Abstract
Toll-like receptor 4 (TLR4), together with MD-2, binds bacterial endotoxins (E) with high affinity, triggering formation of the activated homodimer (E-MD-2-TLR4)2. Activated TLR4 induces intracellular signaling leading to activation of transcription factors that result in cytokine and chemokine production and initiation of inflammatory and immune responses. TLR4 also responds to endogenous ligands called danger associated molecular patterns (DAMPs). Increased sensitivity to infection and a variety of immune pathologies have been associated with either too little or too much TLR4 activation. We review here the molecular mechanisms of TLR4 activation (agonism) or inhibition (antagonism) by small organic molecules of both natural and synthetic origin. The role of co-receptors MD-2 and CD14 in the TLR4 modulation process is also discussed. Recent achievements in the field of chemical TLR4 modulation are reviewed, with special focus on non-classical TLR4 ligands with a chemical structure different from lipid A.
Introduction
Toll-like receptors (TLRs) play a critical role in the recognition of conserved pathogen-associated molecular patterns (PAMPs) derived from various microbial pathogens including viruses, bacteria, protozoa and fungi, and in the subsequent initiation of innate immune responses.1 Among TLRs, TLR4 selectively responds to bacterial endotoxin (E), Gram-negative bacterial lipopolysaccharides (LPS) or lipooligosaccharides (LOS).2,3 In addition, TLR4 recognizes a broad variety of substances from viruses, fungi, and mycoplasma.4 TLR4 is also activated by endogenous factors, generally known as danger (or damage) associated molecular patterns (DAMPs).5 Typical DAMPs acting as TLR4 agonists are endogenous substances which are released as a consequence of injury and inflammation. They include β-defensin, high-mobility group protein 1 (HMGB1), heat shock proteins (HSP), hyaluronic acid, heparin sulfate, substance P, and others. It remains a challenge to provide unequivocal evidence that DAMP proteins are direct TLR4 ligands, as the DAMP proteins used in experiments are often contaminated with endotoxin and other TLR ligands introduced during protein expression and/or purification. Targeting activation of TLR4 by bacterial endotoxin (LPS) is important in order to develop drugs active against acute sepsis and septic shock derived from excessive and deregulated TLR4 activation and signaling.6 The inhibition of TLR4 stimulation by endogenous factors could be used to contrast a wide range of inflammatory and autoimmune disorders associated to the release of Reactive Oxygen or Nitrogen Species (ROS/RNS) and inflammatory cytokines following “sterile inflammations” induced by DAMPs. A recent review on TLR literature emphasizes TLR4 as an emerging molecular target related to an impressively broad spectrum of modern day disorders6 including asthma, cardiovascular disorder, diabetes, obesity, metabolic syndrome, autoimmune disorders, neuroinflammatory disorders, neuropathic pain, CNS disorders such as amyotrophic lateral sclerosis (ALS) and Alzheimer Disease (AD), psychiatric diseases, skin inflammations (dermatitis), psoriasis and some tumors. As the majority of these pathologies still lack specific pharmacological treatment, small molecules active in inhibiting TLR4 activation have attracted increasing interest in a wide range of possible clinical settings.
The TLR4 activation process
The mechanism by which LPS activates TLR47 is the one we understand best. Activation of TLR4 by LPS is a complex process and depends on LPS binding protein (LBP)8-catalyzed extraction and transfer of individual LPS molecules from aggregated LPS to cluster of differentiation 14 (CD14),9 and then from CD14 to myeloid differentiation protein 2 (MD-2),10 followed by engagement and dimerization of TLR4 thus forming the activated complex (LPS.MD-2.TLR4)2.11 Crystal structures of the human11 and murine12 (LPS.MD-2.TLR4)2 complex reveal that five of six acyl chains of lipid A moiety of LPS insert into the hydrophobic binding pocket of the MD-2 bound to TLR4. It is clear from X-ray structures that the sixth acyl chain stays on the rim of the cavity, and binds to a second TLR4 molecule that is also part of an LPS.MD-2.TLR4 complex.11,12 However, recently published NMR studies indicate that protrusion of one of the six fatty acyl chains of endotoxin bound to MD-2 precedes interaction with TLR4 when endotoxin is bound to MD-2. According to NMR data, the presence of a protruding fatty acyl chain is not necessarily a distinguishing feature of TLR4-activating LPS.MD-2 complexes and so is not sufficient for driving TLR4 activation.13 The intracellular signal triggered by TLR4 dimerization consists of two different pathways, the myeloid differentiating primary response gene 88 (MyD88)-dependent and the MyD88-independent pathway based on the TRIF and TRAM effectors. TLR4 is unique among the TLRs in triggering two distinct signal/transduction pathways.
While the structural data of endotoxin-receptor complexes allowed the rational design of synthetic molecules able to compete with endotoxin (lipid A and LPS) for the binding to TLR4.MD-2 complex, very little is still known about the molecular details of the interaction between TLR4 and different DAMPs. The high chemical diversity of DAMPs structures probably reflects different modes of interaction with TLR4 and/or TLR4.MD-2 heterodimer. The role of accessory proteins CD14 and MD-2 is still controversial in the case of DAMP stimulation, and it has recently been proposed that, while CD14 might be a universal adaptor for PAMPs and DAMPs, MD-2 would selectively recognize only exogenous PAMPs.14
Structure-activity relationship in lipid A variants: from agonism to antagonism
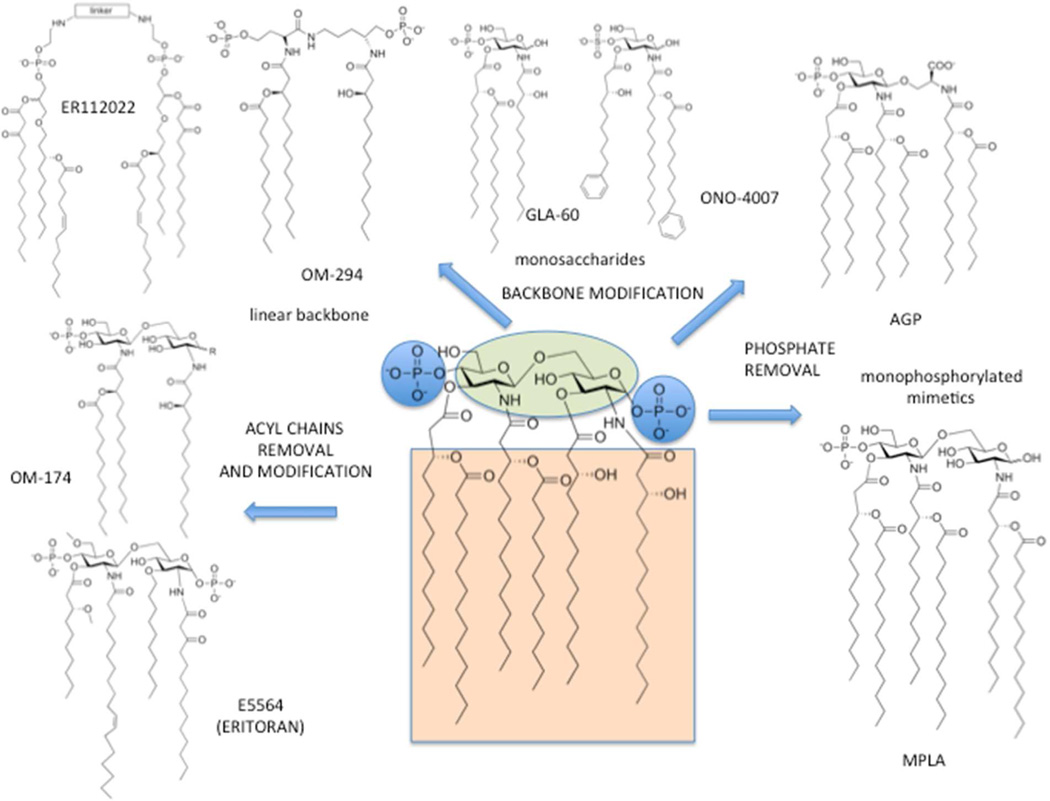
The membrane-anchoring moiety of LPS, lipid A, is also the biologically active (toxic) part of the entire LPS and is composed of a glucosamine disaccharide core with two negatively charged phosphate esters at C1 and C4’ positions and six fatty acid (FA) acyl chains linked to C2, C3, C2’ and C3’ disaccharide positions. While lipid A from E. coli (Figure 1) is considered the typical chemical structure associated to endotoxic properties, a variety of natural lipid A variants exist with modifications in the number and disposition of FA chains and with other covalent modifications that impact on pathogenesis, bacterial physiology and bacterial interactions with the host immune system.15
Figure 1.
TLR4 agonists and antagonists obtained by chemical elaboration of E. coli lipid A structure.
The chemical modification of the lipid A structure is now a well-established approach to TLR4 active molecules (Figure 1). Modifications of fatty acid (FA) acyl chains of lipid A, namely the removal of two or more FA chains, give the so-called underacylated lipid A variants containing less than six acyl chains. These natural or synthetic lipid A variants can block or inhibit the TLR4 activation, thus acting as antagonists (Figure 1). The crystal structure of TLR4.MD-2 homodimer bound to the tetracylated antagonist Eritoran16 revealed that Eritoran inserts all FA chains into the hydrophobic pocket of MD-2; however, in contrast to lipid A, Eritoran fails to induce TLR4 dimerization and activation, thus acting as a TLR4 antagonist. Interestingly, the tetraacylated lipid IVa, a biosynthetic precursor of lipid A, acts as antagonist on human but as an agonist on mouse TLR4. When bound to mouse MD-2 (mMD-2), lipid IVa presents one lipid chain on the surface of MD-2, which reveals striking similarity to the crystal structure of the agonist hexaacylated with human MD-2 (hMD-2) in the activated complex (lipid A.hMD-2.TLR4)2.12 This species specific activity of lipid IVa is attributed, among other factors, to the dissimilarities in the shape of the hydrophobic binding pocket of h- and mMD-2 and to the variations in the electrostatic potentials at the rim of the binding cavity of MD-2 and at the dimerization interface.12
Lipid A dephosphorylation or replacement of the reducing-end sugar with an aminoacid allowed non toxic TLR4 stimulants to be obtained, with agonist action. Synthetic low-toxicity TLR4 agonists monophosphoryl lipid As (MPLA) and aminoalkyl glucosaminide 4-phosphates (AGPs) are in use as vaccine adjuvants and in cancer immunotherapy (Figure 1). The low toxicity and adjuvant function is associated with a TRIF-selective signaling instead of MyD88-dependent stimulation for both AGPs17 and MPLAs.18
Other TLR4 agonists and antagonists are shown in Figure 1. They are obtained by lipid A structure simplification, including monosaccharides (GLA and ONO compounds), and mimetics with a linear backbone (ER112022). These compounds have been reviewed elsewere.19,20
The TLR4 agonists and antagonists depicted in Figure 1, with a structure derived from E. coli lipid A, are amphiphilic molecules with a polar (charged) head linked to a variable number of lipophilic chains. The amphiphilic character results in the formation of micelles in an aqueous environment above their critical micellar concentration (CMC) that is normally very low (nM range). As a consequence, aggregated forms of synthetic lipid A mimetic, and of natural lipid A and LPS, should predominate in the concentration range relevant for biological responses and for biochemical characterization in vitro. Accordingly, variables in the aggregation state and in the 3D form of endotoxin or synthetic molecule aggregates may directly influence the kinetics and potency of TLR4 activation and signaling.21 Differences in lipid A/LPS molecular structure may therefore influence the kinetics and potency of TLR4 activation by affecting the 3-D structure of aggregates of these molecules to which LBP, albumin, and CD14 must first interact to yield the monomeric endotoxin. protein (CD14; albumin) complexes that provide endotoxin monomer to MD-2 and MD-2/TLR4. Moreover, aggregates structure influences the geometry of monomeric endotoxin.MD-2.TLR4 complexes that determine TLR4 agonist or antagonist function.
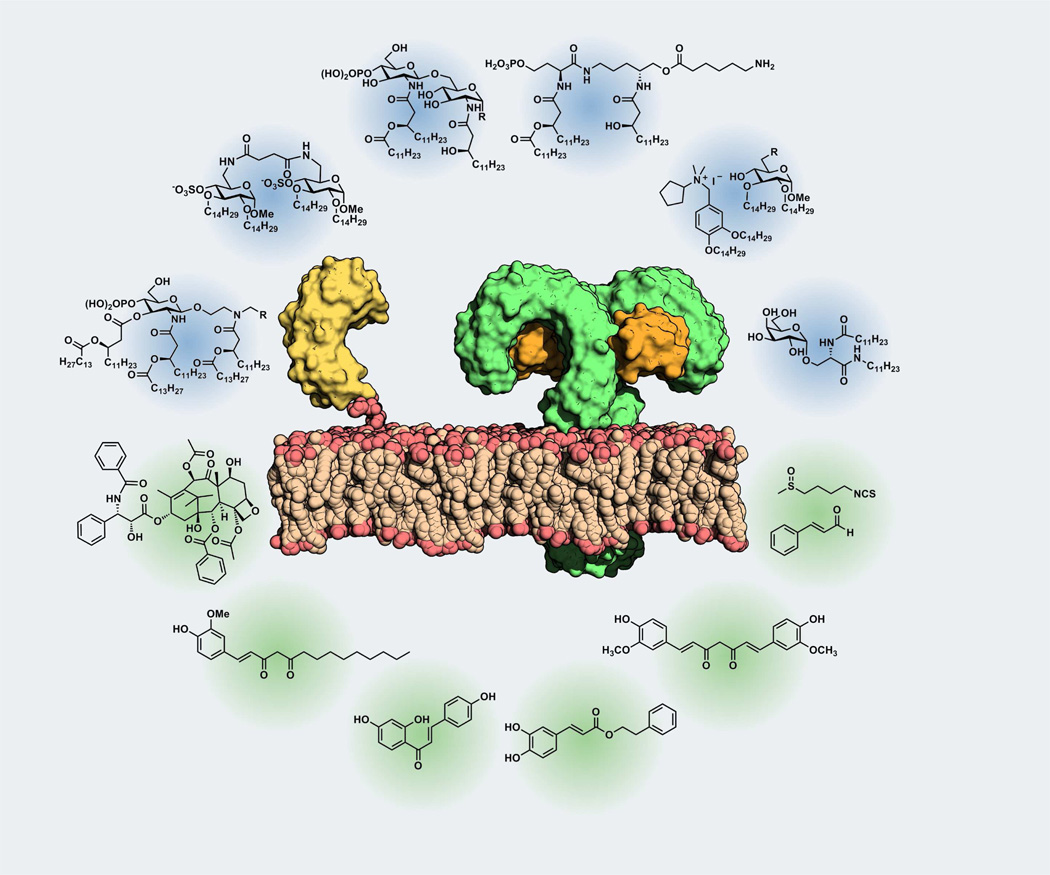
While the aggregates conformation in solution has been related to bioactivity in the case of natural TLR4 ligands, very few studies have been done so far with synthetic ligands. Another important consequence of the amphiphilic character of these molecules is their low water solubility which sometimes complicates their handling in vitro and in vivo and it is associated to poor distribution (pharmacokinetic) properties. This Mini-Perspective article presents the most recent achievements in the development of TLR4-active synthetic and natural small-molecules,19, 20 focusing on the mechanism of action and on the interaction with specific targets (TLR4, MD-2, and CD14 receptors). The extreme variety of chemical structures of small molecule TLR4-active compounds, especially in the case of natural compounds, is intriguing (Figure 2). As a consequence, these compounds target TLR4 and/or MD-2 and CD14 differently. However, the final common effect is the inhibition of the formation of the activated endotoxin.MD-2.TLR4 homodimer. Development of new molecular structures inspired by TLR4-active natural compounds, with improved water solubility, bioavailability and reduced toxicity compared to lipid A analogues, is one of the most promising future directions in this field, as will be discussed in this Perspective article.
Figure 2.
Pictorial representation of the TLR4 receptor complex (including MD2 and CD14 co-receptors) and some natural (green background) and synthetic (blue background) TLR4 ligands.
Synthetic TLR4 modulators
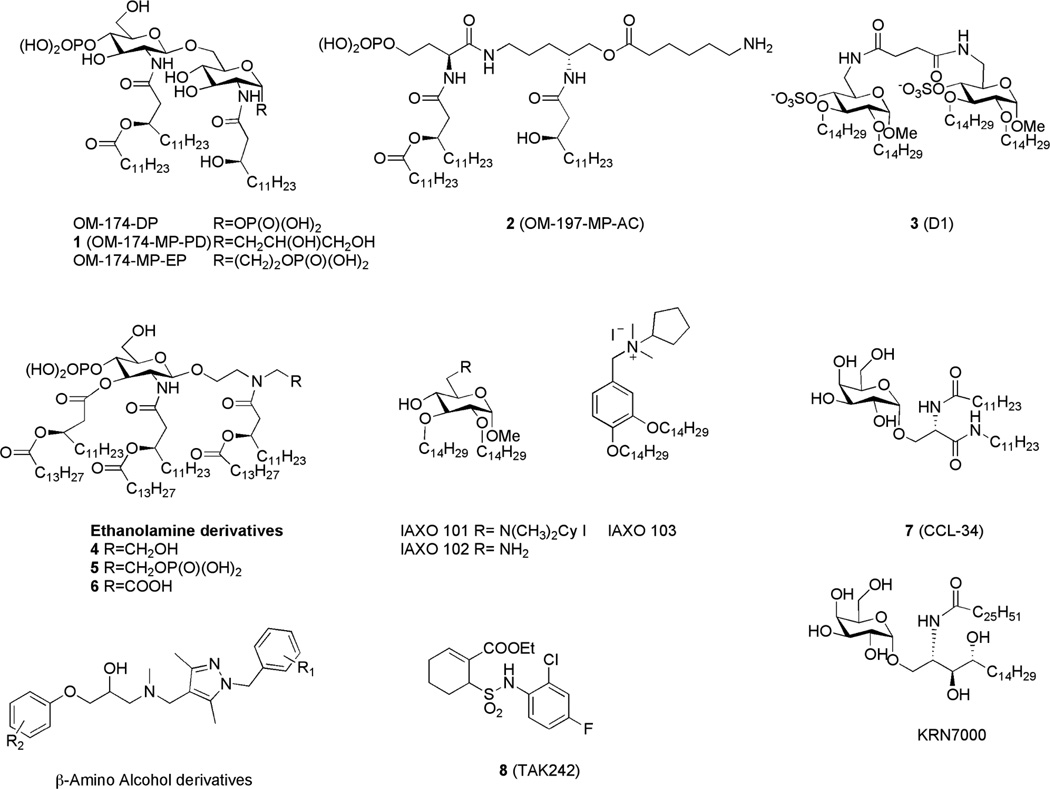
Great synthetic effort has been produced through the years to explore the structure-activity relationship of different lipid A analogues and mimetics. Several TLR4-active compounds have been synthesized varying the nature, the position and the length of FA acyl chains and the number of phosphate groups, or using phosphate bioisoteres. Eritoran (Figure 1), with four acyl chains of different length and nature, is representative of this class of compounds. The rationale of antagonism of Eritoran relies on the capacity of this molecule to insert into the hydrophobic MD-2 binding cavity and occupy MD-2 without promoting the formation of TLR4.MD-2 heterodimer. Antagonist activity is shared by other underacylated lipid A variants with four fatty acid (FA) chains, as the biosynthetic precursor lipid IVa.22
Triacylated lipid A mimetics constituted by a diglucosamine core (Figure 3, compounds OM-174 type), or a pseudodipeptide backbone (compound 2, OM-197-MP-AC,23 Figure 3) with one or two phosphates have been recently synthesized. These compounds turned out to be active in modulating the activity of both TLR2 and TLR4. Diphosphorylated compound OM-174-DP23 (Figure 3) showed antagonist action on TLR4 in HEK-BlueTM cells. The NF-κB activation in response to TLR4 stimulation can be measured in HEK-BlueTM hTLR4 cells, which are stably transfected with human TLR4, CD14, MD-2 and a secreted embryonic alkaline phosphatase (SEAP) reporter gene. TLR4 antagonism was accompanied by an agonist effect on TLR2 with the level of activation of TLR2-expressing cells comparable to that observed with the classical TLR2 ligand Pam3CSK4. The change of anomeric substituents of compounds 1 (OM-174-MP-PD) and OM-174-MP-EP23 (Figure 3) led to a significant lower activity as TLR4 antagonists. Otherwise compound 2 (Figure 3) showed an agonistic activity on TLR4, much lower than E. coli LPS, combined to a low endotoxic activity, thus representing a promising new vaccines adjuvant.
Figure 3.
Recently developed synthetic TLR4 modulators (agonists and antagonists).
Compound 3,24 (D1, Figure 3), with four ether chains, is a non-classical lipid A mimetic in which two sulfate groups are linked to a core containing two α-d-glucopyranoside units bridged through a (C6-C6’) succinic diamide linker. The sulfate groups, in anionic form at neutral pH, are bioisosteres of lipid A phosphates. In contrast to natural lipid A, 3 is symmetric with two-fold rotational symmetry (C2), and four linear ether chains (C14H29) replace the acyl esters (COC13H27 or COC11H23) found in E. coli lipid A and other synthetic antagonists. 3 inhibited in a dose-dependent manner the LPS-stimulated TLR4 activation in stable transformants of HEK293 cells expressing human TLR4 (HEK-TLR4 cells) supplemented with soluble MD-2, LBP and CD14.24 3 suppressed the formation of the (LPS.MD-2.TLR4)2 complex in vitro and inhibited the transfer of tritiated LOS monomers from a preformed LOS-CD14 complex to MD-2, thus suggesting a high affinity binding of the synthetic compound with both CD14 and MD-2.
Lipid A mimetics in which the reducing-end glucosamine is replaced by an acylated diethanolamine moiety have been synthesized and showed a dose-dependent immunostimulatory activity in the human monocytic cell line THP-1.25 Depending on the amine substituent, the molecules showed different potency in stimulating cytokines production, with compound 6 (Figure 3) containing a carboxylic acid group as a phosphate analogue exhibiting the greatest activity. A competitive inhibition study with lipid IVa gave indirect evidence that these compounds are TLR4 ligands, suggesting an interaction with the TLR4.MD-2 complex similar to that of the natural antagonist lipid IVa.25
In a further simplification of lipid A structure, different series of monosaccharidic glycolipids were found to activate or inhibit LPS-TLR4 signaling. Positively charged glycolipids with two C14 alkyl chains and a protonatable amine group on position C-6 of a methyl-α-D-glucopyranoside (IAXO 101 and IAXO 102, Figure 3) and aromatic compound IAXO 103 (Figure 3), inhibited TLR4 signaling in vitro26 and suppressed some TLR4-related pathologies such as acute sepsis26 and neuropathic pain27 in mice. Experiments with purified receptors and NMR binding studies showed that IAXO compounds bind CD14 with higher affinity than MD-2.28 Despite the striking structural difference with negatively charged lipid A, cationic IAXO compounds are active as TLR4 antagonists.
Synthetic serine-based analogues of α-galactosyl ceramide, in particular compounds 729 (CCL-34, Figure 3), stimulated TLR4-dependent TNF-α production, while the natural α-galactosyl ceramide turned out to be inactive. Some variants of this molecule have been recently synthesized, to unravel the relationship between their chemical structure and immunostimulating properties. While the anomeric α-configuration is essential to the biological activity, the galactose moiety can be replaced by other monosaccharides (α-fucose, α- or β-glucose, α-galacturonic acid) retaining the TLR4-stimulating activity, as assessed by experiments on HEK cells stably transfected with TLR4, MD-2 and CD14 receptors.30 The antitumor potential of TLR4 agonist 7 was studied: macrophages activated by the synthetic ceramide induced cancer cell death via the apoptotic pathway and treatment with 7 suppressed tumor growth and increased the survival rate in TLR4-functional C3H/HeN mice but not in TLR4-defective C3H/HeJ mice.31 Other natural and synthetic α-GalCer are known to possess immunostimulant activities, molecule KRN700032 (Figure 3), that is a simplified analogue of glycosphingolipids of marine origin is capable of activating natural killer T (NKT) cells. In the light of the recently observed TLR4 activity of 7, the previously reported immunostimulating activity of some natural and synthetic α-GalCer is very likely due to TLR4 activation.
An alternative strategy to target TLR4 is to design low molecular weight inhibitors with a chemical structure totally different from lipid A, as in the case of compound 833 (TAK-242, Figure 3) that, as Eritoran, reached phase III clinical trials as an antisepsis agent. 8 selectively inhibits TLR4 signal by covalently binding Cys747 in the intracellular domain of TLR4 and probably reacting with Cys thiol as a Michael acceptor, thus forming a covalent adduct with TLR4.34 Yin and coworkers recently identified some β-aminoalcohol derivatives (Figure 3), as virtual candidates to disrupt the MD-2-TLR4 interaction. A panel of racemic and optically pure compounds derived from this scaffold inhibited TLR4-dependent nitric oxide (NO) production in RAW 264.7 macrophages and cytokine production in human whole blood with µM potency.35 These compounds showed no cytotoxic activity at concentrations up to 300 µM, and the easily modified and scalable synthesis could provide more potent antagonists, studying a different arrangement of the aromatic substituents.36
In the course of an high-throughput screening designed to identify activators of innate immunity, a series of pyrimido[5,4-b]indoles,37 with a structure totally unrelated to lipid A, were recently discovered as selective TLR4 activators (agonists). These agonists were active in stimulating the TLR4 signal in HEK293 reporter cell lines, and inactive on HEK cells expressing other TLRs. Interestingly, the activities of these compounds seem to be CD14-independent, as assessed by the capacity to stimulate cytokine production also in CD14-defective macrophages.37 The direct binding to MD-2 of one of these compounds has been simulated by docking studies. Peptides are structurally versatile compounds that can be designed to interact in a complementary way with protein targets. Short peptides have been synthesized corresponding to the minimal TLR4-binding region of MD-2, and it was reported that they act as antagonists, inhibiting LPSinduced TLR4-dependent expression of proinflammatory cytokines both in human and in murine cells.38 Peptide-based TLR4 agonists have also been developed recently, by screening a 7-mer phage-display peptide library.39 The activity of the peptides in activating TLR4 signaling was assessed by NF-κB nuclear translocation analyses in HEK-blue™ cells. Furthermore, the peptides were capable of inducing inflammatory cytokine secretion from RAW264.7 cells.
Natural TRL4 modulators
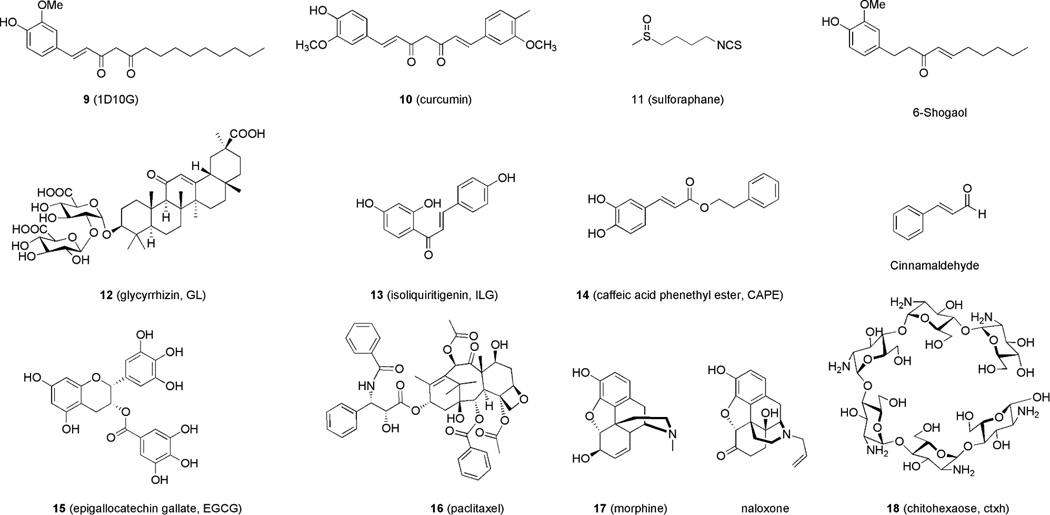
Plant secondary metabolism provides an endless source of chemically diverse bioactive and pharmacologically active compounds. Plant-based extracts present a large source of natural immune modulators, many of which have been used in traditional medicine for centuries. Recent research efforts have identified plant extracts as potential modulators of TLRs.40 The understanding of the molecular mechanisms of TLR4 activation promoted the study of TLR4 activity of some plant and herbal extracts with focus on the molecular mechanism of action of active principles.4 Interestingly, many herbs used in Traditional Chinese medicine (TCM) and Ayurvedic medicine seem to be rich in molecules that interfere with TLR4 activation and signaling. These include green tea, Glycyrrhiza uralensis, better known as licorice, Magnolia officinalis, ginger (Zingiber officinalis), Salvia miltiorrhiza (red sage), and curcumin. The chemical structures of natural compounds whose TLR4 activity has been recently investigated are depicted in Figure 4. With some important exceptions, such as paclitaxel, morphine, opioids and sulforaphane, the TLR4-active compounds depicted in Figure 4 are polyphenolic and aromatic compounds sharing interesting similarities in their chemical structures and in their mechanism of action.
Figure 4.
Some recently studied TLR4-active compounds from natural sources.
In particular, cinnamaldehyde, 1-dehydro-10-gingerdione (1D10G), 6-shogaol, isoliquiritigenin, curcumin and caffeic acid ester (Figure 4) contain α,β-unsaturated carbonyl moieties that can easily react as carbon electrophile with the nucleophilic thiol of a cysteine, thus forming Michael adducts with protein targets. The disruption of TLR4.MD-2 heterodimer by formation of covalent adducts with solvent-exposed MD-2 and/or TLR4 cysteines has been proposed as the main mechanism of action of 1341 (isoliquiritigenin), 6-shoagol,42 caffeic acid phenetyl ester 14 (CAPE)43 and cinnamaldehyde.44 Despite the presence of the same α, β-unsaturated carbonyl functionality, curcumin does not seem to form a covalent bond with MD-2,45 in contrast with a previously suggested mechanism of action.46 Also compound 9 (1D10G) seems to inhibit the formation of TLR4 activated complex by interacting non-covalently with MD-2 and competing with endotoxin for MD-2 binding.47 Sulforaphane contains a different electrophilic group, the isothiocyanato moiety, that forms covalent adducts with MD-2 exposed cysteine thiols, as recently observed by mass analysis.48 The properties of the above-mentioned natural compounds on TLR4 signaling have been characterized through experiments on purified receptors, the molecular details will be discussed separately for each compound.
The molecular mechanism explaining the anti-inflammatory action of cinnamaldehyde (the major constituent of cinnamon bark from Cinnamomum trees, Figure 4), has recently been discovered.44 It has been observed that cinnamaldehyde is active in inhibiting the activation of NFκB and IRF3 induced by LPS while being inactive in inhibiting NF-κB activation by downstream effectors such as MyD88 and IKKβ, thus suggesting an upstream effect on the extracellular TLR4.MD-2 complex. Furthermore, cinnamaldehyde inhibited TLR4 oligomerization induced by LPS in co-immunoprecipitation experiments. The observation that the saturated analogue of cinnamaldehyde, the 2-phenylpropionaldehyde, was inactive in inhibiting LPS-induced signaling, and the reactivity of cinnamaldehyde with other cysteine-exposing proteins suggested to the authors the formation of a covalent adduct cinnamaldehyde/MD-2. However, this adduct was not directly observed.44 Curcumin (Figure 4) is a polyphenolic compound isolated from the rhizomes of the plant Curcuma longa (turmeric) that has profound anti-inflammatory activity and is used in the treatment of sprain and swelling caused by injury in traditional Indian and Chinese medicine. It has been shown that curcumin inhibits not only the IκB kinase (IKK)β in the MyD88 signaling pathway but also the MyD88-independent pathway upstream of Toll/IL-1R domain-containing adaptor-inducing IFN-β(TRIF).46 The binding between curcumin 10 and purified human MD-2 was investigated experimentally.45 Fluorescence of 10 increased significantly upon addition of MD-2 and 10 inhibited in a dose-dependent manner the interaction between immobilized LPS and MD-2 in an ELISA-type assay, thus suggesting that LPS and 10 very likely compete for the same MD-2 binding pocket.45 In this study authors also observe that 10 does not form a covalent linkage with MD-2 (in particular with Cys133 exposed on the inner surface of the MD-2 hydrophobic pocket), because denaturation of MD-2 subsequent to the formation of complex with 10, followed by organic solvent extraction caused the complete transfer of 10 into the organic phase, demonstrating the noncovalent nature of the interaction with MD-2.45
Sulforaphane 11 (SFN, 1-isothiocyanato-4-(methylsulfinyl)-butane, Figure 4) is an organosulfur compound obtained from cruciferous vegetables such as broccoli or cabbages. Several studies documented that the well-established anti-inflammatory activity of 11 depends on suppression of TLR4 signaling. 11 attenuates LPS-induced nitric oxide synthase (iNOS), cyclooxigenase-2 (COX-2) and tumor necrosis factor-α (TNF-α) production in macrophage cell lines,48 and inhibits NFκB translocation and IκBα degradation in LPS-stimulated endothelial cells and suppress LPS-induced expression of inflammatory genes.48 Recently, it has been observed that 11 preferentially forms adducts with Cys133 in the hydrophobic pocket of MD-2, blocking the interaction with LPS and lipid IVa.48 In Ba/F3 cells expressing TLR4 and Flag-tagged MD-2, treated with biotinylated LPS, 11 was observed to inhibit the association of LPS with MD-2 in a dose-dependent manner, as determined by immunoblot analysis. LC-MS/MS analysis after incubation of recombinant MD-2 with SFN showed the SFN covalently bound to Cys133 which is a free cysteine residue, but not to Cys95 and Cys105 which are engaged in the formation of intramolecular disulfide bonds.48
Ginger, the rhizome of the herb Zingiber officinalis has been traditionally used in Ayurvedic medicine to treat several inflammatory diseases and ginger extracts have anti-inflammatory and anti-cancer effects. The major molecular components that account for ginger’s anti-inflammatory effects are gingerols and their dehydratation products, shogaols. The activity on TLR4 of some of these components, namely 9 (Figure 4), 6-, and 10-gingerdione, 4-, 6- and 10-shogaol (Figure 4) and gingerenone A was recently compared.47 9 is the most potent inhibitor of LPS-MD-2 interaction as determined by an ELISA test, the 6-gingerol and 10-shogaol were also active in inhibiting the interaction, while, surprisingly, the 6-shogaol (Figure 4), that has been described by some authors as the most active component of ginger, presented a weaker activity in this experiment.47 The most effective inhibitor 9 also inhibited the interaction of MD-2 with its fluorescent ligand bis-ANS and suppressed both MyD88-dependent NF-κB and AP1 activation (for the expression of TNF-α and IL-1β) and TRIF-dependent pathway for IRF3 activation and expression of IFN-β and IP-10 in LPS-stimulated macrophages.47 Despite the low activity for interfering with LPS-MD-2 interaction reported in this paper, the anti-inflammatory activity of 6-shogaol is well established. It has been reported recently that 6-shogaol inhibits LPS-triggered TLR4 homodimerization,42 and targets TBK1 kinase in the TRIF-dependent pathway.49
Roots and rhizomes of Glycyrrhiza plants (licorice) have been used as herbal medicine worldwide for over 4000 years. Among Glycyrrhiza plants, G. uralensis is one of the mostly used in traditional medicines in Japan, and various components have been isolated from G. uralensis, including triterpene saponins, flavonoids, isoflavonoids, and chalcones. Glycyrrhizin 12 (GL, Figure 4), a triterpene saponin, and chalcone isoliquiritigenin 13 (ILG, Figure 4), are the major biological active components of licorice. 12 and 13 were reported to suppress TLR4.MD-2-mediated NF-κB and MAPK activation, resulting in decreased production of proinflammatory cytokines.50 Also, 12 treatment down-regulated the TLR4 internalization upon LPS stimulation.51 In a recent paper, it was reported that 12 and 13 modulate the initial steps of TLR4 signaling with different mechanisms.52 While both compounds were active in inhibiting LPS-induced TLR4.MD-2 homodimerization, only 12 and not 13 inhibited the formation of the LPS.MD-2.TLR4 complex in an experiment of coprecipitation with biotinylated LPS.52 The activity of 13 in inhibiting TLR4 dimerization in a dose-dependent manner was described in another report where the authors propose the formation of a covalent adduct between 13 and MD-2 and/or TLR4 as mechanism of action of ILG.41 Caffeic acid phenethyl ester 14 (CAPE,3 Figure 4), first isolated as an active phenolic constituent of honeybee propolis potently inhibits activation of the NF-κB signaling. It has been recently reported that 14 significantly inhibits TNF-induced IP-10 expression in intestinal epithelial cells55 and that the NF-κB inhibition is due to the formation of a covalent adduct with Cys133 in MD-2 hydrophobic pocket.43
Green tea polyphenolic fraction possess anti-inflammatory and chemopreventive effects, and contains several active compounds which are catechin, (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and 15 (−)-epigallocatechin-3-gallate (EGCG). Among these 15 (Figure 4) is known to possess the most potent anti-oxidative and chemopreventive properties, and it has been demonstrated not to prevent LPS-induced dimerization of TLR4, but to inhibit the TLR4 signal downstream, blocking both MyD88- and TRIF-dependent signal pathways by targeting IKKβ and TBK1 kinases respectively. The parent catechin molecule, showed no LPS induced inhibition of NF-κB activation, thus suggesting an anti-inflammatory property of the galloyl group.55
Paclitaxel 16 (PTX,56 Figure 4) is a potent anti-cancer agent derived from the Pacific yew tree, which acts through over-stabilization of cellular microtubules. Although the anti-cancer mechanism of action of 16 has long been attributed to effects at the level of β-tubulin, growing evidence supports innate immune activation and possibly antitumor effects of 16 mediated through TLR4-MD-2. Activation of the TLR4.MD-2 pathway by 16 has been demonstrated using murine macrophages, transfected cell lines, mouse cancer cell and direct evidence of 16 binding to MD-2 has been collected by in vitro experiments.56 In contrast, cells transfected with human TLR4 and human MD-2 fail to mount a pro-inflammatory response to 16. On the contrary, recent data show that 16 can block NF-κB activation and cytokines expression by binding to human MD-2 as an antagonist, thus ameliorating TLR4-dependent pathologies such as LPS-induced kidney injury.57 The species-specific action of 16 has been explained in terms of different binding to MD-2.58 16 binding to murine MD-2 causes a shift of 123–130 loop , including Phe126, outward the protein surface thus creating a binding interface for TLR4 dimerization and formation of the activated TLR4.MD-2 dimer. This repositioning of the loop does not occur when PTX binds to human MD-2, leading to antagonism.58
It is known that opioids such as morphine (Figure 4) induce neuroinflammatory responses in CNS, Hutchinson and Watkins recently associated the neuroinflammatory action of morphine to activation of TLR4 signaling. In an elegant work, the molecular mechanism underlying the TLR4 agonist action of morphine 17 has been investigated.59 Biotinylated morphine immobilized on plate bound to human MD-2 in a dose-dependent manner and LPS competed with immobilized morphine for binding to MD-2. 17 induced TLR4 oligomerization in Ba-F3 cells and proinflammatory cytokines production in TLR4-expressing CNS immunocompetent cells. Interestingly, the co-administration of TLR4.MD-2 antagonists inhibited morphine-induced proinflammatory effect in vitro and in vivo. The same authors associated the TLR4 agonist action of 17 and opioids to reinforcing/rewarding effects that contribute to the widespread abuse of these drugs.60, 61 Molecular modeling and docking calculations suggested that, similarly to LPS and 16, 17 binding to human MD-2 would induce conformational rearrangement of the Phe126 loop, resulting in the formation of the TLR4 oligomerization interface.59
The same research group showed that the TLR4 antagonist action of both enantiomers of opioids naloxone (Figure 4) and naltrexone can be used to efficiently contrast acute62 and chronic63 neuropathic pain in animal models associated with microglial TLR4 activation.
Chitohexaose 18 (chtx, Figure 4), a small molecular weight oilgosaccharide which is a constituent of some nematode surface glycoproteins, inhibited LPS-induced production of inflammatory mediators by murine macrophages and human monocytes.64 The authors started from the observation that soluble TLR4 present in membrane lysates of human and murine macrophages binds to solid-phase immobilized parasites of Setaria digitata (FAg). This suggested the presence of conserved TLR4 binding components on the surface of nematodes. The hexasaccharide 18, being a main constituent of helminths surface glycans, was therefore selected as a potential, high-affinity, low-molecular weight TLR4 ligand. Co-administration of 18 blocked LPS-induced lethal endotoxemia in mice, and chtx reversed inflammation and protected mice even 24/48 hrs after onset of endotoxemia. The authors suggest that 18 acts on TLR4 signaling, competing with LPS as an antagonist. Interestingly, it seems that this compound interacts directly with TLR4 without need of MD-2.64
A growing number of publications during recent years strongly suggest that the biological activity of some pure natural compounds or extracts from natural sources is related to a specific interaction with MD-2 and/or TLR4.MD-2. The property of the anticancer macrocylic lactone bryostatin (bryo-1) to induce cytokine production in dendritic cells has been recently related to TLR4 agonism,65 while TLR4 antagonism can explain the inhibitory effect on nitric oxide synthase (iNOS) expression of polysaccharides extracted from pu-erh tea.66 The anti-inflammatory effect of phenolic compounds such as hydroxytyrosol and oleuropein aglycon extracted from olives and olive oil is also very likely related with TLR4 antagonism.67 The identification of active principles in natural extracts and the clarification of the molecular details of the interaction between these molecules and TLR4.MD-2 will provide a variety of chemical scaffolds for developing new, nontoxic, TLR4 modulators.
Conclusions and future directions
The wealth of information available on the structure-activity relationship of natural and synthetic lipid A analogues and the availability of the structures of the human11 and murine12 (LPS.MD-2.TLR4)2 complexes, enable the rational design of TLR4 agonists and antagonists based on rational modification of the lipid A structure. This procedure based on LPS (lipid A) mimicry afforded efficient and non-toxic TLR4 agonists MPLAs18 and AGPs17 in use as vaccine adjuvants. However rationally designed TLR4 antagonist Eritoran failed in phase III clinical trials.68 Compound 8 (TAK-242), a small molecule with a chemical structure different from lipid A, and directly targeting TLR4 with a non-classical mechanism of action, also failed to suppress cytokines levels in patients in a phase III clinical trial. The lack of activity in septic patients of both these TLR4 antagonists, that were very efficient in preclinical phase on animal models, may imply that the current approaches to target the TLR4 receptor should be part of a more general approach to the sepsis. Expression of TLR4 varies in different stages of sepsis, in particular TLR4 expression on neutrophils in septic shock patients is down-regulated in the acute phase.69 Very recently, a TLR4-independent mechanism for innate immune recognition of LPS and LPS-induced inflammation has been observed.70 These data could explain the lack of efficacy of TLR4 inhibition in septic patients. Novel, integrated, multi-targeted pharmacological strategies are therefore urgently needed to regulate the innate immune response.
From a chemical point of view, the amphiphilic character of all synthetic and natural lipid A analogues is often associated with low solubility in aqueous media. In this respect, the natural TLR4-active compounds reviewed in this article have better water solubility and bioavailability. The chemical modification of compounds such as caffeic acid esters, isoliquiritigenin, shogaol, gingerdione, curcumin, opioids and taxanes should therefore afford new generations of TLR4-active compounds with improved pharmacokinetic properties and reduced toxicity. Synthetic modification of natural compounds is required 1) to increase target-specificity, and 2) to improve water stability and resistance to enzyme hydrolysis.
Detailed molecular modeling and docking studies are available on the interaction of MD-2 with agonists 1658 and 17.59 These models suggest a common mechanism based on the displacement of Cys126 loop to trigger TLR4 dimerization and activation. The ligand-MD-2 complex structures from these studies should guide the design of new MD-2 ligands with agonist or antagonist activity based on natural scaffolds.
Some natural compounds presented in this review act as disruptors of the TLR4.MD-2 interaction by forming covalent bonds with Cys133 of MD-2. An appealing and still unexplored way to have access to novel, high-affinity TLR4 antagonists, would be the incorporation of thiol (cysteine)-reactive electrophile groups into synthetic or natural TLR4 ligands. This would combine the high target specificity with increased affinity due to covalent bond formation. Finally, no natural ligands have been so far co-crystallized with the MD-2.TLR4 complex. The use of NMR for the determination of ligand.TLR4.MD-2 complexes structure has so far been limited by the availability of pure and functional TLR4 and MD-2 receptors and by the poor aqueous solubility of both receptors and ligands at the high concentrations required for NMR experiments. Once addressed the practical issues cited above, NMR experiments such as Saturation Transfer Difference (STD) and transfer-NOE would provide atomic-level structural information thus allowing to map the regions of the synthetic or natural ligand directly interacting with MD-2.TLR4 (epitope mapping) and vice-versa the residues of MD-2.(TLR4) involved in ligand binding and recognition. Some recent reports on the determination of the structures of13C-enriched lipooligosacharide (LOS) with wild-type MD-2 or MD-2 mutants13 suggest the possibility to prepare13C-enriched synthetic or natural molecules for similar binding studies.
A multidisciplinary effort is needed to gain information on the atomic structure of different ligand-MD-2.TLR4 complexes, thus enabling the rational design of non-classical TLR4 modulators.
Aknowledgements
We thank the US National Institutes of Health (NIH)/ National Institute of Allergy and Infectious Diseases (NIAID), project: “Regulation of MD-2 function and expression” (1R01AI059372); COST action BM 1003 “Microbial cell surface determinants of virulence as targets for new therapeutics for Cystic Fybrosis”; COST Action CM1102 MultiGlycoNano; Italian Ministry of University and Research (MIUR), PRIN 2010–11, project: “Italian network for the development of multivalent nanosystems”. We thank Roberto Cighetti for assistance in figures preparation.
Abbreviations
- AGP
-
aminoalkyl glucosaminide 4-phosphates
Ba/F3 cells
- CD14
cluster differentiation antigen (14)
- CNS
central nervous system
- DAMP
Danger Associated Molecular Pattern
- E
endotoxin
- FA
-
fatty acid
HEK cells
- HMGB1
high-mobility group protein 1
- HSP
heat shock protein
- LBP
lipopolysaccharide binding protein
- LOS
lipooligosaccharide
- LPS
lipopolysaccharide
- MD-2
myeloid differentiation protein (2)
- MPL
monophosphoryl lipid A
- MyD88
myeloid differentiation primary response gene (88)
- NF-κ
nuclear factor kappa-light-chain-enhancer of activated B cells
- TNF-α
tumor necrosis factor alpha
- PAMP
pathogen associated molecular pattern.
Biographies
Francesco Peri
Francesco Peri was born in San Francisco (USA) in 1968. He received a first PhD in Chemistry at the University of Parma (Italy) in 1998 and a second PhD in Bioorganic Chemistry at the University of Lausanne (Switzerland) in 2001. Since 2007 he is Professor of Organic Chemistry at the Department of Biotechnology and Biosciences, University of Milano-Bicocca (Italy). He is visiting professor at the Ecole Normale Superieure of Lyon (France) and at the Department of Chemistry, University of Davis (CA). His research interests span the disciplines of organic, bioorganic and medicinal chemistry with an emphasis of study of the interactions between small molecules and protein targets. At present, his research is focused on the study of molecular mechanisms of innate immunity and inflammation.
Valentina Calabrese
Valentina Calabrese graduated in Chemistry in 2005 at the University of Milan, in 2008 she received the PhD degree in Industrial Chemistry. In 2009 she was appointed as post-doc in the research group of F. Peri at the University of Milano-Bicocca. Her research focuses on the synthesis and biological characterization of synthetic TLR4-active compounds with the aim to develop new anti-sepsis drugs (TLR4 antagonists) and vaccines adjuvants (TLR4 agonists). Several active compounds have been developed with good activity in TLR4 modulation through selective CD14 and MD-2 co-receptors targeting, and low in vitro toxicity. Some of these new compounds are in preclinical phase as anti-inflammatory and anti-neuropathic pain drugs.
References
- 1.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Poltorak A, He X, Smirnova I, Liu M, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Du X, Poltorak A. Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. J Endotoxin Res. 2001;7:277–280. [PubMed] [Google Scholar]
- 4.Jeong E, Lee JY. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med J. 2011;52:379–392. doi: 10.3349/ymj.2011.52.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas K, Maes M. Role of the Toll Like Receptor (TLR) Radical Cycle in Chronic Inflammation: Possible Treatments Targeting the TLR4 Pathway. Mol Neurobiol. 2013;48:190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cribbs S, Martin G. Expanding the global epidemiology of sepsis. Crit Care Med. 2007;35:2646–2648. doi: 10.1097/01.CCM.0000288082.99980.90. [DOI] [PubMed] [Google Scholar]
- 7.Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007;297:353–363. doi: 10.1016/j.ijmm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Schumann R, Leong S, Flaggs G, Gray P, Wright S, Mathison J, Tobias P, Ulevitch R. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 9.Wright S, Ramos R, Tobias P, Ulevitch R, Mathison J. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 10.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park B, Song D, Kim H, Choi B, Lee H, Lee J. The structural basis of lipopolysaccharide recognition by the TLR4.MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 12.Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc Natl Acad Sci U S A. 2012;109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L, Phillips RL, Zhang D, Teghanemt A, Weiss JP, Gioannini TL. NMR studies of hexaacylated endotoxin bound to wild-type and F126A mutant MD-2 and MD-2·TLR4 ectodomain complexes. J Biol Chem. 2012;287:16346–16355. doi: 10.1074/jbc.M112.343467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun KH, Seong SY. CD14 but not MD-2 transmit signals from DAMP. Int Immunopharmacol. 2010;10:98–106. doi: 10.1016/j.intimp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Needham BD, Trent MS. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol. 2013;11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Park B, Kim J, Kim S, Lee J, Oh S, Enkhbayar P, Matsushima N, Lee H, Yoo O, Lee J. Crystal structure of the TLR4.MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Bowen WS, Minns LA, Johnson DA, Mitchell TC, Hutton MM, Evans JT. Selective TRIF-dependent signaling by a synthetic toll-like receptor 4 agonist. Sci Signal. 2012;5:13–20. doi: 10.1126/scisignal.2001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 19.Peri F, Piazza M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol Adv. 2012;30:251–260. doi: 10.1016/j.biotechadv.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Smith C, Yin H. Targeting Toll-like receptors with small molecule agents. Chem Soc Rev. 2013;42:4859–4866. doi: 10.1039/c3cs60039d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schromm A, Howe J, Ulmer A, Wiesmüller K, Seyberth T, Jung G, Rössle M, Koch M, Gutsmann T, Brandenburg K. Physicochemical and biological analysis of synthetic bacterial lipopeptides: validity of the concept of endotoxic conformation. J Biol Chem. 2007;282:11030–11037. doi: 10.1074/jbc.M700287200. [DOI] [PubMed] [Google Scholar]
- 22.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 23.Dunn-Siegrist I, Tissieres P, Drifte G, Bauer J, Moutel S, Pugin J. Toll-like receptor activation of human cells by synthetic triacylated lipid A-like molecules. J Biol Chem. 2012;287:16121–16131. doi: 10.1074/jbc.M112.348383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piazza M, Calabrese V, Damore G, Cighetti R, Gioannini T, Weiss J, Peri F. A synthetic lipid A mimetic modulates human TLR4 activity. ChemMedChem. 2012;7:213–217. doi: 10.1002/cmdc.201100494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewicky JD, Ulanova M, Jiang ZH. Improving the immunostimulatory potency of diethanolamine-containing lipid A mimics. Bioorg Med Chem. 2013;21:2199–2209. doi: 10.1016/j.bmc.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Piazza M, Rossini C, Della Fiorentina S, Pozzi C, Comelli F, Bettoni I, Fusi P, Costa B, Peri F. Glycolipids and Benzylammonium Lipids as Novel Antisepsis Agents: Synthesis and Biological Characterization. Journal of Medicinal Chemistry. 2009;52:1209–1213. doi: 10.1021/jm801333m. [DOI] [PubMed] [Google Scholar]
- 27.Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, Costa B. Glial TLR4 receptor as new target to treat neuropathic pain: Efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia. 2008;56:1312–1319. doi: 10.1002/glia.20699. [DOI] [PubMed] [Google Scholar]
- 28.Piazza M, Yu L, Teghanemt A, Gioannini T, Weiss J, Peri F. Evidence of a Specific Interaction between New Synthetic Antisepsis Agents and CD14. Biochemistry. 2009;48:12337–12344. doi: 10.1021/bi901601b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung LC, Lin CC, Hung SK, Wu BC, Jan MD, Liou SH, Fu SL. A synthetic analog of alpha-galactosylceramide induces macrophage activation via the TLR4-signaling pathways. Biochem Pharmacol. 2007;73:1957–1970. doi: 10.1016/j.bcp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Huang LD, Lin HJ, Huang PH, Hsiao WC, Reddy LV, Fu SL, Lin CC. Synthesis of serine-based glycolipids as potential TLR4 activators. Org Biomol Chem. 2011;9:2492–2504. doi: 10.1039/c0ob00990c. [DOI] [PubMed] [Google Scholar]
- 31.Lin YS, Huang LD, Lin CH, Huang PH, Chen YJ, Wong FH, Lin CC, Fu SL. In vitro and in vivoanticancer activity of a synthetic glycolipid as Toll-like receptor 4 (TLR4) activator. J Biol Chem. 2011;286:43782–43792. doi: 10.1074/jbc.M111.285171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 33.Yamada M, Ichikawa T, Ii M, Sunamoto M, Itoh K, Tamura N, Kitazaki T. Discovery of novel and potent small-molecule inhibitors of NO and cytokine production as antisepsis agents: synthesis and biological activity of alkyl 6-(N-substituted sulfamoyl)cyclohex-1-ene-1-carboxylate. J Med Chem. 2005;48:7457–7467. doi: 10.1021/jm050623t. [DOI] [PubMed] [Google Scholar]
- 34.Takashima K, Matsunaga N, Yoshimatsu M, Hazeki K, Kaisho T, Uekata M, Hazeki O, Akira S, Iizawa Y, Ii M. Analysis of binding site for the novel small-molecule TLR4 signal transduction inhibitor TAK-242 and its therapeutic effect on mouse sepsis model. Br J Pharmacol. 2009;157:1250–1262. doi: 10.1111/j.1476-5381.2009.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bevan DE, Martinko AJ, Loram LC, Stahl JA, Taylor FR, Joshee S, Watkins LR, Yin H. Selection, Preparation, and Evaluation of Small- Molecule Inhibitors of Toll-Like Receptor 4. ACS Med Chem Lett. 2010;1:194–198. doi: 10.1021/ml100041f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chavez SA, Martinko AJ, Lau C, Pham MN, Cheng K, Bevan DE, Mollnes TE, Yin H. Development of β-amino alcohol derivatives that inhibit Toll-like receptor 4 mediated inflammatory response as potential antiseptics. J Med Chem. 2011;54:4659–4669. doi: 10.1021/jm2003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan M, Hayashi T, Mathewson RD, Nour A, Hayashi Y, Yao S, Tawatao RI, Crain B, Tsigelny IF, Kouznetsova VL, Messer K, Pu M, Corr M, Carson DA, Cottam HB. Identification of substituted pyrimido[5,4-b]indoles as selective Toll-like receptor 4 ligands. J Med Chem. 2013;56:4206–4223. doi: 10.1021/jm301694x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slivka P, Shridhar M, Lee G, Sammond D, Hutchinson M, Martinko A, Buchanan M, Sholar P, Kearney J, Harrison J, Watkins L, Yin H. A peptide antagonist of the TLR4.MD-2 interaction. Chembiochem. 2009;10:645–649. doi: 10.1002/cbic.200800769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanmugam A, Rajoria S, George AL, Mittelman A, Suriano R, Tiwari RK. Synthetic Toll like receptor-4 (TLR-4) agonist peptides as a novel class of adjuvants. PLoS One. 2012;7:e30839. doi: 10.1371/journal.pone.0030839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chahal DS, Sivamani RK, Rivkah Isseroff R, Dasu MR. Plant-Based Modulation of Toll-like Receptors: An Emerging Therapeutic Model. Phytother Res. 2013;27:1423–1438. doi: 10.1002/ptr.4886. [DOI] [PubMed] [Google Scholar]
- 41.Park SJ, Youn HS. Suppression of homodimerization of toll-like receptor 4 by isoliquiritigenin. Phytochemistry. 2010;71:1736–1740. doi: 10.1016/j.phytochem.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Ahn SI, Lee JK, Youn HS. Inhibition of homodimerization of toll-like receptor 4 by 6-shogaol. Mol Cells. 2009;27:211–215. doi: 10.1007/s10059-009-0026-y. [DOI] [PubMed] [Google Scholar]
- 43.Kim SY, Koo JE, Seo YJ, Tyagi N, Jeong E, Choi J, Lim KM, Park ZY, Lee JY. Suppression of Toll-like receptor 4 activation by caffeic acid phenethyl ester is mediated by interference of LPS binding to MD-2. Br J Pharmacol. 2013;168:1933–1945. doi: 10.1111/bph.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, Lee JY. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. 2008;75:494–502. doi: 10.1016/j.bcp.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 45.Gradisar H, Keber M, Pristovsek P, Jerala R. MD-2 as the target of curcumin in the inhibition of response to LPS. J Leukoc Biol. 2007;82:968–974. doi: 10.1189/jlb.1206727. [DOI] [PubMed] [Google Scholar]
- 46.Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol. 2006;72:62–69. doi: 10.1016/j.bcp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Park SH, Kyeong MS, Hwang Y, Ryu SY, Han SB, Kim Y. Inhibition of LPS binding to MD-2 co-receptor for suppressing TLR4-mediated expression of inflammatory cytokine by 1-dehydro-10-gingerdione from dietary ginger. Biochem Biophys Res Commun. 2012;419:735–740. doi: 10.1016/j.bbrc.2012.02.091. [DOI] [PubMed] [Google Scholar]
- 48.Koo JE, Park ZY, Kim ND, Lee JY. Sulforaphane inhibits the engagement of LPS with TLR4/MD-2 complex by preferential binding to Cys133 in MD-2. Biochem Biophys Res Commun. 2013;434:600–605. doi: 10.1016/j.bbrc.2013.03.123. [DOI] [PubMed] [Google Scholar]
- 49.Park SJ, Lee MY, Son BS, Youn HS. TBK1-targeted suppression of TRIF-dependent signaling pathway of Toll-like receptors by 6-shogaol, an active component of ginger. Biosci Biotechnol Biochem. 2009;73:1474–1478. doi: 10.1271/bbb.80738. [DOI] [PubMed] [Google Scholar]
- 50.Kim JY, Park SJ, Yun KJ, Cho YW, Park HJ, Lee KT. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages. Eur J Pharmacol. 2008;584:175–184. doi: 10.1016/j.ejphar.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 51.Schröfelbauer B, Raffetseder J, Hauner M, Wolkerstorfer A, Ernst W, Szolar OH. Glycyrrhizin, the main active compound in liquorice, attenuates pro-inflammatory responses by interfering with membrane-dependent receptor signaling. Biochem J. 2009;421:473–482. doi: 10.1042/BJ20082416. [DOI] [PubMed] [Google Scholar]
- 52.Honda H, Nagai Y, Matsunaga T, Saitoh S, Akashi-Takamura S, Hayashi H, Fujii I, Miyake K, Muraguchi A, Takatsu K. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J Leukoc Biol. 2012;91:967–976. doi: 10.1189/jlb.0112038. [DOI] [PubMed] [Google Scholar]
- 53.Natarajan K, Singh S, Burke TR, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mapesa JO, Waldschmitt N, Schmoeller I, Blume C, Hofmann T, Mahungu S, Clavel T, Haller D. Catechols in caffeic acid phenethyl ester are essential for inhibition of TNF-mediated IP-10 expression through NF-κB-dependent but HO-1- and p38-independent mechanisms in mouse intestinal epithelial cells. Mol Nutr Food Res. 2011;55:1850–1861. doi: 10.1002/mnfr.201100105. [DOI] [PubMed] [Google Scholar]
- 55.Youn HS, Lee JY, Saitoh SI, Miyake K, Kang KW, Choi YJ, Hwang DH. Suppression of MyD88- and TRIF-dependent signaling pathways of Toll-like receptor by (−)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem Pharmacol. 2006;72:850–859. doi: 10.1016/j.bcp.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Resman N, Gradisar H, Vasl J, Keber M, Pristovsek P, Jerala R. Taxanes inhibit human TLR4 signaling by binding to MD-2. FEBS Lett. 2008;582:3929–3934. doi: 10.1016/j.febslet.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 57.Zhang D, Li Y, Liu Y, Xiang X, Dong Z. Paclitaxel ameliorates lipopolysaccharide-induced kidney injury by binding myeloid differentiation protein-2 to block Toll-like receptor 4-mediated nuclear factor-κB activation and cytokine production. J Pharmacol Exp Ther. 2013;345:69–75. doi: 10.1124/jpet.112.202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmer SM, Liu J, Clayton JL, Stephens DS, Snyder JP. Paclitaxel binding to human and murine MD-2. J Biol Chem. 2008;283:27916–27926. doi: 10.1074/jbc.M802826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hutchinson MR, Loram LC, Zhang Y, Shridhar M, Rezvani N, Berkelhammer D, Phipps S, Foster PS, Landgraf K, Falke JJ, Rice KC, Maier SF, Yin H, Watkins LR. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience. 2010;168:551–563. doi: 10.1016/j.neuroscience.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis SS, Loram LC, Hutchinson MR, Li CM, Zhang Y, Maier SF, Huang Y, Rice KC, Watkins LR. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J Pain. 2012;13:498–506. doi: 10.1016/j.jpain.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panda SK, Kumar S, Tupperwar NC, Vaidya T, George A, Rath S, Bal V, Ravindran B. Chitohexaose activates macrophages by alternate pathway through TLR4 and blocks endotoxemia. PLoS Pathog. 2012;8:e1002717. doi: 10.1371/journal.ppat.1002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ariza ME, Ramakrishnan R, Singh NP, Chauhan A, Nagarkatti PS, Nagarkatti M. Bryostatin-1, a naturally occurring antineoplastic agent, acts as a Toll-like receptor 4 (TLR-4) ligand and induces unique cytokines and chemokines in dendritic cells. J Biol Chem. 2011;286:24–34. doi: 10.1074/jbc.M110.135921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y, Wang G, Li C, Zhang M, Zhao H, Sheng J, Shi W. Pu-erh Tea Reduces Nitric Oxide Levels in Rats by Inhibiting Inducible Nitric Oxide Synthase Expression through Toll-Like Receptor 4. Int J Mol Sci. 2012;13:7174–7185. doi: 10.3390/ijms13067174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang HL, Lai JT, Lin WC. Inhibitory effect of olive oil on fibrosis induced by carbon tetrachloride in rat liver. Clin Nutr. 2008;27:900–907. doi: 10.1016/j.clnu.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Barochia A, Solomon S, Cui X, Natanson C, Eichacker PQ. Eritoran tetrasodium (E5564) treatment for sepsis: review of preclinical and clinical studies. Expert Opin Drug Metab Toxicol. 2011;7:479–494. doi: 10.1517/17425255.2011.558190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martins PS, Brunialti MK, Martos LS, Machado FR, Assuncao MS, Blecher S, Salomao R. Expression of cell surface receptors and oxidative metabolism modulation in the clinical continuum of sepsis. Crit Care. 2008;12:R25. doi: 10.1186/cc6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]