Abstract
Background
The Korean version of the Michigan Hand Outcomes Questionnaire (K-MHQ) was recently validated; however, the questionnaire's responsiveness as well as the degree to which the instrument is sensitive to change has not been thoroughly evaluated in a specific condition in Koreans. We evaluated the responsiveness of the K-MHQ in a homogenous cohort of patients with carpal tunnel syndrome (CTS) and we compared it with that of the Korean version of the Disability of the Arm, Shoulder, and Hand Questionnaire (K-DASH), which was found to have a large degree of responsiveness after carpal tunnel release for Korean patients with CTS.
Methods
Thirty-seven patients with CTS prospectively completed the K-MHQ and the K-DASH before and 6 months after surgery. The responsiveness statistics were assessed for both the K-MHQ and the K-DASH by using the standardized response mean (SRM), which was defined as the mean change of the original scores after surgery divided by the standard deviation of the change.
Results
All domains of the K-MHQ significantly improved after carpal tunnel release (p < 0.001). The SRM for all scales but one (the aesthetics scale) showed large responsiveness of ≥ 0.8. The aesthetics scale showed medium responsiveness of 0.6. The combined function/symptom scale of the K-DASH significantly improved after surgery (p < 0.001). The SRM of the K-DASH revealed large responsiveness of 0.9.
Conclusions
The K-MHQ was found to have a large degree of responsiveness after carpal tunnel release for Korean patients with CTS, which is comparable not only to the K-DASH, but also to the original version of the MHQ. The region-specific K-MHQ can be useful for outcomes research related to carpal tunnel surgery, especially for research comparing CTS with various other hand and wrist health conditions.
Keywords: Responsiveness, K-MHQ, K-DASH, Carpal tunnel syndrome
Health and functional status questionnaires have been increasingly used to assess the effectiveness of medical treatment or surgery. In hand surgery, physicians have used these instruments to evaluate patient outcomes for specific hand conditions such as carpal tunnel syndrome (CTS), distal radius fracture, and rheumatoid arthritis.1,2)
The Michigan Hand Outcomes Questionnaire (MHQ) is one of the most widely used hand-specific surveys that measures health status relevant to patients with hand disorders.3) The MHQ assesses the patient's perception for six different scales, including function, activities, pain, work, satisfaction, and asthetics.4) The validity, reliability, and responsiveness of the MHQ have been reported for a variety of upper extremity conditions such as CTS, distal radius fracture, and rheumatoid arthritis.5,6,7) The MHQ has also been translated into Korean: the validity and reliability of the Korean version of MHQ (K-MHQ) were recently assessed for arm, shoulder, and hand musculoskeletal conditions.8) However, the responsiveness of K-MHQ, and the degree to which the instrument is sensitive to change, has not been thoroughly evaluated for a specific medical condition in Koreans.
CTS is the most common compressive neuropathy in the upper extremity.9) The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire is a well-known and frequently used tool that was developed for the assessment of patients with upper extremity conditions. The Korean version of DASH (K-DASH) has been validated for the assessment of upper extremity conditions; it was also found to be responsive to carpal tunnel release.10,11) The purpose of this study was to evaluate the responsiveness of the K-MHQ to carpel tunnel release, and to compare the results with those of the K-DASH in a homogenous cohort of patients with CTS.
METHODS
Subjects
Eighty-four consecutive patients with CTS scheduled for surgery at our institution (an urban tertiary referral hospital) were prospectively recruited for the study between June 2011 and February 2012. All patients were referred by primary care physicians, general orthopaedic surgeons, neurologists, or rehabilitation physicians. We excluded those from the study with any other upper extremity problem besides CTS, such as a history of forearm fracture or malunion, cervical radiculopathy, and cubital tunnel syndrome. We also excluded those from the study with systemic comorbidities, such as rheumatoid arthritis, diabetes mellitus, thyroid disease, and chronic renal failure. Concurrent disorders and loss of follow-up led to a total of forty-seven patients being excluded from the final data analysis. Therefore, data analysis was performed for the remaining thirty-seven patients. The patients included consisted of 1 man and 36 women; their ages ranged from 28 years old to 73 years old (average, 53.5 years old). This study was approved by the Institutional Review Board of the authors' hospital; informed consent was obtained from all patients.
CTS was diagnosed based on clinical symptoms, such as tingling sensation of the hand. Electrophysiologic studies were conducted for all patients to confirm the diagnosis; only those with positive findings were included for analysis. We used the classification developed by Bland12): the classification consists of 7 grades from grade 0 (normal) to grade 6 (extremely severe) based on conduction time and amplitude. All patients underwent either unilateral or simultaneous bilateral open carpal tunnel release by a single surgeon (HSG) under local anesthesia.
K-MHQ and K-DASH
Evaluations using the K-MHQ and K-DASH were performed preoperatively and six months postoperatively. The six-month interval period was chosen due to findings from a previous study: the study found that patients who have carpal tunnel release tend to plateau in functional and symptom improvement six months after surgery when assessed via questionnaire.13)
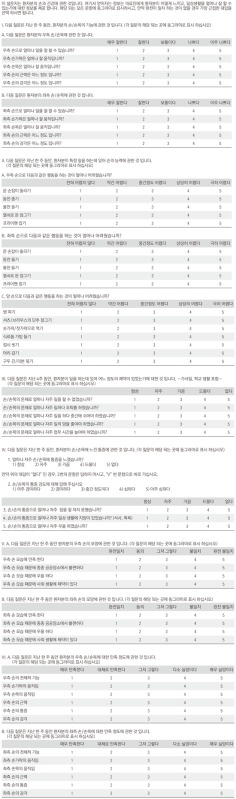
The K-MHQ is a 57-item hand-specific outcomes questionnaire that contains 6 domains: (1) function, (2) activities of daily living, (3) pain, (4) work performance, (5) aesthetics, and (6) patient satisfaction. Patients are asked to answer each question for the relevant domain using a scale of 1 to 5. Each domain is assessed on a score from 0 to 100. All scales except for work and pain assess each hand separately and are scored according to the affected hand or as an average for bilaterally affected hands. There is no scoring adjustment for hand dominance. The K-MHQ was translated by two of the authors (HSG and YHR), approved by the original developer (Kevin Chung, University of Michigan, USA), and was validated for its reliability and cross-cultural adaptation for common hand disorders. The instrument is presented in the Appendix 1.8)
The K-DASH questionnaire primarily consists of a 30 item scale concerning the patient's health status for the preceding week. The items ask about the following issues: degree of difficulty in performing various physical activities because of an arm; shoulder or hand problem (21 items); the severity of each symptom of pain, activity-related pain, tingling, weakness, and stiffness (5 items); the problem's effect on social activities, work and sleep; and its psychological impact (4 items). Each item has five response options, ranging from 1 to 5. If at least 27 of the 30 items are completed, then a score ranging from 0 (no disability) to 100 (the most severe disability) can be calculated. The two optional scales of K-DASH (sport/music and work) were excluded from this study.
Data Analysis
Statistical analysis was performed using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). Paired t-tests were used when comparing the preoperative versus postoperative scores. Two-sample Student t-tests were performed when comparing scores for patients who had unilateral carpal tunnel release versus patients who had simultaneous bilateral carpal tunnel release and comparing scores for the mild (Bland grade 1-2) group versus moderate to severe (Bland grade 3-5) group. The level of significance chosen for the analysis was p = 0.05.
The responsiveness of each questionnaire was evaluated in this study using a distribution-based methodology: This was performed by calculating the standardized response mean (SRM).14) The SRM was defined as the mean change between pre- and postoperative scores divided by the standard deviation of the total change. The higher the SRM, the greater the level of responsiveness is. Values ≤ 0.5, between 0.5 and 0.8, and ≥ 0.8 were considered to represent small, moderate, and large degrees of responsiveness, respectively.15)
For the sample size calculation, we needed a total of 56 patients to achieve 90% power. We initially recruited 84 patients for this study. However, due to concurrent disorders and a loss of follow-up, data analysis was performed for only 37 patients. However, as the study results were positive, retrospective power analysis indicated that the statistical power was adequate.
RESULTS
Comparison of Pre- and Postoperative Scores
All domains of the K-MHQ (function, activities of daily living, work, pain, aesthetics, and satisfaction) revealed significant postoperative improvement (p < 0.001) (Table 1). Comparing the unilateral and bilateral surgery group, there were no statistically significant differences preoperatively and postoperatively in mean function scores (p = 0.71 and 0.53, respectively), and in mean pain scores (p = 0.85 and 0.96, respectively). Comparing the mild (Bland grade 1-2) and moderate to severe (Bland grade 3-5) group, there were no significant differences in mean preoperative and postoperative K-MHQ scores (function: p = 0.33 and 0.51; pain: p = 0.59 and 0.25, respectively).
Table 1.
Preoperative vs. 6-month Postoperative K-MHQ Scores
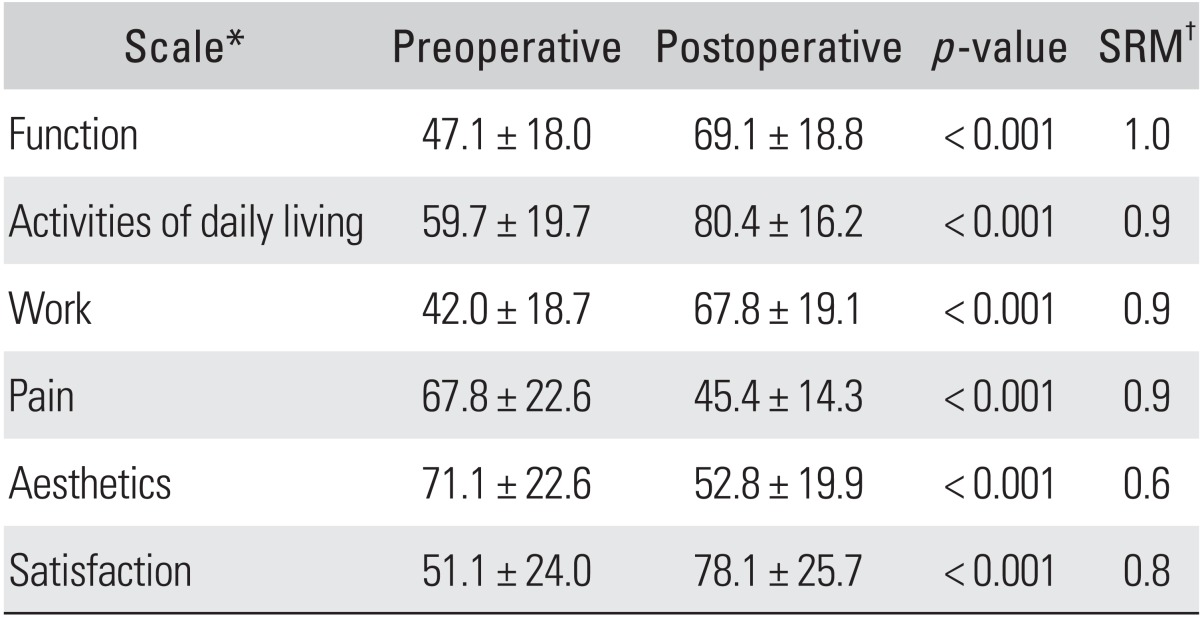
Values are presented as mean ± SD.
K-MHQ: Korean version of the Michigan Hand Outcomes Questionnaire, SRM: standardized response mean.
*All of the K-MHQ scales are based on a score from 0 to 100. For all of the scales except pain, a higher score translates into better performance for the patient's hand. For the pain scale, the relationship is inverse: the lower the score, the less pain the patient experiences, which signifies a better outcome.
†Mean difference between preoperative and postoperative scores/standard deviation of mean difference. An SRM of 0.2 is considered small, 0.5 is considered medium, and 0.8 is considered large.
K-DASH scores decreased by 19 points, revealing a significant postoperative improvement (p < 0.001) (Table 2). There were no significant differences between unilateral and bilateral patients in mean preoperative and postoperative K-DASH scores (p = 0.82 and 0.54, respectively). There were no significant differences between mild and moderate to severe group in mean preoperative or postoperative K-DASH scores, either (p = 0.22 and 0.35, respectively).
Table 2.
Preoperative vs. 6-month Postoperative Scores of the K-DASH Questionnaire
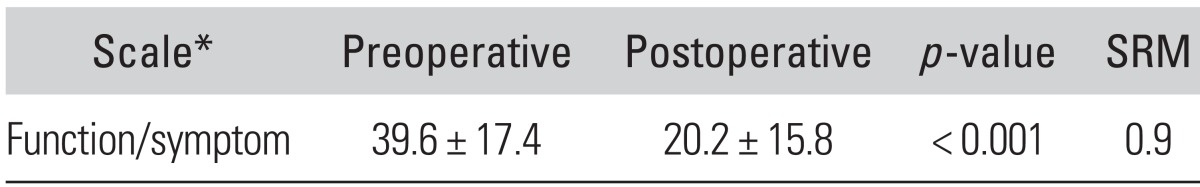
Values are presented as mean ± SD.
K-DASH: Korean version of the Disability of the Arm, Shoulder, and Hand Questionnaire, SRM: standardized response mean.
*The K-DASH is based on a score from 0 to 100. A lower score indicates less disability.
Responsiveness of Outcome Scores
The SRM for the K-MHQ ranged from medium (0.6) for the aesthetics scales, to large (0.8-1.0) for the pain, satisfaction, activities of daily living, work, and function scales. For patients who had unilateral surgery, the SRMs for the work and pain scales were 0.8 and 0.9, respectively. For patients who had bilateral surgery. the SRMs for the work and pain scales were 1.0 and 1.1, respectively.
The SRM for K-DASH was 0.9. For patients who had unilateral surgery, the SRM for the K-DASH was 0.8. For patients who had bilateral surgery, the SRM for the K-DASH was 1.1.
DISCUSSION
In this study, we evaluated the responsiveness of the K-MHQ for CTS and compared it with that of the K-DASH. We found that the K-MHQ had a level of responsiveness similar to that of the K-DASH in the assessment of CTS outcomes.
Several previous studies have evaluated the responsiveness of the MHQ for CTS patients. Kotsis and Chung16) reported that the SRM varied from 0.5 to 0.6 for the activity subscale and from 0.9 to 1.1 for the pain and satisfaction scales. The study by Chatterjee and Price7) showed the SRM varied from 0.78 to 1.30 for the pain, aesthetics, and function scales and from 0.79 to 0.80 for the satisfaction, activity, and work scales (Table 3). Compared with these studies, the K-MHQ was found to have a sufficient degree of responsiveness for assessing and comparing outcomes for Korean patients with CTS.
Table 3.
The Responsiveness of Various Studies of the MHQ after Carpal Tunnel Release
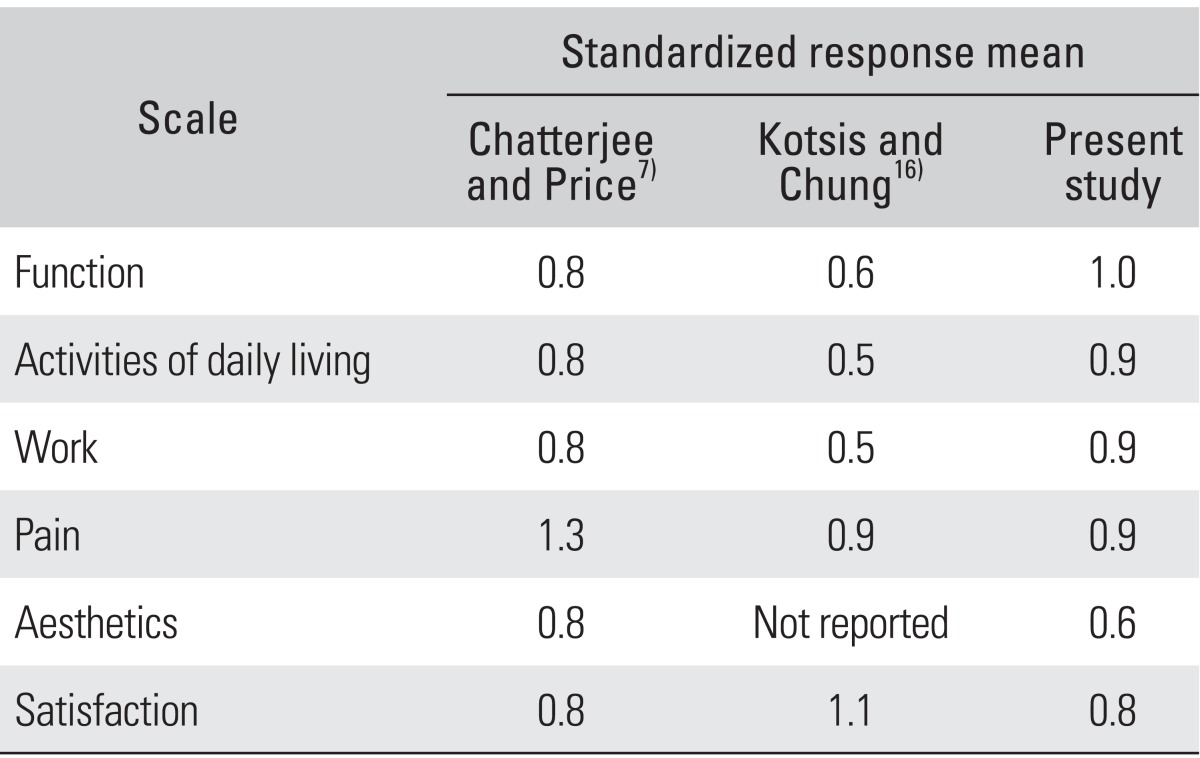
MHQ: Michigan Hand Outcomes Questionnaire.
Previous studies found that the pain domain seemed to be the most responsive for the MHQ, but as shown in Table 3, our study found that the function domain appeared to be the most responsive. The reason for this difference is presumed that the high proportion (83.8%) of moderate to severe patients (Bland grade 3-5) who had a sensory or motor deficit would experience comprehensive improvement in hand functioning.
The aesthetics domain may not be pertinent to carpal tunnel release.17) In addition, aesthetics outcomes may worsen because of the scar after open carpal tunnel release. In this study, however, there was a significant improvement in the aesthetic domain after surgery, which concurs with previous study by the Chatterjee and Price.7) They suggested that patients may have improved their self-image secondary to better function following carpal tunnel release and their perception of their hand aesthetics therefore improved.
In the present study, we compared scores between the K-DASH and-MHQ. The K-DASH is a more general questionnaire with questions that assess the collective arm, shoulder, and hand conditions. The combined function/symptom scale of the K-DASH limits the measurement of symptom and function improvement after carpal tunnel surgery because symptoms are quicker to improve than functional outcomes.18,19,20,21) Furthermore, the K-DASH outcomes are not scored separately for each hand; thus, it is difficult to interpret the K-DASH outcome in conditions that often involve both hands, such as CTS. Compared with the K-DASH, the K-MHQ contains multiple domains, each of which can be scored individually. All domains (except for work and pain) assess the right and left hand separately, making it possible to assess both hands separately, and also allowing for scores of the affected hand to be compared with an unaffected control hand if only one hand is affected. In addition, the K-MHQ is more region-specific than the K-DASH in that it has questions relating to the hand only.
Our study has several limitations that require consideration. First, our study did not have a balanced sex ratio: the study had an overabundance of female patients (97.3%). This limits the generalizability of our results to the population, but it is not likely to affect the conclusions of our study because outcomes of CTS were not found to vary by gender.13) Furthermore, similar demographics have been used in other prospective CTS studies.18) Second, we lacked other general health measurement questionnaires that can be used for comparison; we also did not analyze any clinical factors such as physical findings that may influence the responsiveness of each score. Third, we did not compare the K-MHQ with the Boston carpal tunnel scores, which is a disease specific scale and is known to have a greater responsiveness than K-DASH in CTS.11)
In conclusion, the K-MHQ was found to have a large degree of responsiveness after carpal tunnel release for Korean patients with CTS, which is comparable not only to the K-DASH, but also to the original version of the MHQ. The region-specific K-MHQ can be used for outcomes research related to carpal tunnel surgery, especially for research comparing CTS with various other hand and wrist conditions.
Appendix 1
Korean version of the Michigan Hand Outcomes Questionnaire (K-MHQ)
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Chung KC, Hamill JB, Walters MR, Hayward RA. The Michigan Hand Outcomes Questionnaire (MHQ): assessment of responsiveness to clinical change. Ann Plast Surg. 1999;42(6):619–622. doi: 10.1097/00000637-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Pincus T, Yazici Y, Bergman MJ. Patient questionnaires in rheumatoid arthritis: advantages and limitations as a quantitative, standardized scientific medical history. Rheum Dis Clin North Am. 2009;35(4):735–743. doi: 10.1016/j.rdc.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Waljee JF, Kim HM, Burns PB, Chung KC. Development of a brief, 12-item version of the Michigan Hand Questionnaire. Plast Reconstr Surg. 2011;128(1):208–220. doi: 10.1097/PRS.0b013e318218fc51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23(4):575–587. doi: 10.1016/S0363-5023(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 5.Chung KC, Kotsis SV. Outcomes of multiple microvascular toe transfers for reconstruction in 2 patients with digitless hands: 2- and 4-year follow-up case reports. J Hand Surg Am. 2002;27(4):652–658. doi: 10.1053/jhsu.2002.33706. [DOI] [PubMed] [Google Scholar]
- 6.Kotsis SV, Lau FH, Chung KC. Responsiveness of the Michigan Hand Outcomes Questionnaire and physical measurements in outcome studies of distal radius fracture treatment. J Hand Surg Am. 2007;32(1):84–90. doi: 10.1016/j.jhsa.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee JS, Price PE. Comparative responsiveness of the Michigan Hand Outcomes Questionnaire and the Carpal Tunnel Questionnaire after carpal tunnel release. J Hand Surg Am. 2009;34(2):273–280. doi: 10.1016/j.jhsa.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Roh YH, Yang BK, Noh JH, Baek GH, Song CH, Gong HS. Cross-cultural adaptation and validation of the Korean version of the Michigan hand questionnaire. J Hand Surg Am. 2011;36(9):1497–1503. doi: 10.1016/j.jhsa.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263–265. doi: 10.1136/jnnp.2005.066696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JY, Lim JY, Oh JH, Ko YM. Cross-cultural adaptation and clinical evaluation of a Korean version of the disabilities of arm, shoulder, and hand outcome questionnaire (K-DASH) J Shoulder Elbow Surg. 2008;17(4):570–574. doi: 10.1016/j.jse.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Jeon SH, Lee JH, Chung MS, et al. Responsiveness of the Korean version of the disabilities of the arm, shoulder and hand questionnaire (K-DASH) after carpal tunnel release. Clin Orthop Surg. 2011;3(2):147–151. doi: 10.4055/cios.2011.3.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23(8):1280–1283. doi: 10.1002/1097-4598(200008)23:8<1280::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Losina E, Amick BC, 3rd, Fossel AH, Bessette L, Keller RB. Predictors of outcomes of carpal tunnel release. Arthritis Rheum. 2001;44(5):1184–1193. doi: 10.1002/1529-0131(200105)44:5<1184::AID-ANR202>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Hays RD, Hadorn D. Responsiveness to change: an aspect of validity, not a separate dimension. Qual Life Res. 1992;1(1):73–75. doi: 10.1007/BF00435438. [DOI] [PubMed] [Google Scholar]
- 15.Atroshi I, Johnsson R, Sprinchorn A. Self-administered outcome instrument in carpal tunnel syndrome: reliability, validity and responsiveness evaluated in 102 patients. Acta Orthop Scand. 1998;69(1):82–88. doi: 10.3109/17453679809002363. [DOI] [PubMed] [Google Scholar]
- 16.Kotsis SV, Chung KC. Responsiveness of the Michigan Hand Outcomes Questionnaire and the Disabilities of the Arm, Shoulder and Hand questionnaire in carpal tunnel surgery. J Hand Surg Am. 2005;30(1):81–86. doi: 10.1016/j.jhsa.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Weber RA, Rude MJ. Clinical outcomes of carpal tunnel release in patients 65 and older. J Hand Surg Am. 2005;30(1):75–80. doi: 10.1016/j.jhsa.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Katz JN, Fossel KK, Simmons BP, Swartz RA, Fossel AH, Koris MJ. Symptoms, functional status, and neuromuscular impairment following carpal tunnel release. J Hand Surg Am. 1995;20(4):549–555. doi: 10.1016/S0363-5023(05)80265-5. [DOI] [PubMed] [Google Scholar]
- 19.Reale F, Ginanneschi F, Sicurelli F, Mondelli M. Protocol of outcome evaluation for surgical release of carpal tunnel syndrome. Neurosurgery. 2003;53(2):343–350. doi: 10.1227/01.neu.0000073421.92105.62. [DOI] [PubMed] [Google Scholar]
- 20.Brown RA, Gelberman RH, Seiler JG, 3rd, et al. Carpal tunnel release: a prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265–1275. doi: 10.2106/00004623-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Gellman H, Kan D, Gee V, Kuschner SH, Botte MJ. Analysis of pinch and grip strength after carpal tunnel release. J Hand Surg Am. 1989;14(5):863–864. doi: 10.1016/s0363-5023(89)80091-7. [DOI] [PubMed] [Google Scholar]


