Abstract
Rationale
Attention-Deficit Hyperactivity Disorder (ADHD) is associated with a higher prevalence of smoking, which may be related to potential therapeutic effects of nicotine on ADHD symptoms. Whereas nicotine offers robust improvements in sustained attention, the effects of nicotine on impulsivity are unclear.
Objectives
The present study examined the effects of nicotine on the response inhibition capacity of spontaneously hypertensive rats (SHR), an animal model of ADHD, compared to that of a normotensive control Wistar Kyoto (WKY) using the Fixed Minimum Interval (FMI) schedule of reinforcement.
Methods
Tests were conducted following acute injections of subcutaneous nicotine (0.1 – 0.6 mg/kg). On each FMI trial, the first lever press initiated an inter-response time (IRT); a head entry into a food receptacle terminated the IRT. IRTs longer than 6 s were intermittently reinforced with sucrose.
Results
A model that assumes that only a proportion of IRTs are sensitive to the timing contingencies of the FMI provided a close fit to the data, regardless of strain or treatment. No baseline difference in FMI performance was observed between SHR and WKY. Nicotine reduced the duration of timed IRTs and the duration of latencies to the IRT-initiating lever press similarly for both strains. Nicotine dose-dependently increased the proportion of timed IRTs; the dose-response curve was shifted leftwards in SHR relative to WKY.
Conclusions
These results suggest that nicotine (a) reduces response-inhibition capacity (b) enhances the reinforcement efficacy of sucrose, and (c) dose-dependently enhances attention-like sensitivity to contingencies of reinforcement, through mechanisms that are yet unknown.
Keywords: Nicotine, Impulsivity, Reinforcement, Attention deficit hyperactivity disorder, Spontaneously hypertensive rat, Fixed Minimum Interval schedule, Temporal Regulation, Response inhibition, Motivation
Introduction
Attention-deficit hyperactivity disorder (ADHD) is among the most common childhood psychiatric disorders (Froehlich et al. 2007; Skounti et al. 2007). Barkley (1997) suggested that the core features of ADHD are deficits in response inhibition capacity and sustained attention. Response inhibition capacity refers to the ability to withhold a reinforced response; it is typically assessed in humans using Go/No-Go tasks (e.g., Wodka et al. 2007). Sustained attention refers to the effortful maintenance of responding to a stimulus; it is typically assessed using vigilance tasks (e.g., Stins et al. 2005).
ADHD is associated with tobacco smoking during adolescence (Lambert and Hartsough 1998; Milberger et al. 1997). This link may reflect the ameliorating effect of tobacco on some ADHD-related deficits (Blume et al. 2000; Gehricke et al. 2007; Khantzian 1997; Pomerleau et al. 2000). Because nicotine is the main psychoactive ingredient of tobacco, it is possible that nicotine enhances response inhibition and/or sustained attention.
Effects of nicotine on response inhibition capacity
The effects of nicotine on response inhibition capacity in humans are somewhat disparate. Although acute nicotine appears to enhance the capacity to withhold an ongoing response regardless of smoking or ADHD status (Potter et al. 2012; Potter and Newhouse 2008; Potter and Newhouse 2004), these findings are inconsistent with other data from non-clinical smokers and non-smokers (Bekker et al. 2005; Wignall and de Wit 2011). The effects of acute nicotine on estimates of response inhibition capacity observed in vigilance tasks are also inconsistent: whereas some studies report substantial nicotine-induced enhancement in vigilance-related response inhibition capacity (Myers et al. 2013; Myers et al. 2008), other studies report very mild effects (Levin et al. 1998), effects related only to irrelevant stimuli (Dawkins et al. 2007), and dose-dependent effects (Bekker et al. 2005). Inconsistent effects of acute nicotine have also been observed in biased visual discrimination tasks (Barr et al. 2008; Wignall and de Wit 2011) that are discriminative of ADHD status (Tripp and Alsop 1999).
Unlike humans, acute nicotine administration in rodents consistently decreases response inhibition capacity in a wide range of tasks, including the 5-CSRTT (Bizarro et al. 2004; Blondel et al. 2000; Hahn et al. 2002; Semenova et al. 2007), the differential reinforcement of low rates (DRL) schedule of reinforcement (Kirshenbaum et al. 2011; Kirshenbaum et al. 2009; Kirshenbaum et al. 2008; Popke et al. 2000a; Popke et al. 2000b), the stop-signal task (Kirshenbaum et al. 2011), and the go/no-go discrimination (Kolokotroni et al. 2011). Only the temporal response differentiation task, which involves holding down a lever for a target interval, appears to be insensitive to nicotine-induced premature responding (Popke et al. 2000b).
Effects of nicotine on sustained attention
Acute nicotine appears to reliably enhance sustained attention in individuals with and without ADHD (Conners et al. 1996; Gehricke et al. 2006; Levin et al. 1998; Levin et al. 2000, 2001; Warburton 1992; for review see Heishman et al. 2010). Measures obtained from rats in the five-choice serial reaction time task (5-CSRTT) suggest that acute nicotine improves sustained attention in outbred rodent models as well (Bizarro et al. 2004; Blondel et al. 2000; Day et al. 2007; Hahn et al. 2002; Mirza and Stolerman 1998; Semenova et al. 2007). Results from a visual signal detection task, another test of vigilance, also support the hypothesis that nicotine improves attention in rodents (Rezvani et al. 2002; Rezvani and Levin 2004).
Human and animal studies are consistent in supporting the ameliorating effects of nicotine on sustained attention, but not on response inhibition capacity. In this study, we consider an explanation to the latter negative findings. Nicotine may not appear to enhance response inhibition capacity in past studies due to (a) the animal models used, and/or (b) the method by which response inhibition capacity was assessed in these models.
An animal model of ADHD
The negative effects of nicotine on response inhibition capacity may be due to the choice of animal model in which these effects were tested. Subjects in these studies typically are outbred rats that may not have inhibitory deficits to rescue. The effect of nicotine on response inhibition capacity may be different in animals that model ADHD-related deficits. The spontaneously hypertensive rat (SHR) is the most widely studied animal model of ADHD (Sagvolden 2000; Sagvolden et al. 2009). Despite the prevalent use of this strain, the majority of evidence suggests that sustained attention is not compromised in SHR (Van den Bergh et al. 2006; Thanos et al. 2010; but see Sagvolden and Xu 2008). In contrast, performance in response-withholding paradigms, such as the DRL schedule, consistently shows a reduced response inhibition capacity in SHR compared to normoactive controls (Evenden and Meyerson 1999, Ferguson et al. 2007, Orduña et al. 2009; Sagvolden and Berger 1996, Sanabria and Killeen 2008; van den Bergh et al. 2006). Therefore, despite its limitations as an animal model of ADHD, SHR appears to be an ideal model to test whether or not nicotine enhances response inhibition capacity.
Assessing response inhibition capacity
In tasks like the DRL, the ability to withhold a response for an incentive is confounded with the reinforcing efficacy of the incentive (Doughty and Richards 2002; Hill et al. 2012). Nicotine may appear to reduce response inhibition capacity when in reality it is enhancing the efficacy of a reinforcer. The present study implements a novel response inhibition paradigm that empirically isolates response inhibition capacity from reinforcer efficacy: the fixed minimum interval (FMI) schedule of reinforcement (Hill et al. 2012).
In the FMI schedule, reinforcement is contingent upon withholding a response for a programmed time interval. Intervals are initiated with a lever press (initial response) and terminated with a head entry into the food hopper (terminal response). Head entries made after the programmed interval elapses are reinforced. Although this arrangement is similar to the DRL, a key difference is that in the FMI the topography of the initial response and the terminal response are different. In this way, the FMI dissociates the capacity to withhold the terminal response (inferred from the intervals between initial and terminal responses) from the efficacy of the reinforcer in maintaining initial responses (inferred from the intervals between trial onset and initial response). Such dissociation is not possible with DRL schedules, because initial and terminal responses are identical. Put simply, motivation significantly affects presumed measures of response inhibition capacity in the DRL, but not in the FMI. This difference between FMI and DRL may explain why methylphenidate (MPH), which enhances response inhibition capacity in individuals with ADHD (Aron et al. 2003; Boonstra et al. 2005; DeVito et al. 2009), also enhances response-withholding performance of rats in the FMI (Hill et al. 2012), but not in the DRL (Van den Bergh et al. 2006; Ferguson et al. 2007; Hill et al. 2012; Orduña et al. 2009). In the FMI, acute MPH selectively reduces the interval between trial onset and initial response (Hill et al., 2012); because this interval and the interval between initial and terminal response are confounded in DRL schedules, MPH often induces short intervals between consecutive responses (Emmett-Oglesby et al. 1980; Ferguson et al. 2007; Orduña et al. 2009; Pearl and Seiden 1976; Seiden et al. 1979; but see Hill et el. 2012). We hypothesize that a similar confound explains nicotine-induced response-withholding deficits inferred from DRL performance. Thus, the FMI schedule was expected to reveal a lower baseline response inhibition capacity in SHR relative to a control strain. Also, in agreement with the symptom-amelioration hypothesis of ADHD-related smoking, the FMI was expected to reveal a nicotine-induced enhancement of reinforcer efficacy and inhibitory capacity.
Methods
Subjects
Twelve Spontaneously Hypertensive Rats (SHR/NCrl) served as experimental subjects; 11 Wistar Kyoto rats (WKY/NHsd) served as normoactive controls. Rats arrived on post-natal day (PND) 25, pair-housed by strain. Initially, 10 rats of each strain were received, but 3 WKY died within 3 days of arrival. One rat, left without a cagemate, was added to another pair to make one cage of 3 WKY. Before examining any performance data, a second cohort (4 WKY, 2 SHR) was added to compensate for the attrition. Both cohorts experienced identical procedures throughout the experiment. Shortly upon arrival, the duration of access to food was reduced daily from 24 h to 18 h, 12 h, and finally 1 h. For both SHR and WKY, this feeding regimen yielded weights at the beginning of each session that were, on average, 75.5% of mean ad libitum weights estimated from growth charts provided by the breeders. The average difference in weight between cagemates under food restriction was approximately 9% of their estimated mean ad libitum weights, which was similar to the 10% difference observed when food was freely available. Water was always available in the home cages ad libitum.
Apparatus
Experiments were conducted in 10 MED Associates (St. Albans, VT) modular test chambers (three chambers were 305 mm long, 241 mm wide, and 210 mm high; seven chambers were 305 mm long, 241 mm wide, and 292 mm high), each enclosed in a sound- and light-attenuating box equipped with a ventilating fan. The front and back walls and the ceiling of the test chambers were made of Plexiglas; the front wall was hinged and served as a door to the chamber. One of the two aluminum side panels served as a test panel. The floor consisted of thin metal bars positioned above a catch pan. A square opening (51 mm sides) located 15 mm above the floor and centered on the test panel provided access to the hopper (MED Associates, ENV-200-R2M) and was furnished with a head entry detector (ENV-254-CB). Each activation of a dispenser delivered a single 45-mg sucrose pellet (TestDiet, Richmond, IN) to the hopper. A multiple tone generator (MED Associates, ENV-223) was used to produce 3 kHz tones at approximately 75 dB through a speaker (MED Associates, ENV-224AM) centered on the top of the wall opposite to the test panel, 240 mm above the floor of the chamber. Two retractable levers (ENV-112CM) flanked the food hopper, and three-color light stimuli (ENV-222M) were mounted above each lever and could be illuminated yellow, green, and red. Lever presses were recorded when a force of approximately 0.2 N was applied to the end of the lever. The ventilation fan mounted on the rear wall of the sound-attenuating chamber provided masked noise of approximately 60 dB. The test chambers could be dimly illuminated by a houselight located behind the wall opposite to the test panel. Experimental events were arranged via a Med-PC® interface connected to a PC controlled by Med-PC IV® software.
Procedure
Figure 1 describes the sequence of events rats were exposed to. Sessions were conducted once daily, 7 days a week. Training initiated with autoshaping, consisting of pairing lever insertion with the delivery of a sucrose pellet. Once all rats were responding reliably to the lever, FMI training began. During FMI sessions, reinforcement was contingent upon the rat successfully waiting a given interval of time. The waiting interval was initiated by a lever press and terminated with a head entry into the food hopper. All terminal responses resulted in the retraction of the lever and a 5.5-s blackout period, after which the lever was reinserted and the next trial began. Each session ended after 60 min or after 150 sucrose pellets were delivered, whichever happened first. For a detailed description of training and the arrangement of sessions, see Mika et al. (2012).
Fig. 1.
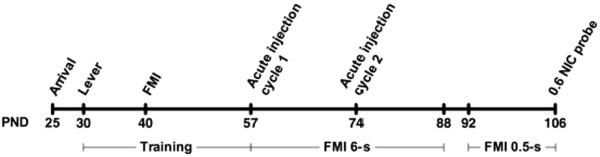
Timeline of events. Rats arrived at the facility on PND 25; after an acclimation period, they began lever training on PND 30. FMI training started on PND 40, and once performance stabilized on the FMI 6-s schedule, the treatment phase began on PND 57. During treatment, rats were injected with saline, three different doses of nicotine (0.1, 0.3, 0.6 mg/kg), and pre-fed in counterbalanced order. Rats were subjected to each treatment twice, with the second cycle beginning on PND 74. On PND 92, the criterial waiting time for the FMI schedule was reduced from 6 s to 0.5 s. After 14 days of experience with FMI 0.5-s, all animals received 0.6 mg/kg nicotine immediately prior to session (PND 106).
At the onset of FMI training, the criterial waiting time (t) was set to 0.5 s. Each correct response increased t by 1.25%; t was carried over from one session to the next until t = 6 s, and remained constant thereafter. Once the 6-s criterion had been established, a conjunctive variable interval (VI) 9-s schedule was introduced and gradually increased across sessions, to a VI 30-s schedule. The conjunctive VI 30-s schedule was implemented to reduce the between-subject variability in rate of reinforcement that would otherwise result from unequal performance (for details, see footnote 1). When there were no noticeable upward or downward trends in the proportion of correct responses in four consecutive days, as determined by visual inspection of the data, the treatment phase began.
Nicotine and pre-feeding treatments
Prior to PND 52, when treatment commenced, all rats where drug naïve. Treatment consisted of either a subcutaneous (s.c.) injection of saline, 0.1, 0.3, or 0.6 mg/kg of nicotine, 10 min prior to session, or ad libitum access to food in the home cage during the hour leading up to session start. Treatment was implemented twice per week, with at least 2 rest days between treatments. This drug regimen was chosen because neither tolerance nor sensitization effects were evidenced in a similar regimen implemented by Kirshenbaum et al. (2008) using DRL 4.5-s and Sprague Dawley rats. Within this range of doses, acute s.c. nicotine disrupts DRL performance (Kirshenbaum et al., 2008), timing (Hinton and Meck, 1996), improves performance in 5-CSRTT (Bizarro and Stolerman 2003; Hahn et al. 2003), induces place preference, and enhances social rewards (Thiel et al. 2009).
FMI 6-s sessions were conducted daily. (−)Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in saline, and sodium hydroxide was added until the pH of the solution was approximately 7.2. Nicotine dose was calculated as the base, and injection volume was based on body weight at the time of injection. All animals experienced two determinations of each dose and two pre-feeding (PF) sessions. Each animal received each treatment (dose or PF) once (cycle 1) and then again in the same order (cycle 2). Treatment order within each cycle was counterbalanced across animals.
FMI with minimal delay
Once the two cycles of treatment were completed, two daily FMI 6-s sessions were conducted in the absence of nicotine. Immediately following, 15 daily sessions were conducted in which the criterial time was reduced to 0.5 s (FMI 0.5-s). No injections were administered until the 15th day, when all rats received 0.6 mg/kg nicotine immediately prior to the FMI session. The purpose of this condition was to determine whether selected effects of nicotine on FMI 6-s performance were dependent on the 6-s waiting period.
Dependent measures
The primary dependent measures were median latency to initial lever press, and selected parameters of the distribution of inter-response times (IRTs), computed for each rat over individual sessions. Latencies are the intervals between lever presentation (start of the trial) and initial lever press. Latencies were classified into two categories based on the outcome of the previous trial: (1) post-R latencies: those following correct reinforced trials, and (2) post-N latencies: those following non-reinforced trials, including those following trials in which a correct response went unreinforced because the conjunctive VI had not elapsed, as well as those following incorrect responses (i.e., IRT < t).
IRT refers to the time elapsed between the initial lever press and the terminal head entry. The Temporal Regulation model was applied to estimate parameters of the distribution of IRTs (Mika et al. 2012; Sanabria and Killeen 2008). The model assumes that, at the beginning of every trial, rats sometimes enter a timing state with probability P. When in a timing state, rats produce IRTs that are gamma-distributed, centered close to the criterial FMI interval (here, 6 s). When rats are not in a timing state, they produce IRTs at a constant average rate, and as such, non-timing IRTs are exponentially distributed. Thus, according to the Temporal Regulation model, a mixture of two distributions, one gamma and one exponential, underlie the distribution of IRTs:
| (1) |
In Equation 1, the probability of entering a timing state, P, is the mixture weight of a gamma distribution with shape parameter n and scale parameter c. Both distributions, gamma and exponential, are shifted rightwards to account for the minimum time required to complete an IRT, δ. Thus, the mean duration of timed IRTs is nc + δ and the mean duration of non-timed IRTs is K + δ. Our analysis was primarily concerned with estimates of P and of the rescaled mean of the gamma distribution, θ = (nc + δ) / 6 s. If the mean timed IRT is shorter or longer than the 6-s criterial time, estimates of θ are, respectively, less than or greater than 1. Estimates of θ served as indices of response inhibition capacity (Sanabria and Killeen, 2008).
Data Analysis
IRTs and latencies were collected on every treatment day, on the day preceding each treatment (no-treatment days), and on the last 2 experimental days (FMI 0.5-s on no-treatment and on 0.6 mg/kg nicotine). Median latencies and estimates of θ were log-transformed. The proportion of correct IRTs (i.e., IRT > 6 s), the proportion of correct IRTs reinforced (i.e., reinforced IRTs / total IRTs), and estimates of P were log-odds transformed. These transformations follow suggestions on the estimation of population parameters in a similar model by Cheung, Neisewander and Sanabria (2012). All dependent measures are reported as back-transformed mean ± SEM. P and θ were estimated for each rat using the method of maximum likelihood (Myung 2003).
Analysis of variance (ANOVA)
ANOVA was implemented to establish the statistical significance of the effects of strain (SHR vs. WKY) and nicotine dose (vehicle vs. 0.1 vs. 0.3 vs. 0.6 mg/kg) on proportions of correct IRTs and correct IRTs reinforced. The effects of strain, nicotine dose, pre-feeding, schedule (FMI 6-s vs. 0.5-s) and treatment cycle (first vs. second) were examined on rate of reinforcement, median latencies and estimates of P and θ, using a significance threshold of α = .05. When sphericity was violated according to Mauchly's test, a Huynh-Feldt correction was implemented. Only significant main effects or interaction effects were followed by post hoc 2-tailed t-tests. Significant cycle effects were followed by a separate ANOVA in each cycle. Only significant effects are reported.
Nicotine effects
Separate 2 × 2 × 4 (strain × cycle × dose) mixed-design ANOVAs were conducted on median latencies and estimates of P and θ to establish the dose effects of nicotine on these dependent measures, and whether those effects were modulated by strain and treatment cycle.
Pre-feeding effects
Separate 2 × 2 × 2 (strain × cycle × feeding status: not pre-fed at vehicle vs. pre-fed) mixed-design ANOVAs were conducted on median latencies and estimates of P and θ to establish the effects of pre-feeding on these dependent measures, and whether such effects were modulated by strain and treatment cycle. This analysis was intended to identify effects related to a potential nicotine-induced reduction in appetite (Dandekar et al. 2011; Wellman et al. 2005). Nicotine effects that matched pre-feeding effects were discounted as potentially related to appetite reduction and not to performance enhancement.
Schedule effects on latencies
Separate 2 × 2 × 2 (strain × schedule: FMI 0.5-s vs. 6-s on 0.6 mg/kg in second cycle; treatment: no-treatment vs. treatment day) mixed-design ANOVAs were conducted on post-R and post-N latencies to establish whether strain and nicotine effects on latency were modulated by the length of the criterial waiting time. Only the second cycle of 0.6 mg/kg FMI 6-s treatment was used in this comparison to minimize the confound between schedule and order effects (FMI 6-s was implemented before FMI 0.5-s, see Figure 1).
Stability of rate of reinforcement
Rate of reinforcement was measured as the number of reinforcers obtained per 60-min session. Although the maximum number of reinforcers obtainable in a session was 150 pellets, no rat reached this limit in any experimental session. The conjunctive VI schedule was expected to keep rate of reinforcement relatively constant across experimental manipulations. Stability of rate of reinforcement was tested by conducting a 2 × 2 × 4 (strain × cycle × dose) and a 2 × 2 × 2 (strain × cycle × feeding status) mixed-design ANOVAs, with reinforcers per session as the dependent measure.
Coefficient of variation
The estimation of the coefficient of variation (CV) of timed IRTs is often included as part of the analysis of FMI performance (Sanabria and Killeen 2008). Higher CV is indicative of less precise timing (Sanabria and Killeen 2008). In this study we monitored CV across manipulations, but do not report it because baseline timing is not less precise in SHR than in WKY (Orduña et al. 2009; Orduña et al. 2008; Sanabria and Killeen 2008). This null finding was replicated here in the vehicle condition, CVSHR = 0.18 +/− 0.01, CVWKY = 0.20 +/− 0.01. Thus, SHR does not appear to be an adequate model of timing deficits in ADHD (Toplak et al. 2006); effects of nicotine in this domain are, therefore, uninterpretable.
Results
Temporal Regulation Parameters
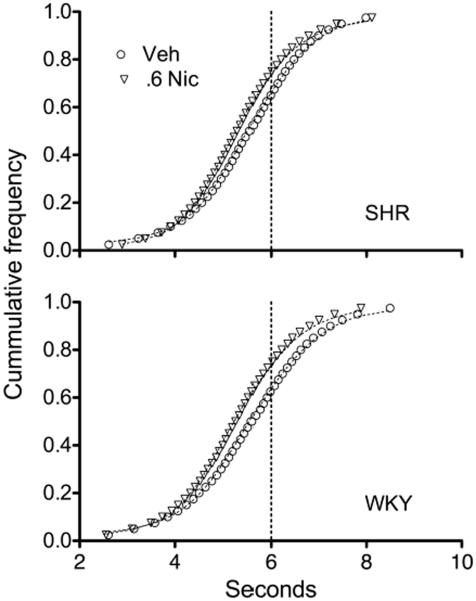
In all conditions the distribution of IRTs were well described by a mixture of two underlying distributions, one gamma (timed IRTs) and one exponential (non-timed IRTs) (Eq. 1). Figure 2 illustrates the goodness-of-fit of this model to performance under vehicle and under the highest dose of nicotine, 0.6 mg/kg.
Fig. 2.
Mean cumulative frequency distributions of IRTs produced by SHR (top) and WKY (bottom) after s.c. injections of vehicle (circles) and 0.6 mg/kg nicotine (downward triangles). Data are organized in 39 bins, each containing approximately equal number of IRTs. Dotted lines are fits of the Temporal Regulation (TR) model of response inhibition capacity (Eq. 1).
Nicotine effects
A significant main effect of nicotine dose was observed on the proportion of correct IRTs, F(3,63) = 4.23, p = .009. Post hoc paired-sample t-tests revealed that all doses of nicotine reduced the proportion of correct IRTs, from an average of 51% at baseline to 33–36% under nicotine; t(22) ranged between 2.25, p = .035, and 3.00, p = .007. No significant effect of nicotine dose or strain was observed on the proportion of correct IRTs reinforced, which was on average 54%.
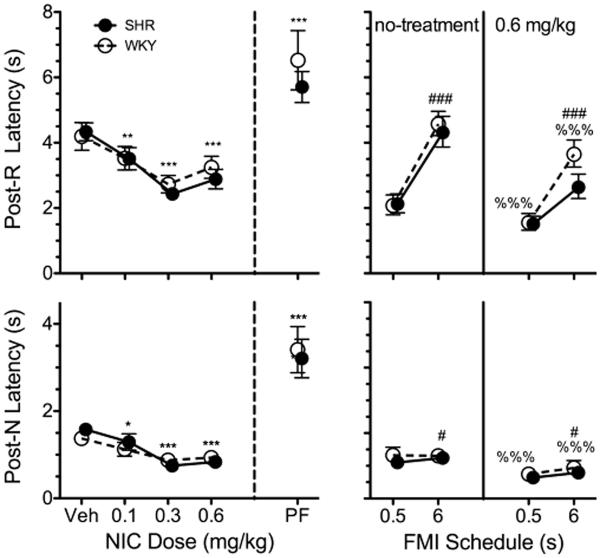
Figure 3 shows the effects of strain and dose on estimates of θ and P. A significant main effect of dose on θ estimates was observed, F(3, 63) = 4.64, p = .005. Post hoc paired-samples t-tests revealed that, relative to vehicle, all doses of nicotine significantly reduced θ; t(22) ranged between 2.58, p = .017, and 3.31, p = .003. Significant strain × dose interaction effects on estimates of P were observed, F(3, 63) = 5.05, p = .003. Post hoc t-tests on P revealed that it was (a) significantly higher for SHR than WKY on 0.1 mg/kg nicotine, t(21) = 2.33, p = .030, (b) significantly higher for SHR on 0.1 and 0.3 mg/kg nicotine than on vehicle, t(11) = 4.41, p = .001, and t(11) = 4.05, p = .002, respectively, and (c) significantly higher for WKY on 0.3 and 0.6 than on vehicle, t(10) = 2.79, p = .019, and t(10) = 2.64, p = .024. Overall, these results suggest that nicotine reduced response inhibition capacity, but dose-dependently increased the sensitivity to the temporal contingencies of reinforcement, requiring a lower dose for the latter improvement in SHR.
Fig. 3.
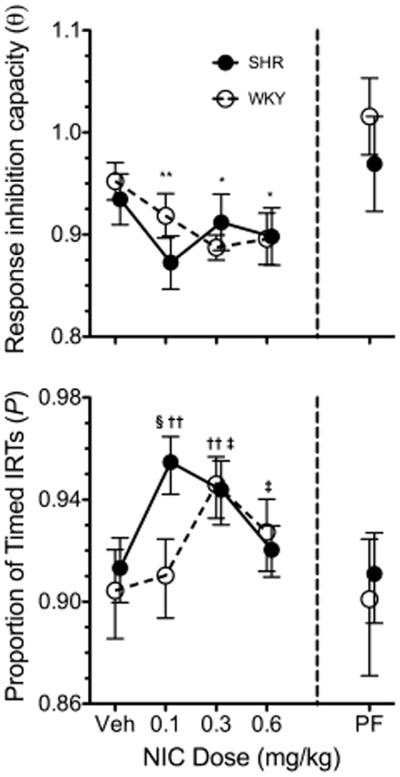
Mean estimates of θ (top) and P (bottom) for SHR (solid circles) and WKY (open circles) rats across vehicle (Veh), 0.1, 0.3, and 0.6 mg/kg s.c. nicotine (left of vertical dashed line) and pre-feeding (PF; right of vertical dashed line). *Significant difference between dose and Veh. §Significant simple main effect of strain at a dose level. †Significant difference between dose and Veh in SHR. ‡Significant difference between dose and Veh in WKY. Symbols are repeated with lower p-values (i.e., *p < .050, **p < .010, ***p < .001). A significant strain × cycle × feeding status interaction effect was observed on θ, but no significant main or interaction effect of strain or feeding status on θ were observed in either cycle. All doses of nicotine reduced estimates of θ relative to Veh. Nicotine increased estimates of P in a dose-dependent manner and differentially across strains. The nicotine dose-response curve of P was shifted leftwards in SHR relative to WKY.
Pre-feeding effects
Figure 3 shows the effects of pre-feeding (PF) on estimates of θ and P. A significant strain × cycle × feeding status interaction effect was observed on θ estimates, F(1, 21) = 5.61, p = .028. A separate analysis was conducted on θ estimates at each cycle. No significant effect of strain or feeding status was observed on θ in either cycle, suggesting that the 3-way interaction effect on θ was an effect of cycle modulated by strain and feeding status. No significant effects of strain, cycle, or feeding status were observed on P.
Latencies
Nicotine effects
Figure 4 (left) shows the effects of strain and dose on post-R and post-N latencies. Significant effects of dose on post-R and post-N latencies were observed [post-R: F(3, 63) = 19.83, p < .001; post-N: F(3, 63) = 28.16, p < .001]. Post hoc paired-sample t-tests revealed that all doses of nicotine significantly shortened post-R and post-N latencies relative to vehicle; t(22) ranged between 2.36, p = .027, and 8.21, p < .001. No significant effect of strain was observed. Thus, regardless of strain or of the outcome in the preceding trial, even the lowest dose of nicotine was effective in reducing the time between trial onset and first lever press.
Fig. 4.
Mean median latencies to the initial lever press across nicotine and pre-feeding (PF) treatment conditions (left panel) and across FMI schedules (right panel; FMI 6-s data is from cycle 2) for SHR (solid circles) and WKY (open circles) rats. “Post-R” denotes the latencies following reinforced trials; “Post-N” denotes latencies following non-reinforced trials. *Significant difference between dose and Veh. #Significant main effect of FMI schedule, %Significant main effect of nicotine relative to no-treatment (day preceding injection). Symbols are repeated with lower p-values (i.e., *p < .050, **p < .010, ***p < .001). Nicotine and shorter FMI target time reduced median post-R and post-N latencies; pre-feeding increased median post-R and post-N latencies.
Pre-feeding effects
Figure 4 (PF) shows the effects of strain and feeding status on post-R and post-N latencies. Significant main effects of feeding status on post-R and post-N latencies were observed [post-R: F(3, 63) = 20.08, p < .001; post-N: F(3, 63) = 62.20, p < .001]. All latencies were roughly 2 s longer when rats were pre-fed. No significant strain × feeding status interaction effect was observed. Thus, regardless of strain or of the outcome of the preceding trial, pre-feeding increased the time between trial onset and first lever press.
Schedule effects
Figure 4 (right) shows the effects of an s.c. injection of 0.6 mg/kg on FMI 0.5-s and FMI 6-s performance. Significant main effects of schedule and nicotine were observed in both post-R [respectively: F(1, 21) = 61.56, p < .001; F(1,21) = 27.92, p < .001] and post-N latencies [respectively: F(1,21) = 4.58, p = .044; F(1,21) = 51.06, p < .001]. No significant effect of strain or interaction effect was observed. These results suggest that the nicotine-induced shortening of latencies was not schedule-dependent, and was observable even when reinforcement was minimally delayed.
Stability of Rate of Reinforcement
A significant strain × dose effect on rate of reinforcement was observed, F(3,63) = 2.84, p = .045. Post hoc paired-sample t-tests revealed that WKY obtained fewer reinforcers under 0.6 mg/kg nicotine than under vehicle, t(10) = 3.29, p = .008. This means that the effect of nicotine on WKY performance was confounded with the effects of reduced rate of reinforcement only at the highest dose. At other doses in WKY and all doses in SHR, these effects were not confounded.
A significant strain × cycle × feeding status effect on rate of reinforcement was observed, F(1,21) = 310.42, p = .004. Post hoc t-tests were conducted separately in each cycle. Post hoc comparisons revealed that, in the second cycle, pre-feeding substantially reduced the rate of reinforcement of WKY relative to no pre-feeding, t(10) = 2.98, p = .014, and relative to pre-fed SHR, t(21) = 2.97, p = .007.
Discussion
Nicotine and response inhibition capacity
The acute administration of nicotine reduced estimates of θ similarly for SHR and WKY (Figure 3). This result suggests that acute nicotine reduces response inhibition capacity in both strains. Although this finding is inconsistent with our expectations, it is consistent with nicotine-induced reductions in IRTs observed in DRL studies using Sprague Dawley rats (Kirshenbaum et al. 2008, 2009, 2011). Performance in DRL schedules cannot be readily interpreted in terms of response inhibition capacity, because it is also sensitive to reinforcer-efficacy manipulations (e.g., reinforcer magnitude; Doughty and Richards 2002). In contrast, estimates of θ are not significantly sensitive to changes in reinforcer efficacy via pre-feeding (Figure 3). Yet, nicotine also appears to reduce θ, suggesting that the effect of nicotine on DRL performance may not be solely explained on the basis of enhanced reinforcer efficacy.
An alternative account of our results would suggest that reduced estimates of θ resulted from a faster internal clock under acute nicotine (Hinton and Meck 1996). Intervals trained without nicotine may be perceived as being longer under nicotine, thus yielding shorter timed IRTs when nicotine was acutely administered. The accelerative effects of nicotine on timing, however, have only been demonstrated using the peak interval method (Hinton and Meck 1996; Meck 2007), which is vulnerable to confounding motivational effects (Galtress and Kirkpatrick 2009; Ludvig et al. 2011; Plowright et al. 2000; Sanabria et al. 2009). Timing estimates that are more robust to motivational manipulations, such as those obtained from the temporal bisection procedure (Galtress and Kirkpatrick, 2010), do not suggest an accelerative effect of nicotine on the internal clock (Ward et al. 2009). These findings do not support an explanation of nicotine-induced effects on θ based on timing mechanisms.
Neither changes in reinforcer efficacy nor changes in rate of reinforcement can explain nicotine-induced reductions in estimates of θ. Pre-feeding did not have a significant effect on estimates of θ (Figure 3), but it increased latencies, an effect opposite that of nicotine. The robustness of θ to the pre-feeding manipulation suggests that, consistent with prior findings (Mechner and Guevrekian 1962), mean timed IRTs are robust against changes in reinforcer efficacy. Relative to baseline, rate of reinforcement was significantly lower only for WKY at the highest dose of nicotine. The stability of rate of reinforcement in both strains across most conditions was primarily due to the conjunctive VI schedule of reinforcement that imposed a minimum (but variable) amount of time between reinforcers. The limited effect of nicotine on rate of reinforcement cannot account for reductions in estimates of θ in both strains at every dose of nicotine. Thus, it appears that nicotine-induced reductions in estimates of θ reflect a nicotine-induced reduction in the capacity of both SHR and WKY to withhold a reinforced response.
Given that nicotine reduces response inhibition capacity so consistently in rodent models, it is unclear why the effects of nicotine on response inhibition capacity appear to vary so much among human studies. The key to these inconsistencies may be the underlying processes assessed by divergent methodologies. For instance, nicotine-induced improvements in human response-inhibition capacity are primarily observed in the stop-signal task and in the Stroop task (Potter et al. 2012; Potter and Newhouse 2008; Potter and Newhouse 2004; Wignall and de Wit 2011). In these tasks, the behavior to be withheld is prepotent because it is either already initiated (stop signal task) or because it is strongly associated with a present stimulus (Stroop task). In contrast, most rodent paradigms, including DRL and FMI schedules, are based on behavior that is potentiated by its consequences. Nicotine has detrimental effects on performance in analogous tasks in humans, such as the biased visual discrimination task used by Barr et al. (2008).
The absence of strain effects on estimates of θ at baseline tempers our interpretation of the measure of response inhibition capacity obtained from FMI performance. To the extent that θ reflects response inhibition capacity, and SHR models inhibitory deficits associated with ADHD, lower estimates of θ would be expected in SHR relative to WKY. This difference between strains has been observed systematically in the DRL (Ferguson et al. 2007, Orduña et al. 2009; Sagvolden and Berger 1996, Sanabria and Killeen 2008; van den Bergh et al. 2006), but was not observed in the present study using the FMI. It is thus likely that procedural differences between FMI and DRL schedules are responsible for these divergent results. Various features of the FMI—the longer resting period between withholding trials, the response-initiated nature of these trials, the separation of initial and terminal responses—may facilitate the response-withholding performance of SHR.
Nicotine and reinforcer efficacy
Mechner and Guevrekian (1962) demonstrated that FMI latencies, but not IRTs, are sensitive to reinforcer deprivation. Our results are consistent with those findings, showing that pre-feeding increased latencies regardless of strain and of the outcome of the preceding trial (i.e., post-R vs. post-N; Figure 4, left). These results support the interpretation of changes in latencies as reflecting changes in reinforcer efficacy. The reduction in latencies following nicotine administration (Figure 4, left) thus suggests that nicotine enhanced the reinforcing efficacy of sucrose pellets. These data are consistent with reports of nicotine-induced enhancement of reinforcer efficacy for appetitive reinforcers or food-related cues (see Donny et al. 2011) in both rats (Grimm et al. 2012; Raiff and Dallery 2006; Wing and Shoaib 2010) and humans (Epstein et al. 1992; Perkins 1992).
In principle, however, it is possible that the reduction in latencies following nicotine administration reflects a nicotine-induced reduction in the sensitivity to the delay of reinforcement. Prior research (Morgan 1972) and the positive relationship between latencies and FMI target time (0.5 s vs. 6 s; Figure 4), support the notion that latencies are sensitive to delay of reinforcement. If nicotine effects on latencies were mediated by changes in sensitivity to delay of reinforcement, it would be expected that the elimination of the delay of reinforcement would also eliminate the effect of nicotine on latencies. Contrary to that expectation, however, nicotine produced shorter latencies even in FMI 0.5 s, when delay to reinforcement was minimal (Figure 4, right panels). Such effect supports the hypothesis that changes in latencies induced by nicotine reflect changes in reinforcer efficacy and not in sensitivity to the delay of reinforcement.
Nicotine and sensitivity to timing contingencies
In all conditions the distribution of IRTs were well described by a mixture of two underlying distributions, one gamma (timed IRTs) and one exponential (non-timed IRTs) (Eq. 1, Figure 2). Generally, more than 90% of the IRTs were timed, signified by parameter P. The remaining 10% of intervals (1-P) appear to be produced absent of the control of the timing contingencies, as they are produced at a constant rate. Although it has not been explicitly evaluated in the FMI schedule, this loss of control by the contingencies of reinforcement may be attributed to lapses in attention (Killeen et al. 2013; Sagvolden et al. 1998).
In the present study, the proportion of timed intervals did not differ significantly at baseline (vehicle) between SHR and WKY (Figure 3, bottom). This lack of significant baseline differences has also been observed in paradigms designed to assess sustained attention in rodents, such as the 5-CSRTT (van den Bergh et al. 2006) and the visual stimulus position discrimination task (Thanos et al. 2010). The evidence available suggests that the SHR is not an adequate model of ADHD-related deficits in sustained attention.
Nevertheless, the effects of nicotine on P may be informative. It was observed that P increased following nicotine administration, tracing an inverted-U dose response function that peaked at a lower dose for SHR (0.1 mg/kg) than for WKY (0.3 mg/kg; Figure 3). This effect suggests that, relative to WKY, SHR required a lower dose of nicotine to increase the proportion of timed IRTs. If P were to be interpreted in terms of attentional processes, these results would be the opposite of what would be expected. This is because the SHR, compared to WKY, has fewer α4β2 nicotinic receptors (Wigestrand et al. 2011), which appear to mediate the enhancement of sustained attention in rodents induced by nicotine (Rezvani et al. 2011; Young et al. 2013). Other nicotinic receptor subunits potentially involved in attentional processes, such as α7 (Leiser et al. 2009), do not vary significantly in number between SHR and WKY (Wigestrand et al. 2011) and may therefore mediate the effect of nicotine on estimates of P. Nonetheless, the involvement of α7 receptors in sustained attention in rats is disputable (Grottick and Higgins 2000; Shoaib and Bizarro 2005). Furthermore, estimates of P do not appear to increase with MPH treatment in rats (Hill et al. 2012), even though past research has shown that MPH enhances sustained attention in both rats (Paine et al. 2007) and humans (Epstein et al. 2006; Riccio et al. 2001). A potential explanation to these seemingly contradictory findings requires that nicotine and MPH enhance sustained attention through different mechanisms (Levy and Hobbes 1996; McGaughy et al. 1999), and P indexes a nicotine-sensitive attention-like process that is not necessarily mediated by α4β2 nicotinic receptors. Although it is yet unclear what neural processes are indexed by P, these processes are likely to have implications in the research and treatment of attentional deficits.
Conclusion
This investigation provides the first evidence, in an animal model of ADHD, for nicotine-induced reduction in response inhibition capacity that is not confounded with reinforcer-efficacy effects. In particular, our data suggests that nicotine hinders the capacity of rats to withhold a response that has been instrumentally reinforced. This result undermines the hypothesis that smoking among individuals with ADHD is facilitated by an ameliorating effect of nicotine on response inhibition deficits.
Acute nicotine increased the degree to which rats responded to reinforcement contingencies. This effect peaked at a lower dose for the animal model of ADHD (0.1 mg/kg) than for its control (0.3 mg/kg). Along with observations from past research and pre-feeding manipulations, it suggests that the mechanism by which nicotine enhances the sensitivity to reinforcement contingencies involve neither α4β2 nicotinic receptors—which are often involved in sustained attention—nor motivational mechanisms on which MPH appear to operate (Levy and Hobbes 1996). Future research may unveil the specific mechanisms supporting this effect.
Acknowledgements
This study was supported by funds from the National Institutes of Health. The authors wish to acknowledge M. Foster Olive and Natalie Peartree for their support, and Raul Garcia, Cameron Gibbons, Fritzgerald Jerome, Tara Mahmood, Jennifer May, Alexandra Paul, Marie Simonsen, Alexander Spitzer, Charles Wilson, and Alex Zoloto for assistance in data collection.
This study was funded by two grants from the National Institutes of Health (DA032632, MH094562)
Footnotes
No conflicts of interest declared
The VI schedule was implemented as follows: A timer ran throughout the session. Reinforcement became available when the timer completed a specified interval, with one exception: if the interval elapsed after the initial response, reinforcement was not available until the subsequent trial. After each reinforcer, the timer was reset and a new interval was specified. If a correct response was made before reinforcement became available, the rat was exposed to the 3kHz tone, but sucrose reinforcement was withheld. Intervals were specified by sampling without replacement from a 12-item Fleschler-Hoffman distribution (Fleshler & Hoffman, 1962). The VI-schedule requirement progressed in daily succession (9, 15, 20, 30 s), until all rats were performing at VI 30-s and t = 6 s.
The VI schedule was implemented to reduce the between-subject variability in rate of reinforcement that would otherwise result from unequal performance. With this control in place, differences in performance could be reliably attributed to the experimental manipulation and not to differences in rate of reinforcement.
References
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. doi: 10.1016/S0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, et al. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biological psychiatry. 2008;63:1061–5. doi: 10.1016/j.biopsych.2007.09.015. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker EM, Böcker KBE, Van Hunsel F, et al. Acute effects of nicotine on attention and response inhibition. Pharmacology, biochemistry, and behavior. 2005;82:539–48. doi: 10.1016/j.pbb.2005.10.009. doi: 10.1016/j.pbb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Van den Bergh FS, Bloemarts E, Chan JSW, et al. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacology, biochemistry, and behavior. 2006;83:380–90. doi: 10.1016/j.pbb.2006.02.018. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. 2004;15:195–206. doi: 10.1097/01.fbp.0000131574.61491.50. [PubMed] [Google Scholar]
- Bizarro L, Stolerman IP. Attentional effects of nicotine and amphetamine in rats at different levels of motivation. Psychopharmacology. 2003;170:271–7. doi: 10.1007/s00213-003-1543-6. doi: 10.1007/s00213-003-1543-6. [DOI] [PubMed] [Google Scholar]
- Blondeau C, Dellu-Hagedorn F. Dimensional analysis of ADHD subtypes in rats. Biological psychiatry. 2007;61:1340–50. doi: 10.1016/j.biopsych.2006.06.030. doi: 10.1016/j.biopsych.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Blondel A, Sanger DJ, Moser PC. Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology. 2000;149:293–305. doi: 10.1007/s002130000378. [DOI] [PubMed] [Google Scholar]
- Blume A, Schmaling K, Marlatt G. Revisiting the self-medication hypothesis from a behavioral perspective. Cognitive and Behavioral Practice. 2000;7:379–384. [Google Scholar]
- Boonstra AM, Kooij JJS, Oosterlaan J, et al. Does methylphenidate improve inhibition and other cognitive abilities in adults with childhood-onset ADHD? Journal of clinical and experimental neuropsychology. 2005;27:278–98. doi: 10.1080/13803390490515757. doi: 10.1080/13803390490515757. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. American journal of public health. 1996;86:214–20. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung THC, Neisewander JL, Sanabria F. Extinction under a behavioral microscope: isolating the sources of decline in operant response rate. Behavioural processes. 2012;90:111–23. doi: 10.1016/j.beproc.2012.02.012. doi: 10.1016/j.beproc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Levin ED, Sparrow E, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacology. 1996;32:67–73. [PubMed] [Google Scholar]
- Dandekar MP, Nakhate KT, Kokare DM, Subhedar NK. Effect of nicotine on feeding and body weight in rats: involvement of cocaine- and amphetamine-regulated transcript peptide. Behavioural brain research. 2011;219:31–8. doi: 10.1016/j.bbr.2010.12.007. doi: 10.1016/j.bbr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, et al. A double-blind placebo-controlled experimental study of nicotine: II--Effects on response inhibition and executive functioning. Psychopharmacology. 2007;190:457–67. doi: 10.1007/s00213-006-0634-6. doi: 10.1007/s00213-006-0634-6. [DOI] [PubMed] [Google Scholar]
- Day M, Pan JB, Buckley MJ, et al. Differential effects of ciproxifan and nicotine on impulsivity and attention measures in the 5-choice serial reaction time test. Biochemical pharmacology. 2007;73:1123–34. doi: 10.1016/j.bcp.2006.12.004. doi: 10.1016/j.bcp.2006.12.004. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Clark L, et al. Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD) Psychopharmacology. 2009;202:531–9. doi: 10.1007/s00213-008-1337-y. doi: 10.1007/s00213-008-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Weaver MT, et al. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiology & behavior. 2011;104:143–8. doi: 10.1016/j.physbeh.2011.04.043. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty AH, Richards JB. Effects of reinforcer magnitude on responding under differential-reinforcement-of-low-rate schedules of rats and pigeons. Journal of the experimental analysis of behavior. 2002;78:17–30. doi: 10.1901/jeab.2002.78-17. doi: 10.1901/jeab.2002.78-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Taylor KE, Dafter RE. Differential effects of methylphenidate on signalled and non-signalled reinforcement. Pharmacology, biochemistry, and behavior. 1980;13:467–70. doi: 10.1016/0091-3057(80)90257-9. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Conners CK, Hervey AS, et al. Assessing medication effects in the MTA study using neuropsychological outcomes. Journal of child psychology and psychiatry, and allied disciplines. 2006;47:446–56. doi: 10.1111/j.1469-7610.2005.01469.x. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Caggiula AR, Perkins KA, et al. Abstinence from smoking decreases habituation to food cues. Physiology & behavior. 1992;52:641–6. doi: 10.1016/0031-9384(92)90391-e. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Paule MG, Cada A, et al. Baseline behavior, but not sensitivity to stimulant drugs, differs among spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rat strains. Neurotoxicology and teratology. 2007;29:547–61. doi: 10.1016/j.ntt.2007.07.001. doi: 10.1016/j.ntt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Archives of pediatrics & adolescent medicine. 2007;161:857–64. doi: 10.1001/archpedi.161.9.857. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Galtress T, Kirkpatrick K. Reward value effects on timing in the peak procedure. Learning and Motivation. 2009;40:109–131. doi: 10.1016/j.lmot.2008.05.004. [Google Scholar]
- Gehricke J-G, Loughlin SE, Whalen CK, et al. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine & tobacco research. 2007;9(Suppl 4):S523–36. doi: 10.1080/14622200701685039. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Gehricke J-G, Whalen CK, Jamner LD, et al. The reinforcing effects of nicotine and stimulant medication in the everyday lives of adult smokers with ADHD: A preliminary examination. Nicotine & tobacco research. 2006;8:37–47. doi: 10.1080/14622200500431619. doi: 10.1080/14622200500431619. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behavioural Brain Research. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Grimm JWJ, Ratliff C, North K, et al. Nicotine increases sucrose self-administration and seeking in rats. Addiction biology. 2012;17:623–633. doi: 10.1111/j.1369-1600.2012.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology. 2002;162:129–37. doi: 10.1007/s00213-002-1005-6. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–69. doi: 10.1007/s00213-010-1848-1. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and Smoking : A Review of Effects on Human Performance. Experimental and Clinical Psychopharmacology. 1994;2:345–395. [Google Scholar]
- Hill JC, Covarrubias P, Terry J, Sanabria F. The effect of methylphenidate and rearing environment on behavioral inhibition in adult male rats. Psychopharmacology. 2012;219:353–62. doi: 10.1007/s00213-011-2552-5. doi: 10.1007/s00213-011-2552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton S, Meck W. Increasing the speed of an internal clock: the effects of nicotine on interval timing. Drug development research. 1996;38:204–211. [Google Scholar]
- Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. The American journal of psychiatry. 2006;163:716–23. doi: 10.1176/appi.ajp.163.4.716. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The Self-Medication Hypothesis of Substance Use Disorders: A Reconsideration and Recent Applications. Harvard review of psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Russell V a, Sergeant J a. A behavioral neuroenergetics theory of ADHD. Neuroscience and biobehavioral reviews. 2013;37:625–57. doi: 10.1016/j.neubiorev.2013.02.011. doi: 10.1016/j.neubiorev.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AP, Brown SJ, Hughes DM, Doughty AH. Differential-reinforcement-of-low-rate-schedule performance and nicotine administration: a systematic investigation of dose, dose-regimen, and schedule requirement. Behavioural pharmacology. 2008;19:683–97. doi: 10.1097/FBP.0b013e328315ecbb. doi: 10.1097/FBP.0b013e328315ecbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Jackson ER, Brown SJ, et al. Nicotine-induced impulsive action: sensitization and attenuation by mecamylamine. Behavioural pharmacology. 2011;22:207–21. doi: 10.1097/FBP.0b013e328345ca1c. doi: 10.1097/FBP.0b013e328345ca1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Johnson MWM, Schwarz SL, Jackson ER. Response disinhibition evoked by the administration of nicotine and nicotine-associated contextual cues. Drug and alcohol dependence. 2009;105:97–108. doi: 10.1016/j.drugalcdep.2009.06.018. doi: 10.1016/j.drugalcdep.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology. 2011;217:455–73. doi: 10.1007/s00213-011-2296-2. doi: 10.1007/s00213-011-2296-2. [DOI] [PubMed] [Google Scholar]
- Koschack J, Kunert HJ, Derichs G, et al. Impaired and enhanced attentional function in children with attention deficit/hyperactivity disorder. Psychological Medicine. 2003;33:481–489. doi: 10.1017/s0033291702007067. doi: 10.1017/S0033291702007067. [DOI] [PubMed] [Google Scholar]
- Lambert N, Hartsough C. Prospective Study of Tobacco Smoking and Substance Dependencies among Samples of ADHD and Non-ADHD Participants. Journal of Learning Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Bowlby MR, Comery TA, et al. A cog in cognition: How the α7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacology & Therapeutics. 2009;122:302–311. doi: 10.1016/j.pharmthera.2009.03.009. doi:10.1016/j.pharmthera.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, et al. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140:135–41. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, et al. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Experimental and Clinical Psychopharmacology. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. doi: 10.1037//1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB, Conners CK. Nicotine in psychiatry: psychopathology and emerging therapeutics Wiley. New York: 2000. Nicotine effects and attention deficit disorder; pp. 203–214. [Google Scholar]
- Levy F, Hobbes G. Does haloperidol block methylphenidate? Psychopharmacology. 1996;126:70–74. doi: 10.1007/BF02246413. [DOI] [PubMed] [Google Scholar]
- Loitfelder M, Fazekas F, Petrovic K, et al. Reorganization in cognitive networks with progression of multiple sclerosis Insights from fMRI. Neurology. 2011;76:526–533. doi: 10.1212/WNL.0b013e31820b75cf. [DOI] [PubMed] [Google Scholar]
- Ludvig EA, Balci F, Spetch ML. Reward magnitude and timing in pigeons. Behavioural processes. 2011;86:359–63. doi: 10.1016/j.beproc.2011.01.003. doi: 10.1016/j.beproc.2011.01.003. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology. 1999;144:175–82. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- Mechner F, Guevrekian L. Effects of deprivation upon counting and timing in rats. Journal of the experimental analysis of behavior. 1962;5:463–466. doi: 10.1901/jeab.1962.5-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH. Acute ethanol potentiates the clock-speed enhancing effects of nicotine on timing and temporal memory. Alcoholism, clinical and experimental research. 2007;31:2106–13. doi: 10.1111/j.1530-0277.2007.00540.x. doi: 10.1111/j.1530-0277.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- Mika A, Mazur GJ, Hoffman AN, et al. Chronic Stress Impairs Prefrontal Cortex-Dependent Response Inhibition and Spatial Working Memory. Behavioral neuroscience. 2012 doi: 10.1037/a0029642. doi: 10.1037/a0029642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone S, et al. ADHD is associated with early initiation of cigarette smoking in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology. 1998;138:266–74. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Morgan M. Fixed-ratio performance under conditions of delayed reinforcement. Journal of the experimental analysis of behavior. 1972:95–98. doi: 10.1901/jeab.1972.17-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–98. doi: 10.1038/sj.npp.1301425. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Salmeron BJ, et al. Nicotine Enhances Alerting, but not Executive, Attention in Smokers and Nonsmokers. Nicotine & tobacco research. 2013;15:277–81. doi: 10.1093/ntr/nts108. doi: 10.1093/ntr/nts108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung IJ. Tutorial on maximum likelihood estimation. Journal of Mathematical Psychology. 2003;47:90–100. doi: 10.1016/S0022-2496(02)00028-7. [Google Scholar]
- Navarra R, Graf R, Huang Y, et al. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Orduña V, García A, Menez M, et al. Performance of spontaneously hypertensive rats in a peak-interval procedure with gaps. Behavioural brain research. 2008;191:72–6. doi: 10.1016/j.bbr.2008.03.012. doi: 10.1016/j.bbr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Orduña V, Valencia-Torres L, Bouzas A. DRL performance of spontaneously hypertensive rats: dissociation of timing and inhibition of responses. Behavioural brain research. 2009;201:158–65. doi: 10.1016/j.bbr.2009.02.016. doi: 10.1016/j.bbr.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA. Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biological psychiatry. 2007;62:687–93. doi: 10.1016/j.biopsych.2006.11.017. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Parry AMM, Scott RB, Palace J, et al. Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain. 2003;126:2750–60. doi: 10.1093/brain/awg284. doi: 10.1093/brain/awg284. [DOI] [PubMed] [Google Scholar]
- Pearl R, Seiden L. The existence of tolerance to and cross-tolerance between d-amphetamine and methylphenidate for their effects on milk consumption and on differential-reinforcement-of-low-rate performance in the rat. Journal of pharmacology and experimental therapeutics. 1976;198:635–647. [PubMed] [Google Scholar]
- Perkins KA. Effects of tobacco smoking on caloric intake. British journal of addiction. 1992;87:193–205. doi: 10.1111/j.1360-0443.1992.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Plowright C, Church D, Behnke P, Silverman a. Time estimation by pigeons on a fixed interval: the effect of pre-feeding. Behavioural processes. 2000;52:43–48. doi: 10.1016/s0376-6357(00)00110-8. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerleau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine & tobacco research. 2000;2:275–80. doi: 10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. Journal of substance abuse. 1995;7:373–8. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Fogle CM, Paule MG. Ethanol Enhances Nicotine's Effects on DRL Performance in Rats. Pharmacology Biochemistry and Behavior. 2000a;66:819–826. doi: 10.1016/s0091-3057(00)00275-6. doi: 10.1016/S0091-3057(00)00275-6. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Mayorga AJ, Fogle CM, Paule MG. Effects of acute nicotine on several operant behaviors in rats. Pharmacology, biochemistry, and behavior. 2000b;65:247–54. doi: 10.1016/s0091-3057(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Potter AS, Bucci DJ, Newhouse PA. Manipulation of nicotinic acetylcholine receptors differentially affects behavioral inhibition in human subjects with and without disordered baseline impulsivity. Psychopharmacology. 2012;220:331–40. doi: 10.1007/s00213-011-2476-0. doi: 10.1007/s00213-011-2476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacology, biochemistry, and behavior. 2008;88:407–17. doi: 10.1016/j.pbb.2007.09.014. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology. 2004;176:182–94. doi: 10.1007/s00213-004-1874-y. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Puumala T, Ruotsalainen S, Jäkälä P, et al. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiology of learning and memory. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Experimental and clinical psychopharmacology. 2006;14:296–305. doi: 10.1037/1064-1297.14.3.296. doi: 10.1037/1064-1297.14.3.296. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Bushnell PJ, Levin ED. Effects of nicotine and mecamylamine on choice accuracy in an operant visual signal detection task in female rats. Psychopharmacology. 2002;164:369–75. doi: 10.1007/s00213-002-1221-0. doi: 10.1007/s00213-002-1221-0. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Sexton H, et al. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor ligand: effects on dizocilpine and scopolamine-induced attentional impairments in female Sprague-Dawley rats. Psychopharmacology. 2011;215:621–30. doi: 10.1007/s00213-010-2161-8. doi: 10.1007/s00213-010-2161-8. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-antipsychotic drug interactions and attentional performance in female rats. European journal of pharmacology. 2004;486:175–82. doi: 10.1016/j.ejphar.2003.12.021. doi: 10.1016/j.ejphar.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. The Journal of neuropsychiatry and clinical neurosciences. 2001;13:326–35. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neuroscience and biobehavioral reviews. 2000;24:31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behavioural brain research. 1998;94:61–71. [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Wøien G, et al. The spontaneously hypertensive rat model of ADHD--the importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–26. doi: 10.1016/j.neuropharm.2009.08.004. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Xu T. l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD) Behavioral and brain functions : BBF. 2008;4:3. doi: 10.1186/1744-9081-4-3. doi: 10.1186/1744-9081-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria F, Killeen PR. Evidence for impulsivity in the Spontaneously Hypertensive Rat drawn from complementary response-withholding tasks. Behavioral and brain functions : BBF. 2008;4:7. doi: 10.1186/1744-9081-4-7. doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria F, Thrailkill E, Killeen PR. Timing with opportunity cost: Concurrent schedules of reinforcement improve peak timing. Learning & Behavior. 2009;37:217–229. doi: 10.3758/LB.37.3.217. doi:10.3758/LB.37.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden LS, Andresen J, MacPhail RC. Methylphenidate and d-amphetamine: effects and interactions with alphamethyltyrosine and tetrabenazine on DRL performance in rats. Pharmacology, biochemistry, and behavior. 1979;10:577–84. doi: 10.1016/0091-3057(79)90236-3. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacology, biochemistry, and behavior. 2007;87:360–8. doi: 10.1016/j.pbb.2007.05.009. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood N. Effects of nicotine on human psychomotor performance. Human Psychopharmacology. 1993;8:155–184. [Google Scholar]
- Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology. 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Skounti M, Philalithis A, Galanakis E. Variations in prevalence of attention deficit hyperactivity disorder worldwide. European journal of pediatrics. 2007;166:117–23. doi: 10.1007/s00431-006-0299-5. doi: 10.1007/s00431-006-0299-5. [DOI] [PubMed] [Google Scholar]
- Stins JF, Tollenaar MS, Dorine IE, et al. Sustained Attention and Executive Functioning Performance in Attention-Deficit/Hyperactivity Disorder. Child neuropsychology. 2005:285–294. doi: 10.1080/09297040490916938. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Ivanov I, Robinson JK, et al. Dissociation between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats in baseline performance and methylphenidate response on measures of attention, impulsivity and hyperactivity in a visual stimulus position discrimination task. Pharmacology, biochemistry, and behavior. 2010;94:374–9. doi: 10.1016/j.pbb.2009.09.019. doi: 10.1016/j.pbb.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology. 1999;28:366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: findings to date and new methods. Journal of neuroscience methods. 2006;151:15–29. doi: 10.1016/j.jneumeth.2005.09.018. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Warburton DM. Nicotine as a cognitive enhancer. Progress in neuro-psychopharmacology & biological psychiatry. 1992;16:181–91. doi: 10.1016/0278-5846(92)90069-q. [DOI] [PubMed] [Google Scholar]
- Ward RD, Barrett ST, Johnson RN, Odum AL. Nicotine does not enhance discrimination performance in a temporal bisection procedure. Behavioural pharmacology. 2009;20:99–108. doi: 10.1097/FBP.0b013e3283242fc2. doi: 10.1097/FBP.0b013e3283242fc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ, Bellinger LL, Cepeda-Benito A, et al. Meal patterns and body weight after nicotine in male rats as a function of chow or high-fat diet. Pharmacology, biochemistry, and behavior. 2005;82:627–34. doi: 10.1016/j.pbb.2005.11.002. doi: 10.1016/j.pbb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wigestrand MB, Mineur YS, Heath CJ, et al. Decreased α4β2 nicotinic receptor number in the absence of mRNA changes suggests post-transcriptional regulation in the spontaneously hypertensive rat model of ADHD. Journal of neurochemistry. 2011;119:240–50. doi: 10.1111/j.1471-4159.2011.07415.x. doi: 10.1111/j.1471-4159.2011.07415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall ND, De Wit H. Effects of nicotine on attention and inhibitory control in healthy nonsmokers. Experimental and clinical psychopharmacology. 2011;19:183–91. doi: 10.1037/a0023292. doi: 10.1037/a0023292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. A second-order schedule of food reinforcement in rats to examine the role of CB1 receptors in the reinforcement-enhancing effects of nicotine. Addiction biology. 2010;15:380–92. doi: 10.1111/j.1369-1600.2009.00203.x. doi: 10.1111/j.1369-1600.2009.00203.x. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, et al. Evidence that response inhibition is a primary deficit in ADHD. Journal of clinical and experimental neuropsychology. 2007;29:345–56. doi: 10.1080/13803390600678046. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behavioural brain research. 2013;240:119–133. doi: 10.1016/j.bbr.2012.11.028. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]