Abstract
Having excluded common structural, inflammatory and vascular causes of a spastic paraparesis, the diagnostic yield of further clinical investigations is low. Here, we show that testing for rare metabolic and genetic causes can have important implications for both the patient and their family.
INTRODUCTION
Patients with a spastic paraparesis often report non-specific symptoms, and the neurological syndrome may not be obvious from the referral letter. However, a characteristic gait may emerge if a patient can walk through the door, and a systematic neurological examination usually localises the pathology. The likely underlying cause depends on the age at presentation, mode of onset, progression and associated symptoms. The priority is to identify a structural, inflammatory or vascular cause. When the imaging and cerebrospinal fluid (CSF) findings are normal, it is tempting to stop there, reassuring the patient that all treatable causes have been excluded. Here, we describe four women who presented with non-specific symptoms and a spastic paraparesis developing over many years and with normal neuroaxis MRI. Identifying the underlying cause had clear treatment implications for other family members.
Patient 1
A 64-year-old woman presented with increasing stiffness in both legs and difficulty walking. She had always walked ‘duck-toed’, but had noted a progressive decline in her mobility within the last decade with associated urinary urgency and urge incontinence. MRI of her lumbosacral spine led to a diagnosis of degenerative lumbosacral disc disease several years before her referral.
Her daughter had initially been fit and well, while her eldest son and brother both had ‘wobbly knees’. The patient’s younger son suffered from cri du chat syndrome due to a de novo deletion of 5p14; she had characteristic facial features, severe learning difficulties and was a wheelchair user. Cytogenetic analysis was normal in patient 1.
On examination, she had a spastic gait, normal cranial nerves and upper limbs, hypertonia and hyper-reflexia in the lower limbs with extensor plantars. There were no cerebellar signs. Brain and spine MRI was normal, as was CSF examination, with no oligoclonal bands.
Plasma very-long-chain fatty acid (VLCFA) levels showed a high concentration of hexacosanoic acid (C26:0) and high C26:0/C22:00 and C24:0/C22:0 ratios. Subsequent DNA sequencing indicated a heterozygous c1679C>T (p.Pro560Leu) mutation in exon 7 of the ABCD1 gene.
The patient’s daughter had previously presented to another neurology department with a spastic paraparesis; she had been diagnosed as having primary progressive multiple sclerosis. It was only following her mother’s diagnosis that her plasma VLCFA testing showed greatly raised hexacosanoic acid (C26:0) and mildly raised docosanoic acid (C22:0) and tetracosanoic acid (C24:0).
The patient’s two sons consented to a neurological examination and had tetracosactide (Synacthen) tests. The eldest son (Son A) was found to have a spastic paraparesis, and both sons had a degree of adrenal insufficiency (Son A: pre-Synacthen cortisol: 83 nmol/L, 30 min post-Synacthen: 89 nmol/L; Son B: pre-Synacthen cortisol: 241 nmol/L, 30 min post-Synacthen: 263 nmol/L; N.B. cortisol <350 nmol/L measured 30 min after Synacthen indicates a deficiency). Both now receive corticosteroid replacement.
Patient 2
A 54-year-old woman gave a 2-year history of difficulty walking and unsteadiness due to stiffness, sharp shooting pains and spasms in both feet. Twenty years previously she had been involved in a car crash, sustaining a whiplash injury causing neck pain. Two years before presenting, there had been a series of falls.
On examination, she had a spastic gait with normal cranial nerve and upper limb function. There was pyramidal weakness in the lower limbs with hyper-reflexia, bilateral extensor plantars and seven beats of clonus in the right ankle. There were no cerebellar or sensory signs. Brain and spinal cord MRI was normal, as were CSF examination and visual-evoked potentials.
Plasma VLCFA levels showed a moderately elevated C26:0 level and borderline elevated C26:0/C22:0 and C26:0/C24:0 ratios. This test was repeated, and the same results were confirmed. Molecular genetic analysis showed a heterozygous c.1771C>T (p.Arg591Trp) mutation in the ABCD1 gene. She was treated with physiotherapy and baclofen. In the subsequent months, she developed urinary urgency and frequency we referred her to urogynaecology. Adrenal function was preserved. She had no offspring or affected family members.
Patient 3
A 49-year-old woman was referred from primary care with spastic diplegia. She had been investigated for knee pain by orthopaedics 5 years before, and subsequently found her gait becoming increasingly stiff. She described learning difficulties as a child and a tendency for her knees to knock and to catch her toes while walking. Her mother had 13 pregnancies, with 4 miscarriages, 8 healthy offspring and a child with spina bifida who died aged 8 years. The patient described urinary symptoms, including urgency, frequency and occasional incontinence of urine.
On examination, she had a spastic gait and bilaterally increased tone in the lower limbs, clonus of both ankles and bilateral brisk reflexes with no sensory or cerebellar signs. Her cranial nerve and upper limb function were normal. MR scans of brain and spinal cord were normal, as were visual-evoked potentials and thyroid function.
Plasma VLCFA showed a high hexacosanoic acid (C26:0) and high C26:0/C22:00 and C24:0/C22:0 ratios. Genetic testing identified a heterozygous c.742G>C (p. Gly248Arg) mutation in exon 1 of the ABCD1 gene. We offered genetic counselling to her and her family.
Patient 4
A 69-year-old woman with worsening gait required two sticks to walk. She had been diagnosed with an unspecified rheumatological disorder in her late 50s, with progressive deterioration and further leg stiffness. She presented now with limited mobility and urinary urgency and frequency. Genetic testing showed a heterozygous c.761C>T (p.Thr254Met) mutation of the ABCD1 gene. A Synacthen test showed no evidence of adrenal failure. Her condition progressed with further stiffening of the legs, treated with exercise and increasing doses of baclofen. We offered genetic counselling to the family. We assessed her offspring, who had no symptoms and no mutation on molecular genetic testing.
DISCUSSION
We describe four unrelated women with a late-onset progressive spastic paraparesis and heterozygous mutations in the ABCD1 gene. Mutations in ABCD1 can cause X-linked adrenoleukodystrophy presenting in boys or adrenomyeloneuropathy in men and women. The frequency of heterozygous female carriers is estimated at 1 in 14 000, with 20%–50% manifesting with symptoms.1 Due to X-inactivation, female heterozygotes can compensate to some degree by skewed expression of the ABCD1 gene in favour of the normal X chromosome. Affected males have a single X chromosome, and therefore have a single defective copy of the ABCD1 gene. For this reason, female heterozygotes present later with a milder adrenomyeloneuropathy-like phenotype compared with the adrenoleukodystrophy phenotype of severe cerebral and adrenal involvement seen in male counterparts. Clinical features include gradual onset of vague symptoms, including gait abnormalities and urinary symptoms, often leading to a misdiagnosis of multiple sclerosis or hereditary spastic paraparesis. A relevant family history provides a clue, but is often absent; adrenal involvement is rare, and MR brain and spine imaging is frequently normal.
The diagnosis can be made biochemically by measuring plasma VLCFAs and detecting a high concentration of hexacosanoic acid (C26:0), as well as abnormalities between the ratios of C26:0 and tetracosanoic acid (C24:0) to docosanoic acid (C22:0) (see figure 1).1 However, 15% of heterozygous females have equivocal results, as did two out of our five patients who required repeat VLCFA measurements. For this reason, we recommend genetic counselling and molecular testing to confirm the diagnosis in patients and to screen for affected family members. Knowing the genetic diagnosis is also very valuable when counselling other family members because of the risk of severe childhood-onset adrenoleukodystrophy in boys in subsequent generations, which is preventable.
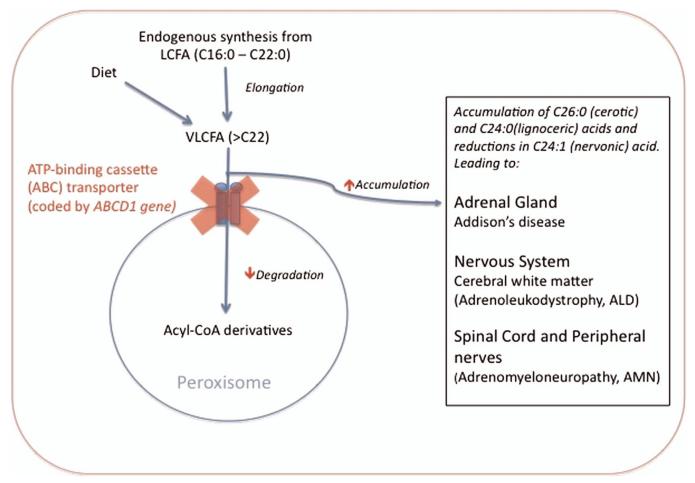
Figure 1. Simplified schematic showing disruption of the β-oxidation of very-long-chain fatty acids (VLCFAs) in adrenoleukodystrophy/adrenomyeloneuropathy.
VLCFAs are produced within all cells by the elongation of long-chain fatty acids (LCFA) and through dietary intake. Following transport of VLCFA via the membrane ATP-binding cassette (ABC) transporter into the peroxisome, the degradation into acyl coenzyme A (acyl CoA) derivatives occurs. Mutations in the ABCD1 gene cause dysfunction of the ABC transporter, resulting in accumulation of VLCFA (>C22 length, particularly C24:0, C24:1 and C26:0) leading to dysfunction within the adrenal gland and nervous system (box). LCFA, long-chain-fatty acid; VLCFA, very-long-chain fatty acid; Acyl-CoA, acyl coenzyme A; ABCD1, ATP-binding cassette domain 1; Cx, fatty acid with x carbon chain length.
The clinical management of adrenomyeloneuropathy is currently limited to supportive treatment, but there are experimental therapies currently under scrutiny, with antioxidant therapies showing the most promising results.2 Short Synacthen testing of adrenal function is crucial to identify adrenal insufficiency, which, although rare in female carriers, can occur in hemizygous male family members. Corticosteroid replacement therapy is required both to treat symptoms and prevent future adrenal crises.
For patients presenting with a spastic paraplegia, it is important not to be satisfied prematurely with a normal MR scan of brain (figure 2) and CSF. The diagnosis of adrenomyeloneuropathy has treatment implications for the patient and their family and should not be missed.
Figure 2. MR scan of spinal cord showing no abnormalities in a woman with adrenomyeloneuropathy.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Commissioned; externally peer reviewed. This paper was reviewed by Angela O’Neal, Boston, USA.
REFERENCES
- 1.Jangouk P, Zackowski KM, Naidu S, et al. Adrenoleukodystrophy in female heterozygotes: underrecognized and undertreated. Mol Genet Metab. 2012;105:180–5. doi: 10.1016/j.ymgme.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Erauskin J, Fourcade S, Galino J, et al. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann Neurol. 2011;70:84–92. doi: 10.1002/ana.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]