cells in the absence of ligand-dependent phosphorylation of the receptor (Fan et al., 2001b). To determine whether CXCR2 phosphorylation is required for the receptor association with Rab11-FIP2 and myosin Vb, HEK293 cells stably expressing CXCR2 or CXCR2–331T were transfected with plasmids for EGFP-myosin Vb or EGFP-Rab11-FIP2. CXCR2 or CXCR2–331T receptors were immunoprecipitated with an antibody raised against the N-terminal domain of the receptor, and coprecipitated EGFP-myosin Vb or EGFP-Rab11-FIP2 was detected by Western blotting. As shown in Figure 4, E and F, myosin Vb and Rab11-FIP2 were coimmunoprecipitated with both the full-length CXCR2 and CXCR2–331T after ligand stimulation. These data indicate that receptor phosphorylation is not required for the association of CXCR2 with myosin Vb and Rab11-FIP2 and that the association of CXCR2 with these proteins does not require the C-terminal domain of the receptor.
Involvement of Myosin Vb and Rab11-FIP2 in CXCR2 Recycling
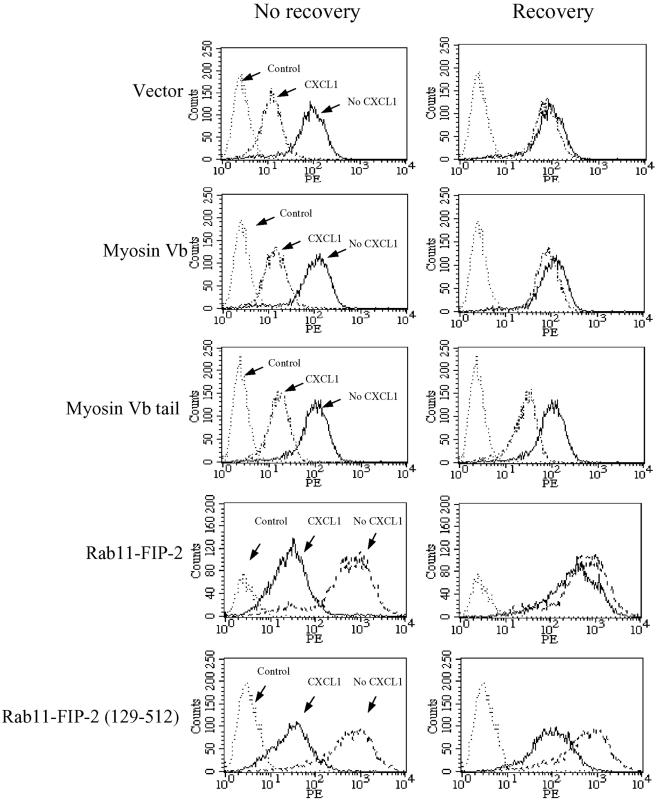
Previous studies in neutrophils and HEK293 cells have shown that internalized CXCR2 is recycled back to the plasma membrane when free-ligand (CXCL1) is removed (Ray and Samanta 1997; Fan et al., 2003). The colocalization of CXCR2 with myosin Vb and Rab11-FIP2 in the Rab11a-positive vesicles suggests that both proteins may play a role in CXCR2 recycling. To test this hypothesis, we transfected HEK293 cells stably expressing CXCR2 with plasmids for vector alone as a control, full-length myosin Vb, myosin Vb tail, full-length Rab11-FIP2, or Rab11-FIP2 (129–512). After CXCL1 exposure for 60 min followed by removal of the ligand and incubation of the cells at 37°C for 60 min, receptor recycling was analyzed by FACS. As shown in Figure 5, without recovery, the internalized receptor levels were comparable among all the cells transfected with different plasmids. After removal of the agonist followed by incubation for 60 min, CXCR2 was gradually reexpressed on the cell membrane. Recovery of receptor expression to 88.6 ± 7.2% was observed after 1 h of incubation in the cells transfected with the vector alone. Overexpression of the full-length myosin Vb or Rab11-FIP2 did not affect the reexpression rate of CXCR2 (88.4 ± 8.5% for myosin Vb and 80.7 ± 9.2% for Rab11-FIP2 after 1 h of recovery). In contrast, the recovery of CXCR2 expression at the membrane was significantly slowed (p < 0.01) in myosin Vb tail- and Rab11-FIP2 (129–512)–expressing cells (36.9 ± 6.8% for myosin Vb tail and 30.6 ± 7.4% for Rab11-FIP2 (129–512) after 1 h of incubation).
Figure 5.
Effect of myosin Vb and Rab11-FIP2 on CXCR2 recycling. HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for vector, full-length myosin Vb, myosin Vb tail, full-length Rab11-FIP2, or Rab11-FIP2 (129–512). Cells were treated with or without CXCL1 (200 ng/ml) at 37°C for 60 min. Cells were washed, followed by continued incubation in ligand-free medium at 37°C for 60 min. For the staining of the cell surface receptor, cells were incubated with a phycoerythrin-conjugated CXCR2 antibody (1:100 dilution) at 4°C for 60 min. HEK293 cells without CXCR2 expression were used as a control. Cells were washed, fixed in 2% formaldehyde in PBS, and analyzed in FACScan. Shown are representatives of three independent experiments with similar results.
Role of Receptor Recycling in the Resensitization of CXCR2
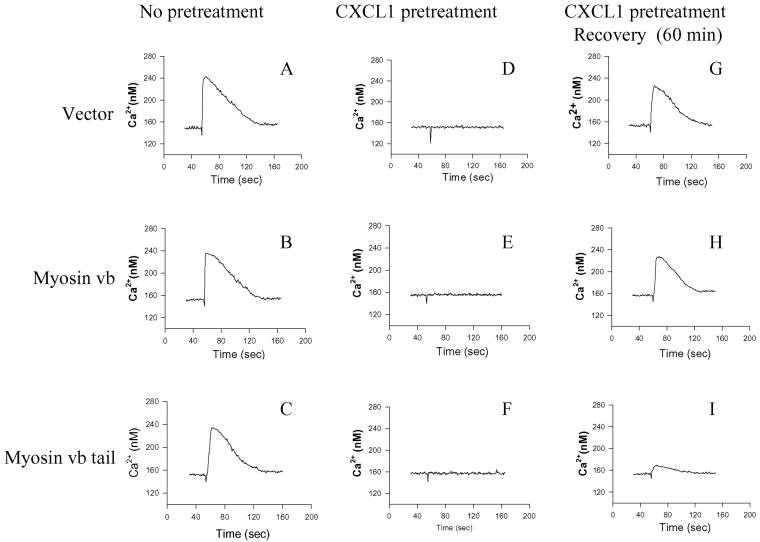
Because receptor recycling is required for the receptor resensitization, a process essential for the reestablishment of the functional responsiveness of the chemokine receptors, we sought to determine whether blocking CXCR2 recycling by overexpressing myosin Vb tail affects the resensitization of CXCR2. HEK293 cells stably expressing CXCR2 were transfected with plasmids for vector alone (control), full-length myosin Vb, or myosin Vb tail. CXCL1-induced Ca2+ mobilization response, and the desensitization and resensitization of CXCR2-mediated Ca2+ mobilization response were determined as described in MATERIAL AND METHODS. Stimulation of the cells with CXCL1 (200 ng/ml) resulted in a similar potency of Ca2+ mobilization response among the control cells, cells expressing full-length myosin Vb, and cells expressing myosin Vb tail (Figure 6, A–C). Preincubation of cells with CXCL1 (200 ng/ml) caused substantial desensitization of CXCR2 in all three groups of cells (Figure 6, D–F). These data suggest that CXCR2 signaling and desensitization are not affected by the expression of full-length myosin Vb or myosin Vb tail. After removal of the ligand and recovery of the cells, the Ca2+ mobilization response was mostly resumed in the cells expressing vector alone (control) and the cells expressing full-length myosin Vb (Figure 6, G and H), with comparable potencies and t0.80 values (potency of 84.66 ± 6.53 and 80 ± 5.54 nM; t0.80 of 72.46 ± 4.67 and 70.57 ± 5.32 s for the control and the full-length myosin Vb-expressing cells, respectively). In contrast, the resumption of CXCR2-mediated Ca2+ mobilization was remarkably reduced in the cells expressing myosin Vb tail (Figure 6I), with a potency of 30.88 ± 2.47 nM and a t0.80 of 34.66 ± 3.75 s. Statistical analysis demonstrated that the resensitization of CXCR2 was significantly reduced by the expression of myosin Vb tail (p < 0.01 compared with the control and full-length myosin Vb-expressing cells, Student's two-tailed t test).
Figure 6.
Effect of myosin Vb on CXCR2 resensitization. HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for vector alone, myosin Vb, or myosin Vb tail. Cells were loaded with Fluo-3 (2.5 μM) for 30 min (A–C) or pretreated with CXCL1 (200 ng/ml) for 30 min while being loaded with Fluo-3 (D–F), or pretreated with CXCL1 (200 ng/ml) for 30 min and recovered for 60 min in ligand-free buffer before being loaded with Fluo-3 (G–I). CXCL1 (200 ng/ml)-induced intracellular Ca2+ mobilization was measured as described under MATERIALS AND METHODS. Shown are representatives of three independent experiments.
Role of Receptor Recycling in CXCR2 and CXCR4-mediated Chemotaxis
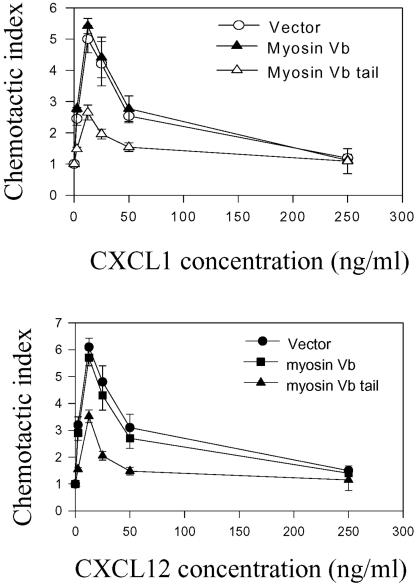
Like other members of the chemokine receptor family, CXCR2 can stimulate cell migration in response to gradient concentrations of ligand (Loetscher et al., 1994; Wolf et al., 1998; Wuyts et al., 1998; Feniger-Barish et al., 2000). Previous studies have demonstrated that vesicle recycling is necessary for cell migration (Bretscher 1992; Hopkins et al., 1994). We examined whether receptor recycling is required for the receptor-mediated chemotaxis. We first compared the random migration of cells expressing either the EGFP-myosin Vb or DsRed-myosin Vb tail by measuring the migration distance of cells over a period of 120 min. No significant difference in the random migration was observed between the full-length myosin Vb and the myosin Vb tail-expressing cells (our unpublished data). We then evaluated the effect of the full-length myosin Vb or the myosin Vb tail on CXCL1-induced chemotaxis. The modified Boyden chamber assay was used in these experiments. As shown in Figure 7A, cells expressing CXCR2 exhibited a typical biphasic response to CXCL1, with a maximal response at 12.5 ng/ml. Overexpression of full-length myosin Vb did not affect the cell chemotaxis, whereas overexpression of the myosin Vb tail significantly reduced the receptor-mediated chemotaxis in comparison with the chemotactic response of the control cells (Figure 7A). To determine whether other chemokine receptor-mediated chemotaxis is regulated by myosin Vb, we cotransfected the cDNAs of CXCR4 and either full-length myosin Vb or myosin Vb tail into HEK293 cells stably expressing CXCR2, and determined CXCL12-induced chemotaxis. As shown in Figure 7B, CXCL12-induced chemotactic response was not affected by the expression of full-length myosin Vb but was significant reduced by the expression of myosin Vb tail. These data suggest that receptor recycling plays an important role in CXCR2-mediated chemotaxis.
Figure 7.
Effect of myosin Vb on CXCR2 and CXCR4-mediated chemotaxis. (A) HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for vector alone, myosin Vb, or myosin Vb tail. Chemotactic responses of the cells to different concentrations of CXCL1 (0–250 ng/ml) were determined as described under MATERIALS AND METHODS. (B) HEK293 cells stably expressing CXCR2 were transiently cotransfected with plasmids for CXCR4 and vector, or CXCR4 and full-length myosin Vb, or CXCR4 and myosin Vb tail. Chemotactic responses of the cells to different concentrations of CXCL12 (0–250 ng/ml) were determined as described above. Values represent the mean ± S.E. of three independent experiments performed in triplicate. The data were analyzed using Student's paired t test (**p < 0.01).
DISCUSSION
Individual Rab proteins localized on discrete vesicle populations regulate unique trafficking pathways of CXCR2 and other GPCRs (Seachrist et al., 2000; Volpicelli et al., 2002; Fan et al., 2003). Rab proteins may organize multiprotein complexes associated with specific vesicle-trafficking pathways. For example, the association of myosin Vb with Rab11a and the inhibitory effect of myosin Vb tail on transferrin and IgA trafficking indicate the importance of myosin Vb in plasma membrane recycling system (Lapierre et al., 2001). Similarly, the association of Rab11a with a family of Rab11 interacting proteins (Hales et al., 2001), and the interaction between Rab11-FIP2 and myosin Vb (Hales et al., 2002), indicate the complexity in the regulation of membrane recycling system. Following these studies, we have demonstrated for the first time that myosin Vb and Rab11-FIP2 coimmunoprecipitated with the chemokine receptor CXCR2 in a ligand-dependent manner. After ligand stimulation, the internalized CXCR2 colocalizes with myosin Vb and Rab11-FIP2 in Rab11a-positive vesicles. CXCR2 also colocalizes with myosin Vb tail and Rab11-FIP2 (129–512) but in a highly condensed manner. The interaction between CXCR2 and myosin Vb or Rab11-FIP2 is important for the receptor recycling because expression of EGFP-tagged myosin Vb tail or Rab11-FIP2 (129–512) significantly retards the recycling of CXCR2. Moreover, we provide evidence that inhibition of the receptor recycling by overexpressing the myosin Vb tail attenuates the resensitization of CXCR2 and reduces the receptor-mediated chemotaxis. These data indicate that Rab11-FIP2 and myosin Vb play important roles in the CXCR2 receptor recycling, resensitization, and the receptor-mediated chemotaxis.
The coimmunoprecipitation of myosin Vb and Rab11-FIP2 with CXCR2 implicates a complex formed by these proteins in response to ligand stimulation of the receptor expressing cells. The associations of CXCR2 with myosin Vb tail and Rab11-FIP2 (129–512) suggest that the interaction occurs in the C-terminal domain of myosin Vb or Rab11-FIP2. Based on the previous data that Rab11a interacts with both myosin Vb and Rab11-FIP2 (Lapierre et al., 2001; Hales et al., 2001), we propose that Rab11a is included in the complex formed by CXCR2, myosin Vb and Rab11-FIP2. However, we failed to pull down Rab11a together with CXCR2 in our coimmunoprecipitation experiments. We have noted the difficulty in demonstrating biochemical association of Rab11a with myosin Vb in our previous coimmunoprecipitation experiments (Lapierre et al., 2001). We have hypothesized that the interaction of Rab11a with myosin Vb is labile in the detergent lysis conditions required for extraction of Rab11a from the membrane (Lapierre et al., 2001). The fact that CXCR2 did not coimmunoprecipitate with Rab11a suggests that Rab11a does not play a role in linking CXCR2 with myosin Vb or Rab11-FIP2. Because myosin Vb and Rab11-FIP2 form a direct complex with each other (Hales et al., 2002), it is not clear whether CXCR2 interacts with either or both of myosin Vb and Rab11-FIP2. Although CXCR2 interacts with myosin Vb and Rab11-FIP2 in in vivo experiments, we had difficulty in demonstrating the binding of CXCR2 with myosin Vb or Rab11-FIP2 in in vitro (glutathione S-transferase pull-down) experiments. We propose that either a conformational change of CXCR2 upon ligand-induced activation is required for the receptor association with myosin Vb and Rab11-FIP2 in intact cells, or a third protein is involved in the interaction. It is known that GPCRs undergo conformational changes after ligand stimulation of the receptor expressing cells (Baneres et al., 2003; Swaminath et al., 2004). The interaction of CXCR2 with myosin Vb or Rab11-FIP2 only occurs after ligand stimulation, suggesting that a conformational change of CXCR2 is required for the interaction. In addition, in our yeast two-hybrid studies, we identified a number of proteins interacting with CXCR2 (our unpublished data), but many of them have not been characterized functionally. Therefore, we cannot exclude the possibility for the involvement of other proteins in the interaction between CXCR2 and myosin Vb or Rab11-FIP2.
The results demonstrating that the internalized CXCR2 colocalized with myosin Vb, Rab11-FIP2, and Rab11a provide additional evidence for the complex formed by these proteins. We noticed that the internalized CXCR2 only partially colocalized with myosin Vb or Rab11-FIP2. The incomplete colocalization is likely due to the dynamic trafficking of CXCR2 among different endosomal compartments, including early, recycling, and late endosomes, such that only a proportion of CXCR2 is localized in the recycling vesicles at the given time points. This hypothesis is supported by our previous data showing that CXCR2 partially colocalized with Rab11a, Rab5, and Rab7, which are associated with the recycling vesicles and early and late endosomes, respectively, after ligand stimulation for different lengths of time (Fan et al., 2003). Interestingly, compared with the expression of the full-length myosin Vb or Rab11-FIP2, which did not affect the distribution of the recycling system, expression of myosin Vb tail or Rab11-FIP2 (129–512) apparently impairs the recycling system, because it resulted in high concentration of CXCR2 in Rab11a-positive vesicles. Similarly, previous data have shown that overexpression of the myosin Vb tail or Rab11-FIP2 (129–512) induced concentration of transferrin receptor or M4 muscarinic receptor in Rab11a-positive vehicle (Lapierre et al., 2001; Hales et al., 2002; Volpicelli et al., 2002). These data suggest that both myosin Vb and Rab11-FIP2 are important for the maintenance of the Rab11a-positive recycling system. However, expression of the DsRed-myosin Vb tail apparently induced more rigorous condensation of the Rab11a-positive vesicles than that induced by the expression of EGFP-Rab11-FIP2 (129–512), suggesting different functions of myosin Vb and Rab11-FIP2 in the regulation of the recycling system. Another interesting observation in the present study is that the internalized CXCR2 exhibited constant colocalization with the myosin Vb tail and Rab11a after prolonged ligand treatment (4 h), whereas in the same time frame the colocalization of CXCR2 with the full-length myosin Vb and Rab11a was greatly reduced. We have provided evidence in the previous studies showing that the internalized CXCR2 was trafficked to late endosomes/lysosomes after prolonged ligand challenge (Fan et al., 2003). These results suggest that the continued recycling is required for eventual shunting of CXCR2 into degradation pathway after prolonged ligand stimulation.
We have demonstrated previously that the internalized CXCR2 was recycled back to the cell surface after removal of the ligand and recovery of the cells (Fan et al., 2003). Rab11a plays an important role in the recycling of CXCR2 (Fan et al., 2003). The present studies provide further evidence that myosin Vb and Rab11-FIP2, the Rab11a interacting proteins, are involved in the regulation of CXCR2 recycling, because expression of myosin Vb tail or Rab11-FIP2 (129–512) reduced the recycling of CXCR2. These results are consistent with the previous data showing the inhibition of the recycling of several other receptors, such as transferrin receptor and M4 muscarinic receptor, by the expression of myosin Vb tail or Rab11-FIP2 (129–512) (Lapierre et al., 2001; Hales et al., 2002; Volpicelli et al., 2002). The inhibition of the receptor recycling is likely due to the condensation of the recycling system induced by the expression of the mutant myosin Vb or Rab11-FIP2 secondary to the exit from the recycling system.
One of the most important functions of chemokine receptors is to mediate the chemotactic response of the chemokine receptor-expressing cells (Mehrad et al., 1999), which is essential for the recruitment of leukocytes to inflammatory site (Garcia-Ramallo et al., 2002; Mariani and Panina-Bordignon, 2003) and is likely important for the metastasis of cancer cells (Loukinova et al., 2000; Muller et al., 2001; Mashino et al., 2002). Vesicle recycling of chemokine receptors, including CXCR2, has been implicated in chemotaxis (Perez et al., 1986, 1987, 1989; Zaslaver et al., 2001). In this study, we demonstrate that expression of a C-terminal fragment of myosin Vb (myosin Vb tail) does not affect the random cell migration (our unpublished data) but significantly reduces the chemotactic response to CXCL1 of the CXCR2-expressing cells, suggesting the specific involvement of myosin Vb in the chemokine receptor-mediated chemotaxis. In addition, we have obtained evidence that Rab11 interacting proteins, including myosin Vb and Rab11-FIP2, are expressed in neutrophils in which CXCR2 and many other chemokine receptors are predominantly expressed (our unpublished data). Therefore, we propose that myosin Vb and Rab11-FIP2 may play a role in regulation of leukocyte chemotaxis. Many chemokine receptors undergo recycling after ligand-induced internalization (Forster et al., 1998; Feniger-Barish et al., 2000; Signoret et al., 2000; Mueller et al., 2002; Fan et al., 2003). Based on the colocalization of Rab11a with CXCR4 (our unpublished data), and the reduction of CXCR4-mediated chemotaxis by myosin Vb tail, we propose that chemotaxis mediated through other chemokine receptors is also regulated by myosin Vb or Rab11-FIP2. In fact, recent studies have shown that overexpression of either a dominant negative mutant of Rab11a (Rab11aS25N) or a C-terminal fragment of Rab11-binding protein/Rabphilin 11 reduce migration of Madin-Darby canine kidney cells and HeLa cells, respectively (Mammoto et al., 1999). The involvement of a functionally intact mechanism for receptor recycling in migratory responses suggests that chemokine receptors are subjected to a specialized regulation, under which both receptor recycling and cell migration depend on an interdependent mechanism.
In summary, we have demonstrated that, in response to ligand stimulation, CXCR2 forms a complex with myosin Vb and Rab11-FIP2. The internalized CXCR2 colocalizes with myosin Vb and Rab11-FIP2 in Rab11a-positive recycling system. Expression of either myosin Vb tail or Rab11-FIP2 (129–512) results in condensation of the recycling system and retardation of CXCR2 recycling, suggesting that both myosin Vb and Rab11-FIP2 are involved in the recycling of CXCR2. Finally, we have demonstrated that expression of myosin Vb tail attenuates CXCR2 resensitization and the receptor-mediated chemotaxis.
Acknowledgments
We thank Dr. Sam Wells and Sean B. Schaffer (Vanderbilt University Ingram-Cancer Center) for confocal analysis and image quantification, and we also thank Catherine Allen, Lynn Butler, and Melanie Smith (Nashville VA Medical Center) for assistance with FACS analysis. In addition, we thank Linda W. Horton and Yingchun Yu in our laboratory for technical help. This work was supported by a career scientist grant from the Department of Veterans Affairs (to A.R.), by grant CA-34590 from National Institutes of Health (to A.R.), and by Vanderbilt-Ingram Cancer Center Support grant CA68485. J.R.G. is supported by National Institutes of Health grants DK-48370, DK-38063, and AR-49311 and a VA Merit Review grant.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–09–0706. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–09–0706.
Abbreviations used: FACS, fluorescence-activated cell sorting; GPCR, G protein-coupled receptor; HEK, human embryonic kidney; Rab11-FIP2, Rab11-family interacting protein 2; Rab11-FIP1, Rab11-family interacting protein 1; Rab11-FIP3, Rab11-family interacting protein 3; RBL, rat basophilic leukemia.
References
- Asagoe, K., Yamamoto, K., Takahashi, A., Suzuki, K., Maeda, A., Nohgawa, M., Harakawa, N., Takano, K., Mukaida, N., and Matsushima, K. (1998). Down-regulation of CXCR2 expression on human polymorphonuclear leukocytes by TNF-alpha. J. Immunol. 160, 4518–4525. [PubMed] [Google Scholar]
- Anborgh, P.H., Seachrist, J.L., Dale, L.B., and Ferguson, S.S. (2000). Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol. Endocrinol. 14, 2040–2053. [DOI] [PubMed] [Google Scholar]
- Baneres, J.L., Martin, A., Hullot, P., Girard, J.P., Rossi, J.C., and Parello, J. (2003). Structure-based analysis of GPCR function: conformational adaptation of both agonist and receptor upon leukotriene B4 binding to recombinant BLT1. J. Mol. Biol. 329, 801–814. [DOI] [PubMed] [Google Scholar]
- Belperio, J.A., Keane, M.P., Arenberg, D.A., Addison, C.L., Ehlert, J.E., Burdick, M.D., and Strieter, R.M. (2000). CXC chemokines in angiogenesis. J. Leukoc. Biol. 68, 1–8. [PubMed] [Google Scholar]
- Berger, E.A., Murphy, P.M., and Farber, J.M. (1999). Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17, 657–700. [DOI] [PubMed] [Google Scholar]
- Bretscher, M.S. (1992). Cells can use their transferrin receptors for locomotion. EMBO J. 11, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, T.T., Deacon, H.W., Reczek, D., Bretscher, A., and von Zastrow, M. (1999). A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401, 286–290. [DOI] [PubMed] [Google Scholar]
- Chuntharapai, A., and Kim, K.J. (1995). Regulation of the expression of IL-8 receptor A/B by IL-8, possible functions of each receptor. J. Immunol. 155, 2587–2594. [PubMed] [Google Scholar]
- Fan, G.H., Lapierre, L.A., Goldenring, J.R., and Richmond, A. (2003). Differential regulation of CXCR2 trafficking by Rab GTPases. Blood 101, 2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, G.H., Yang, W., Sai, J., and Richmond, A. (2001a). Phosphorylation-independent association of CXCR2 with the protein phosphatase 2A core enzyme. J. Biol. Chem. 276, 16960–16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, G.H., Yang, W., Wang, X.J., Qian, Q., and Richmond, A. (2001b). Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry 40, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feniger-Barish, R., Belkin, D., Zaslaver, A., Gal, S., Dori, M., Ran, M., and Ben-Baruch, A. (2000). GCP-2-induced internalization of IL-8 receptors: hierarchical relationships between GCP-2 and other ELR(+)-CXC chemokines and mechanisms regulating CXCR2 internalization and recycling. Blood 95, 1551–1559. [PubMed] [Google Scholar]
- Forster, R., Kremmer, E., Schubel, A., Breitfeld, D., Kleinschmidt, A., Nerl, C., Bernhardt, G., and Lipp, M. (1998). Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J. Immunol. 160, 1522–1531. [PubMed] [Google Scholar]
- Ganju, R.K., Brubaker, S.A., Meyer, J., Dutt, P., Yang, Y., Qin, S., Newman, W., and Groopman, J.E. (1998). The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J. Biol. Chem. 273, 23169–23175. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramallo, E., Marques, T., Prats, N., Beleta, J., Kunkel, S.L., and Godessart, N. (2002). Resident cell chemokine expression serves as the major mechanism for leukocyte recruitment during local inflammation. J. Immunol. 169, 6467–6473. [DOI] [PubMed] [Google Scholar]
- Hales, C.M., Griner, R., Hobdy-Henderson, K.C., Dorn, M.C., Hardy, D., Kumar, R., Navarre, J., Chan, E.K., Lapierre, L.A., and Goldenring, J.R. (2001). Identification and characterization of a family of Rab11-interacting proteins. J. Biol. Chem. 276, 39067–39075. [DOI] [PubMed] [Google Scholar]
- Hales, C.M., Vaerman, J.P., and Goldenring, J.R. (2002). Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 277, 50415–50421. [DOI] [PubMed] [Google Scholar]
- Hopkins, C.R., Gibson, A., Shipman, M., Strickland, D.K., and Trowbridge, I.S. (1994). In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J. Cell Biol. 125, 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorati, G., Le Gouill, C., Balamotis, M., and Birnbaumer, M. (2001). The long and the short cycle. Alternative intracellular routes for trafficking of G-protein-coupled receptors. J. Biol. Chem. 276, 13096–13103. [DOI] [PubMed] [Google Scholar]
- Khandaker, M.H., Xu, L., Rahimpour, R., Mitchell, G., DeVries, W.E., Pickering, J.G., Singhal, S.K., Feldman, R.D., and Kelvin, D.J. (1998). CXCR1 and CXCR2 are rapidly down-modulated by bacterial endotoxin through a unique agonist-independent, tyrosine kinase-dependent mechanism. J. Immunol. 161, 1930–1938. [PubMed] [Google Scholar]
- Krudewig, R., Langer, B., Vogler, O., Markschies, N., Erl, M., Jakobs, K.H., and van Koppen, C.J. (2000). Distinct internalization of M2 muscarinic acetylcholine receptors confers selective and long-lasting desensitization of signaling to phospholipase C. J. Neurochem. 74, 1721–1730. [DOI] [PubMed] [Google Scholar]
- Lapierre, L.A., Kumar, R., Hales, C.M., Navarre, J., Bhartur, S.G., Burnette, J.O., Provance, D.W., Jr., Mercer, J.A., Bahler, M., and Goldenring, J.R. (2001). Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, A.J., Hendrick, A.G., Cantalupo, G., Senic-Matuglia, F., Goud, B., Bucci, C., and McCaffrey, M.W. (2002). Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J. Biol. Chem. 277, 12190–12199. [DOI] [PubMed] [Google Scholar]
- Loetscher, P., Seitz, M., Clark-Lewis, I., Baggiolini, M., and Moser, B. (1994). Both interleukin-8 receptors independently mediate chemotaxis: Jurkat cells transfected with IL-8R1 or IL-8R2 migrate in response to IL-8, GRO and NAP-2. FEBS Lett. 341, 187–192. [DOI] [PubMed] [Google Scholar]
- Loukinova, E., Dong, G., Enamorado-Ayalya, I., Thomas, G.R., Chen, Z., Schreiber, H., and Van Waes, C. (2000). Growth regulated oncogene-alpha expression by murine squamous cell carcinoma promotes tumor growth, metastasis, leukocyte infiltration and angiogenesis by a host CXC receptor-2 dependent mechanism. Oncogene 19, 3477–3486. [DOI] [PubMed] [Google Scholar]
- Luster, A.D. (1998). Chemokines-chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 12, 436–445. [DOI] [PubMed] [Google Scholar]
- Mack, M., et al. (1998). (Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 187, 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto, A., Ohtsuka, T., Hotta, I., Sasaki, T., and Takai, Y. (1999). Rab11BP/Rabphilin-11, a downstream target of rab11 small G protein implicated in vesicle recycling. J. Biol. Chem. 274, 25517–25524. [DOI] [PubMed] [Google Scholar]
- Mariani, M., and Panina-Bordignon, P. (2003). Analysis of homing receptor expression on infiltrating leukocytes in disease states. J. Immunol. Methods. 273, 103–114. [DOI] [PubMed] [Google Scholar]
- Mashino, K., Sadanaga, N., Yamaguchi, H., Tanaka, F., Ohta, M., Shibuta, K., Inoue, H., and Mori, M. (2002). Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 62, 2937–2941. [PubMed] [Google Scholar]
- Mehrad, B., Strieter, R.M., Moore, T.A., Tsai, W.C., Lira, S.A., and Standiford, T.J. (1999). CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J. Immunol. 163, 6086–6094. [PubMed] [Google Scholar]
- Mellado, M., Rodriguez-Frade, J.M., Aragay, A., del Real, G., Martin, A.M., Vila-Coro, A.J., Serrano, A., Mayor F., Jr., and Martinez, A.C. (1998). The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J. Immunol. 161, 805–813. [PubMed] [Google Scholar]
- Mueller, A., Kelly, E., and Strange, P.G. (2002). Pathways for internalization and recycling of the chemokine receptor CCR5. Blood 99, 785–791. [DOI] [PubMed] [Google Scholar]
- Muller, A., et al. (2001). Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56. [DOI] [PubMed] [Google Scholar]
- Mueller, S.G., Schraw, W.P., and Richmond, A. (1995). Activation of protein kinase C enhances the phosphorylation of the type B interleukin-8 receptor and stimulates its degradation in non-hematopoietic cells. J. Biol. Chem. 270, 10439–10448. [DOI] [PubMed] [Google Scholar]
- Murphy, P.M., Baggiolini, M., Charo, I.F., Hebert, C.A., Horuk, R., Matsushima, K., Miller, L.H., Oppenheim, J.J., and Power, C.A. (2000). International union of pharmacology, XXII: nomenclature for chemokine receptors. Pharmacol. Rev. 52, 145–176. [PubMed] [Google Scholar]
- Nagasawa, T., et al. (1996). Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638. [DOI] [PubMed] [Google Scholar]
- Perez, H.D., Elfman, F., Lobo, E., Sklar, L., Chenoweth, D., and Hooper, C. (1986). A derivative of wheat germ agglutinin specifically inhibits formylpeptide-induced polymorphonuclear leukocyte chemotaxis by blocking reexpression (or recycling) of receptors. J. Immunol. 136, 1803–1812. [PubMed] [Google Scholar]
- Perez, H.D., Elfman, F., and Lobo, E. (1987). Removal of human polymorphonuclear leukocyte surface sialic acid inhibits re-expression (or recycling) of formyl peptide receptors. A possible explanation for its effect on formyl peptide-induced polymorphonuclear leukocyte chemotaxis. J. Immunol. 139, 1978–1984. [PubMed] [Google Scholar]
- Perez, H.D., Elfman, F., Marder, S., Lobo, E., and Ives, H.E. (1989). Formyl peptide-induced chemotaxis of human polymorphonuclear leukocytes does not require either marked changes in cytosolic calcium or specific granule discharge. Role of formyl peptide receptor reexpression (or recycling). J. Clin. Investig. 83, 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris, R., Klumperman, J., and Scheller, R.H. (2000). A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol. Cell. 6, 1437–1448. [DOI] [PubMed] [Google Scholar]
- Ray, E., and Samanta, A.K. (1997). Receptor-mediated endocytosis of IL-8, a fluorescent microscopic evidence and implication of the process in ligand-induced biological response in human neutrophils. Cytokine 9, 587–596. [DOI] [PubMed] [Google Scholar]
- Sabroe, I., Williams, T.J., Hebert, C.A., and Collins, P.D. (1997). Chemoattractant cross-desensitization of the human neutrophil IL-8 receptor involves receptor internalization and differential receptor subtype regulation. J. Immunol. 158, 1361–1369. [PubMed] [Google Scholar]
- Seachrist, J.L., Anborgh, P.H., and Ferguson, S.S. (2000). Beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J. Biol. Chem. 275, 27221–27228. [DOI] [PubMed] [Google Scholar]
- Signoret, N., et al. (1997) Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J. Cell Biol. 139, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret, N., Pelchen-Matthews, A., Mack, M., Proudfoot, A.E., and Marsh, M. (2000). Endocytosis and recycling of the HIV coreceptor CCR5. J. Cell Biol. 151, 1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminath, G., Xiang, Y., Lee, T.W., Steenhuis, J., Parnot, C., and Kobilka, B.K. (2004). Sequential binding of agonists to the beta 2 adrenoceptor: kinetic evidence for intermediate conformational states. J. Biol. Chem. 279, 686–691. [DOI] [PubMed] [Google Scholar]
- Trejo, J., and Coughlin, S.R. (1999). The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomes versus recycling. J. Biol. Chem. 274, 2216–2224. [DOI] [PubMed] [Google Scholar]
- Tsao, P.I., and von Zastrow, M. (2000). Type-specific sorting of G protein-coupled receptors after endocytosis. J. Biol. Chem. 275, 11130–11140. [DOI] [PubMed] [Google Scholar]
- Venkatesan, S., Rose, J.J., Lodge, R., Murphy, P.M., and Foley, J.F. (2003). Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4. Mol. Biol. Cell 14, 3305–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares, M., Cabrero, J.R., Rey, M., Perez-Martinez, M., Ursa, A., Itoh, K., and Sanchez-Madrid, F. (2002). A role for the Rho-p160 Rho coiled-coil kinase axis in the chemokine stromal cell-derived factor-1alpha-induced lymphocyte actomyosin and microtubular organization and chemotaxis. J. Immunol. 168, 400–410. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares, M., et al. (1999). Involvement of phosphatidylinositol 3-kinase in stromal cell-derived factor-1 alpha-induced lymphocyte polarization and chemotaxis. J. Immunol. 163, 4001–4012. [PubMed] [Google Scholar]
- Volpicelli, L.A., Lah, J.J., Fang, G., Goldenring, J.R., and Levey, A.I. (2002). Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J. Neurosci. 22, 9776–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, D.M., Lindsay, A.J., Hendrick, A.G., and McCaffrey, M.W. (2002). Rab11-FIP4 interacts with Rab11 in a GTP-dependent manner and its overexpression condenses the Rab11 positive compartment in HeLa cells. Biochem. Biophys. Res. Commun. 299, 770–779. [DOI] [PubMed] [Google Scholar]
- Wang, X., Kumar, R., Navarre, J., Casanova, J.E., and Goldenring, J.R. (2000). Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J. Biol. Chem. 275, 29138–29146. [DOI] [PubMed] [Google Scholar]
- Wolf, M., Delgado, M.B., Jones, S.A., Dewald, B., Clark-Lewis, I., and Baggiolini, M. (1998). Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur. J. Immunol. 28, 164–170. [DOI] [PubMed] [Google Scholar]
- Wu, D., LaRosa, G.J., and Simon, M.I. (1993). G protein-coupled signal transduction pathways for interleukin-8. Science 261, 101–103. [DOI] [PubMed] [Google Scholar]
- Wu, X., Zhao, X., Baylor, L., Kaushal, S., Eisenberg, E., and Greene, L.E. (2001). Clathrin exchange during clathrin-mediated endocytosis. J. Cell Biol. 155, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuyts, A., Proost, P., Lenaerts, J.P., Ben-Baruch, A., Van Damme, J., Wang, J.M. (1998). Differential usage of the CXC chemokine receptors 1 and 2 by interleukin-8, granulocyte chemotactic protein-2 and epithelial-cell-derived neutrophil attractant-78. Eur. J. Biochem. 255, 67–73. [DOI] [PubMed] [Google Scholar]
- Yang, W., Wang, D., and Richmond, A. (1999). Role of clathrin-mediated endocytosis in CXCR2 sequestration, resensitization, and signal transduction. J. Biol. Chem. 274, 11328–11333. [DOI] [PubMed] [Google Scholar]
- Zaslaver, A., Feniger-Barish, R., and Ben-Baruch, A. (2001). Actin filaments are involved in the regulation of trafficking of two closely related chemokine receptors, CXCR1 and CXCR2. J. Immunol. 166, 1272–1284. [DOI] [PubMed] [Google Scholar]
- Zeng, J., et al. (1999). Identification of a putative effector protein for rab11 that participates in transferrin recycling. Proc. Natl. Acad. Sci. USA 96, 2840–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik, A., and Yoshie, O. (2000). Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127. [DOI] [PubMed] [Google Scholar]